Abstract
Vitamin D plays a central role in calcium homeostasis; however, its relationship with bone turnover during pregnancy remains unclear due to a lack of studies that have rigorously controlled for other nutrients known to influence bone metabolism. Similarly, prior investigations of the effect of pregnancy on bone turnover relative to the nonpregnant state may have been confounded by varying intakes of these nutrients. Nested within a controlled intake study, the present investigation sought to quantify associations between maternal vitamin D biomarkers and biochemical markers of bone turnover among pregnant (versus nonpregnant) women and their fetuses under conditions of equivalent and adequate intakes of vitamin D and related nutrients. Changes in markers of bone turnover across the third trimester were also examined. Healthy pregnant (26–29 wk gestation; n=26) and nonpregnant (n=21) women consumed 511IU vitamin D/d, 1.6g calcium/d, and 1.9g phosphorus/d for 10 weeks while participating in a controlled feeding study featuring two choline doses. Based on linear mixed models adjusted for influential covariates (e.g., BMI, ethnicity, and season), pregnant women had 50–150% higher (P<0.001) concentrations of bone resorption markers than nonpregnant women. Among pregnant women, increases in maternal 25(OH)D across the study period were associated (P<0.020) with lower osteocalcin and deoxypyridinoline at study-end, and higher fetal osteocalcin. In addition, maternal free 25(OH)D, 1,25(OH)2D, and 24,25(OH)2D tended to be negatively associated (P≤0.063) with maternal NTx at study-end, and maternal free 25(OH)D and 24,25(OH)2D were positively associated (P≤0.021) with fetal CTx. Similarly, maternal 3-epi-25(OH)D3 was negatively related (P≤0.037) to maternal NTx and deoxypyridinoline at study-end. These declines in bone resorption markers resulting from higher vitamin D biomarker concentrations among pregnant women coincided with increases in their albumin-corrected serum calcium concentrations, indicating that calcium transfer to the fetus was uncompromised. Notably, none of these associations achieved statistical significance among nonpregnant women. Overall, our study findings suggest that achieving higher maternal concentrations of vitamin D biomarkers might attenuate third-trimester bone resorption while ensuring sufficient calcium delivery to the fetus.
Keywords: Vitamin D, 25-hydroxyvitamin D, Bone turnover, Bone metabolism, Calcium homeostasis, Pregnancy
INTRODUCTION
Pregnancy is characterized by a high maternal demand for calcium secondary to the laying down of bone in the fetus. This demand for calcium is mostly met by enhanced intestinal absorption of calcium which has been shown to double during pregnancy [1]. Increased calcium mobilization from the bone is another mechanism to ensure adequate calcium to the developing fetus. However, maternal bone loss may ensue, particularly when the dominant adaptation of enhanced maternal intestinal calcium absorption does not fully meet calcium demands [2].
Calcium homeostasis is regulated by vitamin D which influences intestinal calcium absorption, bone resorption and renal calcium absorption in a normal nonpregnant state. In pregnancy, a 2–3 times increase in 1,25-dihydroxyvitamin D [1,25(OH)2D] concentrations contributes, in part, to the doubling of intestinal calcium absorption [2]. However, whether vitamin D plays a critical role in calcium mobilization from the bone during gestation is unclear. Animal studies have reported normal calcium homeostasis in fetuses from mothers that were either severely depleted in vitamin D [3,4], or deficient in the vitamin D receptor (VDR) [5,6], suggesting that fetal bone mineralization is independent of maternal vitamin D [7]. In humans, data are mixed with some, but not all [8–10] studies, reporting an impact of maternal vitamin D status (25-hydroxyvitamin D [25(OH)D]) on fetal bone development [11–15] and maternal bone turnover [16,17]. These differences across studies may be secondary to diverse maternal intakes of calcium and phosphorus from food and supplements, which like vitamin D, play essential roles in bone biology and thus are important confounders [18–20].
In addition to 25(OH)D, other forms of vitamin D may have critical roles in bone health. 24,25-dihydroxyvitamin D [24,25(OH)2D] has been associated with bone mineralization in chicks and mice recovering from skeletal fracture [21,22]. Free 25(OH)D was also shown to be associated with bone mineral density in nonpregnant populations [23,24]. Similarly, the C-3α epimer of 25(OH)D3 [3-epi-25(OH)D3] was reported to have calcemic effects in cell culture studies [25,26]. Nonetheless, very few studies have examined the relationship between these other biomarkers of vitamin D and markers of bone turnover during human pregnancy.
As part of a controlled feeding study, our research group recently demonstrated that vitamin D metabolism varies among women in different reproductive states consuming adequate and equivalent amounts of vitamin D, calcium, and phosphorus. Specifically, higher circulating concentrations of several vitamin D biomarkers including 25(OH)D, 1,25(OH)2D, vitamin D binding protein (DBP), and 3-epi-25(OH)D3 were observed among pregnant women as compared to nonpregnant control women [27]. As an extension of this feeding study, the present study sought to examine the relationships between maternal vitamin D biomarkers [i.e., 25(OH)D, 1,25(OH)2D, 24,25(OH)2D, free 25(OH)D, 3-epi-25(OH)D3] and biochemical markers of bone turnover among pregnant, nonpregnant women, and the newborns of the pregnant women. In addition, changes in bone turnover markers across the third trimester were examined in pregnant women who consumed adequate and equivalent intakes of vitamin D, calcium, and phosphorus.
MATERIALS AND METHODS
Participants
The study participants were twenty-six singleton pregnant women who entered the study at 26–29 weeks gestation and twenty-one nonpregnant control women of childbearing age. The women were recruited from Ithaca, New York (latitude 42.4 N) during 2009–2010 [28,29] and were 21–40 years old, non-smokers, and in good general health based on responses to a health-history questionnaire, a blood chemistry profile, and a complete blood count. Pregnant women reported no use of alcohol throughout their prenatal period, and all women abstained from alcohol during the feeding study. The study protocol was reviewed and approved by the Institutional Review Board for Human Study Participant Use at Cornell University and the Cayuga Medical Center (the hospital where pregnant women delivered their infants). Written informed consent was obtained from all participants prior to study entry.
Study Design
The current study was nested within a controlled feeding study in which the study participants consumed a 7-day rotational menu, and either 100 or 550 mg/d of supplemental choline [28]. In addition, both pregnant and nonpregnant women consumed a prenatal multivitamin supplement containing vitamin D and calcium (daily; Pregnancy Plus [Fairhaven Health LLC]), a DHA supplement (200mg/d; Neuromins [Nature’s Way Products]), and a potassium and magnesium supplement (3 times/wk; General Nutrition Corp). During the week, pregnant and nonpregnant women consumed one meal and their daily supplements each day at the onsite location under supervision. For offsite and weekend meals, food, beverages, and supplements were provided as carry-outs.
All pregnant and nonpregnant participants consumed an average daily intake of 511±48 IU vitamin D (311±48 IU from food, 200 IU from a supplement), 1622±414 mg calcium (1582±414 mg from food and 40 mg from a supplement), and 1877±280 mg phosphorus for 10 weeks. Food estimates were based on the USDA National Nutrient Database for Standard Reference Release 28.
Sample Collection and Processing
Fasting (10-h) EDTA venous blood and serum, as well as 24-h urine samples, were collected from all participants at study baseline (study week 0; corresponding to “the beginning of the third trimester”) and at study-end (study week 10; corresponding to “near term” before delivery). Fetal cord blood samples (n = 23) were also obtained at delivery in EDTA-coated tubes. Three cord blood samples were not retrieved: one participant delivered without notifying the research team, and two participants gave birth at home. The samples were processed and stored at −80°C until analysis [28].
Analytical Measurements
Vitamin D Biomarkers and Calcitropic Hormones in Pregnant and Nonpregnant Women
Vitamin D biomarkers and calcitropic hormones were quantified in blood samples from pregnant and nonpregnant women. Serum 25(OH)D (i.e., the sum of 25(OH)D2 and 25(OH)D3), serum 3-epi-25(OH)D3, and plasma 24,25(OH)2D were quantified using isotope dilution liquid chromatography-mass spectrometry methodology [30,31] with modifications based on our instrumentation as previously described [27]. Assay precision and accuracy were assessed by using the National Institute of Standards and Technology SRM and participating in the Vitamin D External Quality Assessment Scheme. Measurements of plasma 1,25(OH)2D (Immunodiagnostic Systems, Inc., Scottsdale, AZ) and DBP (R&D Systems, Minneapolis, MN) were conducted using ELISA kits, and free 25(OH)D was estimated by an equation [32]. Plasma intact parathyroid hormone (iPTH) was quantified by a Siemens Immulite 2000 automated immunoassay.
Biochemical Markers of Bone Turnover in Pregnant and Nonpregnant Women
Bone Resorption Markers
ELISA kits were used to measure CTx (Immunodiagnostic Systems, Inc., Scottsdale, AZ), and NTx (MyBiosource, San Diego, CA) in plasma samples from pregnant and nonpregnant women. DPD was measured in urinary samples from pregnant and nonpregnant women using ELISA kits (Quidel Corporation, Athens, OH) and was subsequently expressed on the basis of creatinine concentrations.
Bone Formation Markers
OC was quantified in plasma samples from pregnant and nonpregnant women using ELISA kits (R&D Systems, Minneapolis, MN), while ALP was measured in serum samples from pregnant and nonpregnant women using an automated chemistry analyzer (Dimension Xpand Plus; Siemens Healthcare Diagnostics).
Serum Calcium and Phosphorus
An automated chemistry analyzer was used to measure serum total calcium, albumin, and phosphorus. Then, serum calcium concentrations were corrected for albumin concentrations.
Biochemical Markers of Bone Turnover in Neonates (Cord Blood)
CTx and OC were measured in cord plasma obtained at delivery using ELISA kits mentioned above.
Genotyping in Pregnant and Nonpregnant Women
Genotypes of three SNPs in the vitamin D binding protein gene (i.e., GC rs7041) and 1-alpha-hydroxylase gene (i.e, CYP2R1 rs12794714 and CYP2R1 rs10741657) that are known to be associated with 25(OH)D concentrations [33] were determined among pregnant and nonpregnant women as previously described [27].
Statistical Analysis
Data were analyzed by JMP Pro 11 (SAS Institute, Inc., Cary, NC). Differences in demographic characteristics and concentrations of vitamin D biomarkers between pregnant and nonpregnant women were assessed using independent t-tests (normally distributed continuous variables); Wilcoxon Rank Sum tests (non-normally distributed continuous variables); and Chi-squared or Fisher’s exact tests (categorical variables). Changes in vitamin D biomarkers throughout the study within each group were tested using paired t-tests except for the change in 3-epi-25(OH)D3 by Wilcoxon Signed-Rank test due to non-normal distribution. Because of different statistical approaches to assess vitamin D biomarkers, values reported in Table 1 are slightly different from the values in our previous report [27].
Table 1.
| Pregnant (n = 26) | Nonpregnant (n = 21) | P-value | |
|---|---|---|---|
| Age, y | 280 ± 3 | 29 ± 5 | 0.791 |
| Pre-pregnancy or baseline BMI, kg/m2 | 23.7 ± 3.1 | 23.5 ± 2.8 | 0.813 |
| Ethnicity, n | 0.716 | ||
| White/Non-White | 16/10 | 14/7 | |
| Multivitamin supplement uses before study entry, n | <0.001 | ||
| Yes/No | 22/4 | 7/14 | |
| CYP2R1 rs10741657 A>G polymorphism, n | 0.012 | ||
| AA/AG/GG, n | 2/20/4 | 2/8/11 | |
| CYP2R1 rs12794714 A>G polymorphism, n | 0.153 | ||
| AA/AG/GG | 1/20/5 | 4/11/6 | |
| GC rs7041 G>T polymorphism, n | 0.873 | ||
| GG/GT/TT | 9/11/6 | 6/9/6 | |
| Season at study entry, n | 0.920 | ||
| April–September/October–March | 14/12 | 11/10 | |
| Serum 25(OH)D, nmol/L | |||
| Baseline | 88.7 ± 28.5 | 63.9 ± 24.7 | 0.004 |
| Study-end | 97.8 ± 32.1* | 78.3 ± 25.3** | 0.028 |
| Plasma 1,25(OH)2D, geometric means (95% CIs), pmol/L | |||
| Baseline | 283 (232, 344) | 151 (129, 178) | <0.001 |
| Study-end | 303 (252, 364) | 163 (139, 191) | <0.001 |
| Plasma 24,25(OH)2D, geometric means (95% CIs), nmol/L | |||
| Baseline | 9.6 (7.5, 12.4) | 9.1 (6.7, 12.5) | 0.784 |
| Study-end | 11.4 (9.5, 13.8) | 11.7 (9.2, 15.0)** | 0.867 |
| Plasma DBP, geometric means (95% CIs), μg/mL | |||
| Baseline | 405 (319, 515) | 204 (164, 254) | <0.001 |
| Study-end | 370 (291, 470)** | 205 (166, 255) | <0.001 |
| Serum 3-epi-25(OH)D3, nmol/L | |||
| Baseline | 3.2 ± 2.1 | 1.9 ± 1.2 | 0.018 |
| Study-end | 4.5 ± 3.6* | 2.5 ± 2.2 | 0.035 |
| Free 25(OH)D, geometric means (95% CIs), pmol/L | |||
| Baseline | 16.0 (12.3, 20.8) | 19.5 (14.9, 25.5) | 0.291 |
| Study-end | 19.2 (15.1, 24.4)* | 25.7 (20.8, 31.7)* | 0.074 |
Data are presented as means ±SDs, unless otherwise indicated. The P value in each row indicates a significant difference between pregnant and nonpregnant women. An asterisk beside a vitamin D biomarker indicates a significant difference between baseline and study-end within either pregnant or nonpregnant women: *, P < 0.05. **, P < 0.01.
CYP2R1, 25-hydroxylase gene; DBP, vitamin D binding protein; 3-epi-25(OH)D3, C3 epimer of 25-hydroxyvitamin D3; GC, vitamin D binding protein gene; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 24,25(OH)2D, 24,25-dihydroxyvitamin D.
Linear mixed models (LMMs) were used to examine differences/changes in bone turnover markers between the two groups at baseline and study-end. Reproductive state and time (i.e., baseline and study-end) were included as fixed factors, while participant identifier was entered as a random factor. Initial models included several covariates such as age, ethnicity/race, BMI (pre-pregnancy or baseline), education, pre-study multivitamin supplement use, season at study entry, genetic variants in vitamin D metabolism, and choline intake (480 or 930 mg/d). Covariates achieving statistical significance (P<0.05) were retained in the final models. Bonferroni corrections were performed for post hoc comparisons between pregnant and nonpregnant groups at each study time point, and between baseline and study-end within each group.
The relationships of maternal vitamin D biomarkers with maternal and fetal bone turnover markers were also assessed using LMMs. Candidate covariates for initial models included the variables mentioned above along with variables related to gestation and neonatal anthropometric outcomes [i.e., gestational age at birth, gestational weight gain, mode of delivery, parity, season at birth, neonate’s gender, birth weight, head circumference, and length (Supplemental Table 1)]. Final models retained the covariates that achieved statistical significance (P<0.05). LMMs were also used to assess differences in each bone marker between pregnant women and their fetuses.
In all analyses, data that did not satisfy the normality and homogeneity of variance criteria were log (ln)-transformed, and influencing data that had studentized residuals greater than 3 were excluded (i.e., two ALP values). When covariates were retained in final models, data are presented as predicted means. P values <0.05 for two-tailed tests were considered statistically significant.
RESULTS
Participant characteristics and concentrations of blood vitamin D biomarkers
Demographic variables and vitamin D measures of the study participants (26 pregnant and 21 nonpregnant women) are presented in Table 1. Briefly, no differences between pregnant and nonpregnant groups were detected in age, pre-pregnancy/baseline BMI, ethnicity, or season at study entry, whereas a greater portion of pregnant women had taken multivitamin supplements as compared to nonpregnant women prior to study entry. Pregnant women had higher 25(OH)D, 1,25(OH)2D, DBP, and 3-epi-25(OH)D3 concentrations than nonpregnant women at baseline and study-end. Conversely, 24,25(OH)2D and free 25(OH)D concentrations did not differ between pregnant and nonpregnant women at either study time point. Over the course of the study (i.e., baseline to study-end), 25(OH)D, 3-epi-25(OH)D3, and free 25(OH)D increased, and 24,25(OH)2D tended to increase (P=0.057) among pregnant women. Increases in 25(OH)D, 24,25(OH)2D and free 25(OH)D were observed among nonpregnant women.
Associations of maternal vitamin D biomarkers with bone turnover markers in pregnant women and their fetuses
Serum 25(OH)D was not associated with bone turnover markers at either baseline or study-end. However, increases in serum 25(OH)D across the study period (i.e., concentration at study-end – concentration at baseline) were associated negatively with maternal OC and DPD/Cr at study-end, and positively with fetal OC at delivery (Table 2). For 1,25(OH)2D, positive associations were observed at baseline and study-end with maternal OC and DPD/Cr, whereas negative associations were observed at these study time points with maternal NTx (Table 2). No associations with 24,25(OH)2D and maternal or fetal bone markers were detected at baseline. However, study-end 24,25(OH)2D was positively related to fetal CTx at delivery, and tended to be negatively related to study-end maternal NTx (Table 2). Baseline free 25(OH)D was associated negatively with baseline maternal iPTH and ALP, and positively with fetal CTx (Table 2). In addition, study-end free 25(OH)D was negatively related to study-end maternal ALP and NTx (Table 2). Finally, baseline 3-epi-25(OH)D3 was negatively associated with study-end maternal ALP, while study-end 3-epi-25(OH)D3 was negatively related to study-end maternal DPD/Cr and ALP (Table 2). Moreover, the increase in 3-epi-25(OH)D3 through time was negatively associated with study-end maternal NTx (Table 2).
Table 2.
Associations of maternal vitamin D biomarkers with biochemical markers of bone turnover in mothers (n = 26) and their fetuses (n = 23)1–3
| Maternal vitamin D markers | R2 | β (95% CI) | P | Significant Covariates4,5 | |
|---|---|---|---|---|---|
|
| |||||
| Maternal bone markers at baseline | |||||
| 1,25(OH)2D at baseline | NTx | 0.30 | −0.002 (−0.0043, −5.0e-6) | 0.050 | BMI |
| 1,25(OH)2D at baseline | DPD/Cr | 0.38 | 0.001 (0.0004, 0.002) | 0.010 | rs10741657 |
| 1,25(OH)2D at baseline | OC | 0.34 | 0.003 (0.0003, 0.006) | 0.035 | season, rs10741657 |
| Free 25(OH)D at baseline | iPTH | 0.54 | −0.020 (−0.040, −0.001) | 0.040 | rs10741657, education |
| Free 25(OH)D at baseline | ALP | 0.31 | 0.012 (−0.023, −0.0005) | 0.042 | rs7041 |
|
|
|||||
| Maternal bone markers at study-end | |||||
|
|
|||||
| Δ 25(OH)D6 | DPD/Cr | 0.49 | −0.009 (−0.016, −0.002) | 0.020 | age, rs10741657 |
| Δ 25(OH)D6 | OC | 0.74 | −0.031 (−0.046, −0.017) | < 0.001 | BMI, gestational weight gain, rs10741657, rs12794714 |
| 1,25(OH)2D at baseline | DPD/Cr | 0.60 | 0.001 (0.0001, 0.002) | 0.033 | season, age, rs10741657 |
| 1,25(OH)2D at baseline | OC | 0.46 | 0.004 (0.002, 0.007) | 0.002 | season, rs10741657 |
| 1,25(OH)2D at study-end | NTx | 0.31 | −0.002 (−0.003, 4.4e-5) | 0.056 | BMI |
| 24,25(OH)2D at study-end | NTx | 0.68 | −0.061 (−0.126, 0.004) | 0.063 | ethnicity, gestational weight gain, rs10741657 |
| Free 25(OH)D at study-end | NTx | 0.30 | −0.018 (−0.038, 0.001) | 0.062 | BMI |
| Free 25(OH)D at study-end | ALP | 0.90 | −0.011 (−0.018, −0.004) | 0.004 | BMI, education, infant weight, infant length, rs7041 |
| 3-epi-25(OH)D3 at baseline | ALP | 0.92 | −0.078 (−0.119, −0.037) | 0.001 | BMI, ethnicity, season, infant weight, infant length, mode of delivery |
| 3-epi-25(OH)D3 at study-end | DPD/Cr | 0.56 | −0.040 (−0.075, −0.004) | 0.031 | age, infant length |
| 3-epi-25(OH)D3 at study-end | ALP | 0.49 | −0.058 (−0.093, −0.023) | 0.002 | BMI |
| Δ 3-epi-25(OH)D36 | NTx | 0.72 | −0.104 (−0.020, −0.007) | 0.037 | BMI, rs10741657, rs7041, infant weight, infant head circumference |
|
|
|||||
| Fetal bone markers at delivery | |||||
|
|
|||||
| Δ 25(OH)D6 | OC | 0.83 | 0.042 (0.015, 0.068) | 0.005 | BMI, gestational weight gain, rs10741657, rs7041 |
| 24,25(OH)2D at study-end | CTx | 0.70 | 0.031 (0.007, 0.055) | 0.018 | BMI, ethnicity, gestational weight gain, infant weight, rs10741657, prestudy supplement use |
| Free 25(OH)D at baseline | CTx | 0.53 | 0.008 (0.001, 0.015) | 0.021 | gestational weight gain, infant weight, infant length, rs10741657 |
Data were derived using linear mixed models which controlled for covariates.
All biochemical markers of bone turnover were log(ln)-transformed except for fetal CTx.
None of these associations achieved statistical significance among nonpregnant women.
Influential covariates which achieved statistical significance (P < 0.05) were retained in the final model of each outcome.
Two intake levels of dietary choline of this study did not achieve a statistical significance, and consequently, were not included in any of the final models.
Calculated by subtracting baseline values from study-end values.
Effect of pregnancy on biochemical markers of bone turnover and their response through time
Plasma Intact Parathyroid Hormone
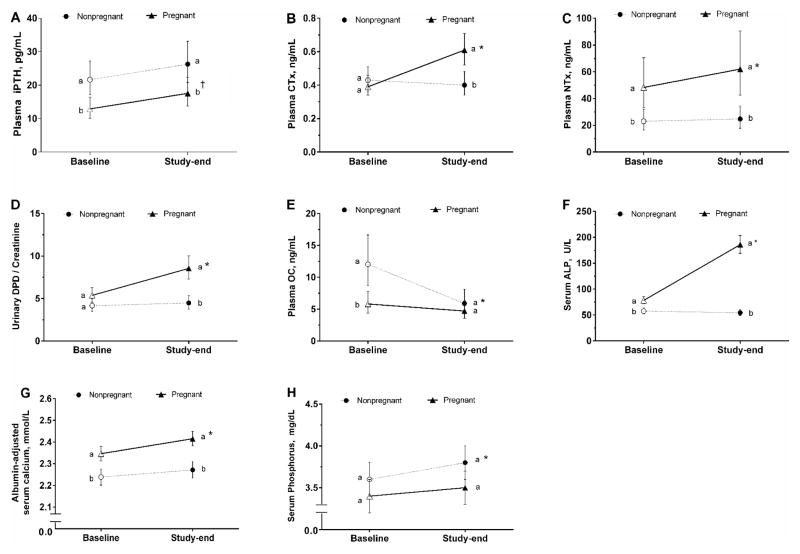
Pregnant women had lower (P=0.005) iPTH concentrations than nonpregnant women at baseline (Figure 1A). Reproductive state did not interact with time (P=0.533) to affect circulating iPTH concentrations. However, iPTH concentrations increased by a mean of 38% (P=0.054) among pregnant women throughout the study, but not among nonpregnant women whose concentrations remained stable (P=0.594; Figure 1A). Similar to baseline, iPTH concentrations were 31% lower (P=0.043) among pregnant women (18 pg/mL) than nonpregnant women (26 pg/mL) at study-end after adjustment for covariates (reproductive state, time, and CYP2R1 rs12794714) (Figure 1A).
Figure 1.
Biochemical markers of bone turnover in pregnant and nonpregnant women consuming equivalent and adequate amounts of vitamin D, calcium, and phosphorus for 10 weeks. Values are predicted means after adjusted for covariates. Baseline values are represented as open shapes, while study-end values are represented as closed shapes. Different letters (i.e., a>b>c) indicate significant differences (P<0.05) in bone markers between pregnant and nonpregnant groups at each study time point. An asterisk (*) indicates a significant change (P<0.05) in the concentrations of bone markers within each group throughout the study, and a long cross symbol (†) indicates a borderline significant change (P=0.054). (A) iPTH, intact parathyroid hormone; (B) CTx, carboxy-terminal cross-linking telopeptide of type 1 collagen; (C) NTx, amino-terminal cross-linking telopeptide of type 1 collagen; (D) Urinary DPD/Creatinine, urinary deoxypyridinoline/creatinine; (E) OC, osteocalcin; (F) ALP, alkaline phosphatase; (G) Albumin-adjusted serum calcium; (H) Phosphorus.
Plasma Carboxy-Terminal Cross-Linking Telopeptide of Type 1 Collagen
CTx concentrations were not different (P>0.9) between pregnant and nonpregnant women at baseline (Figure 1B). Reproductive state interacted with time (P<0.001) to influence circulating CTx response. Specifically, pregnant women experienced a mean 55% increase in CTx (P<0.001) throughout the study period (Figure 1B), whereas no change (P>0.9) in CTx was detected in nonpregnant women. Consequently, pregnant women had 50% higher (P=0.003) CTx concentrations (0.61 ng/mL) than nonpregnant women (0.40 ng/mL) at study-end after adjustment for covariates (reproductive state and time) (Figure 1B).
Plasma Amino-Terminal Cross-Linking Telopeptide of Type 1 Collagen
Pregnant women had higher (P=0.006) NTx concentrations than nonpregnant women at baseline (Figure 1C). Reproductive state interacted with time (P=0.006) to affect circulating NTx concentrations, with a mean 28% increase (P<0.001) observed among pregnant women (Figure 1C), but not among nonpregnant women (P=0.459). Similar to baseline, NTx concentrations in pregnant women (62 ng/mL) were 150% higher (P<0.001) than nonpregnant women (25 ng/mL) at study-end after adjustment for covariates (reproductive state, time, CYP2R1 rs12794714, season, BMI, and education) (Figure 1C).
Urinary Deoxypyridinoline/Creatinine
Urinary DPD/Cr values were not different (P=0.136) between pregnant and nonpregnant women at baseline (Figure 1D). Reproductive state interacted with time (P<0.001) to influence DPD/Cr. Pregnant women exhibited a mean 59% increase (P<0.001) in DPD/Cr, although no change (P>0.9) was detected in nonpregnant women (Figure 1D). As a result, pregnant women showed approximately 100% higher (P<0.001) DPD/Cr (8.6 nmol/mmol) than nonpregnant women (4.5 nmol/mmol) at study-end after adjustment for covariates (reproductive state and time) (Figure 1D).
Plasma Osteocalcin
Pregnant women had lower (P=0.005) OC concentrations that nonpregnant women at baseline (Figure 1E). Reproductive state interacted with time (P=0.007) to influence circulating OC concentrations. Specifically, nonpregnant women experienced a mean 50% reduction (P<0.001) in OC throughout the study period, whereas OC remained stable among pregnant women (P=0.331). At study-end, there was no difference (P=0.267) between pregnant (4.7 ng/mL) and nonpregnant (5.9 ng/mL) women after adjustment for covariates (reproductive state and time) (Figure 1E).
Serum Alkaline Phosphatase
Pregnant women had higher (P<0.001) ALP concentrations than nonpregnant women at baseline (Figure 1F). Reproductive state interacted with time (P<0.001) to affect circulating ALP response. ALP concentrations among pregnant women more than doubled (P<0.001) over the course of the study (Figure 1F), whereas nonpregnant women showed stable concentrations (P=0.705). Similar to baseline, pregnant women (186 U/L) had 240% higher (P<0.001) ALP concentrations than nonpregnant women (54 U/L) at study-end after adjustment for covariates (reproductive state, time, and GC rs7041) (Figure 1F).
Albumin-adjusted Serum Calcium and Phosphorus
Pregnant women showed higher (P<0.001) albumin-adjusted serum calcium concentrations (Figure 1G) and non-different (P=0.646) phosphorus concentrations (Figure 1H) as compared to nonpregnant women at baseline. Reproductive state did not interact with time (P≥0.202) to influence albumin-adjusted serum calcium and phosphorus concentrations. However, pregnant women experienced a significant increase (P=0.002) in albumin-adjusted serum calcium through time (Figure 1G), whereas albumin-adjusted serum calcium remained stable (P>0.452) among nonpregnant women. While no change (P=0.545) in serum phosphorus occurred among pregnant women, a significant increase (P=0.030) in phosphorus was detected in nonpregnant women over the course of the study (Figure 1H). Similar to baseline, pregnant women showed higher (P<0.001) albumin-adjusted serum calcium concentrations than nonpregnant women at study-end after adjustment for covariates (reproductive state and time) (Figure 1G), whereas phosphorus did not differ (P=0.084) between the two groups after adjustment for covariates (reproductive state and time) (Figure 1H).
Comparison of fetal bone markers to maternal markers
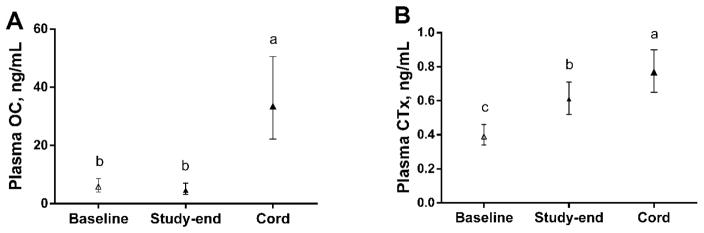
Fetal OC concentrations at delivery (33.5 ng/mL) were 6–7 times higher (P<0.001) than OC concentrations in pregnant women at baseline (5.8 ng/mL) and study-end (4.7 ng/mL) (Figure 2A). Similarly, fetal CTx concentrations (0.77 ng/mL) were 30–100% higher (P≤0.034) than CTx concentrations in pregnant women at baseline (0.39 ng/mL) and study-end (0.61 ng/mL) (Figure 2B).
Figure 2.
(A, B) Bone turnover markers, OC and CTx, in pregnant women (baseline and study-end) and their fetuses (delivery). Values are means (95% CIs). Different letters (i.e., a>b>c) indicate significant differences (P<0.05) in OC and CTx between pregnant women and their fetuses.
DISCUSSION
To the best of our knowledge, this is the first controlled feeding study to assess associations of vitamin D biomarkers with bone turnover during pregnancy. Participants consumed equivalent amounts of vitamin D along with calcium and phosphorus which can influence bone metabolism during pregnancy [19,34]. The intake levels of all essential nutrients aligned with, or exceeded, current dietary recommendations [1]. Our findings show associations between several vitamin D biomarkers and a panel of biochemical markers of maternal and fetal bone metabolism. Moreover, our data suggest that achieving higher concentrations of maternal vitamin D biomarkers including 25(OH)D (>90 nmol/L) across the last third of pregnancy may be a nutritional strategy for reducing pregnancy-induced maternal bone loss.
Higher maternal concentrations of vitamin D biomarkers are associated with reduced maternal bone resorption
We found that increases in serum 25(OH)D across the third trimester of pregnancy were associated with decreases in bone turnover markers (i.e., DPD/Cr and OC). In addition, higher concentrations of 24,25(OH)2D, which rise in parallel with 25(OH)D, were associated with lower NTx concentrations among pregnant women at study-end (near term) although statistical significance was not achieved (P=0.063). This latter finding is consistent with previous studies in vitamin D-replete animals showing reduced bone resorption during the administration of pharmacological doses of 24,25(OH)2D [35,36]. Of note, free 25(OH)D (but not protein-bound 25(OH)D) was negatively associated with PTH in pregnant women at baseline, suggesting that low concentrations of free 25(OH)D may increase PTH secretion, possibly resulting in greater calcium loss from maternal bone at the beginning of the third trimester. Furthermore, lower concentrations of free 25(OH)D at study-end were also associated with higher study-end concentrations of the bone resorption marker NTx. Although 1,25(OH)2D was positively associated with DPD/Cr throughout the study period, it was also negatively related to NTx. In addition, higher 1,25(OH)2D concentrations were associated with higher OC concentrations at both baseline and study-end.
Overall, data from these functional bone health outcomes suggest that higher concentrations of maternal vitamin D biomarkers including 25(OH)D (> 90 nmol/L) might reduce calcium mobilization from maternal bone. However, while this reduction may be of benefit to maternal bone, it raises questions as to the adequacy of calcium supply to the developing fetus. Notably, albumin-adjusted serum calcium concentrations increased throughout the third trimester of our pregnant participants, and this increase was not influenced by any of the biomarkers of vitamin D status even after adjustments. These findings suggest that net calcium transfer from the maternal to the fetal compartment was uncompromised by vitamin D-associated declines in maternal bone resorption. In contrast, a recent randomized controlled trial in Iran that administered a high vitamin D dose (2,000 IU) throughout the third trimester [37] reported no difference in maternal bone measurements between the supplement and placebo groups. However, the supplementation group showed low serum 25(OH)D concentrations (mean of 45 nmol/L) over the course of the study, indicating that the high dose of vitamin D supplementation did not yield sufficient vitamin D status among the pregnant women. These data collectively suggest that concentrations of vitamin D biomarkers [e.g., 25(OH)D], rather than the assigned vitamin D dose, might be an important discriminatory factor when ascertaining the effects of maternal vitamin D on bone health.
Maternal vitamin D biomarkers are associated with fetal bone metabolism
Increases in maternal serum 25(OH)D across the third trimester were associated with increases in fetal OC. This finding suggests that achieving higher maternal 25(OH)D concentrations during pregnancy may promote bone formation in fetal skeleton. Maternal 25(OH)D readily crosses the placenta [38]; thus greater transfer of 25(OH)D to the fetal compartment could enhance fetal OC synthesis by raising fetal 1,25(OH)2D which is known to stimulate OC production [39]. Alternatively, because achieving higher maternal 25(OH)D concentrations was coincidentally associated with lower maternal OC levels among pregnant women in this study, it is also possible that maternal 25(OH)D has a putative a role in the transplacental movement of maternal OC, which has been shown to cross the placenta [40].
In addition to bone formation, mineralization of the fetal skeleton encompasses bone resorption. In the present study, maternal free 25(OH)D and 24,25(OH)2D were positively associated with fetal CTx, a marker of bone resorption. Thus, a higher maternal vitamin D status appears to promote fetal bone turnover (i.e., bone formation + bone resorption) which may be favorable when mineralization of the fetal skeleton is accelerated. However, due to a lack of calcium and other bone resorption marker measurements in cord blood as well as amniotic fluid, we cannot exclude the possibility that higher fetal bone resorption may be indicating fetal hypocalcemia and fetal skeleton demineralization. Nonetheless, this appears unlikely within the context of this study because fetal demineralization resulting from elevated fetal bone resorption generally transpires secondary to diminished maternal calcium transfer.
In contrast to the other vitamin D biomarkers, maternal 3-epi-25(OH)D3 did not show any relationship with bone markers in fetuses, suggesting that maternal 3-epi-25(OH)D3 may not influence fetal bone metabolism. Unlike 25(OH)D, 3-epi-25(OH)D3 may not readily cross the placenta to the fetus, and consequently, would not participate in fetal bone development. Indeed, no significant correlation was detected between maternal and fetal 3-epi-25(OH)D3 in a recently published study [41].
Our findings of associations between maternal vitamin D biomarkers and fetal markers of bone metabolism appear to be inconsistent with two recent randomized controlled trials [42,43] which demonstrated no effects of maternal vitamin D3 supplementation (1,000 IU/d [43] and 200 IU/d [42]) on fetal bone measurements as compared to a placebo group. The reasons for these inconsistencies are unclear but may be due to differences in the intake of bone-related nutrients. For example, dietary calcium was not reported in one study [43] and the supplementation group had very low vitamin D intake (234 IU/d from food and the supplement) in the other study [42]. In addition, genetic variants that might alter bone metabolism were not assessed in either study which may obscure study findings. For example, in the present study, CYP2R1 rs10741657 A>G polymorphism emerged as the most common significant covariate in the linear mixed models examining associations between vitamin D biomarkers and maternal/fetal bone turnover markers. In addition, others have reported that polymorphisms in the VDR gene influenced changes in bone measurements among adolescent pregnant women consuming supplemental vitamin D and calcium [44].
Pregnancy induced a net negative balance of bone metabolism even under conditions of sufficient intake of bone-related nutrients
All of the bone resorption markers (i.e., CTx, NTx, and urinary DPD/Cr) increased by 28–60% throughout the third trimester of pregnancy and were 50–150% higher than those of nonpregnant women consuming equivalent amounts of bone-related nutrients, even after controlling for another confounding factors such as BMI, ethnicity/race, season, and vitamin D-related genetic variants. In contrast, concentrations of the bone formation marker OC did not change among pregnant women, nor did it differ from those of nonpregnant women at study-end, which aligns with prior work [45–47]. This finding of stable and non-different OC concentrations among pregnant women remained (P>0.9) even after removing the effects of hemodilution by adjusting for albumin concentrations. Further, although ALP increased and remained higher among pregnant women, this increase is partly attributable to the placental contribution and may not be an accurate reflection of maternal bone formation. Overall, our findings are consistent with other longitudinal studies that have reported high levels of bone resorption among third-trimester women [45–48].
In agreement with some [47,49] but not all [50,51] previous work, pregnant women maintained lower PTH concentrations than nonpregnant women throughout their third trimester. Moreover, although PTH is well-known for its role in stimulating the production of 1,25(OH)2D in nonpregnant state, 1,25(OH)2D was not associated (P≥0.293) with PTH at any study time-point among pregnant women. Taken together, the divergent relationship between these hormones supports prior work indicating that 1,25(OH)2D is not under the influence of PTH during pregnancy [52].
Study limitations
This feeding study provided a single dose of vitamin D and thus prohibited comparisons among dosing levels. In addition, the study sample size was relatively small and may have reduced the statistical power of our study. Finally, bone mass parameters, indicative of quantitative bone changes, were not obtained.
CONCLUSION
Overall data from the present study suggest that higher concentrations of maternal vitamin D biomarkers including 25(OH)D (> 90 nmol/L) might have functional benefits for pregnant women by reducing maternal bone resorption without compromising fetal calcium supply. Large-scale randomized controlled trials with higher doses of vitamin D supplementation are needed to confirm the potential functional benefits (i.e., less reduction in maternal bone mass) within the context of adequate dietary calcium and phosphorus intake.
Supplementary Material
Highlights.
Pregnancy induces a negative bone balance under adequate nutrient intakes.
Higher vitamin D associates with lower maternal bone resorption during pregnancy.
This reduction in maternal bone resorption does not influence fetal calcium supply.
Higher vitamin D biomarkers may have benefits for bone health of pregnant women.
Acknowledgments
The present study is supported by the USDA NIFA AFRI-ELI Predoctoral Fellowship (2016-67011-25174); NIH (1R03HD080824-01A1); the Gerber Foundation (1843-3882); the American Egg Board/Egg Nutrition Center; the Beef Checkoff, through the National Cattlemen’s Beef Association and Nebraska Beef Council. The authors wish to thank Cornell Statistical Consulting Unit at Cornell University for providing guidance in the statistical analyses of our data.
Footnotes
Supported by USDA NIFA AFRI-ELI Predoctoral Fellowship [2016-67011-25174]; NIH [1R03HD080824-01A1]; the Gerber Foundation [1843-3882]; the American Egg Board/Egg Nutrition Center; the Beef Checkoff, through the National Cattlemen’s Beef Association and Nebraska Beef Council. The funding sources had no role in the study design, interpretation of the data, and/or publication of results.
Abbreviations used: ALP, alkaline phosphatase; CTx, carboxy-terminal cross-linking telopeptide of type 1 collagen; CYP2R1, 25-hydroxylase gene; CYP24A1, 24-hydoxylase; DBP, vitamin D binding protein; DPD, deoxypyridinoline; GC, vitamin D binding protein gene; iPTH, intact parathyroid hormone; LMM, linear mixed model; NTx, amino-terminal cross-linking telopeptide of type 1 collagen; OC, osteocalcin; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 24,25(OH)2D, 24,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; 3-epi-25(OH)D3, C3 epimer of 25-hydroxyvitamin D3.
Disclosures
All authors state that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ross AC, Taylor CL, Yaktine AL, Valle HD Institute of Medicine. Dietary reference intakes for calcium and vitamin D. National Academies Press; Washington DC: 2011. [PubMed] [Google Scholar]
- 2.Kovacs CS. Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiol Rev. 2016;96:449–547. doi: 10.1152/physrev.00027.2015. [DOI] [PubMed] [Google Scholar]
- 3.Halloran BP, De Luca HF. Effect of vitamin D deficiency on skeletal development during early growth in the rat. Arch Biochem Biophys. 1981;209:7–14. doi: 10.1016/0003-9861(81)90251-4. [DOI] [PubMed] [Google Scholar]
- 4.Miller S, Halloran B, DeLuca H, Jee W. Studies on the role of vitamin D in early skeletal development, mineralization, and growth in rats. J Bone Miner Res. 1983;35:455–60. doi: 10.1007/BF02405076. [DOI] [PubMed] [Google Scholar]
- 5.Kovacs CS, Woodland ML, Fudge NJ, Friel JK. The vitamin D receptor is not required for fetal mineral homeostasis or for the regulation of placental calcium transfer in mice. Am J Physiol Endocrinol Metab. 2005;289:E133–44. doi: 10.1152/ajpendo.00354.2004. [DOI] [PubMed] [Google Scholar]
- 6.Fudge NJ, Kovacs CS. Pregnancy up-regulates intestinal calcium absorption and skeletal mineralization independently of the vitamin D receptor. Endocrinology. 2010;151:886–95. doi: 10.1210/en.2009-1010. [DOI] [PubMed] [Google Scholar]
- 7.Kovacs CS. The role of vitamin D in pregnancy and lactation: insights from animal models and clinical studies. Annu Rev Nutr. 2012;32:97–123. doi: 10.1146/annurev-nutr-071811-150742. [DOI] [PubMed] [Google Scholar]
- 8.Akcakus M, Koklu E, Budak N, Kula M, Kurtoglu S, Koklu S. The relationship between birthweight, 25-hydroxyvitamin D concentrations and bone mineral status in neonates. Ann Trop Paediatr. 2006;26:267–75. doi: 10.1179/146532806X152782. [DOI] [PubMed] [Google Scholar]
- 9.Prentice A, Jarjou LMa, Goldberg GR, Bennett J, Cole TJ, Schoenmakers I. Maternal plasma 25-hydroxyvitamin D concentration and birthweight, growth and bone mineral accretion of Gambian infants. Acta Paediatr. 2009;98:1360–2. doi: 10.1111/j.1651-2227.2009.01352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olausson H, Goldberg GR, Ann Laskey M, Schoenmakers I, Jarjou LMA, Prentice A. Calcium economy in human pregnancy and lactation. Nutr Res Rev. 2012;25:40–67. doi: 10.1017/S0954422411000187. [DOI] [PubMed] [Google Scholar]
- 11.Morley R, Carlin JB, Pasco JA, Wark JD. Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab. 2006;91:906–12. doi: 10.1210/jc.2005-1479. [DOI] [PubMed] [Google Scholar]
- 12.Mahon P, Harvey N, Crozier S, Inskip H, Robinson S, Arden N, Swaminathan R, Cooper C, Godfrey K. Low maternal vitamin D status and fetal bone development: cohort study. J Bone Miner Res. 2010;25:14–9. doi: 10.1359/jbmr.090701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viljakainen HT, Saarnio E, Hytinantti T, Miettinen M, Surcel H, Mäkitie O, Andersson S, Laitinen K, Lamberg-Allardt C. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010;95:1749–57. doi: 10.1210/jc.2009-1391. [DOI] [PubMed] [Google Scholar]
- 14.Brooke OG, Brown IR, Bone CD, Carter ND, Cleeve HJ, Maxwell JD, Robinson VP, Winder SM. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. Br Med J. 1980;280:751–4. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalra P, Das V, Agarwal A, Kumar M, Ramesh V, Bhatia E, Gupta S, Singh S, Saxena P, Bhatia V. Effect of vitamin D supplementation during pregnancy on neonatal mineral homeostasis and anthropometry of the newborn and infant. Br J Nutr. 2012;108:1052–1058. doi: 10.1017/S0007114511006246. [DOI] [PubMed] [Google Scholar]
- 16.Gambacciani M, Spinetti A, Gallo R, Cappagli B, Teti GC, Facchini V. Ultrasonographic bone characteristics during normal pregnancy: Longitudinal and cross-sectional evaluation. Am J Obstet Gynecol. 1995;173:890–893. doi: 10.1016/0002-9378(95)90361-5. [DOI] [PubMed] [Google Scholar]
- 17.Hashemipour S, Lalooha F, Zahir Mirdamadi S, Ziaee A, Dabaghi Ghaleh T. Effect of vitamin D administration in vitamin D-deficient pregnant women on maternal and neonatal serum calcium and vitamin D concentrations: a randomised clinical trial. Br J Nutr. 2013;110:1611–6. doi: 10.1017/S0007114513001244. [DOI] [PubMed] [Google Scholar]
- 18.Zeni SN, Soler CRO, Lazzari A, López L, Suarez M, Di Gregorio S, Somoza JI, De Portela ML. Interrelationship between bone turnover markers and dietary calcium intake in pregnant women: A longitudinal study. Bone. 2003;33:606–613. doi: 10.1016/S8756-3282(03)00203-5. [DOI] [PubMed] [Google Scholar]
- 19.Calvo MS, Tucker KL. Is phosphorus intake that exceeds dietary requirements a risk factor in bone health? Ann N Y Acad Sci. 2013;1301:29–35. doi: 10.1111/nyas.12300. [DOI] [PubMed] [Google Scholar]
- 20.Bonjour J-P, Kohrt W, Levasseur R, Warren M, Whiting S, Kraenzlin M. Biochemical markers for assessment of calcium economy and bone metabolism: application in clinical trials from pharmaceutical agents to nutritional products. Nutr Res Rev. 2014;27:252–67. doi: 10.1017/S0954422414000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.St-Arnaud R. CYP24A1-deficient mice as a tool to uncover a biological activity for vitamin D metabolites hydroxylated at position 24. J Steroid Biochem Mol Biol. 2010;121:254–256. doi: 10.1016/j.jsbmb.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 22.St-Arnaud R, Naja RP. Vitamin D metabolism, cartilage and bone fracture repair. Mol Cell Endocrinol. 2011;347:48–54. doi: 10.1016/j.mce.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 23.Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E, Wenger J, Karumanchi SA, Thadhani R, Bhan I. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res. 2011;26:1609–1616. doi: 10.1002/jbmr.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnsen MS, Grimnes G, Figenschau Y, Torjesen PA, Almås B, Jorde R. Serum free and bio-available 25-hydroxyvitamin D correlate better with bone density than serum total 25-hydroxyvitamin D. Scand J Clin Lab Investig. 2014;74:177–183. doi: 10.3109/00365513.2013.869701. [DOI] [PubMed] [Google Scholar]
- 25.Brown AJ, Ritter CS, Slatopolsky E, Muralidharan KR, Okamura WH, Reddy GS. Isolation and Identification of 1 a -Hydroxy-3-epi-Vitamin D 3, a Potent Suppressor of Parathyroid Hormone Secretion. J Cell Biochem. 1999;73:106–113. doi: 10.1002/jcb.20553. [DOI] [PubMed] [Google Scholar]
- 26.Kamao M, Tatematsu S, Hatakeyama S, Sakaki T, Sawada N, Inouye K, Ozono K, Kubodera N, Reddy GS, Okano T. C-3 epimerization of vitamin D3 metabolites and further metabolism of C-3 epimers: 25-Hydroxyvitamin D3 is metabolized to 3-epi-25-hydroxyvitamin D3 and subsequently metabolized through C-1a or C-24 hydroxylation. J Biol Chem. 2004;279:15897–15907. doi: 10.1074/jbc.M311473200. [DOI] [PubMed] [Google Scholar]
- 27.Park H, Brannon PM, West AA, Yan J, Jiang X, Perry CA, Malysheva OV, Mehta S, Caudill MA. Vitamin D metabolism varies among women in different reproductive states consuming the same intakes of vitamin D and related nutrients. J Nutr. 2016;146:1537–1545. doi: 10.3945/jn.116.229971.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan J, Jiang X, West AA, Perry CA, Malysheva OV, Devapatla S, Pressman E, Vermeylen F, Stabler SP, Allen RH, Caudill MA. Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am J Clin Nutr. 2012:1060–1071. doi: 10.3945/ajcn.111.022772.INTRODUCTION. [DOI] [PubMed] [Google Scholar]
- 29.West AA, Yan J, Perry CA, Jiang X, Malysheva OV, Caudill MA. Folate-status response to a controlled folate intake in nonpregnant, pregnant, and lactating women. Am J Clin Nutr. 2012:789–800. doi: 10.3945/ajcn.112.037523.2. [DOI] [PubMed] [Google Scholar]
- 30.Schleicher RL, Encisco SE, Chaudhary-Webb M, Paliakov E, McCoy LF, Pfeiffer CM. Isotope dilution ultra performance liquid chromatography-tandem mass spectrometry method for simultaneous measurement of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3 and 3-epi-25-hydroxyvitamin D3 in human serum. Clin Chim Acta. 2011;412:1594–1599. doi: 10.1016/j.cca.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Kaufmann M, Gallagher JC, Peacock M, Schlingmann KP, Konrad M, DeLuca HF, Sigueiro R, Lopez B, Mourino A, Maestro M, St-Arnaud R, Finkelstein JS, Cooper DP, Jones G. Clinical utility of simultaneous quantitation of 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D by LC-MS/MS involving derivatization with DMEQ-TAD. J Clin Endocrinol Metab. 2014;99:2567–2574. doi: 10.1210/jc.2013-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolland MJ, Grey AB, Ames RW, Horne AM, Mason BH, Wattie DJ, Gamble GD, Bouillon R, Reid IR. Age-, gender-, and weight-related effects on levels of 25-hydroxyvitamin D are not mediated by vitamin D binding protein. Clin Endocrinol (Oxf) 2007;67:259–264. doi: 10.1111/j.1365-2265.2007.02873.x. [DOI] [PubMed] [Google Scholar]
- 33.Bu F-X, Armas L, Lappe J, Zhou Y, Gao G, Wang H-W, Recker R, Zhao L-J. Comprehensive association analysis of nine candidate genes with serum 25-hydroxy vitamin D levels among healthy Caucasian subjects. Hum Genet. 2010;128:549–56. doi: 10.1007/s00439-010-0881-9. [DOI] [PubMed] [Google Scholar]
- 34.Ettinger AS, Lamadrid-Figueroa H, Mercado-García A, Kordas K, Wood RJ, Peterson KE, Hu H, Hernández-Avila M, Téllez-Rojo MM. Effect of calcium supplementation on bone resorption in pregnancy and the early postpartum: a randomized controlled trial in Mexican women. Nutr J. 2014;13:116. doi: 10.1186/1475-2891-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura T, Kurokawa T, Orimo H. Increased of bone volume in vitamin D-repleted rats by massive administration of 24R,25(OH)2D3. Calcif Tissue Int. 1988;43:235–243. doi: 10.1007/BF02555140. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura T, Suzuki K, Hirai T, Kurokawa T, Orimo H. Increased bone volume and reduced bone turnover in vitamin D-replete rabbits by the administration of 24R,25-dihydroxyvitamin D3. Bone. 1992;13:229–236. doi: 10.1016/8756-3282(92)90202-8. [DOI] [PubMed] [Google Scholar]
- 37.Vaziri F, Dabbaghmanesh MH, Samsami A, Nasiri S, Shirazi PT. Vitamin D supplementation during pregnancy on infant anthropometric measurements and bone mass of mother-infant pairs: A randomized placebo clinical trial. Early Hum Dev. 2016;103:61–68. doi: 10.1016/j.earlhumdev.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Brannon PM, Picciano MF. Vitamin D in pregnancy and lactation in humans. Annu Rev Nutr. 2011;31:89–115. doi: 10.1146/annurev.nutr.012809.104807. [DOI] [PubMed] [Google Scholar]
- 39.Hlaing TT, Compston JE. Biochemical markers of bone turnover - uses and limitations. Ann Clin Biochem. 2014;51:189–202. doi: 10.1177/0004563213515190. [DOI] [PubMed] [Google Scholar]
- 40.Oury F, Khrimian L, Denny CA, Gardin A, Chamouni A, Goeden N, Huang YY, Lee H, Srinivas P, Gao XB, Suyama S, Langer T, Mann JJ, Horvath TL, Bonnin A, Karsenty G. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell. 2013;155:228–241. doi: 10.1016/j.cell.2013.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bailey D, Perumal N, Yazdanpanah M, Al Mahmud A, Baqui AH, Adeli K, Roth DE. Maternal-fetal-infant dynamics of the C3-epimer of 25-hydroxyvitamin D. Clin Biochem. 2014;47:816–822. doi: 10.1016/j.clinbiochem.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 42.Diogenes MEL, Bezerra FF, Rezende EP, Donangelo CM. Calcium Plus Vitamin D Supplementation During the Third Trimester of Pregnancy in Adolescents Accustomed to Low Calcium Diets Does Not Affect Infant Bone Mass at Early Lactation in a Randomized Controlled Trial. J Nutr. 2015;145:1515–1523. doi: 10.3945/jn.114.208140. [DOI] [PubMed] [Google Scholar]
- 43.Cooper C, Harvey NC, Bishop NJ, Kennedy S, Papageorghiou AT, Schoenmakers I, Fraser R, Gandhi SV, Carr A, D’Angelo S, Crozier SR, Moon RJ, Arden NK, Dennison EM, Godfrey KM, Inskip HM, Prentice A, Mughal MZ, Eastell R, Reid DM, Javaid MK, Robinson S, Cantle J, McGill K, Barron L, Davill V, Morgan B, Macey S, Hammond J, Collins S, Taylor C, Higginbottom S, Hart K, Wood S, Alexander E, Johnson W, Standfield S, Horsfall T, Coakley P, Cox V, Mahon P, Nisbet C, Taylor P, Doré C, Hedger D, Symmons D, Francis R, Philips M, Roberts C. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): A multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2016;4:393–402. doi: 10.1016/S2213-8587(16)00044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Normando P, Diogenes MEL, Cabello PH, Cabello GM, Donangelo CM, Bezerra FF. Calcium plus vitamin D supplementation during pregnancy interacts with polymorphisms in the promoter region of the VDR gene to affect postpartum bone mass of Brazilian adolescent mothers: A randomized controlled trial. Nutrition. 2015;32:1068–1074. doi: 10.1016/j.nut.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Ulrich U, Miller PB, Eyre DR, Chesnut CH, Schlebusch H, Soules MR. Bone remodeling and bone mineral density during pregnancy. Arch Gynecol Obstet. 2003;268:309–316. doi: 10.1007/s00404-002-0410-8. [DOI] [PubMed] [Google Scholar]
- 46.Paoletti AM, Orrù M, Floris L, Guerriero S, Ajossa S, Romagnino S, Melis GB. Pattern of bone markers during pregnancy and their changes after delivery. Horm Res. 2003;59:21–29. doi: 10.1159/000067935. [DOI] [PubMed] [Google Scholar]
- 47.Møller UK, Streym S, Mosekilde L, Heickendorff L, Flyvbjerg A, Frystyk J, Jensen LT, Rejnmark L. Changes in calcitropic hormones, bone markers and insulin-like growth factor I (IGF-I) during pregnancy and postpartum: A controlled cohort study. Osteoporos Int. 2013;24:1307–1320. doi: 10.1007/s00198-012-2062-2. [DOI] [PubMed] [Google Scholar]
- 48.Haliloglu B, Ilter E, Aksungar FB, Celik A, Coksuer H, Gunduz T, Yucel E, Ozekici U. Bone turnover and maternal 25(OH) vitamin D3 levels during pregnancy and the postpartum period: Should routine vitamin D supplementation be increased in pregnant women? Eur J Obstet Gynecol Reprod Biol. 2011;158:24–27. doi: 10.1016/j.ejogrb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 49.Naylor KE, Iqbal P, Fledelius C, Fraser RB, Eastell R. The effect of pregnancy on bone density and bone turnover. J Bone Miner Res. 2000;15:129–137. doi: 10.1359/jbmr.2000.15.1.129. [DOI] [PubMed] [Google Scholar]
- 50.Cross NA, Hillman LS, Allen SH, Krause GF, Vieira NE. Calcium homeostasis pregnancy, lactation, and bone metabolism during and postweaning : a longitudinal. 1995:514–523. doi: 10.1093/ajcn/61.3.514. [DOI] [PubMed] [Google Scholar]
- 51.More C, Bhattoa HP, Bettembuk P, Balogh A. The effects of pregnancy and lactation on hormonal status and biochemical markers of bone turnover. Eur J Obstet Gynecol Reprod Biol. 2003;106:209–13. doi: 10.1016/s0301-2115(02)00237-3. [DOI] [PubMed] [Google Scholar]
- 52.Kirby BJ, Ma Y, Martin HM, Buckle Favaro KL, Karaplis AC, Kovacs CS. Upregulation of calcitriol during pregnancy and skeletal recovery after lactation do not require parathyroid hormone. J Bone Miner Res. 2013;28:1987–2000. doi: 10.1002/jbmr.1925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.