Highlights
-
•
Bilateral adrenal metastases can be lead to adrenal insufficiency.
-
•
A rapid ACTH test is useful to diagnose adrenal insufficiency.
-
•
Adrenal crisis may be fatal if not promptly recognized and treated.
Keywords: Rectal cancer, Adrenal failure, Bilateral adrenal metastases, Rapid ACTH test
Abstract
Introduction
It is rare for a patient to present with adrenal insufficiency secondary to bilateral adrenal metastases from a malignant colorectal tumor.
Case presentation
An 82-year-old Japanese man presented to our hospital with high fever and malaise. He was receiving oral chemotherapy for the treatment of rectal cancer with multiple metastases. Computed tomography showed new bilateral adrenal gland metastases. A rapid adrenocorticotropic hormone (ACTH) test showed adrenal insufficiency. Treatment with hydrocortisone provided immediate symptom improvement.
Discussion
Adrenal insufficiency secondary to bilateral adrenal metastases from rectal cancer is rare. A rapid ACTH test is useful to diagnose adrenal insufficiency.
Conclusion
The incidence of adrenal insufficiency may be underestimated in patients with multiple metastasis. Appropriate therapy with adrenal corticosteroid hormone supplementation may lead to a significant improvement in the patient’s symptoms and quality of life.
1. Introduction
Addison’s disease, the result of adrenal insufficiency, has various etiologies [1]. It develops when >90% of adrenal tissue is destroyed [2]. It is rare for a patient to present with Addison’s disease secondary to bilateral adrenal gland metastases from a malignant colorectal tumor; only five cases have been published in the English literature [3], [4], [5], [6], [7]. Herein, we describe a sixth case of adrenal insufficiency in a patient with bilateral adrenal gland metastasis from rectal cancer, based on Surgical Case Report (SCARE) Guidelines [8].
2. Case presentation
In May 2016, an 82-year-old man presented with a 2-week history of a high fever and malaise. His medical history was significant for rectal cancer (pT3, pN1a, pM0, pStage IIIB), diagnosed in 2015, for which he underwent a laparoscopic ultra-low anterior resection. He developed metastases in the liver, lung, right adrenal gland, right fourth rib, and right ilium in February 2016. Subsequently, oral chemotherapy (capecitabine 2400 mg/day) was begun. He had been receiving oral chemotherapy until admission on May 25, 2016, at which point it was interrupted.
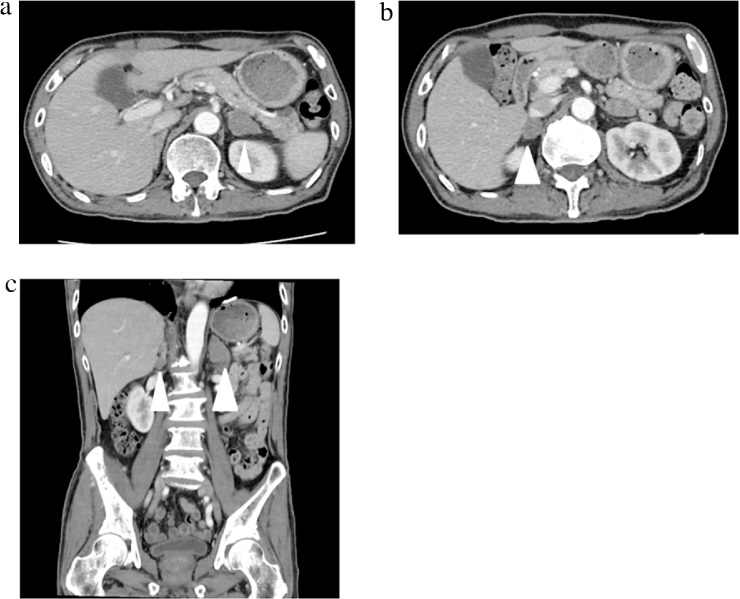
Physical examination revealed a tired-looking man with a body weight of 52 kg. His blood pressure was 141/91 mmHg; pulse rate, 101 beats/min; and body temperature, 37.2 °C. No abnormalities were noted on physical examination. The results of his laboratory investigations are shown in Table 1. Blood and urine culture tests revealed no bacteria. Computed tomography (CT) of the abdomen revealed that the normal tissue of both adrenal glands had been replaced by tumor tissue (Fig. 1a–c). Unfortunately, the patient refused further invasive examination, including the biopsy.
Table 1.
Results of laboratory investigations.
| WBC | 8900/μl | PCT | negative |
| (neutrophils 65.5% eosinophils 16%) | β-D glucan | negative | |
| RBC | 349×104/μl | CEA | 1903 ng/ml |
| Hb | 11.5g/dl | CA19-9 | 477 mA U/ml |
| Ht | 33.8% | Cortisol | 7.8 μg/dL |
| PLT | 47.5×104/μl | ACTH | 28 pg/mL |
| TP | 6.5 g/dl | Blood sugar | 86 mg/dl |
| Alb | 2.6 g/dl | ||
| T-Bil | 0.4 mg/dl | ||
| AST | 53U/1 | ||
| ALT | 36U/1 | ||
| ALP | 1554U/l | ||
| LDH | 884U/l | ||
| Ch-E | 154U/1 | ||
| BUN | 23.4 mg/dl | ||
| Cr | 0.93 mg/dl | ||
| Na | 141 mEq/l | ||
| K | 4.9 mEq/l | ||
| Cl | 101 mEq/1 | ||
| CRP | 17.32 mg/dl | ||
WBC, white blood cells; Hb, hemoglobin; Ht, hematocrit; PLT, platelets; TP, total protein; Alb, albumin; T-bil, total bilirubin; AST, aspartamine aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; Ch-E, cholinesterase; BUN, blood urea nitrogen; Cr, serum creatinine; Na, serum sodium; K, serum potassium; Cl, serum chloride; CRP, C-reactive protein; PCT, procalcitonin test; CEA, carcinoembryonic antigen; ACTH, adrenocorticotropic hormone.
Fig. 1.
(a) Computed tomography (CT) scans showing metastases in the left adrenal gland (25 × 23 mm); (b) CT scans showing metastases in the right adrenal gland (19 × 13 mm); (c) Coronal CT scans showing new bilateral adrenal gland metastasis.
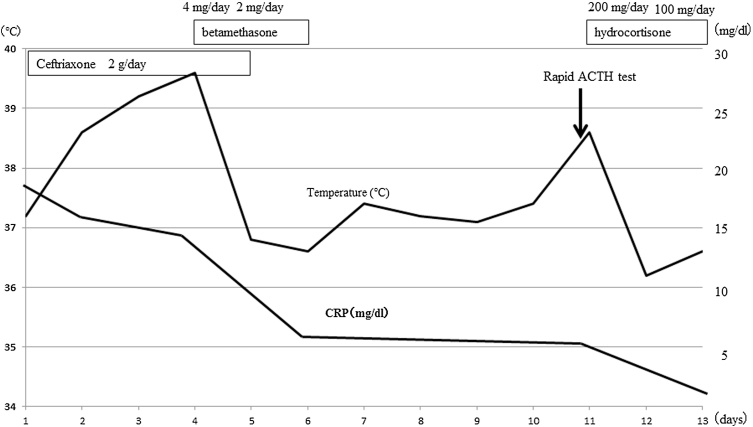
The patient developed a fever (>39 °C) despite administration of intravenous antibiotic therapy (ceftriaxone 2 g/day) and antipyretic analgesics. On the fourth day of hospitalization, betamethasone 4 mg was administered intravenously, and his fever immediately resolved. Ten days after admission, using a sample obtained early in the morning, the adrenocorticotropic hormone (ACTH) level was found to be normal (28 pg/mL) and the cortisol level was low (7.8 μg/dL), but other hormone levels, such as catecholamines, aldosterone, and renin, were normal. A rapid ACTH stimulation test was performed 11 days after admission; the result indicated primary adrenal failure, with a basal cortisol level of 14.8 μg/dL and a maximal increase to only 18.1 μg/dL (Table 2). Hydrocortisone therapy (200 mg/day) was begun, with tapering every second day. The patient’s fever and elevated C-reactive protein levels gradually resolved (Fig. 2). He was discharged 25 days after admission and prescribed hydrocortisone 20 mg/day.
Table 2.
Rapid adrenocorticotropic hormone test results.
| pre | 30 min | 60 min | |
|---|---|---|---|
| Cortisol(μg/dl) | 14.8 | 17.1 | 18.1 |
| ACTH(ng/dl) | 35.4 | – | – |
ACTH, adrenocorticotropic hormone.
Fig. 2.
Body temperature and CRP levels before and after hydrocortisone therapy.
On July 19, 2016, he was re-admitted for management of cancer-related pain. At that point, he had no symptoms of adrenal failure, but the cancer had progressed, with evidence of new brain metastases. The patient died on September 3, 2016. His death was unrelated to the adrenal insufficiency.
3. Discussion
Adrenal insufficiency secondary to any type of metastatic cancer has been reported in fewer than 100 cases in the literature [9]. It is rare for a patient to present with adrenal insufficiency secondary to bilateral adrenal gland metastasis from a malignant colorectal tumor [3], [4], [5], [6], [7]. The survival rate of patients with cancer is increasing, and improvements in imaging techniques have resulted in increased antemortem recognition of adrenal gland metastases. An autopsy series reported adrenal metastases in 14–20% of patients with stomach or colon cancer [10]. Hence, the incidence of adrenal insufficiency may be underestimated in patients with multiple metastases.
Generally, adrenal insufficiency is diagnosed based on a low early morning serum cortisol level ( < 3 μg/dL) with a high basal plasma ACTH level [11], and on the results of a cosyntropin stimulation test [12]. Cortisol levels of 3–15 μg/dL may indicate adrenal insufficiency. Adrenal insufficiency is defined as failure of the serum cortisol level to increase to at least 5 μg/dL above its baseline level [13]. The cut-off value for cortisol after rapid ACTH tests in various reports is 18–20 μg/dL [14], [15], [16]. The criterion of serum cortisol levels <4.4 μg/dL before and 1 h after administration of cosyntropin has also been reported to be clinically important [10].
This patient’s main complaints were high fever and lassitude, and it seemed unlikely that these were due to bacterial infection or cancer cachexia. Adrenal insufficiency seemed more likely in view of the low white blood cell count, absence of calcitonin elevation, the lack of effect of non-steroidal anti-inflammatory drugs, and the significant response to a corticosteroid. There were no physical signs of adrenal insufficiency, such as hyperpigmentation, hair loss, low blood pressure, shock, hyponatremia, or hypoglycemia.
4. Conclusion
We described a case of adrenal insufficiency caused by bilateral adrenal gland metastases from rectal cancer. In general, patients with multiple metastases due to advanced cancer should be investigated for organ failure. However, the incidence of adrenal insufficiency can be underestimated. Appropriate adrenal corticosteroid hormone supplementation may lead to a significant improvement in the patient's symptoms and quality of life. Moreover, adrenal crisis may be fatal if not promptly recognized and treated.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
We have no sponsors involving this paper.
Ethical approval
Approval to publish this case report was waived by the institution.
Consent
Written informed consent for publication of this case report and accompanying images was obtained from the patient. A copy of the written consent is available for review by the Editor-in-Chief of this journal, on request.
Author contribution
YI drafted the manuscript and acquired the data. FK revised the manuscript and has given final approval of the version to be published. All authors read and approved the final manuscript.
Guarantor
Yuki Imaoka.
Acknowledgements
Not applicable.
Contributor Information
Yuki Imaoka, Email: ub044982kkr@yahoo.co.jp.
Fumito Kuranishi, Email: ishikai@beach.ocn.ne.jp.
Yoshiteru Ogawa, Email: iishikai@beach.ocn.ne.jp.
Hiroshi Okuda, Email: hokuda.2nd-s@go5.enjoy.ne.jp.
Masahiro Nakahara, Email: hokuda.2nd-s@go5.enjoy.ne.jp.
References
- 1.Addison T. Samuel Highley; London: 1855. On the Constitutional and Local Effects of Disease of the Suprarenal Capsules. [Google Scholar]
- 2.Kronenberg H.M., Melmed S., Polonsky K.S. 11th edition. Saunders; Philadelphia: 2007. Williams Textbook of Endocrinology; pp. 477–485. [Google Scholar]
- 3.Cedarmark B.J., Blumenson L.E., Pickren J.W., Holyoke D.E., Elias E.G. The significance of metastasis to the adrenal glands in adenocarcinoma of the colon and rectum. Surg. Gynecol. Obstet. 1977;144:537–546. [PubMed] [Google Scholar]
- 4.Black R.M., Daniels G.H., Coggins C.H., Muella P.R., Data R.E., Lichtenstein N. Adrenal insufficiency from metastatic colonic carcinoma masquerading as isolated aldosterone deficiency. Acta Endocrinol. 1981;98:586–591. doi: 10.1530/acta.0.0980586. [DOI] [PubMed] [Google Scholar]
- 5.Khristov V., Kolebinov N., Tsanev A., Nedialkov N., Nesheva P. Bilateral adrenal metastases with the clinical manifestations of hypocorticism in rectal cancer. Vutr. Boles. 1990;29:97–101. [PubMed] [Google Scholar]
- 6.Omoigui N.A., Cave W.T., Jr., Chang A.Y. Adrenal insufficiency: A rare initial sign of metastatic colon carcinoma. J. Clin. Gastroenterol. 1987;9:470–474. [PubMed] [Google Scholar]
- 7.Crisci A., Cartei G., De Antoni P., Giannarini G., Moro U., Selli C. Surgical management of isolated bilateral adrenal metastases from colon carcinoma causing adrenal insufficiency. Urol. Int. 2001;67:113–116. doi: 10.1159/000050963. [DOI] [PubMed] [Google Scholar]
- 8.Agha R.A., Fowler A.J., Saeta A., Barai I., Rajmohan S., Orgill D.P. The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Ross I.L., Marais S., Raubenheimer P., Abratt R., Isaacs S., Soule S. Overt hypoadrenalism is uncommon in patients with stage 3 and 4 bronchogenic carcinoma. S. Afr. Med. J. 2003;93:695–699. [PubMed] [Google Scholar]
- 10.Orth D.N., Kovacs W.J. The adrenal cortex. In: Wilson J.D., Foster D.W., Kronenberg H.M., Larsen P.R., editors. Williams Textbook of Endocrinology. 9th ed. WB Saunders; Philadelphia: 1998. pp. 517–664. [Google Scholar]
- 11.Faulhaber G.A., Borges F.K., Ascoli A.M., Seligman R., Furlanetto T.W. Adrenal failure due to adrenal metastasis of lung cancer: a case report. Case Rep. Oncol. Med. 2011:326815. doi: 10.1155/2011/326815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longui C.A., Vottero A., Harris A.G., Chrousos G.P. Plasma cortisol responses after intramuscular corticotropin 1–24 in healthy men. Metabolism. 1998;47:1419–1422. doi: 10.1016/s0026-0495(98)90316-x. [DOI] [PubMed] [Google Scholar]
- 13.Manu P., Howland T. Best conditions for the rapid 1–24 corticotropin stimulation test of adrenocortical function. Clin. Chem. 1983;29:1450–1451. [PubMed] [Google Scholar]
- 14.Oelkers W. Adrenal insufficiency. N. Engl. J. Med. 1996;335:1206–1212. doi: 10.1056/NEJM199610173351607. [DOI] [PubMed] [Google Scholar]
- 15.Maghnie M., Uga E., Temporini F., Di Iorgi N., Secco A. Evaluation of adrenal function in patients with growth hormone deficiency and hypothalamic- pituitary disorders: comparison between insulin-induced hypoglycemia, low-dose ACTH, standard ACTH and CRH stimulation tests. Eur. J. Endcrinol. 2005;152:735–741. doi: 10.1530/eje.1.01911. [DOI] [PubMed] [Google Scholar]
- 16.Dorin R.I., Qualls C.R., Crapo L.M. Diagnosis of adrenal insufficiency. Ann. Intern. Med. 2003;139:194–204. doi: 10.7326/0003-4819-139-3-200308050-00009. [DOI] [PubMed] [Google Scholar]