Abstract
Although several studies have demonstrated that facial-affect recognition impairment is common following moderate-severe traumatic brain injury (TBI), and that there are diffuse alterations in large-scale functional brain networks in TBI populations, little is known about the relationship between the two. Here, in a sample of 26 participants with TBI and 20 healthy comparison participants (HC) we measured facial-affect recognition abilities and resting-state functional connectivity (rs-FC) using fMRI. We then used network-based statistics to examine (A) the presence of rs-FC differences between individuals with TBI and HC within the facial-affect processing network, and (B) the association between inter-individual differences in emotion recognition skills and rs-FC within the facial-affect processing network. We found that participants with TBI showed significantly lower rs-FC in a component comprising homotopic and within-hemisphere, anterior-posterior connections within the facial-affect processing network. In addition, within the TBI group, participants with higher emotion-labeling skills showed stronger rs-FC within a network comprised of intra- and inter-hemispheric bilateral connections. Findings indicate that the ability to successfully recognize facial-affect after TBI is related to rs-FC within components of facial-affective networks, and provide new evidence that further our understanding of the mechanisms underlying emotion recognition impairment in TBI.
Keywords: Traumatic brain injury, Emotion recognition, Facial affect, Emotion labeling, Resting state, Network-based statistics
Highlights
-
•
Emotion recognition deficits are common following severe TBI.
-
•
TBI patients show reduced rs-FC within affect processing network.
-
•
Affect processing network rs-FC correlates with emotion recognition skills.
-
•
Rs-FC disruption as possible mechanism of emotion recognition deficit
1. Introduction
Among the many sequelae of traumatic brain injury (TBI), difficulties in social functioning are major predictors of overall outcome, posing a challenge for patients and clinicians (Morton and Wehman, 1995, Spikman et al., 2013). Several studies have linked overall social and communication impairment to the ability to successfully identify others' emotions from their facial expressions (Knox and Douglas, 2009, McDonald and Flanagan, 2004, Pettersen, 1991, Watts and Douglas, 2006), suggesting that poor interpersonal skill might be attributed—at least partially—to deficits in emotion perception and interpretation. Emotion recognition abilities show marked individual differences both within healthy (Germine and Hooker, 2011, Palermo et al., 2013, Tamamiya and Hiraki, 2013) and brain injury populations (Babbage et al., 2011, Rigon et al., 2016b, Rosenberg et al., 2014). Indeed, among groups of individuals with TBI that would otherwise be defined as homogeneous (i.e., as “moderate”, “severe”, or “moderate-severe” (Malec et al., 2007)), great variability in facial-affect recognition skills has been reported (Rigon et al., 2016b, Rosenberg et al., 2014), leading to a considerable challenge for clinicians attempting to predict deficit profiles and long-term interpersonal outcomes.
Successful treatment of social impairment represents an additional challenge: as traditional rehabilitation strategies have shown little success in improving social competence following TBI (McDonald et al., 2008, Ylvisaker et al., 2005), current research has attempted to develop complementary treatments that, instead of simply targeting and training a specific impaired behavior, focus on the additional improvement of the functionality of large-scale brain networks mapping onto cognitive, affective or motor functions through brain stimulation and lifestyle interventions (Barbey et al., 2015).
Several studies have attempted to link facial-affect recognition skills with site of focal brain lesions (e.g., frontal or medial temporal lobe lesions), with mixed results (Green et al., 2004, Martins et al., 2012, Spikman et al., 2012). However, recent work suggests that a better understanding of individual differences in TBI populations might be achieved by adopting a view of brain function as the product of functional communication between nodes of integrated networks, and not only of the structure of a specific brain region (Barbey et al., 2015). Indeed, TBI is a condition characterized by widespread axonal damage leading to disconnection within and between regions included in brain networks supporting different cognitive processes (Adams et al., 1982, Graham et al., 1988, Sharp et al., 2014). Structural connectivity following TBI has traditionally been examined using diffusion tensor imaging (DTI), which provides a way to assess integrity and directionality of white matter tracts traveling between nodes of a given brain network (Hulkower et al., 2013, Kennedy et al., 2009, Sharp and Ham, 2011). However, in the past decade several studies have employed resting state functional connectivity (rs-FC) measured with functional magnetic resonance imaging (fMRI) to investigate integrity of brain networks in TBI populations (Arenivas et al., 2014, Bonnelle et al., 2011, Marquez de la Plata et al., 2011, Palacios et al., 2013, Rigon et al., 2016a, Sharp et al., 2011). Rs-FC measures the correlation between fluctuations in the hemodynamic fMRI signal between regions throughout the brain that form large-scale brain networks (Biswal et al., 1995, Fox et al., 2005). A growing body of work supports a relationship between the functionality of these intrinsic networks assessed at rest and cognitive (e.g., executive functioning, processing speed, personality variable) and behavioral (e.g., tasks-modulation) processes in both healthy and clinical populations (Cox et al., 2012, Ham and Sharp, 2012, Hampson et al., 2006, Seeley et al., 2007).
To date, a wealth of studies have found abnormalities in both white matter integrity (Hulkower et al., 2013, Sidaros et al., 2008) and patterns of rs-FC in several large-scale brain networks in TBI populations (Rigon et al., 2016a, Stevens et al., 2012), as well as correlations between these measures and behavioral performance (Bonnelle et al., 2011, Ham et al., 2014, Sours et al., 2014, Rigon et al., 2016c). However, only one study has explored the relationship between white matter integrity and facial-affect recognition ability in individuals with moderate-severe TBI: Genova and colleagues (Genova et al., 2015) found that performance on emotion recognition tasks was positively correlated with fractional anisotropy of the inferior fronto-occipital and inferior longitudinal fasciculus, which connect visual regions with temporal and prefrontal areas involved in affective processing and decision making. Although no work has examined the relationship between rs-FC and facial-affect recognition abilities, a recent study by Neumann and colleagues compared blood oxygen level-dependent (BOLD) response during an emotion-labeling task between individuals with TBI and a matched comparison group (Neumann et al., 2015). The authors reported that individuals with TBI who had facial-affect recognition impairment showed less activation in the fusiform gyrus during an emotion-labeling task than those in the comparison group. This finding suggests that deficits in facial-affect recognition secondary to TBI might be related to functional abnormalities in specific brain areas associated with processing faces. However, participants with TBI who did not have facial affect recognition impairments also had lower scores than the comparison group, albeit not significantly, suggesting that changes in the fusiform gyrus alone might not explain all of the variance in affect recognition among adults with TBI. Given that structural disconnection is the hallmark of TBI, it is likely that functional disconnection between regions involved in the perception and interpretation of facial affects also plays a role in impaired facial-affect processing.
The current study aims to expand the work carried out to date on the structural and functional neural correlates on emotion recognition impairment following TBI by adopting a large-scale network perspective and by focusing on the neural mechanisms related to inter-individual differences within TBI individuals. Our analysis combines behavioral data (Rigon et al., 2016b) and rs-FC, measured using fMRI, in a sample of adults with moderate-severe TBI. Out of the scanner participants completed a dynamic emotion-labeling task. Our analysis focused on rs-FC within a set of brain regions that have been consistently found to be involved in processing facial affect as reported by a recent meta-analysis (Sabatinelli et al., 2011) (See Table 1). We hypothesized that there would be (A) differences between individuals with TBI and healthy comparison participants in rs-FC within regions involved in processing facial affect, and (B) an association between inter-individual differences in emotion recognition skills and rs-FC within regions involved in processing facial affect.
Table 1.
ROI coordinates for the facial affect processing network.
| ROIs |
Original coordinates |
Modified coordinates |
Role in facial affect processing |
||||
|---|---|---|---|---|---|---|---|
| Axis | x | y | z | x | y | z | |
| Medial prefrontal cortex | 4 | 47 | 7 | Emotional/reward processing | |||
| Right inferior frontal gyrus | 42 | 25 | 3 | Processing of emotional stimuli | |||
| Left inferior frontal gyrus | − 42 | 25 | 3 | ||||
| Right middle frontal gyrus | 48 | 17 | 29 | Emotion regulation | |||
| Left middle frontal gyrus | − 42 | 13 | 27 | ||||
| Superior frontal gyrus | − 2 | 8 | 59 | ||||
| Right amygdala | 20 | − 4 | − 15 | 22 | − 8 | − 14 | Multimodal emotion processing, perception of arousing stimuli, facial identification |
| Left amygdala | − 20 | − 6 | − 15 | − 26 | − 10 | − 14 | |
| Right middle temporal gyrus | 53 | − 50 | 4 | Discrimination of expressive faces | |||
| Left parahippocampal gyrus | − 20 | − 33 | − 4 | Basic perception of human faces (increased activation for emotional than for neutral faces) | |||
| Right parahippocampal gyrus | 14 | − 33 | − 7 | 18 | − 38 | − 8 | |
| Right fusiform gyrus | 38 | − 55 | − 20 | 38 | − 56 | − 16 | |
| Left fusiform gyrus | − 40 | − 55 | − 22 | − 40 | − 56 | − 18 | |
| Right posterior fusiform gyrus | 38 | − 76 | − 16 | ||||
| Left posterior fusiform gyrus | − 40 | − 78 | − 21 | − 42 | − 74 | − 18 | |
Original coordinates were reported by Sabatinelli et al. (2011), and reflect the activation peak for the contrast emotional faces > neutral faces obtained by a 100 studies activation likelihood estimation analysis. Coordinates are reported in mm and in standard MNI space. Coordinates were modified when maintain the original peak would result in a 7 mm-radius seed partially overlapping with cerebrospinal fluid, taking care that the newly centered seed would still include the original peak. ROI = Region of Interest. When no values are reported in the ‘Modified coordinates’ column the original coordinates were used. The “Role in Facial Affect Processing” column was compiled according to the interpretations of the meta-analysis findings of Sabatinelli et al.
2. Methods
2.1. Participants
Twenty-eight participants with TBI and twenty normal healthy comparison participants (HC) were recruited for this experiment. Individuals with TBI were recruited among the community of the University of Iowa. All individuals with TBI were in the chronic phase of their injury (> 6 months), and they had sustained a moderate-severe brain injury. TBI severity was determined through a combination of medical records and participant self-report, and assessed using the Mayo Classification Scale (Malec et al., 2007). Participants were defined as moderate-severe if one of the following criteria was met: (a) Glasgow Coma Scale < than 12, (b) presence of positive acute computer tomography findings or chronic intracranial abnormality defined as focal lesions visible on MRI, (c) loss of consciousness > 30 min, and d) post traumatic amnesia > 24 h (See Table 2). HCs were excluded if they had any previous history of head or brain injury, loss of consciousness or psychiatric and/or neurological problems per self-report. The groups did not differ for age, education or sex composition (all ps > 0.11, see Table 3). Within the TBI group, five participants reported a history of mood and/or anxiety disorders.
Table 2.
Severity characteristics of participants with TBI.
| ID | Etiology | GCS | PTA | LOC | Acute CT findings |
|---|---|---|---|---|---|
| 1 | Fall | 7 | 24 h | Duration unclear | Right temporal EDH, left temporal and frontal contusions, right tentorial SDH |
| 2 | Fall | N/A | N/A | N/A | Temporal and parietal fractures and contusions, right SDH and SAH |
| 3 | MVA | 3 | N/A | 10 days | Right frontal EDH, SAH, multiple fractures |
| 4 | Fall | N/A | 30 min | Minutes | Left SAH |
| 5 | Fall | 3 | 20 days | 2 weeks | SAH (required craniotomy) |
| 6 | Fall | 8 | 2 days | N/A | Bifrontal contusions (required craniotomy) |
| 7 | Fall | N/A | 1 day | 2 days | Basilar skull fracture |
| 8 | Fall | N/A | A few minutes | N/A | SAH |
| 9 | Fall | 15 | A few minutes | N/A | SDH |
| 10a | Fall/MVA | N/A/15 | N/A/N/A | 1 day/none | N/A/N/A |
| 11 | MVA | ||||
| 6 | Duration unclear | Duration unclear | SAH | ||
| 12 | Fall | N/A | No | A few minutes | SAH |
| 13 | Fall | 15 | A few minutes | A few minutes | Hemorrhagic contusions |
| 14 | Fall | N/A | A few hours | N/A | SAH |
| 15 | Fall | 15 | N/A | Duration unclear? | EDH, right temporal bone fracture (required craniotomy) |
| 16 | Assault | N/A | 2 months | 5 min | SAH, occipital skull fracture |
| 17 | Fall | N/A | 12 h | 4–5 h | SAH |
| 18 | MVA | N/A | N/A | Several hours | Intracranial hemorrhage (require craniotomy) |
| 19 | NVA | N/A | 2 weeks | A few minutes | N/A |
| 20 | NVA | N/A | 2 weeks | 20 min | Negative |
| 21 | MVA | 13 | N/A | 3–5 min | SAH |
| 22 | MVA | 15 | 2 weeks | Duration unclear | SAH |
| 23 | Fall | 3 | 2 days | Duration unclear | SDH |
| 24 | Fall | 15 | 3 h | Duration unclear | ICH |
| 25 | Fall | N/A | N/A | Duration unclear | SDH |
| 26 | MVA | 5 | N/A | 10 days (medically induced coma) | SAH |
| 27 | Fall | N/A | 2 days | 1 h | SAH |
| 28 | MVA | N/A | Several minutes | None | SDH |
MVA = motored vehicle accident; NVA = non-motored vehicle accident; PTA = post Traumatic Amnesia; LOC = loss of consciousness; SAH = subarachnoid hemorrhage; EDH = epidural hemorrhage; SDH = subdural hemorrhage.
Sustained two TBIs in two different occasions.
Table 3.
Demographic characteristics of participants.
| N | Age (mean ± SD) | Sex (males) | Education (mean ± SD) | Chronicity (months, mean ± SD) | |
|---|---|---|---|---|---|
| TBI | 26 | 50.92 ± 15.09 | 16 | 14.32 ± 2.25 | 73.54 ± 101.55 |
| HC | 20 | 52.65 ± 16.07 | 9 | 15.3 ± 1.66 | N/A |
| Group difference (P-value) | 0.71 | 0.56 | 0.11 | N/A |
TBI = traumatic brain injury; HC = normal healthy comparison participants; SD = standard deviation; N/A = not available.
Both participants with TBI and HCs were part of a larger-sample behavioral study examining the relationship between sex, neuropsychological impairment and emotion recognition skills following TBI (Rigon et al., 2016b).
2.2. Facial affect labeling task
The ability to label facial affect was tested using the short version of the Emotion Recognition Task (ERT). The ERT (Kessels et al., 2014, Montagne et al., 2007) is a computer-based facial-affect recognition task, in which participants are asked to label the affect of videos of faces first appearing neutral then gradually morphing to express one of six basic emotions (afraid, angry, disgusted, happy, neutral, sad, surprised). In this version of the test, ninety-six items (four for each emotion) morph from neutral to four levels of emotion intensities (0 to 40%, 60%, 80%, and 100%), with a fixed presentation order incrementally increases from lowest to highest intensities. For each morph, participants select the correct response between choices listed to the right of the stimulus (afraid, angry, disgusted, happy, sad, surprised). For the analysis here reported, the dependent variable was obtained by summing the number of accurate responses for each intensity and emotion. Group differences were calculated using a one-way analysis of covariance (ANCOVA), which allowed us to covary for presence of mood/anxiety disorders (because having had a psychiatric diagnosis constituted an exclusionary criterion for the HC group but not for participants with TBI, and mood and anxiety disorders have been previously associated with emotion recognition deficits (Bourke et al., 2010, Demenescu et al., 2010)).
2.3. Neuroimaging data acquisition
All neuroimaging data were collected at the University of Iowa Magnetic Resonance Facilities, on a 3T whole-body MRI scanner (Magnetom TIM Trio, Siemens Healthcare, Erlangen, Germany) with a 12-channel RF head receive coil.
High resolution T1-weighted brain images were acquired using a 3D Magnetization Prepared Rapid Gradient Echo Imaging (MPRAGE) protocol with 208 contiguous coronal slices, echo time (TE) = 3.04 ms, repetition time (TR) = 2530 ms, field of view (FOV) = 256 mm2, voxel size = 1 mm3 and flip angle = 10°.
T2*-weighted resting state data were collected with a fast echo planar imaging (EPI sequence) with BOLD contrast (6 min, TR = 2000 ms, TE = 30 ms, 31 slices acquired in ascending order, voxel size = 3.4 × 3.4 × 3.5 mm, 64 × 64 matrix, flip angle = 75°). Participants were instructed to keep their eyes closed and let their mind wander without falling asleep. Two participants with TBI were excluded from further analysis because of pronounced artifacts in the T1 image or very large regions of signal dropout in the EPI image, bringing the final TBI sample to N = 26.
2.4. Rs-FC data analysis
fMRI data preprocessing was carried out using FSL 5.0.4 (Smith et al., 2004), and included motion correction, brain extraction, spatial smoothing (FWHM = 6.0 mm) and denoising of EPI data (single-subject ICA with two raters, resulting in Cohen's kappa = 0.79, ‘Excellent’ (Fleiss and Chilton, 1983)), temporal filtering (0.008 < f < 0.08 Hz), nuisance regression (motion parameters, WM, CSF, and global signal) and motion scrubbing (Power et al., 2012)). EPI data were transformed into standard MNI 2-mm space by concatenating the nonlinear transform of the T1 image to MNI space (using the FSL Non-Linear Registration Tool) with the transform of EPI to the T1 image (using the boundary-based registration algorithm (Greve and Fischl, 2009)).
As the aim of this study was to examine the neural correlates of facial-affect recognition abilities following TBI, regions of interest (ROIs; also referred to here as seeds) were selected based on their relevance for facial-affect processing. Seeds were created by generating spherical ROIs (14 mm diameter) centered on the peak coordinates for the contrast emotional faces > neutral faces identified in a 100-study Activation Likelihood Estimation meta-analysis (Sabatinelli et al., 2011). For brevity, here we refer to the resulting group of fifteen seeds as the “facial-affect processing network”; the network includes prefrontal, medial temporal lobe, and occipital regions (for further detail on the specific role of each region in facial-affect processing, see Sabatinelli et al., 2011, and Adolphs, 2002). For six ROIs, preserving the reported peak MNI coordinates would have resulted in the 7 mm radius ROIs partially overlapping with tentorial space and/or being located partially outside of the brain. For this reason, coordinates were slightly shifted, always ensuring that the spherical seed would eventually include the original coordinates reported in the meta-analysis (see Table 1).
To calculate pairwise ROI-ROI correlations, for each subject MATLAB was used to compute the Pearson correlation coefficient between the timeseries of all possible ROI pairs, and to apply a Fisher's r-to-z transformation to convert each coefficient into Fisher's Z estimates (Zar, 1996). We then used the network based statistics (NBS) algorithm (Zalesky et al., 2012, Zalesky et al., 2010a) to identify components, or sub-systems, of connected ROI pairs with reliably different rs-FC between the TBI and HC groups, and for which individual differences in rs-FC were related to emotion recognition abilities within each group. Briefly, the NBS is a well-validated nonparametric statistical method used to control the family-wise error rate (FWER) when conducting a large amount of univariate statistics. In the present case, given the large number of possible connections (or edges) between 15 ROIs, NBS was chosen over more traditional multiple comparison correction methods (e.g., Bonferroni correction). The NBS method consists of three steps: (1) mass univariate testing (at every possible connection) occurs; (2) a primary threshold is chosen, and only connections exceeding said threshold are carried on for further testing; (3) topological clusters (not based on physical proximity but on their topological configuration) are identified among supra-threshold connections applying a FWER threshold. The creators of NBS emphasize how different primary thresholds can reveal different information about the nature of an effect (e.g., lower thresholds, such as Z = 1.65, will reveal widespread but weaker effects, while higher thresholds, such as Z = 3.11, reveal strong and very focal effects), and encourage researchers to experiment with different values. For this reason, here we report results obtained with three different primary test statistics (Z > 1.65, Z > 2.33 and Z > 3.1), and a subsequent FWER correction of p < 0.05; all results were obtained generating randomized data with 10,000 permutations.
The purpose of the NBS is to reveal specific networks (i.e., groups of links) associated with an experimental effect; however, the NBS does not test the relationship between single connections and said effects. For this reason, we accompanied each NBS analysis with a false discovery rate (FDR) procedure (Genovese et al., 2002), which examines the relationship between an experimental effect and each pairwise connection (setting significance at p < 0.05), in order to investigate the presence of local effects involving small numbers of ROI pairs.
As the TBI group included participants with a history of mood and anxiety disorders (which have been found to be associated with emotion recognition deficits) group differences in FC within the facial-affect processing network were calculated controlling for psychiatric diagnosis. Correlational analysis between FC and ERT were calculated both separately for TBI and HC, in order to examine whether neural correlated of facial-affect recognition differed between the two groups, and across-groups. Correlations between FC and ERT performance were corrected for psychiatric diagnosis as well as sex and age, which have been found to affect emotion recognition both in healthy (Kessels et al., 2014, Montagne et al., 2005) and TBI populations (Rigon et al., 2016b).
Here, the NBS Connectome toolbox (version 1.2) was used to perform all NBS and FDR analysis (https://www.nitrc.org/projects/nbs/). NBS results and functional connectivity maps are visualized with BrainNet Viewer (Xia et al., 2013).
3. Results
3.1. Facial-affect recognition
A one-way ANCOVA revealed that the TBI group (mean = 51.89, SD = 8.55) performed significantly worse than HCs (mean = 57.65, SD = 8.20) on the ERT (F1,47 = 5.77, p = 0.02, η2p = 0.11). There was not a significant effect of psychiatric diagnosis (F1,47 = 5.77, p = 0.54, η2p = 0.008). This result is consistent with our previous report on the larger sample in which the current participants were included (Rigon et al., 2016b) (see Fig. 1).
Fig. 1.
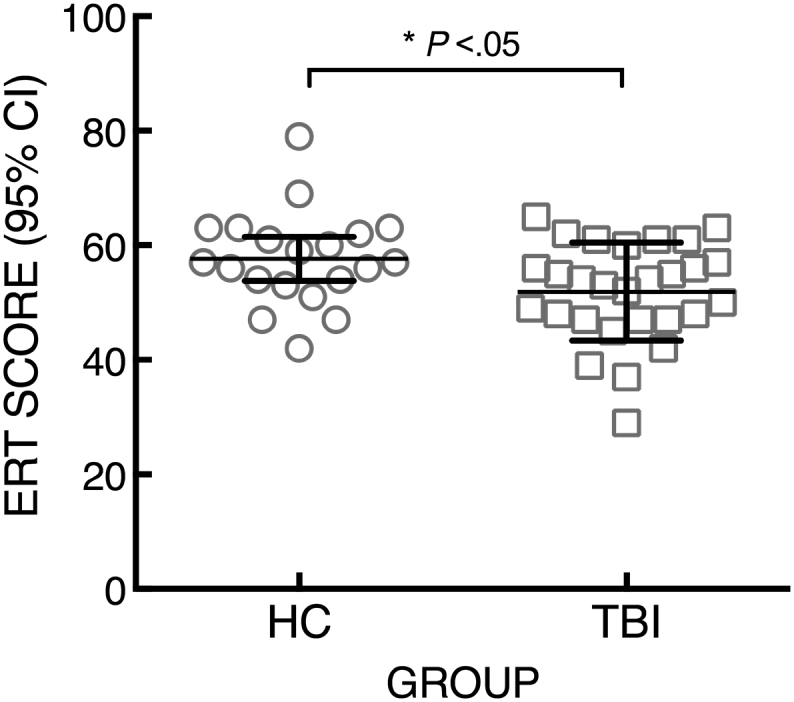
ERT performance of HC and TBI participants shows the distribution of ERT scores within the TBI and HC groups: a one-way ANCOVA, correcting for presence of mood disorders, revealed that participants with TBI showed significantly lower scores than HCs.
3.2. Functional connectivity analysis – group comparison
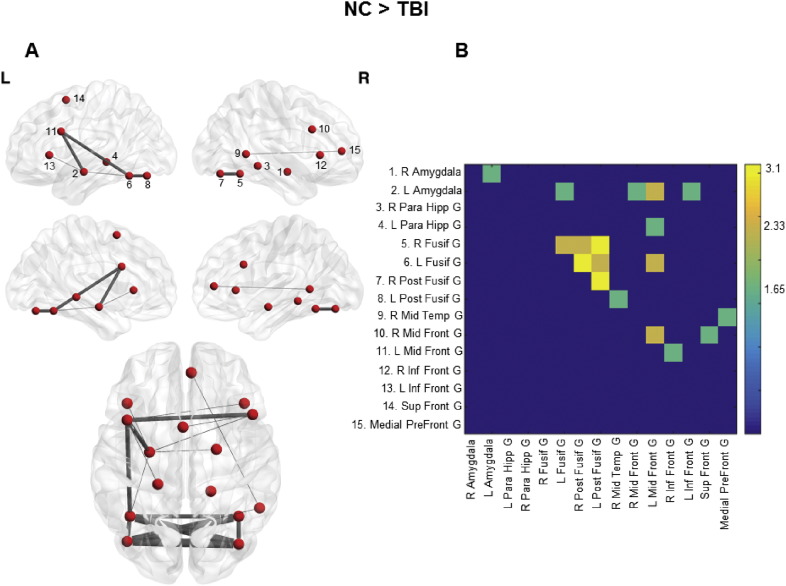
The NBS revealed one network component within the facial-affect processing network showing significantly lower FC in the TBI group when compared to the HC group. When the primary threshold was set to Z > 1.65, the disconnected sub-network included fourteen nodes and eighteen edges (p < 0.004). A visual inspection of the edges revealed that the component included homotopic connections (i.e., edges connecting the left and right fusiform gyri, amygdalae, and middle frontal gyri), as well as connections between posterior and anterior regions of the left hemisphere (e.g., connecting the left fusiform gyrus with the left amygdala and prefrontal cortex). Raising the primary threshold to Z > 2.33 revealed a more focal component (seven nodes, nine edges) comprising frontal and occipital homotopic connections as well as left fronto-occipital edges (p < 0.001). Lastly, the highest threshold (Z > 3.11) yielded a very restricted component, encompassing 4 nodes and 3 edges, all inter-hemispheric connections between anterior and posterior fusiform regions (p = 0.001) (see Fig. 2 for the specific links surviving each threshold).
Fig. 2.
NBS derived rs-FC component within the facial affect processing network. A shows the component obtained by using the NBS algorithm with the contrast HC > TBI (10,000 permutations, p < 0.05). The thickness of the edges represents the primary threshold: thinner edges are part of the widespread component that resulted by setting Z > 1.65 as threshold, while thicker lines represent the connections surviving a threshold of Z > 3.11. 2B displays the adjacency matrix for each threshold.
When we examined group differences on a link-by-link bases (using FDR correction), we found that three edges were significantly weaker within the TBI group than the HC group: right to left posterior fusiform gyrus, right posterior fusiform gyrus to left fusiform gyrus, and left posterior fusiform gyrus to right fusiform gyrus.
NBS and FDR analyses revealed no components or links showing significantly higher rs-FC in the TBI group.
3.3. Functional connectivity analysis – within group correlations
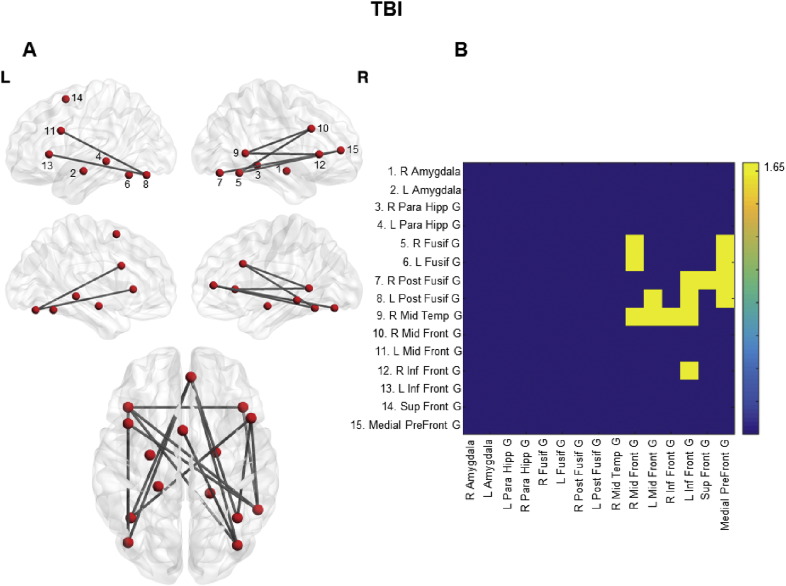
Within the TBI group, when the primary threshold was set to Z > 1.65 the NBS revealed a component showing higher FC in participants with higher emotion recognition abilities (p = 0.01). The component included eleven nodes and fifteen edges: visual inspection revealed a large number of intra- and inter-hemispheric connections between anterior and posterior nodes of the facial-affect processing network. When the primary threshold was raised to 2.33 no components associated with ERT performance survived, indicating a widespread but weak effect. Similarly, pairwise link analysis using FDR correction revealed no pairs of nodes whose strength of FC significantly correlated with emotion recognition abilities. Lastly, both the NBS algorithm and the application of FDR correction revealed no components or single connections for which greater FC corresponded to lower ERT performance (see Fig. 3 for the specific links surviving each threshold). Within the HC group, there were no components or pairwise links showing a positive or negative correlation with ERT performance.
Fig. 3.
Correlation between emotion recognition abilities and rs-FC in the facial-affect processing network within the TBI group. A shows the component correlating with ERT performance obtained by using the NBS algorithm within the TBI group (10,000 permutations, p < 0.05), setting Z > 1.65 as preliminary threshold. No component survived with higher thresholds. 2B displays the adjacency matrix for the component depicted.
The same analyses were carried out across the TBI and NC groups, using ERT as a continuous regressor and adding group as a covariate of no interest. When TBI and NC groups were considered together, there were no components or links showing a correlation between strength of FC and emotion recognition abilities.
As relative head motion has been related to spurious rs-FC correlations (Power et al., 2012), we repeated all above analyses adding subject mean relative motion as a covariate of no interest. We found that for all analyses NBS results were unchanged. For the NC > TBI contrast, correcting for head motion revealed an additional surviving FDR corrected link: the edge between the right anterior and posterior fusiform gyri.
4. Discussion
Although impairment in the ability to recognize emotions has been frequently reported in TBI populations, very little is known about its relationship with network functionality. The aim of the current study was to examine how differences in large-scale network rs-FC correspond to facial-affect recognition abilities in individuals with and without TBI. We asked how individuals with TBI and demographically matched healthy comparison participants differed in rs-FC within what we refer to as the facial-affect processing network, a set of regions found to be involved in emotion recognition both by studies focusing on BOLD activation peaks (Fusar-Poli et al., 2009, Sabatinelli et al., 2011), and on functional connectivity during task performance (Hariri et al., 2000). We used the NBS algorithm, as it allowed us to examine the association between an effect of interest and connectivity between nodes by taking into account the large-scale networks in which said nodes participate (Zalesky et al., 2010b). We found that within the facial-affect processing network participants with TBI showed significantly lower rs-FC in a component comprising homotopic and within-hemisphere, anterior-posterior bidirectional connections. We then separately examined the relationship between facial-affect recognition performance and rs-FC within the TBI and the HC groups. Although we found no correlations between rs-FC and ERT performance within the HC group, within the TBI group participants with higher emotion labeling skills also showed stronger FC within a network formed of intra- and inter-hemispheric bilateral connections. Below we discuss each finding in depth.
To date, several studies have reported lower rs-FC within and between resting-state networks in chronic moderate-severe TBI populations (Han et al., 2016, Rigon et al., 2016a, Sharp et al., 2014). Most of this work has focused on examining the state of traditionally defined intrinsic networks, such as the default mode, salience, and executive networks (Smith et al., 2009). Here, we focused on rs-FC between regions that have been found to be specifically involved in facial-affect processing by task-related fMRI studies. In particular, occipito-temporal areas, such as the fusiform gyrus and the middle temporal gyrus, play a role in face perception (Haxby et al., 2000, Haxby et al., 2002), and presentation of emotion stimuli (as opposed to neutral faces) increases activation in these regions (Sabatinelli et al., 2011). The key role of the medial temporal lobe, and of the amygdalae in particular, in the interpretation of emotional faces has been well documented (Adolphs, 2002, Fusar-Poli et al., 2009, Hariri et al., 2000, Hariri et al., 2002). Last, prefrontal regions have been found to be especially involved in appraisal, reward, and conscious semantic aspects of facial-affect discrimination (Hariri et al., 2000, Sabatinelli et al., 2007).
Disruption of the structural connections among secondary visual areas and the amygdala and prefrontal cortex, in the form of the inferior longitudinal and fronto-occipital fasciculi, has been associated with emotion recognition skills both in individuals with TBI (Genova et al., 2015) and in other neurological populations (Philippi et al., 2009). Here, we found that rs-FC in a network comprising ipsilateral and contralateral connections between bilateral fusiform gyri seeds and bilateral and medial prefrontal cortex seeds was positively associated with emotion labeling performance within the TBI group. These findings mirror the structural results in DTI and lesion studies (Genova et al., 2015, Philippi et al., 2009). This association between poor performance on emotion recognition tasks and connectivity between visual and prefrontal regions suggests that abnormal rs-FC might serve as one of the mechanisms underlying the disruption of facial-affect recognition skills.
Interestingly, edges showing significantly lower rs-FC within the TBI group did not fully correspond to the edges correlating with ERT performance. In agreement with previous findings (Han et al., 2016, Marquez de la Plata et al., 2011, Sours et al., 2014), the TBI group displayed significantly lower inter-hemispheric FC between homotopic regions (the amygdalae, the fusiform gyri and the bilateral middle prefrontal gyri); and higher primary thresholds, as well as FDR correction, indicated that inter-hemispheric connectivity between the fusiform gyri represented the highest focus of lower rs-FC when comparing the TBI group with healthy comparison participants. The importance of the structural and functional integrity of secondary visual regions for facial-affect discrimination following TBI has previously been noted. In a structural study, Genova and colleagues used Voxel Based Morphometry to demonstrate that reduced gray matter volume in the parahippocampal and lingual gyrus was significantly related to emotion recognition skills in TBI (Genova et al., 2015); on a functional level, Neumann and colleagues found that in participants with TBI with facial-affect recognition impairment the only region showing significantly lower activation during an emotion labeling task was the fusiform gyrus (Neumann et al., 2015). In our study, the discrepancy of the results obtained by the contrast and the correlational analysis might indicate that a decrease in rs-FC within homotopic nodes of the affective network following TBI could lead to a reorganization of the connections supporting emotion recognition skills. It should also be noted that the disconnected subnetwork with lower FC within TBI also included intra-hemispheric connections among the fusiform gyrus, the amygdala, and the lateral prefrontal cortex: the interplay among these regions has been found to be specifically important for facial-affect labeling (as opposed, for example, to facial-affect matching) (Hariri et al., 2000). Our findings indicate that the breakdown of a specific sub-network is associated with emotion recognition deficits following TBI, and suggests that this network might be a target of future treatments such as non-invasive brain stimulation or lifestyle interventions.
Interestingly, we found no correlations between ERT performance and FC within the HC group. One possible explanation is that the neural correlates of emotion recognition abilities differ after TBI, because of functional reorganization in response to injury. Within the TBI group we were able to isolate one component for which rs-FC positively correlated with ERT scores. This component was not present when a higher significance threshold (p < 0.01) was applied, revealing a widespread but weak correlation with the experimental effect. Although the focus of the current study was to examine rs-FC within the facial-affect processing network, it is possible that some of the variance in ERT performance can be explained by rs-FC between regions of this network and nodes included in other systems. For example, the salience network, anchored in the anterior insula, has been found to be important in monitoring external stimuli (Menon and Uddin, 2010, Seeley et al., 2007), and the dorsal and ventral attention networks are respectively responsible for orienting one's focus and responding to relevant environmental events (Corbetta and Shulman, 2002). These systems might work in collaboration with areas involved in facial affect processing to successfully recognize emotions. Similarly, within healthy populations, who on average show better emotion labeling skills than individuals with TBI, inter-individual variance in these abilities might not be associated with within facial-affect processing network rs-FC, but with re-FC between this network and other large-scale systems. Future task-related studies should explore the role of inter-network functional connectivity using techniques such as psychophysiological interaction.
4.1. Conclusions
The current study examined how individual differences in rs-FC are associated with facial-affect labeling skills in adults with TBI. Compared to demographically matched comparison participants, individuals with TBI showed lower rs-FC within specific nodes of the facial-affect processing network, and in particular in homotopic and anterior-posterior pathways among secondary visual areas, the amygdalae, and lateral prefrontal cortices. In addition, we found that within the TBI group individuals with better emotion recognition skills showed higher rs-FC between ipsi- and contralateral occipital and prefrontal regions. These findings advance our understanding of the basic neural mechanisms underlying facial-affect recognition impairment in adults with TBI.
Acknowledgments
This work was supported by NICHD/NCMRR grant R01 HD071089, the University of Iowa Magnetic Resonance Research Facilities and a research grant from the University of Iowa Graduate and Professional Student Government. The authors wish to thank Ruth Hanson, Katherine Jones and Kristina Warndahl for their help in scheduling participants.
References
- Adams J.H., Graham D.I., Murray L.S., Scott G. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann. Neurol. 1982;12:557–563. doi: 10.1002/ana.410120610. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Neural systems for recognizing emotion. Curr. Opin. Neurobiol. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Arenivas A., Diaz-Arrastia R., Spence J., Cullum C.M., Krishnan K., Bosworth C., Culver C., Kennard B., Marquez de la Plata C. Three approaches to investigating functional compromise to the default mode network after traumatic axonal injury. Brain Imaging Behav. 2014;8:407–419. doi: 10.1007/s11682-012-9191-2. [DOI] [PubMed] [Google Scholar]
- Babbage D.R., Yim J., Zupan B., Neumann D., Tomita M.R., Willer B. Meta-analysis of facial affect recognition difficulties after traumatic brain injury. Neuropsychology. 2011;25:277–285. doi: 10.1037/a0021908. [DOI] [PubMed] [Google Scholar]
- Barbey A.K., Belli A., Logan A., Rubin R., Zamroziewicz M., Operskalski J.T. Network topology and dynamics in traumatic brain injury. Curr. Opin. Behav. Sci. 2015;4:92–102. [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bonnelle V., Leech R., Kinnunen K.M., Ham T.E., Beckmann C.F., De Boissezon X., Greenwood R.J., Sharp D.J. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J. Neurosci. 2011;31:13442–13451. doi: 10.1523/JNEUROSCI.1163-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke C., Douglas K., Porter R. Processing of facial emotion expression in major depression: a review. Aust. N. Z. J. Psychiatry. 2010;44:681–696. doi: 10.3109/00048674.2010.496359. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox C.L., Uddin L.Q., Di Martino A., Castellanos F.X., Milham M.P., Kelly C. The balance between feeling and knowing: affective and cognitive empathy are reflected in the brain's intrinsic functional dynamics. Soc. Cogn. Affect. Neurosci. 2012;7:727–737. doi: 10.1093/scan/nsr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demenescu L.R., Kortekaas R., den Boer J.A., Aleman A. Impaired attribution of emotion to facial expressions in anxiety and major depression. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiss J.L., Chilton N.W. The measurement of interexaminer agreement on periodontal disease. J. Periodontal Res. 1983;18:601–606. doi: 10.1111/j.1600-0765.1983.tb00397.x. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Placentino A., Carletti F., Landi P., Allen P., Surguladze S., Benedetti F., Abbamonte M., Gasparotti R., Barale F., Perez J., McGuire P., Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Genova H.M., Rajagopalan V., Chiaravalloti N., Binder A., Deluca J., Lengenfelder J. Facial affect recognition linked to damage in specific white matter tracts in traumatic brain injury. Soc. Neurosci. 2015;10:27–34. doi: 10.1080/17470919.2014.959618. [DOI] [PubMed] [Google Scholar]
- Genovese C.R., Lazar N.A., Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Germine L.T., Hooker C.I. Face emotion recognition is related to individual differences in psychosis-proneness. Psychol. Med. 2011;41:937–947. doi: 10.1017/S0033291710001571. [DOI] [PubMed] [Google Scholar]
- Graham D.I., Adams J.H., Gennarelli T.A. Mechanisms of non-penetrating head injury. Prog. Clin. Biol. Res. 1988;264:159–168. [PubMed] [Google Scholar]
- Green R.E., Turner G.R., Thompson W.F. Deficits in facial emotion perception in adults with recent traumatic brain injury. Neuropsychologia. 2004;42:133–141. doi: 10.1016/j.neuropsychologia.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Greve D.N., Fischl B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham T.E., Sharp D.J. How can investigation of network function inform rehabilitation after traumatic brain injury? Curr. Opin. Neurol. 2012;25:662–669. doi: 10.1097/WCO.0b013e328359488f. [DOI] [PubMed] [Google Scholar]
- Ham T.E., Bonnelle V., Hellyer P., Jilka S., Robertson I.H., Leech R., Sharp D.J. The neural basis of impaired self-awareness after traumatic brain injury. Brain. 2014;137:586–597. doi: 10.1093/brain/awt350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M., Driesen N.R., Skudlarski P., Gore J.C., Constable R.T. Brain connectivity related to working memory performance. J. Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K., Chapman S.B., Krawczyk D.C. Disrupted intrinsic connectivity among default, dorsal attention, and frontoparietal control networks in individuals with chronic traumatic brain injury. J. Int. Neuropsychol. Soc. 2016;22:263–279. doi: 10.1017/S1355617715001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R., Bookheimer S.Y., Mazziotta J.C. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri A.R., Tessitore A., Mattay V.S., Fera F., Weinberger D.R. The amygdala response to emotional stimuli: a comparison of faces and scenes. NeuroImage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Haxby J.V., Hoffman E.A., Gobbini M.I. The distributed human neural system for face perception. Trends Cogn. Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Haxby J.V., Hoffman E.A., Gobbini M.I. Human neural systems for face recognition and social communication. Biol. Psychiatry. 2002;51:59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- Hulkower M.B., Poliak D.B., Rosenbaum S.B., Zimmerman M.E., Lipton M.L. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am. J. Neuroradiol. 2013;34:2064–2074. doi: 10.3174/ajnr.A3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M.R., Wozniak J.R., Muetzel R.L., Mueller B.A., Chiou H.H., Pantekoek K., Lim K.O. White matter and neurocognitive changes in adults with chronic traumatic brain injury. J. Int. Neuropsychol. Soc. 2009;15:130–136. doi: 10.1017/S1355617708090024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels R.P., Montagne B., Hendriks A.W., Perrett D.I., de Haan E.H. Assessment of perception of morphed facial expressions using the emotion recognition task: normative data from healthy participants aged 8–75. J. Neuropsychol. 2014;8:75–93. doi: 10.1111/jnp.12009. [DOI] [PubMed] [Google Scholar]
- Knox L., Douglas J. Long-term ability to interpret facial expression after traumatic brain injury and its relation to social integration. Brain Cogn. 2009;69:442–449. doi: 10.1016/j.bandc.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Malec J.F., Brown A.W., Leibson C.L., Flaada J.T., Mandrekar J.N., Diehl N.N., Perkins P.K. The mayo classification system for traumatic brain injury severity. J. Neurotrauma. 2007;24:1417–1424. doi: 10.1089/neu.2006.0245. [DOI] [PubMed] [Google Scholar]
- Marquez de la Plata C.D., Garces J., Shokri Kojori E., Grinnan J., Krishnan K., Pidikiti R., Spence J., Devous M.D., Sr., Moore C., McColl R., Madden C., Diaz-Arrastia R. Deficits in functional connectivity of hippocampal and frontal lobe circuits after traumatic axonal injury. Arch. Neurol. 2011;68:74–84. doi: 10.1001/archneurol.2010.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins A.T., Faisca L., Esteves F., Simao C., Justo M.G., Muresan A., Reis A. Changes in social emotion recognition following traumatic frontal lobe injury. Neural Regen. Res. 2012;7:101–108. doi: 10.3969/j.issn.1673-5374.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald S., Flanagan S. Social perception deficits after traumatic brain injury: interaction between emotion recognition, mentalizing ability, and social communication. Neuropsychology. 2004;18:572–579. doi: 10.1037/0894-4105.18.3.572. [DOI] [PubMed] [Google Scholar]
- McDonald S., Tate R., Togher L., Bornhofen C., Long E., Gertler P., Bowen R. Social skills treatment for people with severe, chronic acquired brain injuries: a multicenter trial. Arch. Phys. Med. Rehabil. 2008;89:1648–1659. doi: 10.1016/j.apmr.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne B., Kessels R.P., Frigerio E., de Haan E.H., Perrett D.I. Sex differences in the perception of affective facial expressions: do men really lack emotional sensitivity? Cogn. Process. 2005;6:136–141. doi: 10.1007/s10339-005-0050-6. [DOI] [PubMed] [Google Scholar]
- Montagne B., Kessels R.P., De Haan E.H., Perrett D.I. The emotion recognition task: a paradigm to measure the perception of facial emotional expressions at different intensities. Percept. Mot. Skills. 2007;104:589–598. doi: 10.2466/pms.104.2.589-598. [DOI] [PubMed] [Google Scholar]
- Morton M.V., Wehman P. Psychosocial and emotional sequelae of individuals with traumatic brain injury: a literature review and recommendations. Brain Inj. 1995;9:81–92. doi: 10.3109/02699059509004574. [DOI] [PubMed] [Google Scholar]
- Neumann D., McDonald B.C., West J., Keiski M.A., Wang Y. Neurobiological mechanisms associated with facial affect recognition deficits after traumatic brain injury. Brain Imaging Behav. 2015 doi: 10.1007/s11682-015-9415-3. [DOI] [PubMed] [Google Scholar]
- Palacios E.M., Sala-Llonch R., Junque C., Roig T., Tormos J.M., Bargallo N., Vendrell P. Resting-state functional magnetic resonance imaging activity and connectivity and cognitive outcome in traumatic brain injury. JAMA Neurol. 2013;70:845–851. doi: 10.1001/jamaneurol.2013.38. [DOI] [PubMed] [Google Scholar]
- Palermo R., O'Connor K.B., Davis J.M., Irons J., McKone E. New tests to measure individual differences in matching and labelling facial expressions of emotion, and their association with ability to recognise vocal emotions and facial identity. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen L. Sensitivity to emotional cues and social behavior in children and adolescents after head injury. Percept. Mot. Skills. 1991;73:1139–1150. doi: 10.2466/pms.1991.73.3f.1139. [DOI] [PubMed] [Google Scholar]
- Philippi C.L., Mehta S., Grabowski T., Adolphs R., Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. J. Neurosci. 2009;29:15089–15099. doi: 10.1523/JNEUROSCI.0796-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigon A., Duff M.C., McAuley E., Kramer A.F., Voss M.W. Is traumatic brain injury associated with reduced inter-hemispheric functional connectivity? A study of large-scale resting state networks following traumatic brain injury. J. Neurotrauma. 2016;33:977–989. doi: 10.1089/neu.2014.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigon A., Turkstra L., Mutlu B., Duff M. The female advantage: sex as a possible protective factor against emotion recognition impairment following traumatic brain injury. Cogn. Affect. Behav. Neurosci. 2016 doi: 10.3758/s13415-016-0437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigon A., Voss M.W., Turkstra L.S., Mutlu B., Duff M.C. Frontal and temporal structural connectivity is associated with social communication impairment following traumatic brain injury. J. Int. Neuropsychol. Soc. 2016;22(7):705–716. doi: 10.1017/S1355617716000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H., McDonald S., Dethier M., Kessels R.P., Westbrook R.F. Facial emotion recognition deficits following moderate-severe traumatic brain injury (TBI): re-examining the valence effect and the role of emotion intensity. J. Int. Neuropsychol. Soc. 2014;20:994–1003. doi: 10.1017/S1355617714000940. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D., Bradley M.M., Lang P.J., Costa V.D., Versace F. Pleasure rather than salience activates human nucleus accumbens and medial prefrontal cortex. J. Neurophysiol. 2007;98:1374–1379. doi: 10.1152/jn.00230.2007. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D., Fortune E.E., Li Q., Siddiqui A., Krafft C., Oliver W.T., Beck S., Jeffries J. Emotional perception: meta-analyses of face and natural scene processing. NeuroImage. 2011;54:2524–2533. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D.J., Ham T.E. Investigating white matter injury after mild traumatic brain injury. Curr. Opin. Neurol. 2011;24:558–563. doi: 10.1097/WCO.0b013e32834cd523. [DOI] [PubMed] [Google Scholar]
- Sharp D.J., Beckmann C.F., Greenwood R., Kinnunen K.M., Bonnelle V., De Boissezon X., Powell J.H., Counsell S.J., Patel M.C., Leech R. Default mode network functional and structural connectivity after traumatic brain injury. Brain. 2011;134:2233–2247. doi: 10.1093/brain/awr175. [DOI] [PubMed] [Google Scholar]
- Sharp D.J., Scott G., Leech R. Network dysfunction after traumatic brain injury. Nat. Rev. Neurol. 2014;10:156–166. doi: 10.1038/nrneurol.2014.15. [DOI] [PubMed] [Google Scholar]
- Sidaros A., Engberg A.W., Sidaros K., Liptrot M.G., Herning M., Petersen P., Paulson O.B., Jernigan T.L., Rostrup E. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain. 2008;131:559–572. doi: 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sours C., Rosenberg J., Kane R., Roys S., Zhuo J., Shanmuganathan K., Gullapalli R.P. Associations between interhemispheric functional connectivity and the Automated Neuropsychological Assessment Metrics (ANAM) in civilian mild TBI. Brain Imaging Behav. 2014 doi: 10.1007/s11682-014-9295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spikman J.M., Timmerman M.E., Milders M.V., Veenstra W.S., van der Naalt J. Social cognition impairments in relation to general cognitive deficits, injury severity, and prefrontal lesions in traumatic brain injury patients. J. Neurotrauma. 2012;29:101–111. doi: 10.1089/neu.2011.2084. [DOI] [PubMed] [Google Scholar]
- Spikman J.M., Boelen D.H., Pijnenborg G.H., Timmerman M.E., van der Naalt J., Fasotti L. Who benefits from treatment for executive dysfunction after brain injury? Negative effects of emotion recognition deficits. Neuropsychol. Rehabil. 2013;23:824–845. doi: 10.1080/09602011.2013.826138. [DOI] [PubMed] [Google Scholar]
- Stevens M.C., Lovejoy D., Kim J., Oakes H., Kureshi I., Witt S.T. Multiple resting state network functional connectivity abnormalities in mild traumatic brain injury. Brain Imaging Behav. 2012;6:293–318. doi: 10.1007/s11682-012-9157-4. [DOI] [PubMed] [Google Scholar]
- Tamamiya Y., Hiraki K. Individual differences in the recognition of facial expressions: an event-related potentials study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0057325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts A.J., Douglas J.M. Interpreting facial expression and communication competence following severe traumatic brain injury. Aphasiology. 2006;20:707–722. [Google Scholar]
- Xia M., Wang J., He Y. BrainNet viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylvisaker M., Turkstra L.S., Coelho C. Behavioral and social interventions for individuals with traumatic brain injury: a summary of the research with clinical implications. Semin. Speech Lang. 2005;26:256–267. doi: 10.1055/s-2005-922104. [DOI] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Bullmore E. Network-based statistic: identifying differences in brain networks. NeuroImage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Bullmore E.T. Network-based statistic: identifying differences in brain networks. NeuroImage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Zalesky A., Cocchi L., Fornito A., Murray M., Bullmore E. Connectivity differences in brain networks. NeuroImage. 2012;60:1055–1062. doi: 10.1016/j.neuroimage.2012.01.068. [DOI] [PubMed] [Google Scholar]
- Zar J. Prentice-Hall; Upper Saddle River, NJ: 1996. Biostatistical Analysis. [Google Scholar]