Abstract
We report the first case of a postoperative wound infection caused by Vibrio metschnikovii on the lower right leg of a patient after saphenectomy. Compared to the healing of an uninfected site, that of the right leg was delayed, and a cure was achieved by intensified wound care. Several swabs taken from the infected site grew a gram-negative rod in pure culture that was identified as V. metschnikovii by the VITEK 2 system. The source of the infection was not detected; however, the absence of putative risk factors (exposure to water or shellfish or an episode of diarrhea), the profession of the patient (butcher), and the isolation of V. metschnikovii in a variety of farm animals (chicken, cattle, swine, and horses) suggest that infections caused by V. metschnikovii may be regarded as zoonotic.
CASE REPORT
Vibrio metschnikovii was isolated from a saphenous vein donor site on the lower right leg of a 64-year-old male patient. Ten weeks earlier, the patient had undergone cardiac surgery with saphenectomy performed on both legs. After 8 weeks of an uneventful postoperative course, the healing of the explantation site of the right leg was noted to be delayed in comparison to that of the left leg. Signs of local inflammation included erythema and discharge of exudate after pressure but no pain. Multiple swabs were taken from the two explantation sites, but only swabs from the wound of the right leg yielded polymorphonuclear leukocytes and gram-negative, slightly curved rods in great numbers. The same gram-negative rods were also demonstrated in follow-up samples. When the infection became apparent, topical wound care was intensified and resulted in spontaneous healing of the wound after another 8 weeks without administration of systemic antimicrobial treatment.
Microbiology.
The isolates appeared as catalase-positive and oxidase-negative, gram-negative, slightly curved rods and produced good growth of grayish, opaque colonies 2 to 3 mm in diameter with complete hemolysis on Columbia sheep blood agar after 24 h. Growth on MacConkey agar was poor with positive lactose utilization. Reduction of nitrate to nitrite was negative. The isolates were identified as V. metschnikovii by the VITEK 2 system (with excellent identification) (VITEK ID-GNB card, VITEK software version WSVT2-R03.01; bioMérieux Vitek, Inc., Hazelwood, Mo.). PCR amplification of the complete 16S rRNA gene was performed with genomic DNA in accordance with a previously published protocol (17). Amplification products were subjected to direct sequencing, and a 100% match to GenBank sequence accession number X74712.1 of V. metschnikovii was noted (19). Additional confirmatory tests demonstrated the characteristic movement of Vibrio spp. in hanging-drop preparations, a positive reaction with sickle-shaped-cell hemolysis in the CAMP test performed under an aerobic atmosphere (11), and dependence on salt for growth. Antibiotic susceptibility was determined in accordance with NCCLS guidelines for disk diffusion (16), and the isolates were found to be susceptible to mezlocillin, piperacillin, piperacillin-sulbactam, carbapenems, expanded- and broad-spectrum cephalosporins, aztreonam, fluoroquinolones, trimethoprim, fosfomycin, tetracycline, and aminoglycosides but resistant to ampicillin and sulfamethoxazole.
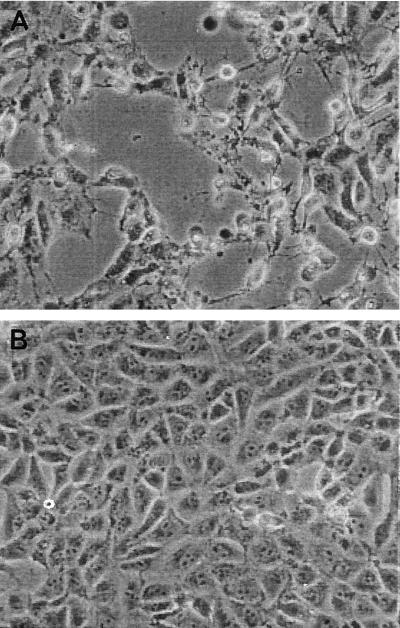
The cytotoxic activity of the V. metschnikovii isolate was investigated by the Vero cell toxicity assay. The assay was performed with bacteria grown overnight in Trypticase soy broth at 37°C as described previously (1). The Shiga toxin (Stx)-producing Escherichia coli strain H19 (O26:H11; Stx1) and Stx-negative E. coli strain KK11/6 were taken as positive and negative controls. The toxic activity of V. metschnikovii became clearly visible after 5 h of incubation of Vero cell layers with V. metschnikovii culture fluid (Fig. 1). In contrast, the deleterious effect of Shiga toxins on Vero cells became visible only after 24 h of incubation of Vero cells with H19 culture supernatant. The addition of serum obtained from the patient 9 months after the infection did not neutralize the cytotoxic effect. Possible reasons for the failure to detect a neutralizing effect were that (i) no antibodies were present, due either to the superficial nature of the infection or to antibody titers being insufficient or lacking a neutralizing effect, and (ii) V. metschnikovii had no role in the infection.
FIG. 1.
(A) Vero cell lawn after 5 h of incubation with culture supernatant of the V. metschnikovii isolate from the patient; (B) undamaged Vero cell control.
Discussion.
V. metschnikovii can be found in various aquatic habitats, including streams, lakes, marine waters, and sewage, as well as in shellfish (3, 13). Human infections by V. metschnikovii, in contrast, are a rare event: so far, only one case of cholecystitis (10), three cases of septicemia (8, 9), and cases of diarrhea (5, 14, 15) have been reported. The possible sources or routes of transmission in these cases remain obscure. The present report describes the first case of a wound infection by V. metschnikovii in a male 64-year-old patient as a complication of surgery, leading to delayed wound healing. Efforts were made to identify the reservoir. The patient denied any contact with fresh water, seawater, or seafood or any episode of diarrhea. Postoperatively, both vein donor sites were treated with topical agents (a mixture of streptodornase and streptokinase [Varidase; Riemser, Greifswald, Germany] by medical staff and several preparations containing Aloe vera by the patient), raising the suspicion of contamination; however, both Varidase and A. vera preparations were found negative for V. metschnikovii by culture or PCR using the universal eubacterial 16S rRNA gene-specific primers 27f and 1492r (2). After discharge from the hospital, the patient had returned to his profession as a butcher and slaughtered calves, swine, and deer but no fowl. He had worn protective clothing at all times and is not aware of any direct contact with blood or other bodily fluids with his legs. Interestingly, V. metschnikovii isolates with cytotoxin production indistinguishable from our isolate have recently been cultivated from aborted cattle, swine, and horses as well as the brain tissue of ducks and geese in different parts of Germany (12, 20). V. metschnikovii has also been found in the intestines of chickens and together with E. coli in cases of infectious hepatitis of fowl (6, 7). These observations suggest that V. metschnikovii may be a zoonotic organism and can be transmitted to humans via the food chain.
Vibrio spp. are well known to produce a variety of toxins and hemolysins (4). Miyake et al. described a cytolysin specific for V. metschnikovii (15) with hemolytic properties; however, its role in infections caused by V. metschnikovii is unclear. Our isolate clearly demonstrated hemolytic activity, especially after 2 days of growth and after enhancement in the CAMP test. The strain showed a different verocytotoxic activity than the Shiga toxin of E. coli did, and PCR for Stx genes was negative (protocol in accordance with reference 18; data not shown). The finding that the hemolysin and the cytotoxin were produced at a physiological temperature points to their possible contribution to the pathological process. The presence of large plasmids in V. metschnikovii was described by Dalsgaard et al. (5). Using a standard plasmid preparation procedure (QIAfilter plasmid midi kit, catalogue number 12243; QIAGEN, Hilden, Germany), we were also able to demonstrate a plasmid of about 340 kb in our isolate.
In conclusion, this is the first report to describe V. metschnikovii as the cause of a postoperative wound infection in a human, possibly linked to the handling and slaughtering of animals. The culture and identification were unproblematic due to robust growth on routine medium and a unique biochemical profile. The risk for human infections due to contact with colonized or infected animals and the putative virulence factors hemolysin and cytotoxin should be further investigated.
REFERENCES
- 1.Beutin, L., S. Zimmermann, and K. Gleier. 1996. Rapid detection and isolation of Shiga-like toxin (verocytotoxin)-producing Escherichia coli by direct testing of individual enterohemolytic colonies from washed sheep blood agar plates in the VTEC-RPLA assay. J. Clin. Microbiol. 34:2812-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blok, H. J., A. M. Gohlke, and A. D. Akkermans. 1997. Quantitative analysis of 16S rDNA using competitive PCR and the QPCR System 5000. BioTechniques 22:700-704. [DOI] [PubMed] [Google Scholar]
- 3.Caldini, G., A. Neri, S. Cresti, V. Boddi, G. M. Rossolini, and E. Lanciotti. 1997. High prevalence of Vibrio cholerae non-O1 carrying heat-stable-enterotoxin-encoding genes among Vibrio isolates from a temperate-climate river basin of central Italy. Appl. Environ. Microbiol. 63:2934-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakraborty, S., G. B. Nair, and S. Shinoda. 1997. Pathogenic vibrios in the natural aquatic environment. Rev. Environ. Health 12:63-80. [DOI] [PubMed] [Google Scholar]
- 5.Dalsgaard, A., A. Alarcon, C. F. Lanata, T. Jensen, H. J. Hansen, F. Delgado, A. I. Gil, M. E. Penny, and D. Taylor. 1996. Clinical manifestations and molecular epidemiology of five cases of diarrhoea in children associated with Vibrio metschnikovii in Arequipa, Peru. J. Med. Microbiol. 45:494-500. [DOI] [PubMed] [Google Scholar]
- 6.Gerlach, H., and I. Gylstorff. 1967. Studies on biochemical properties, pathogencity and resistance spectrum against antibiotics in Vibrio metschnikovi. Berl. Muench. Tieraerztl. Wochenschr. 80:153-155. [PubMed] [Google Scholar]
- 7.Gerlach, H., and I. Gylstorff. 1967. Tests made on biochemical properties, pathogenicity and resistance spectrum against antibiotics in Vibrio metschnikovi. Berl. Muench. Tieraerztl. Wochenschr. 80:161-164. [PubMed] [Google Scholar]
- 8.Hansen, W., J. Freney, H. Benyagoub, M.-N. Letouzey, J. Gigi, and G. Wauters. 1993. Severe human infections caused by Vibrio metschnikovii. J. Clin. Microbiol. 31:2529-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardardottir, H., K. Vikenes, A. Digranes, J. Lassen, and A. Halstensen. 1994. Mixed bacteremia with Vibrio metschnikovii in an 83-year-old female patient. Scand. J. Infect. Dis. 26:493-494. [DOI] [PubMed] [Google Scholar]
- 10.Jean-Jacques, W., K. R. Rajashekaraiah, J. J. Farmer III, F. W. Hickman, J. G. Morris, and C. A. Kallick. 1981. Vibrio metschnikovii bacteremia in a patient with cholecystitis. J. Clin. Microbiol. 14:711-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohler, W. 1988. CAMP-like phenomena of Vibrios. Zentbl. Bakteriol. Mikrobiol. Hyg. A 270:35-40. [DOI] [PubMed] [Google Scholar]
- 12.Kuehn, T. 2001. Isolation procedure for halophilic vibrios from cattle, p. 168-169. In Proceedings of the Xth International Symposium of Veterinary Laboratory Diagnosticians and OIE Seminar on Biotechnology, 4-7 July, Salsomaggiore, Italy.
- 13.Lee, J. V., T. J. Donovan, and A. L. Furniss. 1978. Characterization, taxonomy, and emended description of Vibrio metschnikovii. Int. J. Syst. Bacteriol. 28:99-111. [Google Scholar]
- 14.Magalhaes, V., A. Branco, L. R. de Andrade, and M. Magalhaes. 1996. Vibrio metschnikovii among diarrheal patients during cholera epidemic in Recife Brazil. Rev. Inst. Med. Trop. Sao Paulo 38:1-3. [DOI] [PubMed] [Google Scholar]
- 15.Miyake, M., T. Honda, and T. Miwatani. 1988. Purification and characterization of Vibrio metschnikovii cytolysin. Infect. Immun. 56:954-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 17.Neubauer, H., U. Reischl, J. Kostler, S. Aleksic, E. J. Finke, and H. Meyer. 1999. Variations in the 16S rRNA gene sequence of Yersinia enterocolitica isolates influence the specificity of molecular identification systems. Zentbl. Bakteriol. 289:329-337. [DOI] [PubMed] [Google Scholar]
- 18.Reischl, U., M. T. Youssef, J. Kilwinski, N. Lehn, W. L. Zhang, H. Karch, and N. A. Strockbine. 2002. Real-time fluorescence PCR assays for detection and characterization of Shiga toxin, intimin, and enterohemolysin genes from Shiga toxin-producing Escherichia coli. J. Clin. Microbiol. 40:2555-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruimy, R., V. Breittmayer, P. Elbaze, B. Lafay, O. Boussemart, M. Gauthier, and R. Christen. 1994. Phylogenetic analysis and assessment of the genera Vibrio, Photobacterium, Aeromonas, and Plesiomonas deduced from small-subunit rRNA sequences. Int. J. Syst. Bacteriol. 44:416-426. [DOI] [PubMed] [Google Scholar]
- 20.Stephan, R., K. Taureck, D. Knabner, T. Shimada, J. L. Larsen, and C. Kruppe. 2002. Zum Vorkommen von halophilen und humanpathogenen Vibrionen in deutschen Nutztierbeständen. Tierärztl. Prax. 30:69-74. [Google Scholar]