Abstract
In the yeast Saccharomyces diastaticus, expression of the STA1 gene, which encodes an extracellular glucoamylase, is activated by the specific DNA-binding activators Flo8, Mss11, Ste12, and Tec1 and the Swi/Snf chromatin-remodeling complex. Here we show that Flo8 interacts physically and functionally with Mss11. Flo8 and Mss11 bind cooperatively to the inverted repeat sequence TTTGC-n-GCAAA (n = 97) in UAS1-2 of the STA1 promoter. In addition, Flo8 and Mss11 bind indirectly to UAS2-1 of the STA1 promoter by interacting with Ste12 and Tec1, which bind to the filamentation and invasion response element (FRE) in UAS2-1. Furthermore, our findings indicate that the Ste12, Tec1, Flo8, and Mss11 activators and the Swi/Snf complex bind sequentially to the STA1 promoter, as follows: Ste12 and Tec1 bind first to the FRE, whereby they recruit the Swi/Snf complex to the STA1 promoter. Next, the Swi/Snf complex enhances Flo8 and Mss11 binding to UAS1-2. In the final step, Flo8 and Mss11 directly promote association of RNA polymerase II with the STA1 promoter to activate STA1 expression. In the absence of glucose, the levels of Flo8 and Tec1 are greatly increased, whereas the abundances of two repressors, Nrg1 and Sfl1, are reduced, suggesting that the balance of transcriptional regulators may be important for determining activation or repression of STA1 expression.
Expression of eukaryotic genes is regulated by a complex array of transcription factors acting through cis-binding elements within promoters. In the yeast Saccharomyces cerevisiae, among several other well-characterized transcriptional regulation mechanisms, the molecular mechanism by which transcription and chromatin remodeling factors bind sequentially to the HO promoter serves as a paradigm for the exploration of potentially related transcriptional regulation mechanisms, including that which controls STA gene expression in Saccharomyces diastaticus (4, 8).
In S. diastaticus, three unlinked homologous STA genes (STA1, STA2, and STA3) encode glucoamylase isozymes (GAI, GAII, and GAIII) that degrade starch to glucose. Several positive regulators, such as Flo8, Mss10/Msn1, Mss11, Ste12, and Tec1, are known to activate the transcription of STA1 in the absence of glucose (13, 19, 45). Furthermore, it has been reported that two upstream regions, UAS1-2 and UAS2-1, play critical roles in the activation of STA1 expression in response to glucose depletion (20). In the presence of glucose, the two repressors Nrg1 and Sfl1 bind to UAS1-1 and UAS2-2 of the STA1 promoter, respectively, to repress STA1 transcription (20).
The two independent signal pathways, cyclic AMP (cAMP)-protein kinase A (PKA) and mitogen-activated protein (MAP) kinase, are involved in transcriptional control of the FLO11 gene, which encodes a cell surface glycoprotein critical for invasive growth and pseudohyphal differentiation (27, 33, 38). The cAMP-PKA pathway is activated by increased levels of cAMP, and Tpk2, a catalytic subunit of PKA, regulates transcription factors that play an important role in the activation of FLO11 transcription. Tpk2 phosphorylates Flo8 to promote its binding to the FLO11 promoter, whereas it inhibits multimerization and DNA binding of Sfl1, a repressor of FLO11. Flo8 is a putative transcriptional activator of FLO1, which encodes a lectin-like protein (22). A flo8Δ mutant has reduced glucoamylase activity, loses flocculence, and displays defects in haploid invasion and pseudohyphal differentiation (13, 19, 34). Mss11 was originally isolated as a multicopy suppressor of STA10, a negative regulator of STA1 expression, and it is also implicated in the activation of FLO11 expression as a downstream activator of Flo8 (13, 14, 45). Furthermore, the increased gene dosage of MSS11 suppresses the defects in glucoamylase production and haploid invasion of a flo8Δ mutant (13). It has been suggested that the functions of Flo8 and Mss11 are closely related because mutation of these two genes show very similar phenotypes (19).
The MAP kinase (MAPK) pathway is also involved in haploid invasion and pseudohyphal differentiation and activates the transcription factors Ste12 and Tec1 through the Ste11 (MAPK kinase kinase), Ste7 (MAPK kinase), and Kss1 (MAPK) MAPK cascade (14, 29, 38). Once activated, Ste12 and Tec1 bind cooperatively to the filamentation and invasion response element (FRE), a composite DNA sequence (TGAAACA and CATTCC) in the FLO11 promoter, to activate FLO11 gene expression (27, 29). Although the Flo8, Mss11, Ste12, and Tec1 activators regulated by two independent signaling pathways are known to be required for FLO11 expression, their precise modes of action are unclear.
The 5′ upstream region of the STA1 gene is similar to that of the FLO11 gene, and these genes are coregulated in response to environmental signals (13). Therefore, it was possible, although unproven, that the cAMP-PKA pathway (via Flo8 and Mss11) and the MAPK signaling pathway (via Ste12 and Tec1) converge on the STA1 promoter to activate STA1 expression.
Remodeling of the chromatin structure also influences eukaryotic transcription (48). The Swi/Snf remodeling complex and SAGA histone acetyltransferase complex regulate gene expression by remodeling chromatin structure and altering histone acetylation patterns (21, 36, 48). The Swi/Snf complex is capable of transplacing histone octamers and forming nucleosome-free regions to which transcriptional activators can bind (28, 37, 47). Gam1/Snf2, Gam3/Swi1, Snf5, and Snf6—all components of the Swi/Snf complex—were reported to be involved in STA1 transcription (25, 50). However, it is not known if the Swi/Snf complex directly regulates STA1 expression. This complex is also involved in the regulation of FLO1 expression and remodels the FLO1 promoter (11). Furthermore, the fact that the transcriptional activator Flo8 is critical for FLO1 expression raises the possibility that the function of Flo8 may be closely related to that of the Swi/Snf complex and that Flo8-dependent activation of STA1 expression may require the Swi/Snf complex.
In this study, we demonstrate the molecular mechanism that activates STA1 expression under derepressed conditions. We find that both UAS1-2 and UAS2-1 of the STA1 promoter are the target sites for Flo8, Mss11, Ste12, and Tec1. These four transcriptional activators and the Swi/Snf complex bind sequentially to the STA1 promoter to activate STA1 expression. Furthermore, we demonstrate that the MAP kinase pathway, but not the cAMP-PKA pathway, activates STA1 expression by regulating the function of the Ste12 and Tec1 activators.
MATERIALS AND METHODS
Strains and plasmids.
Yeast strains and plasmids are listed in Tables 1 and 2. To construct the hemagglutinin (HA)-tagged strains, pRS305-SNF6-HA and pRS305-RPB3-HA were linearized with SphI and NsiI, respectively, and the linearized DNA fragments were integrated into their original loci. Appropriate integrations were confirmed by PCR analysis, glucoamylase assay, and Western blot analysis with HA antibody. Mutant strains were constructed by replacing the open reading frames with TRP1, HIS3, or URA3 by PCR-mediated disruption and confirmed by PCR. To construct STA1 promoter-lacZ strains, pLG-STA1-1a was linearized with XbaI and integrated into the original STA1 locus. pLG-FLO11-1 (UAS1-2), which contains an inverted repeat sequence (TTTGC-n-GCAAA), was constructed by PCR with Pfu polymerase(Stratagene) and mutagenic primer pairs 5′-AGTGATGGTTCTCACCACCGCAAAACGGTCACTTGCATG-3′ and 5′-CATGCAAGTGACCGTTTTGCGGTGGTGAGAACCATCACT-3′). The construct generated was confirmed by sequencing.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| KHS 182 | mataSTA1 leu2 his3 trp1 ura3 | 19 |
| KHS 182-1 | mataSTA1 leu2 his3 trp1 ura3 flo8Δ::TRP1 | 19 |
| KHS 182-2 | mataSTA1 leu2 his3 trp1 ura3 mss11Δ::TRP1 | 19 |
| KHS 182-4 | mataSTA1 leu2 his3 trp1 ura3 ste12Δ::HIS3 | 19 |
| KHS 182-5 | mataSTA1 leu2 his3 trp1 ura3 tec1Δ::HIS3 | 19 |
| KHS 182-6 | mataSTA1 leu2 his3 trp1 ura3 tpk1Δ::TRP1 | This study |
| KHS 182-7 | mataSTA1 leu2 his3 trp1 ura3 tpk2Δ::TRP1 | This study |
| KHS 182-8 | mataSTA1 leu2 his3 trp1 ura3 tpk3Δ::TRP1 | This study |
| KHS 182-9 | mataSTA1 leu2 his3 trp1 ura3 ste7Δ::TRP1 | This study |
| KHS 182-10 | mataSTA1 leu2 his3 trp1 ura3 snf5Δ::TRP1 | This study |
| KHS 182-10-1 | mataSTA1 leu2 his3 trp1 ura3 snf6Δ::TRP1 | This study |
| KHS 182-30 | mataSTA1 leu2 his3 trp1 ura3 FLO8-HA::LEU2 | 20 |
| KHS 182-30a | mataSTA1 leu2 his3 trp1 ura3 FLO8-HA::LEU2 STA1-lacZ::URA3 | This study |
| KHS 182-30-1 | mataSTA1 leu2 his3 trp1 ura3 FLO8-HA::LEU2 mss11Δ::TRP1 | This study |
| KHS 182-30-1a | mataSTA1 leu2 his3 trp1 ura3 FLO8-HA::LEU2 mss11Δ::TRP1 STA1-lacZ::URA3 | This study |
| KHS 182-30-2 | mataSTA1 leu2 his3 trp1 ura3 FLO8-HA::LEU2 ste12Δ::HIS3 | This study |
| KHS 182-30-2a | mataSTA1 leu2 his3 trp1 ura3 FLO8-HA::LEU2 ste12Δ::HIS3 STA1-lacZ::URA3 | This study |
| KHS 182-30-3 | mataSTA1 leu2 his3 trp1 ura3 FLO8-HA::LEU2 tec1Δ::HIS3 | This study |
| KHS 182-30-3a | mataSTA1 leu2 his3 trp1 ura3 FLO8-HA::LEU2 tec1Δ::HIS3 STA1-lacZ::URA3 | This study |
| KHS 182-30-4 | mataSTA1 leu2 his3 trp1 ura3 FLO8-HA::LEU2 snf6Δ::TRP1 | This study |
| KHS 182-30-5 | mataSTA1 leu2 his3 trp1 ura3 FLO8-HA::LEU2 ste7Δ::TRP1 | This study |
| KHS 182-31 | mataSTA1 leu2 his3 trp1 ura3 MSS11-HA::LEU2 | 20 |
| KHS 182-31-1 | mataSTA1 leu2 his3 trp1 ura3 MSS11-HA::LEU2 flo8Δ::TRP1 | This study |
| KHS 182-31-2 | mataSTA1 leu2 his3 trp1 ura3 MSS11-HA::LEU2 ste12Δ::HIS3 | This study |
| KHS 182-31-3 | mataSTA1 leu2 his3 trp1 ura3 MSS11-HA::LEU2 tec1Δ::HIS3 | This study |
| KHS 182-31-4 | mataSTA1 leu2 his3 trp1 ura3 MSS11-HA::LEU2 snf6Δ::TRP1 | This study |
| KHS 182-32 | mataSTA1 leu2 his3 trp1 ura3 STE12-HA::LEU2 | 20 |
| KHS 182-32-1 | mataSTA1 leu2 his3 trp1 ura3 STE12-HA::LEU2 flo8Δ::TRP1 | This study |
| KHS 182-32-2 | mataSTA1 leu2 his3 trp1 ura3 STE12-HA::LEU2 mss11Δ::TRP1 | This study |
| KHS 182-32-3 | mataSTA1 leu2 his3 trp1 ura3 STE12-HA::LEU2 snf6Δ::TRP1 | This study |
| KHS 182-32-4 | mataSTA1 leu2 his3 trp1 ura3 STE12-HA::LEU2 ste7Δ::TRP1 | This study |
| KHS 182-33 | mataSTA1 leu2 his3 trp1 ura3 TEC1-HA::LEU2 | 20 |
| KHS 182-33-1 | mataSTA1 leu2 his3 trp1 ura3 TEC1-HA::LEU2 flo8Δ::TRP1 | This study |
| KHS 182-33-2 | mataSTA1 leu2 his3 trp1 ura3 TEC1-HA::LEU2 mss11Δ::TRP1 | This study |
| KHS 182-33-3 | mataSTA1 leu2 his3 trp1 ura3 TEC1-HA::LEU2 snf6Δ::TRP1 | This study |
| KHS 182-34 | mataSTA1 leu2 his3 trp1 ura3 SNF6-HA::LEU2 | This study |
| KHS 182-34-1 | mataSTA1 leu2 his3 trp1 ura3 SNF6-HA::LEU2 flo8Δ::TRP1 | This study |
| KHS 182-34-2 | mataSTA1 leu2 his3 trp1 ura3 SNF6-HA::LEU2 mss11Δ::TRP1 | This study |
| KHS 182-34-3 | mataSTA1 leu2 his3 trp1 ura3 SNF6-HA::LEU2 ste12Δ::HIS3 | This study |
| KHS 182-34-4 | mataSTA1 leu2 his3 trp1 ura3 SNF6-HA::LEU2 tec1Δ::HIS3 | This study |
| KHS 182-35 | mataSTA1 leu2 his3 trp1 ura3 RPB3-HA::LEU2 | This study |
| KHS 182-35-1 | mataSTA1 leu2 his3 trp1 ura3 RPB3-HA::LEU2 flo8Δ::TRP1 | This study |
| KHS 182-35-2 | mataSTA1 leu2 his3 trp1 ura3 RPB3-HA::LEU2 mss11Δ::TRP1 | This study |
TABLE 2.
Plasmids used in this study
| Plasmid | Description | Reference |
|---|---|---|
| pGEX-NRG1 | NRG1 ORFa in pGEX4T-1 | 35 |
| pGEX-FLO8 | Replacement of NRG1 with FLO8 ORF of pGEX-NRG1 | This study |
| pGXE-MSS11 | Replacement of NRG1 with MSS11 ORF of pGEX-NRG1 | This study |
| pRS305 | LEU2 | 40 |
| pRS305-HA | Triple HA tag in pRS305(SalI/XhoI) | This study |
| pRS305-SNF6-HA | SNF6 in pRS305-HA | This study |
| pRS305-RPB3-HA | RPB3 in pRS305-HA | This study |
| pRS325 | 2μm LEU2 | 6 |
| pRS325-HA | Triple HA tag in pRS325(SalI/XhoI) | This study |
| pRS325-ADH1p-HA | ADH1 promoter (−1500 to −1) in pRS325-HA(SacI/NotI) | 20 |
| pRS325-MSS11-HA | MSS11 in pRS325-ADH1p-HA | This study |
| pRS325-STE12-HA | STE12 in pRS325-ADH1p-HA | This study |
| pRS325-TEC1-HA | TEC1 in pRS325-ADH1p-HA | This study |
| pRS326 | 2μm URA3 | 6 |
| pRS326-HA | Triple HA tag in pRS326(SalI/XhoI) | This study |
| pRS326-FLO8-HA | 3.5-kb fragment containing FLO8 ORF in pRS326-HA | This study |
| pRS326-MSS11-HA | 3.7-kb fragment containing MSS11 ORF in pRS326-HA | This study |
| pLG 670-Z | 2μm UAS3 CYC1-lacZ | 15 |
| pLG-STA1-1 | −2105 to −1 region of the STA1 promoter in pLG-670z | 20 |
| pLG-STA1-1a | Deletion of SpeI fragment in pLG-STA1-1 | This study |
| pLG-UAS1-2 | −1905 to −1642 region of the STA1 promoter in pLG-670Z | 20 |
| pLG-UAS2-1 | −1380 to −1147 region of the STA1 promoter in pLG-670Z | 20 |
| pLG-FLO11(UAS1-2) | −1993 to −1730 region of the FLO11 promoter in pLG-670Z | This study |
| pLG-FLO11-1(UAS1-2) | Substitution of CCAAA with GCAAA in pLG-FLO11(UAS1-2) | This study |
| pLG-FLO11b | −1435 to −1202 region of the FLO11 promoter in pLG-670Z | This study |
| pRS425-NRG1-HA | 2.0-kb fragment containing NRG1 ORF in pRS425-HA | 20 |
| pRS425-SFL1-HA | 3.5-kb fragment containing SFL1 ORF in pRS425-HA | 20 |
| pLexA-MIG1 | MIG1 in pSH2-1 | 42 |
| pLexA-FLO8 | FLO8 in pLexA-Mig1 (BamHI/XhoI) | This study |
| pLexA-MSS11 | MSS11 in pLexA-Mig1 (BamHI/SalI) | This study |
| pLexA-STE12 | STE12 in pLexA-Mig1 (BamHI/XhoI) | This study |
| pLexA-TEC1 | TEC1 in pLexA-Mig1 (BamHI/XhoI) | This study |
| pSH18-34 | 2μm URA3 8lexAop-GAL1TATA-lacZ | 10, 46 |
ORF, open reading frame.
Glucoamylase assay and β-galactosidase assay.
The glucoamylase assay were performed as described previously (35). β-Galactosidase was assayed as described previously (2).
Coimmunoprecipitation.
HA-tagged or LexA-fused activators were expressed from the ADH1 promoter. Cells containing each plasmid were grown in 2% glucose to an optical density at 600 nm of approximately 1.0, and protein extracts were prepared. The preparation of whole-cell extracts and coimmunoprecipitation were performed as described previously (49).
ChIP assay.
A chromatin immunoprecipitation (ChIP) assay was performed essentially as described by Hecht et al. (16) with minor modifications. To identify specific regions for Flo8 and Mss11 binding, cells bearing pLG-UAS1-2 and pLG-UAS2-1 were used. Cells were grown in synthetic media containing 2% glucose or 2% glycerol-ethanol to an optical density at 600 nm of 1.0 and treated with formaldehyde (1%) to cross-link DNA and proteins. Total extracts were prepared and sonicated, and then equal amounts of extract were incubated with mouse anti-HA antibody (Santa Cruz) at 4°C overnight. GammaBind G Sepharose beads (Amersham) were added to precipitate DNA-HA tagged proteins, and then the beads were washed four times. Elution buffer was added and incubated at 65°C overnight. DNA fragments were purified with a QiaQuick PCR column (Qiagen). The immunoprecipitated DNAs were amplified by 30 cycles of PCR to detect the upstream regions of the STA1 promoter in the pLG vector series and the endogenous STA1 promoter with the following primer pairs: CYC1 (5′-GAAAGGAAAGCAGGAAAGG-3′ and 5′-TATACACGCCTGGCGGATCTG-3′), UAS1-2 (5′-CCTATTCTCATCGAGAGCCGAG-3′ and 5′-CAAGTACTGCAGTGCATGTCC-3′), UAS2-1 (5′-GGTAAGATTTGTTCTATG-3′ and 5′-GAACTTTCCAGGCTCACC-3′), UAS2-2 (5′-GGTGTGCCTGGAAAGTTC-3′ and 5′-GAGCAATCAGCAGTTCTTTG-3′), TATA (5′-CTTAACAAATATGTTCAAGC-3′ and 5′-TGGATTTTTGAGGCCTACC-3′), and KSS1 (F-GAGGGCTAAAGAGTATATAGC and R-TAGTTCATCGTAAAGCATGTC). The KSS1 and CYC1 primer pairs were used to detect the nonspecific background and upstream activation sequences (UASs) of the STA1 promoter in the pLG vector series, respectively. The PCR products were separated by 2% agarose gel electrophoresis and photographed.
RESULTS
Transcriptional activators interact with specific regions of STA1 promoter.
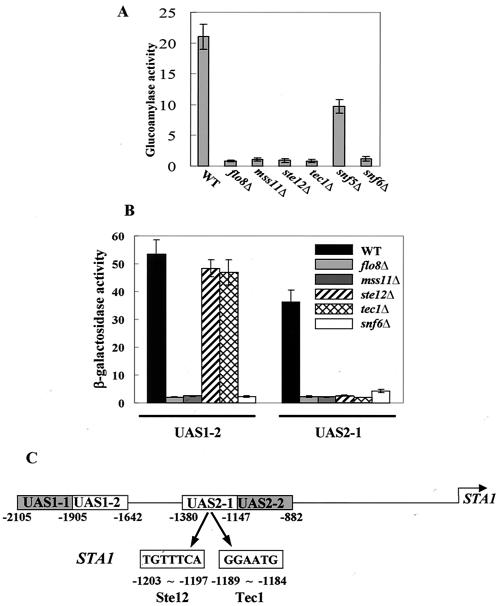
It was reported that four transcriptional activators, such as Flo8, Mss11, Ste12, and Tec1, and components of the Swi/Snf chromatin-remodeling complex, including Swi1, Snf2, Snf5, and Snf6, are involved in the activation of STA gene expression (13, 14, 19, 45, 50). We confirmed that these factors were required for STA1 expression. STA1 expression is abolished completely in flo8Δ, mss11Δ, ste12Δ, tec1Δ, and snf6Δ mutants (from 21.5 to <1 U) (Fig. 1A). As previously reported, two UASs of the STA1 promoter, UAS1-2 and UAS2-1 (Fig. 1C), are critical for the activation of STA1 expression (20). Therefore, we investigated the effects of the transcriptional activators and Snf6 on these UASs. To this end, we determined the β-galactosidase activity of plasmid-based UASSTA1-CYC1TATA-lacZ reporter constructs transformed into deletion mutants and wild-type strains. lacZ expression mediated by both UAS1-2 and UAS2-1 was greatly reduced in flo8Δ, mss11Δ, and snf6Δ mutants (Fig. 1B). This result suggests that both UAS1-2 and UAS2-1 contain specific sequences through which Flo8 and Mss11 may function. In contrast, ste12Δ and tec1Δ mutations did not affect lacZ expression from UAS1-2. Instead, they blocked expression from UAS2-1 (Fig. 1B), suggesting that binding sites for Ste12 and Tec1 may be located in UAS2-1.
FIG. 1.
Transcriptional activators act on specific regions of the STA1 promoter. (A) Effects of transcriptional activators on STA1 expression. The wild type (WT) and mutants were grown in synthetic medium containing 2% glycerol-ethanol as a carbon source. Glucoamylase activities are averages of the results from three independent experiments. (B) Effects of transcriptional activators on lacZ expression mediated by UAS1-2 and UAS2-1. pLG-UAS1-2 and pLG-UAS2-1 were transformed into the wild type and each mutant, and three independent transformants of each were tested for β-galactosidase activity under derepressing conditions (2% glycerol-ethanol). (C) The STA1 promoter is composed of two UASs, UAS1-2 and UAS2-1. The FRE for Ste12 and Tec1 binding in the STA1 promoter is indicated. UAS1-1 and UAS2-2, to which two major repressors, NRG1 and SFL1, for glucose repression of STA1 expression bind, respectively, are also represented (20).
We therefore analyzed the nucleotide sequences of the UASs and found that UAS2-1 contains an FRE known to be important for cooperative binding of Ste12 and Tec1 to the FLO11 promoter (29). In the STA1 promoter, the two half sites of the element are separated by 7 bp and are an exact match to the FRE consensus sequence (Fig. 1C). This finding suggests that Ste12 and Tec1 bind directly to UAS2-1 of the STA1 promoter. As described above, Flo8 and Mss11 act on both UAS1-2 and UAS2-1. However, it is unlikely that Flo8 and Mss11 directly bind to both UASs because there is no sequence homology between the two promoter sequences. We therefore tested the possibility that Flo8 and Mss11 act directly on UAS1-2 but indirectly on UAS2-1 by interacting with Ste12 and Tec1.
Flo8 and Mss11 cooperatively interact with the inverted repeat sequence in UAS1-2.
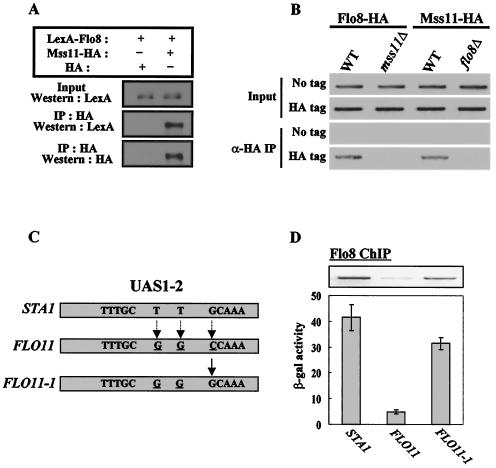
The flo8Δ and mss11Δ mutants have very similar phenotypes. These include reduced glucoamylase activity, a defect in invasive growth, and loss of flocculation ability, suggesting that their functions may be closely related (12, 13, 19). We first examined whether Flo8 interacts physically with Mss11. Flo8 was tagged with the LexA epitope on its N terminus, Mss11 was tagged with a triple HA epitope on its C terminus, and these fusion proteins were expressed from the ADH1 gene promoter. Plasmids expressing LexA-Flo8 or Mss11-HA were transformed into a wild-type strain to examine the physical interaction between them. LexA-Flo8 coprecipitated with Mss11-HA but not with HA itself, indicating that Flo8 interacts physically with Mss11 in vivo (Fig. 2A). The Flo8-Mss11 interaction was confirmed by glutathione S-transferase (GST) pull-down and two hybrid assays (data not shown).
FIG. 2.
Flo8 and Mss11 bind to the inverted repeat sequence in UAS1-2. (A) Physical interaction between Flo8 and Mss11. Cells carrying plasmids expressing either LexA-Flo8 and Mss11-HA or LexA-Flo8 and HA were grown in selective medium containing 2% glucose. Protein extracts (500 μg) were immunoprecipitated (IP) with anti-HA antibody. Input extracts (50 μg) and the precipitates were separated by SDS-8% PAGE and analyzed by blotting with LexA antibody. The membrane was stripped and reprobed with anti-HA antibody. +, present; −, absent. (B) Flo8 and Mss11 bind cooperatively to UAS1-2. FLO8-HA wild-type (WT), FLO8-HA mss11Δ, MSS11-HA wild-type, MSS11-HA flo8Δ, or untagged strains bearing pLG-UAS1-2 were grown in synthetic medium containing 2% glycerol-ethanol to mid-log phase and treated with formaldehyde to cross-link DNA and proteins. Anti-HA (α-HA) chromatin immunoprecipitation was performed with 1 mg of total extracts, and the UAS1-2 region was PCR amplified with the purified DNA to determine the binding of Flo8 and Mss11 to UAS1-2 in pLG-UAS1-2. (C) Comparison of nucleotide sequences in UAS1-2 of the STA1 and the corresponding region of the FLO11 promoter. STA1, the inverted repeat sequence within UAS1-2 of the STA1 promoter is indicated. FLO11, the GCAAA sequence is altered to CCAAA in the FLO11 promoter. Three different bases of the UAS1-2 sequence between the STA1 and FLO11 promoters are underlined. FLO11-1, the CCAAA sequence in the FLO11 promoter was replaced with GCAAA by site-directed mutagenesis. (D) Effect of the inverted repeat sequence on Flo8 binding and lacZ expression. Transformants carrying plasmids pLG-UAS1-2[STA1], pLG-UAS1-2[FLO11], or pLG-UAS1-2[FLO11-1] were subjected to β-galactosidase (β-gal) assay under derepressing conditions (2% glycerol-ethanol). The results of a ChIP experiment with the FLO8-HA wild type, which contains each plasmid, are presented in the upper panel.
We next performed a ChIP assay to investigate the binding of Flo8 and Mss11 to UAS1-2. FLO8 and MSS11 tagged with triple HA on their C termini were integrated into their respective genomic loci. The resulting strains showed STA1 expression patterns similar to that of the wild type, indicating that the fusion proteins were functional (data not shown). These strains were transformed with the plasmid pLG-UAS1-2 and were grown in 2% glycerol-ethanol medium to the mid-log phase. After immunoprecipitation with HA antibody, the precipitates were analyzed by PCR with plasmid-specific primers that were designed to amplify only the plasmid-borne UAS1-2. Flo8-HA and Mss11-HA bound to UAS1-2 in wild-type cells, but the binding of Flo8-HA in isogenic mss11Δ mutant cells and the binding of Mss11-HA in flo8Δ mutant cells were completely eliminated (Fig. 2B). This result suggested that Flo8 and Mss11 influence each other's binding to UAS1-2. As controls in the ChIP assay, we confirmed that UAS1-2 was not immunoprecipitated together with untagged Flo8 or Mss11 (Fig. 2B) and that DNA fragments spanning the KSS1 open reading frame, which are unrelated to Flo8 and Mss11, were not immunoprecipitated with either Flo8-HA or Mss11-HA (data not shown).
Next, we compared the levels of Flo8-HA and Mss11-HA in these strains by immunoblotting with anti-HA antibody to determine whether the failure of the binding to UAS1-2 in these mutant cells was due to a reduction in the levels of Flo8-HA or Mss11-HA. The expression of Flo8-HA or Mss11-HA in the mutant cells was comparable to the expression in wild-type cells, indicating that mutation of FLO8 or MSS11 did not affect the level of Mss11-HA or Flo8-HA, respectively (data not shown). Therefore, the inability of Flo8-HA and Mss11-HA to bind to UAS1-2 in the mutant cells was not due to reduced levels of the tagged proteins. The physical interaction of Flo8 and Mss11 and the requirement of both proteins for binding to UAS1-2 imply that they bind cooperatively to DNA.
The UAS1-2 of the STA1 promoter is identical to the corresponding region of the FLO11 promoter except for substitution of 3 bp (Fig. 2C). However, lacZ expression from UAS1-2 of the FLO11 promoter is 10-fold lower than that from the STA1 promoter (Fig. 2D). Consistent with these levels of lacZ expression, the binding of Flo8 to UAS1-2 of the FLO11 promoter was weaker than that observed at the STA1 promoter (Fig. 2D). By sequence analysis of UAS1-2 of the two promoters, we detected an inverted repeat sequence—TTTGC-n-GCAAA (n = 97 bp)—in the STA1 promoter and revealed that this sequence is replaced with TTTGC-n-CCAAA (n = 97 bp) in the FLO11 promoter. To examine the role of this inverted repeat sequence in UAS1-2, we replaced the CCAAA sequences in the FLO11 promoter with GCAAA, as in the STA1 promoter (Fig. 2C). lacZ expression from the resulting plasmid (FLO11-1) was increased approximately sevenfold relative to that of the native FLO11 promoter. In addition, ChIP assays revealed that Flo8 binding to this modified FLO11-1 promoter was increased (Fig. 2D). Furthermore, Mss11 binding to FLO11-1 was increased to the same extent as Flo8 binding (data not shown). These results suggest that all or part of this inverted repeat sequence is important for activation of STA1 expression and that it is probably the region to which Flo8 and Mss11 binds.
Flo8 and Mss11 activate STA1 expression cooperatively.
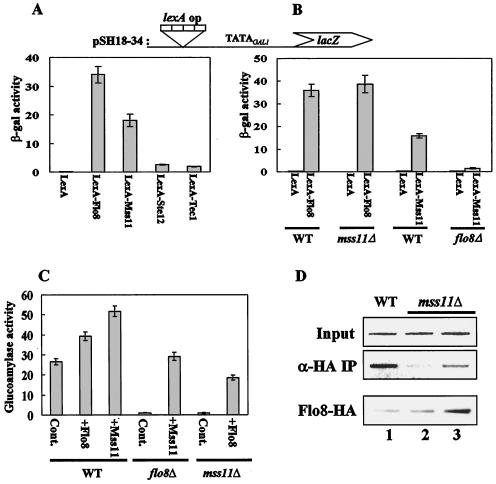
To investigate the functional relationship between Flo8 and Mss11, we examined whether mutations of Flo8 and Mss11 affect each other's ability to activate transcription with a LexA tethering assay. Wild-type and mutant cells were cotransformed with plasmids expressing the LexA DNA-binding domain fused to one of four activator proteins (or LexA alone) from the ADH1 promoter and the plasmid pSH18-34 containing a lacZ reporter under the control of eight lexA operators and the minimal TATA region from the GAL1 promoter (10, 39, 46). In wild-type cells, LexA-Flo8 and LexA-Mss11 each activated lacZ expression, with activation by Flo8 being approximately twofold higher than that by Mss11. In contrast, neither LexA-Ste12 nor LexA-Tec1 activated lacZ expression (Fig. 3A).
FIG. 3.
Flo8 interacts functionally with Mss11. (A) Transcriptional activation by DNA-bound activators. pSH18-34 (lexA operator) and plasmids expressing LexA-Flo8, LexA-Mss11, LexA-Ste12, LexA-Tec1, or LexA were cotransformed into wild-type (WT) cells grown in synthetic medium lacking uracil and histidine and containing 2% glucose. β-Galactosidase (β-gal) activities were tested on three independent colonies. (B) Activation by LexA-Mss11 requires Flo8. pSH18-34 and the plasmids expressing LexA-Flo8, LexA-Mss11, and LexA were cotransformed into the wild type and the flo8Δ and mss11Δ mutants. β-Galactosidase activities were tested on three independent colonies. (C) Suppression of flo8Δ or mss11Δ mutations by multicopy MSS11 or FLO8, respectively. Empty plasmid pRS326 (control [Cont]), pRS326-FLO8-HA (+Flo8), and pRS326-MSS11-HA (+Mss11) were transformed into the wild type and mutants. The resulting transformants were grown in synthetic medium containing 2% glycerol-ethanol as carbon sources and tested for glucoamylase activity. Glucoamylase activities are averages of the results from three independent experiments. (D) FLO8-HA wild type (lane 1), FLO8-HA mss11Δ (lane 2), and FLO8-HA mss11Δ transformed with pRS326-FLO8-HA (lane 3) were subjected to an anti-HA (α-HA) ChIP assay as described for Fig. 2B with 1 mg of total extracts. The bottom panel represents the level of Flo8-HA in the corresponding strains. The same protein extracts (100 μg) as used in the anti-HA ChIP assay were separated by SDS-8% PAGE and analyzed by Western blot analysis with anti-HA antibody.
We then examined whether activation by LexA-Flo8 or LexA-Mss11 requires Mss11 or Flo8, respectively. LexA-Flo8 still activated lacZ expression in mss11Δ cells, whereas LexA-Mss11 failed to activate lacZ expression in flo8Δ cells (Fig. 3B). This result suggests that LexA-Mss11 tethered on lexA operators requires functional Flo8 for activation of the heterologous promoter and that Mss11 is dispensable for transcriptional activation when Flo8 is tethered to the promoter. The physical interaction between Flo8 and Mss11 (see above) and the requirement of functional Flo8 for transcriptional activation by Mss11 support the idea that Flo8 interacts physically and functionally with Mss11.
Gagiano et al. proposed that Mss11 acts downstream of Flo8 because multiple copies of MSS11 were able to restore invasive growth to flo8Δ mutant cells, whereas multiple copies of FLO8 did not restore invasive growth to mss11Δ mutant cells (13). However, we observed that STA1 expression was restored not only by introducing a multicopy plasmid carrying MSS11 into flo8Δ mutant cells but also by introducing a FLO8 multicopy plasmid into mss11Δ mutant cells (Fig. 3C). Interestingly, binding of Flo8 to UAS1-2 was observed in the absence of its partner, Mss11, when Flo8 was overexpressed (Fig. 3D). Similar binding results were observed when Mss11 was overexpressed in the absence of Flo8 (data not shown). The results of these UAS1-2 binding experiments are consistent with the ability of Flo8 and Mss11 overexpression to restore STA1 expression to mss11Δ and flo8Δ mutants, respectively, and suggest that Flo8 and Mss11 may bind to UAS1-2 as homodimers when they are overexpressed in the absence of their usual binding partners. Collectively, our findings indicate that Mss11 is not a downstream effector of Flo8 with regard to STA1 transcription. Rather, Flo8 and Mss11 function cooperatively to activate STA1 expression.
Flo8 and Mss11 act on UAS2-1 via interaction with Ste12 and Tec1.
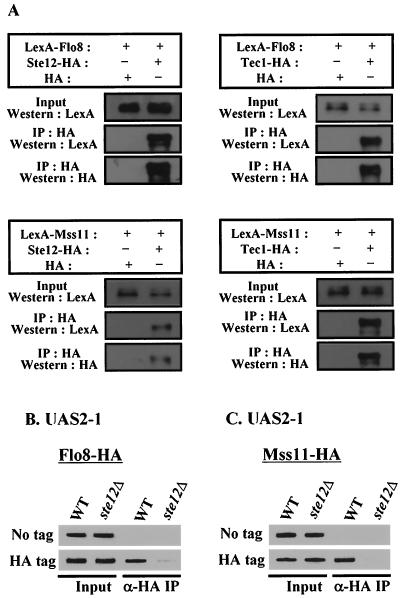
As described above, Flo8 and Mss11 are also required for UAS2-1-mediated transcriptional activation (Fig. 1B). UAS2-1 contains the FRE for Ste12 and Tec1 binding but does not contain the inverted repeat sequence to which Flo8 and Mss11 bind in UAS1-2. Therefore, we considered whether Flo8 and Mss11 may act indirectly at UAS2-1 by interacting with Ste12 and Tec1. To address this possibility, we first tested whether Flo8 and Mss11 coimmunoprecipitate with Ste12 and/or Tec1. Plasmids expressing LexA-Flo8 or LexA-Mss11 and plasmids expressing Ste12-HA or Tec1-HA were cotransformed into wild-type cells, and transformants were grown in 2% glucose medium. Total extracts were immunoprecipitated with monoclonal anti-HA antibody and analyzed as before. Western blot analysis with LexA antibody showed that LexA-Flo8 and LexA-Mss11 coimmunoprecipitated with Ste12-HA and Tec1-HA but not with HA alone (Fig. 4A). These interactions were also confirmed by GST pull-down assay (data not shown). These results support the idea that the four activators interact physically in vivo.
FIG. 4.
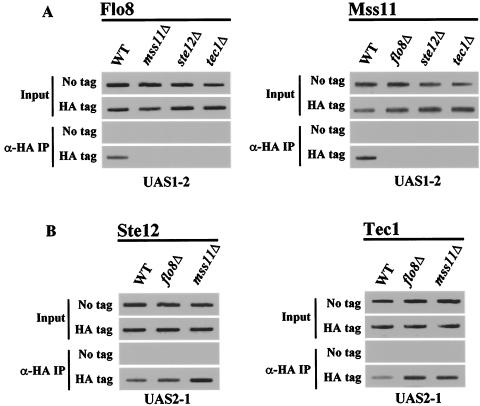
Flo8 and Mss11 act on UAS2-1 by interacting with Ste12 and Tec1. (A) The four activators interact with each other. Cells carrying plasmids which express LexA fusion proteins or HA-tagged proteins were grown in synthetic medium containing 2% glucose. Protein extracts (500 μg) were immunoprecipitated with anti-HA antibody. The precipitates and input extracts (50 μg) were separated by SDS-8 to 10% PAGE and analyzed by Western blot analysis with LexA antibody. The membranes were stripped and reprobed with anti-HA antibody. +, present; −, absent. (B and C) Flo8 and Mss11 binding to UAS2-1 requires Ste12 and Tec1. FLO8-HA wild-type (WT), FLO8-HA ste12Δ, MSS11-HA wild-type, MSS11-HA ste12Δ, or untagged strains bearing pLG-UAS2-1 were grown in synthetic medium containing 2% glycerol-ethanol to mid-log phase and subjected to anti-HA (α-HA) ChIP assays as described for Fig. 2B.
To investigate whether binding of Flo8 and Mss11 to UAS2-1 requires Ste12 and/or Tec1, we performed ChIP assays with the strains in which HA-tagged activator genes are integrated at their loci and which were transformed with the plasmid, pLG-UAS2-1. Plasmid-borne UAS2-1 DNA immunoprecipitated with Flo8-HA and Mss11-HA in wild-type cells, whereas this interaction was abolished in the isogenic ste12Δ mutant (Fig. 4B and C). The lack of Flo8-HA and Mss11-HA binding to UAS2-1 was not due to the reduced level of these fusion proteins in ste12Δ mutant cells because Western blot analysis of these strains revealed that mutation of STE12 does not affect the level of Mss11-HA and Flo8-HA (data not shown). These results demonstrate that cooperative binding of Ste12 and Tec1 to UAS2-1 is required for Flo8 and Mss11 binding to UAS2-1. These findings indicate that UAS2-1-mediated transcriptional activation involves Flo8, Mss11, Ste12, and Tec1.
Binding of Flo8 and Mss11 to the endogenous STA1 promoter requires Ste12 and Tec1.
Flo8 and Mss11 bind directly to plasmid-borne UAS1-2 (Fig. 2B) and activate lacZ reporter expression independently of Ste12 and Tec1, i.e., in ste12Δ and tec1Δ mutants (Fig. 1B). However, the effects of ste12Δ and tec1Δ mutations on plasmid-based UAS1-2::lacZ expression versus endogenous STA1 expression were different. Endogenous STA1 expression is completely abolished in ste12Δ and tec1Δ mutants (Fig. 1A), whereas lacZ expression from the plasmid pLG-UAS1-2 was unaffected in these deletion mutants (Fig. 1B).
To further investigate the interactions of transcriptional activators with specific promoter elements in the context of the endogenous STA1 gene, we performed ChIP assays with integrated HA-tagged strains. Flo8 and Mss11 bind to the endogenous UAS1-2 in wild-type cells but did not in the mss11Δ or flo8Δ mutants, respectively (Fig. 5A). The observation that Flo8 binding to UAS1-2 of the endogenous STA1 promoter requires Mss11 is consistent with the finding that Flo8 and Mss11 bind cooperatively to plasmid-borne UAS1-2. However, unexpectedly, binding of Flo8 and Mss11 to UAS1-2 in the endogenous STA1 promoter is also abolished in ste12Δ and tec1Δ mutants (Fig. 5A). This result provides a possible explanation for why endogenous STA1 expression is completely abolished in ste12Δ or tec1Δ mutants. On the other hand, flo8Δ and mss11Δ do not affect Ste12 and Tec1 binding to UAS2-1 of the STA1 promoter (Fig. 5B). These results suggest that Ste12 and Tec1 bind to the STA1 promoter prior to Flo8 and Mss11 binding and that Ste12 and Tec1 binding to UAS2-1 may function to recruit Flo8 and Mss11 to UAS1-2.
FIG. 5.
Flo8-Mss11 binding to the endogenous STA1 promoter requires Ste12 and Tec1. (A) The ste12Δ and tec1Δ mutations eliminate Flo8 and Mss11 binding to UAS1-2. FLO8-HA wild-type (WT), FLO8-HA mss11Δ, FLO8-HA ste12Δ, FLO8-HA tec1Δ, MSS11-HA wild-type, MSS11-HA flo8Δ, MSS11-HA ste12Δ, MSS11-HA tec1Δ, and untagged strains were grown in synthetic medium containing 2% glycerol-ethanol to mid-log phase, and anti-HA ChIP assays were performed with 1 mg of total extracts. (B) Ste12 and Tec1 binding to UAS2-1 is independent of Flo8 and Mss11. STE12-HA wild-type, STE12-HA flo8Δ, STE12-HA mss11Δ, TEC1-HA wild-type, TEC1-HA flo8Δ, TEC1-HA mss11Δ, and untagged strains were grown in synthetic medium containing 2% glycerol-ethanol to mid-log phase, and anti-HA (α-HA) ChIP assays were performed as described above with 500 μg of total extracts to determine Ste12 and Tec1 binding to UAS2-1.
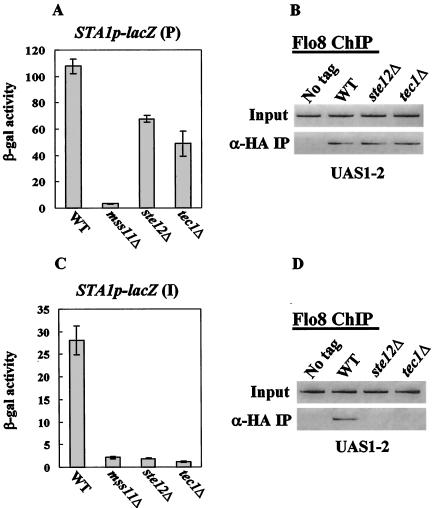
To investigate differences of the effect of Ste12 and Tec1 in the artificial plasmid and the endogenous STA1 promoter, we examined lacZ expression and Flo8 binding to UAS1-2 using the artificial plasmid containing the −2105 to −1 region (both UAS1 and UAS2) of the STA1 promoter and the strain which contains a STA1p-lacZ reporter at the original STA1 locus. lacZ expression from the plasmid containing the −2105 to −1 region of the STA1 promoter was not abolished, but rather reduced by half in both ste12Δ and tec1Δ mutants (Fig. 6A). Furthermore, Flo8 can still bind to plasmid-borne UAS1-2 in the ste12Δ and tec1Δ mutants (Fig. 6B). In contrast, with the STA1p-lacZ reporter integrated at the STA1 locus, both lacZ expression and Flo8 binding were abolished in the ste12Δ and tec1Δ mutants (Fig. 6C and D). These results explain the differential effects of the ste12Δ and tec1Δ mutations on plasmid-based UAS1-2::lacZ expression versus endogenous STA1 expression.
FIG. 6.
Effects of Ste12-Tec1 on binding of Flo8-Mss11 to UAS1-2 in the artificial plasmid or endogenous STA1 promoter. (A) Effect of Ste12 and Tec1 on lacZ expression from the STA1p-lacZ plasmid. The STA1p-lacZ plasmid was transformed into the wild type (WT) and each mutant, and three independent transformants of each were tested for β-galactosidase (β-gal) activity under the derepressed condition (2% glycerol-ethanol). (B) Binding of Flo8 to UAS1-2 in the STA1p-lacZ plasmid. The STA1p-lacZ plasmid was transformed into FLO8-HA wild-type, FLO8-HA ste12Δ, FLO8-HA tec1Δ, and untagged strains. The resulting transformants were grown in synthetic medium containing 2% glycerol-ethanol to mid-log phase, and anti-HA (α-HA) ChIP assays were performed as described above with 1 mg of total extracts to determine Flo8 binding to UAS1-2. (C) Effect of Ste12 and Tec1 on lacZ expression from the endogenous STA1 promoter. The STA1p-lacZ plasmid was integrated into the original STA1 locus. The wild type and isogenic mutants were tested for β-galactosidase activity under the derepressed condition (2% glycerol-ethanol). (D) Binding of Flo8 to UAS1-2 of the endogenous STA1 promoter. FLO8-HA wild-type, FLO8-HA ste12Δ, FLO8-HA tec1Δ, and untagged strains were grown in synthetic medium containing 2% glycerol-ethanol to mid-log phase, and anti-HA ChIP assays were performed as described above with 1 mg of total extracts to determine Flo8 binding to UAS1-2 in the endogenous STA1 promoter.
Ste12 and Tec1 recruit the Swi/Snf complex and thereby promote Flo8 and Mss11 binding to the endogenous STA1 promoter.
As mentioned previously, components of the Swi/Snf complex, such as SNF2/GAM1, SWI1/GAM3, SNF5, and SNF6 are involved in STA1 expression (25, 50). The Swi/Snf complex interacts with several sequence-specific activators, such as Gal4, VP16, and Swi5, and assists some activators in binding to DNA (30-32, 44, 51). Furthermore, Swi5, a sequence-specific activator, recruits the Swi/Snf complex to the HO promoter (4, 8, 23). Therefore, it seemed possible that the Swi/Snf complex is recruited to the STA1 promoter by a gene-specific activator and that the Swi/Snf complex may thereby mediate the binding of additional activators to the STA1 promoter.
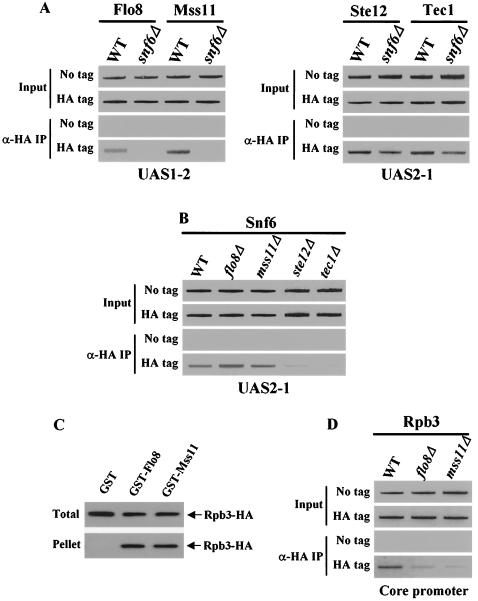
To examine the effect of the Swi/Snf complex on the binding of activators to the STA1 promoter, we performed ChIP assays with the integrated HA-tagged strains and isogenic snf6Δ mutants. The snf6Δ mutant abolished Flo8 and Mss11 binding to UAS1-2, but it did not affect Ste12 and Tec1 binding to UAS2-1 (Fig. 7A). Furthermore, the snf6Δ mutant also blocked binding of Flo8 and Mss11 to UAS2-1, but it did not affect the levels of Flo8 and Mss11 (data not shown). The fact that Ste12 and Tec1 still bind to the STA1 promoter in the snf6Δ mutant suggests that Ste12 and Tec1 may recruit the Swi/Snf complex to the STA1 promoter and that the Swi/Snf complex may then promote the binding of Flo8 and Mss11 to UAS1-2.
FIG. 7.
Ste12 and Tec1 recruit the Swi/Snf complex to promote Flo8 and Mss11 binding to UAS1-2 in the endogenous STA1 promoter. (A) Interactions between activators and the endogenous STA1 promoter in a snf6Δ mutant. FLO8-HA wild-type (WT), FLO8-HA snf6Δ, MSS11-HA wild-type, MSS11-HA snf6Δ, STE12-HA wild-type, STE12-HA snf6Δ, TEC1-HA wild-type, TEC1-HA snf6Δ, and untagged strains were grown in synthetic medium containing 2% glycerol-ethanol to mid-log phase, and anti-HA (α-HA) ChIP assays were performed with 1 mg (for Flo8 and Mss11) and 500 μg (for Ste12 and Tec1) of total extracts to determine the binding of activators to UAS1-2 or UAS2-1. (B) Ste12 and Tec1 recruit the Swi/Snf complex to the STA1 promoter. SNF6-HA wild-type, SNF6-HA flo8Δ, SNF6-HA mss11Δ, SNF6-HA ste12Δ, SNF6-HA tec1Δ, and untagged strains were grown in synthetic medium containing 2% glycerol-ethanol to mid-log phase, and anti-HA ChIP assays were performed as described above with 500 μg of total extracts to determine Snf6 binding to the endogenous STA1 promoter. (C) Flo8 and Mss11 interact with a component of RNA polymerase II. Whole-cell extracts (500 μg) prepared from the integrated RPB3-HA strain were incubated with 5 μg (each) of GST, GST-Flo8, or GST-Mss11. The GST proteins and their interacting proteins were precipitated with glutathione-agarose beads. Fractions of input (1/10) and pellet (1/2) were analyzed by Western blot analysis with anti-HA antibody. (D) Flo8 and Mss11 are required for recruitment of RNA polymerase II. RPB3-HA wild-type, RPB3-HA flo8Δ, RPB3-HA mss11Δ, and untagged strains were grown in synthetic medium containing 2% glycerol-ethanol to mid-log phase, and anti-HA ChIP assays were performed as described above with 500 μg of total extracts to determine RNA polymerase II binding to the core promoter of the endogenous STA1 promoter.
To identify the transcription factors that directly recruit the Swi/Snf complex to the STA1 promoter, we performed ChIP assays with SNF6-HA integrated strains and isogenic flo8Δ, mss11Δ, ste12Δ, and tec1Δ mutants. Snf6 binds to UAS2-1 in wild-type cells, and as expected, Snf6 also binds to UAS2-1 in the flo8Δ and mss11Δ mutants but not in the ste12Δ and tec1Δ mutants (Fig. 7B). This result supports the idea that Ste12 and Tec1 recruit the Swi/Snf complex to the STA1 promoter to promote subsequent Flo8 and Mss11 binding.
Flo8 and Mss11 recruit RNA polymerase II to the STA1 promoter.
It has been reported that Flo8 interacts with Rpb9, a component of RNA polymerase II (18). This result and our finding that STA1 expression is eliminated in flo8Δ and mss11Δ mutants, even though Ste12, Tec1, and the Swi/Snf complex bind to the STA1 promoter in such mutants, suggests that Flo8 and/or Mss11 directly recruits RNA polymerase II to the STA1 promoter. To test this idea, we first performed a GST pull-down assay to investigate whether Flo8 and Mss11 interact with a component of RNA polymerase II by using total extracts prepared from the integrated RPB3-HA strain. As shown in Fig. 7C, Rpb3-HA interacts with GST-Flo8 and GST-Mss11 but not with GST on its own.
To examine whether RNA polymerase II binding to the STA1 core promoter requires Flo8 and Mss11, we next carried out ChIP assays with an integrated RPB3-HA strain and isogenic flo8Δ and mss11Δ mutants. In the derepressed condition, Rpb3 bound to the core promoter of the STA1 gene in wild-type cells, but this binding was eliminated in the flo8Δ and mss11Δ mutants (Fig. 7D). All of these results indicate that Flo8 and/or Mss11 recruit RNA polymerase II to activate STA1 expression.
The MAP kinase pathway is involved in activation of STA1 expression.
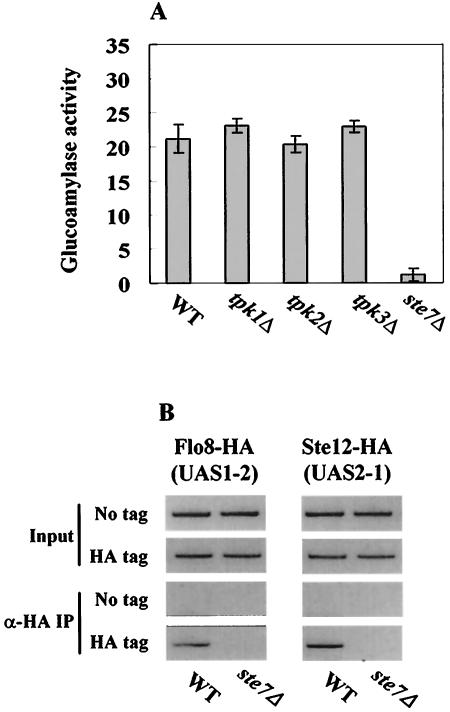
Flo8, Mss11, Ste12, and Tec1 are known to be activated by two independent signaling pathways: cAMP-PKA and MAP kinase (14, 29, 33, 38). We therefore examined whether these pathways are also involved in STA1 expression under the derepressed condition. Although it has been reported that Tpk2 is required for binding of Flo8 to the FLO11 promoter, in the absence of glucose, STA1 expression was not affected by mutations of TPK1, TPK2, and TPK3, three catalytic subunits of the cAMP-PKA pathway (Fig. 8A). In addition, Flo8 still binds to the STA1 promoter in the tpk2Δ mutant (data not shown).
FIG. 8.
MAP kinase pathway is required for activation of STA1 expression. (A) Effect of cAMP-PKA and MAP kinase pathways on STA1 expression. The wild type (WT) and each mutant were grown in synthetic medium containing 2% glycerol-ethanol as carbon sources. Glucoamylase activities are averages of the results from three independent experiments. (B) Mutation of STE7 prevents activators from binding to the STA1 promoter. FLO8-HA wild-type, FLO8-HA ste7Δ, STE12-HA wild-type, STE12-HA ste7Δ, and untagged strains were grown in synthetic medium containing 2% glycerol-ethanol to mid-log phase, and anti-HA (α-HA) ChIP assays were performed as described above with 500 μg or 1 mg of total extracts to determine binding of activators to the STA1 promoter.
In contrast, mutation of STE7, a component of the MAP kinase pathway, reduces glucoamylase activity (Fig. 8A), as do ste12Δ and tec1Δ mutations. We also tested binding of Flo8 and Ste12 to the STA1 promoter in the ste7Δ mutant. As expected, Flo8 and Ste12 did not bind to the STA1 promoter in this mutant (Fig. 8B). Collectively, these results indicate that the MAP kinase pathway, but not the cAMP-PKA pathway, activates STA1 expression through Ste12 and Tec1.
Activation versus glucose repression of STA1 expression.
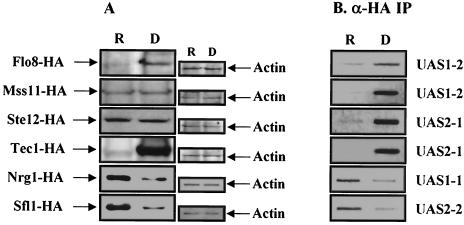
Previously, it was reported that the repressors Nrg1 and Sfl1 bind to UAS1-1 and UAS2-2, respectively, and that the levels of these two repressors were reduced about fourfold in the absence of glucose (20, 35). Furthermore, it was found that Sfl1 directly inhibits FLO8 expression (20). We therefore measured the levels of STA1 activators under repressed and derepressed conditions to examine whether the expression of MSS11, STE12, and TEC1 is also affected by different carbon sources. The integrated HA-tagged strains were grown in synthetic medium containing 2% glucose or 2% glycerol-ethanol. Levels of Flo8 and Tec1 increased in the derepressed condition, whereas the levels of Mss11 and Ste12 were similar in both conditions (Fig. 9A). Previous results and this finding suggest that the reduced levels of Nrg1 and Sfl1 and the increased levels of Flo8 and Tec1 in the absence of glucose lead to activation of STA1 expression (20).
FIG. 9.
The four activators and the Swi/Snf complex occupy the STA1 promoter in the absence of glucose. (A) FLO8 and TEC1 expression is increased, but amounts of Nrg1 and Sfl1 are reduced under derepressing conditions. HA-tagged strains (wild type) were grown in synthetic medium containing 2% glucose (repressed condition [R]) or 2% glycerol-ethanol (derepressed condition [D]) to mid-log phase. Protein extracts (100 μg) prepared from each integrated HA-tagged strain were separated by SDS-PAGE and Western blot analysis with anti-HA (α-HA) antibody. The same membranes were probed with an anti-actin monoclonal antibody. (B) The four activators occupy the STA1 promoter under derepressing conditions. HA-tagged strains (wild type) were grown in synthetic medium containing 2% glucose (R) or 2% glycerol-ethanol (D) to mid-log phase, and anti-HA ChIP assays were performed as described above with 1 mg (for Flo8 and Mss11) and 500 μg (for Ste12, Tec1, Nrg1, and Sfl1) of total extracts to determine the binding of transcription factors to the STA1 promoter.
We also performed a ChIP assay with each strain described above under the same conditions. In glucose-grown cells, an association of Flo8 and Tec1 with the STA1 promoter was not detected, whereas binding of Nrg1 and Sfl1 to the STA1 promoter was observed (Fig. 9B). This result may be due to the reduced levels of Flo8 and Tec1 (Fig. 9A). Furthermore, binding of Mss11 and Ste12 is also abolished in glucose-grown cells, supporting the notion that Mss11 and Ste12 require Flo8 and Tec1 for STA1 promoter binding. In the absence of glucose, instead of the Nrg1 and Sfl1 repressors, all four activators bind strongly to the STA1 promoter. These results suggest that STA1 expression may be activated by the increased levels of the Flo8 and Tec1 activators and the reduced levels of Nrg1 and Sfl1 repressors.
DISCUSSION
The STA1 promoter has an unusually large upstream region containing multiple regulatory elements (20). Here, we show that two heterodimeric proteins, Flo8-Mss11 and Ste12-Tec1, known to be regulated by the cAMP-PKA and MAP kinase signaling pathways, bind to UAS1-2 and UAS2-1, respectively, of the STA1 promoter to activate STA1 expression. We have also shown how these activators function together with the Swi/Snf complex to regulate STA1 expression.
Transcriptional activators and the Swi/Snf complex are involved in activation of STA1 expression.
STA1 expression is activated by multiple transcriptional activators with different functions. Several findings support the idea that Flo8 and Mss11 play a principle role in activating STA1 expression. First, glucoamylase assays and ChIP assays show that STA1 expression is abolished in flo8Δ and mss11Δ mutants even though Ste12, Tec1, and the Swi/Snf complex still bind to the STA1 promoter in these mutants. Second, Flo8 and Mss11 have more strong activation activity than Ste12 and Tec1. Third, Flo8 and Mss11 interact physically with RNA polymerase II and are required for recruiting RNA polymerase II to the STA1 promoter.
Ste12, Tec1, and the Swi/Snf complex are also important for activating STA1 expression. However, they appear to be accessory factors that assist Flo8 and Mss11 binding. It has been reported that gene-specific activators, including Swi5, recruit the Swi/Snf complex to the target promoter and that the Swi/Snf complex increases the accessibility of nucleosomal DNA to DNase I to promote binding of transcription factors (9, 43). Our ChIP assays showed that Ste12 and Tec1 are required to recruit the Swi/Snf complex to the STA1 promoter and that the Swi/Snf complex is required for Flo8 and Mss11 to bind to the STA1 promoter. From these results, we conclude that Ste12 and Tec1 recruit the Swi/Snf complex to the STA1 promoter and that the Swi/Snf complex in turn facilitates Flo8 and Mss11 binding to STA1 promoter to activate STA1 transcription.
Flo8 and Mss11 play a critical role as a heterodimer in activating STA1 expression.
The activation of STA1 expression in the absence of glucose requires the cooperation of several activators. Several lines of evidence strongly suggest that two of them, Flo8 and Mss11, function as a heterodimer to activate STA1 expression. First, mutations of FLO8 and MSS11 have similar phenotypes. Second, Flo8 and Mss11 coimmunoprecipitate in vivo. Third, both proteins have Lissencephaly type 1-like homology (LisH) motifs at their N termini (Flo8, amino acids 73 to 105; Mss11, amino acids 50 to 83). Fourth, Flo8 and Mss11 bind cooperatively to an inverted repeat sequence in UAS1-2 of the STA1 promoter. Furthermore, we show here that this inverted repeat sequence is also present in the promoters of the FLO1 and FLO11 genes, which are also activated by Flo8 and Mss11 (Fig. 2C and unpublished data). This finding indicates that Flo8 and Mss11 cooperatively activate STA1, FLO11, and FLO1 expression.
The LisH motif is an alpha-helical motif present in Lis1, Nopp140, LEUNIG, and several WD40 repeat-containing proteins that contribute to the regulation of microtubule dynamics by promoting dimerization (1, 17, 26, 41). These reports and our findings suggest that Flo8 and Mss11 may form heterodimers via the LisH motif. In addition, we have shown that Flo8 or Mss11 is able to bind to the STA1 promoter in mss11Δ or flo8Δ mutants, respectively, when they are overexpressed, suggesting that both proteins can form homodimers capable of binding to UAS1-2 in the absence of their usual partner. Although Gagiano et al. showed that Mss11 acted as a downstream promoter of Flo8 in enhancing haploid invasion (13), our findings demonstrate that Flo8 cooperates with Mss11 to activate STA1 expression.
Transcriptional activators, the Swi/Snf complex, and RNA polymerase II may bind sequentially to the STA1 promoter.
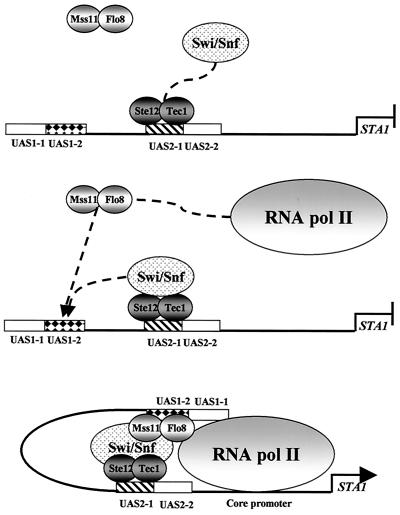
The molecular mechanism of HO gene expression has been particularly well studied. Multiple factors, including Swi5, SBF, Swi/Snf, and the SAGA complex are involved in this regulation and bind sequentially to the HO promoter (4, 7, 8). We attempted to perform a kinetic analysis of the association of transcription factors at STA1 but were unsuccessful due to the slow derepression of STA1 transcription when cells are shifted from glucose to glycerol-ethanol media. However, our findings suggest that multiple DNA-binding activators, the Swi/Snf complex, and RNA polymerase II bind sequentially to the STA1 promoter. We provide evidence that Ste12 and Tec1 bind to UAS2-1 and recruit the Swi/Snf complex. Next, the Swi/Snf complex facilitates Flo8 and Mss11 binding to UAS1-2. Finally, Flo8 and Mss11 recruit RNA polymerase II to the STA1 promoter (Fig. 10).
FIG. 10.
Model for activation of STA1 transcription. In the absence of glucose, among the four activators, Ste12 and Tec1 first bind cooperatively to UAS2-1 of the STA1 promoter and then recruit the Swi/Snf complex. The Swi/Snf complex then facilitates cooperative binding of Flo8 and Mss11 to UAS1-2 and the formation of a looped DNA domain (3). Finally, Flo8 and Mss11 recruit RNA polymerase II to the core promoter of STA1.
Differences in regulation of STA1 and FLO11 expression.
It has been reported that expressions of STA1 and FLO11 are coregulated in response to nutrient signals, since the 5′ upstream regions of the two genes are similar (13). In fact, the upstream regions of STA1 and FLO11 show a strong similarity (94.6%) except for two inserts (of 20 and 64 bp), small substitutions, and deletions. However, lacZ expression driven by the entire FLO11 promoter is approximately 20-fold lower than that of the STA1 promoter (unpublished data). We suggest that these differences may be due to two reasons. First, the inverted repeat sequence, TTTGC-n-GCAAA, a probable binding site of Flo8 and Mss11 in UAS1-2, is replaced with TTTGC-n-CCAAA in the FLO11 promoter (Fig. 2C). Second, the FLO11 promoter has an incomplete FRE due to the insertion of two bases, TGCCTTTCA (−1269 to −1261), in the Ste12 binding site. These findings suggest that Flo8 and Mss11 bind less efficiently to the FLO11 promoter than to the STA1 promoter (Fig. 2D) and that Ste12 and Tec1 may not bind directly to the FLO11 promoter (data not shown). Previously, Lo and Dranginis suggested that the sequence TGCAACA-n-CATTCT(n = 14), located at −725 to −699 of the FLO11 promoter, may be a target site for cooperative binding of Ste12 and Tec1 despite a 1-base mismatch relative to the consensus sequence (27). However, Ste12 binding to this region was not observed in vivo (data not shown). Although it was reported that a ste12Δ mutation reduces invasive growth and pseudohyphal differentiation, these results suggest that Ste12 may not be involved in FLO11 expression under certain conditions. Previous reports that Ste12 and Tec1 are not required for activation of FLO11 expression in response to amino acid starvation support this interpretation (5).
Transcription factors regulated by two signaling pathways are required for activation of STA1 expression.
As previously reported, two independent pathways, MAP kinase and cAMP-PKA, are known to be involved in the regulation of FLO11 transcription (14, 29, 33, 34, 38), and transcription factors activated by these two pathways, Flo8 and Ste12-Tec1, play important roles in STA1 expression (13, 14, 19). The wild-type TPK2 gene is required for Flo8 binding to the FLO11 promoter. However, we show here that mutation of TPK2 does not affect STA1 expression under derepressing conditions (Fig. 8A). Recently, Pan and Heitman suggested that Tpk1 or Tpk3 may regulate Flo8 binding in tpk2Δ mutants because Tpk1 can phosphorylate Flo8 in vitro (34). However, we failed to obtain any evidence that Tpk1 and Tpk3 are involved in STA1 expression. Instead of Tpk2, it is possible that another kinase regulates the binding of Flo8 to the STA1 promoter. A possible candidate is Snf1 because the Snf1 kinase is critical for STA1 and FLO11 expression in response to glucose limitation in haploids (24). We also showed that mutation of STE7, a component of the MAP kinase pathway, blocked not only Ste12 binding to UAS2-1 but also binding of Flo8 to UAS1-2 of the STA1 promoter (Fig. 8B). These results indicate that the MAP kinase pathway activates STA1 expression through the downstream targets Ste12 and Tec1.
Although the cAMP-PKA pathway is not involved in the activation of STA1 expression, our findings describe how Flo8, Mss11, Ste12, and Tec1, regulated by two independent signaling pathways, control STA1 expression. The mechanism of STA1 transcriptional regulation thus provides an excellent example of how multiple signaling pathways converge to control eukaryotic RNA polymerase II-mediated gene expression.
Activation versus repression of STA1 expression.
NRG1 and SFL1, two major repressors for glucose repression of STA1 expression, were initially identified as multicopy inhibitors of the STA1 promoter under the derepressed condition (20, 35). This implies that the increased gene dosage of NRG1 and SFL1 reduces STA1 expression, even though cells are grown in derepressed conditions. Furthermore, the introduction of a multicopy plasmid containing FLO8 or MSS11 increases STA1 expression even under repressed conditions (data not shown). These results suggest that the relative abundance of transcriptional regulators involved in STA1 expression may be essential for determining activation or repression of STA1 expression.
We therefore measured the levels of transcriptional regulators under repressed and derepressed conditions by Western blot analysis. We found that the levels of two repressors, Nrg1 and Sfl1, were reduced approximately fourfold in the absence of glucose, whereas the levels of Flo8 and Tec1 were increased. These results suggest that activation of STA1 expression results from the increased levels of Flo8 and Tec1 plus the reduced levels of Nrg1 and Sfl1 repressors under derepressed conditions.
The binding of various transcriptional regulators to the STA1 promoter appears to be correlated with the concentrations of the regulators under different growth conditions. In the presence of glucose, the levels of Nrg1 and Sfl1 are high and they can occupy the STA1 promoter and repress transcription. However, in the absence of glucose, the levels of Nrg1 and Sfl1 fall, they are removed from the STA1 promoter, and they are replaced by the transcriptional activators Ste12, Tec1, Flo8, and Mss11. However, we cannot exclude the possibility that the function of transcriptional repressors and activators is also regulated at the posttranscriptional level, even though the levels of activators or repressors are correlated with the binding of them to DNA. Also, we cannot rule out the possibility that these differences according to conditions reflect a secondary feedback of changes in signaling on the levels of regulators.
Acknowledgments
This work was supported by a grant from the Korea Science and Engineering Foundation (R01-2002-000-00221-0 [2002]) given to H.S.K. T.S.K. and H.Y.K. are supported by a BK21 Research Fellowship from the Ministry of Education and Human Resources Development.
REFERENCES
- 1.Ahn, C., and N. R. Morris. 2001. Nudf, a fungal homolog of the human LIS1 protein, functions as a dimer in vivo. J. Biol. Chem. 276:9903-9909. [DOI] [PubMed] [Google Scholar]
- 2.Ahn, J. H., S. H. Park, and H. S. Kang. 1995. Inactivation of the UAS1 of STA1 by glucose and STA10 and identification of two loci, SNS1 and MSS1, involved in STA10-dependent repression in Saccharomyces cerevisiae. Mol. Gen. Genet. 246:529-537. [DOI] [PubMed] [Google Scholar]
- 3.Bazett-Jones, D. P., J. Cote, C. C. Landel, C. L. Peterson, and J. L. Workman. 1999. The SWI/SNF complex creates loop domains in DNA and polynucleosome arrays and can disrupt DNA-histone contacts within these domains. Mol. Cell. Biol. 19:1470-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhoite, L. T., Y. Yu, and D. J. Stillman. 2001. The Swi5 activator recruits the Mediator complex to the HO promoter without RNA polymerase II. Genes Dev. 15:2457-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braus, G. H., O. Grundmann, S. Bruckner, and H. U. Mosch. 2003. Amino acid starvation and Gcn4p regulate adhesive growth and FLO11 gene expression in Saccharomyces cerevisiae. Mol. Biol. Cell 14:4272-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christianson, T. W., R. S. Sikorski, M. Dante, J. H. Shero, and P. Hieter. 1992. Multifunctional yeast high-copy-number shuttle vectors. Gene 110:119-122. [DOI] [PubMed] [Google Scholar]
- 7.Cosma, M. P. 2002. Ordered recruitment: gene-specific mechanism of transcription activation. Mol. Cell 10:227-236. [DOI] [PubMed] [Google Scholar]
- 8.Cosma, M. P., T. Tanaka, and K. Nasmyth. 1999. Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle- and developmentally regulated promoter. Cell 97:299-311. [DOI] [PubMed] [Google Scholar]
- 9.Cote, J., J. Quinn, J. L. Workman, and C. L. Peterson. 1994. Stimulation of GAL4 derivative binding to nucleosomal DNA by the yeast SWI/SNF complex. Science 265:53-60. [DOI] [PubMed] [Google Scholar]
- 10.Estojak, J., R. Brent, and E. A. Golemis. 1995. Correlation of two-hybrid affinity data with in vitro measurements. Mol. Cell. Biol. 15:5820-5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleming, A. B., and S. Pennings. 2001. Antagonistic remodelling by Swi-Snf and Tup1-Ssn6 of an extensive chromatin region forms the background for FLO1 gene regulation. EMBO J. 20:5219-5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagiano, M., M. Bester, D. van Dyk, J. Franken, F. F. Bauer, and I. S. Pretorius. 2003. Mss11p is a transcription factor regulating pseudohyphal differentiation, invasive growth and starch metabolism in Saccharomyces cerevisiae in response to nutrient availability. Mol. Microbiol. 47:119-134. [DOI] [PubMed] [Google Scholar]
- 13.Gagiano, M., D. Van Dyk, F. F. Bauer, M. G. Lambrechts, and I. S. Pretorius. 1999. Divergent regulation of the evolutionarily closely related promoters of the Saccharomyces cerevisiae STA2 and MUC1 genes. J. Bacteriol. 181:6497-6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagiano, M., D. van Dyk, F. F. Bauer, M. G. Lambrechts, and I. S. Pretorius. 1999. Msn1p/Mss10p, Mss11p and Muc1p/Flo11p are part of a signal transduction pathway downstream of Mep2p regulating invasive growth and pseudohyphal differentiation in Saccharomyces cerevisiae. Mol. Microbiol. 31:103-116. [DOI] [PubMed] [Google Scholar]
- 15.Guarente, L., and M. Ptashne. 1981. Fusion of Escherichia coli lacZ to the cytochrome c gene of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 78:2199-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hecht, A., S. Strahl-Bolsinger, and M. Grunstein. 1996. Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature 383:92-96. [DOI] [PubMed] [Google Scholar]
- 17.Hirokawa, N. 1998. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279:519-526. [DOI] [PubMed] [Google Scholar]
- 18.Ito, T., T. Chiba, R. Ozawa, M. Yoshida, M. Hattori, and Y. Sakaki. 2001. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. USA 98:4569-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, T. S., J. Y. Ahn, J. H. Yoon, and H. S. Kang. 2003. STA10 repression of STA gene expression is caused by a defective activator, flo8, in Saccharomyces cerevisiae. Curr. Genet. 44:261-267. [DOI] [PubMed] [Google Scholar]
- 20.Kim, T. S., S. B. Lee, and H. S. Kang. 2004. Glucose repression of STA1 expression is mediated by the Nrg1 and Sfl1 repressors and the Srb8-11 complex. Mol. Cell. Biol. 24:7695-7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kingston, R. E., and G. J. Narlikar. 1999. ATP-dependent remodeling and acetylation as regulators of chromatin fluidity. Genes Dev. 13:2339-2352. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi, O., H. Yoshimoto, and H. Sone. 1999. Analysis of the genes activated by the FLO8 gene in Saccharomyces cerevisiae. Curr. Genet. 36:256-261. [DOI] [PubMed] [Google Scholar]
- 23.Krebs, J. E., M. H. Kuo, C. D. Allis, and C. L. Peterson. 1999. Cell cycle-regulated histone acetylation required for expression of the yeast HO gene. Genes Dev. 13:1412-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuchin, S., V. K. Vyas, and M. Carlson. 2002. Snf1 protein kinase and the repressors Nrg1 and Nrg2 regulate FLO11, haploid invasive growth, and diploid pseudohyphal differentiation. Mol. Cell. Biol. 22:3994-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuchin, S. V., N. N. Kartasheva, and S. V. Benevolensky. 1993. Genes required for derepression of an extracellular glucoamylase gene, STA2, in the yeast Saccharomyces. Yeast 9:533-541. [DOI] [PubMed] [Google Scholar]
- 26.Leventer, R. J., C. Cardoso, D. H. Ledbetter, and W. B. Dobyns. 2001. LIS1: from cortical malformation to essential protein of cellular dynamics. Trends Neurosci. 24:489-492. [DOI] [PubMed] [Google Scholar]
- 27.Lo, W. S., and A. M. Dranginis. 1998. The cell surface flocculin Flo11 is required for pseudohyphae formation and invasion by Saccharomyces cerevisiae. Mol. Biol. Cell 9:161-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorch, Y., M. Zhang, and R. D. Kornberg. 1999. Histone octamer transfer by a chromatin-remodeling complex. Cell 96:389-392. [DOI] [PubMed] [Google Scholar]
- 29.Madhani, H. D., and G. R. Fink. 1997. Combinatorial control required for the specificity of yeast MAPK signaling. Science 275:1314-1317. [DOI] [PubMed] [Google Scholar]
- 30.Natarajan, K., B. M. Jackson, H. Zhou, F. Winston, and A. G. Hinnebusch. 1999. Transcriptional activation by Gcn4p involves independent interactions with the SWI/SNF complex and the SRB/mediator. Mol. Cell 4:657-664. [DOI] [PubMed] [Google Scholar]
- 31.Neely, K. E., A. H. Hassan, C. E. Brown, L. Howe, and J. L. Workman. 2002. Transcription activator interactions with multiple SWI/SNF subunits. Mol. Cell. Biol. 22:1615-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neely, K. E., A. H. Hassan, A. E. Wallberg, D. J. Steger, B. R. Cairns, A. P. Wright, and J. L. Workman. 1999. Activation domain-mediated targeting of the SWI/SNF complex to promoters stimulates transcription from nucleosome arrays. Mol. Cell 4:649-655. [DOI] [PubMed] [Google Scholar]
- 33.Pan, X., and J. Heitman. 1999. Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:4874-4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan, X., and J. Heitman. 2002. Protein kinase A operates a molecular switch that governs yeast pseudohyphal differentiation. Mol. Cell. Biol. 22:3981-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park, S. H., S. S. Koh, J. H. Chun, H. J. Hwang, and H. S. Kang. 1999. Nrg1 is a transcriptional repressor for glucose repression of STA1 gene expression in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:2044-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterson, C. L., and J. L. Workman. 2000. Promoter targeting and chromatin remodeling by the SWI/SNF complex. Curr. Opin. Genet. Dev. 10:187-192. [DOI] [PubMed] [Google Scholar]
- 37.Phelan, M. L., G. R. Schnitzler, and R. E. Kingston. 2000. Octamer transfer and creation of stably remodeled nucleosomes by human SWI-SNF and its isolated ATPases. Mol. Cell. Biol. 20:6380-6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rupp, S., E. Summers, H. J. Lo, H. Madhani, and G. Fink. 1999. MAP kinase and cAMP filamentation signaling pathways converge on the unusually large promoter of the yeast FLO11 gene. EMBO J. 18:1257-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato, T., M. Hanada, S. Bodrug, S. Irie, N. Iwama, L. H. Boise, C. B. Thompson, E. Golemis, L. Fong, H. G. Wang, et al. 1994. Interactions among members of the Bcl-2 protein family analyzed with a yeast two-hybrid system. Proc. Natl. Acad. Sci. USA 91:9238-9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith, D. S., M. Niethammer, R. Ayala, Y. Zhou, M. J. Gambello, A. Wynshaw-Boris, and L. H. Tsai. 2000. Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nat. Cell Biol. 2:767-775. [DOI] [PubMed] [Google Scholar]
- 42.Treitel, M. A., and M. Carlson. 1995. Repression by SSN6-TUP1 is directed by MIG1, a repressor/activator protein. Proc. Natl. Acad. Sci. USA 92:3132-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Utley, R. T., J. Cote, T. Owen-Hughes, and J. L. Workman. 1997. SWI/SNF stimulates the formation of disparate activator-nucleosome complexes but is partially redundant with cooperative binding. J. Biol. Chem. 272:12642-12649. [DOI] [PubMed] [Google Scholar]
- 44.Wallberg, A. E., K. E. Neely, A. H. Hassan, J. A. Gustafsson, J. L. Workman, and A. P. Wright. 2000. Recruitment of the SWI-SNF chromatin remodeling complex as a mechanism of gene activation by the glucocorticoid receptor τ1 activation domain. Mol. Cell. Biol. 20:2004-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webber, A. L., M. G. Lambrechts, and I. S. Pretorius. 1997. MSS11, a novel yeast gene involved in the regulation of starch metabolism. Curr. Genet. 32:260-266. [DOI] [PubMed] [Google Scholar]
- 46.West, R. W., Jr., R. R. Yocum, and M. Ptashne. 1984. Saccharomyces cerevisiae GAL1-GAL10 divergent promoter region: location and function of the upstream activating sequence UASG. Mol. Cell. Biol. 4:2467-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whitehouse, I., A. Flaus, B. R. Cairns, M. F. White, J. L. Workman, and T. Owen-Hughes. 1999. Nucleosome mobilization catalysed by the yeast SWI/SNF complex. Nature 400:784-787. [DOI] [PubMed] [Google Scholar]
- 48.Workman, J. L., and R. E. Kingston. 1998. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu. Rev. Biochem. 67:545-579. [DOI] [PubMed] [Google Scholar]
- 49.Yang, X., E. J. Hubbard, and M. Carlson. 1992. A protein kinase substrate identified by the two-hybrid system. Science 257:680-682. [DOI] [PubMed] [Google Scholar]
- 50.Yoshimoto, H., and I. Yamashita. 1991. The GAM1/SNF2 gene of Saccharomyces cerevisiae encodes a highly charged nuclear protein required for transcription of the STA1 gene. Mol. Gen. Genet. 228:270-280. [DOI] [PubMed] [Google Scholar]
- 51.Yudkovsky, N., C. Logie, S. Hahn, and C. L. Peterson. 1999. Recruitment of the SWI/SNF chromatin remodeling complex by transcriptional activators. Genes Dev. 13:2369-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]