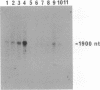
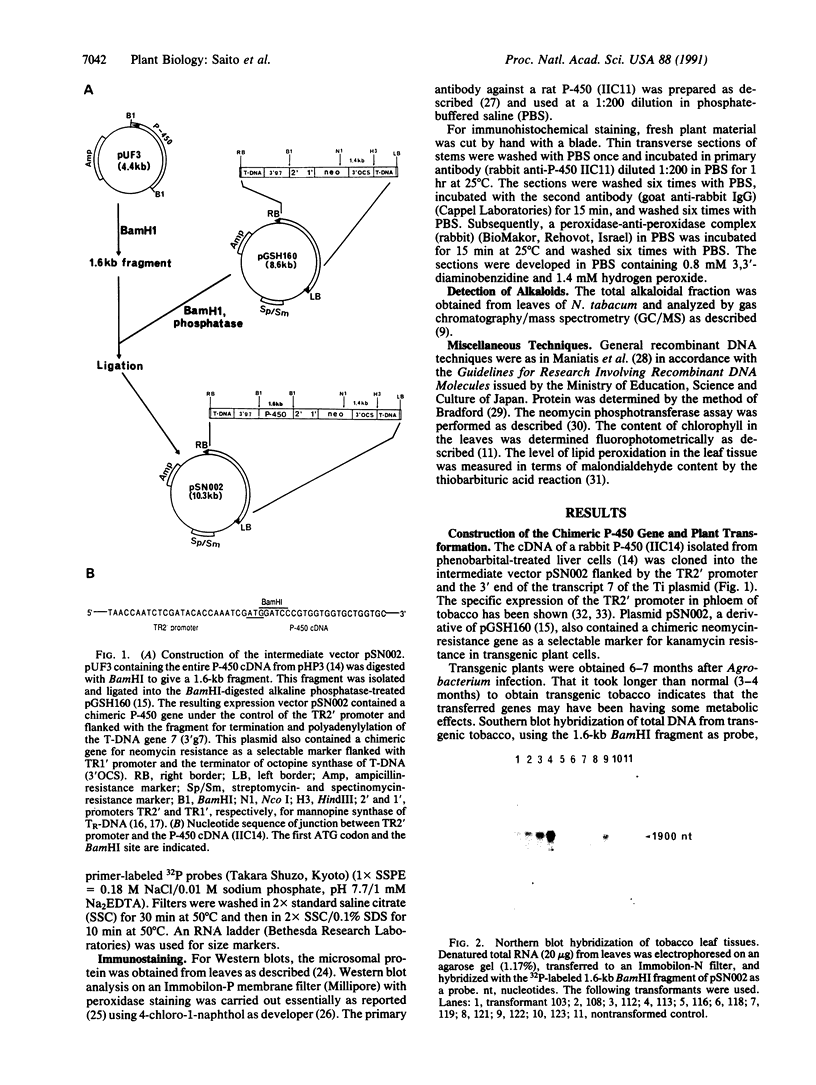
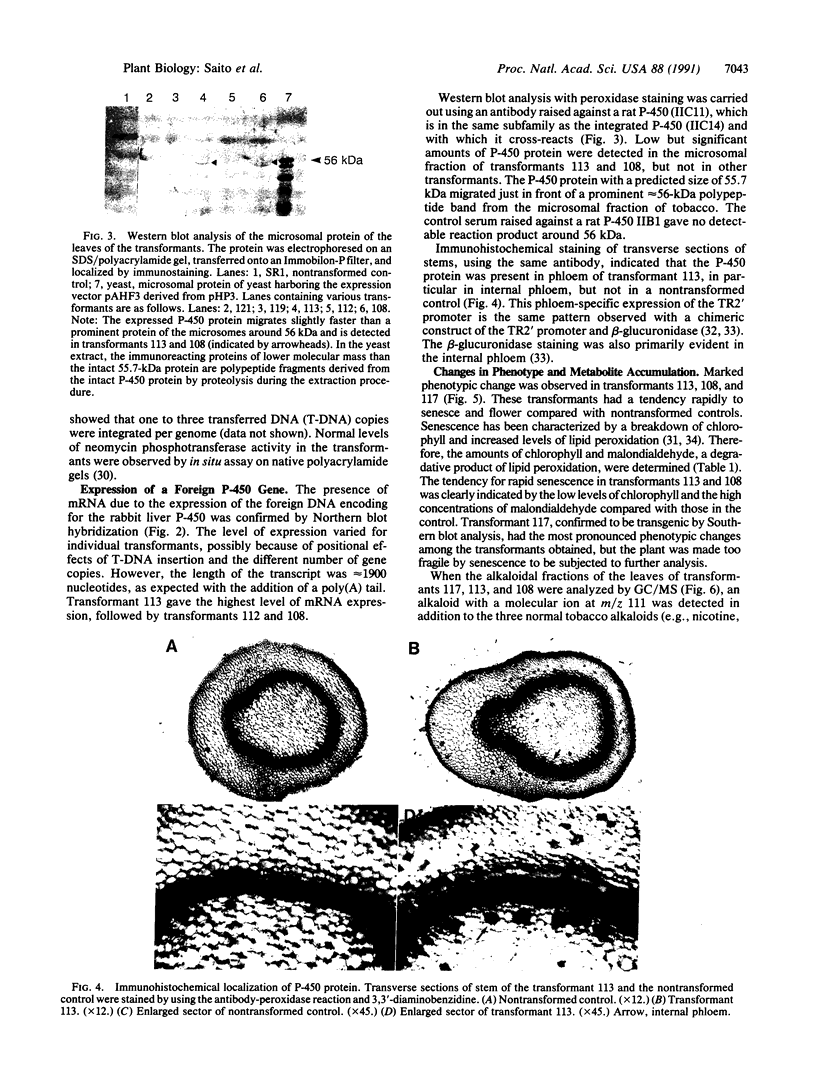
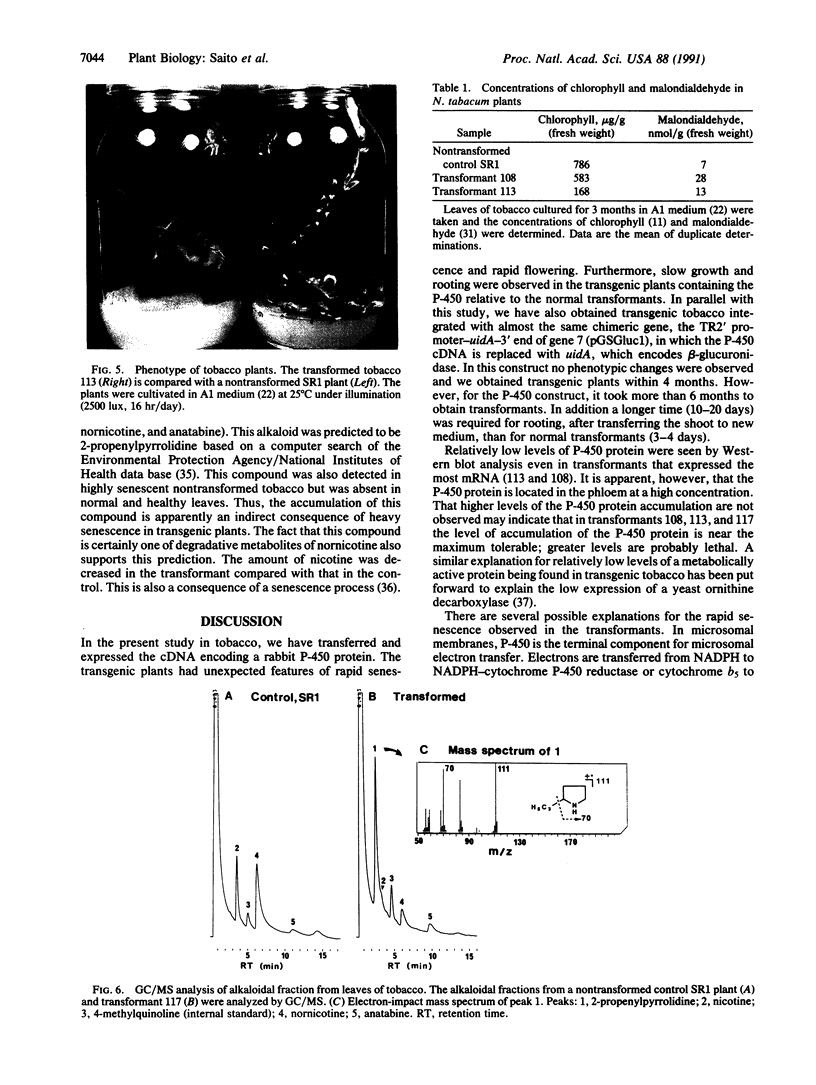
Abstract
Cytochrome P-450 is involved in the oxidative metabolism of a broad range of substrates. We have made a chimeric construct, pSN002, containing the cDNA for rabbit liver cytochrome P-450 (IIC14) under the control of the TR2' promoter for mannopine synthase in the Agrobacterium Ti plasmid. Nicotiana tabacum was transformed with Agrobacterium tumefaciens harboring a cointegrated plasmid pSN002::pGV2260. The presence of mRNA and of the translated protein from the chimeric cytochrome P-450 gene in transgenic plants was confirmed by RNA blot hybridization and by Western blot and immunohistochemical analyses, respectively. The transformants in which the foreign cytochrome P-450 protein is expressed show marked phenotypic changes, notably a tendency rapidly to senesce. We detected 2-propenylpyrrolidine, a degradative metabolite of nicotine alkaloids, in transgenic tobacco showing this pronounced phenotypic change. Such metabolism is likely to be due to the effect of senescence and not directly to the presence of the cytochrome P-450.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Block M. D., Botterman J., Vandewiele M., Dockx J., Thoen C., Gosselé V., Movva N. R., Thompson C., Montagu M. V., Leemans J. Engineering herbicide resistance in plants by expression of a detoxifying enzyme. EMBO J. 1987 Sep;6(9):2513–2518. doi: 10.1002/j.1460-2075.1987.tb02537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozak K. R., Yu H., Sirevåg R., Christoffersen R. E. Sequence analysis of ripening-related cytochrome P-450 cDNAs from avocado fruit. Proc Natl Acad Sci U S A. 1990 May;87(10):3904–3908. doi: 10.1073/pnas.87.10.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Deblaere R., Bytebier B., De Greve H., Deboeck F., Schell J., Van Montagu M., Leemans J. Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res. 1985 Jul 11;13(13):4777–4788. doi: 10.1093/nar/13.13.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaese P., De Greve H., Decraemer H., Schell J., Van Montagu M. Rapid mapping of transposon insertion and deletion mutations in the large Ti-plasmids of Agrobacterium tumefaciens. Nucleic Acids Res. 1979 Dec 11;7(7):1837–1849. doi: 10.1093/nar/7.7.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo A., Marco R. Visualization under ultraviolet light enhances 100-fold the sensitivity of peroxidase-stained blots. Anal Biochem. 1989 Oct;182(1):176–181. doi: 10.1016/0003-2697(89)90738-0. [DOI] [PubMed] [Google Scholar]
- Eckes P., Schmitt P., Daub W., Wengenmayer F. Overproduction of alfalfa glutamine synthetase in transgenic tobacco plants. Mol Gen Genet. 1989 Jun;217(2-3):263–268. doi: 10.1007/BF02464891. [DOI] [PubMed] [Google Scholar]
- Gillette J. R. The problem of chemically reactive metabolites. Drug Metab Rev. 1982;13(6):941–961. doi: 10.3109/03602538208991371. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Nebert D. W. Evolution of the P450 gene superfamily: animal-plant 'warfare', molecular drive and human genetic differences in drug oxidation. Trends Genet. 1990 Jun;6(6):182–186. doi: 10.1016/0168-9525(90)90174-5. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Wang P., Davidson N. K. Estimation of isozymes of microsomal cytochrome P-450 in rats, rabbits, and humans using immunochemical staining coupled with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochemistry. 1982 Mar 30;21(7):1698–1706. doi: 10.1021/bi00536a035. [DOI] [PubMed] [Google Scholar]
- Hamill J. D., Robins R. J., Parr A. J., Evans D. M., Furze J. M., Rhodes M. J. Over-expressing a yeast ornithine decarboxylase gene in transgenic roots of Nicotiana rustica can lead to enhanced nicotine accumulation. Plant Mol Biol. 1990 Jul;15(1):27–38. doi: 10.1007/BF00017721. [DOI] [PubMed] [Google Scholar]
- Imai Y. Cytochrome P-450 related to P-4504 from phenobarbital-treated rabbit liver: molecular cloning of cDNA and characterization of cytochrome P-450 obtained by its expression in yeast cells. J Biochem. 1987 May;101(5):1129–1139. doi: 10.1093/oxfordjournals.jbchem.a121977. [DOI] [PubMed] [Google Scholar]
- Imai Y., Nakamura M. Point mutations at threonine-301 modify substrate specificity of rabbit liver microsomal cytochromes P-450 (laurate (omega-1)-hydroxylase and testosterone 16 alpha-hydroxylase). Biochem Biophys Res Commun. 1989 Feb 15;158(3):717–722. doi: 10.1016/0006-291x(89)92780-0. [DOI] [PubMed] [Google Scholar]
- Kamataki T., Maeda K., Yamazoe Y., Nagai T., Kato R. Sex difference of cytochrome P-450 in the rat: purification, characterization, and quantitation of constitutive forms of cytochrome P-450 from liver microsomes of male and female rats. Arch Biochem Biophys. 1983 Sep;225(2):758–770. doi: 10.1016/0003-9861(83)90087-5. [DOI] [PubMed] [Google Scholar]
- Lagrimini L. M., Bradford S., Rothstein S. Peroxidase-Induced Wilting in Transgenic Tobacco Plants. Plant Cell. 1990 Jan;2(1):7–18. doi: 10.1105/tpc.2.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge W. H., Fitzgerald K. J., Koncz C., Schell J., Szalay A. A. Dual promoter of Agrobacterium tumefaciens mannopine synthase genes is regulated by plant growth hormones. Proc Natl Acad Sci U S A. 1989 May;86(9):3219–3223. doi: 10.1073/pnas.86.9.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann J., Schell J., Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987 May 15;163(1):16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Napoli C., Lemieux C., Jorgensen R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in trans. Plant Cell. 1990 Apr;2(4):279–289. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Adesnik M., Coon M. J., Estabrook R. W., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F., Kemper B. The P450 superfamily: updated listing of all genes and recommended nomenclature for the chromosomal loci. DNA. 1989 Jan-Feb;8(1):1–13. doi: 10.1089/dna.1.1989.8.1. [DOI] [PubMed] [Google Scholar]
- O'keefe D. P., Leto K. J. Cytochrome P-450 from the Mesocarp of Avocado (Persea americana). Plant Physiol. 1989 Apr;89(4):1141–1149. doi: 10.1104/pp.89.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleman J., Saito K., Cottyn B., Engler G., Seurinck J., Van Montagu M., Inzé D. Structure and expression analyses of the S-adenosylmethionine synthetase gene family in Arabidopsis thaliana. Gene. 1989 Dec 14;84(2):359–369. doi: 10.1016/0378-1119(89)90510-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingen B. A., Buege J. A., O'Neal F. O., Aust S. D. The mechanism of NADPH-dependent lipid peroxidation. The propagation of lipid peroxidation. J Biol Chem. 1979 Jul 10;254(13):5892–5899. [PubMed] [Google Scholar]
- Velten J., Schell J. Selection-expression plasmid vectors for use in genetic transformation of higher plants. Nucleic Acids Res. 1985 Oct 11;13(19):6981–6998. doi: 10.1093/nar/13.19.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velten J., Velten L., Hain R., Schell J. Isolation of a dual plant promoter fragment from the Ti plasmid of Agrobacterium tumefaciens. EMBO J. 1984 Dec 1;3(12):2723–2730. doi: 10.1002/j.1460-2075.1984.tb02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. E., Coon M. J. Oxygen activation by cytochrome P-450. Annu Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]
- van der Krol A. R., Mur L. A., Beld M., Mol J. N., Stuitje A. R. Flavonoid genes in petunia: addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell. 1990 Apr;2(4):291–299. doi: 10.1105/tpc.2.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]