Abstract
Much diseased human myocardial tissue is fibrotic and stiff, which increases the work that the ventricular myocytes must perform to maintain cardiac output. The hypothesis tested is that the increased load due to greater stiffness of the substrata drives sarcomere assembly of cells, thus strengthening them. Neonatal rat ventricular myocytes (NRVM) were cultured on polyacrylamide or polydimethylsiloxane substrates with stiffness of 10 kPa, 100 kPa, or 400 kPa, or glass with stiffness of 61.9 GPa. Cell size increased with stiffness. Two signaling pathways were explored, phosphorylation of focal adhesion kinase (p-FAK) and lipids by phosphatidylinositol 4,5-bisphosphate (PIP2). Subcellular distributions of both were determined in the sarcomeric fraction by antibody localization, and total amounts were measured by Western or dot blotting, respectively. More p-FAK and PIP2 distributed to the sarcomeres of NRVM grown on stiffer substrates. Actin assembly involves the actin capping protein Z (CapZ). Both actin and CapZ dynamic exchange were significantly increased on stiffer substrates when assessed by fluorescence recovery after photobleaching (FRAP) of green fluorescent protein tags. Blunting of actin FRAP by FAK inhibition implicates linkage from mechano-signalling pathways to cell growth. Thus, increased stiffness of cardiac disease can be modeled with polymeric materials to understand how the microenvironment regulates cardiac hypertrophy.
Keywords: mechano-transduction, focal adhesion kinase, lipid signaling, actin assembly, substrate stiffness
Résumé
Une bonne partie du tissu myocardique pathologique chez l’homme est fibrotique et rigide, ce qui augmente le travail imposé aux myocytes ventriculaires pour maintenir le débit cardiaque. Nous avons testé l’hypothèse selon laquelle l’augmentation de la charge provoquée par l’augmentation de la rigidité des substrats commande l’assemblage des cellules en sarcomères, ce qui les fortifie. Nous avons mis des myocytes ventriculaires de rats nouveau-nés (MVRN) en culture sur un substrat de polyacrylamide ou de polydiméthylsiloxane avec des rigidités de 10 kPa, de 100 kPa ou de 400 kPa, ou sur du verre dont la rigidité était de 61,9 GPa. La taille des cellules augmentait en fonction de la rigidité. Nous avons étudié 2 voies de signalisation : celle de la phosphorylation de la p-FAK (<< focal adhesion kinase >>) et celle des lipides par le PIP2 (phosphatidylinositol 4,5-bisphosphate). Nous avons établi la répartition subcellulaire de chacune d’elles dans la fraction sarcomérique a` l’aide de la technique de localisation d’anticorps et nous avons mesuré leur quantité totale a` l’aide des techniques de buvardage de western et << en point >> (<< dot blot >>), respectivement. Plus de p-FAK et de PIP2 étaient répartis dans les sarcomères de MVRN sur des substrats plus rigides. L’assemblage de l’actine met en jeu la protéine de coiffage de l’actine CapZ (pour << actin capping protein Z >>). L’évaluation de la récupération de la fluorescence après photoblanchiment (FRAP pour << fluorescence recovery after photobleaching >>) de marqueurs protéiques verts fluorescents nous a permis d’observer que les échanges dynamiques de l’actine comme de la CapZ augmentaient de façon marquée sur des substrats plus rigides. L’atténuation de la FRAP de l’actine par l’inhibition de la FAK trace un lien entre les voies de signalisation mécaniques et la croissance des cellules. Par conséquent, l’augmentation de la rigidité observée en cas de maladie cardiaque peut être modélisée a` l’aide de matériaux polymères en vue de comprendre comment le microenvironnement assure la régulation de l’hypertrophie cardiaque. [Traduit par la Rédaction]
Introduction
The mechanical properties of the local microenvironment influence the function of cells (Yang et al. 2014). This is particularly critical following a myocardial infarction, in which stiff, fibrotic scar tissue replaces the normally compliant ventricle with adverse functional consequences. The ventricular myocytes must work harder to maintain cardiac output, which they mainly accomplish by cell hypertrophy. Thus, the processes that link mechanosensing of increased load to the strengthening of myocytes by cell hypertrophy are of major significance in heart diseases. Multiple mechanosensors detect increased mechanical loading (Hoshijima 2006), but the feedback linking sensing to local actin filament assembly is not yet fully understood. Here, we culture cardiac myocytes on substrata of defined stiffness to analyze cell responses.
The study of mechanisms of cell growth requires altering the load in a controlled manner, which is difficult to do at the cellular level because cells are usually cultured on hard, plastic surfaces, which poorly mimic the external forces existing in living tissue. Stiffness of the surface on which cells grow significantly affects maturation and differentiation into myocytes (Jacot et al. 2010) and also force generation (Bhana et al. 2010; Broughton and Russell 2015). The stiffness in the heart can vary from embryonic and neonatal of 5–10 kPa (Bhana et al. 2010; de Tombe 2003) to the normal adult rat myocardium of 10–70 kPa (Borbély et al. 2005). Infarct stiffness and collagen content increased with time post-infarct up to 400 kPa (Fomovsky and Holmes 2010; Fomovsky et al. 2010; Holmes et al. 2005). Therefore, we fabricated novel substrata out of polyacrylamide (PAA) (Engler et al. 2008) and polydimethylsiloxane (PDMS) in the physiologic and pathologic range (10–400 kPa) (Broughton and Russell 2015) to model loading of cardiac myocytes and resultant signaling pathways.
In muscle, focal adhesions are the primary biomechanical sensors found at the Z-discs where integrins are anchored at the costamere to the extracellular matrix. The focal adhesion kinase (FAK) binds to the cytoskeletal domain of the integrin complex and responds to mechanical stimuli (Senyo et al. 2007). Mechanical stimulation rapidly phosphorylates and activates FAK possibly by unfolding of the protein to expose a phosphorylation site (Chu et al. 2011; Franchini et al. 2000). Interestingly, FAK is activated by cyclic strain at Tyr397 and distributes along the myofilaments (Torsoni et al. 2003), which might suggest that the distribution of phosphorylation of FAK (p-FAK) regulates actin assembly. Another actin assembly site is at the Z-disc where the actin capping protein (CapZ) is able to slow down filament assembly (Edwards et al. 2014). On mechanical stimulation of myocytes, the CapZβ1 C-terminus may control capping of the actin filament (Lin et al. 2013). Moreover, mechano-transduction arising from stress or strain may also modify the function of CapZ by phosphatidylinositol 4,5-bisphosphate (PIP2), a phospholipid (Li and Russell 2013).
In this report, cell signaling and thin filament assembly was assessed by fluorescence recovery after photobleaching (FRAP) to determine the capping dynamics of CapZ and actin. Understanding how fibrotic stiffness in the microenvironment regulates cardiac hypertrophy may be important in cardiac disease states.
Materials and methods
Substrata fabrication
Polymers with varying stiffness were used to coat glass surfaces with a layer approximately 100 μm thick. The goals were to attain the stiffness range from physiologic (10 kPa) to pathologic (400 kPa) while retaining cell adhesion for culture, good optics for immunolocalization, and protein isolation for Western and dot blotting. PDMS is viscoelastic below 100 kPa, requiring the use of PAA at lower stiffnesses (Wei et al. 2015). Cells can be scraped for Western and dot blotting from stiff PDMS but not from soft PAA below 100 kPa. To control for potential differences in material properties, 100 kPa surfaces were produced with both PDMS and PAA.
Preparation of glass base for application of polymer layer
Cell culture glass-bottomed dishes and 10 mm circular coverslips were treated using a modified protocol (Poellmann and Wagoner Johnson 2013; Tse and Engler 2010). The center portions of glass-bottomed dishes were treated for 30 min with 10 mol/L NaOH to expose hydroxyl groups, then washed thoroughly with deionized water. The glass was silanated with 3-(trimethoxysilyl) propyl methacrylate (Cat# M6514; Sigma–Aldrich, USA) for 90 min. Dishes were washed 3 times with 70% EtOH and dried on a 100 °C hotplate. Coverslips necessary for creating flat surfaces were washed with 70% EtOH in petri dishes, air-dried, then silanated by placing them in a desiccator with 20 μL of tridecafluoro-(1,1,2,2-tetrahydrooctyl)-1-trichlorosilane (UCT T2492) for 90 min. Following treatment, coverslips were washed with 70% EtOH and dried on a 100 °C hotplate.
Polyacrylamide substrata
Unpolymerized acrylamide (40%, Cat#161–0140; Bio-Rad, USA) and 2% Bis solution (Cat#161–0142, Bio-Rad, USA) were diluted in water to concentrations necessary to develop 10 kPa (final concentration 5% acrylamide, 0.3% Bis) and 100 kPa (final concentration 30% acrylamide, 0.3% Bis) substrata, respectively. Ammonium persulfate was added at 1% by volume and tetraethylmethylenediamine at 0.1% by volume to begin the polymerization reaction. Solutions (10 μL) were then added to glass-bottomed dishes and each covered with a treated coverslip. Substrata were allowed to polymerize for 10 min, then coverslips were gently pried up, leaving behind a flat, circular substrate. Dishes were washed 3 times in deionized water for 10 min at a time to remove unpolymerized acrylamide.
To functionalize polyacrylamide substrata for cell adhesion, they were treated twice by drying sulfo-SANPAH (Cat#22589; Thermo Fisher, USA) in HEPES (50 mmol/L, pH 8.5) on each sub-stratum for 90 min at 57 °C. A UV cross-linker (UV Crosslinker; Spectronics Corporation, USA) with a 365 nm bulb, placed approximately 10 cm away for 10 min, was then used to cross-link sulfo-SANPAH to the substrate surface. Substrates were washed with HEPES between treatments. Following sulfo-SANPAH treatment, substrates were washed 3 times using 50 mmol/L HEPES, then HEPES containing fibronectin (10 μg/mL) was added to dishes and incubated at 37 °C for 2 h before UV-sterilizing in water for 20 min.
Polydimethylsiloxane substrata
Polydimethylsiloxane (PDMS) (DowCorning, Midland, Michigan, USA) was mixed in a 10:1 (400 kPa) or 50:1 (100 kPa) elastomer base to curing agent ratio for approximately 10 min. PDMS mixtures were degassed using a vacuum desiccator for approximately 30 min. PDMS was then spun onto cell culture glass bottom dishes (In Vitro Scientific, California, USA), creating a PDMS thickness of approximately 50 μm, or added to 6-well plates. PDMS substrata were then cured for 24 h in a 57 °C oven. After curing, PDMS was cooled and ready for activation for fibronectin coating. PDMS substrata were treated with 5% (3-aminopropyl) triethoxysilane in 90.25% ethanol solution for 10 min, then washed with 100% ethanol. Substrata were placed in a 57 °C oven for 20 min, washed with 95% ethanol and twice with PBS, then coated with 10 μg/mL fibronectin in DMEM for 2 h at 37 °C.
Measurement of stiffness
Stiffness of both materials was measured by Young’s Modulus using atomic force microscopy. For PDMS, our lab found 50:1 ratio to yield a Young’s Modulus of 98.4 ± 7.36 kPa, while a 10:1 ratio yields a 397.4 ± 30.79, which are rounded off to 100 and 400 kPa, respectively (Broughton and Russell 2015); for PAA, mixtures of 5%–0.3% and 30%–0.3% acrylamide–Bis yielded ~9.3 ± 0.26 and 101.4 ± 1.6 kPa, respectively, by atomic force microscopy (Asylum 1-D; Asylum Research; Santa Barbara, California, USA) and indented by a pyramid-tipped probe (Veeco; Santa Barbara, California, USA) (data provided by Adam Engler at University of California at San Diego, Engler et al. 2006). The glass stiffness was reported as 61.9 GPa (Wang 2012).
Neonatal rat ventricular myocyte (NRVM) culture
Primary heart cultures were obtained from neonatal rats according to the Institutional Animal Care and Use Committee at the University of Illinois at Chicago and National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011) guidelines that are equivalent to the Canadian Council on Animal Care (CCAC) regulations. Hearts were removed and cells isolated from 1–2 day old Sprague-Dawley rats with collagenase type II (Worthington, Lakewood, New Jersey, USA) as previously described (Boateng et al. 2003). NRVMs were re-suspended, filtered through a metal sieve to remove large material, and plated at high density (1000 cells/mm2) in PC–1 medium (Lonza Group Ltd., USA). Unattached cells were removed by aspiration and PC–1 media was replenished. Myocytes were plated on fibronectin-coated (10 μg/mL) dishes at 1 560 cells/mm2 for Western blotting or 520 cells/mm2 for immunostaining. Stiffnesses used for PAA were 10 and 100 kPa; for PDMS were 100 and 400 kPa; and on glass-bottomed dishes (61.9 GPa). Cells were cultured for 3 days in a 5% CO2 incubator.
Localization of p-FAK (Tyr397) or PIP2 in the cytoskeletal fraction by immunostaining and microscopy
For isolation of the cytoskeletal fraction, the Calbiochem ProteoExtract Subcellular Proteome Extraction Kit was used (EMD Millipore, Billerica, Massachusetts, USA), following a previously described detergent-based protocol (Boateng et al. 2007). The remaining myofibrillar cytsokeleton was immunostained with a-actinin antibody (1:200, Cell Signaling Technology, Inc., Danvers, Massachusetts, USA), and either with a p-FAK (Tyr397) antibody (1:200, Cell Signaling Technology, Inc., Danvers, Massachusetts, USA) or a PIP2 antibody (1:200, mouse IgG, Abcam, Cambridge, Massachusetts, USA).
Cardiomyocytes were observed by microscopy (Observer Z1, Zeiss), and by confocal microscopy (LSM 710; Zeiss). Cell surface areas were measured by ImageJ software. In each case, 3 independent experiments were performed, values were calculated, and 20 cells from each condition were randomly chosen and used to calculate cell areas. Experiments were repeated at least 3 times on PDMS (100 kPa, 400 kPa) or glass. The selective extraction method for the subcellular components on the polyacrylamide hydrogels detached the cells from the surface. Efforts to retain cell attachment by shortening the exposure times and altering the detergents were unsuccessful.
PIP2 levels by dot blots
Whole cell lysates extracted from NRVMs grown on 100 kPa, 400 kPa, PDMS substrata, or 61.9 GPa (glass) were spotted onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, California, USA). These were probed with PIP2 antibody (mouse IgG, Abcam, Cambridge, Massachusetts, USA) at a 1:500 dilution and detected using a horseradish peroxidase conjugated secondary antibody (anti-mouse, HRP, Cell Signaling Technology, Boston, Massachusetts, USA) and enhanced chemiluminescence. To exclude any non-specific binding of the PIP2 antibodies, we detected the signal with a positive control PIP2 (Echelon Biosciences, Cat#P-4516) (data not shown). Experiments were repeated at least 3 times. Cell scraping for traditional dot blots could not be performed on PAA (10 kPa or 100 kPa) due to the structurally weak nature of polyacrylamide hydrogels.
FRAP for actin dynamics
Microscopic techniques, such as FRAP, have yielded quantitative information about the processes that regulate actin polymerization in living myocytes. The methods and analysis for FRAP of actin-GFP were described by our lab (Lin et al. 2013). NRVMs were treated with a FAK inhibitor PF–573228 (30 μmol/L) (Cat#PZ0117, Sigma–Aldrich, USA) (Slack-Davis et al. 2007) for 1 h prior to the FRAP experiment. In the present study, 5 myocytes were analyzed per culture, and at least 3 separate cultures were studied for 100 kPa, 400 kPa, and glass. Stiffness affects embryonic cardiomyocyte structure and contractility (Engler et al. 2008). We also found very rapid beating of the neonatal myocytes on 10 kPa substrates, which did not permit the imaging quality and time resolution necessary for FRAP analysis.
Statistics
Data are presented as mean ± SEM. Statistical significance was determined by one-way ANOVA with Tukey’s multiple comparison tests. Significance was taken as p < 0.05.
Results
NRVM cell size increase with substrata stiffness
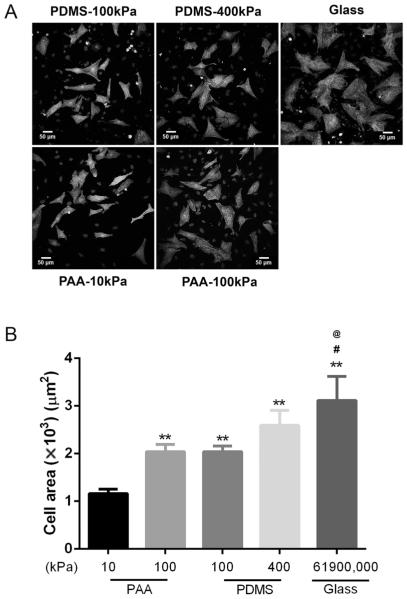
NRVMs grown for 3 days were beating well in culture on the various substrata and had good sarcomere striations (Fig. 1A). The NRVM area was greatest for cells grown on the hard glass surface and least for cells on PAA substrates of 10 kPa. The ratios of cell area normalized to the 10 kPa were 1.76, 1.80, 2.23, and 2.69 for stiffness of PAA substrates of 100 kPa, PDMS substrates of 100 kPa and 400 kPa, and glass (61.9 GPa), respectively, with statistical significance levels as shown in Fig. 1B. Also, the area of NRVMs on glass were significantly increased by 1.52 and 1.50 times, respectively, see Fig. 1B, compared with that on 100 kPa PAA and 100 kPa PDMS. The areas of myocytes were similar when grown on the same stiffness (100 kPa) but on 2 different polymers (PAA and PDMS).
Fig. 1.
Cardiac myocyte hypertrophy is increased with the stiffness of the culture substrata. (A) Neonatal rat ventricular myocytes (NRVMs) differed significantly in area after 3 days in culture on polyacrylamide (PAA; 10 kPa and 100 kPa), polydimethylsiloxane (PDMS; 100 and 400 kPa), or glass (61.9 GPa). (B) The areas of NRVMs were significantly lower on the softest PAA (10 kPa) than on all other substrata. Correspondingly, the areas of NRVMs on glass were increased compared with 100 kPa PAA or 100 kPa PDMS. There were no significant changes between cell areas for the 2 polymers (PAA and PDMS) of the same stiffness (100 kPa). Mean ± SEM, **p < 0.01 compared with 10 kPa PAA, #p < 0.05 compared with 100 kPa PAA, @p < 0.05 compared with 100 kPa PDMS, n = 20. Scale bars = 50 μm.
FAK activation by substrate stiffness
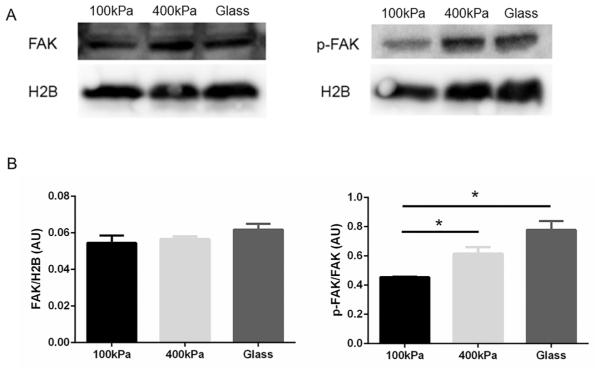
The tyrosine residue Tyr397 is phosphorylated via an autophosphorylation process, leading to an increase in FAK enzymatic activity (Chen et al. 1996). The effect of the stiffness on FAK activity was assessed by Western blotting, which was detected with a phosphospecific antibody against the autophosphorylation site of FAK (FAK-Tyr397). The level of FAK phosphorylation at Tyr397 was significantly increased (p < 0.05, n = 3) in myocytes on stiffer PDMS substrata (400 kPa) and glass (61.9 GPa), compared with PDMS (100 kPa), (Fig. 2A). The protein level of total FAK in all the groups was not significantly changed when normalized to histone 2B (H2B) (Fig. 2B).
Fig. 2.
Phosphorylation of focal adhesion kinase (p-FAK) in cultured neonatal rat ventricular myocytes (NRVMs) is increased with higher stiffness of the substrata. (A) The ratio of p-FAK (Y397) to total focal adhesion kinase (FAK) was quantified by Western blotting. (B) The level of total FAK was not significantly changed when normalized to histone 2B (H2B) intensity. p-FAK significantly increased in NRVM cultured for 3 days on the 400 kPa polydimethylsiloxane (PDMS) or 61.9 GPa glass, compared with 100 kPa PDMS. Mean ± SEM. *p < 0.05, n = 3.
PIP2 increases with substrate stiffness
The effect of stiffness on total PIP2 abundance in cultured NRVMs was measured by dot blotting (Fig. 3). After 3 days of culture, the total abundance of PIP2 in NRVMs varied with the stiffness of the underlying PDMS or glass substratum. The PIP2 level was significantly increased (p < 0.05) in PDMS (400 kPa) and glass (61.9 GPa) substrates compared with the softer PDMS (100 kPa).
Fig. 3.
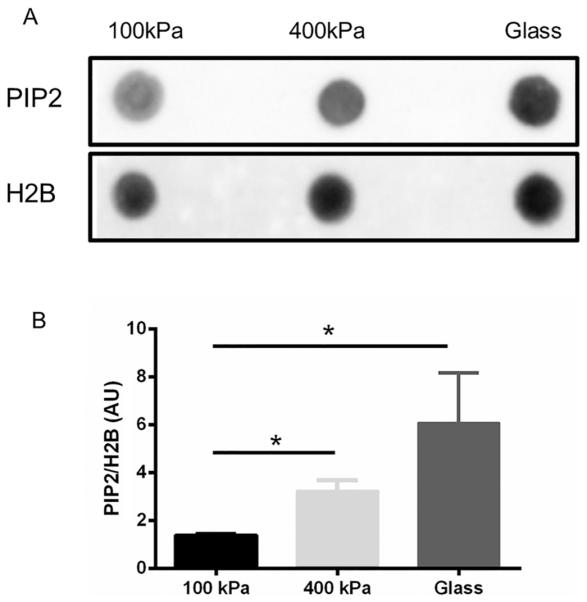
Increased phosphatidylinositol 4,5-bisphosphate (PIP2) production in cultured neonatal rat ventricular myocytes (NRVMs) with substrata stiffness (A). The PIP2 level was analyzed by the intensity of the dot blots and (B) was significantly increased in the 400 kPa polydimethylsiloxane (PDMS) or glass, compared with 100 kPa. Histone 2B (H2B) was used to normalize the dot blot density. Mean ± SEM. *p < 0.05, n = 4.
Sarcomeric distribution of phosphorylated FAK with substrate stiffness
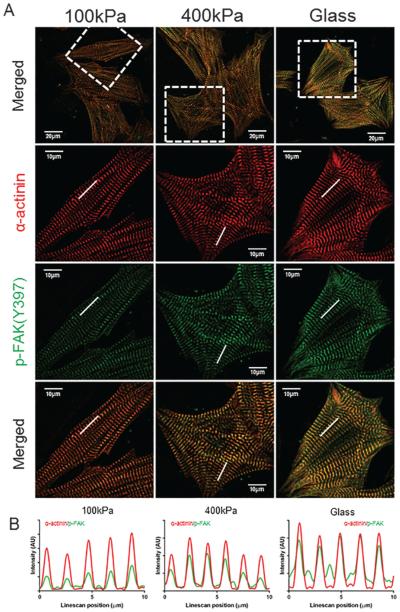
The sarcomeric distribution p-FAK in NRVMs was evaluated by confocal microscopy using anti-p-FAK (Tyr397), Fig. 4. An a-actinin antibody was used to identify the repeating sarcomeric structure of the Z-discs. Overall, there appeared to be less staining for p-FAK on PDMS (100 kPa), and the most staining intensity for NRVM grown on glass (61.9 GPa) but immunostaining is a semiquantitative method (Fig. 4A). However, line scans provide a quantitative method for analysis of the distribution of the immuno-label in the various sarcomere bands (Fig. 4B). At all stiffnesses, the peaks of the a-actinin at the Z-disc coincided with the peaks of p-FAK. Line scans intensity also suggested that the p-FAK was lowest in NRVM on the PDMS (100 kPa) substrata. The glass (61.9 GPa) had the highest amount of p-FAK signaling, which accumulated at the Z-disc.
Fig. 4.
Sarcomeric distribution of phosphorylated focal adhesion kinase (p-FAK) in neonatal rat ventricular myocytes (NRVMs) with varied substrata stiffness. Immunofluorescence images of the cytoskeletal/myofibrillar fraction of NRVMs grown for 3 days on 100 kPa polydimethylsiloxane (PDMS), 400 kPa PDMS, or 61.9 GPa glass (A). NRVM were stained for p-FAK (Tyr397) (green) and a-actinin (red). A greater intensity of green striations from p-FAK is seen by NRVM grown on glass (61.9 GPa) than on the 100 and 400 kPa PDMS substrata. Line scans quantify the proteins along the myofibril (10 μm long white line) (B). Note the peaks at the 2 μm spacing of the sarcomeric repeat in the a-actinin (red line scan) of the Z-disc. The peaks of pFAK (green lines) in the line scan colocalize with the Z-disc. Note the intensity of the p-FAK and ratio of pFAK to a-actinin (red) is lower in the 100 kPa and 400 kPa cells than in those grown on glass (61.9 GPa). Taken together, these findings suggest that the FAK is involved in the pathway for signaling stiffness. Scale bars = 20 μm in low magnification (top row). Scale bars = 10 μm in high magnification (bottom 3 rows). [Colour online.]
Sarcomeric distribution of PIP2 with substrate stiffness
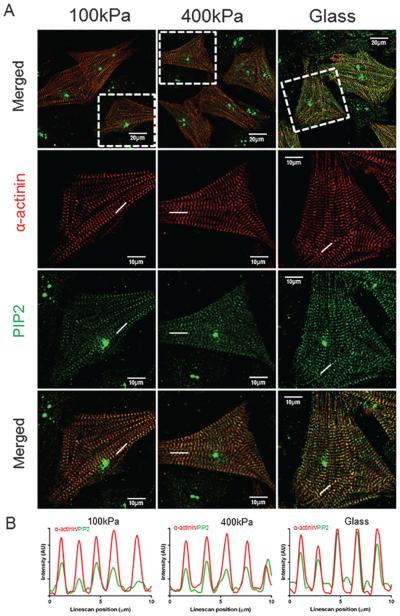
Similarly, the sarcomeric distribution of PIP2 in NRVMs was evaluated by confocal microscopy with a-actinin for identification of the Z-disc and with double antibody staining for PIP2 signaling (Fig. 5). Here too, the typical sarcomeric pattern was seen for NRVM on all stiffnesses. Overall, the PIP2 was higher on glass than for the 100 kPa or 400 kPa PDMS substrata. Furthermore, line scans showed clear co-localization of the peak intensity of PIP2 with the a-actinin at the Z-discs in myocytes (Fig. 5B).
Fig. 5.
Subcellular distribution of phosphatidylinositol 4,5-bisphosphate (PIP2) to the sarcomeres of in neonatal rat ventricular myocytes (NRVMs) with stiffness. Immunofluorescence images of the cytoskeletal/myofibrillar fraction of NRVM grown for 3 days on 100 kPa polydimethylsiloxane (PDMS), 400 kPa PDMS, or 61.9 GPa glass (A). NRVM were stained for PIP2 (green) and a-actinin (red). More PIP2 (green line) co-localized with a-actinin (red line) in the Z-disc in the 61.9 GPa group, as quantified by line scan (B). Glass (61.9 GPa) has greater intensity of PIP2 staining, suggesting that the PIP2 is involved in the stiffness-signaling pathway. Scale bars = 20 μm in low magnification (top row). Scale bars = 10 μm in high magnification (bottom 3 rows). [Colour online.]
CapZβ1 and actin dynamics increase with substrate stiffness that FAK inhibition blunts
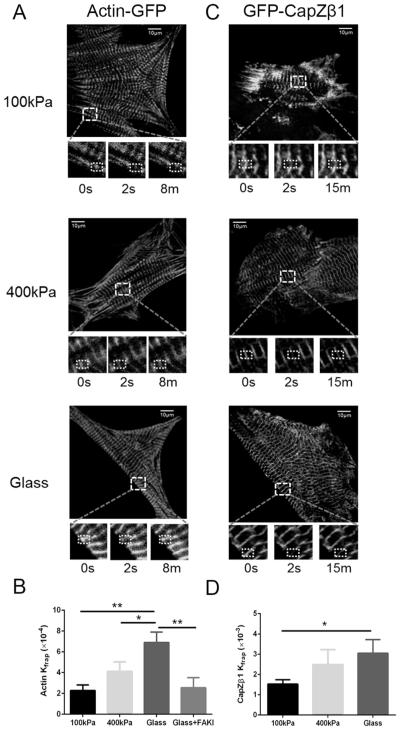
FRAP experiments revealed differences in protein dynamics on different substrates. The actin-GFP and GFP-CapZβ1 had strong striations in NRVMs and signals were detected in a 10 μm× 10 μm square region of interest (Fig. 6). Actin-GFP had a faster dynamic protein exchange in myocytes on glass compared with those on 100 kPa PDMS substrata (6.90 ± 0.99 vs. 2.28 ± 0.54 × 10−4·s−1, p < 0.01) (Figs. 6A and 6B). Consistently, the dynamics of GFP-CapZβ1 was significantly higher in myocytes on glass compared with those on 100 kPa substrata (3.05 ± 0.67 vs. 1.52 ± 0.22 × 10−3·s−1, p < 0.05) (Figs. 6C and 6D), meaning that a faster protein exchange was occurring in myocytes working against the increased load due to the stiffness of the substrata.
Fig. 6.
Actin and CapZβ1 dynamics measured by fluorescence recovery after photobleaching (FRAP) in myocytes cultured on different stiffnesses. Neonatal rat ventricular myocytes (NRVMs) were plated on polydimethylsiloxane (PDMS; 100 kPa, 400 kPa) and glass (61.9 GPa) substrates for 3 days. (A) The increased dynamics of actin was measured by FRAP. Microscopic images of whole living NRVMs infected with actin-GFP in myocytes on 100 kPa, 400 kPa, and 61.9 GPa. The enlarged inset below shows FRAP of the region of interest (10 μm × 10 μm box of dashed white lines) for actin-GFP before, immediately after, and 8 min after photobleaching. Scale bars = 10 μm. (B) Kfrap of actin-GFP on glass had increased kinetic rates compared with 100 kPa PDMS. The FAK inhibitor abolished the high Kfrap of increased actin-GFP on the glass surface. Mean ± SEM, *p < 0.05, **p < 0.01, n = 15. (C) The inset below shows FRAP of the region of interest (10 μm × 10 μm box of dashed white lines) for GFP-CapZβ1 before, immediately after, and 15 min after photobleaching. (D) Kfrap of CapZB1-GFP on glass had increased kinetic rates compared with 100 kPa PDMS. Mean ± SEM, *p < 0.05, n = 15.
Furthermore, to study the role of p-FAK at Z-disc, NRVMs grown on glass were treated with 30 μmol/L FAK inhibitor PF–573228 for 1 h, then FRAP experiments were performed. The increased actin dynamics dramatically decreased (2.55 ± 0.97 vs. 6.90 ± 0.99 × 10−4·s−1, p < 0.01), suggesting that increased p-FAK was involved in regulating actin dynamics.
Discussion
Our novel bioengineering approaches with 2 polymers (PAA and PDMS) permitted fabrication of substrata with varying physiologic or pathologic stiffness for the study of NRVM in culture. Hence, we could determine how sarcomere assembly of cardiomyocytes depends on chronic loading. Interestingly, cell size depended on the stiffness, not on the polymer itself, suggesting that stiffness is more important than other polymer-specific properties. Cardiac hypertrophy measured by cell size increased with the stiffness of the culture substrata. Analysis showed significant changes with stiffness for 2 components of the signaling pathways, namely phosphorylation of FAK at Y397 and the PIP2 production level. Furthermore, both the FAK and PIP2 signaling molecules dramatically increased at the Z-disc of the myofibrils. Moreover, the actin and CapZβ1 dynamics increased with substrata stiffness as measured by FRAP but actin dynamics were blunted by FAK inhibition implicating linkage from mechano-signalling to cell growth. Taken together, results suggest that increased levels of p-FAK and PIP2 at the Z-disc are related to actin assembly and, hence, to myocyte hypertrophy.
Integrin transmembrane receptors on the cell surface regulates attachment to substrate or matrix, resulting in activation and focal adhesion formation. With suitable functionalization of the fibronectin binding, we found the surface chemistry of the PAA and PDMS polymers was excellent for NRVM adhesion in culture. However, cell detachment during processing for extraction of the membrane fraction differed and we were unable to process cells for the cytoskeletal distribution on the PAA substrata. FAK was activated downstream of integrins, and its signaling was especially important for cardiac hypertrophy (Clemente et al. 2012; Peng et al. 2008). For example, tyrosine-phosphorylated FAK dramatically increased in the isolated rat heart perfused to produce pressure loading (Domingos et al. 2002). p-FAK at Tyr397 redistributed to the Z-disc when myocytes were activated by mechanical stretch (Torsoni et al. 2003). FAK was also involved in increasing adhesion strength, particularly in response to tension forces (Wang et al. 2001). Our data confirm similar activation of p-FAK by chronic loading with increased stiffness.
Mechanical stimulation also engaged PIP2 signaling, which was a key determinant of sarcomeric assembly (Li and Russell 2013). PIP2 is the most abundant of the phosphoinositides that binds cellular proteins and accounts for approximately 1% of lipid content in the plasma membrane of a typical mammalian cell because PIP2 has a phosphinositol head and 2 fatty acid chains, making it highly hydrophobic and lipophilic (Lemmon 2008). However, PIP2 also binds to hydrophobic pockets found within proteins, such as CapZ. Thus, PIP2 binding may result in a reduction in binding affinity of CapZ dimers to the actin filament (Hartman et al. 2009; Li and Russell 2013). We have shown that there is more PIP2 at Z-disc when the cell size is bigger (Li and Russell 2013; Li et al. 2014). Both actin and CapZ dynamics were also greater with hypertrophy. Neomycin, the PIP2 scavenger, abolished the increased cell size. The multi-step relationship between PIP2 and cell size is proposed so that PIP2 binds CapZ, which loosens the cap enabling more actin filaments to form resulting in cell hypertrophy. In this study, PIP2 antibody staining of fixed cells showed PIP2 increased at the Z-discs with chronic mechanical loading by stiffness (Fig. 5). Also, actin and CapZβ1 FRAP data showed their significantly increased dynamics (Fig. 6), which was consistent with the increased PIP2 localization at the Z-discs. These results suggest that the increased PIP2 at Z-discs may bind CapZβ1, which regulates actin filament assembly.
The present study demonstrates that the effect of stiffness of the microenvironment in heart tissue is very important in regulating actin assembly in cardiac myocytes. Changes in FAK and PIP2 signaling at the Z-disc indicate involvement as mechanical sensors in response to the stiffer environment in load-induced cardiac myocyte hypertrophy. These 2 signaling pathways may have independent targets or they may interact. Indeed, FAK interacts with PIP2, which affects FAK clustering on the lipid membrane (Chen et al. 1996; Goñi et al. 2014). PIP2 is produced by PIP5K, an important mediator of the integrin/FAK signaling link (Goñi et al. 2014). Additionally, activated FAK might phosphorylate and activate local PIP5K (Brancaccio et al. 2006). The latter could lead to local synthesis of high amounts of PIP2 binding to CapZβ1, resulting in the high assembly and destabilization of the actin filaments (Xu et al. 2014). FAK might interact directly or indirectly with PIP2. Therefore, we interpret our data to suggest that FAK/PIP2 signaling pathways interact both upstream at the membrane as well described previously (Li and Russell 2013) but also down-stream in the sarcomere, leading to integrated mechanical transduction for increased filament assembly resulting from chronic loading by external stiffness. Many other signaling pathways are known to be involved in mechanotransduction signaling leading to induced cardiac hypertrophy, such as calcineurin, a cytoplasmic Ca2+/calmodulin-dependent protein phosphatase (Liu et al. 2010). CapZ is critical for actin capping but is not itself calcium dependent (Li and Russell 2013). However, partner signaling proteins and lipids do have calcium dependence.
Changes in actin capping dynamics with stiffness further suggest that thin filament assembly depends on cell tension, which feeds back via signaling pathways onto sarcomere assembly. It seems likely that filaments are built to serve the functional work being demanded by the myocyte, and that local mechanical conditions ultimately regulate filament assembly and muscle mass. Despite the vast amount of knowledge of the multi-protein complexes of the costamere and Z-disc, there is currently no clinical strategy to reduce or prevent the maladaptive cardiac remodeling that occurs within each myocyte of the heart. Our findings may provide a better understanding of fundamental processes in local fibrotic or stiff scar tissue based on modeling of physiologic and pathologic stiffness modeled with substrata for myocyte culture.
Acknowledgements
We gratefully acknowledge Jennifer Wen in the laboratory of Adam Engler, University of California at San Diego, for conducting atomic force microscopy for stiffness assessment of the polyacrylamide substrata. We thank Sagar Dommaraju for cell area measurements. This work was supported by a National Institutes of Health grant (NIH HL62426).
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest, financial or otherwise, associated with this work.
Contributor Information
Jieli Li, Department of Physiology and Biophysics, Center for Cardiovascular Research, University of Illinois at Chicago, 835 S. Wolcott Ave, Chicago, IL 60612, USA.
Michael A. Mkrtschjan, Department of Bioengineering, University of Illinois at Chicago, 851 South Morgan Street, Chicago, IL 60607, USA
Ying-Hsi Lin, Department of Physiology and Biophysics, Center for Cardiovascular Research, University of Illinois at Chicago, 835 S. Wolcott Ave, Chicago, IL 60612, USA.
Brenda Russell, Department of Physiology and Biophysics, Center for Cardiovascular Research, University of Illinois at Chicago, 835 S. Wolcott Ave, Chicago, IL 60612, USA; Department of Bioengineering, University of Illinois at Chicago, 851 South Morgan Street, Chicago, IL 60607, USA.
References
- Bhana B, Iyer RK, Chen WL, Zhao R, Sider KL, Likhitpanichkul M, et al. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol. Bioeng. 2010;105:1148–1160. doi: 10.1002/bit.22647. PMID:20014437. [DOI] [PubMed] [Google Scholar]
- Boateng SY, Hartman TJ, Ahluwalia N, Vidula H, Desai TA, Russell B. Inhibition of fibroblast proliferation in cardiac myocyte cultures by surface microtopography. Am. J. Physiol.: Cell Physiol. 2003;285:C171–C182. doi: 10.1152/ajpcell.00013.2003. doi: 10.1152/ajpcell.00013.2003. PMID:12672651. [DOI] [PubMed] [Google Scholar]
- Boateng SY, Belin RJ, Geenen DL, Margulies KB, Martin JL, Hoshijima M, et al. Cardiac dysfunction and heart failure are associated with abnormalities in the subcellular distribution and amounts of oligomeric muscle LIM protein. Am. J. Physiol.: Heart Circ. Physiol. 2007;292:H259–H269. doi: 10.1152/ajpheart.00766.2006. PMID:16963613. [DOI] [PubMed] [Google Scholar]
- Borbély A, van der Velden J, Papp Z, Bronzwaer JGF, Edes I, Stienen GJM, Paulus WJ. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–781. doi: 10.1161/01.CIR.0000155257.33485.6D. doi:10.1161/01.CIR.0000155257.33485.6D. PMID:15699264. [DOI] [PubMed] [Google Scholar]
- Brancaccio M, Hirsch E, Notte A, Selvetella G, Lembo G, Tarone G. Integrin signalling: the tug-of-war in heart hypertrophy. Cardiovasc. Res. 2006;70:422–433. doi: 10.1016/j.cardiores.2005.12.015. doi:10.1016/j.cardiores.2005.12.015. PMID:16466704. [DOI] [PubMed] [Google Scholar]
- Broughton KM, Russell B. Cardiomyocyte subdomain contractility arising from microenvironmental stiffness and topography. Biomech. Model. Mechanobiol. 2015;14:589–602. doi: 10.1007/s10237-014-0624-2. doi:10.1007/s10237-014-0624-2. PMID:25273278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H-C, Appeddu PA, Isoda H, Guan J-L. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J. Biol. Chem. 1996;271:26329–26334. doi: 10.1074/jbc.271.42.26329. doi:10.1074/jbc.271.42.26329. PMID:8824286. [DOI] [PubMed] [Google Scholar]
- Chu M, Iyengar R, Koshman YE, Kim T, Russell B, Martin JL, et al. Serine-910 phosphorylation of focal adhesion kinase is critical for sarcomere reorganization in cardiomyocyte hypertrophy. Cardiovasc. Res. 2011;92:409–419. doi: 10.1093/cvr/cvr247. doi:10.1093/cvr/cvr247. PMID:21937583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente CFMZ, Xavier-Neto J, Dalla Costa AP, Consonni SR, Antunes JE, Rocco SA, et al. Focal adhesion kinase governs cardiac concentric hypertrophic growth by activating the AKT and mTOR pathways. J. Mol. Cell. Cardiol. 2012;52:493–501. doi: 10.1016/j.yjmcc.2011.10.015. doi:10.1016/j.yjmcc.2011.10.015. PMID: 22056317. [DOI] [PubMed] [Google Scholar]
- de Tombe PP. Cardiac myofilaments: mechanics and regulation. J. Biomech. 2003;36:721–730. doi: 10.1016/s0021-9290(02)00450-5. doi:10.1016/S0021-9290(02)00450-5. PMID:12695002. [DOI] [PubMed] [Google Scholar]
- Domingos PP, Fonseca PM, Nadruz W, Jr., Franchini KG. Load-induced focal adhesion kinase activation in the myocardium: role of stretch and contractile activity. Am. J. Physiol.: Heart Circ. Physiol. 2002;282:H556–H564. doi: 10.1152/ajpheart.00534.2001. PMID:11788403. [DOI] [PubMed] [Google Scholar]
- Edwards M, Zwolak A, Schafer DA, Sept D, Dominguez R, Cooper JA. Capping protein regulators fine-tune actin assembly dynamics. Nat. Rev. Mol. Cell. Biol. 2014;15:677–689. doi: 10.1038/nrm3869. doi:10.1038/nrm3869. PMID:25207437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang H-Y, Speicher DW, et al. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J. Cell. Sci. 2008;121:3794–3802. doi: 10.1242/jcs.029678. doi:10.1242/jcs.029678. PMID:18957515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. doi:10.1016/j.cell. 2006.06.044. PMID:16923388. [DOI] [PubMed] [Google Scholar]
- Fomovsky GM, Holmes JW. Evolution of scar structure, mechanics, and ventricular function after myocardial infarction in the rat. Am. J. Physiol.: Heart Circ. Physiol. 2010;298:H221–H228. doi: 10.1152/ajpheart.00495.2009. PMID:19897714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomovsky GM, Thomopoulos S, Holmes JW. Contribution of extracellular matrix to the mechanical properties of the heart. J. Mol. Cell. Cardiol. 2010;48:490–496. doi: 10.1016/j.yjmcc.2009.08.003. doi:10.1016/j.yjmcc.2009.08.003. PMID:19686759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini KG, Torsoni AS, Soares PHA, Saad MJA. Early activation of the multicomponent signaling complex associated with focal adhesion kinase induced by pressure overload in the rat heart. Circ. Res. 2000;87:558–565. doi: 10.1161/01.res.87.7.558. doi:10.1161/01.RES.87.7.558. PMID:11009560. [DOI] [PubMed] [Google Scholar]
- Goñi GM, Epifano C, Boskovic J, Camacho-Artacho M, Zhou J, Bronowska A, et al. Phosphatidylinositol 4,5-bisphosphate triggers activation of focal adhesion kinase by inducing clustering and conformational changes. Proc. Natl. Acad. Sci. U.S.A. 2014;111:E3177–E3186. doi: 10.1073/pnas.1317022111. doi:10.1073/pnas.1317022111. PMID:25049397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman TJ, Martin JL, Solaro RJ, Samarel AM, Russell B. CapZ dynamics are altered by endothelin-1 and phenylephrine via PIP2- and PKC-dependent mechanisms. Am. J. Physiol.: Cell Physiol. 2009;296:C1034–C1039. doi: 10.1152/ajpcell.00544.2008. doi: 10.1152/ajpcell.00544.2008. PMID:19295171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annu. Rev. Biomed. Eng. 2005;7:223–253. doi: 10.1146/annurev.bioeng.7.060804.100453. doi:10.1146/annurev.bioeng.7.060804.100453. PMID:16004571. [DOI] [PubMed] [Google Scholar]
- Hoshijima M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am. J. Physiol.: Heart Circ. Physiol. 2006;290:H1313–H1325. doi: 10.1152/ajpheart.00816.2005. PMID:16537787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacot JG, Martin JC, Hunt DL. Mechanobiology of cardiomyocyte development. J. Biomech. 2010;43:93–98. doi: 10.1016/j.jbiomech.2009.09.014. doi:10.1016/j.jbiomech.2009.09.014. PMID: 19819458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat. Rev. Mol. Cell. Biol. 2008;9:99–111. doi: 10.1038/nrm2328. doi:10.1038/nrm2328. PMID:18216767. [DOI] [PubMed] [Google Scholar]
- Li J, Russell B. Phosphatidylinositol 4,5-bisphosphate regulates CapZβ1 and actin dynamics in response to mechanical strain. Am. J. Physiol.: Heart Circ. Physiol. 2013;305:H1614–H1623. doi: 10.1152/ajpheart.00477.2013. doi:10.1152/ajpheart.00477.2013. PMID: 24043251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Tanhehco EJ, Russell B. Actin dynamics is rapidly regulated by the PTEN and PIP2 signaling pathways leading to myocyte hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2014;30(11):H1618–H1625. doi: 10.1152/ajpheart.00393.2014. doi:10.1152/ajpheart.00393.2014. PMID:25260617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-H, Li J, Swanson ER, Russell B. CapZ and actin capping dynamics increase in myocytes after a bout of exercise and abates in hours after stimulation ends. J. Appl. Physiol. 2013;114:1603–1609. doi: 10.1152/japplphysiol.01283.2012. doi:10.1152/japplphysiol.01283.2012. PMID:23493359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H-B, Yang B-F, Dong D-L. Calcineurin and electrical remodeling in pathologic cardiac hypertrophy. Trends. Cardiovasc. Med. 2010;20(5):148–153. doi: 10.1016/j.tcm.2010.12.003. doi:10.1016/j.tcm.2010.12.003. PMID:21742270. [DOI] [PubMed] [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. 8th. National Academies Press; Washington, D.C.: 2011. PMID:21595115. [PubMed] [Google Scholar]
- Peng X, Wu X, Druso JE, Wei H, Park AY-J, Kraus MS, et al. Cardiac developmental defects and eccentric right ventricular hypertrophy in cardiomyocyte focal adhesion kinase (FAK) conditional knockout mice. Proc. Natl. Acad. Sci. U.S.A. 2008;105:6638–6643. doi: 10.1073/pnas.0802319105. doi:10.1073/pnas.0802319105. PMID:18448675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poellmann MJ, Wagoner Johnson AJ. Characterizing and patterning polyacrylamide substrates functionalized with N-hydroxysuccinimide. Cell. Mol. Bioeng. 2013;6:299–309. doi:10.1007/s12195-013-0288-5. [Google Scholar]
- Senyo SE, Koshman YE, Russell B. Stimulus interval, rate and direction differentially regulate phosphorylation for mechanotransduction in neonatal cardiac myocytes. FEBS Lett. 2007;581:4241–4247. doi: 10.1016/j.febslet.2007.07.070. doi:10.1016/j.febslet.2007.07.070. PMID:17698065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack-Davis JK, Martin KH, Tilghman RW, Iwanicki M, Ung EJ, Autry C, et al. Cellular characterization of a novel focal adhesion kinase inhibitor. J. Biol. Chem. 2007;282:14845–14852. doi: 10.1074/jbc.M606695200. doi:10.1074/jbc.M606695200. PMID: 17395594. [DOI] [PubMed] [Google Scholar]
- Torsoni AS, Constancio SS, Nadruz W, Jr., Hanks SK, Franchini KG. Focal adhesion kinase is activated and mediates the early hypertrophic response to stretch in cardiac myocytes. Circ. Res. 2003;93:140–147. doi: 10.1161/01.RES.0000081595.25297.1B. doi:10.1161/01.RES.0000081595.25297.1B. PMID:12805241. [DOI] [PubMed] [Google Scholar]
- Tse JR, Engler AJ. Unit 10.16 Preparation of hydrogel substrates with tunable mechanical properties. Curr. Protoc. Cell. Biol. 2010;47:10.16.1–10.16.16. doi: 10.1002/0471143030.cb1016s47. doi:10.1002/0471143030.cb1016s47. PMID:20521229. [DOI] [PubMed] [Google Scholar]
- Wang WH. The elastic properties, elastic models and elastic perspectives of metallic glasses. Prog. Mater. Sci. 2012;57:487–656. doi:10.1016/j.pmatsci.2011.07.001. [Google Scholar]
- Wang H-B, Dembo M, Hanks SK, Wang Y-L. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc. Natl. Acad. Sci. U.S.A. 2001;98:11295–11300. doi: 10.1073/pnas.201201198. doi:10.1073/pnas.201201198. PMID:11572981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat. Cell. Biol. 2015;17:678–688. doi: 10.1038/ncb3157. doi:10.1038/ncb3157. PMID:25893917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J-X, Si M, Zhang H-R, Chen X-J, Zhang X-D, Wang C, et al. Phosphoinositide kinases play key roles in norepinephrine- and angiotensin II-induced increase in phosphatidylinositol 4,5-bisphosphate and modulation of cardiac function. J. Biol. Chem. 2014;289:6941–6948. doi: 10.1074/jbc.M113.527952. doi:10.1074/jbc.M113.527952. PMID:24448808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 2014;13:645–652. doi: 10.1038/nmat3889. doi:10.1038/nmat3889. PMID:24633344. [DOI] [PMC free article] [PubMed] [Google Scholar]