Abstract
Objectives
The aim was to describe the contribution of basal ganglia (BG) thalamo-cortical circuitry to the whole-brain functional connectivity in focal epilepsies.
Methods
Interictal resting-state fMRI recordings were acquired in 46 persons with focal epilepsies. Of these 46, 22 had temporal lobe epilepsy: 9 left temporal (LTLE), 13 right temporal (RTLE); 15 had frontal lobe epilepsy (FLE); and 9 had parietal/occipital lobe epilepsy (POLE). There were 20 healthy controls. The complete weighted network was analyzed based on correlation matrices of 90 and 194 regions. The network topology was quantified on a global and regional level by measures based on graph theory, and connection-level changes were analyzed by the partial least square method.
Results
In all patient groups except RTLE, the shift of the functional network topology away from random was observed (normalized clustering coefficient and characteristic path length were higher in patient groups than in controls). Links contributing to this change were found in the cortico-subcortical connections. Weak connections (low correlations) consistently contributed to this modification of the network. The importance of regions changed: decreases in the subcortical areas and both decreases and increases in the cortical areas were observed in node strength, clustering coefficient and eigenvector centrality in patient groups when compared to controls. Node strength decreases of the basal ganglia, i.e. the putamen, caudate, and pallidum, were displayed in LTLE, FLE, and POLE. The connectivity within the basal ganglia–thalamus circuitry was not disturbed; the disturbance concerned the connectivity between the circuitry and the cortex.
Significance
Focal epilepsies affect large-scale brain networks beyond the epileptogenic zones. Cortico-subcortical functional connectivity disturbance was displayed in LTLE, FLE, and POLE. Significant changes in the resting-state functional connectivity between cortical and subcortical structures suggest an important role of the BG and thalamus in focal epilepsies.
Keywords: Functional magnetic resonance imaging, Functional connectivity, Epilepsy, Network analysis, Partial least square analysis
Highlights
-
•
Focal epilepsies affect large-scale brain networks beyond the epileptogenic zones.
-
•
The functional network topology shifted away from random in focal epilepsies.
-
•
Subcortico-cortical connectivity decreased in epilepsy due to changes in weak links.
-
•
Basal ganglia–thalamus circuitry connectivity was not disturbed in focal epilepsy.
-
•
The connectivity between basal ganglia-thalamus circuitry and cortex was affected.
1. Introduction
Increasing interest in brain connectivity in epilepsy reflects growing evidence that focal epilepsies affect large neural networks involved in normal brain function (Chiang and Haneef, 2014, Kramer and Cash, 2012, Richardson, 2012, Van Diessen et al., 2013). Localized and generalized epilepsies are disorders involving impairments of both anatomical and functional connections on large scales, which may be present either on the side of the epileptogenic zone or bilaterally (Bettus et al., 2010, Engel et al., 2013, Frings et al., 2009, Chiang et al., 2014, Laufs et al., 2007, Morgan et al., 2010, Spencer, 2002). The spread of ictal activity resulting in the clinical expression of focal seizures requires the involvement of large-scale brain networks (Richardson, 2012) that are considered important for seizure onset and termination (Keller et al., 2014).
Brain impairment distant from the epileptic focus and not revealed by underlying neuronal substrate damage, e.g. cognitive decline, may be related to disturbances of brain connectivity. An excitotoxic effect of seizures can contribute to the decline of connectivity (Bonilha et al., 2010).
Resting-state functional magnetic resonance imaging (rs fMRI) is a procedure that requires little from the participant and allows for exploration of the integrity and organization of cerebral networks. Functional resting-state network studies could identify abnormal brain organization in epilepsy, including disturbed connectivity of the basal ganglia (BG) with large scale cortical networks (Rektor et al., 2013) or disruptions within basal ganglia networks identified by independent component analysis (Luo et al., 2012). Based on direct ictal recordings from the BG (Rektor et al., 2002, Rektor et al., 2012) and anterior nucleus of thalamus (Rektor et al., 2016) via depth electrodes, we have suggested an important role of subcortical structures in focal seizures. Interest in the role of the BG in epilepsy has increased recently in terms of their prospective use as deep brain stimulation targets for treating seizures. The BG form a very complex system of nuclei and pathways. The BG may act as an integrated system due to the unidirectional character of the major connections, the information being transformed and transmitted from the cortex via the BG into the cortex. The cortico-BG-thalamocortical circuits promote the execution of cortical programs and exercise a control function in suppressing unwanted elements. Based on a large body of experimental data, it has been proposed that the BG nuclei are structures controlling cortical seizure activity (for review, see Rektor et al., 2012).
Even though many studies have shown disruptions in brain connectivity caused by epilepsy (Englot et al., 2015, Haneef et al., 2014a, Luo et al., 2011, Maneshi et al., 2014, Voets et al., 2012), the mechanisms by which the subcortical structures are related to the cortical epileptic processes are largely unknown. We hypothesize that the BG-thalamic system might modulate large scale cortical networks and this modulation might be disturbed in epilepsy.
In this study, we evaluated the contribution of subcortical structures (BG and thalamus) to the whole-brain functional connectivity changes in focal epilepsies. To achieve this goal, we used network analysis to describe the topological differences on global and regional levels; we next employed the partial least squares analysis to identify the connections contributing the most to the difference. We focused on complete connectivity matrices (weighted networks) and evaluated systematic relations between low-value correlation coefficients and network topology, especially the connectivity of subcortical structures. In addition to the existing literature, we show the advantages of combining network and partial least squares analyses and the importance of low correlations, and we provide better insight into the regional distribution of alterations and the role of subcortical structures in whole-brain functional connectivity in epilepsy.
2. Materials and methods
2.1. Subjects
The study examined 46 patients with epilepsy (median age of 32.3 years; 21 males) and 20 controls (median age of 29.5 years; 14 males). The patients were epilepsy surgery candidates with pharmaco-resistant focal epilepsy. The diagnostic of the type of epilepsy was based on presurgical evaluation, including ictal video-EEG and advanced imaging methods. A MRI lesion was identified in 23 patients, while 23 patients were MR negative. In MR negative (non-lesional) patients the exact diagnosis was based on converging results of other methods, including intracranial recordings and/or surgery in some cases. All patients were investigated under their current antiepileptic medication. The study was approved by the local ethics committee; all patients were informed and agreed to participate in the study. The patients underwent a complex neuropsychological examination with Wechsler Memory Scale - III, Wechsler Adult Intelligence Scale - R, Trail Making Test (A, B), Rey-Osterrieth complex figure, Stroop test, Verbal fluency test, Behavioral Dyscontrol Scale, Test laterality, Five Digit Test, and Boston Naming Test. No patient manifested a serious cognitive impairment.
The patients were divided into 4 groups according to epileptogenic zone localization: 9 left temporal lobe epilepsy (LTLE; median age of 40.2 years; 2 males), 13 right temporal lobe epilepsy (RTLE; median age of 40.9 years; 5 males), 15 frontal lobe epilepsy (FLE; median age of 26.6 years; 10 males) and 9 parietal/occipital lobe epilepsy (POLE; median age of 30.2 years; 4 males). Based on the literature (Bernhardt et al., 2011, Chiang et al., 2014, Liu et al., 2014) and our preliminary analysis, we handled the RTLE and LTLE separately. In the preliminary analysis, we divided patients into left and right temporal epilepsy groups, and left and right epilepsy groups; as the right and left extratemporal epilepsies did not significantly differ, we pooled the data from the two sided epilepsies to the frontal and parieto-occipital lobe epilepsy groups. Among the 5 groups, there was a trend in age difference (Kruskal-Wallis H(4.66) = 8.67, p = 0.07) and a trend in a gender difference (Pearson Chi-square = 8.24, df = 4, p = 0.08). Among the 4 patient groups, there were non-significant differences in disease duration (Kruskal-Wallis H(3.44) = 0.93, p = 0.82) and significant differences in seizure frequency (Kruskal-Wallis H(3.44) = 9.57, p = 0.02, post-hoc test revealed significant difference between FLE and LTLE, p = 0.04). A higher frequency of seizure occurrence in FLE is usual. For details, see Table 1. Epileptogenic zone localization, neurocognitive impairment in specific lobe functions, and the antiepileptic drugs used are presented in the Supplementary material in Tables X1–X4.
Table 1.
Subjects' characteristics.
| Number of subjects | Agea [years] | Gender (males/females) | Disease durationa [years] | Seizure frequencya [1/month] | |
|---|---|---|---|---|---|
| Controls | 20 | 29.5; 26.7 ÷ 40.4 | 14/6 | – | – |
| LTLE | 9 | 40.2; 25.6 ÷ 65.1 | 2/7 | 23.0; 5 ÷ 41 | 1.0; 0.5 ÷ 4 |
| RTLE | 13 | 40.9; 22.4 ÷ 61.1 | 5/8 | 14.0; 3 ÷ 57 | 2.0; 0.3 ÷ 40 |
| FLE | 15 | 26.6; 19.7 ÷ 51.8 | 10/5 | 16.0b; 3 ÷ 48 | 7.0b; 1 ÷ 56 |
| POLE | 9 | 30.2; 19.3 ÷ 47.9 | 4/5 | 18.0; 3 ÷ 41 | 1.0; 0.5 ÷ 10 |
LT/RT/F/PO LE … left temporal/right temporal/frontal/parietal or occipital lobe epilepsy.
Median; range.
Data from 13 subjects available.
2.2. Data acquisition and preprocessing
Imaging was performed with a 1.5 T Siemens Symphony MR scanner. We acquired resting-state data for each subject using a gradient echo, echoplanar imaging sequence, 15 min, 300 scans (TR = 3 s; TE = 40 ms; FOV = 220 mm; FA = 90°; matrix size 64 × 64; slice thickness = 3.5 mm; 32 transversal slices which covered the whole brain excluding the cerebellum). Subjects were instructed to lie still with their eyes closed and not to fall asleep. Following functional measurements, anatomical T1-weighted images were acquired using an MPRAGE sequence with 160 sagittal slices, matrix size 256 × 256 resampled to 512 × 512; TR = 1700 ms; TE = 3.96 ms; FOV = 246 mm; FA = 15°; and slice thickness = 1.17 mm. Simultaneously, the ECG signal was acquired with the MR-compatible BrainProducts ExG system, sampled with 5 kHz.
The ECG signal was corrected for gradient artifacts (Allen et al., 2000). We used the BrainVision Analyzer 2.0 tool to identify the positions of R-waves. We applied the same preprocessing pipeline to the fMRI data from each subject: realignment of functional scans, removing the physiological noise related to the heartbeat using timing of ECG R-waves and Retroicor method (Glover et al., 2000), slice timing correction, normalization to the standard stereotactic space, removing the residual physiological noise by regressing out the signals derived from white matter and ventricles (Weissenbacher et al., 2009), and smoothing by spatial filter with Gaussian kernel (FWHM = 8 mm) to improve the signal-to-noise ratio. The spatial extent of the filter was set sufficiently small to avoid artificial correlations among regions of interest. We did not restrict the fMRI time-series to any frequency band as there is evidence that functional connectivity is manifested in the whole frequency range of fMRI signals (Lin et al., 2015). We did not regress out the global signal. The whole brain (except the cerebellum and middle brain) was parceled into 90 regions of interest (ROI) according to the AAL atlas (Tzourio-Mazoyer et al., 2002). The time-series of each ROI was averaged and cross-correlated using Pearson's correlation coefficient to form a 90 × 90 correlation matrix for each subject. To remove the nuisance effects on correlation coefficients, the matrices from all subjects (66) were concatenated to form a single 3D matrix, and the effects of age and gender were regressed out from each vector along the subject dimension.
2.3. Network analysis
We described the functional connectivity with the help of network analysis on both global and regional levels. The AAL atlas was used for global and local level analyses. For more robust network analysis results, we also parceled the brain into 194 ROIs according to the Craddock atlas (Craddock et al., 2012), with the temporal correlation as the similarity metric; subject-level similarity matrices were averaged and then submitted to clustering (tcorr05_mean variant). The Craddock atlas parcellation was used only for global network analysis; the 194 × 194 connectivity matrix was constructed in the same way as for the AAL atlas.
The correlation matrix defines the network; network nodes are the regions of interest, the edges represent the relations between ROIs (Bullmore and Sporns, 2009) – we used the Fisher's Z-transformed Pearson's correlation coefficients (Lowe et al., 1998). The subjects' correlation matrices can be thresholded and binarized to create adjacency matrices, or can stay intact and represent weighted network. We focused on a weighted approach because it does not discard possibly useful information as the thresholded approach does (Kaiser, 2011); however, we replaced negative correlations with zeros. To describe network topology, we investigated normalized characteristic path length λ and normalized clustering coefficient γ. For better insight into the network structure, we computed the regional level of these properties: node strength w, clustering coefficient C, and eigenvector centrality EC. The Brain Connectivity Toolbox was used (Rubinov and Sporns, 2010), normalization assured by dividing the property value by the average property values of 100 random networks created with preserved strength, weight, and degree distributions.
A node strength wi refers to a sum of edge weights incident to a given node, edge weight wij represents the relation (Fisher's Z-transformed correlation coefficient) between two ROIs. The characteristic path length L between two nodes is computed as the lowest sum of weights (from the distance matrix which we obtain from the correlation matrix by element-wise inversion) of links that need to be traversed to reach one node from another; from a global point of view it assesses functional integration (for more details on network measures, see (Bullmore and Sporns, 2009, Rubinov and Sporns, 2010)). Functional segregation can be expressed by clustering coefficient C, which is computed for each node separately and takes into account the connections between neighboring nodes, global value averages regional values of C. Higher values of average clustering imply better connectedness of neighbors; low values suggest weaker connectedness with, in extreme, a star-like topology. The small-world coefficient σ = γ / λ was used on a global level to relate the network topology to the small-world model and possible shifts towards more random or regular structures (Watts and Strogatz, 1998). We have further concentrated on second-level measure of a node's importance in the network – eigenvector (EC) centrality. The EC quantifies a node's influence on the network in the context of the influences of its neighboring nodes, taking their connectivity strengths into account (Bonacich, 1987, Bonacich, 2007, Van Diessen et al., 2013). The inter-group differences were determined separately for each pair control–patient-type by the Mann-Whitney U test because of the non-Gaussian distributions of measured properties.
2.4. Partial least square analysis
We used a partial least square method (PLS) to find inter-group differences in the brain functional connectivity on the level of particular connections. This method was used on connectivity matrices based on the AAL atlas. The PLS method (McIntosh and Lobaugh, 2004) belongs to a huge family of multivariate statistical analysis methods and therefore it has some advantages when compared to univariate methods (e.g., two-sample t-test). The PLS takes into account the patterns of connections among all the nodes which increase the sensitivity to subtle differences between groups that are spread over many connections. At the same time, it significantly reduces the number of tests, i.e. the multiple testing problem. Both positive and negative correlations were considered in the ensuing statistical analysis. The subjects' correlation matrices Mi (adjusted for age and gender) were stacked to form a single I ∗ s matrix M, where I indicates the number of subjects and s indicates the number of relevant coefficients in the correlation matrix (upper triangular part without diagonal). The rows of matrix M belonging to each group j (total of Nj rows) were averaged, resulting in J ∗ s matrix M*, where J is a number of groups entering the PLS analysis.
The M* was then mean-corrected and subjected to PLS, which enables the finding of common group-related differences in correlation coefficients spread over respective pairs of ROIs. The PLS employs a singular value decomposition of matrix M* which produces orthogonal latent variables (LV).
The matrices V and U are composed by vectors v and u and S is a diagonal matrix. The triplets u, v, and s (the respective entry of the S diagonal) compose the LVs. Each LV is then characterized by three features: vector v shows group-related differences, vector u (saliences) indicates how much each pair of ROIs contributes to the effect depicted by v, and singular value s indicates the amount (partition) of explained variability in M*. The significance of LVs is assessed by recalculating the PLS with permuted group membership and estimating the likelihood of obtaining a higher singular value than that obtained with the true group membership. The reliability of saliences is determined by calculating the standard error using bootstrap sampling of group members and recalculating the PLS. The level of statistical significance of LVs was set to p < 0.05. To show the most contributing pairs of ROIs, the saliences u of the significant LVs were thresholded with the Z-value corresponding to p < 0.05, FDR corrected.
The PLS was performed within MATLAB (MathWorks Inc., USA), using 5000 permutation steps and 1000 bootstrap steps. Four PLS analyses were performed to compare each patient group to the control group. Each analysis comprised only two groups and therefore it resulted in two LVs, one describing the difference between groups and the other the overall mean. In the Results section, we report the significance of the LV which describes the difference (diffLV).
2.5. Subcortical connectivity
Since our interest lies in BG connectivity, we studied its changes and dependence on network definition. For each subject's network, we set threshold levels from 100% to 0% of the network density with 1% steps; at each step, a threshold value of the correlation coefficient was specified (by replacing all below-threshold values in a correlation matrix with zero, the resulting matrix would be of a given density). The node strengths of the BG and the thalami were computed on this full range of proportional threshold levels and the effects of the contributing edges were studied (Garrison et al., 2015).
To answer our question targeting intra-subcortical connectivity and its changes due to disease, we constructed group-specific networks of 8 regions of interest – the caudate, pallidum, putamen, and thalamus in both hemispheres. We computed the node strengths of these regions and investigated differences between groups. Other network properties were not studied because of their inappropriate use in such small networks. The Mann-Whitney U test was used.
3. Results
3.1. Network analysis
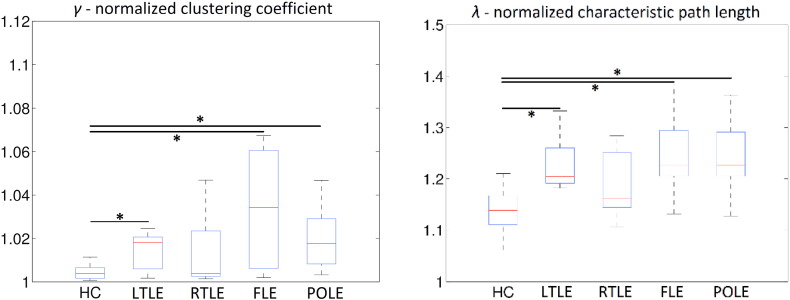
Network measures on the global level showed significant increases (p < 0.05, FDR corrected) in normalized clustering (γ) and path length (λ) in LTLE, FLE, and POLE patient groups as compared to controls, computed from networks based on the AAL atlas and the Craddock atlas. The results for the AAL atlas are presented in detail in boxplots in Fig. 1, the boxplots of metrics for the networks based on the Craddock atlas are presented in Supplementary Fig. X2. These increases led to the altered small-world coefficient which was significantly decreased in all patient groups as compared to controls (p < 0.05, FDR corrected), except the RTLE group with a decrease at 0.1 > p > 0.05, uncorr. We also tested differences between lesional and non-lesional epilepsy and didn't find any significant difference in global network properties in networks based on both AAL and Craddock atlases.
Fig. 1.
Global level properties of examined groups, AAL atlas. Asterisks indicate statistical difference (p < 0.05, FDR corrected) in a given property.
To evaluate regional level intergroup differences, we performed the Mann-Whitney U test separately for each ROI and graph metric (clustering coefficient, node strength, eigenvector centrality). The level of statistical significance was set to p < 0.05, FDR corrected for investigated regional properties. The altered ROIs are shown in Table 2; we highlight the higher eigenvector centrality of the precuneus in RTLE, FLE, and POLE, and of the posterior cingulum in RTLE and FLE. We also observed lower node strengths, clustering as well as EC of occipital areas in FLE and POLE, and inferior temporal areas and fusiform gyri in LTLE, FLE, and POLE. Supplementary Tables X5–X8 present the same results in different settings; Supplementary Figs. X3–X5 visualize regions altered in epilepsy groups in the brain space (with the use of BrainNetViewer (Xia et al., 2013)). Supplementary Figs. X6–X7 show changes in the importance of individual nodes in the patient networks as compared to controls; the importance is defined by EC (Fig. X6) or betweenness centrality (Fig. X7). Since the betweenness centrality is a highly used metric, we show its alterations in patient groups when compared to controls in Supplementary Tables X9–X12. To better understand the changes in the subcortical regions that are the focus of our study, we depict boxplots of node strengths for these regions in Supplementary Fig. X8.
Table 2.
Regional differences between patients with epilepsy and controls. C (clustering coefficient), w (node strength), EC (eigenvector centrality). p < 0.05, FDR corrected. i indicates an increase in the patient group compared to controls, d means a decrease in a given property and region of interest. Results significant after FDR correction to 90 ROIs of the AAL atlas are marked by asterisks.
| LTLE vs. controls |
RTLE vs. controls |
FLE vs. controls |
POLE vs. controls |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | w | EC | C | W | EC | C | w | EC | C | w | EC | |
| Frontal Sup Orb (L) | – | d | – | – | – | – | – | – | – | – | – | – |
| Frontal Mid (L) | – | – | – | – | – | – | – | – | d | – | – | – |
| Frontal Inf Orb (L) | – | – | – | – | – | – | d | d | d | d | – | – |
| Frontal Inf Orb (R) | – | – | – | – | – | – | – | – | – | d | d | – |
| Olfactory (L) | d | d | – | – | – | – | – | – | – | – | – | – |
| Olfactory (R) | d | – | – | – | – | – | – | – | – | – | – | – |
| Cingulum Post (L) | – | – | – | – | – | – | – | – | i* | – | – | – |
| Cingulum Post (R) | – | – | – | – | – | i | – | – | i | – | – | – |
| Parahippocampal (L) | – | – | – | – | – | – | d | d | d | d | d | d |
| Amygdala (L) | – | – | – | – | – | – | – | – | – | d | d | – |
| Amygdala (R) | – | – | – | – | – | – | d | – | – | d | d | – |
| Calcarine (L) | – | – | – | – | – | – | d | d | – | – | – | – |
| Calcarine (R) | – | – | – | – | – | – | d | – | – | – | – | – |
| Lingual (L) | – | – | – | – | – | – | d | d | d | d | d | – |
| Lingual (R) | – | – | – | – | – | – | d | d | – | – | – | – |
| Occipital Inf (L) | – | – | – | – | – | – | d | d | d | d | d | d |
| Occipital Inf (R) | – | – | – | – | – | – | d | d | – | d | d | d |
| Fusiform (L) | d | d | d* | – | – | d | d | d | d* | d | d | d |
| Fusiform (R) | – | – | – | – | – | – | d | d | d | d | d | d |
| Angular (L) | – | – | i | – | – | – | – | – | – | – | – | – |
| Precuneus (L) | – | – | – | – | – | i | – | – | i* | – | – | i* |
| Precuneus (R) | – | – | – | – | – | i | – | – | i* | – | – | i |
| Caudate (L) | d | d | – | – | – | – | d | d | – | – | – | – |
| Caudate (R) | d | d | – | – | – | – | d | d | – | – | – | – |
| Pallidum (R) | – | – | – | – | – | – | d | d | – | – | – | – |
| Thalamus (L) | – | d | – | – | – | – | – | – | – | – | – | – |
| Thalamus (R) | – | d | – | – | – | – | – | – | – | – | – | – |
| Temporal Inf (L) | d | d | d* | – | – | – | – | – | – | d | d | d |
| Temporal Inf (R) | d | d | – | – | – | – | d | d | d | d | d | d |
3.2. Partial least square analysis
We performed four PLS analyses to assess the differences in whole-brain functional connectivity between each patient group and controls.
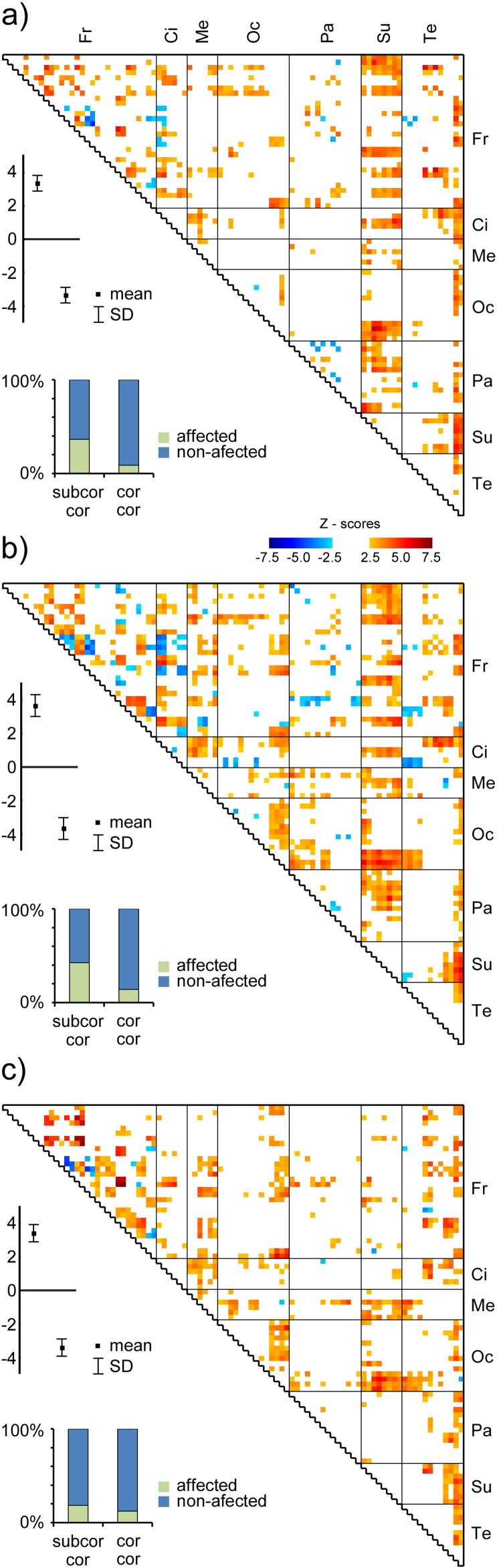
In the LTLE group, the diffLV (the latent variable that describes the difference between groups) reached a significance level (p = 0.0014). Fig. 2a shows the most contributing pairs of ROIs. The same holds for FLE (p = 0.0016, Fig. 2b), and for POLE (p = 0.0008, Fig. 2c). In all three groups, the connectivity of subcortical regions with the cortex was disturbed and this disturbance was most important when compared to the purely cortico-cortical disturbances. For RTLE, the diffLV did not reach a significance level.
Fig. 2.
The difference in functional connectivity between controls and a) LTLE patients, b) FLE patients, c) POLE patients. The warm/cold colors within the triangular graph depicts the Z-scores of the significantly contributing saliences u, i.e. the ROI pairs with significantly higher (cold colors) or lower (warm colors) functional connectivity in patients than in healthy controls. The significance level was set to p < 0.05, corrected for multiple testing. The whisker plot shows the saliences v, i.e. the difference between respective groups. The difference is significant at p < 0.05 for all three comparisons. The bar graphs show the percentage of affected connections either between subcortical and cortical areas (subcor – cor) or between cortical and cortical areas (cor – cor). Fr/Ci/Me/Oc/Pa/Su/Te stands for frontal/cingulate/mesiotemporal/occipital/parietal/subcortical/temporal areas.
3.3. Subcortical connectivity
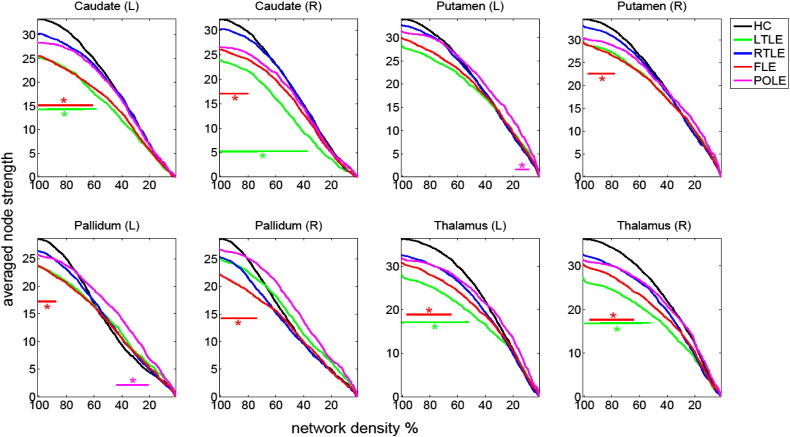
Based on the results from PLS analysis, we concentrated on BG connectivity changes and its dependence on network definition. We found that in all structures of interest, lower values of node strength in patient groups were clearest when the weak correlations (low-weight edges) were included as visible in Fig. 3. When the weaker links were eliminated, the difference diminished. The only structure deviating from this trend was the pallidum, where the node strength in healthy controls was actually lower when at least 40% of the weaker links were eliminated.
Fig. 3.
Comparison of average node strength (negative correlations in absolute values) for subcortical areas as a function of applied threshold for controls and patient groups. Significant differences (p < 0.05) are indicated by horizontal bars in incident colors.
Concerning intra-subcortical connectivity, we observed no statistically significant difference between any epilepsy group and controls. Fig. X9 (see in the Supplementary material) illustrates the average intra/subcortical connectivity in healthy subjects.
4. Discussion
The goal of this study was to identify alterations in the interictal functional networks in patients with focal epilepsies when compared to healthy controls. We used two statistical approaches: complex network and partial least squares analyses. The network analysis tells about node properties and the overall topology of the network under study; the partial least square analysis provides information about altered functional connectivity at the level of connections/edges. The observed connectivity changes indicate altered networks that underlie widespread brain involvement in focal epilepsies, in the temporal LTLE as well as extratemporal FLE and POLE. The most intriguing alteration however concerned the connectivity of the subcortical structures with the cortex, which was observed consistently in all tested groups with significantly lower values in patient groups.
Consistent results were found in TLE, and similar results were also observed in patients with extratemporal epilepsy in a RSN study (Luo et al., 2011). They suggested that the decreased resting state functional connectivity might be a remarkable characteristic of focal epilepsy. In our study, the most notable was the decrease of connectivity between the mesiotemporal structures and the temporal and frontal lateral cortex, insula, and cingulate in LTLE in comparison to controls. In FLE, the connectivity was decreased mainly within the frontal lobe but temporal and posterior structures were also involved. In POLE, the lower values were present mainly between the parietal and frontal structures and fusiform gyrus.
4.1. Network characteristic of FC changes in epilepsy
Several studies have reported disrupted functional connectivity in resting-state functional connectivity in patients with epilepsy (Englot et al., 2015, Haneef et al., 2014a, Luo et al., 2011, Maneshi et al., 2014, Voets et al., 2012). More specifically, works employing the complex network analysis approach have shown increased characteristic path lengths (Horstmann et al., 2010, Chiang and Haneef, 2014, Otte et al., 2012, Vlooswijk et al., 2011) in epilepsy in fMRI as well as in white matter studies, which is in alignment with our results, and confirms the overall disruption of functional connectivity. (For review see (Englot et al., 2015, Chiang and Haneef, 2014, Chiang et al., 2014).) Only one study has reported decreases in path lengths and increased functional connectivity in patients with epilepsy (Liao et al., 2010), possibly because it focused on subjects with bilateral MTLE. Clustering coefficients were reported as increased in epileptic subjects in previous studies (Bartolomei et al., 2013) as in our results; they have also been reported as decreased (Liao et al., 2010); for reviews see (Chiang and Haneef, 2014, Van Diessen et al., 2014). The origins of this discrepancy are unknown and will be the subject of further research.
Combining the effects of higher characteristic path lengths and clustering coefficient in patients shifted the functional network further towards the regular end of the small-world spectrum (Watts and Strogatz, 1998), which corresponds to previous network-oriented studies using different modalities to construct the resting-state functional connectivity of epileptic subjects (cortical thickness correlation by Bernhardt et al., 2011; EEG and MEG by Horstmann et al., 2010). Longer path lengths suggest a loss of links between functionally distant nodes, which together with increased clustering suggests better connectivity structures in close neighborhoods in most of our epilepsy groups as compared to controls. A more regular character of the network suggests decreased efficiency in transferring information between distant regions of the brain, and simultaneous excessive, possibly redundant, connectivity within functional clusters. Interestingly, the regularity of the interictal network was shown to be increasing even further during pre-ictal and ictal periods in EEG studies (Kramer and Cash, 2012, Ponten et al., 2007, Schindler et al., 2008). It is worth repeating that distance here has functional, not anatomical meaning.
The redistribution of important nodes in epilepsy has been reported previously (Bernhardt et al., 2011, Liao et al., 2010) using mostly betweenness centrality. We replicated these results and we showed a similar effect with eigenvector centrality (EC). Both measures indicate the importance of the nodes: the betweenness highlights the nodes serving as information brokers between functionally separated clusters, whereas the EC measures the overall influence of the node on the network (Binnewijzend et al., 2014). In our opinion, the EC better reflects the importance of the node in the functional network, and changes to these influential nodes have a potentially large impact on the brain function. We further highlight a more pronounced influence (as measured by eigenvector centrality) of the precuneus in the networks of RTLE, FLE, and POLE, and of the posterior cingulum in RTLE and FLE. We also observed extensively lowered connectivity of the occipital areas in FLE and POLE, and of the inferior temporal areas and fusiform gyri in LTLE, FLE, and POLE.
The analysis on global and local levels revealed seemingly opposite results; whereas on the global level, clustering in normalized variants increased in the patient groups, on the local level, clustering mostly decreased in comparison to controls. The results are in opposition only at first glance. The ambiguity is probably the result of the choice of a null model for normalization. We explain our reasoning: (i) We used the model preserving node strength distribution. (ii) The average node strength was non-significantly decreased in patient groups as compared to controls. (iii) Average clustering is influenced by connectivity weights – lower edge weights cause lower clustering. Similarly, lower edge weights lead to longer shortest paths making the characteristic path length higher. (iv) Average clustering in real networks is higher than in random networks, and ranges from 0 to 1. (v) Average clustering was non-significantly decreased in patient groups as compared to controls. These observations lead us to believe the following implications: (i) the null model used for patient groups had lower average clustering than the one used for the control group. (ii) The ratio between average clustering in the studied group and in random networks is higher than 1. (iii) In our case, this ratio was higher in patient groups than in controls.
We also showed that the weaker links contribute significantly to the changes in the networks caused by the disease. First, these links should not be simply discarded as random, as they represent mostly long-distance connections in physical space as shown in (Alexander-Bloch et al., 2012, Gießing et al., 2013) and in our data when visually inspecting the localization of these weak connections (see Supplementary Figs. X10–X12). Second, if the group difference is systematically concentrated on weaker links, any thresholding or more sophisticated techniques such as cost-integration (Ginestet et al., 2011) will mask this difference.
4.2. Lateral asymmetry of the connectivity changes
Graph theoretical analysis of anatomical connectivity provided direct evidence of large-scale network disruption in TLE with left hippocampal sclerosis (Liu et al., 2014). We observed that impact on the cortical connectivity depended on the laterality of the epileptogenic zone in TLE. Only LTLE manifested a significant connectivity decline, in contrast to RTLE. The asymmetry in the connectivity patterns of the TLE has been reported in several studies (Billingsley et al., 2001, Dupont et al., 2002); for review see (Vlooswijk et al., 2010). It was also recently reported in a network-oriented study (Chiang et al., 2014). A resting-state fMRI displayed a pattern similar to our finding, i.e. an ipsilesional hippocampal connectivity impairment in LTLE and less marked impairment in RTLE (Pereira et al., 2010). In one study, patients with LTLE displayed a weaker functional connectivity to the network distant from the site of seizure focus (Frings et al., 2009). An increase of the connectivity of the posterior default mode network (DMN) node in LTLE but not RTLE when compared to controls was also described and explained by the increased influence of the contralateral hippocampus on the ipsilesional hippocampus in RTLE, but not in LTLE (Morgan et al., 2010). The innate cerebral asymmetry and dominance pattern could contribute to the difference in connectivity between RTLE and LTLE. The differences between connectivity in LTLE and RTLE could underlie some of the clinical differences, such as cognitive differences, noted between TLE with left- and right-sided epileptogenic zones (Haneef et al., 2014b).
4.3. Role of subcortical structures in epilepsy
The mechanisms by which the subcortical structures influence the cortical epileptic processes were largely unknown. In this study, we explored the connectivity between subcortical structures (SUB) and the cortex as well as the connectivity within the subcortical circuitry. It is interesting to note that the decrease in node strength, i.e. the ability to co-activate within the entire brain, reported in this paper in Table 2 was related to alterations in the domain of low correlation coefficients and not in the domain of strong connections with high correlation coefficients as shown in Fig. 3. This observation may be interpreted in light of the suggested disturbance of short and/or less frequent interactions: possibly remote control and modulation between the BG and cortical areas.
A massive disruption of connectivity between the SUB and most of the cortical regions appears to be a universal feature of the localization of related epilepsies. We observed this pattern in the LTLE as well as in the FLE and POLE. A massive decrease of connectivity between SUB and the lateral frontal cortex, SMA, cingulate, and parietooccipital cortices was observed in TLE, between SUB and the precentral and frontal medial cortices, gyrus rectus, SMA, cingulate, parahippocampal, fusiform, supramarginal and parietooccipital structures in the FLE, and in the POLE between SUB and the parahippocampal, cingulate, and fusiform gyrus, cuneus and lateral occipital cortex. However, no change was observed in the intra-subcortical connectivity. Thus, the functional connectivity within the BG-thalamic circuitry was not disturbed, the impairment concerned the connectivity of BG-thalamic circuitry with the cortical areas.
We do not analyse rich-club or the resilience of subcortical areas since they did not count as hubs in the network (due to their relatively low node strengths/correlation coefficients); these analyses target mostly hubs. Further analysis of the impact of changes in basal ganglia and thalamic functions is needed; however, large-scale network analyses are not the best way to show such detailed information.
Many studies have reported the involvement of the thalamus in various types of epilepsy. In terms of focal epilepsy, a recent study revealed a reduced thalamo-temporal connectivity in patients with TLE. The authors suggested that this network disruption may be considered the anatomic basis of a dysfunction causing seizures (Keller et al., 2014). Considerably fewer studies were devoted to the role of the BG in localization of related epilepsies. In a fMRI resting-state study (Rektor et al., 2013), we observed that the epileptic process reduced the FC between the BG and large-scale resting-state networks. This may be interpreted as a sign of an altered or modified function of the BG in epilepsy.
Epileptic activity can lead to the inhibition of widespread physiological networks via subcortical structures (Federico et al., 2005, Laufs et al., 2007, Norden and Blumenfeld, 2002). In intracerebral EEG studies, we performed direct recordings from the BG during temporal lobe seizures (Rektor et al., 2002, Rektor et al., 2011). The BG did not generate epileptiform activity, but oscillatory activities were modified after the onset of the clinical seizure; the changes persisted after the seizure ended (Rektor et al., 2012). The time course of the oscillatory activities together with the absence of the epileptiform activity, as well as the data in the literature, for review see (Rektor et al., 2012), led us to suggest an inhibitory role of the BG in seizures. The BG circuits may have an inhibitory impact on cortical epileptic activity via their feedback pathways to the cortex. The central position of SUB with afferents and efferents connecting the SUB with nearly all cortical areas may play a substantial role in the large scale connectivity impairment in the epileptic brain.
5. Limitations
The epilepsy groups were homogeneous in their SOZ localization, but the individual participants differed in the underlying etiologies. Another potential confounding factor was the variation in the antiepileptic drugs (AEDs). AEDs probably affect FC directly or via their cognitive or other effects but the large number of AEDs used in the studied cohorts makes it difficult to disentangle the effects of epilepsy from those of AEDs (for details see Supplementary material). More studies with large cohorts of patient groups are needed to elucidate the subcortico-cortical connectivity disturbance in epilepsy in more details.
6. Conclusions
The focal epilepsies affect large-scale brain networks beyond the epileptogenic zones. Our study revealed that all three significantly disturbed groups (LTLE, FLE, and POLE) displayed cortico-subcortical functional connectivity disturbances. Redistribution of functionally important nodes and decreases in the node strength of all three studied BG structures, i.e. the putamen, caudate, and pallidum, were displayed in LTLE, FLE, and POLE. Significant and constant changes in the resting-state functional connectivity between cortical and subcortical structures in patients with epilepsy suggest an important role of the BG and thalamus in focal epilepsies.
To our knowledge, this is the first paper studying the cortico-subcortical connectivity disturbance in the separate groups of LTLE, RTLE, FLE, and POLE using identical methods. This enables our conclusion that the cortico-subcortical connectivity disturbance is a general phenomenon that can be observed in focal epilepsies of various origins.
Methodologically, we would like to emphasize the advantage of combining network and partial least squares analyses, especially when based on complete, not-thresholded, correlation matrices. Considering the results, we report findings similar to those of previously published studies. The similarity of our results to the results of other studies supports our other findings, namely the importance of low correlations, the regional distribution of alterations, and the role of subcortical structures, which bring more insight to the current knowledge of whole-brain functional networks in epilepsy.
Acknowledgement
The study was supported by the Ministry of Education, Youth and Sports of the Czech Republic under the project CEITEC 2020 (LQ1601), grant GACR P103/11/0933, Faculty of Informatics, Masaryk University and grant MUNI/A/0945/2015. The authors wish to thank Prof. Mark Richardson from King's College, London, UK, for his valuable advice and Anne Johnson for grammatical assistance. Access to computing and storage facilities owned by parties and projects contributing to the National Grid Infrastructure MetaCentrum, provided under the programme “Projects of Large Infrastructure for Research, Development, and Innovations” (LM2010005), is greatly appreciated.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2016.12.014.
Contributor Information
Eva Výtvarová, Email: eva.vytvarova@mail.muni.cz.
Radek Mareček, Email: rmarec@med.muni.cz.
Jan Fousek, Email: izaak@mail.muni.cz.
Ondřej Strýček, Email: ondrej.strycek@fnusa.cz.
Ivan Rektor, Email: irektor@med.muni.cz.
Appendix A. Supplementary data
Supplementary material
References
- Alexander-Bloch A.F., Vértes P.E., Stidd R., Lalonde F., Clasen L., Rapoport J., Giedd J., Bullmore E.T., Gogtay N. The anatomical distance of functional connections predicts brain network topology in health and schizophrenia. Cereb. Cortex. 2012 doi: 10.1093/cercor/bhr388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P.J., Josephs O., Turner R. A method for removing imaging artifact from continuous EEG recorded during functional MRI. NeuroImage. 2000;12:230–239. doi: 10.1006/nimg.2000.0599. [DOI] [PubMed] [Google Scholar]
- Bartolomei F., Bettus G., Stam C.J., Guye M. Interictal network properties in mesial temporal lobe epilepsy: a graph theoretical study from intracerebral recordings. Clin. Neurophysiol. 2013;124:2345–2353. doi: 10.1016/j.clinph.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Bernhardt B.C., Chen Z., He Y., Evans A.C., Bernasconi N. Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb. Cortex. 2011;21:2147–2157. doi: 10.1093/cercor/bhq291. [DOI] [PubMed] [Google Scholar]
- Bettus G., Bartolomei F., Confort-Gouny S., Guedj E., Chauvel P., Cozzone P.J., Ranjeva J.-P., Guye M. Role of resting state functional connectivity MRI in presurgical investigation of mesial temporal lobe epilepsy. J. Neurol. Neurosurg. Psychiatry. 2010 doi: 10.1136/jnnp.2009.191460. [DOI] [PubMed] [Google Scholar]
- Billingsley R.L., McAndrews M.P., Crawley A.P., Mikulis D.J. Functional MRI of phonological and semantic processing in temporal lobe epilepsy. Brain. 2001;124:1218–1227. doi: 10.1093/brain/124.6.1218. [DOI] [PubMed] [Google Scholar]
- Binnewijzend M.A.A., Adriaanse S.M., Flier W.M., Teunissen C.E., Munck J.C., Stam C.J., Scheltens P., Berckel B.N.M., Barkhof F., Wink A.M. Brain network alterations in Alzheimer's disease measured by eigenvector centrality in fMRI are related to cognition and CSF biomarkers. Hum. Brain Mapp. 2014;35:2383–2393. doi: 10.1002/hbm.22335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonacich P. Power and centrality: a family of measures. Am. J. Sociol. 1987;92(5):1170–1182. [Google Scholar]
- Bonacich P. Some unique properties of eigenvector centrality. Soc. Networks. 2007;29:555–564. [Google Scholar]
- Bonilha L., Edwards J.C., Kinsman S.L., Morgan P.S., Fridriksson J., Rorden C., Rumboldt Z., Roberts D.R., Eckert M.A., Halford J.J. Extrahippocampal gray matter loss and hippocampal deafferentation in patients with temporal lobe epilepsy. Epilepsia. 2010;51:519–528. doi: 10.1111/j.1528-1167.2009.02506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Chiang S., Haneef Z. Graph theory findings in the pathophysiology of temporal lobe epilepsy. Clin. Neurophysiol. 2014 doi: 10.1016/j.clinph.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang S., Stern J.M., Engel J., Levin H.S., Haneef Z. Differences in graph theory functional connectivity in left and right temporal lobe epilepsy. Epilepsy Res. 2014;108:1770–1781. doi: 10.1016/j.eplepsyres.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock R.C., James G.A., Holtzheimer P.E., Hu X.P., Mayberg H.S. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum. Brain Mapp. 2012;33:1914–1928. doi: 10.1002/hbm.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S., Samson Y., de Moortele P.F., Samson S., Poline J.B., Hasboun D., Le Bihan D., Baulac M. Bilateral hemispheric alteration of memory processes in right medial temporal lobe epilepsy. J. Neurol. Neurosurg. Psychiatry. 2002;73:478–485. doi: 10.1136/jnnp.73.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J., Jr., Thompson P.M., Stern J.M., Staba R.J., Bragin A., Mody I. Connectomics and epilepsy. Curr. Opin. Neurol. 2013;26:186. doi: 10.1097/WCO.0b013e32835ee5b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot D.J., Hinkley L.B., Kort N.S., Imber B.S., Mizuiri D., Honma S.M., Findlay A.M., Garrett C., Cheung P.L., Mantle M., Tarapore P.E., Knowlton R.C., Chang E.F., Kirsch H.E., Nagarajan S.S. Global and regional functional connectivity maps of neural oscillations in focal epilepsy. Brain. 2015 doi: 10.1093/brain/awv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federico P., Archer J.S., Abbott D.F., Jackson G.D. Cortical/subcortical BOLD changes associated with epileptic discharges: an EEG-fMRI study at 3 T. Neurology. 2005;64:1125–1130. doi: 10.1212/01.WNL.0000156358.72670.AD. [DOI] [PubMed] [Google Scholar]
- Frings L., Schulze-Bonhage A., Spreer J., Wagner K. Remote effects of hippocampal damage on default network connectivity in the human brain. J. Neurol. 2009;256:2021–2029. doi: 10.1007/s00415-009-5233-0. [DOI] [PubMed] [Google Scholar]
- Garrison K.A., Scheinost D., Finn E.S., Shen X., Constable R.T. The (in) stability of functional brain network measures across thresholds. NeuroImage. 2015;118:651–661. doi: 10.1016/j.neuroimage.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gießing C., Thiel C.M., Alexander-Bloch A.F., Patel A.X., Bullmore E.T. Human brain functional network changes associated with enhanced and impaired attentional task performance. J. Neurosci. 2013;33:5903–5914. doi: 10.1523/JNEUROSCI.4854-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestet C.E., Nichols T.E., Bullmore E.T., Simmons A. Brain network analysis: separating cost from topology using cost-integration. PLoS One. 2011;6 doi: 10.1371/journal.pone.0021570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover G.H., Li T.-Q., Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn. Reson. Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Haneef Z., Lenartowicz A., Yeh H.J., Engel J., Jr., Stern J.M. Network analysis of the default mode network using functional connectivity MRI in temporal lobe epilepsy. J. Vis. Exp. 2014 doi: 10.3791/51442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneef Z., Lenartowicz A., Yeh H.J., Levin H.S., Engel J., Stern J.M. Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia. 2014;55:137–145. doi: 10.1111/epi.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann M.-T., Bialonski S., Noennig N., Mai H., Prusseit J., Wellmer J., Hinrichs H., Lehnertz K. State dependent properties of epileptic brain networks: comparative graph—theoretical analyses of simultaneously recorded EEG and MEG. Clin. Neurophysiol. 2010;121:172–185. doi: 10.1016/j.clinph.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Kaiser M. A tutorial in connectome analysis: topological and spatial features of brain networks. NeuroImage. 2011;57:892–907. doi: 10.1016/j.neuroimage.2011.05.025. [DOI] [PubMed] [Google Scholar]
- Keller S.S., O'Muircheartaigh J., Traynor C., Towgood K., Barker G.J., Richardson M.P. Thalamotemporal impairment in temporal lobe epilepsy: a combined MRI analysis of structure, integrity, and connectivity. Epilepsia. 2014;55:306–315. doi: 10.1111/epi.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M.A., Cash S.S. Epilepsy as a disorder of cortical network organization. Neuroscience. 2012;18:360–372. doi: 10.1177/1073858411422754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H., Hamandi K., Salek-Haddadi A., Kleinschmidt A.K., Duncan J.S., Lemieux L. Temporal lobe interictal epileptic discharges affect cerebral activity in “default mode” brain regions. Hum. Brain Mapp. 2007;28:1023–1032. doi: 10.1002/hbm.20323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W., Zhang Z., Pan Z., Mantini D., Ding J., Duan X., Luo C., Lu G., Chen H. Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PLoS One. 2010;5:27–29. doi: 10.1371/journal.pone.0008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F.-H., Chu Y.-H., Hsu Y.-C., Lin J.-F.L., Tsai K.W.-K., Tsai S.-Y., Kuo W.-J. Significant feed-forward connectivity revealed by high frequency components of BOLD fMRI signals. NeuroImage. 2015;121:69–77. doi: 10.1016/j.neuroimage.2015.07.036. [DOI] [PubMed] [Google Scholar]
- Liu M., Chen Z., Beaulieu C., Gross D.W. Disrupted anatomic white matter network in left mesial temporal lobe epilepsy. Epilepsia. 2014;55:674–682. doi: 10.1111/epi.12581. [DOI] [PubMed] [Google Scholar]
- Lowe M.J., Mock B.J., Sorenson J.A. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Luo C., Qiu C., Guo Z., Fang J., Li Q., Lei X., Xia Y., Lai Y., Gong Q., Zhou D. Disrupted functional brain connectivity in partial epilepsy: a resting-state fMRI study. PLoS One. 2011;7 doi: 10.1371/journal.pone.0028196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C., Li Q., Xia Y., Lei X., Xue K., Yao Z., Lai Y., Liao W., Zhou D., Valdes-Sosa P.A. Resting state basal ganglia network in idiopathic generalized epilepsy. Hum. Brain Mapp. 2012;33:1279–1294. doi: 10.1002/hbm.21286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneshi M., Vahdat S., Fahoum F., Grova C., Gotman J. Specific resting-state brain networks in mesial temporal lobe epilepsy. Front. Neurol. 2014;5 doi: 10.3389/fneur.2014.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh A.R., Lobaugh N.J. Partial least squares analysis of neuroimaging data: applications and advances. NeuroImage. 2004;23:S250–S263. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Morgan V.L., Gore J.C., Abou-Khalil B. Functional epileptic network in left mesial temporal lobe epilepsy detected using resting fMRI. Epilepsy Res. 2010;88:168–178. doi: 10.1016/j.eplepsyres.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden A.D., Blumenfeld H. The role of subcortical structures in human epilepsy. Epilepsy Behav. 2002;3:219–231. doi: 10.1016/s1525-5050(02)00029-x. [DOI] [PubMed] [Google Scholar]
- Otte W.M., Dijkhuizen R.M., van Meer M.P., van der Hel W.S., Verlinde S.A., van Nieuwenhuizen O., Viergever M.A., Stam C.J., Braun K.P. Characterization of functional and structural integrity in experimental focal epilepsy: reduced network efficiency coincides with white matter changes. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira F.R.S., Alessio A., Sercheli M.S., Pedro T., Bilevicius E., Rondina J.M., Ozelo H.F.B., Castellano G., Covolan R.J.M., Damasceno B.P. Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: evidence from resting state fMRI. BMC Neurosci. 2010;11:66. doi: 10.1186/1471-2202-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponten S.C., Bartolomei F., Stam C.J. Small-world networks and epilepsy: graph theoretical analysis of intracerebrally recorded mesial temporal lobe seizures. Clin. Neurophysiol. 2007;118:918–927. doi: 10.1016/j.clinph.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Rektor I., Kuba R., Brázdil M. Interictal and ictal EEG activity in the basal ganglia: an SEEG study in patients with temporal lobe epilepsy. Epilepsia. 2002;43:253–262. doi: 10.1046/j.1528-1157.2002.28001.x. [DOI] [PubMed] [Google Scholar]
- Rektor I., Kuba R., Brázdil M., Halámek J., Jurák P. Ictal and peri-ictal oscillations in the human basal ganglia in temporal lobe epilepsy. Epilepsy Behav. 2011;20:512–517. doi: 10.1016/j.yebeh.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Rektor I., Kuba R., Brázdil M., Chrastina J. Do the basal ganglia inhibit seizure activity in temporal lobe epilepsy? Epilepsy Behav. 2012;25:56–59. doi: 10.1016/j.yebeh.2012.04.125. [DOI] [PubMed] [Google Scholar]
- Rektor I., Tomčík J., Mikl M., Mareček R., Brázdil M., Rektorová I. Association between the basal ganglia and large-scale brain networks in epilepsy. Brain Topogr. 2013;26:355–362. doi: 10.1007/s10548-012-0272-8. [DOI] [PubMed] [Google Scholar]
- Rektor I., Doležalová I., Chrastina J., Jurák P., Halámek J., Baláž M., Brázdil M. High-frequency oscillations in the human anterior nucleus of the thalamus. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation. 2016;9(4):629–631. doi: 10.1016/j.brs.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Richardson M.P. Large scale brain models of epilepsy: dynamics meets connectomics. J. Neurol. Neurosurg. Psychiatry. 2012:1238–1248. doi: 10.1136/jnnp-2011-301944. [DOI] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Schindler K.A., Bialonski S., Horstmann M.-T., Elger C.E., Lehnertz K. Evolving functional network properties and synchronizability during human epileptic seizures. Chaos. 2008;18:33119. doi: 10.1063/1.2966112. [DOI] [PubMed] [Google Scholar]
- Spencer S.S. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43:219–227. doi: 10.1046/j.1528-1157.2002.26901.x. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Diessen E., Diederen S.J.H., Braun K.P.J., Jansen F.E., Stam C.J. Functional and structural brain networks in epilepsy: what have we learned? Epilepsia. 2013;54:1855–1865. doi: 10.1111/epi.12350. [DOI] [PubMed] [Google Scholar]
- Van Diessen E., Zweiphenning W.J.E.M., Jansen F.E. Brain network organization in focal epilepsy: a systematic review and meta-analysis. PLoS One. 2014;9:1–21. doi: 10.1371/journal.pone.0114606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlooswijk M.C.G., Jansen J.F.A., de Krom M.C., Majoie H.J.M., Hofman P.A.M., Backes W.H., Aldenkamp A.P. Functional MRI in chronic epilepsy: associations with cognitive impairment. Lancet Neurol. 2010;9:1018–1027. doi: 10.1016/S1474-4422(10)70180-0. [DOI] [PubMed] [Google Scholar]
- Vlooswijk M.C.G., Vaessen M.J., Jansen J.F.A., de Krom M., Majoie H.J.M., Hofman P.A.M., Aldenkamp A.P., Backes W.H. Loss of network efficiency associated with cognitive decline in chronic epilepsy. Neurology. 2011;77:938–944. doi: 10.1212/WNL.0b013e31822cfc2f. [DOI] [PubMed] [Google Scholar]
- Voets N.L., Beckmann C.F., Cole D.M., Hong S., Bernasconi A., Bernasconi N. Structural substrates for resting network disruption in temporal lobe epilepsy. Brain. 2012;135:2350–2357. doi: 10.1093/brain/aws137. [DOI] [PubMed] [Google Scholar]
- Watts D.J., Strogatz S.H. Collective dynamics of “small-world”networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Weissenbacher A., Kasess C., Gerstl F., Lanzenberger R., Moser E., Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. NeuroImage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material