Abstract
Non-suicidal and suicidal self-injury are very destructive, yet surprisingly common behaviours. Depressed mood is a major risk factor for non-suicidal self-injury (NSSI), suicidal ideation and suicide attempts. We conducted a genetic risk prediction study to examine the polygenic overlap of depressive symptoms with lifetime NSSI, suicidal ideation, and suicide attempts in a sample of 6237 Australian adult twins and their family members (3740 females, mean age = 42.4 years). Polygenic risk scores for depressive symptoms significantly predicted suicidal ideation, and some predictive ability was found for suicide attempts; the polygenic risk scores explained a significant amount of variance in suicidal ideation (lowest p = 0.008, explained variance ranging from 0.10 to 0.16 %) and, less consistently, in suicide attempts (lowest p = 0.04, explained variance ranging from 0.12 to 0.23 %). Polygenic risk scores did not significantly predict NSSI. Results highlight that individuals genetically predisposed to depression are also more likely to experience suicidal ideation/behaviour, whereas we found no evidence that this is also the case for NSSI.
Keywords: Depression, Suicidal ideation, Suicide attempts, Self-injury, Polygenic risk, Genetics
Intentional self-harm contravenes the fundamental drive of self-preservation. However, both suicidal and non-suicidal self-injurious (NSSI) behaviours (i.e., with and without the direct intention to die, respectively) are surprisingly common in the population. Almost 1 in 10 adults have ever thought about taking their own life, more than 1 in 20 have engaged in NSSI, and more than 1 in 40 have attempted suicide (Nock et al. 2008; Swannell et al. 2014). Moreover, self-harm is the eighth leading cause of death in the US (Rockett and Caine 2015). Recently, both a suicidal behaviour disorder and NSSI disorder were included for the first time in the DSM-5 as separate conditions for further study (American Psychiatric Association 2013).
Several studies indicate that being depressed dramatically increases the risk to engage in both non-suicidal (Glenn and Klonsky 2013; Hankin and Abela 2011; Hawton et al. 2013; Keenan et al. 2014; Nock et al. 2006; Prinstein et al. 2010; Selby et al. 2012) and suicidal self-injurious behaviours (Bernal et al. 2007; Brown et al. 2000; Casey et al. 2006; Fergusson et al. 2005; Hawton et al. 2013; Sokero et al. 2005; Thompson and Light 2011; Tidemalm et al. 2008). A meta-analysis of psychiatric disorders in patients presenting to hospital following self-harm, showed that about 50 % of individuals also suffered from depression (Hawton et al. 2013). In another study of more than 20,000 suicide cases, depression and borderline personality disorder were associated with the greatest increase in suicide risk (Qin 2011). The fact that depression is a major risk factor for self-injury may come as no surprise given that depression often includes symptoms such as hopelessness, negative affect, or recurrent thoughts of death and suicide (American Psychiatric Association 2013). Depression and self-injurious behaviours may be partly influenced by environmental factors, such as stressful life events (e.g., abuse/assault, interpersonal problems or employment difficulties; Haw and Hawton 2008; Kendler et al. 1999). However, genetic factors also contribute heavily to both self-injurious behaviours and depression. Twin studies indicate that between 30 and 60 % of the variance in non-suicidal and suicidal self-injurious behaviours and depression are attributable to genes (Durrett 2006; Kendler et al. 2006; Maciejewski et al. 2014; Sullivan et al. 2000; Voracek and Loibl 2007; Wray and Gottesman 2012). The identification of specific genetic variants for depression and self-injurious behaviours has so far achieved limited success (Galfalvy et al. 2011; Hek et al. 2013; Major Depressive Disorder Working Group of the Psychiatric 2013; Mullins et al. 2014; Schosser et al. 2011; Sokolowski et al. 2014; Willour et al. 2012), although some SNPs have been identified for major depression (Converge Consortium 2015) and depressive symptoms (Okbay et al. 2016).
As both traits are substantially heritable, the association between depression and self-injurious behaviours may be partly due to overlapping genetic risk. Twin studies have reported significant genetic correlations between suicidal ideation and depression (Linker et al. 2012), and between NSSI and internalizing disorders and suicide attempts and internalizing disorders (Durrett 2006). Advances in data availability (due to economically viable genome-wide genotyping) and methodology now allow an alternative means to directly test whether the genetic variance in depression also predicts an increased risk for self-injurious behaviours. Estimating the cumulative risk conferred by multiple risk alleles is referred to as polygenic risk scoring and has become increasingly popular for understanding both the genetic architecture of traits and the covariance between different traits (Dudbridge 2013).
Recently, a study has tested the association of polygenic risk scores for major depressive disorder with suicidal ideation and attempts in several target samples of mood disorder patients (Mullins et al. 2014). The study showed that polygenic risk scores for depression predicted suicidal ideation in a target sample of 747 individuals with up to 1 % explained variance. The prediction results for suicide attempts were inconsistent; the association did not reach significance in the three individual target datasets, and was significant only for two of the five subtests when combining the three datasets (up to 0.3 % explained variance; each subtest used different p value thresholds for inclusion of genetic risk variants for depression to create the polygenic risk scores). Findings suggest that depression and suicidal behaviours share some degree of genetic pleiotropy, but associations with suicide attempts were weak and replication may thus be warranted to strengthen the findings.
In this study we aim to replicate and extend the findings of Mullins et al. (2014) by performing polygenic risk scoring analyses to test whether the aggregated effects of common genetic variants underlying depression symptom count can predict suicidal ideation, suicide attempts and NSSI in a large population-based sample of 6237 individuals. Contrary to Mullins et al. (2014), whose polygenic risk scores were derived from the Psychiatric Genomics Consortium (PGC, N = 9240 mood disorder cases and 9519 controls; Major Depressive Disorder Working Group of the Psychiatric 2013), the polygenic risk scores for the present study were based on results from a genome-wide association study on depressive symptom count (N = 34,549; Hek et al. 2013). We used this continuous measure, because depression lies on a continuum and even sub-threshold depression is associated with increased suicidal risk (Ayuso-Mateos et al. 2010; Fergusson et al. 2005; Lewinsohn et al. 2000) and continuous measures—rather than clinical cut-offs—provide increased statistical power in genetic studies on psychopathology (van der Sluis et al. 2013). We used a considerably larger target sample for suicidal ideation than in the Mullins et al. study (2014; N = 6236 vs N = 747), providing greater power to accurately estimate genetic prediction. Importantly, this is the first study to estimate whether a genetic predisposition to depressive symptoms is associated with increased prevalence of NSSI.
Methods
Sample
The target sample consisted of twins and their family members from the Australian Twin Registry, a population-based twin registry. Between 1992 and 2009 these individuals participated in various semi-structured telephone interviews focused primarily on psychiatric disorders; details of the individual studies can be found elsewhere (Heath et al. 1999, 2011; Knopik et al. 2004). In all studies, the same items about NSSI, suicidal ideation, and suicide attempt were included.
Our final sample with both genotype and phenotype data comprised 6237 participants (2497 males and 3740 females) from 3473 families, including 2115 monozygotic and 2609 dizygotic twins and 1513 other family members. The participant’s age at the time of the survey ranged from 19 to 89 years (M = 42.40, SD = 11.67).
Measures
Lifetime NSSI, suicidal ideation and suicide attempts were assessed as part of the SSAGA (Semi-Structured Assessment for the Genetics of Alcoholism), which assesses alcoholism and related disorders. The SSAGA has been shown to have good reliability and validity (Bucholz et al. 1994; Hesselbrock et al. 1999). The item used to determine lifetime NSSI was: “Did you ever hurt yourself on purpose, for example, by cutting or burning yourself?”1; the item used to determine suicidal ideation was: “Have you ever thought about taking your own life?” and the item used to determine suicide attempts was: “Have you ever tried to take your own life?”. All items were dichotomous (0 = no, 1 = yes). Complete data for NSSI, suicidal ideation, and suicide attempts were available for 4223, 6236, and 6226 individuals, respectively. Note that one cohort did not receive the question on NSSI, which accounts for the differences in sample size.
Genotyping and quality control
DNA samples were collected in accordance with standard protocols and genotyped on various Illumina single nucleotide polymorphism (SNP) platforms (I317 K; I370 K-Duo; I370 K-Quad; I610 K-Quad; I660 K). Standard platform specific quality control procedures (described elsewhere; Medland et al. 2009) were applied before imputation, including checks for ancestry outliers, minor-allele frequency (MAF), Hardy–Weinberg Equilibrium (HWE), Mendelian errors and individual and SNP call rate. SNPs were then imputed using MACH v1 reference data from HapMap (Phases I and II, Release 22 Build 36). Subsequently, we performed a second round of quality control in which we deleted individuals with a call rate <95 % and all SNPs with an imputation quality of r2 < 0.30, MAF < 0.01, HWE test p value < 0.0001, or a call rate <95 %. We also checked for strand-flips and included only SNPs that were present in the summary statistics of the full meta-analysis.
Generation of the polygenic scores and risk prediction analysis
Polygenic risk scores for each individual in the target sample were calculated in PLINK (Purcell et al. 2007) using the p values and z-scores (converted into betas) obtained from the summary statistics from the meta-analysis on depressive symptom count (Hek et al. 2013). The polygenic scores for individuals in the target sample were constructed by multiplying the number of copies of the effect allele at each SNP by the regression beta weight, and summing across SNPs. Scores were generated for nine different p value cut-offs for inclusion of risk variants for depressive symptoms (i.e P values of the association results for the SNPs): 0.001, 0.01, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5 and 1.0. Before generating the polygenic scores, we identified ‘independent’ signals of association in the GWA meta-analysis results, using the linkage disequilibrium based “clumping” procedure as implemented in PLINK (Purcell et al. 2007). We used an LD threshold of r2 = 0.25 within a 250-kb window and the clumping procedure was repeated for all nine different risk prediction cut-offs (i.e. from p < 0.001 to p < 1.0), using the corresponding p value threshold (‘–clump-p1’). The number of SNPs that were retained for the generation of the risk scores ranged from N = 650 (for the significance threshold of p < 0.001) to N = 194,886 (for p = 1).
The risk prediction analysis was performed in SPSS version 23 using generalized estimation equations (GEE) with a logit link function. To account for relatedness of the sample, an exchangeable conditional covariance matrix was used (which allows for correlated residuals between members of the same family) and tests were based on the robust sandwich-corrected standard errors (Minică et al. 2015). We included sex, age, birth cohort (before 1951, 1951–1965, after 1965), and the first ten principal components of genetic variation (to correct for ancestry effects) as covariates in the analyses. Variance explained by the polygenic scores was calculated (in a logistic regression in SPSS) as the Nagelkerke’s pseudo-R 2 of the model including polygenic scores and all covariates minus the Nagelkerke’s pseudo-R 2 of the model including only covariates.
Results
Descriptive statistics
The overall prevalence for NSSI, suicidal ideation, and suicide attempts were 3.2, 27.1, and 4.0 %, respectively. Men and women did not differ on NSSI prevalence, χ2(1) = 0.01, p = 0.95. Men reported significantly more suicidal thoughts, 29.4 % versus 25.6 %, χ2(1) = 10.72, p = 0.001, whereas women reported significantly more suicide attempts, 4.5 versus 3.1 %, χ2(1) = 7.47, p = 0.006. Moreover, lifetime NSSI, suicidal ideation, and suicide attempts were all associated with significantly younger age, all ps < 0.003. The phi coefficients between NSSI and suicidal thoughts and attempts were φ = 0.19 and φ = 0.21 respectively, and φ = 0.33 between suicidal thoughts and suicide attempts (all ps < 0.001).
Prediction
Results from the polygenic risk score analyses are shown in Fig. 1. The polygenic risk scores for depressive symptoms were significantly positively associated with suicidal ideation in our target sample for all significance thresholds from p < 0.05 upwards, with the risk scores explaining between 0.10 and 0.16 % of the variance in suicidal ideation. This indicates that individuals with a genetic predisposition to depression are also significantly more likely to experience suicidal ideation.
Fig. 1.
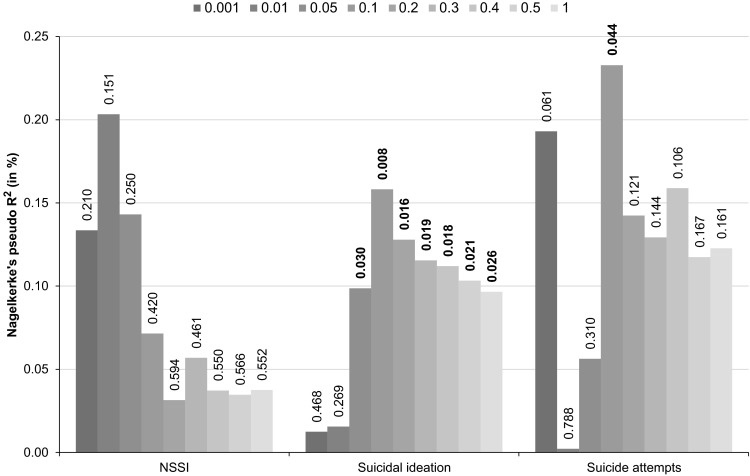
Results of polygenic risk score analysis. Variance explained (Nagelkerke’s pseudo R2) in NSSI, suicidal ideation, and suicide attempts by polygenic risk scores derived from depressive symptoms. Polygenic scores were created using nine significance thresholds for the inclusion of risk variants for depressive symptoms, ranging from p = 0.001 to p = 1.0. Values on the outside end of each bar denote the corresponding p value of the risk score prediction. p values at p < 0.05 are printed in bold
The risk scores also significantly predicted suicide attempts at the significance threshold of p < 0.1, for which the risk scores explained 0.23 % of the variance in suicide attempts. However, prediction did not reach significance for any of the other p value thresholds, even though the variance explained for suicide attempts was between 0.12 and 0.23 % (for the thresholds from p < 0.1 to p < 1.0), which is comparable to the variance explained in suicidal ideation. Depressive symptoms risk scores did not significantly predict variance in NSSI in our target sample under any of the significance thresholds, with estimates of variance explained ranging between 0.03 and 0.20 %.
Discussion
Using a sample of 6,237 adults, we examined the extent to which polygenic scores for depressive symptoms were associated with an increased risk for suicidal ideation, suicide attempts, and NSSI. Polygenic scores for depressive symptoms consistently predicted suicidal ideation (from significance thresholds of p < 0.05 upwards for inclusion of risk variants for depressive symptoms). When including fewer SNPs from the depressive symptom count GWAS (significance threshold of p < 0.001 or p < 0.01), risk scores were not able to predict suicidal ideation or attempt, emphasizing the polygenic architecture of the traits. Some predictive ability was found for suicide attempts, although only one of the significance thresholds reached significance and thus results should be treated with caution. The genetic risk scores explained a comparable amount of variance in suicidal ideation (between 0.10 and 0.16 %) and suicide attempts (between 0.12 and 0.23 %; for significance thresholds of p < 0.1 upwards). However, our sample provided less power for suicide attempts, due to a lower number of cases. Importantly, we estimated for the first time whether a genetic predisposition to depressive symptoms is associated with increased prevalence of NSSI, and we found that polygenic risk scores did not predict NSSI. Altogether, our results suggest that individuals who are genetically vulnerable to depressive symptoms are at higher risk for suicidal thoughts and potentially suicide attempts, whereas no evidence was found for NSSI.
Our results for suicidal ideation and suicide attempt are consistent with findings from another recent study in which risk scores for major depressive disorder were found to be associated with suicidal ideation and attempts in patients with mood disorders, although the association with attempts only reached significance when combining the three target datasets (Mullins et al. 2014). Here we showed that the association with suicidal ideation (and to a lesser extent with suicide attempts) is also present in a population-based sample (as opposed to a sample selected for major depressive disorder) and using genetic risk scores for a continuous measure of depressive symptoms rather than a dichotomous measure of depressive disorder. Although GWA studies have not been very successful in identifying replicable genetic variants for major depressive disorder (Major Depressive Disorder Working Group of the Psychiatric 2013; although see Converge Consortium 2015) or depressive symptoms (Hek et al. 2013), we and others (Mullins et al. 2014) have shown that the aggregate effect of many common genetic variants underlying depression can explain a significant part of individuals’ liability to suicidal self-injurious thoughts and behaviours. This evidence provides additional support for current diagnostic criteria for major depressive disorder, which include suicidal ideation and behaviours.
We performed the first genetic risk prediction study for NSSI. Although twin studies have indicated a genetic overlap between NSSI and internalizing disorders (Durrett 2006), with the genetic risk prediction methodology, we did not find evidence that a genetic predisposition to depressive symptoms was associated with an increased risk for NSSI. Estimates of explained variance (between 0.03 and 0.07 % for significance thresholds of p < 0.1 upwards) are somewhat lower than the explained variance for suicide attempts and ideation. This may indicate a relatively lower genetic covariation between depressive symptoms and NSSI. Indeed, other studies have indicated that, on the phenotypic level, the association of depression with NSSI is somewhat lower than with suicidal behaviours (Claes et al. 2010; Csorba et al. 2009; Dougherty et al. 2009). Additionally, while our target sample was quite large (N = 4223), the sample was smaller than for the other two variables and the prevalence of NSSI was relatively low, so the absence of a significant association may also be the result of reduced power to detect small associations.
Some limitations need to be taken into account. First, the phenotypes were based on single-item questions, which may have introduced measurement error. For instance, with regard to NSSI, we did not have data on the types of NSSI and thus the category may contain also individuals which have engaged in less severe NSSI (e.g., single instances of hair pulling). Similarly, we did not differentiate between brief and sustained suicidal thoughts. These rather heterogeneous categories may therefore limit the power to detect polygenic effects. Indeed, the prevalence of suicidal ideation was relatively high in our sample (27.1 %), higher than in other studies (Nock et al. 2008). However, the prevalences of NSSI and suicide attempts (3.2 and 4.0 %, respectively) were comparable to other population studies in adults (Nock et al. 2008; Swannell et al. 2014). Second, although our risk scores can explain a significant amount of the vulnerability to suicidal self-injurious behaviours, the explained variance was very low, with the highest variance explained being 0.16 % for suicidal ideation and 0.23 % for suicide attempts. This is lower than in the study of Mullins et al. (2014), whose major depressive disorder risk scores explained up to 1 % of the variance in suicidal ideation and 0.3 % in suicide attempts. However, polygenic risk scores for depression can so far only explain up to 1 % variance in depression itself (Converge Consortium 2015; Demirkan et al. 2011; Major Depressive Disorder Working Group of the Psychiatric 2013; Peyrot et al. 2014). The accuracy in risk prediction studies depends largely on the size of the discovery sample from which SNP effects are used to create polygenic scores. Very large discovery samples are needed to accurately estimate single SNP effects (Wray et al. 2013), because summing the estimates of SNP effects also sums the error of those estimates, creating statistical noise. While we used the results from the largest GWAS meta-analysis of depression related phenotypes to date, larger samples will provide more accurate estimates of SNP effects leading to more accurate risk prediction. The Psychiatric Genomics Consortium is currently performing a second major depressive disorder GWA meta-analysis with a larger sample size; future studies using these results may be able to explain a larger part of the variation. Moreover, a recent study suggests that SNP effects for depression are more accurate when using a more homogenous depression measure. This study was the first to identify two loci of major depressive disorder, which the authors attributed to the inclusion of only homogenous cases with severe and recurrent depression episodes (Converge Consortium 2015). However, even a small amount of variance explained in a phenotype can have a critical influence on the development of a particular condition, especially if the genetic predisposition is a necessary precursor to the development of the condition.
Here, the combined effects of thousands of major depressive disorder SNPs only explained a small part of the genetic variation in self-injurious behaviours. It is possible that part of the genetic variation in self-injurious behaviours (and its genetic covariance with depression) is due to genetic variance not taken into account in the current risk-prediction methodology which only focusses on SNPs, i.e. common genetic variants. Future studies may also benefit from accounting for rare genetic variants and non-additive genetic effects. On the other hand, Lubke et al. (2012) showed that the vast majority of genetic variation underlying major depressive disorder could be attributed to common additive genetic effects.
Moreover, NSSI and suicidal ideation are likely to capture genetic liability to several traits in addition to depression. For instance, neuroticism (highly genetically correlated with depression, r g = 0.75; Okbay et al. 2016), anxiety, aggression, impulsivity, psychosis, and schizophrenia may also play an important role in self-harm behaviours (Alaräisänen et al. 2009; Chioqueta and Stiles 2005; Dumais et al. 2005; Haw et al. 2001; Kiekens et al. 2015; Koyanagi et al. 2015; Mc Closkey et al. 2012; Sareen et al. 2005). Therefore, it would be interesting to investigate the genetic association between self-harm and other psychiatric disorders or traits in future risk prediction studies.
Further, environmental factors play a role in depression and self-injurious behaviours (Haw and Hawton 2008; Kendler et al. 1999). Thus, future studies could also examine how the environment moderates the genetic risk for depression to explain more variance in suicidal behaviours. A recent study found that the main effect of polygenic risk scores for depression as well as the interaction between the polygenic risk scores with the environment (childhood trauma) explained a comparable amount of variance in major depressive disorder (both 0.5 %; Peyrot et al. 2014).
In sum, the present study showed that a genetic predisposition to depressive symptoms plays a role in the pathogenesis of suicidal ideation, and possibly also in suicide attempts. We, however, did not find evidence that this is also the case for NSSI. Our results further emphasize the polygenic nature of complex psychiatric traits; like other mental disorders, self-injurious behaviours are likely to be due to the aggregate effect of many genetic variants, each of which on its own has a very small effect size. Future studies based on results from larger discovery samples, using larger target samples, and methodologies taking into account non-additive genetic effects and rare genetic variants as well as interactions between genes and environment may be able to further disentangle the (genetic) correlation between depression and self-injurious behaviours.
Acknowledgments
We thank Anjali Henders, Richard Parker, Soad Hancock, Judith Moir, Sally Rodda, Pieta-Maree Shertock, Heather Park, Jill Wood, Pam Barton, Fran Husband, Adele Somerville, Dixie Statham, David Smyth, Harry Beeby, and Daniel Park. Last, we thank the twins and their families for their participation.
Financial support
Supported by National Institutes of Health Grants AA013326, AA07535, AA0758O, AA07728, AA10249, AA13320, AA13321, AA14041, AA11998, AA17688, DA00272, DA012854, DA07261, DA018267, DA018660, DA23668 and DA019951; by Grants from the Australian National Health and Medical Research Council (241944, 339462, 389927, 389875, 389891, 389892, 389938, 442915, 442981, 496739, 552485, 552498, 628911 and 1047956); by Grants from the Australian Research Council (A7960034, A79906588, A79801419, DP0770096, DP0212016 and DP0343921); and by the 5th Framework Programme (FP-5) GenomEUtwin Project (QLG2-CT-2002-01254). This research was further supported by the Centre for Research Excellence on Suicide Prevention (CRESP-Australia). KJHV is supported in part by a 2014 NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation.
Compliance with Ethical Standards
Conflict of interest
Dominique F. Maciejewski, Miguel E. Renteria, Abdel Abdellaoui, Sarah E. Medland, Lauren R. Few, Scott D. Gordon, Pamela A.F. Madden, Grant Montgomery, Timothy J. Trull, Andrew C. Heath, Dixie J. Statham, Nicholas G. Martin, Brendan P. Zietsch, and Karin J.H. Verweij declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All participants provided verbal informed consent and procedures were approved by the Human Studies Committee at Washington University and the Ethics Committee at Queensland Institute of Medical Research.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation.
Footnotes
If participants endorsed the suicide attempt items, then the NSSI item started with “Other than when you tried to take your own life”.
Edited by Stephen Maxson.
References
- Alaräisänen A, Miettunen J, Räsänen P, Fenton W, Koivumaa-Honkanen H-TJ, Isohanni M. Suicide rate in schizophrenia in the Northern Finland 1966 birth cohort. Soc Psychiatry Psychiatr Epidemiol. 2009;44(12):1107–1110. doi: 10.1007/s00127-009-0033-5. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- Ayuso-Mateos JL, Nuevo R, Verdes E, Naidoo N, Chatterji S. From depressive symptoms to depressive disorders: the relevance of thresholds. Br J Psychiatry. 2010;196(5):365–371. doi: 10.1192/bjp.bp.109.071191. [DOI] [PubMed] [Google Scholar]
- Bernal M, Haro JM, Bernert S, Brugha T, de Graaf R, Bruffaerts R, et al. Risk factors for suicidality in Europe: results from the ESEMED study. J Affect Disord. 2007;101(1–3):27–34. doi: 10.1016/j.jad.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Brown GK, Beck AT, Steer RA, Grisham JR. Risk factors for suicide in psychiatric outpatients: a 20-year prospective study. J Consult Clin Psychol. 2000;68(3):371–377. doi: 10.1037/0022-006X.68.3.371. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock V, Nurnberger J, Jr, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55(2):149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Casey PR, Dunn G, Kelly BD, Birkbeck G, Dalgard OS, Lehtinen V, et al. Factors associated with suicidal ideation in the general population five-centre analysis from the ODIN study. Br J Psychiatry. 2006;189(5):410–415. doi: 10.1192/bjp.bp.105.017368. [DOI] [PubMed] [Google Scholar]
- Chioqueta AP, Stiles TC. Personality traits and the development of depression, hopelessness, and suicide ideation. Pers Individ Differ. 2005;38(6):1283–1291. doi: 10.1016/j.paid.2004.08.010. [DOI] [Google Scholar]
- Claes L, Muehlenkamp J, Vandereycken W, Hamelinck L, Martens H, Claes S. Comparison of non-suicidal self-injurious behavior and suicide attempts in patients admitted to a psychiatric crisis unit. Pers Individ Differ. 2010;48(1):83–87. doi: 10.1016/j.paid.2009.09.001. [DOI] [Google Scholar]
- Converge Consortium Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature. 2015;523(7562):588–591. doi: 10.1038/nature14659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csorba J, Dinya E, Plener P, Nagy E, Páli E. Clinical diagnoses, characteristics of risk behaviour, differences between suicidal and non-suicidal subgroups of hungarian adolescent outpatients practising self-injury. Eur Child Adolesc Psychiatry. 2009;18(5):309–320. doi: 10.1007/s00787-008-0733-5. [DOI] [PubMed] [Google Scholar]
- Demirkan A, Penninx BW, Hek K, Wray NR, Amin N, Aulchenko YS, et al. Genetic risk profiles for depression and anxiety in adult and elderly cohorts. Mol Psychiatry. 2011;16(7):773–783. doi: 10.1038/mp.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh-Richard DM, Prevette KN, Dawes MA, Hatzis ES, et al. Impulsivity and clinical symptoms among adolescents with non-suicidal self-injury with or without attempted suicide. Psychiatry Res. 2009;169(1):22–27. doi: 10.1016/j.psychres.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013;9(3):e1003348. doi: 10.1371/journal.pgen.1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais A, Lesage A, Alda M, Rouleau G, Dumont M, Chawky N, et al. Risk factors for suicide completion in major depression: a case-control study of impulsive and aggressive behaviors in men. Am J Psychiatry. 2005;162(11):2116–2124. doi: 10.1176/appi.ajp.162.11.2116. [DOI] [PubMed] [Google Scholar]
- Durrett C. A behavior genetic study of self-harm, suicidality, and personality in African American and white women [dissertation] Columbia: University of Missouri-Columbia; 2006. [Google Scholar]
- Fergusson DM, Horwood LJ, Ridder EM, Beautrais AL. Subthreshold depression in adolescence and mental health outcomes in adulthood. Arch Gen Psychiatry. 2005;62(1):66–72. doi: 10.1001/archpsyc.62.1.66. [DOI] [PubMed] [Google Scholar]
- Galfalvy H, Zalsman G, Huang Y-Y, Murphy L, Rosoklija G, Dwork AJ, et al. A pilot genome wide association and gene expression array study of suicide with and without major depression. World J Biol Psychiatry. 2011;14(8):574–582. doi: 10.3109/15622975.2011.597875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn CR, Klonsky ED. Nonsuicidal self-injury disorder: an empirical investigation in adolescent psychiatric patients. J Clin Child Adolesc Psychol. 2013;42(4):496–507. doi: 10.1080/15374416.2013.794699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abela JR. Nonsuicidal self-injury in adolescence: prospective rates and risk factors in a 2½ year longitudinal study. Psychiatry Res. 2011;186(1):65–70. doi: 10.1016/j.psychres.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haw C, Hawton K. Life problems and deliberate self-harm: associations with gender, age, suicidal intent and psychiatric and personality disorder. J Affect Disord. 2008;109(1–2):139–148. doi: 10.1016/j.jad.2007.12.224. [DOI] [PubMed] [Google Scholar]
- Haw C, Hawton K, Houston K, Townsend E. Psychiatric and personality disorders in deliberate self-harm patients. Br J Psychiatry. 2001;178(1):48–54. doi: 10.1192/bjp.178.1.48. [DOI] [PubMed] [Google Scholar]
- Hawton K, Saunders K, Topiwala A, Haw C. Psychiatric disorders in patients presenting to hospital following self-harm: a systematic review. J Affect Disord. 2013;151(3):821–830. doi: 10.1016/j.jad.2013.08.020. [DOI] [PubMed] [Google Scholar]
- Heath AC, Madden P, Bucholz K, Dinwiddie S, Slutske W, Bierut L, et al. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol Med. 1999;29(05):1069–1081. doi: 10.1017/S0033291799008909. [DOI] [PubMed] [Google Scholar]
- Heath AC, Whitfield JB, Martin NG, Pergadia ML, Goate AM, Lind PA, et al. A quantitative-trait genome-wide association study of alcoholism risk in the community: findings and implications. Biol Psychiatry. 2011;70(6):513–518. doi: 10.1016/j.biopsych.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hek K, Demirkan A, Lahti J, Terracciano A, Teumer A, Cornelis MC, et al. A genome-wide association study of depressive symptoms. Biol Psychiatry. 2013;73(7):667–678. doi: 10.1016/j.biopsych.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA-a comparison with the SCAN. Addiction. 1999;94(9):1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Keenan K, Hipwell AE, Stepp SD, Wroblewski K. Testing an equifinality model of nonsuicidal self-injury among early adolescent girls. Dev Psychopathol. 2014;26(3):851–862. doi: 10.1017/S0954579414000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am J Psychiatry. 2006;163(1):109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- Kiekens G, Bruffaerts R, Nock MK, Van de Ven M, Witteman C, Mortier P, et al. Non-suicidal self-injury among Dutch and Belgian adolescents: personality, stress and coping. Eur Psychiatry. 2015;30(6):743–749. doi: 10.1016/j.eurpsy.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Madden PA, Bucholz KK, Slutske WS, Nelson EC, et al. Genetic effects on alcohol dependence risk: re-evaluating the importance of psychiatric and other heritable risk factors. Psychol Med. 2004;34(08):1519–1530. doi: 10.1017/S0033291704002922. [DOI] [PubMed] [Google Scholar]
- Koyanagi A, Stickley A, Haro JM. Psychotic-like experiences and nonsuidical self-injury in England: results from a national survey. PLoS One. 2015;10(12):e0145533. doi: 10.1371/journal.pone.0145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Solomon A, Seeley JR, Zeiss A. Clinical implications of “subthreshold” depressive symptoms. J Abnorm Psychol. 2000;109(2):345–351. doi: 10.1037/0021-843X.109.2.345. [DOI] [PubMed] [Google Scholar]
- Linker J, Gillespie NA, Maes H, Eaves L, Silberg JL. Suicidal ideation, depression, and conduct disorder in a sample of adolescent and young adult twins. Suicide Life Threat Behav. 2012;42(4):426–436. doi: 10.1111/j.1943-278X.2012.00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubke GH, Hottenga JJ, Walters R, Laurin C, de Geus EJ, Willemsen G, et al. Estimating the genetic variance of major depressive disorder due to all single nucleotide polymorphisms. Biol Psychiatry. 2012;72(8):707–709. doi: 10.1016/j.biopsych.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejewski DF, Creemers HE, Lynskey MT, Madden PA, Heath AC, Statham DJ, et al. Overlapping genetic and environmental influences on nonsuicidal self-injury and suicidal ideation: different outcomes, same etiology? JAMA Psychiatry. 2014;71(6):699–705. doi: 10.1001/jamapsychiatry.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major Depressive Disorder Working Group of the Psychiatric GC A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry. 2013;18(4):497–511. doi: 10.1038/mp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Closkey MS, Look AE, Chen EY, Pajoumand G, Berman ME. Nonsuicidal self-injury: relationship to behavioral and self-rating measures of impulsivity and self-aggression. Suicide Life Threat Behav. 2012;42(2):197–209. doi: 10.1111/j.1943-278X.2012.00082.x. [DOI] [PubMed] [Google Scholar]
- Medland SE, Zhu G, Martin NG. Estimating the heritability of hair curliness in twins of European ancestry. Twin Res Hum Genet. 2009;12(5):514–518. doi: 10.1375/twin.12.5.514. [DOI] [PubMed] [Google Scholar]
- Minică CC, Dolan CV, Kampert MM, Boomsma DI, Vink JM. Sandwich corrected standard errors in family-based genome-wide association studies. Eur J Hum Genet. 2015;23(3):388–394. doi: 10.1038/ejhg.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins N, Perroud N, Uher R, Butler AW, Cohen-Woods S, Rivera M, et al. Genetic relationships between suicide attempts, suicidal ideation and major psychiatric disorders: a genome-wide association and polygenic scoring study. Am J Med Genet B. 2014;165(5):428–437. doi: 10.1002/ajmg.b.32247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Joiner TE, Jr, Gordon KH, Lloyd-Richardson E, Prinstein MJ. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144(1):65–72. doi: 10.1016/j.psychres.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Nock MK, Borges G, Bromet EJ, Alonso J, Angermeyer M, Beautrais A, et al. Cross-national prevalence and risk factors for suicidal ideation, plans and attempts. Br J Psychiatry. 2008;192(2):98–105. doi: 10.1192/bjp.bp.107.040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okbay A, Baselmans BML, De Neve J-E, Turley P, Nivard MG, Fontana MA, et al. Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat Genet. 2016;48(6):624–633. doi: 10.1038/ng.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrot WJ, Milaneschi Y, Abdellaoui A, Sullivan PF, Hottenga JJ, Boomsma DI, et al. Effect of polygenic risk scores on depression in childhood trauma. Br J Psychiatry. 2014;205:113–119. doi: 10.1192/bjp.bp.113.143081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinstein M, Heilbron N, Guerry J, Franklin J, Rancourt D, Simon V, et al. Peer influence and nonsuicidal self injury: longitudinal results in community and clinically-referred adolescent samples. J Abnorm Child Psychol. 2010;38(5):669–682. doi: 10.1007/s10802-010-9423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P. The impact of psychiatric illness on suicide: differences by diagnosis of disorders and by sex and age of subjects. J Psychiatr Res. 2011;45(11):1445–1452. doi: 10.1016/j.jpsychires.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Rockett IR, Caine ED. Self-injury Is the eighth leading cause of death in the United States: It is time to pay attention. JAMA Psychiatry. 2015;72:1069–1070. doi: 10.1001/jamapsychiatry.2015.1418. [DOI] [PubMed] [Google Scholar]
- Sareen J, Cox BJ, Afifi TO, de Graaf R, Asmundson GJ, ten Have M, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62(11):1249–1257. doi: 10.1001/archpsyc.62.11.1249. [DOI] [PubMed] [Google Scholar]
- Schosser A, Butler AW, Ising M, Perroud N, Uher R, Ng MY, et al. Genomewide association scan of suicidal thoughts and behaviour in major depression. PLoS One. 2011;6(7):e20690. doi: 10.1371/journal.pone.0020690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby EA, Bender TW, Gordon KH, Nock MK, Joiner TE., Jr Non-suicidal self-injury (NSSI) disorder: a preliminary study. Pers Disord. 2012;3(2):167–175. doi: 10.1037/a0024405. [DOI] [PubMed] [Google Scholar]
- Sokero TP, Melartin TK, Rytsälä HJ, Leskelä US, Lestelä-Mielonen PS, Isometsä ET. Prospective study of risk factors for attempted suicide among patients with DSM-IV major depressive disorder. Br J Psychiatry. 2005;186(4):314–318. doi: 10.1192/bjp.186.4.314. [DOI] [PubMed] [Google Scholar]
- Sokolowski M, Wasserman J, Wasserman D. Genome-wide association studies of suicidal behaviors: a review. Eur Neuropsychopharmacol. 2014;24(10):1567–1577. doi: 10.1016/j.euroneuro.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157(10):1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Swannell SV, Martin GE, Page A, Hasking P, St John NJ. Prevalence of nonsuicidal self-injury in nonclinical samples: systematic review, meta-analysis and meta-regression. Suicide Life Threat Behav. 2014;44(3):273–303. doi: 10.1111/sltb.12070. [DOI] [PubMed] [Google Scholar]
- Thompson MP, Light LS. Examining gender differences in risk factors for suicide attempts made 1 and 7 years later in a nationally representative sample. J Adolesc Health. 2011;48(4):391–397. doi: 10.1016/j.jadohealth.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Tidemalm D, Långström N, Lichtenstein P, Runeson B. Risk of suicide after suicide attempt according to coexisting psychiatric disorder: swedish cohort study with long term follow-up. Br Med J. 2008;337:1328–1331. doi: 10.1136/bmj.a2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluis S, Posthuma D, Nivard MG, Verhage M, Dolan CV. Power in GWAS: lifting the curse of the clinical cut-off. Mol Psychiatry. 2013;18(1):2–3. doi: 10.1038/mp.2012.65. [DOI] [PubMed] [Google Scholar]
- Voracek M, Loibl LM. Genetics of suicide: a systematic review of twin studies. Wien Klin Wochenschr. 2007;119(15–16):463–475. doi: 10.1007/s00508-007-0823-2. [DOI] [PubMed] [Google Scholar]
- Willour VL, Seifuddin F, Mahon PB, Jancic D, Pirooznia M, Steele J, et al. A genome-wide association study of attempted suicide. Mol Psychiatry. 2012;17(4):433–444. doi: 10.1038/mp.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Gottesman II. Using summary data from the danish national registers to estimate heritabilities for schizophrenia, bipolar disorder, and major depressive disorder. Front Genet. 2012;3:118. doi: 10.3389/fgene.2012.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Yang J, Hayes BJ, Price AL, Goddard ME, Visscher PM. Pitfalls of predicting complex traits from SNPs. Nat Rev Genet. 2013;14(7):507–515. doi: 10.1038/nrg3457. [DOI] [PMC free article] [PubMed] [Google Scholar]


