Abstract
The purpose of the study was to determine the independent predictors of long-term intraocular pressure (IOP) reduction after cataract surgery with phacoemulsification. This is a retrospective review of uncomplicated cataract surgeries from 2006 to 2008 at the Baltimore VA Medical Center with longitudinal follow-up. Demographic, clinical, biometric, and intraoperative variables including phacoemulsification parameters were recorded. Univariate and multivariate linear regression were used to analyze the relationship between these variables and postoperative IOP, which was the outcome variable. Analysis was performed in 115 eyes of 115 patients who underwent uncomplicated phacoemulsification during the study period. There was an average postoperative IOP reduction through 12, 24, and 36 months of −1.7 ± 3.1, −1.5 ± 3.8, and −1.3 ± 2.6 mmHg, respectively. Higher preoperative IOP (P < 0.001), a more anterior relative lens position (P < 0.05), and longer phaco time (P < 0.05) were significantly associated with greater postoperative decrease in IOP using univariate analysis. Using multivariate analysis, preoperative IOP (P <0.001), and phaco time (P = 0.038) were associated with greater postoperative IOP reduction through 24 months. Phaco time is independently associated with IOP reduction after adjusting for age and preoperative IOP. Higher preoperative IOP is associated with a greater IOP-lowering effect after phacoemulsification.
Keywords: Phacoemulsification cataract surgery, IOP, Glaucoma, Phaco time
Introduction
A large body of evidence has emerged suggesting that cataract surgery with phacoemulsification (phaco) lowers intraocular pressure (IOP) in the long term [1–9]. The mechanism by which IOP is lowered is not well understood, though several hypotheses have been proposed: hyposecretion of aqueous humor due to production of free radicals or irritation [4]; improved trabecular outflow due to deepening of the anterior chamber [10]; or phacoemulsification-induced stress remodeling from the ultrasonic vibrations, an effect similar to that of laser trabeculoplasty [11–13].
Accurate identification of which patients will experience an IOP-lowering response could influence surgical decision-making, for instance determining when a cataract surgery alone would lower IOP adequately, or whether a concurrent glaucoma procedure is indicated [2, 14, 15]. Eyes with highest preoperative IOP have the greatest reduction in IOP in the long term [11]. Whereas multiple studies have evaluated the effect of biometric properties on IOP reduction following phacoemulsification surgery [1–8, 16], few have evaluated the role of modifiable intraoperative factors [2, 17, 18], such as the influence of phacoemulsification energy, on the long-term reduction of IOP.
To further examine these questions in a veteran population, we conducted a retrospective study of intraoperative, biometric, and other clinical predictors of IOP. Detailed information and long-term follow-up were possible as patients received all their follow-up visits in the VA system, and clinical and surgical information was recorded in the VA computerized patient record system (CPRS). The aim of our study was to evaluate various clinical, biometric, and intraoperative predictors of IOP response.
Materials and methods
This study was a retrospective chart review of 115 eyes from 115 patients undergoing cataract surgery who had uncomplicated phacoemulsification with Posterior Chamber Intraocular Lens (PCIOL) implantation between January 2006 and August 2008 at the Baltimore Veterans Administration Medical Center. These surgical cases were performed by multiple surgeons. The institutional review board at University of Maryland, Baltimore and the Research Committee at the Baltimore VA approved this study. One eye per patient was enrolled in the study (or the first operated eye if both eyes underwent cataract surgery). Only patients with uncomplicated phacoemulsification cataract surgery were included. Patients with incomplete records were excluded, such as those who did not have three preoperative IOP values prior to. Patients with a history of prior intraocular surgery or who underwent phacoemulsification combined with another ocular surgery were excluded. Data were collected from 157 eligible charts. Of these, 37 patients were treated with IOP-lowering medications pre- or postoperatively and were omitted from analysis due to potential confounding factors that the medications may have had on IOP. Two patients with implanted sulcus lenses and three with pressures that were not recorded with a Goldmann Applanation Tonometer were also omitted. Analysis was performed in the remaining 115 subjects. All surgeries were performed at the Baltimore VA hospital with an Accurus system (Alcon, Fort Worth, Texas).
Patient charts were reviewed to specifically include demographic factors such as age, sex, and race, clinical information including prior ocular history (e.g., diagnosis of glaucoma, macular degeneration, or ocular surgeries), ocular medications (including number and type of IOP-lowering medications), history of systemic disease, and systemic medications including use of Tamsulosin. All patients underwent a preoperative evaluation including Snellen visual acuity, refraction, slit lamp examination, and dilated funduscopic examinations. To achieve an accurate representation of preoperative baseline IOP, the three most recent separate IOP measurements within 1 year preceding phacoemulsification surgery were averaged together. Biometric information including keratometry, axial length (AL), anterior chamber depth (ACD), and lens thickness (LT) were obtained, the latter three using an immersion A-scan system (Quantel Medical Cedx, France). Relative lens position was calculated as RLP = (0.5 × lens thickness + ACD)/AL [1], where RLP is the lens position relative to the axial length, with smaller RLP values representing more anterior lens positions. Intraoperative variables were recorded in a prospective fashion for each case. These variables included phaco time, percent phacoemulsification, duration of surgery, number of viscoelastic cannulas used, attending surgeon, and number of bottles of balanced salt solution. Finally, each patient’s eye exams were reviewed for 5 years postoperatively and visual acuity, IOP measurements, slit lamp examination (including presence of persistent cell), IOP medications, and mortality were recorded. Due to the large number of deaths and loss to follow-up at 5 years, only data up to 3 years postoperatively were included in the analysis.
Statistical analysis
Statistical analysis was performed using the mean preoperative IOP from three separate IOP measurements. The mean postoperative IOP for each patient was determined from 1 month through 12, 24, and 36 months postoperatively. The absolute change in postoperative IOP from mean preoperative IOP is defined as
To examine the relative change in IOP as a fraction of the baseline IOP, we also calculated the percent change in postoperative IOP as:
IOP lowering was assessed as both a continuous and categorical variable. As a categorical variable, IOP lowering was dichotomized to IOP lowering of greater than 20 % or IOP lowering of 20 % or less for each time period. Continuous demographic (e.g., age), clinical, biometric, and intraoperative predictors of Δ%IOP were determined by univariate linear regression analysis. Categorical predictors (e.g., sex, race, inter-surgeon differences) were compared using Student’s t test. Multivariate models adjusting for age were constructed using significant univariate variables by regression analysis. Two multivariate models were used, one using IOP as a continuous variable and the other using IOP as a dichotomized variable (IOP lowering greater than 20 %). Predictors of greater phaco time were also determined using univariate analysis. Statistical software (version 21, SPSS, Inc., Chicago, IL) was used to calculate r2 and statistical significance (P value). A P value less than 0.05 was considered statistically significant.
To determine whether preoperative IOP has an effect on the postoperative course, patients were divided into upper and lower 50 percentiles of mean preoperative IOPs and the differences in their postoperative IOP were analyzed.
Results
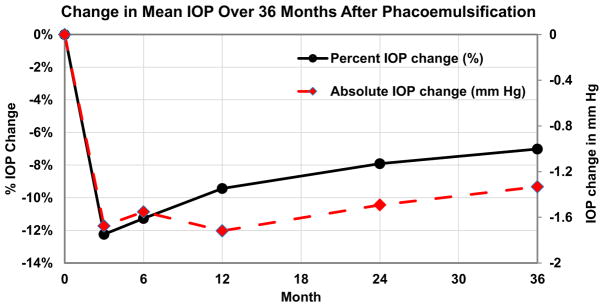
The analysis included 115 eyes of 115 patients. 110 patients (95.7 %) were male, 62 (53.9 %) were white, and the mean age was 70.4 ± 11 years. Table 1 shows the baseline demographic, clinical, biometric, and intraoperative characteristics of the subjects. The mean postoperative IOP (in mmHg) was lower than the mean preoperative IOP for all postoperative follow-up intervals (P < 0.01). Figure 1 shows the percent change in mean postoperative IOP through 36 months, indicating that the reduction in IOP (both as percentage change and absolute value in mmHg) persisted over time. IOP reduction was defined as a postoperative IOP measurement less than mean pre-operative IOP.
Table 1.
Baseline patient characteristics in (N = 115)
| Clinical parameters | Patient characteristics |
|---|---|
| Mean age (years) | 70.4 ± 11.0 |
| Sex | 4.3 % (5/115) females |
| Race | 53.9 % (62/115) white |
| 35.6 % (41/115) African American | |
| 10.4 % (12/115) unknown | |
| Medical history | |
| Diabetes | 57.4 % (66/115) |
| Hypertension | 67.8 % (78/115) |
| Ocular history | |
| Nuclear sclerosis grade 3+ or greater | 56.2 % (41/73) |
| Biometric (preoperative) | |
| Anterior chamber depth (mm) | 3.20 ± 0.47 |
| Lens thickness (mm) | 4.61 ± 0.57 |
| Axial length (mm) | 23.72 ± 0.94 |
| Spherical equivalent (D) | −0.88 ± 2.89 |
| Average keratometry (D) | 43.36 ± 1.56 |
| RLP | 0.23 ± 0.02 |
| Intraoperative | |
| Phaco time (seconds) | 18.83 ± 23.28 |
| Phaco percentage (%) | 10.18 ± 6.3 |
| Surgery duration (minutes) | 43.44 ± 21.10 |
| Lens power (D) | 20.88 ± 2.56 |
| Number of viscoelastic cannulas | |
| 1 | 5.2 % (6/115) |
| 2 | 87.8 % (101/115) |
| 3 | 3.5 % (4/115) |
| Mean preoperative IOP, mm Hg* | 14.9 ± 2.8 |
| Change in postoperative IOP** | |
| 1–12 months | −1.7 ± 3.1 (−9.4 ± 18.3 %) |
| 1–24 months | −1.5 ± 3.8 (−8.0 ± 19.0 %) |
| 1–36 months | −1.3 ± 2.6 (−7.0 ± 18.0 %) |
| Postoperative IOP reduction > 20 % of mean preoperative IOP | 31 patients |
Preoperative IOP was the mean IOP of 3 measurements within 1 year before the surgery date
The postoperative IOP for each patient was assessed from 1 month through 36 months postoperatively (e.g., 3, 6, 12, 24 months). Percent change in postoperative IOP from preoperative IOP is calculated as (postoperative IOP – preoperative IOP)/preoperative IOP ×100 %
Fig. 1.
Change in mean IOP over 36 months after phacoemulsification. The IOP reduction [expressed as both percentage change (%) and absolute change (mm Hg)] persisted through 36 months in subjects without the use of IOP-lowering medications (N = 115)
Univariate predictors of postoperative IOP reduction
Table 2 shows results from a univariate analysis comparing clinical, demographic, biometric, and intraoperative variables associated with percent change in mean postoperative IOP through 24 and 36 months. In terms of clinical predictors, high preoperative IOP is significantly correlated with postoperative IOP reduction (Pearson correlation coefficient r = −0.59 with r2 = 0.37 through 36 months). Patients in the lower 50th percentile of mean preoperative IOP (57 patients, average of 12.7 mmHg) had an average postoperative IOP reduction of 0.1 mmHg, whereas those in the upper 50 % of preoperative IOP (58 patients, average of 17.1 mmHg) had an average postoperative IOP reduction of 2.6 mmHg through 36 months. In other words, patients with a higher baseline IOP experience greater IOP reduction than those with a lower baseline IOP.
Table 2.
Univariate predictors of the percent change in mean postoperative IOP through 36 months (Δ%IOP), with clinical, biometric, and intraoperative parameters
| Clinical, biometric, intraoperative predictors of Δ%IOP, 1–36 months | r2 (24 months) | P value (24 months) | r2 (36 months) | P value (36 months) |
|---|---|---|---|---|
| Categorical variables | ||||
| Sex | 0.62 | 0.54 | ||
| Race | 0.59 | 0.49 | ||
| Diabetes | 0.81 | 0.50 | ||
| Hypertension | 0.79 | 0.44 | ||
| Nuclear sclerosis grade 3+ or greater | 0.73 | 0.28 | ||
| Number of viscoelastic cannulas | 0.15 | 0.17 | ||
| Inter-surgeon differences | 0.56 | 0.38 | ||
| Continuous variables | ||||
| Age | 0.03 | 0.06 | 0.02 | 0.11 |
| Mean preoperative IOP (mm Hg) | 0.35 | <0.001** | 0.37 | <0.001** |
| Anterior chamber depth (mm) | 0.02 | 0.16 | 0.02 | 0.16 |
| Lens thickness (mm) | < 0.01 | 0.79 | < 0.01 | 0.57 |
| Axial length (mm) | 0.02 | 0.15 | 0.02 | 0.17 |
| keratometry (D) | < 0.01 | 0.64 | < 0.01 | 0.58 |
| Relative lens position (RLP) | 0.05 | 0.029* | 0.04 | 0.08 |
| Mean preoperative spherical equivalent (D) | 0.01 | 0.29 | 0.01 | 0.28 |
| Phaco percentage (%) | < 0.01 | 0.99 | < 0.01 | 0.86 |
| Surgery duration (min) | 0.02 | 0.13 | 0.01 | 0.32 |
| Lens power (D) | 0.01 | 0.25 | 0.01 | 0.23 |
| Phaco time (s) | 0.05 | 0.018* | 0.04 | 0.049* |
P value is significant at the 0.05 level
P value is significant at the 0.01 level
Increased phaco time is also significantly correlated with greater IOP reduction through 12, 24, and 36 months (Pearson correlation coefficient r = −0.20, P < 0.05 at 36 months). A more anterior relative lens position is also correlated with greater postoperative percent IOP reduction through 24 months (Pearson correlation coefficient r = 0.23, P < 0.05 at 24 months) though not at 36 months.
Preoperative IOP and phaco time as significant variables associated with postoperative IOP reduction in multivariate analysis
After multivariate analysis adjusting for age and other significant univariate predictors (RLP and preoperative IOP) through 12 and 24 months postoperatively, phaco time was found to be significantly associated with percentage change in postoperative IOP reduction (P = 0.038 at 24 months). This association becomes insignificant through 36 months (P = 0.090 at 36 months). No association was found for RLP (P = 0.14 at 36 months). In a separate multivariate analysis, using IOP as a dichotomized variable, preoperative IOP was associated with IOP reduction greater than 20 % through 36 months (P <0.01 at 36 months), while phaco time was only significant through 24 but not 36 months (P = 0.041 at 24 months and P = 0.12 at 36 months, respectively), and no association was found for RLP (P = 0.17 at 36 months) (Table 3).
Table 3.
Multivariate analysis of clinical predictors of postoperative IOP reduction
|
P value
|
|||
|---|---|---|---|
| 1 year | 2 years | 3 years | |
| A. Predictors associated with postoperative IOP reduction (1–36 months) (N = 115) (Model r2 = 0.37) | |||
| Age | 0.63 | 0.66 | 0.62 |
| Preoperative IOP | <0.01** | <0.01** | <0.01** |
| Relative lens position (RLP) | 0.084 | 0.054 | 0.14 |
| Phaco time | 0.043* | 0.038* | 0.090 |
| B. Predictors associated with IOP reduction of 20 % or greater (1–36 months) (N = 115) (Model r2 = 0.22) | |||
| Age | 0.38 | 0.27 | 0.61 |
| Preoperative IOP | <0.01** | <0.01** | <0.01** |
| RLP | 0.75 | 0.53 | 0.17 |
| Phaco time | 0.026* | 0.041* | 0.12 |
RLP relative lens position
Correlation is significant at the 0.05 level (2-tailed)
Correlation is significant at the 0.01 level (2-tailed)
Phaco time itself was found to be significantly higher for patients with denser cataracts (defined as nuclear sclerosis grade 3+ or greater, P = 0.028), greater age (P = 0.047), as well as greater phacoemulsification percentage (P = 0.008), and longer surgery duration (P <0.001). However, no significant association was found between phaco time and lens characteristics such as AL (P = 0.46) and RLP (P = 0.65).
Discussion
Multiple studies have examined the effect of phacoemulsification cataract surgery on IOP, ACD, and angle opening [1–8, 17], but few have studied the effects of clinical and intraoperative phacoemulsification variables [2, 17, 18]. A unique finding of this work is that phaco time is an independent intraoperative variable associated with postoperative IOP reduction.
Prior research assessing the impact of phaco parameters on IOP has yielded conflicting results. Lee et al. found no association between the amount of CDE and postoperative IOP up to 3 months after surgery [17]. The differences in our findings may be due to the different racial makeup of the patient populations, with the majority of patients in Lee’s study being Asian (89 out of 161), while a substantial fraction of our patients are African American. Pradhan et al. also did not find a statistically significant relationship between phaco time and long-term IOP [2]. Our study had a longer follow-up time than the studies of Lee and Pradhan. Damji et al. found associations between irrigation volume and IOP lowering after cataract surgery in patients with pseudoexfoliation, with a trend toward higher mean phaco time and IOP lowering [18]. While pseudoexfoliation patients may have a different response to phaco than normals, this is consistent with our findings.
The exact mechanism of IOP reduction after phacoemulsification remains unclear. One possible explanation is that lens extraction opens up the angle, thereby improving the aqueous outflow facility, especially in eyes with low ACD or occludable angles [1]. Our study did find that a more anterior RLP was associated with greater IOP reduction in univariate analysis, which may also support this hypothesis, although this was not significant in multivariate analysis and none of the patients studied had occludable angles. Our study lends support to another potential mechanism for IOP lowering after cataract surgery: a laser trabeculoplasty-like effect on the trabecular meshwork related to ultrasound energy. The theoretical explanation for such an effect is that a longer phacoemulsification delivers more ultrasound energy, activating the cytokine pathway in trabecular meshwork, thereby increasing outflow and decreasing IOP. This explanation is supported by in vitro experiments on human trabecular meshwork by Wang et al. [12]. Similar to laser trabeculoplasty, this effect wears off over time [19]. This proposed mechanism is further supported by the observation that phacoemulsification may provide more optimal long-term IOP control than extracapsular surgery [12, 20]. Phaco time may thus be a modifiable risk factor for IOP lowering after cataract surgery. While increased phaco time is also linked to loss of corneal endothelial cells [21], the patients in this study required phaco times well within the typical range [2, 21]. For most patients, modern phacoemulsification surgery is well within the range considered safe for the cornea [22].
The strongest factor associated with IOP lowering after cataract surgery is high preoperative IOP, which is consistent with the findings in our study [2, 4, 8, 11]. While regression to the mean may explain this finding [23], the ocular hypertension treatment study (OHTS) showed that the proportional IOP response held true even after instituting measures to prevent regression to the mean, including multiple IOP measurements and testing according to strict guidelines [8].
This study has certain limitations inherent to a retrospective chart review such as missing or incomplete data. In our study, phacoemulsification was performed with the Accurus system, which could not directly calculate the cumulative dissipated energy (CDE) for each surgery. However, our analysis showed that increased phaco time was correlated with a denser cataract, in which one would expect greater CDE. Irrigation volume was also not recorded at the time of cataract surgery and is thus missing from this analysis.
The setting of the study in the VA system also allowed for certain strengths and limitations. Many veterans generally seek all their optometry, general ophthalmology, and subspecialty care in the VA system, and hence these data were readily available for use in this study, decreasing the potential for selection or referral bias. Although the VA population is biased toward males, numerous studies on cataract surgery performed in the VA system [2, 5] have been conducted and considered applicable to the general population. Furthermore, there is no known effect of gender on IOP lowering after cataract surgery. We are currently conducting a prospective study to examine the relationships between CDE, phaco time, and postoperative IOP.
Acknowledgments
This study was supported in part by the 2015 ASCRS Foundation Research Grant. Dr. Saeedi is funded through an NIH Career Development Grant (K23EY025014-01A1).
Footnotes
Presented in part at the 2015 ASCRS Annual Meeting, April 18th 2015, San Diego, CA (Best Paper of Session, Glaucoma Outcomes).
Author contribution
Dr. DeVience takes full responsibility for the integrity of the data.
Compliance with ethical standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Contributor Information
Eva DeVience, Department of Ophthalmology and Visual Sciences, University of Maryland School of Medicine, 419 W Redwood St, Suite 470, Baltimore, MD 21201, USA.
Sona Chaudhry, Department of Ophthalmology, Boston University School of Medicine, Boston, MA, USA.
Osamah J. Saeedi, Department of Ophthalmology and Visual Sciences, University of Maryland School of Medicine, 419 W Redwood St, Suite 470, Baltimore, MD 21201, USA
References
- 1.Shin HC, Subrayan V, Tajunisah I. Changes in anterior chamber depth and intraocular pressure after phacoemulsification in eyes with occludable angles. J Cataract Refract Surg. 2010;36:1289–1295. doi: 10.1016/j.jcrs.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 2.Pradhan S, Leffler CT, Wilkes M, Mahmood MA. Preoperative iris configuration and intraocular pressure after cataract surgery. J Cataract Refract Surg. 2012;38:117–123. doi: 10.1016/j.jcrs.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 3.Poley BJ, Lindstrom RL, Samuelson TW. Long-term effects of phacoemulsification with intraocular lens implantation in normotensive and ocular hypertensive eyes. J Cataract Refract Surg. 2008;34:735–742. doi: 10.1016/j.jcrs.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 4.Poley BJ, Lindstrom RL, Samuelson TW, Schulze R. Intraocular pressure reduction after phacoemulsification with intraocular lens implantation in glaucomatous and nonglaucomatous eyes: evaluation of a causal relationship between the natural lens and open-angle glaucoma. J Cataract Refract Surg. 2009;35:1946–1955. doi: 10.1016/j.jcrs.2009.05.061. [DOI] [PubMed] [Google Scholar]
- 5.Guan H, Mick A, Porco T, Dolan BJ. Preoperative factors associated with IOP reduction after cataract surgery. Optom Vis Sci. 2013;90:179–184. doi: 10.1097/OPX.0b013e31827ce224. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki R, Kuroki S, Fujiwara N. Ten-year follow-up of intraocular pressure after phacoemulsification and aspiration with intraocular lens implantation performed by the same surgeon. Ophthalmologica. 1997;211:79–83. doi: 10.1159/000310763. [DOI] [PubMed] [Google Scholar]
- 7.Jahn CE. Reduced intraocular pressure after phacoemulsification and posterior chamber intraocular lens implantation. J Cataract Refract Surg. 1997;23:1260–1264. doi: 10.1016/s0886-3350(97)80325-2. [DOI] [PubMed] [Google Scholar]
- 8.Mansberger SL, Gordon MO, Jampel H, et al. Reduction in intraocular pressure after cataract extraction: the Ocular Hypertension Treatment Study. Ophthalmology. 2012;119:1826–1831. doi: 10.1016/j.ophtha.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shingleton BJ, Pasternack JJ, Hung JW, O’Donoghue MW. Three and five year changes in intraocular pressures after clear corneal phacoemulsification in open angle glaucoma patients, glaucoma suspects, and normal patients. J Glaucoma. 2006;15:494–498. doi: 10.1097/01.ijg.0000212294.31411.92. [DOI] [PubMed] [Google Scholar]
- 10.Husain R, Gazzard G, Aung T, et al. Initial management of acute primary angle closure: a randomized trial comparing phacoemulsification with laser peripheral iridotomy. Ophthalmology. 2012;119:2274–2281. doi: 10.1016/j.ophtha.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Slabaugh MA, Chen PP. The effect of cataract extraction on intraocular pressure. Curr Opin Ophthalmol. 2014;25:122–126. doi: 10.1097/ICU.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 12.Wang N, Chintala SK, Fini ME, Schuman JS. Ultrasound activates the TM ELAM-1/IL-1/NF-kappaB response: a potential mechanism for intraocular pressure reduction after phacoemulsification. Investig Ophthalmol Vis Sci. 2003;44:1977–1981. doi: 10.1167/iovs.02-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tumminia SJ, Mitton KP, Arora J, Zelenka P, Epstein DL, Russell P. Mechanical stretch alters the actin cytoskeletal network and signal transduction in human trabecular meshwork cells. Investig Ophthalmol Vis Sci. 1998;39:1361–1371. [PubMed] [Google Scholar]
- 14.Shrivastava A, Singh K. The impact of cataract surgery on glaucoma care. Curr Opin Ophthalmol. 2014;25:19–25. doi: 10.1097/ICU.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 15.Lam DS, Leung DY, Tham CC, et al. Randomized trial of early phacoemulsification versus peripheral iridotomy to prevent intraocular pressure rise after acute primary angle closure. Ophthalmology. 2008;115:1134–1140. doi: 10.1016/j.ophtha.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 16.Tanito M, Ohira A, Chihara E. Factors leading to reduced intraocular pressure after combined trabeculotomy and cataract surgery. J Glaucoma. 2002;11:3–9. doi: 10.1097/00061198-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Lee RY, Chen RI, Kasuga T, Cui QN, Porco TC, Lin SC. The effect of cumulative dissipated energy on changes in intraocular pressure after uncomplicated cataract surgery by phacoemulsification. J Glaucoma. 2015 doi: 10.1097/IJG.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damji KF, Konstas AG, Liebmann JM, Hodge WG, Ziakas NG, Giannikakis S, Ritch R. Intraocular pressure following phacoemulsification in patients with and without exfoliation syndrome: a 2 year prospective study. Br J Ophthalmol. 2006;90:1014–1018. doi: 10.1136/bjo.2006.091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Buskirk EM. Pathophysiology of laser trabeculoplasty. Surv Ophthalmol. 1989;33:264–272. doi: 10.1016/0039-6257(82)90152-7. [DOI] [PubMed] [Google Scholar]
- 20.Casson RJ, Riddell CE, Rahman R, Byles D, Salmon JF. Long-term effect of cataract surgery on intraocular pressure after trabeculectomy: extracapsular extraction versus phacoemulsification. J Cataract Refract Surg. 2002;28:2159–2164. doi: 10.1016/s0886-3350(02)01501-8. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien PD, Fitzpatrick P, Kilmartin DJ, Beatty S. Risk factors for endothelial cell loss after phacoemulsification surgery by a junior resident. J Cataract Refract Surg. 2004;30:839–843. doi: 10.1016/S0886-3350(03)00648-5. [DOI] [PubMed] [Google Scholar]
- 22.Bourne RR, Minassian DC, Dart JK, Rosen P, Kaushal S, Wingate N. Effect of cataract surgery on the corneal endothelium: modern phacoemulsification compared with extracapsular cataract surgery. Ophthalmology. 2004;111:679–685. doi: 10.1016/j.ophtha.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Shrivastava A, Singh K. The effect of cataract extraction on intraocular pressure. Curr Opin Ophthalmol. 2010;21:118–122. doi: 10.1097/ICU.0b013e3283360ac3. [DOI] [PubMed] [Google Scholar]