Abstract
Background
Chronic rhinosinusitis with nasal polyps (CRSwNP) is often characterized by tissue eosinophilia that is associated with poor prognosis. Recent findings that proton pump inhibitors (PPIs) directly modulate expression of eotaxin-3, an eosinophil chemoattractant, in eosinophilic diseases suggest therapeutic potential for PPIs in CRSwNP.
Objective
We assessed the effect of type-2 mediators, particularly IL-13 and eotaxin-3, on tissue eosinophilia and disease severity in CRS. Further investigation focused on PPI suppression of eotaxin-3 expression in vivo and in vitro with exploration of underlying mechanisms.
Methods
Type-2 mediator levels in nasal tissues and secretions were measured by multiplex immunoassay. Eotaxin-3 and other chemokines expressed in IL-13-stimulated human sinonasal epithelial cells (HNECs) and BEAS-2Bs with or without PPIs was assessed by using ELISA, Western blot, real-time PCR, and intracellular pH (pHi) imaging.
Results
Nasal tissues and secretions from CRSwNP patients had increased IL-13, eotaxin-2 and eotaxin-3 levels, and these were positively correlated with tissue ECP and radiographic scores in CRS (P<.05). IL-13-stimulation of HNECs and BEAS-2Bs dominantly induced eotaxin-3 expression, which was significantly inhibited by PPIs (P<.05). CRS patients taking PPIs also showed lower in vivo eotaxin-3 levels compared with those without PPIs (P<.05). Using pHi imaging and by altering extracellular [K+], we found that IL-13 enhanced H+,K+-exchange, which was blocked by PPIs and the mechanistically unrelated H,K-ATPase inhibitor, SCH-28080. Furthermore, knockdown of ATP12A (gene for the non-gastric H,K-ATPase [ngH,K-ATPase]) significantly attenuated IL-13-induced eotaxin-3 expression in HNECs. PPIs also had effects on accelerating IL-13-induced eotaxin-3 mRNA decay.
Conclusion
Our results demonstrated that PPIs reduce IL-13-induced eotaxin-3 expression by airway epithelial cells. Furthermore, mechanistic studies suggest that the ngH,K-ATPase is necessary for IL-13-mediated epithelial responses, and its inhibitors, including PPIs, may be of therapeutic value in CRSwNP by reducing epithelial production of eotaxin-3.
Keywords: Chronic rhinosinusitis, Eotaxin-1/CCL11, Eotaxin-2/CCL24, Eotaxin-3/CCL26, Omeprazole, Proton pump inhibitors, Interleukin-13, Epithelial cells, H(+)-K(+)-Exchanging ATPase, Eosinophils
Introduction
Chronic rhinosinusitis (CRS) is characterized by local inflammation of the sinonasal mucosa with symptoms persisting for at least 12 weeks.1 It is further classified into 2 clinical phenotypes: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP).1-3 In Western populations, CRSwNP is frequently associated with type-2 inflammation and tissue eosinophilia.4 Since tissue eosinophilia has been implicated in increased post-surgical recurrence rates5, 6 and decreased improvements in quality of life outcomes7, strategies for blocking eosinophil recruitment could improve treatment for CRSwNP.
Eosinophil recruitment is generally regulated by type-2 cytokines (e.g., IL-4, IL-5, and IL-13) and CC chemokines (e.g., eotaxin-1/CCL11, eotaxin-2/CCL24, eotaxin-3/CCL26, and MCP-4/CCL13).8-10 Among the cytokines, IL-13 appears to drive epithelial responses including barrier dysfunction, mucus overproduction, and production of chemokines in type-2 inflammatory airway diseases.11-14 The induced eotaxins are ligands for the CC chemokine receptor 3 (CCR3) that is highly expressed on eosinophils.9, 15-19 Of the eotaxins expressed by humans, recent studies increasingly emphasize a critical role for eotaxin-3 in eosinophilic diseases, showing greater and more sustained eosinophil recruitment in asthma8, 20 and strong associations with susceptibility to eosinophilic esophagitis (EoE).21 In CRSwNP, increased levels of type-2 mediators and type-2 cytokine-producing cells, like Th2 cells and group 2 innate lymphoid cells (ILC2s) are found in nasal mucosa,22-25 supporting a crucial role of tissue eosinophilia in CRSwNP pathogenesis. However, whether eotaxin-3 is associated with eosinophilic responses in CRSwNP is yet to be established.
While novel monoclonal antibodies like mepolizumab (anti-IL-5) and dupilumab (anti-IL-4Rα) have shown promise for CRSwNP treatment,26, 27 cost, parenteral administration and lack of clinical approval for a CRSwNP indication will foreseeably limit access to these agents.28 Interestingly, recent studies have shown that proton pump inhibitors (PPIs), used traditionally for treating gastroesophageal reflux disease (GERD), suppress IL-13-induced eotaxin-3 production in esophageal squamous cells29-31 and have clinically relevant anti-eosinophil effects in EoE, even in patients without coexisting GERD.31, 32 Since PPIs are not used for treating CRSwNP, mechanistic evidence that PPIs may also directly suppress IL-13 responses in the upper airway may open new avenues for treating this common chronic inflammatory condition.
Given the biological parallels between EoE and CRSwNP,33 we characterized the relationship of type-2 mediators, particularly IL-13 and eotaxin-3, with tissue eosinophilia and disease severity in CRSwNP. We further evaluated the relative production of eotaxins by IL-13-stimulated airway cells in vitro and explored the efficacy of PPIs on inhibiting eotaxin-3 expression in vivo and in vitro. Finally, we investigated potential mechanisms by which PPIs suppressed IL-13-induced eotaxin-3 expression in airway epithelial cells.
Methods
Subjects and sample collection
Healthy controls and patients with CRS2, 34 were recruited from the Otolaryngology and Allergy-Immunology Clinics at Northwestern Medicine. Computed Tomography (CT) scans were graded according to the methods defined by Okushi et al.35 and history of taking PPIs listed in preoperative anesthesia records on the day of sinus surgery was obtained. Subject characteristics are included in Table E1. All subjects provided informed consent. The Institutional Review Board of Northwestern University-Feinberg School of Medicine approved this study. Tissue specimens including uncinate tissue (UT) and nasal polyp (NP), nasal lavage fluid, and epithelial scrapings from inferior turbinate (IT) and NP were obtained from subjects and prepared, as previously described.36, 37 Further details are provided in this article's Online Repository at www.jacionline.org.
Measurement of cytokines, eotaxins, and ECP in specimens
We measured IL-4, IL-13, eotaxin-1, eotaxin-2, and eotaxin-3 levels using the Milliplex Map kit (EMD Millipore, Billerica, MA) with a Luminex 200 instrument (Life Technologies, Gaithersburg, MD). We measured eosinophil cationic protein (ECP) levels using the Mesacup ECP Test (MBL International, Woburn, MA). Tissue concentrations of these mediators were normalized to the total protein concentration measured by the Bicinchoninic acid Protein Assay (Thermo Fisher Scientific, Watham, MA).
Cell culture
BEAS-2B, a human bronchial epithelial cell line transformed with a hybrid adenovirus 12-simian virus 40 was obtained from ATCC (CRL-9609, Manassas, VA). Primary HNECs were collected by epithelial scraping of IT and NP and cultured. For cytokine (Peprotech, Rocky Hill, NJ) stimulation, submerged cultured cells were treated with 1-100ng/ml IL-13, 10ng/ml IFN-γ, 100ng/ml TNF or 50ng/ml IL-17 for 6h or 48h. To study the effects of PPIs (Sigma-Aldrich, St Louis, MO) on cytokine-induced chemokines, cells were pretreated for 2h with acid-activated omeprazole (0.1-50μM) or other PPIs: lansoprazole, rabeprazole, pantoprazole, and esomeprazole (1-50μM) prior to stimulation with 5ng/ml IL-13. Additionally, SCH-28080 (1-50μM; Sigma-Aldrich) was used with the same protocol. In experiments altering extracellular K+ concentration ([K+]e), modified Ringer's solution that contained different contents of K+ (0-11.2mM KCl, Table E2) was used as culture media. For mRNA stability assessment, actinomycin D (3μg/ml, Sigma-Aldrich) was used and eotaxin-3 mRNA was measured using real-time PCR. Supernatants, whole cell lysates, and total RNAs were harvested for further analysis. Further detailed methods are described in this article's Online Repository.
ELISA
Eotaxin-1, eotaxin-2, and eotaxin-3 protein concentrations in supernatants were determined with the appropriate ELISA kits, as detailed in Online Repository.
Real-Time PCR and Western blot
mRNA levels of eotaxin-1, eotaxin-2, eotaxin-3, CXCL1, CXCL10, ATP12A, and ATP4A in total RNAs isolated from cells were measured using quantitative real-time PCR. Western blots were performed to assess total signal transducer and activator of transcription 6 (STAT6), phosphorylated-STAT6 (pSTAT6) and ATP12A protein in whole cell lysates.38 Further details are described in this article's Online Repository.
Intracellular pH (pHi) Imaging
The pH-sensitive dye, pHrodo® Green AM intracellular pH indicator (Life Technologies) that increases its fluorescence with decreasing pHi was used.39 Cells cultured in glass bottom microwell dishes (MatTek, Ashland, MA) were pre-treated with omeprazole or vehicle prior to 6h IL-13 stimulation. Then cells were incubated with dye (5 M) with live cell imaging solution (Life Technologies) at 37°C for 30 minutes per manufacturer's instructions. Spinning disk confocal microscopy for live cells imaging was performed with Andor XDi Revolution (Andor Technologies, Belfast, UK). Fluorescence intensity was measured in 150 cells using Image J software (National Institutes of Health, Bethesda, MD). For kinetic experiments, fluorescence intensity of cells cultured in 96-well plates with omeprazole, SCH-28080 or matched vehicle was measured at various times before and after IL-13 stimulation up to 1h using the SpectraMax® Gemini EM Microplate Spectrofluorometer (Molecular devices, Sunnyvale, CA) at 485/538nm (excitation/emission).
Small interfering RNA (siRNA) transfection
At 30–50% confluence, HNECs were transfected with 25pmol ON-TARGETplus ATP12A siRNA or non-targeting negative control siRNA (Dharmacon™; GE Healthcare Life Sciences) in Lipofectamine RNAiMAX reagent (Life Technologies) per manufacturer's instructions. At 96h post-transfection, cells were treated with omeprazole or vehicle, followed by IL-13 stimulation for 6h. Knockdown efficiency was confirmed by using real-time PCR and Western blots.
Statistical analyses
All data are reported as mean ± SEM, unless otherwise noted. A P-value less than .05 was considered significant. Further details are described in this article's Online Repository.
Results
Levels of type-2 inflammatory mediators and their relationship with tissue eosinophilia and radiographic severity
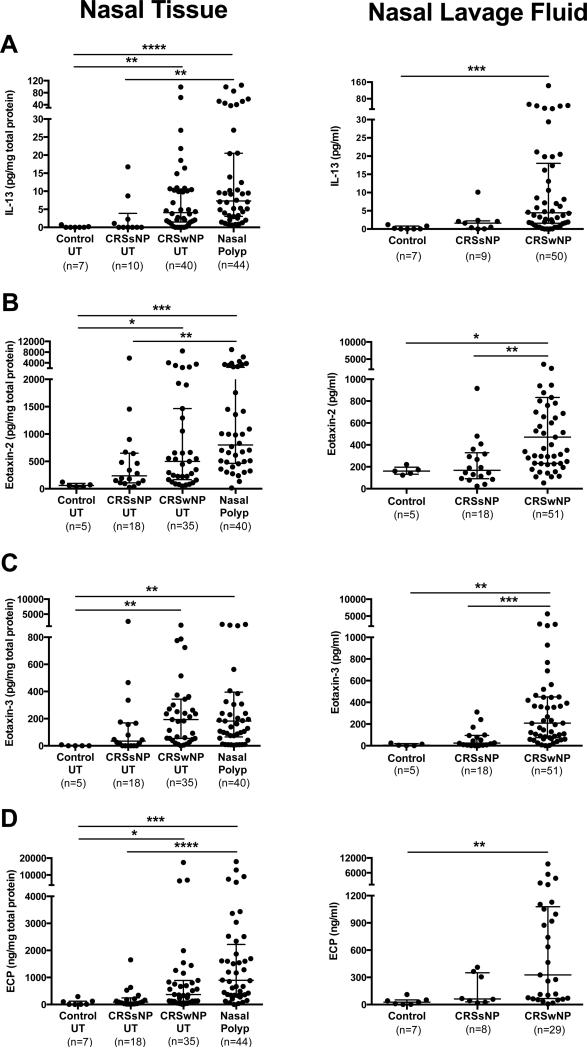
We first assessed whether type-2 mediators in vivo levels were increased in patients with CRSwNP. Consistent with our recent study,25 IL-13 levels, but not IL-4 (data not shown), were significantly elevated in CRSwNP UT and NP compared with control UT, with similar profiles in nasal lavage fluid (Fig 1, A). Among the eotaxins, eotaxin-2 (Fig 1, B) and eotaxin-3 (Fig 1, C) were significantly increased in tissues (UT and NP) and lavage fluid of CRSwNP compared with those of control. Eotaxin-1 levels were significantly elevated in NP only compared with control UT (median 61.0 versus 12.9 pg/mg total protein, respectively, P<.05). ECP levels were significantly elevated in nasal tissues and secretions of CRSwNP compared with control (Fig 1, D).
Figure 1.
Increased levels of type-2 inflammatory mediators in nasal tissues and secretions of CRSwNP. Protein levels of A, IL-13, B, eotaxin-2, C, eotaxin-3, and D, ECP were measured in UT, nasal polyp, and nasal lavage fluid. Dot plots illustrate individual data points, and solid lines represent median with interquartile range. *P<.05, **P<.01, ***P<.001, ****P<.0001.
We next evaluated the correlations between tissue eosinophilia, as determined by ECP, and levels of type-2 mediators. ECP levels were significantly correlated with eotaxin-2, eotaxin-3, and IL-13 levels in UT and in lavage fluid among all subjects (Table 1). We further correlated radiographic severity35 with these mediators in CRSwNP patients, and found that all eotaxins, IL-13 and ECP levels in UT were significantly correlated with CT scores (Table 1). Tissue and lavage eotaxin-2 and eotaxin-3 levels were also moderately correlated with UT IL-13 levels (Table E3). However, correlations carried out on type-2 mediators measured in NP were uncorrelated with local eosinophilia and radiographic severity (Table E4).
Table 1.
Correlations between type-2 inflammatory mediators and tissue eosinophilia or radiographic severity
| Type-2 inflammatory mediators | ECP in UT (Total Subjects*) | CT scores (Patients with CRSwNP†) | |||
|---|---|---|---|---|---|
| r | P-value | r | P-value | ||
| in UT | |||||
| IL-13 | 0.84 | <0.0001 | 0.49 | 0.002 | |
| Eotaxin-1 | 0.19 | 0.31 | 0.34 | 0.04 | |
| Eotaxin-2 | 0.70 | <0.0001 | 0.60 | 0.0002 | |
| Eotaxin-3 | 0.54 | 0.002 | 0.34 | 0.049 | |
| ECP | - | - | 0.58 | 0.0003 | |
| in Nasal Lavage Fluid | |||||
| IL-13 | 0.55 | 0.001 | 0.11 | 0.41 | |
| Eotaxin-1 | 0.12 | 0.52 | 0.09 | 0.49 | |
| Eotaxin-2 | 0.51 | 0.003 | 0.15 | 0.29 | |
| Eotaxin-3 | 0.49 | 0.004 | 0.14 | 0.31 | |
| ECP | 0.26 | 0.30 | 0.05 | 0.81 | |
ECP, eosinophil cationic protein; UT, uncinate tissue; CT, computed tomography; CRSwNP, chronic rhinosinusitis with nasal polyps
N= 32 for correlations between ECP in UT with measures in UT and nasal lavage fluid
N= 34 and 55 for correlations between CT scores with measures in UT and nasal lavage fluid respectively; UT tissue was not always available in instances of revision surgery.
Eotaxin-3 was the dominant eotaxin induced by IL-13 in airway epithelial cells
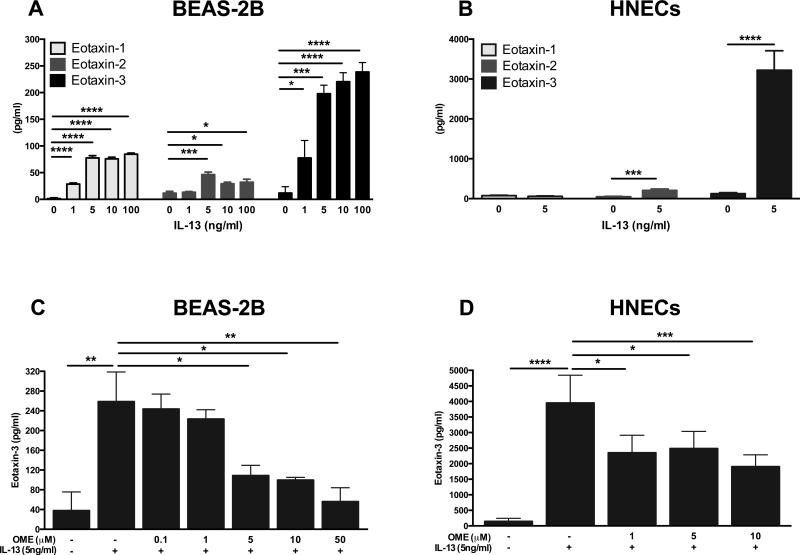
Given our in vivo findings, we evaluated the effect of IL-13 on production of the eotaxins in airway epithelial cells including HNECs and BEAS-2Bs in vitro. We found that IL-13 significantly increased protein levels of all eotaxins in BEAS-2Bs (Fig 2, A) and HNECs (Fig 2, B). Notably, eotaxin-3 protein (Fig 2, A and B) and mRNA (Fig E1, A and B) expression were profoundly and concentration-dependently induced by IL-13 in both cell types. Considering that eotaxin-3 was most profoundly induced in vitro, and was highly expressed and positively correlated with surrogate markers of tissue eosinophilia in vivo, we focused further experiments using eotaxin-3 as our target mediator for stimulation with IL-13.
Figure 2.
IL-13-induced eotaxins protein secretion and inhibitory effects of omeprazole in airway epithelial cells. A, BEAS-2Bs and B, HNECs were stimulated for 48h with IL-13. C, BEAS-2Bs and D, HNECs were pretreated with omeprazole for 2h and stimulated for 48h with IL-13. Eotaxins (A and B) and eotaxin-3 (C and D) levels in supernatants were measured by using ELISA. Data represent means ± SEMs of three (A and C), fifteen (B) or nine (D) independent experiments. *P<.05, **P<.01, ***P<.001, ****P<.0001.
Omeprazole inhibited IL-13-induced eotaxin-3 production in airway epithelial cells
Next, we investigated whether recent findings that suggest omeprazole could inhibit IL-13-induced eotaxin-3 in esophageal squamous cells29 could be replicated in airway epithelial cells. We found IL-13-induced eotaxin-3 protein secretion was significantly inhibited in BEAS-2Bs and HNECs treated with omeprazole at concentrations as low as 5μM and 1μM, respectively (Fig 2, C and D). A similar pattern was observed in mRNA expression (Fig E1, C and D).
To ensure that the observed effect was specific to IL-13-induced eotaxin-3 and not a result of general inhibition of gene expression, we measured mRNA expression of other chemokines (CXCL10, eotaxin-1, and CXCL1) in response to IFN-γ, TNF-α, and IL-17, respectively, with or without omeprazole pre-treatment. These chemokines were significantly induced by their respective cytokines as previously described,16, 40, 41 but their expression was not inhibited by omeprazole or other tested PPIs in BEAS-2Bs (Fig E2).
Association of PPI use and in vivo eotaxins levels in CRS patients
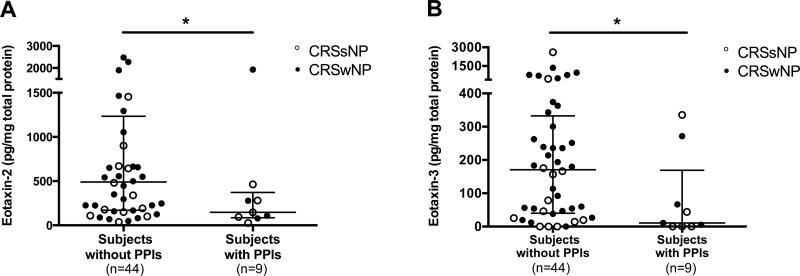
Since we found the inhibitory effect of omeprazole on IL-13-induced eotaxin-3 expression in airway epithelial cells, we sought to determine if in vitro findings might have in vivo effects. Upon medical record review, nine (17%) of our CRS patients were identified as taking PPIs including omeprazole (n=5), esomeprazole (n=1), lansoprazole (n=2), and rabeprazole (n=1) at the time of sinus surgery. Interestingly, subjects taking PPIs had significantly lower eotaxin-2 and eotaxin-3 levels in UT compared with subjects without PPIs (Fig 3). Similar trends were observed in tissue eotaxin-1 and ECP levels, although these did not achieve statistical significance (data not shown).
Figure 3.
Eotaxin-2 and eotaxin-3 levels were decreased in CRS patients taking PPIs at the time of sinus surgery. Protein levels of A, eotaxin-2 and B, eotaxin-3 in UT of CRS patients taking PPIs and those without PPIs were measured by using Luminex. Dot plots illustrate individual data points, and solid lines represent median with interquartile range. *P<.05.
Other PPIs and SCH-28080 inhibited IL-13-induced eotaxin-3 expression
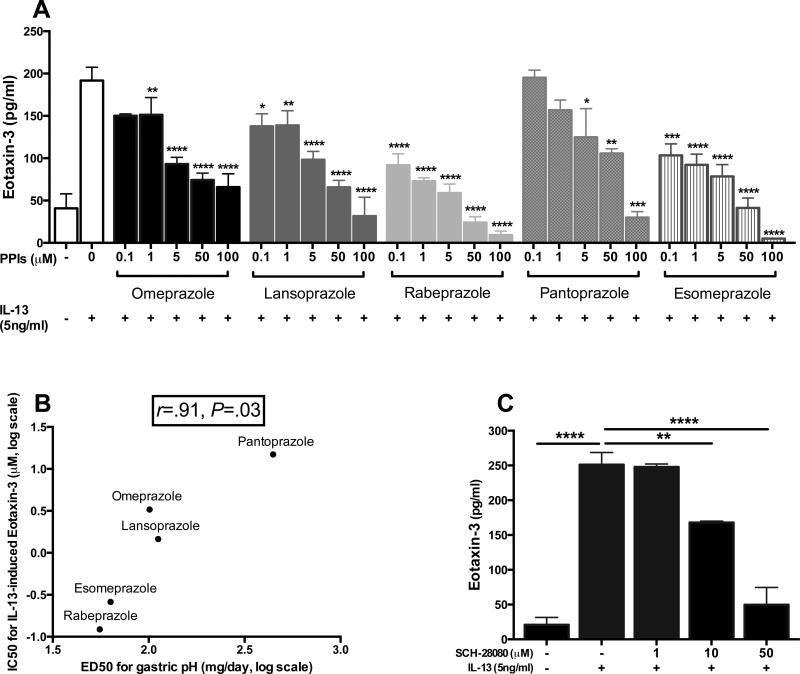
Like omeprazole, other PPIs, including lansoprazole, rabeprazole, pantoprazole, and esomeprazole, showed dose-dependent inhibitory effects on IL-13-induced eotaxin-3 protein secretion, indicating a class effect of PPIs (Fig 4, A). Moreover, when the extrapolated relative potencies of PPIs for inhibiting IL-13-induced eotaxin-3, were compared with their published potencies as inhibitors of gastric acid secretion42, there was a strong positive correlation between these two different effects (r=.91, P=.03; Fig 4, B). We further found that SCH-28080 also significantly inhibited IL-13-induced eotaxin-3 levels (Fig 4, C). SCH-28080 is mechanistically unrelated to PPIs in that it inhibits H,KATPases via competitive interactions with K+,43, 44 while PPIs function via binding to sulfhydryl groups of the H,K-ATPase.43 Given these findings, we postulated that H,K-ATPase activity might regulate IL-13-induced eotaxin-3 expression.
Figure 4.
H,K-ATPase inhibitors decreased IL-13-induced eotaxin-3 protein secretion. A, BEAS-2Bs were pretreated for 2h with PPIs followed by IL-13 stimulation for 48h. Eotaxin-3 levels in supernatants were measured by using ELISA. B, Correlations between the measured IC50 of PPIs for IL-13-induced eotaxin-3 with published ED50 of PPIs for gastric pH42. C, SCH-28080 was used with the same protocol as A. Data represent means ± SEMs of three independent experiments. *P<.05, **P<.01, ***P<.001, ****P<.0001, compared with vehicle-treated/IL-13-stimulated cells (A and C).
Non-gastric H,K-ATPase: Implication for IL-13-induced responses and effect of PPIs
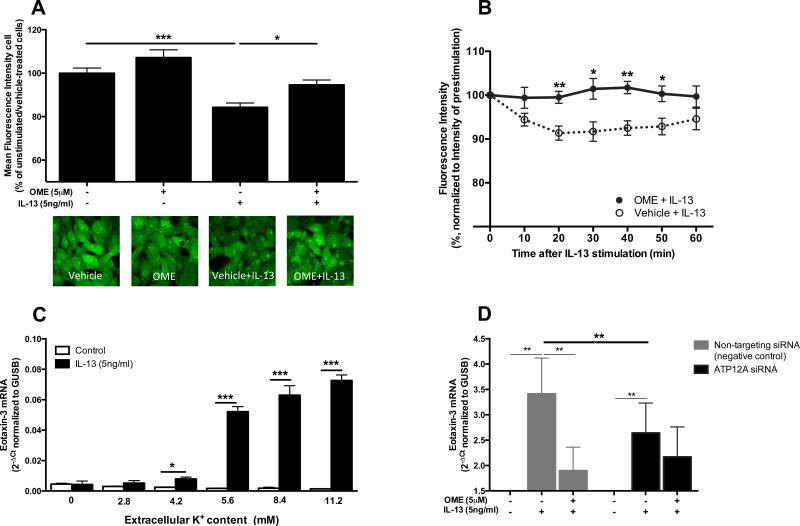
In humans, P-type ATPases comprise numerous ion-pumps but only two H,KATPases have been described. The gastric H,K-ATPase (gH,K-ATPase, encoded by the ATP4A gene), is the classic target of PPIs in the stomach but was not expressed by airway epithelial cells (data not shown). In contrast, the non-gastric H,K-ATPase (ngH,K-ATPase, encoded by the ATP12A gene) has been found in kidney, prostate, lung and nasal epithelium, and represented a possible candidate.45-47 We confirmed the presence of the catalytic α-subunit of ngH,K-ATPase in BEAS-2Bs and HNECs (Fig E3). Given that the ngH,K-ATPase exchanges extracellular K+ for intracellular H+,44 activated ngH,KATPase might induce intracellular alkalinization, To test this, we measured pHi and found that IL-13-stimulated cells showed significantly decreased fluorescence compared with unstimulated cells, indicating IL-13-induced increased intracellular pH (Fig 5, A). Moreover, omeprazole significantly attenuated this effect compared with vehicle (Fig 5, A). In kinetic studies, intracellular alkalinization became apparent as early as 20 minutes after IL-13 stimulation and was blunted in omeprazole- or SCH-28080-treated cells (Figs 5, B and E4, respectively).
Figure 5.
IL-13-induced responses are mediated by the ngH,K-ATPase. A, After 6h IL-13 stimulation with omeprazole or vehicle in BEAS-2Bs, fluorescence intensity was measured in confocal microscopic images (60x objective). B, Time course changes in fluorescence intensity in omeprazole- or vehicle-pretreated BEAS-2Bs after IL-13 stimulation. IL-13-induced eotaxin-3 mRNA expression was measured in C, BEAS-2Bs cultured in various [K+]e-containing solution, and D, ATP12A or non-targeting siRNA-transfected HNECs. Data represent means ± SEMs of three (C) or eight (D) independent experiments. *P<.05, **P<.01, ***P<.001.
Additionally, we hypothesized that IL-13-mediated responses would depend on [K+]e to facilitate ngH,K-ATPase activity. As demonstrated in Fig 5C, IL-13-mediated eotaxin-3 mRNA induction was influenced by [K+]e and was completely eliminated in [K+]e-free conditions, further supporting the role of ngH,K-ATPase in mediating IL-13-induced gene expression.
Knockdown of ATP12A
To reinforce the observed findings, we directly disrupted the expression of ATP12A by using a siRNA knockdown approach. Overall knockdown efficiency for ATP12A mRNA was 71% in HNECs (Fig E5). As hypothesized, induction of eotaxin-3 by IL-13 was significantly reduced in ATP12A siRNA-transfected cells compared with non-targeting siRNA-transfected cells (P<.01), but no additive effect of omeprazole were observed in ATP12A siRNA-transfected cells (Fig 5, D).
Effect of omeprazole on STAT6 phosphorylation and eotaxin-3 mRNA stability
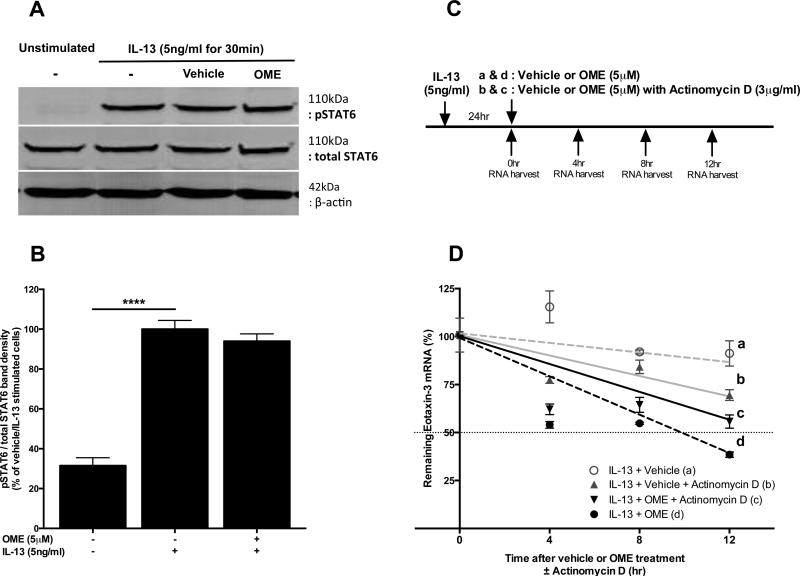
Since transcriptional regulation of IL-13-induced eotaxin-3 mRNA is known to be mediated via STAT6 signaling,17, 48 we evaluated the effect of omeprazole on STAT6 phosphorylation. IL-13-induced pSTAT6 was not significantly inhibited by omeprazole (Fig 6, A and B).
Figure 6.
Effects of omeprazole on IL-13-induced STAT6 phosphorylation and eotaxin-3 mRNA stability. A, In IL-13-stimulated BEAS-2Bs with omeprazole or vehicle, pSTAT6 and total STAT6 protein expression were measured by using Western blots. B, Semi-quantitative densitometry data for A (Mean ± SEM, n=3-6). C, Experimental protocol for eotaxin-3 mRNA stability assessment using real-time PCR. D, Relative eotaxin-3 mRNA expression levels following treatment with actinomycin D and/or omeprazole (Data represents Means ± SEM, n=3 each). ***P<.001
We next assessed whether omeprazole influenced IL-13-induced eotaxin-3 mRNA stability by utilizing actinomycin D, which inhibits de novo transcription (Fig 6, C).48 IL-13-induced eotaxin-3 mRNA expression was relatively stable without omeprazole or actinomycin D (Fig 6, D, line a). Omeprazole significantly accelerated decline of eotaxin-3 mRNA levels over the following 12h (Fig 6, D, lines a vs. d, P<.001 at 12h). In the presence of actinomycin D, omeprazole had a lesser effect but still enhanced eotaxin-3 mRNA decay compared to vehicle (Fig 6, D, lines c vs. b, P<.05 all at each time-point), suggesting post-transcriptional regulation by omeprazole. However, when comparing the effect of omeprazole with or without actinomycin D, a lesser magnitude of eotaxin-3 mRNA decay was observed in the presence of actinomycin D (Fig 6, D, line c) compared to that of omeprazole alone (Fig 6, D, line d, P<.05 after 8h), indicating that inhibition of eotaxin-3 mRNA by omeprazole might in part be related to decreased de novo transcription as well as increased post-transcriptional degradation.
Discussion
It is well established that enhanced tissue eosinophilia plays a role in both pathogenesis and prognosis of CRSwNP.5, 6 Thus, recent pharmacotherapeutic approaches are focused on controlling type-2 inflammatory mediators.26, 28, 49 In this study, we showed that eotaxin-3 is a potential biomarker for tissue IL-13 levels, eosinophilia and radiographic severity in CRS (Tables 1 and E3). We then comprehensively evaluated in vitro profiles of the eotaxins by IL-13-stimulated HNECs and BEAS-2Bs, and found that both cell types, but particularly HNECs, predominantly expressed eotaxin-3 (Fig 2). Given recent findings that PPIs had direct anti-eosinophilic effects in esophageal conditions, we hypothesize and confirmed that PPIs had similar inhibitory effects on IL-13-induced eotaxin-3 expression by HNECs in vitro (Fig 2), and that PPIs may have similar effects on patients taking these medications (Fig 3). Furthermore, we provide the first demonstration that potential mechanisms underlying the observed effect of PPIs might occur through inhibition of ngH,K-ATPase activity that is activated by IL-13 (Fig 5).
To date, there are only a few recent reports evaluating the eotaxins in CRS.50-52 These studies reported that tissue eotaxin-3 mRNA expression was correlated with clinical symptoms and eosinophilia,50 and that eotaxin-2 levels in nasal secretions correlated with radiographic and endoscopic scores.51 Our in vivo analysis supports these studies, but also demonstrates that eotaxin-2, -3 and IL-13 levels were intercorrelated in tissues and secretions, and further positively correlated with tissue eosinophilia and radiographic severity in CRS (Tables 1). Additionally, we found that the eotaxins could be measured in nasal secretions and significantly reflected tissue eosinophilia (Table 1) and IL-13 levels (Table E3), suggesting their potential value as non-invasive biomarkers. Although these measures were increased in both UT and NP in CRSwNP, and were actually higher in NP, the significant correlations between mediators and radiographic and eosinophilic severity were only found within UT (Tables 1 and E4). This suggests that the extent of type-2 inflammation in UT may be more reliably representative of disease burden of CRS. The reasons for the discrepancies in NP are unclear, but one possible hypothesis is that the dense fibrin deposition in the stroma of NP may alter chemotaxis resulting in discordance between measures derived from different cellular sources (e.g., ILC2-derived IL-13 and eosinophil-produced ECP).53
Using in vitro experiments, we found that eotaxin-3 was the predominant eotaxin produced by HNECs (Fig 2, A and B). While eotaxin-2 in vivo levels were highly elevated in CRSwNP tissue extracts, it was only modestly induced in IL-13-stimulated HNECs. This suggests that the majority of eotaxin-2 may be attributable to non-epithelial inflammatory cells, which has been previously reported in other diseases.16, 54 Among the eotaxins, recent studies converge on a critical role for eotaxin-3 in human eosinophilic diseases. Provost et. al. found that effects of eotaxin-3 on eosinophil migration were greater than the other eotaxins in asthmatics.20 Another study reported that eotaxin-3 was the only CC chemokine to be highly induced by IL-13-treated human bronchial epithelial cells (HBECs) and correlations between eotaxin-3 levels and eosinophil counts within the sputum were significant, supporting our observations.8 Although this study also showed that IL-13-stimulated eotaxin-3 release by HBECs from severe asthmatics was increased compared to control HBECs, we do not find similar differences comparing HNECs from control versus CRSwNP patients.8 In EoE, eotaxin-3 was shown to be the most highly upregulated gene (53-fold) compared with control, while eotaxin-2 and eotaxin-1 were only modestly induced (< 2-fold) in a genome-wide microarray analysis,55 and its protein levels strongly correlated with eosinophilia.21 Altogether, given that eotaxin-3 was most highly induced in vitro, was highly expressed in vivo and positively correlated with surrogate markers of disease severity, we postulated that therapeutic approaches modulating HNECs-produced eotaxin-3 may improve CRSwNP management.
Safe systemic options for long-term medical management of CRSwNP are currently lacking. Although corticosteroids are the mainstay of medical management in CRSwNP, their effects are short lived and long-term treatment is limited by systemic side effects.28, 56, 57 Recent innovative biologics targeting type-2 mediators have demonstrated promising therapeutic benefits,26-28 but access still limits their availability as options for treating CRSwNP.26-28 In eosinophilic esophageal conditions, PPIs are increasingly recognized to have anti-eosinophil properties. They currently serve as first-line therapy in patients with symptomatic esophageal eosinophilia, leading to histological remission with greater than 50% efficacy.55, 58 PPIs are thought to block the gH,K-ATPase in parietal cells and have a well-established record as orally available medications for GERD.42, 59 Their anti-eosinophil effects in the esophagus were previously assumed to result from PPIs suppression in gastric acid and GERD resolution. However, the greatest resolution of eosinophilia was observed in the proximal esophagus, where gastroesophageal reflux is less likely to reach, and patients who respond to PPIs frequently did not show abnormal esophageal pH.31 Furthermore, PPIs blocked IL-4/IL-13-induced eotaxin-3 expression in esophageal epithelial cells.29, 30 Together, these observations have raised the possibility that anti-eosinophil effects of PPIs might be through mechanisms that are more direct and unrelated to GERD resolution.
We showed here that IL-13-induced eotaxin-3 protein secretion was reduced 57.9% in BEAS-2Bs and 37.1% in HNECs by 5μM omeprazole (Fig 2, C and D) in vitro. Notably, these in vitro anti-inflammatory effects were specific to type-2 cytokine-mediated responses (Fig E2). Furthermore, we made striking observations that CRS patients who were taking PPIs at the time of surgery showed significantly lower levels of eotaxin-3 and eotaxin-2 in nasal tissue compared with patients not receiving PPIs (Fig 3). These results show promise that our in vitro results might be replicated in vivo but further studies including clinical trials are needed to prospectively evaluate their efficacy in CRSwNP. Prior studies have shown mixed efficacy of PPIs for treating asthma, but analysis was targeted at comorbid GERD resolution, but not for type-2 asthma.60-62
We also present novel evidence indicating that the mechanism by which PPIs inhibit IL-13-induced eotaxin-3 involves inhibition of ngH,K-ATPase activity. Specifically, PPIs inhibited IL-13-induced eotaxin-3 expression with the same rank order as inhibition of gastic acid secretion42, suggesting a near-perfect structure-activity relationships of PPIs for these two effects (r=.91, Fig 4,B) and further, IL-13-induced eotaxin-3 expression was suppressed by SCH-28080, a mechanistically distinct H,K-ATPase inhibitor (Fig 4,C). Since the gH,K-ATPase, the known target of PPIs, is not expressed in airway epithelium, our data led us to consider the ngH,K-ATPase, the only other P-type ATPase with H+,K+-antiporting activity. It should be noted that the ngH,K-ATPase shares approximately 65% sequence homology with the gH,K-ATPase and Na,K-ATPase, and is moderately sensitive to their inhibitors.44, 63-65 Although the inhibitory effects of PPIs on P-type ATPases besides gH,K-ATPase are largely unknown, a recent study demonstrates that omeprazole blocked another P-type ATPase, ATP7A (Menkes protein) in human epidermal melanocytes, supporting our hypothesis.66 Additionally, our results may explain recent findings that PPI-responsiveness in esophageal biopsies of EoE patients was strongly associated with expression of KCNJ2 (gene encoding the K+ channel, Kir2.1) that is colocalizes with and counteracts H,K-ATPase activity.55
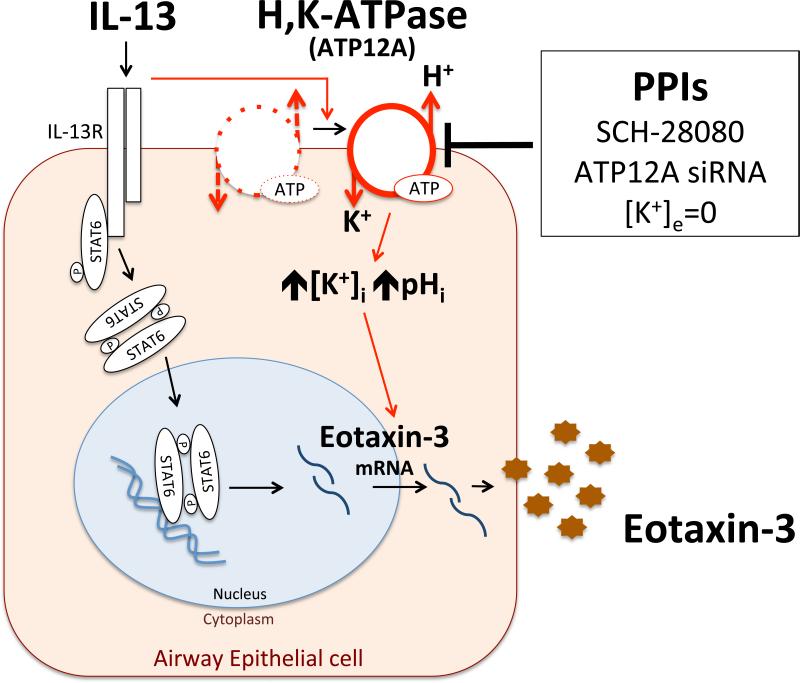
Another major finding is that expression of IL-13-responsive genes, like eotaxin-3, might require ngH,K-ATPase activity for optimal expression (summarized in Fig 7). This hypothesis is supported by findings that IL-13 stimulation induced rapid intracellular alkalization, that was blocked by omeprazole (Fig 5, A and B) and SCH-28080 (Fig E4); eotaxin-3 mRNA induction by IL-13 was highly sensitive to [K+]e, and was completely eliminated in [K+]e-free solution; and knockdown of ATP12A significantly blunted IL-13-induction of eotaxin-3 mRNA (Fig 5, D). While the ngH,K-ATPase exists in airway epithelium and plays a role in airway surface liquid acidification67, its role in IL-13 signaling is unknown. A fascinating recent study demonstrates that humans normally express 10-100-fold higher baseline levels of airway ngH,K-ATPase than mice and the pH gradient generated by this ion-pump is counteracted by CFTR secreting bicarbonate. Overexpression of the ngH,K ATPase in CFTR−/− mice, led to uncompensated airway acidification that increased bacteria at the airway surface giving these mice a phenotype closer to the human disease.68 Further investigation is needed to evaluate if IL-4 and IL-13 similarly acidifies the airway surface, but prior reports respectively demonstrate that these cytokines induce basolateral secretion of of H+ by glomerular epithelial cells69 and reduced K+ secretion by HBECs.70
Figure 7.
Hypothetical model showing potential role of the ngH,K-ATPase in facilitating inhibitory effects of PPIs on IL-13-medicated eotaxin-3 expression. In addition to the canonical IL-13/STAT6 pathway, IL-13-mediated eotaxin-3 expression may be affected by the ngH,K-ATPase activity. The ngH,K-ATPase can be blocked by PPIs and other inhibitors including SCH-28080, ATP12A siRNA, and [K+]e-free solution, resulting in H+,K+-flux and pHi changes, which may affect expression of IL-13-mediated eotaxin-3.
Limitations of our data are that we have not yet established direct mechanisms by which the IL-13 signaling pathway, intracellular alkalinization, eotaxin-3 expression, and ngH,K-ATPase interact. It should be noted that pHi or [K+]e can affect cytokine-induced gene expression, transcription factor DNA binding activity or cellular enzyme activity.71, 72 These ionic effects in airway epithelial cells may explain the previously reported decrease in STAT6 binding to the eotaxin-3 promoter30, although detailed biochemical studies of the effects of pHi and [K+]i on promoter binding will be required to directly implicate this mechanism. Other limitations include noteworthy findings that eotaxin-3 protein adheres to cell surfaces and may only be fully released by different cell extraction protocols73 from those utilized in the numerous previous studies8, 18, 29, 30, 48 including our own. These studies may thus underestimate the total amount of eotaxin-3 released by cells. Other potential limitations to the value of PPIs for treating CRSwNP include their decreased bioavailability and reduced potency outside acidic spaces like the stomach.59 However, given the availability of extended release PPI formulations and evidence that airway inflammatory conditions, including CRS, lead to airway acidification74-76 may make these surmountable concerns. We also note that the peak concentrations of omeprazole utilized for our in vitro studies are achievable in vivo using conventional oral dosing of omeprazole, with published peak mean plasma concentrations ranging from 3.2μM (20mg/day for 8 days) to 6.0μM (60mg/day for 7days).77, 78 Additionally, other previous studies have shown significant improvement in postnasal drip, a component symptom of CRS by lansoprazole79 or rabeprozole80 compared with placebo, thus reinforcing the potential therapeutic benefit for PPIs outside the stomach.
Taken together, we suggest here that inhibitors of the ngH,K-ATPase may be of significant therapeutic value in the IL-13-mediated responses found in CRSwNP and further studies are needed to elucidate their potential.
Supplementary Material
Key Messages.
Tissue levels of type-2 inflammatory mediators, including IL-13, eotaxin-2, and eotaxin-3,were correlated with tissue eosinophilia and radiographic severity in CRS.
Eotaxin-3, the most highly induced eotaxin following IL-13 stimulation in human airway epithelial cells, was inhibited by PPIs in vitro. Lower in vivo levels of eotaxin-3 were observed in CRS patients taking PPIs compared with those without PPIs.
The inhibitory effect of PPIs in vitro occurred via multiple mechanisms, including inhibition of ngH,K-ATPase activity.
Capsule Summary.
PPIs reduced IL-13-stimulated eotaxin-3 expression by airway epithelial cells in vitro and were associated with lower in vivo levels in CRS tissue. The non-gastric H,K-ATPase may be involved in this response, suggesting that it is a therapeutic target in CRSwNP.
Acknowledgements
We thank Dina Arvanitis, Daniela Janevska, and S.Marina Casalino-Matsuda for their technical assistance. Imaging work was performed at the Northwestern University Center for Advanced Microscopy supported by NCI CCSG P30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center. Spinning disk confocal microscopy was performed on an Andor XDI Revolution microscope, purchased through the support of NCRR 1S10 RR031680-01. Some of the results of this study have been reported previously in the form of abstracts presented at the American Academy of Allergy, Asthma and Immunology Annual Meetings.81, 82
Funding: This work was supported by NIH grant K23DC012067 (B.K.T), Chronic Rhinosinusitis Integrative Studies Program (CRISP) U19 AI106683 (B.K.T, R.C.K, K.E.H, A.K., R.P.S), R01 HL078860, R01 HL068546, R01 AI072570 and R01 AI104733, the Triological Society/ American College of Surgeons (B.K.T) and the Ernest S. Bazley Foundation.
List of Abbreviations
- CRS
Chronic rhinosinusitis
- CRSsNP
CRS without nasal polyps
- CRSwNP
CRS with nasal polyps
- gH,K-ATPase
Gastric H,K-ATPase
- ngH,K-ATPase
Non-gastric H,K-ATPase
- NP
Nasal polyp
- UT
Uncinate tissue
- IT
Inferior turbinate
- EoE
Eosinophilic esophagitis
- PPIs
Proton Pump Inhibitors
- HNECs
Human sinonasal epithelial cells
- STAT6
Signal transducer and activator of transcription 6
- HBECs
Human bronchial epithelial cells
- GERD
Gastroesophageal reflux disease
- CCR3
CC chemokine receptor 3
- ILC2
group 2 innate lymphoid cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
References
- 1.Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125:S103–15. doi: 10.1016/j.jaci.2009.12.989. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114:155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi-Peters A, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol. 2008;22:549–59. doi: 10.2500/ajr.2008.22.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Crombruggen K, Zhang N, Gevaert P, Tomassen P, Bachert C. Pathogenesis of chronic rhinosinusitis: inflammation. J Allergy Clin Immunol. 2011;128:728–32. doi: 10.1016/j.jaci.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 5.Tokunaga T, Sakashita M, Haruna T, Asaka D, Takeno S, Ikeda H, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy. 2015 doi: 10.1111/all.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakayama T, Yoshikawa M, Asaka D, Okushi T, Matsuwaki Y, Otori N, et al. Mucosal eosinophilia and recurrence of nasal polyps - new classification of chronic rhinosinusitis. Rhinology. 2011;49:392–6. doi: 10.4193/Rhino10.261. [DOI] [PubMed] [Google Scholar]
- 7.Soler ZM, Sauer D, Mace J, Smith TL. Impact of mucosal eosinophilia and nasal polyposis on quality-of-life outcomes after sinus surgery. Otolaryngol Head Neck Surg. 2010;142:64–71. doi: 10.1016/j.otohns.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larose MC, Chakir J, Archambault AS, Joubert P, Provost V, Laviolette M, et al. Correlation between CCL26 production by human bronchial epithelial cells and airway eosinophils: Involvement in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303–10. doi: 10.1016/j.jaci.2007.03.048. quiz 11-2. [DOI] [PubMed] [Google Scholar]
- 10.Lamkhioued B, Garcia-Zepeda EA, Abi-Younes S, Nakamura H, Jedrzkiewicz S, Wagner L, et al. Monocyte chemoattractant protein (MCP)-4 expression in the airways of patients with asthma. Induction in epithelial cells and mononuclear cells by proinflammatory cytokines. Am J Respir Crit Care Med. 2000;162:723–32. doi: 10.1164/ajrccm.162.2.9901080. [DOI] [PubMed] [Google Scholar]
- 11.Schleimer RP, Kato A, Peters A, Conley D, Kim J, Liu MC, et al. Epithelium, inflammation, and immunity in the upper airways of humans: studies in chronic rhinosinusitis. Proc Am Thorac Soc. 2009;6:288–94. doi: 10.1513/pats.200808-088RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laoukili J, Perret E, Willems T, Minty A, Parthoens E, Houcine O, et al. IL-13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J Clin Invest. 2001;108:1817–24. doi: 10.1172/JCI13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–9. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 14.Pope SM, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI, et al. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J Allergy Clin Immunol. 2001;108:594–601. doi: 10.1067/mai.2001.118600. [DOI] [PubMed] [Google Scholar]
- 15.Kelly M, Hwang JM, Kubes P. Modulating leukocyte recruitment in inflammation. J Allergy Clin Immunol. 2007;120:3–10. doi: 10.1016/j.jaci.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Komiya A, Nagase H, Yamada H, Sekiya T, Yamaguchi M, Sano Y, et al. Concerted expression of eotaxin-1, eotaxin-2, and eotaxin-3 in human bronchial epithelial cells. Cell Immunol. 2003;225:91–100. doi: 10.1016/j.cellimm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Blanchard C, Durual S, Estienne M, Emami S, Vasseur S, Cuber JC. Eotaxin-3/CCL26 gene expression in intestinal epithelial cells is up-regulated by interleukin-4 and interleukin-13 via the signal transducer and activator of transcription 6. Int J Biochem Cell Biol. 2005;37:2559–73. doi: 10.1016/j.biocel.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Heiman AS, Abonyo BO, Darling-Reed SF, Alexander MS. Cytokine-stimulated human lung alveolar epithelial cells release eotaxin-2 (CCL24) and eotaxin-3 (CCL26). J Interferon Cytokine Res. 2005;25:82–91. doi: 10.1089/jir.2005.25.82. [DOI] [PubMed] [Google Scholar]
- 19.Neilsen CV, Bryce PJ. Interleukin-13 directly promotes oesophagus production of CCL11 and CCL24 and the migration of eosinophils. Clin Exp Allergy. 2010;40:427–34. doi: 10.1111/j.1365-2222.2009.03419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Provost V, Larose MC, Langlois A, Rola-Pleszczynski M, Flamand N, Laviolette M. CCL26/eotaxin-3 is more effective to induce the migration of eosinophils of asthmatics than CCL11/eotaxin-1 and CCL24/eotaxin-2. J Leukoc Biol. 2013;94:213–22. doi: 10.1189/jlb.0212074. [DOI] [PubMed] [Google Scholar]
- 21.Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116:536–47. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013;188:432–9. doi: 10.1164/rccm.201212-2227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 24.Beck LA, Stellato C, Beall LD, Schall TJ, Leopold D, Bickel CA, et al. Detection of the chemokine RANTES and endothelial adhesion molecules in nasal polyps. J Allergy Clin Immunol. 1996;98:766–80. doi: 10.1016/s0091-6749(96)70126-4. [DOI] [PubMed] [Google Scholar]
- 25.Stevens WW, Ocampo CJ, Berdnikovs S, Sakashita M, Mahdavinia M, Suh L, et al. Cytokines in Chronic Rhinosinusitis: Role in Eosinophilia and Aspirin Exacerbated Respiratory Disease. Am J Respir Crit Care Med. 2015 doi: 10.1164/rccm.201412-2278OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gevaert P, Van Bruaene N, Cattaert T, Van Steen K, Van Zele T, Acke F, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128:989–95. e1–8. doi: 10.1016/j.jaci.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 27.Bachert C, Mannent L, Naclerio RM, Mullol J, Ferguson BJ, Gevaert P, et al. Effect of Subcutaneous Dupilumab on Nasal Polyp Burden in Patients With Chronic Sinusitis and Nasal Polyposis: A Randomized Clinical Trial. JAMA. 2016;315:469–79. doi: 10.1001/jama.2015.19330. [DOI] [PubMed] [Google Scholar]
- 28.Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: Focus on nasal polyposis. J Allergy Clin Immunol. 2015;136:1431–40. doi: 10.1016/j.jaci.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Cheng E, Zhang X, Huo X, Yu C, Zhang Q, Wang DH, et al. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut. 2013;62:824–32. doi: 10.1136/gutjnl-2012-302250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Cheng E, Huo X, Yu C, Zhang Q, Pham TH, et al. Omeprazole blocks STAT6 binding to the eotaxin-3 promoter in eosinophilic esophagitis cells. PLoS One. 2012;7:e50037. doi: 10.1371/journal.pone.0050037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park JY, Zhang X, Nguyen N, Souza RF, Spechler SJ, Cheng E. Proton pump inhibitors decrease eotaxin-3 expression in the proximal esophagus of children with esophageal eosinophilia. PLoS One. 2014;9:e101391. doi: 10.1371/journal.pone.0101391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucendo AJ, Arias A, Molina-Infante J. Efficacy of Proton Pump Inhibitor Drugs for Inducing Clinical and Histologic Remission in Patients With Symptomatic Esophageal Eosinophilia: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 33.Padia R, Curtin K, Peterson K, Orlandi RR, Alt J. Eosinophilic esophagitis strongly linked to chronic rhinosinusitis. Laryngoscope. 2015 doi: 10.1002/lary.25798. [DOI] [PubMed] [Google Scholar]
- 34.Pearlman AN, Conley DB. Review of current guidelines related to the diagnosis and treatment of rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2008;16:226–30. doi: 10.1097/MOO.0b013e3282fdcc9a. [DOI] [PubMed] [Google Scholar]
- 35.Okushi T, Nakayama T, Morimoto S, Arai C, Omura K, Asaka D, et al. A modified Lund-Mackay system for radiological evaluation of chronic rhinosinusitis. Auris Nasus Larynx. 2013;40:548–53. doi: 10.1016/j.anl.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:1198–206. e1. doi: 10.1016/j.jaci.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008;121:1385–92. 92, e1–2. doi: 10.1016/j.jaci.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chustz RT, Nagarkar DR, Poposki JA, Favoreto S, Jr., Avila PC, Schleimer RP, et al. Regulation and function of the IL-1 family cytokine IL-1F9 in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2011;45:145–53. doi: 10.1165/rcmb.2010-0075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arppe R, Nareoja T, Nylund S, Mattsson L, Koho S, Rosenholm JM, et al. Photon upconversion sensitized nanoprobes for sensing and imaging of pH. Nanoscale. 2014;6:6837–43. doi: 10.1039/c4nr00461b. [DOI] [PubMed] [Google Scholar]
- 40.Sauty A, Dziejman M, Taha RA, Iarossi AS, Neote K, Garcia-Zepeda EA, et al. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol. 1999;162:3549–58. [PubMed] [Google Scholar]
- 41.Huang F, Kao CY, Wachi S, Thai P, Ryu J, Wu R. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-kappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J Immunol. 2007;179:6504–13. doi: 10.4049/jimmunol.179.10.6504. [DOI] [PubMed] [Google Scholar]
- 42.Kirchheiner J, Glatt S, Fuhr U, Klotz U, Meineke I, Seufferlein T, et al. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur J Clin Pharmacol. 2009;65:19–31. doi: 10.1007/s00228-008-0576-5. [DOI] [PubMed] [Google Scholar]
- 43.Shin JM, Munson K, Vagin O, Sachs G. The gastric HK-ATPase: structure, function, and inhibition. Pflugers Arch. 2009;457:609–22. doi: 10.1007/s00424-008-0495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Modyanov NN, Mathews PM, Grishin AV, Beguin P, Beggah AT, Rossier BC, et al. Human ATP1AL1 gene encodes a ouabain-sensitive H-K-ATPase. Am J Physiol. 1995;269:C992–7. doi: 10.1152/ajpcell.1995.269.4.C992. [DOI] [PubMed] [Google Scholar]
- 45.Coakley RD, Grubb BR, Paradiso AM, Gatzy JT, Johnson LG, Kreda SM, et al. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci U S A. 2003;100:16083–8. doi: 10.1073/pnas.2634339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altman KW, Kinoshita Y, Tan M, Burstein D, Radosevich JA. Western blot confirmation of the H+/K+-ATPase proton pump in the human larynx and submandibular gland. Otolaryngol Head Neck Surg. 2011;145:783–8. doi: 10.1177/0194599811415589. [DOI] [PubMed] [Google Scholar]
- 47.Pestov NB, Korneenko TV, Shakhparonov MI, Shull GE, Modyanov NN. Loss of acidification of anterior prostate fluids in Atp12a-null mutant mice indicates that nongastric H-K-ATPase functions as proton pump in vivo. Am J Physiol Cell Physiol. 2006;291:C366–74. doi: 10.1152/ajpcell.00042.2006. [DOI] [PubMed] [Google Scholar]
- 48.Heller NM, Matsukura S, Georas SN, Boothby MR, Rothman PB, Stellato C, et al. Interferon-gamma inhibits STAT6 signal transduction and gene expression in human airway epithelial cells. Am J Respir Cell Mol Biol. 2004;31:573–82. doi: 10.1165/rcmb.2004-0195OC. [DOI] [PubMed] [Google Scholar]
- 49.Tajiri T, Matsumoto H, Hiraumi H, Ikeda H, Morita K, Izuhara K, et al. Efficacy of omalizumab in eosinophilic chronic rhinosinusitis patients with asthma. Ann Allergy Asthma Immunol. 2013;110:387–8. doi: 10.1016/j.anai.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 50.Lam M, Hull L, McLachlan R, Snidvongs K, Chin D, Pratt E, et al. Clinical severity and epithelial endotypes in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013;3:121–8. doi: 10.1002/alr.21082. [DOI] [PubMed] [Google Scholar]
- 51.De Corso E, Baroni S, Romitelli F, Luca L, Di Nardo W, Passali GC, et al. Nasal lavage CCL24 levels correlate with eosinophils trafficking and symptoms in chronic sino-nasal eosinophilic inflammation. Rhinology. 2011;49:174–9. doi: 10.4193/Rhino10.133. [DOI] [PubMed] [Google Scholar]
- 52.Gu Z, Jin M, Cao Z. Role of eotaxin-3 in chronic rhinosinusitis with nasal polyps. Otolaryngol Head Neck Surg. 2011;145:324–6. doi: 10.1177/0194599811403077. [DOI] [PubMed] [Google Scholar]
- 53.Takabayashi T, Kato A, Peters AT, Hulse KE, Suh LA, Carter R, et al. Increased expression of factor XIII-A in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2013;132:584–92. e4. doi: 10.1016/j.jaci.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watanabe K, Jose PJ, Rankin SM. Eotaxin-2 generation is differentially regulated by lipopolysaccharide and IL-4 in monocytes and macrophages. J Immunol. 2002;168:1911–8. doi: 10.4049/jimmunol.168.4.1911. [DOI] [PubMed] [Google Scholar]
- 55.Wen T, Dellon ES, Moawad FJ, Furuta GT, Aceves SS, Rothenberg ME. Transcriptome analysis of proton pump inhibitor-responsive esophageal eosinophilia reveals proton pump inhibitor-reversible allergic inflammation. J Allergy Clin Immunol. 2015;135:187–97. doi: 10.1016/j.jaci.2014.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamilos DL. Chronic rhinosinusitis: epidemiology and medical management. J Allergy Clin Immunol. 2011;128:693–707. doi: 10.1016/j.jaci.2011.08.004. quiz 8-9. [DOI] [PubMed] [Google Scholar]
- 57.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012:3. preceding table of contents, 1-298. [PubMed] [Google Scholar]
- 58.Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108:679–92. doi: 10.1038/ajg.2013.71. quiz 93. [DOI] [PubMed] [Google Scholar]
- 59.Shin JM, Kim N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J Neurogastroenterol Motil. 2013;19:25–35. doi: 10.5056/jnm.2013.19.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Littner MR, Leung FW, Ballard ED, 2nd, Huang B, Samra NK, Lansoprazole Asthma Study G Effects of 24 weeks of lansoprazole therapy on asthma symptoms, exacerbations, quality of life, and pulmonary function in adult asthmatic patients with acid reflux symptoms. Chest. 2005;128:1128–35. doi: 10.1378/chest.128.3.1128. [DOI] [PubMed] [Google Scholar]
- 61.Calabrese C, Fabbri A, Areni A, Scialpi C, Zahlane D, Di Febo G. Asthma and gastroesophageal reflux disease: effect of long-term pantoprazole therapy. World J Gastroenterol. 2005;11:7657–60. doi: 10.3748/wjg.v11.i48.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.American Lung Association Asthma Clinical Research C. Mastronarde JG, Anthonisen NR, Castro M, Holbrook JT, Leone FT, et al. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487–99. doi: 10.1056/NEJMoa0806290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Modyanov N, Pestov N, Adams G, Crambert G, Tillekeratne M, Zhao H, et al. Nongastric H,K-ATPase: structure and functional properties. Ann N Y Acad Sci. 2003;986:183–7. doi: 10.1111/j.1749-6632.2003.tb07158.x. [DOI] [PubMed] [Google Scholar]
- 64.Modyanov NN, Petrukhin KE, Sverdlov VE, Grishin AV, Orlova MY, Kostina MB, et al. The family of human Na,K-ATPase genes. ATP1AL1 gene is transcriptionally competent and probably encodes the related ion transport ATPase. FEBS Lett. 1991;278:91–4. doi: 10.1016/0014-5793(91)80091-g. [DOI] [PubMed] [Google Scholar]
- 65.Swarts HG, Koenderink JB, Willems PH, De Pont JJ. The non-gastric H,K-ATPase is oligomycin-sensitive and can function as an H+,NH4(+)-ATPase. J Biol Chem. 2005;280:33115–22. doi: 10.1074/jbc.M504535200. [DOI] [PubMed] [Google Scholar]
- 66.Matsui MS, Petris MJ, Niki Y, Karaman-Jurukovska N, Muizzuddin N, Ichihashi M, et al. Omeprazole, a gastric proton pump inhibitor, inhibits melanogenesis by blocking ATP7A trafficking. J Invest Dermatol. 2015;135:834–41. doi: 10.1038/jid.2014.461. [DOI] [PubMed] [Google Scholar]
- 67.Smith JJ, Welsh MJ. Fluid and electrolyte transport by cultured human airway epithelia. J Clin Invest. 1993;91:1590–7. doi: 10.1172/JCI116365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shah VS, Meyerholz DK, Tang XX, Reznikov L, Abou Alaiwa M, Ernst SE, et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science. 2016;351:503–7. doi: 10.1126/science.aad5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Den Berg JG, Aten J, Annink C, Ravesloot JH, Weber E, Weening JJ. Interleukin-4 and -13 promote basolateral secretion of H(+) and cathepsin L by glomerular epithelial cells. Am J Physiol Renal Physiol. 2002;282:F26–33. doi: 10.1152/ajprenal.0102.2001. [DOI] [PubMed] [Google Scholar]
- 70.Danahay H, Atherton H, Jones G, Bridges RJ, Poll CT. Interleukin-13 induces a hypersecretory ion transport phenotype in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L226–36. doi: 10.1152/ajplung.00311.2001. [DOI] [PubMed] [Google Scholar]
- 71.Schulz E, Munzel T. Intracellular pH: a fundamental modulator of vascular function. Circulation. 2011;124:1806–7. doi: 10.1161/CIRCULATIONAHA.111.061226. [DOI] [PubMed] [Google Scholar]
- 72.Yoshii K, Tajima F, Ishijima S, Sagami I. Changes in pH and NADPH regulate the DNA binding activity of neuronal PAS domain protein 2, a mammalian circadian transcription factor. Biochemistry. 2015;54:250–9. doi: 10.1021/bi5008518. [DOI] [PubMed] [Google Scholar]
- 73.Yuan Q, Campanella GS, Colvin RA, Hamilos DL, Jones KJ, Mathew A, et al. Membrane-bound eotaxin-3 mediates eosinophil transepithelial migration in IL-4-stimulated epithelial cells. Eur J Immunol. 2006;36:2700–14. doi: 10.1002/eji.200636112. [DOI] [PubMed] [Google Scholar]
- 74.Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, et al. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med. 2000;161:694–9. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- 75.Kostikas K, Papatheodorou G, Ganas K, Psathakis K, Panagou P, Loukides S. pH in expired breath condensate of patients with inflammatory airway diseases. Am J Respir Crit Care Med. 2002;165:1364–70. doi: 10.1164/rccm.200111-068OC. [DOI] [PubMed] [Google Scholar]
- 76.Cho DY, Hajighasemi M, Hwang PH, Illek B, Fischer H. Proton secretion in freshly excised sinonasal mucosa from asthma and sinusitis patients. Am J Rhinol Allergy. 2009;23:e10–3. doi: 10.2500/ajra.2009.23.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li XQ, Andersson TB, Ahlstrom M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004;32:821–7. doi: 10.1124/dmd.32.8.821. [DOI] [PubMed] [Google Scholar]
- 78.Howden CW, Meredith PA, Forrest JA, Reid JL. Oral pharmacokinetics of omeprazole. Eur J Clin Pharmacol. 1984;26:641–3. doi: 10.1007/BF00543502. [DOI] [PubMed] [Google Scholar]
- 79.Vaezi MF, Hagaman DD, Slaughter JC, Tanner SB, Duncavage JA, Allocco CT, et al. Proton pump inhibitor therapy improves symptoms in postnasal drainage. Gastroenterology. 2010;139:1887–93. e1. doi: 10.1053/j.gastro.2010.08.039. quiz e11. [DOI] [PubMed] [Google Scholar]
- 80.Pawar S, Lim HJ, Gill M, Smith TL, Merati A, Toohill RJ, et al. Treatment of postnasal drip with proton pump inhibitors: a prospective, randomized, placebo-controlled study. Am J Rhinol. 2007;21:695–701. doi: 10.2500/ajr.2007.21.3098. [DOI] [PubMed] [Google Scholar]
- 81.Min JY, Kern RC, Ocampo CJ, Homma T, Conley DC, Shintani-Smith S, Huang H, et al. Omeprazole has anti-inflammatory effects on Type-2 cytokine-stimulated human airway epithelial cells. J Allergy Clin Immunol. 2015;135:AB81. [Google Scholar]
- 82.Min JY, Kern RC, Ocampo CJ, Stevens WW, Price CPE, Thompson CF, et al. Proton pump inhibitos (PPIs) may modulate more than just reflux in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2016;137:AB285. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.