Abstract
Background
Current U.S. guidelines recommend the Asthma Control Test (ACT) for assessing disease control and selecting treatment.
Objective
The goal of this study was to prospectively assess the ACT and its component questions for their utility in predicting risk of severe asthma exacerbations.
Methods
Individuals were participants in the Study of Asthma Phenotypes and Pharmacogenomic Interactions by Race-ethnicity (SAPPHIRE), and those included in the current analysis had the following characteristics: age ≥18 years, physician-diagnosed asthma, and longitudinal care received at a large health system in southeastern Michigan. Study participants underwent a baseline evaluation, which included answering the ACT. A severe asthma exacerbation was defined as one requiring oral steroids, an emergency department visit, or inpatient admission. Receiver-operator characteristic curves were used to measure and compare the predictive utility of the ACT and its component questions for severe asthma exacerbations.
Results
Of 1,180 participants, 354 (30.0%) experienced a severe asthma exacerbation within 6 months of their baseline evaluation. When compared with the individual questions that composed the ACT, the composite score was significantly better at predicting severe exacerbations with one exception; the composite ACT score and the question assessing rescue medication use were not significantly different (P=0.580). Pharmacy-based records of metered-dose inhaler short-acting beta-agonist use and asthma severity were also not significantly different from the composite ACT score.
Conclusion
Our study demonstrates that while the ACT is modestly predictive for exacerbations, the composite score may not be superior to assessing rescue medication use alone for predicting risk of severe asthma exacerbations.
Keywords: Asthma Control Test, asthma, short-acting adrenergic beta-2 receptor agonists, bronchodilator agents, asthma exacerbation, asthma rescue medication
INTRODUCTION
Asthma is a chronic disease of the lungs characterized by reversible inflammation of the airways.1 Disease prevalence is rising, affecting 25.7 million people in the United States, and uncontrolled asthma is common, resulting in a high rate of unscheduled office visits, emergency department visits, and hospitalization each year.2, 3
An ideal measure of asthma control must be able to assess both asthma impairment and risk.1 The Asthma Control Test (ACT) is a commonly used self-assessment tool for monitoring asthma control and guiding therapy.4, 5 The ACT has been shown to overlap with other survey instruments in its assessment of control and asthma-related quality of life;6 however, its utility as a predictive tool for asthma exacerbations is less certain. Moreover, since the ACT comprises five separate five-point questions, these component questions may differ in their predictive utility for future asthma exacerbations. As a result, the ACT composite score may be less predictive of asthma exacerbations when compared with its component questions.
The Study of Asthma Phenotypes and Pharmacogenomic Interactions by Race-ethnicity (SAPPHIRE) is a large, prospective and multiethnic study of individuals with asthma.7 All SAPPHIRE participants are asked to complete the ACT at the time of enrollment. By virtue of their health plan membership, SAPPHIRE patients also have detailed prospective information on diagnoses and medication use. Therefore, this is an ideal study population in which to assess longitudinal asthma outcomes after measuring asthma control. The goal of this study was to assess the ACT and its component questions for their ability to predict severe asthma exacerbations.
METHODS
Study setting and patients
This study was approved by the Institutional Review Board of Henry Ford Health System. Written informed consent was required for study enrollment. SAPPHIRE participants had the following characteristics: age 12–56 years, a prior clinical diagnosis of asthma, no prior recorded or reported history of chronic obstructive pulmonary disease and congestive heart disease, and regular outpatient care from a large health system in southeastern Michigan. The participants included in the current analysis also had health care and pharmaceutical coverage through a system-affiliated health provider; this meant that these study individuals had a record of health care utilization, which included insurance claims, encounter diagnoses, prescription fills, and visit types. We restricted the analytic set to adult individuals ≥18 years of age.
At the time of enrollment, participants had a baseline evaluation, which included anthropomorphic measurements, lung function testing, and completion of a study questionnaire. The latter included all of the questions from the ACT (Optum Corp., Eden Prarie, MN), which is 5-question survey including questions about asthma’s impact on daily functioning, frequency of shortness of breath symptoms, frequency of nocturnal asthma symptoms, frequency of rescue inhaler and nebulizer use, and an overall assessment of asthma control. Each question is scored on a 1–5 Likert scale with higher scores denoting better asthma control, such that the resulting composite ACT score ranges from 5–25.4, 5
Exposure and outcome assessment
Short acting beta-agonist (SABA) rescue medication use was measured using pharmacy claims data. National drug codes were recorded with each SABA medication dispensing, and this information was used to estimate the number of doses available for both metered-dose inhaler (MDI) and nebulized SABA prescription fills. We calculated separate measures for MDI and nebulizer usage, as we have previously observed different predictive relationships between the use of these preparations and asthma exacerbations.8 In short, we used the total number of doses dispensed (i.e., the sum of doses in each dispensing) in the 6-month period preceding the baseline assessment to estimate “baseline” SABA MDI and nebulizer use. The baseline number of uses per day was estimated by dividing the total number of doses by 182.5 (i.e., the number of days in half a year).
Prescription claims data and coded diagnoses from clinical visits were used to identify severe asthma exacerbations. A severe asthma exacerbation was defined as the need for burst oral steroids, an emergency department visit for asthma, or an asthma-related hospitalization.
Adapting a method described by Allen-Ramey et al.,9 we also created a baseline asthma severity measure based on medication fills in the year prior to the initial assessment. The most severe group (severity=4) had either ≥3 oral corticosteroid (OCS) fills or 2 OCS fills and >6 SABA fills. The moderate to severe group (severity=3) had either 2 OCS fills or >6 SABA fills or 1 OCS fill and ≥4 SABA fills. The low severity group (severity=1) had no OCS fills and ≤1 SABA fill. All other combinations of SABA and OCS fills made up the low to moderate severity group (severity=2).
Statistical Analysis
We created receiver operating characteristic (ROC) curves for the ACT composite score and each of its component questions for their sensitivity and specificity for a severe asthma exacerbation within the 3 and 6 months following assessment. For each ACT question and the composite score we calculated areas under the curve, which were used to compare the predictive utility for each measure. The primary analysis compared the composite ACT score with each of the ACT component questions for predicting severe asthma exacerbation within 6 months of assessment. Secondary analyses included comparing the ACT composite score with each of the component questions for their ability to predict asthma exacerbations within 3 months of assessment. In other secondary analyses, we performed subgroup analyses within strata defined by sex, race-ethnicity, and degree of ICS use. We also compared the composite ACT score with measures of pharmacy claims-based measures of asthma rescue medication (i.e., both MDI and nebulizer preparations) and to the claims-based measure of asthma severity, as described above and elsewhere.9 For each ROC curve, we calculated the area under the curve (AUC) using the trapezoidal rule; we then assessed differences in the predictive properties of each measure by comparing areas via the non-parametric Mann-Whitley statistic as describe by DeLong et al.10 We used the software package, SAS (SAS Institute, Cary, NC), to analyze the data.11 A p-value <0.05 was considered to be statistically significant for the primary analysis. Since the purpose of the secondary analyses was to assess for consistency rather than statistical significance, there was no type-I error or p-value threshold for these analyses.
RESULTS
This analysis comprised 1,180 individuals enrolled in the SAPPHIRE cohort. The characteristics of those individuals are shown in Table 1. The average age of the participants was 39.5 years (±13.4 years, standard deviation [SD]). Of the 1,180 individuals, 846 (71.7%) were female, 632 (53.6%) reported being African American, and 475 (40.3%) were European American. The mean composite ACT score taken at the time of enrollment (i.e., baseline) was 20.7 (±4.0 SD), and 373 (31.6%) has a baseline ACT score ≤19, suggesting that asthma was not controlled in this latter group. A majority (67.1%) of patients were using an inhaled corticosteroid around the time of their initial visit, as evidenced by a medication possession ratio >0 (a measure of the proportion of days’ supply of ICS in the preceding 6 months). In the 6 months following the initial visit, 354 (30.0%) of the 1,180 participants experienced a severe asthma exacerbation requiring the use of burst systemic corticosteroids, an emergency department visit, or hospitalization.
Table 1.
Characteristics of study participants with 6 months of follow-up (n=1,180)
| Characteristic | |
|---|---|
| Age (years) – mean ± SD | 39.5 ± 13.4 |
| Female sex – no. (%) | 846 (71.7) |
| Race-ethnicity – no (%) | |
| African American | 632 (53.6) |
| European American | 475 (40.3) |
| Other | 73 (6.2) |
| ACT composite score – mean ± SD* | 20.7 ± 4.0 |
| Work domain – mean ± SD† | 4.44 ± 0.86 |
| Shortness of breath domain – mean ± SD† | 4.02 ± 1.07 |
| Symptom frequency domain – mean ± SD† | 4.32 ± 1.13 |
| Rescue medication use domain – mean ± SD† | 3.92 ± 1.18 |
| Self-assessed control domain – mean ± SD† | 3.97 ± 0.90 |
| ACT composite score ≤19 – no (%)* | 373 (31.6) |
| FEV1 percent of predicted – mean ± SD‡ | 88.8 ± 17.4 |
| Inhaled corticosteroid use – no (%)§ | 792 (67.1) |
| Possession ratio = 0 | 388 (32.9) |
| Possession ratio >0 and <0.75 | 604 (51.2) |
| Possession ratio ≥0.75 | 188 (15.8) |
| Asthma severity score – no (%)|| | |
| 1 | 742 (62.8) |
| 2 | 304 (25.8) |
| 3 | 95 (8.1) |
| 4 | 39 (3.3) |
ACT denotes asthma control test; SD, standard deviation, and FEV1, forced expiratory volume in 1 second.
The ACT composite score represents the sum total of its 5 questions, which are each scored using a 5-point Likert Scale. Therefore, the possible composite score ranges from 5–25, where ≤19 is considered not controlled.
Each domain represents a separate question from the ACT. Questions are scored using a 5-point Likert Scale.
Percent of predicted FEV1 values were calculated using the FEV1 values measured from the participants and the predicted values based on age, sex, and race-ethnicity from Hankinson et al.
The inhaled corticosteroid possession ratio was calculated as the total days’ supply of inhaled corticosteroids in the 6-month period following completion of the ACT divided by180 days (i.e., 6 months).
The severity score was based on the methods described by Allen-Ramey et al.9 An individual’s baseline severity (range 1–4) was based on oral corticosteroid fills and SABA fills in the year prior to the initial ACT assessment; higher scores denote more severe asthma.
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the dichotomized, baseline ACT score (i.e., ≤19 [not controlled] vs. ≥20 [controlled]) for predicting a severe asthma exacerbation in the subsequent 6-month period was 42.7%, 73.1%, 40.5%, and 74.8%, respectively. The analogous sensitivity, specificity, PPV, and NPV for predicting a severe exacerbation in the subsequent 3 months was 48.3%, 72.4%, 42.9%, and 76.6%, respectively.
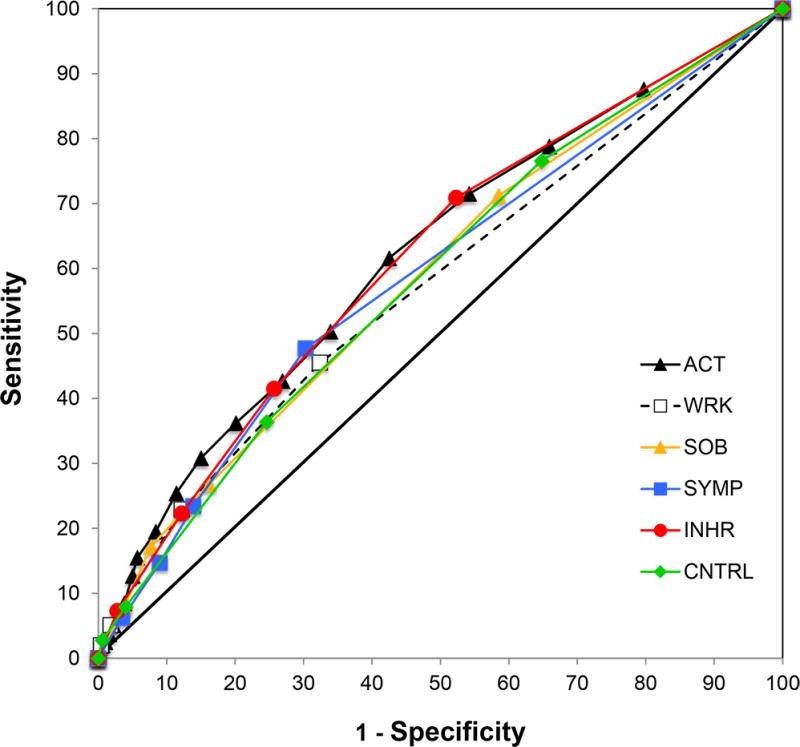
To assess and compare the predictive properties of the ACT score for severe exacerbations, we constructed ROC curves for the composite ACT score and each of its 5-point questions (Figure 1). We used the AUC to compare the predictive ability of each (Table 2). The AUC for the composite ACT score was 0.621. The five component questions of the ACT assessed the effect of asthma over the preceding 4-week period on each of the following domains: 1) limitations at work, school, or home, 2) frequency of shortness of breath, 3) frequency of nocturnal symptoms, 4) use of rescue inhaler or nebulizer medication, and 5) patient-assessed asthma control. The AUCs for these domains were 0.576, 0.587, 0.589, 0.615, and 0.586, respectively. A domain AUC less than the AUC for composite ACT score can be interpreted as being significantly less predictive if the accompanying P-value is <0.05. Therefore, all of the domains performed significantly worse than the composite ACT score in predicting asthma exacerbations with the exception of the question assessing the frequency of rescue inhaler and/or nebulizer use.
Figure 1.
Receiver operator curves for the composite ACT score and its five questions in predicting severe asthma exacerbations in the ensuing 6 months. Performance of the composite ACT (ACT) is show by the solid black line; limitations at work, school, or home (WRK) by the dashed black line; shortness of breath (SOB) by the yellow line; nocturnal symptom frequency (SYMP) by the blue line; rescue inhaler and nebulizer use (INHR) by the red line, and patient-assessed asthma control (CNTRL) by the green line.
Table 2.
Comparison of receiver operator curve performance for the Asthma Control Test and its domains in predicting subsequent asthma exacerbations within 6 months (n=1,180)*
| Strata | Events/total no. | ACT composite score (referent) | Work domain† | Shortness of breath domain† | Symptom frequency domain† | Rescue medication use domain† | Self-assessed control domain† | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC | AUC | P-value | AUC | P-value | AUC | P-value | AUC | P-value | AUC | P-value | ||
| All | 354/1,180 | 0.621 | 0.576 | 0.001 | 0.587 | 0.001 | 0.589 | 0.017 | 0.615 | 0.580 | 0.586 | 0.001 |
| Female | 268/846 | 0.617 | 0.577 | 0.008 | 0.579 | 0.003 | 0.590 | 0.080 | 0.610 | 0.566 | 0.587 | 0.013 |
| Male | 86/334 | 0.624 | 0.558 | 0.029 | 0.597 | 0.205 | 0.580 | 0.128 | 0.623 | 0.965 | 0.577 | 0.036 |
| African Americans | 183/632 | 0.620 | 0.571 | 0.010 | 0.580 | 0.012 | 0.597 | 0.201 | 0.630 | 0.422 | 0.574 | 0.002 |
| European Americans | 145/475 | 0.627 | 0.554 | 0.001 | 0.588 | 0.018 | 0.586 | 0.071 | 0.609 | 0.332 | 0.606 | 0.198 |
| ICS possession ratio = 0‡ | 40/388 | 0.521 | 0.548 | 0.714 | 0.539 | 0.502 | 0.535 | 0.736 | 0.540 | 0.560 | 0.480 | 0.212 |
| ICS possession ratio >0 and <0.75‡ | 182/604 | 0.633 | 0.601 | 0.089 | 0.593 | 0.010 | 0.615 | 0.337 | 0.616 | 0.251 | 0.585 | 0.001 |
| ICS possession ratio ≥ 0.75‡ | 132/188 | 0.613 | 0.577 | 0.277 | 0.581 | 0.243 | 0.559 | 0.064 | 0.596 | 0.540 | 0.596 | 0.565 |
ACT denotes Asthma Control Test; AUC, area under the curve; and ICS, inhaled corticosteroids.
Asthma exacerbations were defined as the need for oral steroid burst treatment, asthma-related emergency department visit, or asthma-related hospitalization in the 6 months following ACT assessment.
Each domain represents one of the 5 questions in the ACT test. P-values for each domain represent the comparison with the ACT composite score in predicting future asthma exacerbations. Domain AUC values that are lower than the ACT composite score AUC and are statistically significant (P-value<0.05) signify that the domain alone performs significantly worse than the composite score in predicting asthma exacerbations.
The possession ratio represents the proportion of days in the 6-month period following ACT administration that the individuals had a supply of ICS (i.e., based on the total days’ supply of ICS provided by prescription fills in the 6-month period).
To better understand the predictive ability of the ACT score and its domains we performed subgroup analyses by sex, race-ethnicity, and level of concurrent ICS use (i.e., the possession ratio). Although the performance of the ACT and its domains varied by stratum, the domain representing rescue inhaler/nebulizer use was the only domain consistently similar in its predictive ability for asthma exacerbations when compared with the composite ACT score (i.e., never statistically different). Using the cut-point of greater than 2–3 uses of rescue inhaler/nebulizer medication per week vs. once a week or fewer times per week (over the preceding 4-weeks) had a sensitivity, specificity, PPV, and NPV for an asthma exacerbation in the ensuing 6 months of 44.4%, 73.3%, 41.6%, and 75.4%, respectively (data not shown).
To explore the relationship between asthma exacerbations and both rescue inhaler and nebulizer use, we used pharmacy records of medication fills to estimate the total number of SABA MDI and SABA nebulizer doses received in the 6-month period before participants competed the ACT (i.e., baseline SABA MDI and baseline SABA nebulizer use, respectively). The predictive utility of these metrics as compared with the baseline ACT for severe exacerbations over a 6-month period is shown in Table 3. With one exception, SABA MDI use was not significantly different from the composite ACT score in predicting severe asthma exacerbations within 6 months. The exception was the group with an ICS possession ratio >0 and <0.75. Based on pharmacy claims, 1.10 SABA MDI uses per day (i.e., 200 doses dispensed over a 6-month period divided by 182.5 days) were associated with the highest combination of sensitivity and specificity – 44.1% and 71.8%, respectively (data not shown).
Table 3.
Receiver operator curve performance for the Asthma Control Test as compared with measured short-acting beta-agonist use and asthma severity score in predicting asthma exacerbations within 6 months (n=1,180)*
| Strata | ACT composite score (referent) | Baseline SABA MDI use† | Baseline SABA nebulizer use† | Baseline asthma severity measure‡ | |||
|---|---|---|---|---|---|---|---|
| AUC | AUC | P-value§ | AUC | P-value§ | AUC | P-value§ | |
| All | 0.621 | 0.585 | 0.077 | 0.521 | 0.001 | 0.647 | 0.224 |
| Female | 0.617 | 0.581 | 0.144 | 0.524 | 0.001 | 0.644 | 0.266 |
| Male | 0.624 | 0.589 | 0.355 | 0.511 | 0.002 | 0.650 | 0.516 |
| African Americans | 0.620 | 0.575 | 0.102 | 0.519 | 0.001 | 0.623 | 0.916 |
| European Americans | 0.627 | 0.611 | 0.620 | 0.518 | 0.001 | 0.681 | 0.095 |
| ICS possession ratio = 0|| | 0.521 | 0.515 | 0.915 | 0.504 | 0.730 | 0.532 | 0.864 |
| ICS possession ratio >0 and <0.75|| | 0.633 | 0.542 | 0.002 | 0.513 | 0.001 | 0.592 | 0.170 |
| ICS possession ratio ≥ 0.75|| | 0.613 | 0.541 | 0.355 | 0.501 | 0.042 | 0.598 | 0.779 |
ACT denotes Asthma Control Test; AUC, area under the curve; SABA, short-acting beta-agonist; MDI, metered dose inhaler; and ICS, inhaled corticosteroids.
Asthma exacerbations were defined as the need for oral steroid burst treatment, asthma-related emergency department visit, or asthma-related hospitalization in the 6 months following ACT assessment.
Baseline metered dose inhaler and nebulizer use represents the total doses/puffs of each short-acting beta-agonist preparation dispensed in the 6 months prior to initial ACT assessment.
Based on the methods described by Allen-Ramey et al.9 An individual’s baseline severity was based on oral corticosteroid fills and SABA fills in the year prior to the initial ACT assessment.
P-value for the comparison with the composite ACT score.
The possession ratio represents the proportion of days in the 6-month period following ACT administration that the individuals had a supply of ICS (i.e., based on the total days’ supply of ICS provided by prescription fills in the 6-month period).
As a post hoc analysis, we repeated the above analyses using a 3-month follow-up window to assess severe asthma exacerbation, SABA MDI use, and SABA nebulizer use (see supplemental materials Figure E1, Table E1, and Table E2). In these analyses, the performance of the ACT question assessing frequency of rescue inhaler and/or nebulizer use was again consistently not significantly different from the AUC of the composite ACT score in predicting severe asthma exacerbations within 3 months (Table E1). However, the claims-based measures of SABA medication use tended to be significantly worse when compared with the composite ACT score in predicting severe asthma exacerbations at 3 months (Table E2). We also assessed another pharmacy claims-based measure of asthma severity developed by others9 and used previously by us.12 This severity measure, which used fills for oral corticosteroids and SABA medication in the year prior to ACT assessment, was not statistically different from the composite ACT score in predicting asthma exacerbations (Table 3 [exacerbations within 6 months] and Table E2 [exacerbations within 3 months]). A severity score of ≥2 was associated with the highest combination of sensitivity and specificity – 55% and 71%, respectively (data not shown). This implied that any OCS fill or ≥2 SABA fills (i.e., either MDI or nebulizer) in the preceding year could be used to identify an increased risk for an asthma exacerbation with a similar predictive ability as the composite ACT score.
Lastly, we repeated our assessment of composite ACT score using a cut-point of <16 vs. ≥16. The sensitivity, specificity, PPV, and NPV for an asthma exacerbation in the ensuing 6 months was 19.5%, 91.7%, 50.0%, and 72%, respectively, demonstrating the tradeoff between sensitivity and specificity when using a more stringent threshold for asthma control. For exacerbations within 3 months of assessment, the composite ACT score with a cut-point of 16 had a sensitivity, specificity, PPV, and NPV of 21.6%, 90.3%, 34.3%, and 83.1%, respectively.
DISCUSSION
Ostensibly, assessing asthma control has two primary aims – to reduce impairment and to mitigate risk. Current U.S. national guidelines include multiple self-assessment tools for patients ≥12 years of age to use in order to monitor their asthma control; the ACT is among these options.1 Both the component questions of the ACT and the composite cut-point score used for assigning disease status were initially selected based on their ability to discriminate “control” as determined by asthma specialists.4 Subsequent studies have found the composite ACT score to be related to the following: specialist assessments of control over time,5 specialist treatment decisions,13 other questionnaire-based measures of asthma control,5, 14 and guideline definitions of control.14, 15 However, Melosini et al. found that while the ACT score was associated with rescue medication use, diary-recorded symptom scores, and peak expiratory flow variability, the score was not associated with other markers of airway inflammation, such as bronchodilator responsiveness, sputum neutrophils or eosinophils, or exhaled nitric oxide.16
Our study found the composite ACT score to be only modestly sensitive and specific (42.7% and 73.1%, respectively) for predicting asthma exacerbations out to 6 months. The score was not substantially better at predicting more proximal exacerbations (i.e., sensitivity and specificity for a severe event within 3 months was 48.3% and 72.4%, respectively). Moreover, in none of the main or subgroup analyses did we find the composite ACT score to be superior to its rescue medication question (i.e., rescue medication use domain) in predicting subsequent asthma exacerbations. These findings were arguably consistent with our assessment of rescue medication use based on pharmacy prescription fill data. SABA MDI use was not significantly different from the composite ACT score in predicting severe asthma exacerbations within 6 months, but the former was significantly worse at predicting asthma exacerbations within 3 months. This may reflect the time course over which SABA MDI use was measured (i.e., 6 months preceding the baseline ACT assessment), which may be useful in identifying an overall increased risk but is less effective in alerting to impending events. In contrast, a claims-based measure of asthma “severity” which employed both SABA and OCS rescue medication fills in the preceding year performed similarly to the composite ACT score in predicting future severe asthma exacerbations. Together, our findings suggest that survey- or claims-based measures of rescue medication use may be comparable to more comprehensive questionnaire assessments of asthma control when predicting risk of severe exacerbations.
In a retrospective study of 78 individuals with asthma, Sato et al. used classification and regression tree (CART) modeling to identify the combined ACT and lung function cut-points to best discriminate whether patients had an asthma exacerbation within a 12-month period.17 An ACT score threshold of ≤23 demonstrated an AUC of 0.613, a sensitivity of 50%, and a specificity of 0.73, which is similar to what we observed. Interestingly, also using a percent of predicted forced expiratory volume at 1 second (FEV1) resulted in an AUC of 0.678, a sensitivity of 44%, and a specificity of 92%. Adding exhaled nitric oxide did not improve the predictive performance of the combined measures. Schatz et al. examined the relationship between the ACT composite score and both asthma exacerbations and SABA use in the 6 months following assessment of asthma control among 2,244 patients in the San Diego Kaiser Permanente asthma database.18 The investigators found the ACT composite score to be significantly inversely associated with both severe exacerbations (i.e., oral corticosteroid dispensing, asthma-related emergency department visit, and asthma-related hospitalization) and SABA dispensing (i.e., dichotomously defined as ≤6 and >6 SABA prescription fills over 6 months). The study did not report the test performance (i.e., AUC, sensitivity, and specificity) of the ACT for these outcomes. In a prospective study of 379 ethnically Chinese individuals from Hong Kong, Ko et al. found that an ACT composite score threshold of ≤19, had an AUC, sensitivity, specificity, PPV, and NPV of 0.71, 74%, 67%, 55%, and 82%, respectively, for asthma exacerbations within 6 months.19 Differences from our study, particularly with regard to ACT sensitivity, may be due in part to their use of patient-reported outcomes and defining asthma exacerbations broadly (i.e., including unscheduled outpatient visits in their exacerbation definition). In contrast, we used asthma events documented in the medical record and employed a consensus definition of severe asthma exacerbations espoused by the American Thoracic Society and European Respiratory Society.20 Another recent study of the ACT in a Chinese cohort demonstrated a higher sensitivity and specificity for predicting asthma exacerbations than that reported here; however, this study also employed a broader definition of asthma exacerbations and relied on patient-reported events.21
Interestingly, the above study by Ko and colleagues also found that neither lung function measures nor quantified exhaled nitric oxide (FeNO) improved upon the predictive ability of the composite ACT score (in terms of AUC) for asthma exacerbations.19 Existing literature suggests that biomarkers, such as FeNO and sputum eosinophils,22, 23 spirometry,24, 25 and other questionnaire assessments of asthma control can be predictive of future asthma exacerbations.26, 27 The predictive utility of risk factors associated with asthma exacerbations may also be influenced by concurrent controller medication use.26 Therefore, additional research is needed to determine whether risk factors and mediators can be combined in a manner that complements their ability to predict exacerbations.
This study had a number of limitations that bear mentioning. First, our data represented the experience of patients at a single, albeit large, health system serving southeast Michigan. While our results may not be generalizable to other patient populations, our study population did reflect the sociodemographic diversity of the Detroit metropolitan area, and we were able to demonstrate similar results when compared with studies performed elsewhere.17 One clear benefit of our patient population and their accompanying health care information was that we had prescription and clinical data (via claims) from both within and outside of our health system. As a result, we were unlikely to miss asthma exacerbations and medication use in our study population.28 A second limitation was that we did not interrogate other survey-based metrics of asthma control. While studies suggest that there is considerable overlap in the content being measured,6, 14 it is possible that these other metrics have different predictive abilities with regard to asthma exacerbations. Despite the large size of the current study, we had limited power to perform subgroup analyses in some groups of interest, such as those with particularly severe asthma. This may be tantamount to studying the predictive properties of the ACT within groups of individuals who at baseline have low composite scores; future work will be needed to examine these subgroups. Lastly, we did not attempt to measure the joint predictive effect of the ACT with other risk factors for asthma attacks. As the primary goal of this analysis was to evaluate the properties of a single well-known metric of asthma control and its component parts, we did not examine whether other variables enhanced the predictive sensitivity or specificity of the ACT. Given the ACT score’s modest performance in predicting exacerbations, this is an obvious additional opportunity for future research.
An undeniable strength of the ACT is its ability to be administered, scored, and interpreted rapidly and inexpensively in the clinic. Our study suggests that for the purposes of predicting future severe asthma exacerbations this already brief questionnaire could be shortened to a single question about rescue medication use without compromising its utility. However, the predictive performance of both the ACT and its rescue medication use domain for asthma exacerbations is modest at best. This in no doubt reflects the difficulties faced when trying to summarize asthma control, since the definition in many ways needs to be tailored to the objective.20 In other words, categorizing control with respect to current functional limitations may differ from defining control with an objective to minimize future asthma attacks. With respect to the latter objective, genetics,29–31 physiologic measures (e.g., lung function),17 and/or biomarkers32–35 may augment what can be gleaned from patient surveys. The importance of the current study may not be in the time saved by shortening exacerbation risk assessment to a single question of rescue medication use but in identifying the important factor to assess in conjunction with other markers of exacerbation risk. The challenge will be in finding additional, independently predictive risk measures that are as accessible as the ACT score is in routine clinical practice.
Supplementary Material
Highlights Box.
1. What is already known about this topic?
The Asthma Control Test (ACT) was initially validated against specialist assessment of control and is a widely used questionnaire for assessing impairment. However, its utility in predicting risk of asthma exacerbation is not well studied.
2.What does this article add to our knowledge?
This study assessed both the ACT and its component questions in a well-characterized, longitudinal cohort study. The ACT and assessments of short-acting beta-agonist use appeared to be comparable in predicting future exacerbations.
3. How does this study impact current management guidelines?
Better tools are needed for determining risk of severe asthma exacerbations. However, for the purposes of rapidly assessing risk clinically, inquiring about rescue medication use alone may be as good as the composite ACT score.
Acknowledgments
This work was supported by grants from the American Asthma Foundation, the Fund for Henry Ford Hospital, and the National Institute of Allergy and Infectious Diseases (R01AI079139) and the National Heart Lung and Blood Institute (R01HL118267 and R01HL079055), National Institutes of Health.
The authors would thank the following organizations which funded this work: the American Asthma Foundation, the Fund for Henry Ford Hospital, and the National Institute of Allergy and Infectious Diseases (R01AI079139) and the National Heart Lung and Blood Institute (R01HL118267 and R01HL079055), National Institutes of Health. We would also like to thank all of the staff members and patients who have participated in the SAPPHIRE cohort.
Abbreviations
- ACT
Asthma Control Test
- AUC
area under curve
- ICS
inhaled corticosteroid
- MDI
metered dose inhaler
- OCS
oral corticosteroid
- ROC
receiver operating characteristic
- SABA
short-acting beta-agonist
- SAPPHIRE
Study of Asthma Phenotypes and Pharmacogenomic Interactions by Race ethnicity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 3.Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, Johnson CA, et al. National Surveillance of Asthma: United States, 2001–2010. Vital and Health Statistics. 2012:3. [PubMed] [Google Scholar]
- 4.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117:549–56. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Schatz M, Mosen DM, Kosinski M, Vollmer WM, Magid DJ, O’Connor E, et al. The relationship between asthma-specific quality of life and asthma control. J Asthma. 2007;44:391–5. doi: 10.1080/02770900701364296. [DOI] [PubMed] [Google Scholar]
- 7.Levin AM, Wang Y, Wells KE, Padhukasahasram B, Yang JJ, Burchard EG, et al. Nocturnal asthma and the importance of race/ethnicity and genetic ancestry. Am J Respir Crit Care Med. 2014;190:266–73. doi: 10.1164/rccm.201402-0204OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paris J, Peterson EL, Wells K, Pladevall M, Burchard EG, Choudhry S, et al. Relationship between recent short-acting beta-agonist use and subsequent asthma exacerbations. Ann Allergy Asthma Immunol. 2008;101:482–7. doi: 10.1016/S1081-1206(10)60286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen-Ramey FC, Bukstein D, Luskin A, Sajjan SG, Markson LE. Administrative claims analysis of asthma-related health care utilization for patients who received inhaled corticosteroids with either montelukast or salmeterol as combination therapy. J Manag Care Pharm. 2006;12:310–21. doi: 10.18553/jmcp.2006.12.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 11.SAS/STAT Users Guide. Cary, NC: SAS Institute Inc; 2008. Version 9.2 ed. [Google Scholar]
- 12.Wells KE, Peterson EL, Ahmedani BK, Severson RK, Gleason-Comstock J, Williams LK. The relationship between combination inhaled corticosteroid and long-acting beta-agonist use and severe asthma exacerbations in a diverse population. J Allergy Clin Immunol. 2012;129:1274–9. doi: 10.1016/j.jaci.2011.12.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ko FW, Leung TF, Hui DS, Chu HY, Wong GW, Wong E, et al. Asthma Control Test correlates well with the treatment decisions made by asthma specialists. Respirology. 2009;14:559–66. doi: 10.1111/j.1440-1843.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- 14.Jia CE, Zhang HP, Lv Y, Liang R, Jiang YQ, Powell H, et al. The Asthma Control Test and Asthma Control Questionnaire for assessing asthma control: Systematic review and meta-analysis. J Allergy Clin Immunol. 2013;131:695–703. doi: 10.1016/j.jaci.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Thomas M, Kay S, Pike J, Williams A, Rosenzweig JR, Hillyer EV, et al. The Asthma Control Test (ACT) as a predictor of GINA guideline-defined asthma control: analysis of a multinational cross-sectional survey. Prim Care Respir J. 2009;18:41–9. doi: 10.4104/pcrj.2009.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melosini L, Dente FL, Bacci E, Bartoli ML, Cianchetti S, Costa F, et al. Asthma control test (ACT): comparison with clinical, functional, and biological markers of asthma control. J Asthma. 2012;49:317–23. doi: 10.3109/02770903.2012.661008. [DOI] [PubMed] [Google Scholar]
- 17.Sato R, Tomita K, Sano H, Ichihashi H, Yamagata S, Sano A, et al. The strategy for predicting future exacerbation of asthma using a combination of the Asthma Control Test and lung function test. J Asthma. 2009;46:677–82. doi: 10.1080/02770900902972160. [DOI] [PubMed] [Google Scholar]
- 18.Schatz M, Zeiger RS, Drane A, Harden K, Cibildak A, Oosterman JE, et al. Reliability and predictive validity of the Asthma Control Test administered by telephone calls using speech recognition technology. J Allergy Clin Immunol. 2007;119:336–43. doi: 10.1016/j.jaci.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 19.Ko FW, Hui DS, Leung TF, Chu HY, Wong GW, Tung AH, et al. Evaluation of the asthma control test: a reliable determinant of disease stability and a predictor of future exacerbations. Respirology. 2012;17:370–8. doi: 10.1111/j.1440-1843.2011.02105.x. [DOI] [PubMed] [Google Scholar]
- 20.Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 21.Wei HH, Zhou T, Wang L, Zhang HP, Fu JJ, Wang L, et al. Current asthma control predicts future risk of asthma exacerbation: a 12-month prospective cohort study. Chin Med J (Engl) 2012;125:2986–93. [PubMed] [Google Scholar]
- 22.Harkins MS, Fiato KL, Iwamoto GK. Exhaled nitric oxide predicts asthma exacerbation. J Asthma. 2004;41:471–6. doi: 10.1081/jas-120033990. [DOI] [PubMed] [Google Scholar]
- 23.Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360:1715–21. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 24.Kitch BT, Paltiel AD, Kuntz KM, Dockery DW, Schouten JP, Weiss ST, et al. A single measure of FEV1 is associated with risk of asthma attacks in long-term follow-up. Chest. 2004;126:1875–82. doi: 10.1378/chest.126.6.1875. [DOI] [PubMed] [Google Scholar]
- 25.Gelb AF, Flynn Taylor C, Shinar CM, Gutierrez C, Zamel N. Role of spirometry and exhaled nitric oxide to predict exacerbations in treated asthmatics. Chest. 2006;129:1492–9. doi: 10.1378/chest.129.6.1492. [DOI] [PubMed] [Google Scholar]
- 26.Bateman ED, Buhl R, O’Byrne PM, Humbert M, Reddel HK, Sears MR, et al. Development and validation of a novel risk score for asthma exacerbations: The risk score for exacerbations. J Allergy Clin Immunol. 2015;135:1457–64. e4. doi: 10.1016/j.jaci.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Zeiger RS, Yegin A, Simons FE, Haselkorn T, Rasouliyan L, Szefler SJ, et al. Evaluation of the National Heart, Lung, and Blood Institute guidelines impairment domain for classifying asthma control and predicting asthma exacerbations. Ann Allergy Asthma Immunol. 2012;108:81–7. doi: 10.1016/j.anai.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Williams LK, Joseph CL, Peterson EL, Wells K, Wang M, Chowdhry VK, et al. Patients with asthma who do not fill their inhaled corticosteroids: a study of primary nonadherence. J Allergy Clin Immunol. 2007;120:1153–9. doi: 10.1016/j.jaci.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 29.Rumpel JA, Ahmedani BK, Peterson EL, Wells KE, Yang M, Levin AM, et al. Genetic ancestry and its association with asthma exacerbations among African American subjects with asthma. J Allergy Clin Immunol. 2012;130:1302–6. doi: 10.1016/j.jaci.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tantisira KG, Lasky-Su J, Harada M, Murphy A, Litonjua AA, Himes BE, et al. Genomewide association between GLCCI1 and response to glucocorticoid therapy in asthma. N Engl J Med. 2011;365:1173–83. doi: 10.1056/NEJMoa0911353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–95. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357:2016–27. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 33.Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012;130:647–54. e10. doi: 10.1016/j.jaci.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gauvreau GM, O’Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370:2102–10. doi: 10.1056/NEJMoa1402895. [DOI] [PubMed] [Google Scholar]
- 35.Krug N, Hohlfeld JM, Kirsten AM, Kornmann O, Beeh KM, Kappeler D, et al. Allergen-induced asthmatic responses modified by a GATA3-specific DNAzyme. N Engl J Med. 2015;372:1987–95. doi: 10.1056/NEJMoa1411776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.