Abstract
Development of antibodies (inhibitors) against coagulation factor VIII (FVIII) is a major complication of intravenous replacement therapy in haemophilia A (HA). Current immune tolerance induction (ITI) regimens are not universally effective. Rituximab, a B cell-depleting antibody against CD20, has shown mixed results for inhibitor reversal in patients. This study aims to develop a combinatorial therapy for inhibitor reversal in HA, using anti-murine CD20 (anti-mCD20) antibody and rapamycin, which targets both B and T cell responses. Additionally, it extensively characterizes the role of the IgG backbone in B cell depletion by anti-CD20 antibodies. For this, inhibitors were generated in BALB/c-HA mice by weekly IV injection of FVIII. Subsequently, anti-mCD20 (18B12) with IgG2a or IgG1 backbone was injected IV in 2 doses 3 weeks apart and B cell depletion and recovery was characterized. Rapamycin was administered orally 3x/week (for 1 month) while continuing FVIII injections. Altering the IgG backbone of anti-mCD20 from IgG2a to IgG1 reduced overall depletion of B cells (including memory B cells), and marginal zone, B-10, and B-1b cells were specifically unaffected. While neither antibody was effective alone, in combination with rapamycin, anti-mCD20 IgG2a but not IgG1 was able to reverse inhibitors in HA mice. This regimen was particularly effective for starting titres of ~10 BU. Although IgG1 anti-mCD20 spared potentially tolerogenic B cell subsets, IgG2a directed sustained hyporesponsiveness when administered in conjunction with rapamycin. This regimen represents a promising treatment for inhibitor reversal in HA, as both of these compounds have been extensively used in human patients.
Keywords: anti-mCD20, factor VIII, haemophilia, inhibitor, rapamycin
Introduction
Haemophilia A (HA) is an X-linked monogenic disorder resulting in a deficiency in blood coagulation due to mutations in coagulation factor VIII (FVIII). Current treatment for HA involves the administration of recombinant or plasma-derived FVIII protein, either prophylactically or on-demand following a bleeding event (1, 2). Although this treatment allows for management of coagulation in many patients, the efficacy of protein replacement therapy can be impaired by the development of inhibitors, which are antibodies against FVIII that neutralize coagulation activity. As many as 30% of severe haemophilia A patients will develop an inhibitor in response to factor replacement therapy, usually within the first 20 days of treatment exposure. Although a number of important risk factors for inhibitor development have been identified, particularly the underlying mutation and MHC haplotype, it is still unknown exactly which patients will respond adversely to FVIII (3–5).
Current treatment for inhibitor patients is less than ideal. The only approved therapy is termed immune tolerance induction (ITI), which involves frequent administration of high doses of FVIII (6). However, ITI is effective in only about 60–70% of patients (6, 7). For those not responsive to ITI, bypass reagents can be used to manage bleeding, but require careful dosing and monitoring. Thus, there is clearly an unmet need for better protocols for the reversal of inhibitors. One potential alternative strategy is B cell depletion using rituximab, an anti-CD20 antibody approved for use in a variety of B cell malignancies and autoimmune diseases. However, clinical results in haemophilia have been mixed and somewhat difficult to interpret due to small sample sizes (8, 9). A recent phase II study tested rituximab in patients with failed ITI with limited success; investigators concluded that an additional drug would be desirable that could work in conjunction with B cell depletion (10). In this regard, preclinical studies using anti-CD20 in HA mice have shown some success, such as hepatic gene transfer or IL-2 complexes (11, 12). In a murine model of ITI, anti-CD20 showed promise when combined with daily FVIII injections, mimicking ITI (13). Although rituximab, like most therapeutic antibodies, has a human IgG1 backbone, there are 3 other subclasses of IgG with different structural and functional properties that may alter the effects of the drug (14). Indeed, a potentially more tolerogenic effect was described using a murine IgG1 as opposed to IgG2a (the murine equivalent to human IgG1) in haemophilia A mice receiving FVIII daily (13).
Most B cells are of the traditionally known follicular B-2 subset, which arise from the bone marrow, traffic to a lymph node, and upon antigen exposure mature and differentiate via the germinal centre and somatic hypermutation into memory B cells and antibody secreting plasma cells. However, marginal zone B cells, another subset of B-2 cells found in the marginal zones of the spleen, can also arise from the transitional B cells, which exit the bone marrow and finish maturing in the periphery. This population, along with B-1 cells (including B-1a and B-1b populations), is considered more innate-like, expressing a more limited B cell receptor (BCR) repertoire, showing less dependence on T cell help, and producing natural antibodies in the absence of antigenic stimulation (15, 16). Interestingly, marginal zone B cells have been reported to be more resistant to depletion by anti-CD20 with an IgG2a backbone than follicular B cells (17). Additionally, regulatory B cells expressing IL-10 (B-10 cells) have also been reported to have a role in immune tolerance; these cells share a number of markers with marginal zone B cells (18).
As inhibitor formation is T cell-dependent (19, 20), a therapy that would target both B cells and T cells may be superior. Rapamycin is an immunosuppressive drug routinely used in organ transplantation that we have previously used to prevent inhibitor formation in HA mice (21). When administered in conjunction with antigen, this drug induces antigen-specific T effector cell deletion and induction of regulatory T cells (Treg) (22). This effect can be further enhanced by co-administration of IL-10 or Flt3L (23, 24). Here, we define the effect of the IgG backbone on B cell depletion with anti-CD20 and demonstrate that rapamycin enhances reversal of FVIII inhibitors in HA mice when combined with IgG2a anti-CD20 (the murine equivalent of rituximab).
Materials and Methods
Mice
All animals used at the onset of the experiments were 8–10 week old male mice of the BALB/c background. BALB/c wt mice were purchased from Jackson Laboratories (Bar Harbor, ME). Haemophilia A mice with a deletion in exon 16 of the F8 gene (BALB/c F8e16−/−) were originally provided by Dr. David Lillicrap (Queens, Ontario, Canada). Animals were housed under special pathogen-free conditions at the University of Florida and treated under Institutional Animal Care and Use Committee-approved protocols.
Reagents
Purified CD16/32 (Fc Block), CD3 (PerCP-Cy5.5), CD21 (BV605), CD44 (V500), CD138 (PE), IgM (V450), IgD (BV605) antibodies were from BD Biosciences (San Jose, CA); CD1d (APC), CD23 (PE Cy7), CD43 (FITC) antibodies were purchased from eBioscience (San Diego, CA); CD5 (BV421), CD19 (APC-Cy7), IgG (APC-Cy7), CD38 (A488) antibodies were from Biolegend (San Diego, CA). Samples were acquired on the LSR II flow cytometer (BD Biosciences) and analyzed using FCS Express 4 (DeNovo Software, Los Angeles, CA). Murine CD20 antibodies of the IgG1 and IgG2a backbone (clone 18B12) and the IgG2a isotype control (2B8) were a kind gift from Biogen (Cambridge, MA). Mouse IgG1 κ isotype control antibody (Clone: P3.6.2.8.1) was from eBioscience (San Diego, CA). Rapamycin was from LC laboratories (Woburn, MA). Keyhole limpet hemocyanin (KLH) was from Sigma-Aldrich (St.Louis, MO). Recombinant human BDD-FVIII (Xyntha) was from Pfizer (New York, NY). FVIII deficient plasma and FIX deficient plasma were from Haematologic Technologies (Essex Junction, VT).
Analysis of plasma samples
Plasma samples were collected by tail bleed and analyzed using a modified activated partial thromboplastin time assay (aPTT). Inhibitory antibodies to FVIII were measured by Bethesda assay as described (25). Measurements were carried out in a Diagnostica Stago STart Hemostasis Analyzer (Parsippany, NJ). Enzyme-linked immunosorbent assay (ELISA)-based measurements of antibodies to FVIII were carried out as described (25).
Inhibitor establishment and reversal
BALB/c F8e16−/− mice received 1IU of BDD-FVIII by weekly tail-vein injections for 1 month. Inhibitors thus established were divided into high titre (50–200 BU/ml) and moderate titre (5–15 BU/ml) groups. Autologous polyclonal murine Treg were isolated and expanded ex vivo as described (26). mCD20 antibody (250µg) of the IgG1 or IgG2a subtype was injected IV, 1 and 3 weeks after the establishment of inhibitors. Rapamycin was administered 3x/week (for 1 month) by oral gavage in 100 µl of sterile PBS at a dose of 4 mg/kg, while continuing BDD-FVIII injections. Mice with moderate titre inhibitors were re-challenged with BDD-FVIII injections 1×/week at 1 IU for an additional month.
Statistical analysis
Statistical significance was determined using 2-way ANOVA or Student’s t-test with GraphPad Prism 5 software (La Jolla, CA). Values at P < 0.05 were deemed significant and indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001.
Results
Treatment of inhibitors with rapamycin and Treg
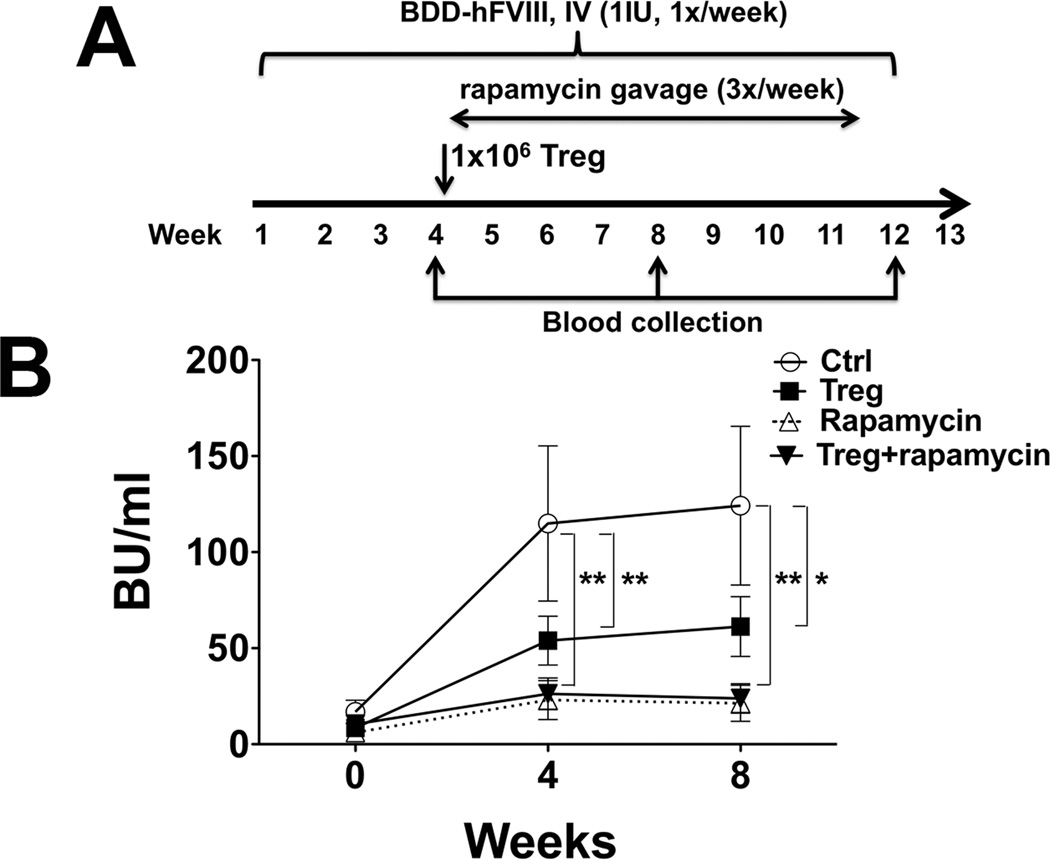
In our prior studies, preventive co-administration of FVIII with rapamycin or with Treg suppressed inhibitor formation to FVIII replacement therapy (21, 26). In order to test for reversal, BALB/c F8e16−/− mice with moderate-titre inhibitors (~10 BU) were generated via IV injection of FVIII. Subsequently, mice received rapamycin 3x per week (for 1 month) by oral gavage in conjunction with continued weekly FVIII injections (1 IU) (Figure 1A). While titres continued to substantially rise in control mice that received FVIII alone, rapamycin and Treg suppressed a further increase of inhibitor formation, with rapamycin being somewhat more effective (Figure 1B). Since expansion of Treg is favored over T effector cells in the presence of rapamycin (22), we hypothesized that a combination of rapamycin and Treg would be synergistic. Disappointingly, the outcome was not improved over rapamycin alone (Figure 1B). Neither protocol reversed inhibitor formation. Therefore, we decided to test B cell depletion with anti-CD20 as an alternative.
Figure 1. Rapamycin and Treg prevent escalation of but fail to reverse inhibitor formation against FVIII in haemophilia A mice with pre-existing response.
A. Experimental timeline. Haemophilia A mice (BALB/c F8e16−/−) received 4 weekly IV injections of BDD-FVIII (1 IU/mouse), resulting in inhibitor formation. Mice were divided into 4 experimental groups (n=6–9/group). “Treg treated” group received IV injection of 1 × 106 expanded Treg. “Control” group received nothing. “Rapamycin” group received oral gavage of rapamycin (4mg/kg, 3X/week). “Treg + rapamycin” group received 1 × 106 expanded Treg followed by rapamycin oral gavage. Weekly BDD-FVIII injections were continued in all animals for 8 more weeks. B. Inhibitor titres (BU/ml) as a function of time. Data are average ±SD. Statistically significant differences are indicated for each time point.
Role of the IgG backbone in anti-CD20-mediated B cell depletion
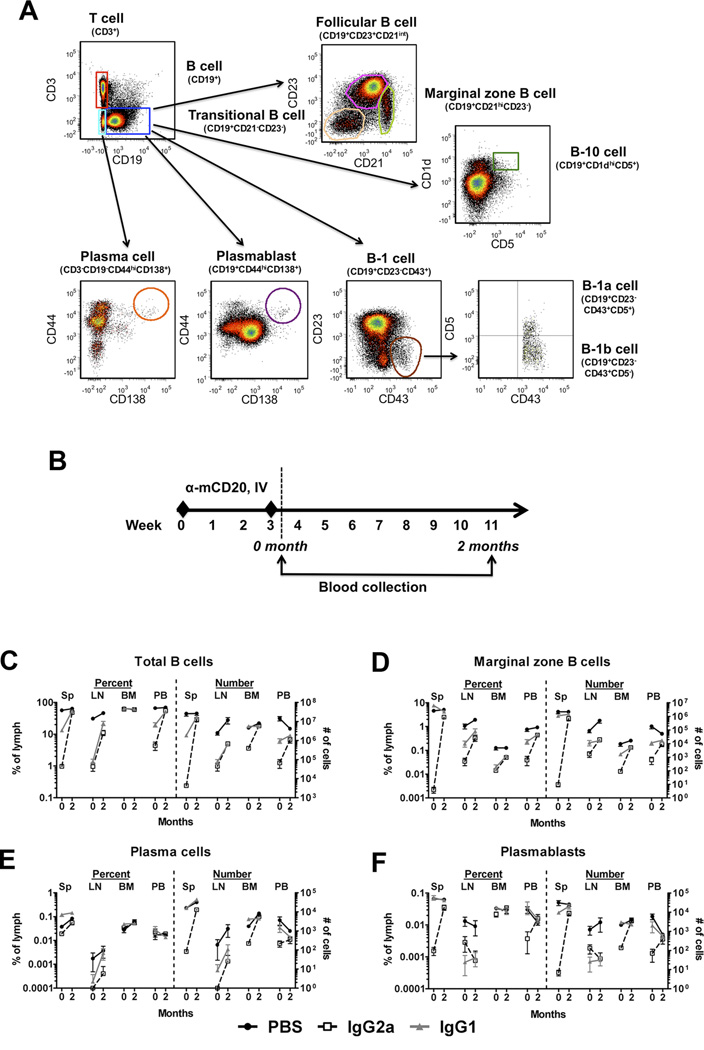
Given a prior report that the Fc region of an anti-CD20 antibody can alter its depletion profile and tolerogenicity in the context of inhibitor reversal in HA, we sought to characterize the depletion and repopulation of B cell subsets in different immune compartments (13). Naïve BALB/c mice were injected IV with two doses of 250µg anti-CD20 IgG2a or IgG1 three weeks apart. One day or two months after the second dose, B cells should be depleted or repopulated, respectively (Figure 2A, B) (11). At both time points, spleen, lymph node (LN), bone marrow (BM), and peripheral blood (PB) were harvested and the frequencies and absolute numbers of several B cell populations were characterized by flow cytometry (Table 1, Supplementary Table S1 and Supplementary Figure S1). Isotype controls to anti-mCD20 IgG1 and IgG2a were used to exclude non-specific depletion (Supplementary Figure S2A, B).
Figure 2. Depletion efficacy of B cell populations by mCD20 antibody depends on IgG backbone.
A. Representative density plots indicating gating scheme for enumeration of B cell subsets from spleen, lymph nodes, bone marrow and peripheral blood of mice receiving mCD20 antibody and controls. Populations of total T cells, total B cells, marginal zone B cells, plasma cells, plasmablasts, follicular B cells, B-1 cells, B-1a cells, and B-10 cells are shown. B. Experimental timeline. Naïve wild-type BALB/c mice (n=5/group) received 2 IV injections of 250µg mCD20 antibody (IgG1 or IgG2a backbone), or PBS on weeks 0 and 3. Blood was collected immediately after, (0 month) and 2 months following the last mCD20 antibody injection. Frequencies of C. Total B cells, D. Marginal zone B cells, E. Plasma cells, and F. Plasmablasts in spleen (Sp), lymph nodes (LN), bone marrow (BM), and peripheral blood (PB) at 0 month and 2 month time points. Data are presented as either % of lymphocytes or total cell numbers/ 108 lymphocytes. Data are average ±SD.
Table 1.
Percent depletion of B cell frequencies in spleen by anti-CD20 IgG2a and IgG1.
| Spleen | ||||
|---|---|---|---|---|
| Population | Markers | IgG2a | IgG1 | Sig. |
| Total B cells | CD19+ CD3− | 98.3 ± 26.7 | 76.3 ± 9.5 | ns |
| Total T cells | CD3+ CD19− | - | - | - |
| Follicular B cells | CD19+ CD23+ CD21int | 99.6 ± 28.0 | 95.2 ± 24.9 | ns |
| Marginal zone B cells | CD19+ CD21hi CD23− | 100.0 ± 62.9 | - | *** |
| Transitional B cells | CD19+ CD21− CD23− | 87.0 ± 36.3 | 60.5 ± 17.5 | ns |
| B-10 cells | CD19+ CD1dhi CD5+ [36] | 99.4 ± 20.6 | - | *** |
| B-1a cells | CD19+ CD23− CD43+ CD5+ | 94.1 ± 42.2 | 23.9 ± 6.0 | ns |
| B-1b cells | CD19+ CD23− CD43+ CD5− | 67.4 ± 27.5 | 6.8 ± 1.5 | ns |
| Plasma cells | CD3− CD19− CD44hi CD138+ | 49.0 ± 16.1 | - | *** |
| Plasmablasts | CD19+ CD44hi CD138+ | 97.7 ± 68.2 | - | ** |
Results show mean percent reduction ± SD of antibody-injected compared to PBS-injected control mice.
- : no depletion observed
P < 0.01,
P < 0.001.
Total B cells were depleted by either anti-CD20 backbone in spleen, LN, and PB at month 0, and largely recovered by month 2, albeit slightly delayed in LN (Figure 2C). Interestingly, while the frequency of total B cells was reduced by about 98% in the spleen by the IgG2a antibody, only about 76% depletion was observed with the IgG1 anti-CD20 (Table 1, Figure 2C). Little if any reduction was observed in the BM, likely due to the continual production and repopulation of B cells within this tissue (Supplementary Table S1, Figure 2C). Due to the reduction in B cell numbers, an increase in the frequency of T cells was observed (Supplementary Figure S1A). In contrast to the frequencies, absolute numbers of T cells were reduced in the spleen with IgG2a, perhaps due to homeostatic interactions between B and T cells (27). Follicular B cells, which make up the majority of the B cell compartment, showed a similar pattern of depletion to the total B cell population: a reduction with both antibodies, and more complete depletion with the IgG2a backbone (Supplementary Figure S1B). This effect was similarly observed for the transitional B cell and B-1a cell compartments (Supplementary Figure S1C,E).
Marginal zone B cells, on the other hand, displayed an alternative outcome. Although these cells were efficiently depleted by the IgG2a antibody in the spleen (Table 1), no alteration of the frequency or absolute numbers was observed in mice that received anti-CD20 IgG1 (Figure 2D). While this phenomenon was less clear in the other tissues (Supplementary Table S1), the vast majority of marginal zone B cells are located in the spleen, so the effect in this tissue is most relevant. This preferential sparing by the IgG1 antibody was also observed for B-10 cells, a B cell population that produces IL-10 and has been associated with immune tolerance in a number of models (Table 1, Figure S1D) (18). B-1a and B-1b cells were also only affected by the IgG2a antibody, although in this case not reaching statistically significant differences between the antibody isotypes (Table 1, Supplementary Table S1, Figure S1E,F).
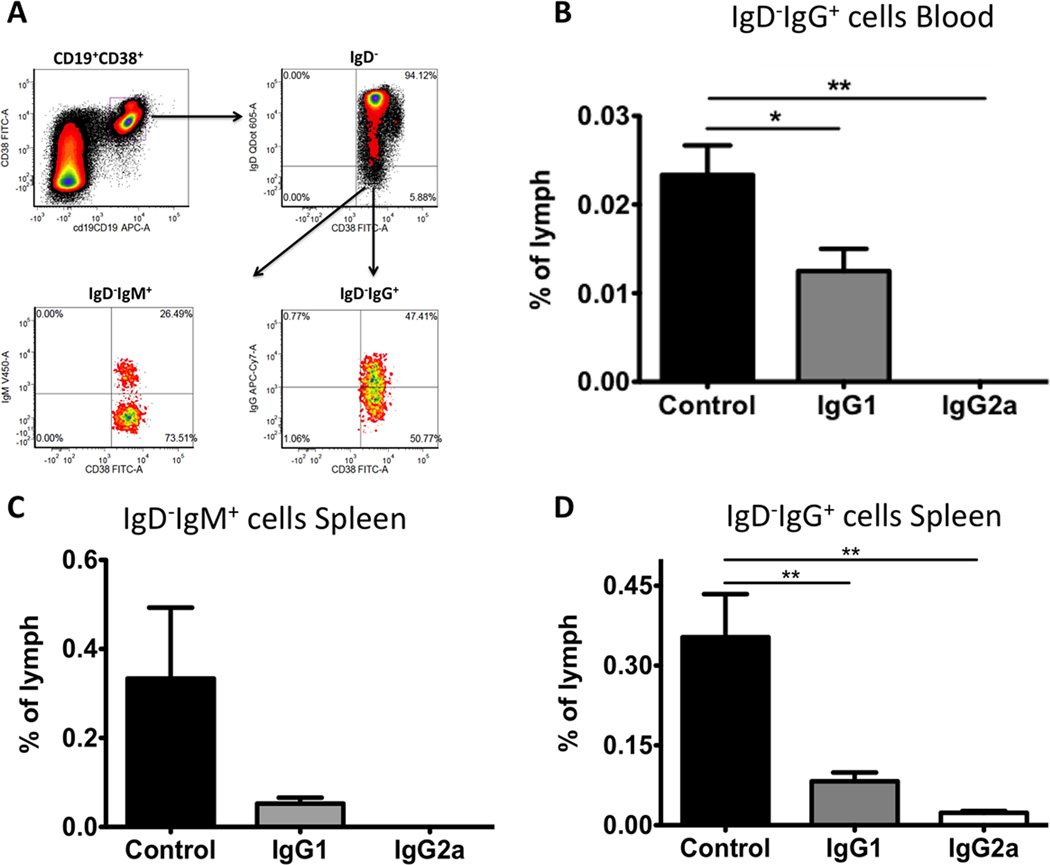
In comparison to the aforementioned B cell populations, the effects of anti-CD20 antibodies on plasma cells were less clear. Plasma cells would not be expected to be depleted by anti-CD20 antibodies, as they lose expression of CD20 as they gain their antibody-secreting status. Indeed, although a partial reduction in plasma cell numbers was observed with the IgG2a antibody, the percentages were only mildly affected (Figure 2E, Table 1). It is probable that any reduction observed was due to disruption of homeostasis by the killing of plasmablast precursors, which were more reliably affected by anti-CD20 IgG2a (Figure 2F, Table 1). We also investigated the effects of these antibodies on memory B cells (Figure 3A). Following anti-CD20 injections, IgD−IgG+ cell frequencies in PB (Figure 3B) or IgD−IgM+ and IgD−IgG+ cell frequencies in spleen (Figure 3C, D) were measured. In both tissues, memory B cells were depleted by either antibody, albeit more effectively by anti-CD20 IgG2a; levels were undetectable in PB and significantly reduced but still measurable in spleen with this antibody.
Figure 3. Depletion efficacy of memory B cells by mCD20 antibody depends on IgG backbone.
A. Representative density plots indicating gating scheme for enumeration of memory B cell subsets from peripheral blood and spleen of mice receiving mCD20 antibody and controls. Naïve BALB/c F8e16−/− mice (n=3–5/group) received 2 IV injections of 250µg mCD20 antibody (IgG1 or IgG2a backbone), or PBS on weeks 0 and 3. 1-day later, frequencies of CD19+CD38+IgD−IgG+ memory cells in peripheral blood or IgD−IgM+ and IgD−IgG+ memory cells in spleen were quantified. Relative percentages of memory B cells in B. peripheral blood, and C, D. spleen are shown. Data are presented as % of lymphocytes. Data are average ±SD.
Inhibitor reversal with anti-CD20 antibodies
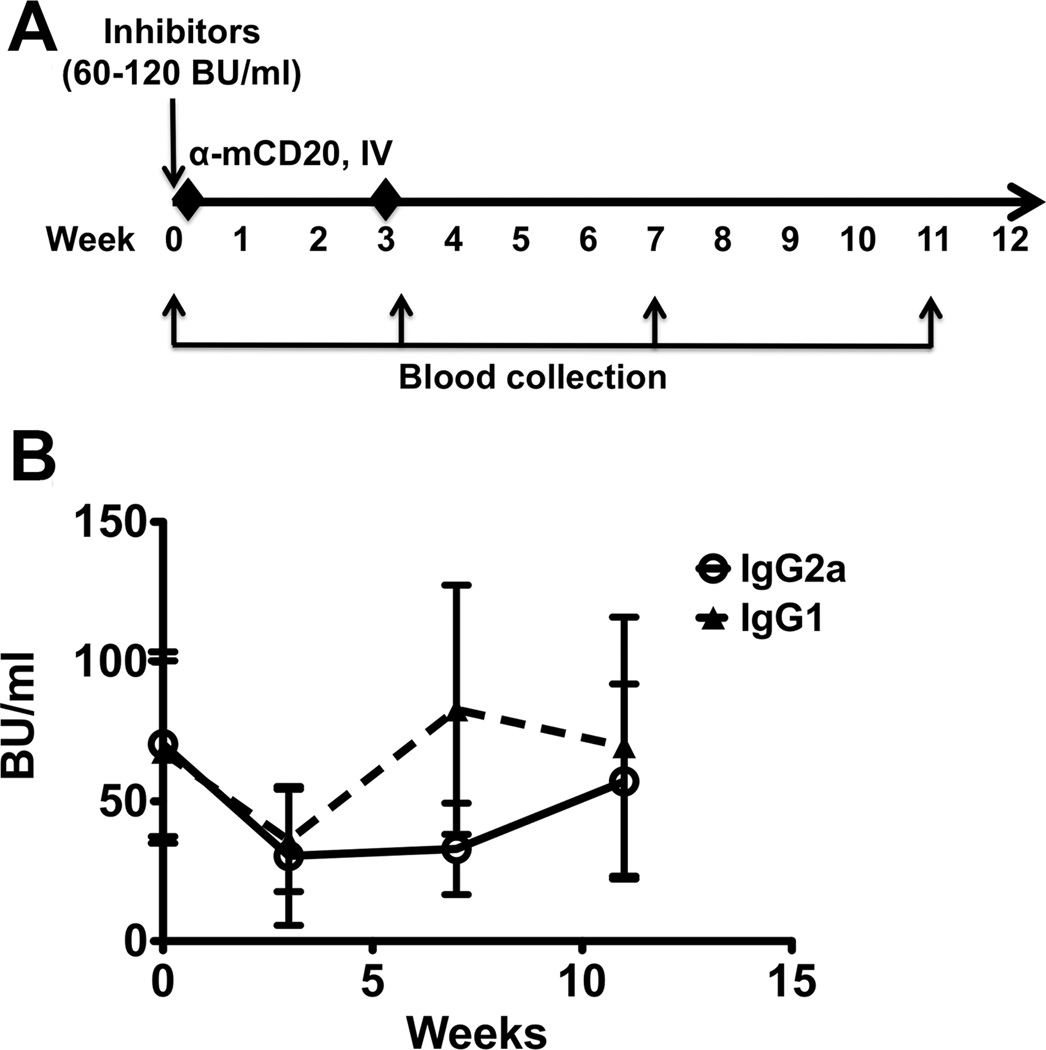
In an initial experiment on attempting to reverse inhibitors with anti-CD20, BALB/c F8e16−/− mice with high-titre inhibitors (BU > 50) were generated by repeated IV FVIII injection. These mice then received 2 injections of anti-CD20 IgG2a or IgG1 (spread 3 weeks apart), and inhibitor titres were followed for 2 months after the second anti-CD20 dose (Figure 4A). Although Bethesda titres dropped by about half by week 3, when B cells should have been most depleted, they still remained around 33 BU at this time point (Figure 4B). As B cells recovered, inhibitor titres recovered completely by week 11. Interestingly, average inhibitors recovered more quickly in mice injected with anti-CD20 IgG1 compared to the IgG2a backbone, possibly due to the less complete B cell depletion observed with this treatment. None of these transient changes in the average inhibitor titres reached statistical significance, and it was clear that neither anti-CD20 antibody was effectively able to reverse inhibitors on its own.
Figure 4. Ineffective reversal of FVIII inhibitors with mCD20 antibody alone.
A. Experimental outline. Inhibitors (60–120 BU/ml) were established in haemophilia A mice (BALB/c F8e16−/−) by 4 weekly IV injections of BDD-FVIII (week 0). Mice with similar inhibitor titres were divided into 3 experimental groups (n=5–7/group). Each group received 2 IV injections of mCD20 antibody (IgG1 or IgG2a backbone), or PBS on weeks 0 and 3. Blood was collected after inhibitor establishment (week 0), and on weeks 3, 7 and 11 to determine effect of mCD20 administration. B. Inhibitor titres (BU/ml) as a function of time. Data are average ±SD. Statistically significant differences are indicated for each time point.
Combination treatment using anti-CD20 and rapamycin
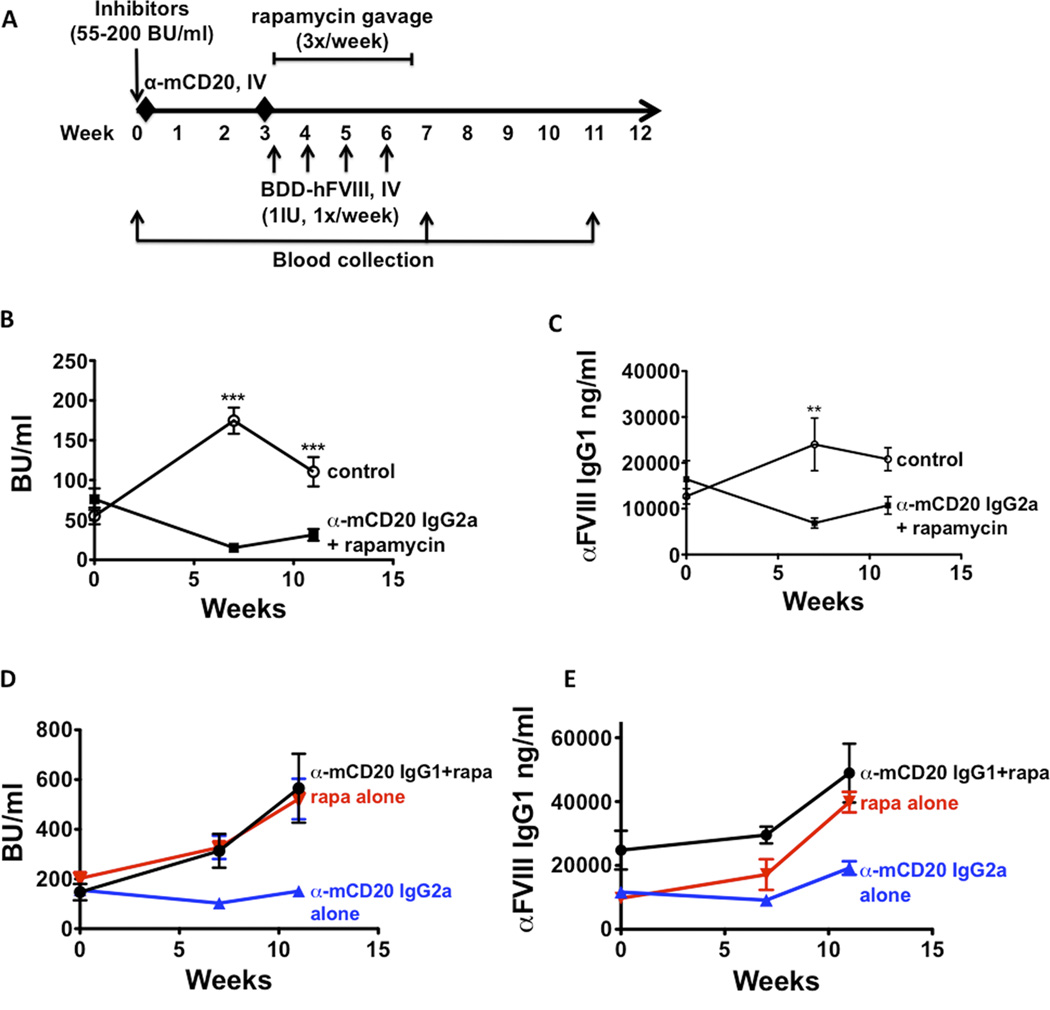
Having demonstrated that neither rapamycin nor anti-CD20 was effectively able to reverse inhibitors in HA mice, we next set out to develop a combinatorial regimen. Following high-titre inhibitor induction (55–220 BU), HA mice received 2 doses of anti-CD20 IgG2a or IgG1, followed by the aforementioned rapamycin/ FVIII regimen immediately after the second injection (Figure 5A). In mice receiving the combination of anti-CD20 IgG2a and rapamycin, inhibitor titres were reduced from ~76 BU/ml to ~15 BU/ml (5-fold reduction) (Figure 5B). Although inhibitors rose to ~31 BU/ml one month after treatment in the absence of further intervention, they were still lower than starting inhibitor titres (2.5-fold reduction). Similar trends were observed in anti-FVIII IgG1 levels in these mice (Figure 5C). IgG2a anti-CD20 alone (i.e. without rapamycin) did not reverse but nonetheless prevented a further rise in antibody formation (Figure 5D, E). In contrast, the combination of IgG1 anti-CD20 and rapamycin or rapamycin alone were not effective. Here, inhibitor titres and anti-FVIII IgG1 levels (the dominant IgG subclass of anti-FVIII in this strain) (28) increased slightly after treatment and continued to rise one month after the end of the therapy (Figure 5D, E).
Figure 5. Combination treatment of IgG2a mCD20 antibody and rapamycin reduces high-titre inhibitors in haemophilia A mice.
A. Experimental timeline. Inhibitors (55–200 BU/ml) were established in haemophilia A mice (BALB/c F8e16−/−) by 4 weekly IV injections of BDD-FVIII (week 0). Mice (n=5–8/group) received 2 IV injections of either mCD20 antibody (IgG2a backbone) or PBS on weeks 0 and 3. This was followed by oral gavage of rapamycin (4mg/kg, 3X/week for 4 weeks) in the group that received mCD20 antibody. Weekly BDD-FVIII injections were continued in all animals from weeks 3–6. Blood was collected on weeks 7 and 11 respectively. B. Inhibitor titres (BU/ml) as a function of time. C. Anti-FVIII IgG titres (ng/ml) as a function of time. D. Inhibitor titres (BU/ml) from control mice that received IgG1 mCD20 antibody and rapamycin (black line), IgG2a mCD20 antibody only (blue line) or rapamycin only (red line). E. Anti-FVIII IgG titres (ng/ml) from control mice that received IgG1 mCD20 antibody and rapamycin (black line), IgG2a mCD20 antibody only (blue line) or rapamycin only (red line). Data are average ±SD. Statistically significant differences are indicated for each time point.
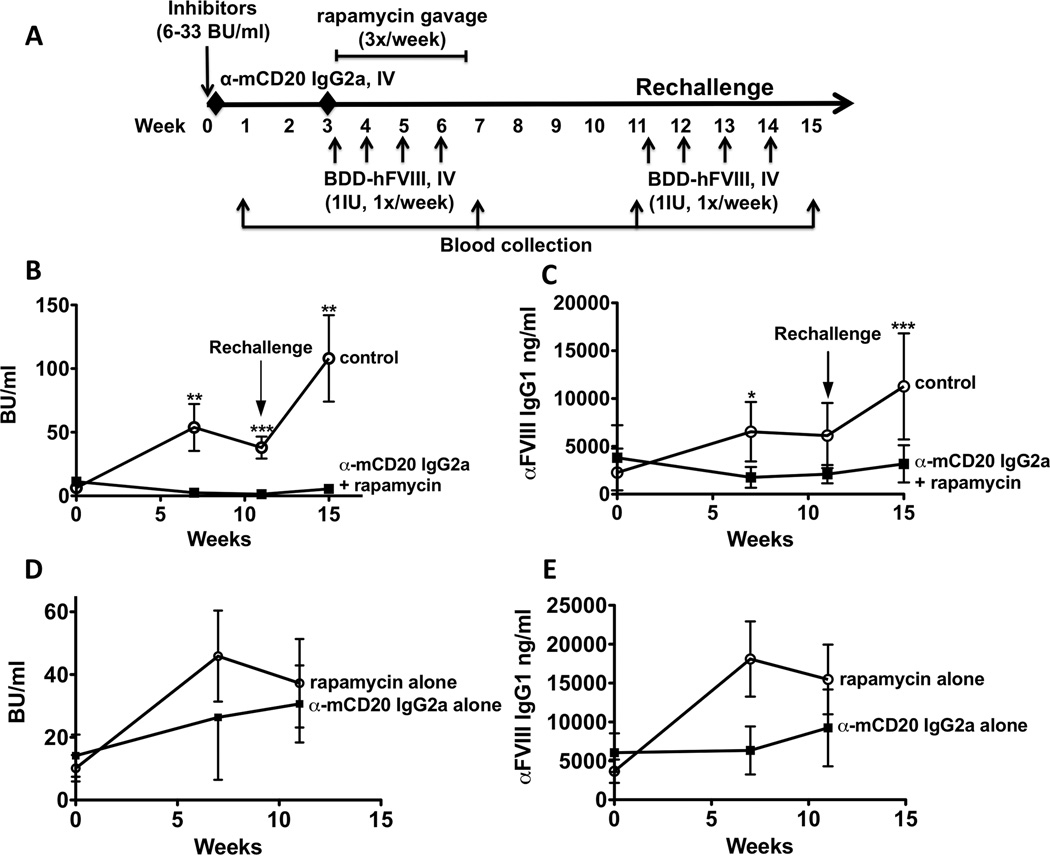
Since the IgG2a combination therapy was effective in reducing existing inhibitor titres, we next investigated the ability of this therapy to reverse moderate-titre inhibitors (Figure 6A). In HA mice with initial BU of ~10, treatment with anti-CD20 IgG2a and rapamycin reduced inhibitors to low titres (0.6–5 BU/ml), and this reduction remained as B cells repopulated (Figure 6B). Subsequent rechallenge of these mice with FVIII minimally increased inhibitor titres (~5 BU/ml), in sharp contrast to the >100 BU titre observed in control mice (>20-fold difference, Figure 6B). Inhibitors were entirely eradicated in 2 out of 5 mice, which withstood rechallenge with FVIII protein. A comparable, albeit less complete, effect was observed in FVIII binding antibodies in these mice (Figure 6C). Control groups that received only mCD20 antibody or rapamycin and FVIII did not have a reduction in inhibitor titres (Figure 6D, E).
Figure 6. Combination treatment of IgG2a mCD20 antibody and rapamycin reverses low titre inhibitors despite subsequent FVIII rechallenge in haemophilia A mice.
Experimental outline. Inhibitors (6–33 BU/ml) were established in haemophilia A mice (BALB/c F8e16−/−) by 4 weekly IV injections of BDD-FVIII (week 0). Mice (n=5–7/group) received 2 IV injections of either mCD20 antibody (IgG2a backbone) or PBS on weeks 0 and 3. This was followed by oral gavage of rapamycin (4mg/kg, 3X/week for 4 weeks) to the group that received mCD20 antibody. Weekly BDD-FVIII injections were continued in all animals from weeks 3–6. Blood was collected on weeks 7 and 11 respectively. Mice were subsequently rechallenged with 4 weekly injections of BDD-FVIII (weeks 11–14). Blood was collected at 1 week following the second round of BDD-FVIII injections (week 15). B. Inhibitor titres (BU/ml) as a function of time. C. Inhibitor titres (BU/ml) from control mice that received mCD20 antibody only or rapamycin only D. Anti-FVIII IgG titres (ng/ml) as a function of time. E. Anti-FVIII IgG titres (ng/ml) from control mice that received mCD20 antibody only or rapamycin only. Data are average ±SD. Statistically significant differences are indicated for each time point.
In enzyme replacement therapy (ERT) for the lysosomal storage disorder Pompe disease, patients are treated with rituximab and rapamycin prophylactically to prevent formation of anti-drug antibodies (29) [ClinicalTrials.gov NCT02240407]. To test effectiveness in haemophilia in a prevention model, naïve BALB/c F8e16−/− mice received 2 injections of anti-CD20 IgG2a, as described above, followed by oral rapamycin (3X /week for 4 weeks) with simultaneous IV FVIII injections (1 IU /week × 4 weeks) (Supplementary Figure S3A). Rapamycin and anti-CD20 IgG2a treatment combination completely prevented inhibitor formation in the treated group (0 BU/ml), while control mice developed inhibitors in response to FVIII administration (~40 BU/ml) (Supplementary Figure S3B).
While we were able to demonstrate that the combination of anti-CD20 IgG2a and rapamycin effectively reduced inhibitor formation in both a prevention and ITI setting, it was important to ensure that rapamycin treatment did not further delay repopulation of immune cell compartments following anti-CD20 mediated depletion. BALB/c mice received 2 doses of anti-CD20 IgG2a spaced 3 weeks apart, followed by the same rapamycin regimen for another 4 weeks. Mice were allowed to recover for an additional month, and total B and T cell frequencies were calculated in the spleen and LN. Total B, CD4+ and CD8+ T cells in the spleen were largely recovered by the end of the combinatorial treatment (Supplementary Figure S4A–C). Although a slight delay in B cell repopulation in the LN was observed, this was similar to what had earlier been observed with anti-CD20 IgG2a treatment alone (Table 1, Figure 2C). Administration of an unrelated protein (keyhole limpet hemocyanin, KLH) to these mice, 1 month after they had been allowed to recover, showed a robust anti-KLH IgG1 antibody response, albeit on average slightly lower than in control mice (Supplementary Figure S4D). Thus, immune competence had largely been regained at these subsequent antigen challenges, and unresponsiveness to FVIII was not due to general immune suppression.
Discussion
Inhibitor reversal in human patients
Attempts to use immunomodulatory therapies in inhibitor patients in addition to or instead of the standard ITI regimen have shown mixed results. Immune suppressive drugs should be used with caution, especially in pediatric patients, but may be needed in inhibitor patients that do not respond to traditional ITI or whose inhibitor titers decline with slow kinetics when FVIII treatment is stopped. Although immune suppression can be effective, particularly in patients with lower initial starting titres, inhibitors are likely to relapse if no specific step for tolerance induction to FVIII is included (10, 30, 31). For example, merely depleting B cells without affecting the memory T cell response or facilitating the generation of FVIII-specific Treg is unlikely to have a lasting effect (30, 32). This is supported by clinical results showing a superior effect of rituximab when given in conjunction with FVIII (33).
Rapamycin and immune tolerance
Rapamycin is an immunosuppressive drug that functions particularly due to its effects on T cells. It suppresses the metabolic mammalian target of rapamycin (mTOR) complex 1 pathway, inhibiting cell cycle progression and inducing effector T cell (Teff) anergy or deletion; Tregs are more resistant than Teffs to mTOR inhibition (34). Additionally, through effects on dendritic cell functionality and cytokine production, rapamycin favors the conversion T cells into Treg rather than effector Th1/2/17 cells (35–40). Rapamycin can effectively suppress deleterious immune responses in several models of autoimmune diseases (39, 41, 42). Additionally, it has previously been used by us and recently by others to great effect in inhibitor prevention in both haemophilia A and B, and - in conjunction with IL-10 - it reversed active immune responses against factor IX (FIX) following gene therapy (21–23, 43). However, when used here for reversal of recombinant protein-induced inhibitors in haemophilia A, rapamycin was at best able to halt the increase in inhibitor titres. When initial titres were increased to >50 BU, rapamycin was unable to suppress a rise in anti-FVIII formation, suggesting that the magnitude of the pre-existing anti-FVIII response can affect the tolerogenic capacity of rapamycin.
Targeting both B and T cells for tolerance induction
Our findings with rapamycin alone in inhibitor reversal suggested that its efficacy might be increased if combined with a therapy that could target antibody-producing B cells. Rituximab, an anti-CD20 antibody which depletes B cells, has been explored clinically for precisely this purpose in inhibitor reversal (8, 10, 33, 44). Mimicking ITI in haemophilia A mice, anti-CD20 had been shown to be partially tolerogenic, preventing a rise in inhibitors (13). However, this only occurred when the backbone was switched from the typical murine IgG2a to IgG1; mice receiving anti-CD20 IgG2a had similar titres to mice treated with control IgG. The authors noted that alteration of backbone changed the pattern of B cell depletion, with IgG1 causing less depletion of marginal zone B cells in the spleen. Here, we expanded on these findings. In addition to less efficiently killing follicular and transitional B cells, IgG1 particularly demonstrated limited toxicity of marginal zone, B-10, B-1b and memory B cells. Interestingly, B-10 cells, which share markers with marginal zone B cells, produce significant levels of the tolerogenic cytokine IL-10 and have been reported to suppress autoimmune disorders including experimental autoimmune encephalitis (18, 45). The persistence of these B-10 cells may explain the enhanced Treg induction after anti-CD20 IgG1 and FVIII administration in HA mice reported by Zhang et al. (13).
However, we find that despite these potential advantages of the IgG1 backbone, the IgG2a anti-CD20 was distinctly superior when combined with rapamycin for inhibitor reversal. We hypothesize that the greater extent of B cell depletion, including memory B cells, more robustly reverses antibody formation and allows FVIII antigen presentation in the context of rapamycin-mediated mTOR inhibition to induce tolerance. Our data show that inhibitors clinically defined as high-titre (>5 BU) can be reversed with this protocol. However, higher-titre inhibitors (>50 BU) are only partially reversed. For this scenario, the protocol needs to be further optimized, or initiation of the regimen should be postponed until titres have fallen over time.
An aspect that remains somewhat unclear is the effect of this regimen on antibody-secreting plasma cells. Our experiments in naïve mice demonstrated some plasma cell depletion in IgG2a-treated mice, though this may be due to a disruption of homeostasis by killing of plasmablast precursors or through another mechanism. Rituximab has been reported to kill short-lived plasma cells in hCD20-transgenic mice (46). In models of haemophilia B, plasma cells in mice can be suppressed by factor IX-specific Tregs, so depletion of these cells is not absolutely required for tolerance induction (47). However, this strategy might further benefit from the addition of a reagent such as bortezomib, a proteasome inhibitor toxic to plasma cells. In a murine model of lupus, a combination of bortezomib and anti-CD20 resulted in sustained disease amelioration by killing long-lived plasma cells and the precursors that repopulate them, respectively (48).
Clinical implications
The combination of anti-CD20 and rapamycin is in use in patients with Pompe disease, a monogenic disorder caused by mutation of acid alpha-glucosidase (GAA) treated via ERT that is complicated by deleterious immune responses against the recombinant protein (49, 50). Although used prophylactically rather than for reversal of an ongoing immune response, treatment with rituximab and rapamycin was able to prevent anti-GAA immune responses in all patients (29). A logical application in haemophilia would be in patients that have failed traditional ITI. Interestingly, the average inhibitor titer when rituximab was given in the RICH trial was 12 BU/ml (10), which is in the range where our protocol was most effective in mice. Given that the combination protocol also directed a substantial decline in higher-titer inhibitor titers in hemophilia A mice, while anti-CD20 alone failed to reduce titers, it is possible that a protocol further optimized in humans could be effective also in patients with higher-titer inhibitors. It should be noted that humans metabolize rapamycin at a different rate compared to rodents, so that doses are different between the two species. Similarly, a dosing schedule of anti-CD20 optimal for B cell depletion in humans would be used. Another potential application is to accelerate the decline of high-titer inhibitors in patients for whom onset of ITI is being delayed. Ideally, patients should have an inhibitor titer of <10 BU/ml at the onset of traditional ITI. However, some patients show a slow spontaneous decline of inhibitors once FVIII replacement therapy was stopped. Such patients may also benefit from anti-CD20/rapamycin treatment, which may accelerate the decline in inhibitor formation.
The combination of anti-CD20 and rapamycin represents a temporary immunosuppressive regimen. B cells repopulate following treatment with rituximab, and in the meantime some degree of humoral immunity can be retained in patients by administration of IVIG. Rapamycin, too, is only administered for a period of about a month, and immune competence returns within ~1 month of the end of rapamycin treatment (21). In this prior work in mice, we found that co-administration of FVIII and rapamycin over a 1-month period could induce long-term tolerance in a preventive model. However, should an anti-CD20/rapamycin/FVIII protocol for inhibitor reversal not reliably prevent relapse of inhibitors, one could consider a more prolonged or perhaps recurring administration of rapamycin. Rituximab, the anti-CD20 currently used in patients, possesses a human IgG1 backbone most similar to the murine IgG2a that was most effective in our studies. This backbone directs both antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity to CD20-expressing cells. Our data support to retain the current IgG backbone when combined with rapamycin. However, there may be other protocols that could benefit from using a backbone that retains potentially tolerogenic B cells subsets as suggested by Zhang et al. (43).
In summary, B cell depletion by mCD20 antibody in combination with T cell suppression by rapamycin resulted in a significant and durable reduction of inhibitor titres in a murine model of haemophilia A. These promising results recommend further exploration of this combination regimen as an effective therapy for inhibitor reversal in haemophilia A.
Supplementary Material
Acknowledgments
The authors thank Biogen for the kind gift of anti-murine CD20 monoclonal antibodies. This work was supported by a sponsored research agreement with Biogen and by NIH grant R01 AI51390 (to RWH).
Footnotes
Author contributions
MB, GLR, AS, and DMM performed experiments. MB, GLR, BJB, DMM, HJ, and RWH designed experiments and interpreted data. MB, GLR, DMM, HJ, and RWH wrote the manuscript. RWH directed the study.
References
- 1.Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. The New England journal of medicine. 2007;357(6):535–544. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 2.Peyvandi F, Mannucci PM, Garagiola I, et al. A Randomized Trial of Factor VIII and Neutralizing Antibodies in Hemophilia A. The New England journal of medicine. 2016;374(21):2054–2064. doi: 10.1056/NEJMoa1516437. [DOI] [PubMed] [Google Scholar]
- 3.Halimeh S, Bidlingmaier C, Heller C, et al. Risk factors for high-titer inhibitor development in children with hemophilia A: results of a cohort study. BioMed research international. 2013;2013:901975. doi: 10.1155/2013/901975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gouw SC, van den Berg HM, Oldenburg J, et al. F8 gene mutation type and inhibitor development in patients with severe hemophilia A: systematic review and meta-analysis. Blood. 2012;119(12):2922–2934. doi: 10.1182/blood-2011-09-379453. [DOI] [PubMed] [Google Scholar]
- 5.Santagostino E, Mancuso ME, Rocino A, et al. Environmental risk factors for inhibitor development in children with haemophilia A: a case-control study. British journal of haematology. 2005;130(3):422–427. doi: 10.1111/j.1365-2141.2005.05605.x. [DOI] [PubMed] [Google Scholar]
- 6.Hay CR, DiMichele DM. The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood. 2012;119(6):1335–1344. doi: 10.1182/blood-2011-08-369132. [DOI] [PubMed] [Google Scholar]
- 7.Mariani G, Siragusa S, Kroner BL. Immune tolerance induction in hemophilia A: a review. Seminars in thrombosis and hemostasis. 2003;29(1):69–76. doi: 10.1055/s-2003-37941. [DOI] [PubMed] [Google Scholar]
- 8.Franchini M, Mannucci PM. Inhibitor eradication with rituximab in haemophilia: where do we stand? British journal of haematology. 2014;165(5):600–608. doi: 10.1111/bjh.12829. [DOI] [PubMed] [Google Scholar]
- 9.Carcao M, St Louis J, Poon MC, et al. Rituximab for congenital haemophiliacs with inhibitors: a Canadian experience. Haemophilia. 2006;12(1):7–18. doi: 10.1111/j.1365-2516.2005.01170.x. [DOI] [PubMed] [Google Scholar]
- 10.Leissinger C, Josephson CD, Granger S, et al. Rituximab for treatment of inhibitors in haemophilia A. A Phase II study. Thrombosis and haemostasis. 2014;112(3):445–458. doi: 10.1160/TH14-01-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sack BK, Merchant S, Markusic DM, et al. Transient B cell depletion or improved transgene expression by codon optimization promote tolerance to factor VIII in gene therapy. PloS one. 2012;7(5):e37671. doi: 10.1371/journal.pone.0037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu CL, Ye P, Lin J, et al. Anti-CD20 as the B-Cell Targeting Agent in a Combined Therapy to Modulate Anti-Factor VIII Immune Responses in Hemophilia a Inhibitor Mice. Frontiers in immunology. 2014;4:502. doi: 10.3389/fimmu.2013.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang AH, Skupsky J, Scott DW. Effect of B-cell depletion using anti-CD20 therapy on inhibitory antibody formation to human FVIII in hemophilia A mice. Blood. 2011;117(7):2223–2226. doi: 10.1182/blood-2010-06-293324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irani V, Guy AJ, Andrew D, et al. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol Immunol. 2015;67(2 Pt A):171–182. doi: 10.1016/j.molimm.2015.03.255. [DOI] [PubMed] [Google Scholar]
- 15.Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013;13(2):118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11(1):34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 17.Gong Q, Ou Q, Ye S, et al. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. Journal of immunology. 2005;174(2):817–826. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- 18.Lykken JM, Candando KM, Tedder TF. Regulatory B10 cell development and function. Int Immunol. 2015 doi: 10.1093/intimm/dxv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bray GL, Kroner BL, Arkin S, et al. Loss of high-responder inhibitors in patients with severe hemophilia A and human immunodeficiency virus type 1 infection: a report from the Multi-Center Hemophilia Cohort Study. Am J Hematol. 1993;42(4):375–379. doi: 10.1002/ajh.2830420408. [DOI] [PubMed] [Google Scholar]
- 20.Qian J, Collins M, Sharpe AH, et al. Prevention and treatment of factor VIII inhibitors in murine hemophilia A. Blood. 2000;95(4):1324–1329. [PubMed] [Google Scholar]
- 21.Moghimi B, Sack BK, Nayak S, et al. Induction of tolerance to factor VIII by transient co-administration with rapamycin. Journal of thrombosis and haemostasis : JTH. 2011;9(8):1524–1533. doi: 10.1111/j.1538-7836.2011.04351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nayak S, Cao O, Hoffman BE, et al. Prophylactic immune tolerance induced by changing the ratio of antigen-specific effector to regulatory T cells. Journal of thrombosis and haemostasis : JTH. 2009;7(9):1523–1532. doi: 10.1111/j.1538-7836.2009.03548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nayak S, Sarkar D, Perrin GQ, et al. Prevention and Reversal of Antibody Responses Against Factor IX in Gene Therapy for Hemophilia B. Frontiers in microbiology. 2011;2:244. doi: 10.3389/fmicb.2011.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biswas M, Sarkar D, Kumar SR, et al. Synergy between rapamycin and FLT3 ligand enhances plasmacytoid dendritic cell-dependent induction of CD4+CD25+FoxP3+ Treg. Blood. 2015;125(19):2937–2947. doi: 10.1182/blood-2014-09-599266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao O, Hoffman BE, Moghimi B, et al. Impact of the underlying mutation and the route of vector administration on immune responses to factor IX in gene therapy for hemophilia B. Mol Ther. 2009;17(10):1733–1742. doi: 10.1038/mt.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarkar D, Biswas M, Liao G, et al. Expanded Autologous Polyclonal Regulatory T Cells Suppress Inhibitor Formation in Hemophilia. Molecular therapy Methods & clinical development. 2014;1 doi: 10.1038/mtm.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lykken JM, DiLillo DJ, Weimer ET, et al. Acute and chronic B cell depletion disrupts CD4+ and CD8+ T cell homeostasis and expansion during acute viral infection in mice. Journal of immunology. 2014;193(2):746–756. doi: 10.4049/jimmunol.1302848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qadura M, Waters B, Burnett E, et al. Immunoglobulin isotypes and functional anti-FVIII antibodies in response to FVIII treatment in Balb/c and C57BL/6 haemophilia A mice. Haemophilia. 2011;17(2):288–295. doi: 10.1111/j.1365-2516.2010.02397.x. [DOI] [PubMed] [Google Scholar]
- 29.Elder ME, Nayak S, Collins SW, et al. B-Cell depletion and immunomodulation before initiation of enzyme replacement therapy blocks the immune response to acid alpha-glucosidase in infantile-onset Pompe disease. The Journal of pediatrics. 2013;163(3):847–854. e1. doi: 10.1016/j.jpeds.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antun A, Monahan PE, Manco-Johnson MJ, et al. Inhibitor recurrence after immune tolerance induction: a multicenter retrospective cohort study. Journal of thrombosis and haemostasis : JTH. 2015;13(11):1980–1988. doi: 10.1111/jth.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laros-van Gorkom BA, Falaise C, Astermark J. Immunosuppressive agents in the treatment of inhibitors in congenital haemophilia A and B--a systematic literature review. Eur J Haematol Suppl. 2014;76:26–38. doi: 10.1111/ejh.12372. [DOI] [PubMed] [Google Scholar]
- 32.Cao O, Loduca PA, Herzog RW. Role of regulatory T cells in tolerance to coagulation factors. Journal of thrombosis and haemostasis : JTH. 2009;7(Suppl 1):88–91. doi: 10.1111/j.1538-7836.2009.03417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins PW, Mathias M, Hanley J, et al. Rituximab and immune tolerance in severe hemophilia A: a consecutive national cohort. Journal of thrombosis and haemostasis : JTH. 2009;7(5):787–794. doi: 10.1111/j.1538-7836.2009.03332.x. [DOI] [PubMed] [Google Scholar]
- 34.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9(5):324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105(12):4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 36.Hackstein H, Taner T, Zahorchak AF, et al. Rapamycin inhibits IL-4--induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101(11):4457–4463. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- 37.Zeiser R, Leveson-Gower DB, Zambricki EA, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111(1):453–462. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delgoffe GM, Kole TP, Zheng Y, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30(6):832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Battaglia M, Stabilini A, Migliavacca B, et al. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. Journal of immunology. 2006;177(12):8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 40.Matsue H, Yang C, Matsue K, et al. Contrasting impacts of immunosuppressive agents (rapamycin, FK506, cyclosporin A, dexamethasone) on bidirectional dendritic cell-T cell interaction during antigen presentation. Journal of immunology. 2002;169(7):3555–3564. doi: 10.4049/jimmunol.169.7.3555. [DOI] [PubMed] [Google Scholar]
- 41.Lui SL, Yung S, Tsang R, et al. Rapamycin prevents the development of nephritis in lupus-prone NZB/W F1 mice. Lupus. 2008;17(4):305–313. doi: 10.1177/0961203307088289. [DOI] [PubMed] [Google Scholar]
- 42.Esposito M, Ruffini F, Bellone M, et al. Rapamycin inhibits relapsing experimental autoimmune encephalomyelitis by both effector and regulatory T cells modulation. Journal of neuroimmunology. 2010;220(1–2):52–63. doi: 10.1016/j.jneuroim.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Zhang AH, Rossi RJ, Yoon J, et al. Tolerogenic nanoparticles to induce immunologic tolerance: Prevention and reversal of FVIII inhibitor formation. Cell Immunol. 2016;301:74–81. doi: 10.1016/j.cellimm.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Streif W, Escuriola Ettingshausen C, Linde R, et al. Inhibitor treatment by rituximab in congenital haemophilia A - Two case reports. Hamostaseologie. 2009;29(2):151–154. [PubMed] [Google Scholar]
- 45.Yanaba K, Bouaziz JD, Haas KM, et al. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28(5):639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 46.Huang H, Benoist C, Mathis D. Rituximab specifically depletes short-lived autoreactive plasma cells in a mouse model of inflammatory arthritis. Proc Natl Acad Sci U S A. 2010;107(10):4658–4663. doi: 10.1073/pnas.1001074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Markusic DM, Hoffman BE, Perrin GQ, et al. Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO molecular medicine. 2013;5(11):1698–1709. doi: 10.1002/emmm.201302859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khodadadi L, Cheng Q, Alexander T, et al. Bortezomib Plus Continuous B Cell Depletion Results in Sustained Plasma Cell Depletion and Amelioration of Lupus Nephritis in NZB/W F1 Mice. PloS one. 2015;10(8):e0135081. doi: 10.1371/journal.pone.0135081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Byrne BJ, Falk DJ, Pacak CA, et al. Pompe disease gene therapy. Human molecular genetics. 2011;20(R1):R61–R68. doi: 10.1093/hmg/ddr174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cousens LP, Mingozzi F, van der Marel S, et al. Teaching tolerance: New approaches to enzyme replacement therapy for Pompe disease. Hum Vaccin Immunother. 2012;8(10):1459–1464. doi: 10.4161/hv.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.