Abstract
Death receptor 3 (DR3; TNFRSF25) and its tumor necrosis factor–like ligand TL1A (TNFSF15) control several processes in inflammatory diseases through the expansion of effector T cells and the induction of proinflammatory cytokines from myeloid and innate lymphoid cells. Using wild-type (DR3+/+) and DR3-knockout (DR3−/−) mice, we show that the DR3/TL1A pathway triggers the release of multiple chemokines after acute peritoneal inflammation initiated by a single application of Staphylococcus epidermidis supernatant, correlating with the infiltration of multiple leukocyte subsets. In contrast, leukocyte infiltration was not DR3 dependent after viral challenge with murine cytomegalovirus. DR3 expression was recorded on connective tissue stroma, which provided DR3-dependent release of chemokine (C-C motif) ligand (CCL) 2, CCL7, CXCL1, and CXCL13. CCL3, CCL4, and CXCL10 production was also DR3 dependent, but quantitative RT-PCR showed that their derivation was not stromal. In vitro cultures identified resident macrophages as a DR3-dependent source of CCL3. Whether DR3 signaling could contribute to a related peritoneal pathology was then tested using multiple applications of S. epidermidis supernatant, the repetitive inflammatory episodes of which lead to peritoneal membrane thickening and collagen deposition. Unlike their DR3+/+ counterparts, DR3−/− mice did not develop fibrosis of the mesothelial layer. Thus, this work describes both a novel function and essential requirement for the DR3/TL1A pathway in acute, resolving, and chronic inflammation in the peritoneal cavity.
Death receptor 3 (DR3; TRAMP, LARD, WSL-1, Apo-3, TR3, TNFRSF25),1, 2, 3, 4, 5, 6 a member of the tumor necrosis factor receptor superfamily (TNFRSF), and its TNFSF ligand TL1A (TNFSF15)7 have emerged as major regulators of inflammation and immunity. Genome-wide association studies have consistently linked polymorphisms around the TNFSF15 gene as a risk factor in inflammatory bowel diseases, such as ulcerative colitis, Crohn's disease, and/or irritable bowel syndrome,8, 9, 10, 11 and in the arthritic disease ankylosing spondylitis,12 with associations also reported with primary biliary cirrhosis.13 Furthermore, variation in the TNFRSF25 gene locus has been suggested as a risk factor in rheumatoid arthritis,14 whereas other genetic studies have associated the TNFSF15 gene with leprosy,15 implicating a further role for the DR3/TL1A axis in pathogenic immunity.
These wide-ranging links to multiple pathologies have been supported by in vitro and in vivo studies in both humans and mice. Levels of serum and tissue TL1A are increased in rheumatoid arthritis,16 inflammatory bowel disease,17 psoriasis,18 primary biliary cirrhosis,19 and ankylosing spondylitis,20 whereas mice overexpressing TL1A develop colitis or ileitis.21, 22, 23 In contrast, mice deficient for DR3 or TL1A, or those expressing dominant negative forms of DR3, are resistant to multiple models of inflammatory and autoimmune disease,24, 25, 26, 27, 28, 29, 30, 31 or exhibit impaired immunity to bacterial or viral pathogens,32, 33 with a more debated impact on immunity to parasitic worms.34, 35 Much of DR3 function has been attributed to its expression on T cells (CD4+ regardless of lineage or CD8+) and the capacity of TL1A, either directly or indirectly, to drive the proliferation of pathogenic effector T-cell numbers.26, 27, 36, 37 A single study also suggests a role in inhibiting proliferation of B cells.38 This has defined significant roles for the DR3/TL1A pathway in adaptive immune responses.
However, DR3 expression is also found on innate immune cell and nonhematopoietic lineages. DR3 signaling on myeloid cells influences myeloid differentiation,30, 39 whereas on natural killer T cells and innate lymphoid cells, triggers effector cytokine release and expansion essential for the development of airway inflammation.25, 34 Furthermore, DR3−/− mice exhibit behavioral defects associated with impaired corticostriatal innervation controlled by DR3 expression on neurones,40 and its expression on tubular epithelial cells regulates renal inflammation and injury caused by reperfusion.41, 42 On osteoblasts, DR3 can trigger apoptosis in vitro43 or control bone mineral apposition in vivo.44 These findings indicate that the DR3/TL1A pathway has intrinsic functions and also controls early events in immune processes in addition to the more established roles in acquired immunity.
In this report, we further investigated the in vivo role of the DR3/TL1A pathway using sterile challenge of Staphylococcus epidermidis supernatant (SES) in the peritoneal cavity.45, 46 As a single application, this system mimics a resolving inflammatory response that returns to baseline levels,45 allowing scrutiny of infiltrating cells and soluble factors in peritoneal washouts within hours of the challenge. Using multiple applications, pathology develops in the form of peritoneal membrane fibrosis,47 mirroring the pathology associated with repeated bacterial infections in renal patients undergoing peritoneal dialysis. Consequently, we show that the DR3/TL1A axis shapes the inflammatory response within the peritoneum and report a previously unappreciated role for DR3 in controlling inflammation via modulation of chemokine expression by both hematopoietic and stromal cells.
Materials and Methods
Animals
Age-matched DR3−/− and DR3+/+ littermates, aged 8 to 12 weeks, on a C57BL/6 background were used in experiments, derived from a DR3het colony that was founded from mice provided by CRUK (London, UK).48 For peritoneal challenge with SES, animals of mixed sex were used, as no significant differences in peritoneal leukocyte content of male and female mice were observed, either at baseline or during the course of experiments (data not shown). The male/female ratio in each DR3+/+ and DR3−/− group for each time point was matched. For challenge with murine cytomegalovirus (MCMV), all animals used were male. All procedures were approved by the local research ethics committee and performed in accordance with Home Office–approved Procedural Project Licenses 30/2401 (for SES) and 30/2442 and 30/2580 (for MCMV).
Immunohistochemistry
Briefly, peritoneal membranes were harvested and fixed in neutral-buffered formal saline and permeated with paraffin wax before dividing into sections (5 μm thick). Sections were rehydrated, and endogenous peroxidase activity was blocked. Antigen unmasking was achieved by incubating the sections in proteinase K (20 μg/mL in Tris/EDTA buffer; Sigma, Gillingham, UK) for 10 minutes. After blocking steps [20% goat serum and streptavidin/biotin blocking kit (Vector Laboratories, Peterborough, UK)], sections were incubated overnight with 4 μg/mL goat biotinylated anti-DR3 (R&D Systems, Abingdon, UK) or isotype controls diluted in phosphate-buffered saline, followed by streptavidin–horse radish peroxidase secondary antibody (Vector Laboratories), as per the manufacturer's instructions. Positive staining was visualized using a diaminobenzidene chromogen (Vector Laboratories). Collagen was visualized using van Gieson stain. Sections were counterstained with hematoxylin, dehydrated, and mounted in DPX. Images were captured using an Olympus N457 (Olympus KeyMed, Southend-on-Sea, UK) or Canon 100D (Canon Europe, Uxbridge, UK) digital camera, and positive staining was analyzed using Adobe Photoshop software version CS3.5 (Adobe Systems Inc., San Jose, CA). Randomly selected areas were used for analysis, with the average from five used to generate values for each section.
RT-qPCR
Total RNA was extracted following the manufacturer's instructions using an RNA easy mini kit (Qiagen, Crawley, UK) from snap-frozen and powdered peritoneal membranes or pelleted macrophages. cDNA synthesis and PCR were performed using systems and primers generated through Primer Design (Southampton, UK) on an iCycler quantitative PCR (qPCR) machine (Bio-Rad, Hemel Hempstead, UK), with β-actin as the housekeeping control gene. Relative quantity was calculated after subtracting the negative control. Primers used were as follows: chemokine ligand (CCL) 2, 5′-CCCAATGAGTAGGCTGGAGA-3′ (forward) and 5′-TCTGGACCCATTCCTTCTTG-3′ (reverse); CCL3, 5′-TTTTGAAACCAGCAGCCTTT-3′ (forward) and 5′-CTGCCTCCAAGACTCTCAGG-3′ (reverse); CCL4, 5′-CCCACTTCCTGCTGTTTCTC-3′ (forward) and 5′-CTCACTGGGGTTAGCACAGA-3′ (reverse); CCL5, 5′-GTGCCCACGTCAAGGAGTAT-3′ (forward) and 5′-CCACTTCTTCTCTGGGTTGG-3′ (reverse); CCL7, 5′-CCCAAGAGGAATCTCAAGAGC-3′ (forward) and 5′-ATAGCCTCCTCGACCCACTT-3′ (reverse); CXCL1, 5′-GCACCCAAACCGAAGTCATA-3′ (forward) and 5′-AGGTGCCATCAGAGCAGTCT-3′ (reverse); CXCL2, 5′-AAAGTTTGCCTTGACCCTGA-3′ (forward) and 5′-CTTTGGTTCTTCCGTTGAGG-3′ (reverse); CXCL5, 5′-GCCCTACGGTGGAAGTCATA-3′ (forward) and 5′-GTGCATTCCGCTTAGCTTTC-3′ (reverse); CXCL10, 5′-TGAATCCGGAATCTAAGACCA-3′ (forward) and 5′-GAGGCTCTCTGCTGTCCATC-3′ (reverse); CXCL13, 5′-TCGTGCCAAATGGTTACAAA-3′ (forward) and 5′-GGTGCAGGTGTGTCTTTTGA-3′ (reverse); and TL1A, 5′-CAGCAGAAGGATGGCAGA-3′ (forward) and 5′-CTCTGGCCTGTGTCTACA-3′ (reverse).
Preparation of SES, Determination of Biological Activity, and the SES Model of Peritoneal Challenge
SES was prepared and used in peritoneal challenge, as described previously.46, 47 Inflammation in the cavity was induced via an i.p. injection of SES. Peritoneal membranes were harvested, fixed, divided into sections, and stained for DR3 expression or analyzed for the relative quantity of TL1A mRNA by RT-qPCR. Supernatants were filtered to remove live bacteria, lyophilized, and stored at −70°C. Biological activity was determined by its capacity to induce IL6 release from RAW 264 cells in a 24-hour culture, measured by enzyme-linked immunosorbent assay (ELISA; BD Pharmingen OpEIA; BD Biosciences, Oxford, UK).
Challenge with MCMV
Mice were challenged i.p. with 5 × 104 plaque-forming units of MCMV Smith strain (ATCC, Manassas, VA), as previously described.49
Flow Cytometry
After peritoneal lavage and centrifugation to isolate leukocytes, samples were treated with Fc block (BD Pharmingen) and then stained at 4°C for 25 minutes with combinations of the following preconjugated monoclonal antibodies: CD19-FITC, CD8a-FITC, NK1.1-PE, Gr-1-PerCP-Cy5.5, CD4-PerCP-Cy5.5, CD11c-PE-Cy7, Ly6G-V450, CD44-V450, CD11b-APC-Cy7, CD3-APC-Cy7 (all BD Biosciences); B220-PE, F4/80-APC (Invitrogen, Life Technologies Ltd., Paisley, UK); Ly6B.2-PE (AbD Serotec, Oxford, UK); TCRαβ-APC (Caltag, Buckingham, UK). Samples were then washed and fixed in 1% paraformaldehyde before analysis on a CyAn ADP Flow Cytometer using Summit software version 4.3 (Beckman Coulter, High Wycombe, UK). Cell subsets were identified using the following criteria: neutrophils (7/4+, Ly6G+), resident macrophages (F4/80+CD11b+), inflammatory macrophages (F4/80intCD11bint), eosinophils (F4/80intCD11bintSSChi), natural killer cells (NK1.1+αβTCR−), natural killer T cells (NK1.1+αβTCR+), T cells (CD3+αβTCR+), and B cells (CD19+B220+).
ELISAs and Chemokine Arrays
ELISAs were performed following manufacturers' instructions. For murine CCL2, CCL3, CCL5, CXCL1, CXCL2, CXCL5, CXCL10, and CXCL13, ELISAs were obtained from R&D Systems. ELISA for murine CCL7 was obtained from Antigenix America (Melville, NY). For determining concentration of chemokines in cultures of resident macrophages, the Legendplex mouse proinflammatory chemokine panel was used following the manufacturer's instructions (Biolegend, London, UK).
Statistical Analysis
Staining readouts were percentages; therefore, nonparametric Mann-Whitney U tests were used for statistical analysis. For protein concentrations from ELISAs and relative quantity from RT-qPCR, Student's t-tests and analyses of variance were used. Analyses were performed on GraphPad Prism version 6 (GraphPad Software Inc., San Diego, CA); P < 0.05 was considered significant, and P < 0.01 was considered highly significant.
Results
DR3 and TL1A Expression in the Peritoneal Cavity after SES Challenge
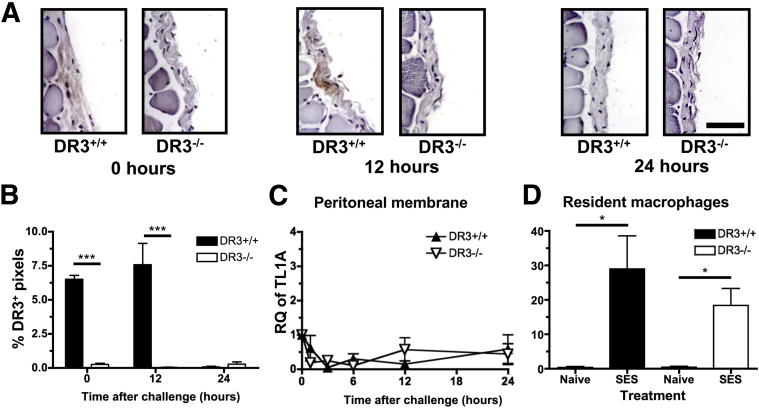
The DR3/TL1A pathway has been shown to be essential in the development of several models of disease involving intraperitoneal priming or challenge,25, 26, 27, 30, 32, 33 but little has been reported about DR3 and TL1A expression patterns in the peritoneal tissue and cavity during this process. DR3 expression in the mesothelial layer of the peritoneal membrane was first investigated, before and after SES challenge by staining of sections using a polyclonal antibody. Anti-DR3 showed minimal staining on DR3−/− peritoneal mesothelial layers (0.25% ± 0.08% positive pixels), but a significant signal was recorded from DR3+/+ samples, in the absence of (6.5% ± 0.3%) and up to 12 hours after challenge (7.6% ± 1.6%) before becoming negative after 24 hours (0.08% ± 0.04%) (Figure 1, A and B). DR3 was also found on CD4+, CD8+, and natural killer T cells within the peritoneal cavity, in agreement with previous reports studying DR3 expression in splenocytes25, 33 (data not shown). These data show, for the first time, that DR3 is present on the stroma of the peritoneal cavity. In addition, expression of TL1A mRNA was measured using RT-qPCR on peritoneal membrane sections, as well as from resident peritoneal macrophages isolated by cell sorting (F4/80+CD11b+) and treated in vitro with SES. Although the mesothelial layers were negative for TL1A throughout the time course (Figure 1C), TL1A mRNA was triggered from resident peritoneal macrophages within 1 hour of SES treatment, irrespective of their derivation from DR3+/+ or DR3−/− mice (Figure 1D). Thus, bacterial challenge in the peritoneal cavity primarily induces TL1A from resident macrophages, not stroma, but responses to TL1A can come from DR3 on both stroma and leukocytes.
Figure 1.
DR3 expression and sources of TL1A in the peritoneal cavity. Inflammation in the cavity was induced via an i.p. injection of Staphylococcus epidermidis supernatant (SES). Peritoneal membranes were harvested, fixed, cut into sections, and stained for DR3 expression or analyzed for the relative quantity (RQ) of TL1A mRNA by quantitative RT-PCR. A: Representative images showing DR3 staining in the mesothelial layer of DR3+/+ mice after 0 and 12 hours but absent after 24 hours of inflammation. B: Summary of DR3 expression in the mesothelial layer. The % positive (brown) pixels within the mesothelial layer of the membrane is shown. C: RQ of TL1A mRNA in the peritoneal membrane of DR3+/+ and DR3−/− mice. Not significantly different by analysis of variance. D: RQ of TL1A mRNA in DR3+/+ and DR3−/− resident peritoneal macrophages 1 hour after stimulation using SES. Macrophages were sorted from other leukocytes found in the peritoneal cavity from 17 pooled DR3+/+ and DR3−/− mice, challenged with SES, and analyzed for RQ of TL1A mRNA. Data shown from four replicate experiments. Data are shown as means ± SEM (B–D). n = 3 to 6 (B, DR3+/+ mice and DR3−/− mice per time point); n = 5 (C, mice per time point); n = 4 (DR3+/+, naive and SES; DR3−/− SES) or 5 (DR3−/− naive) (D). ∗P < 0.05 by t-test assuming unequal variance, ∗∗∗P < 0.001 by one-way analysis of variance. Scale bar = 25 μm (A).
Leukocyte Accumulation Is Impaired in the Absence of DR3
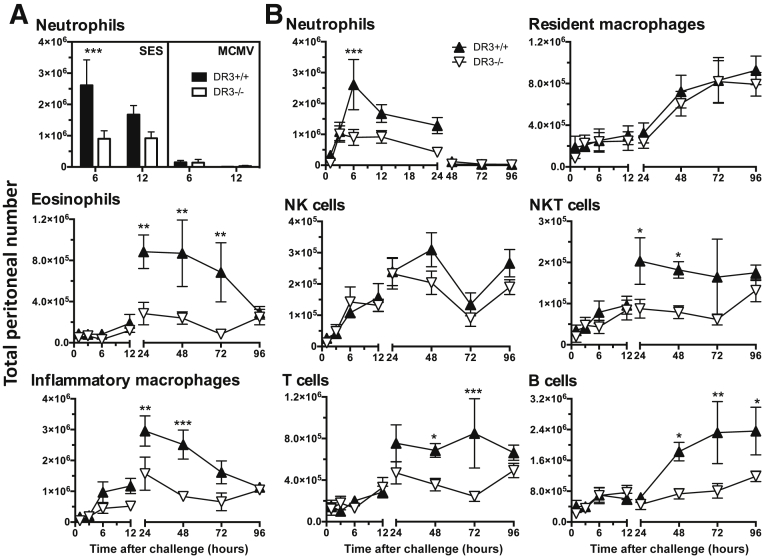
Leukocyte infiltration of the peritoneal cavity after SES challenge was next examined in the presence or absence of DR3. The composition of inflammatory cells in the peritoneal lavage was assessed using multiparameter flow cytometry and two panels of monoclonal antibodies defining myeloid lineages and lymphoid populations (as described in Materials and Methods). Before challenge, there were no differences in the leukocyte content of DR3−/− and DR3+/+ peripheral blood or peritoneal lavage (Supplemental Table S1), showing that the absence of DR3 did not influence the baseline cellular constitution of the circulation or peritoneal cavity. The progression of this model from inflammatory insult to its resolution has been well-characterized, demonstrating a biphasic cellular infiltration predominantly consisting of neutrophils that peaks in the first 6 hours and declines thereafter. Increasing numbers of other cell types follows, peaking after 48 hours, before falling away over 96 hours45, 46 (Figure 2). In DR3−/− mice, significantly lower numbers of neutrophils in the early stage were observed after SES challenge compared to their DR3+/+ counterparts. Interestingly, viral challenge with MCMV induced considerably less neutrophil accumulation and showed no differences between DR3+/+ and DR3−/− mice (Figure 2A), consistent with reports that neutrophil recruitment after MCMV challenge is IL-22 dependent.50 During the second phase after SES challenge, eosinophils, inflammatory macrophages, T cells, B cells, and natural killer T cells were decreased in number (Figure 2B). Although the profile of resident macrophages and natural killer cell numbers was not significantly different between DR3+/+ and DR3−/− mice throughout the time course, peritoneal B-cell numbers remained significantly higher in DR3+/+ animals by 96 hours after challenge (Figure 2B). Overall, there was an intrinsic defect in the accumulation of leukocytes in DR3−/− mice after SES challenge in the peritoneal cavity.
Figure 2.
Leukocyte subset numbers in the peritoneal cavity of DR3+/+ and DR3−/− mice after Staphylococcus epidermidis supernatant (SES) and murine cytomegalovirus (MCMV) induced inflammation. Inflammation in the cavity was induced via an i.p. injection of SES or MCMV. A: Numbers of neutrophils in the peritoneal cavity after the labeled challenges at the indicated times. B: Peritoneal leukocytes were isolated from the cavity by lavage at the indicated times after challenge and numbers of individual cell subsets were calculated using cell counts and flow cytometry. Representative data from one of two experiments. Each symbol represents mean of up to 6 mice per time point. Statistical analysis by analysis of variance and Bonferroni post hoc test showed significant differences between DR3+/+ and DR3−/− mice for individual time points. Overall male/female ratio was 50:50 and matched for DR3+/+ and DR3−/− groups. Error bars correspond to SEM. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. NK, natural killer.
Proliferation Is Unaltered in Leukocytes from DR3−/− Mice
Reduced numbers of effector cells, normally at the site of pathology, have been reported in the absence of DR3 in a variety of different pathological settings, primarily being ascribed to defects in proliferation.25, 26, 27, 32, 33, 34 We, therefore, measured the levels of proliferation of cells from peritoneal lavage using detection of Ki-67 at 48 hours after SES challenge, when there were peak numbers of infiltrating leukocytes. Surprisingly, there were no differences in Ki-67 staining of any of the cell subsets when comparing DR3+/+ and DR3−/− mice (Supplemental Figure S1 and Supplemental Table S2), indicating that at this early stage of the inflammatory process, DR3 was regulating a distinct mechanism of cellular accumulation. Because DR3 contains a death-inducing intracellular domain, apoptosis using annexin-V staining was also measured, but consistent with other reports,33, 51 the absence of DR3 did not alter the levels of cell death in peritoneal leukocytes (data not shown). The DR3/TL1A pathway therefore regulated peritoneal leukocyte numbers early after bacterial challenge with a mechanism that was independent of proliferation and cell death.
Levels of Multiple Chemokines Are Impaired in DR3−/− Mice after SES Challenge
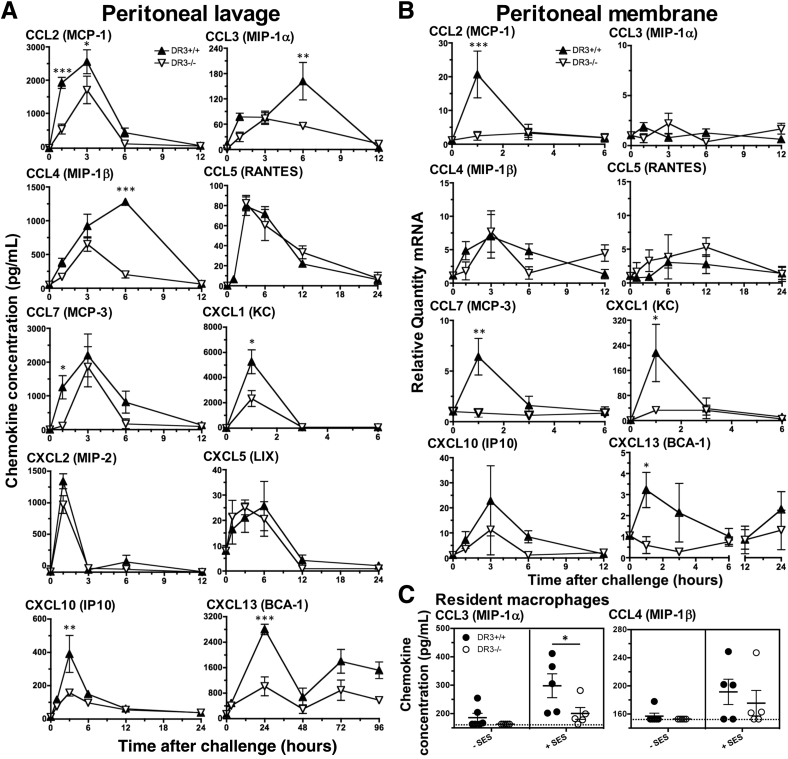
Another possible explanation for the defect in accumulation of cellular infiltrate is that DR3 regulates leukocyte recruitment. To ensure that the absence of DR3 did not adversely influence baseline levels, cell-free supernatants from peritoneal lavages of unchallenged DR3+/+ and DR3−/− mice were tested for a range of chemokines capable of attracting virtually every subset of leukocytes between them. No significant differences were found at baseline, with detectable levels of CCL3, CCL4, CXCL5 (targeting neutrophils and monocytes), CXCL10 (T cells and natural killer cells), and CXCL13 (B cells), whereas CCL2, CCL5, CCL7 (dendritic cells, monocytes, and T cells), CXCL1, and CXCL2 (neutrophils) were lower than levels of detection of their specific kits (Supplemental Figure S2). The kinetics of chemokine release were then investigated in the peritoneal lavage of DR3+/+ and DR3−/− mice challenged with SES. Although some chemokines, including CCL5, CXCL2, and CXCL5, showed no differences between genotypes, the expression of CCL2, CCL3, CCL4, CCL7, CXCL1, CXCL10, and CXCL13 were all significantly impaired in DR3−/−, as compared to DR3+/+ mice, although not necessarily at the same time point as when peak concentrations were observed (Figure 3A). In DR3−/− mice, CCL2, CCL7, and CXCL1 were significantly reduced at 1 hour; CXCL10 at 3 hours; CCL3 and CCL4 at 6 hours; and CXCL13 at 24 hours.
Figure 3.
Chemokine production in the peritoneal cavity of DR3+/+ and DR3−/− mice after Staphylococcus epidermidis supernatant (SES)–induced inflammation. Inflammation in the cavity was induced via an i.p. injection of SES. Levels of the indicated chemokines were measured in the following: cell-free supernatants in peritoneal lavage by enzyme-linked immunosorbent assay (A) and mRNA levels in the peritoneal membrane by quantitative RT-PCR (B). Representative data from one of two experiments. Overall male/female ratio was 50:50 and matched for DR3+/+ and DR3−/− groups. C: Production of chemokine ligand (CCL) 3 and CCL4 by resident macrophages derived from DR3+/+ and DR3−/− mice, stimulated with SES. Each point represents a culture. Values lower than the limit of the assay were assigned the lowest limit for generation of means and statistical testing. Dotted line shows lowest limit of assay. Statistical analysis by analysis of variance and Bonferroni post hoc test showed significant differences between DR3+/+ and DR3−/− mice. Data represent means ± SEM (A–C). n = up to 6 (A and B, DR3+/+ and DR3−/− mice per time point). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. BCA, B-cell attracting chemokine; IP, IFN-γ-inducible protein; KC, keratinocyte chemoattractant; LIX, LPS-induced CXC chemokine; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; RANTES, regulated on activation normal T cell expressed and secreted.
Select Chemokines Are Differentially Produced from the Stroma or Resident Macrophages in a DR3-Dependent Manner
Chemokines can be derived from both stromal and hematopoietic cells. To delineate between these potential sources, RT-qPCR for the messages of all of the above chemokines was performed on RNA extracted from DR3+/+ and DR3−/− mesothelial layers at time points between 1 and 24 hours after SES challenge. Those showing no significant differences in protein levels between DR3−/− and DR3+/+ mice (CCL5, CXCL2, and CXCL5) also showed no significant differences in message. In contrast, of the remaining seven, only CCL2, CCL7, CXCL1, and CXCL13 (attractants of dendritic cells, neutrophils, eosinophils, and memory T and B cells) demonstrated a significant reduction in message from DR3−/− compared to DR3+/+ mesothelial layers (Figure 3B), implicating the stroma as a significant DR3-dependent source of these chemokines and suggesting non-stromal cells are the DR3-dependent source of the others. To further investigate this, resident macrophages (the largest innate immune leukocyte population in the unchallenged peritoneal cavity) (Supplemental Table S1) were extracted from DR3+/+ and DR3−/− mice, pooled, and challenged with SES in vitro. Of 13 chemokines studied, only CCL3 and CCL4 were recorded at levels higher than the detection limit of the array. DR3+/+ resident macrophages produced significantly more CCL3, but not CCL4, compared to DR3−/− resident macrophages in response to SES (Figure 3C). The overall conclusion is that DR3 signaling controls recruitment of multiple leukocyte subsets after bacterial challenge in the peritoneal cavity by a broad release of chemokines, some of which come from stroma, whereas others are derived from non-stromal cell types, including resident macrophages within the peritoneal cavity.
DR3 Deficiency Protects against Pathology of SES-Induced Chronic Inflammation
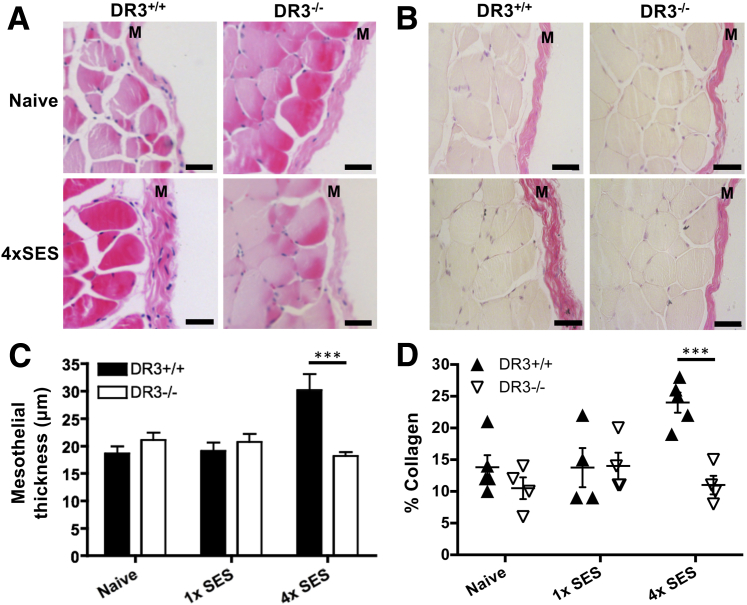
To test whether DR3 signaling could contribute to pathology in a related model of disease, DR3−/− animals were challenged with multiple serial applications of SES, a system designed to mimic the fibrosis of the peritoneal membrane observed in peritoneal dialysis patients after persistent and regular bacterial infections in the peritoneal cavity.47 Thickening of the mesothelial layer and increased deposition of collagen were observed in DR3+/+ mice, but DR3−/− mice showed resistance to this (Figure 4), demonstrating an essential requirement for DR3 in the development of this fibrotic pathology.
Figure 4.
Thickness of the mesothelial layer and collagen deposition in DR3+/+ and DR3−/− mice after inflammation induced by multiple applications of Staphylococcus epidermidis supernatant (SES). Fibrosis of the mesothelial layer was induced by four i.p. injections of SES. Representative micrographs of the peritoneal membranes visualized with hematoxylin and eosin (A) and van Gieson stain (B), from mice treated as indicated. Summary of mesothelial thickness (C) and collagen deposition (D). Statistical analysis by analysis of variance showed significant differences between DR3+/+ and DR3−/− mice. Overall male/female ratio was 50:50 and matched for DR3+/+ and DR3−/− groups. Data represent means ± SEM (C and D). n = up to 6 mice (C and D). ∗∗∗P < 0.001 by Bonferroni post hoc test. Scale bar = 50 μm (A and B). M, mesothelial layer.
Discussion
In recent years, the DR3/TL1A pathway has emerged as a master regulator of inflammatory and autoimmune disease, as well as a coordinator of immune responses to a variety of pathogens. This is in the context of DR3−/− mice showing no impairments in the development of their immune systems, constitution of their immune (or other) organs,48 or intrinsic extrathymic T-cell trafficking.26 Much of the in vivo research in the field has used models of disease involving antigen priming or pathogen challenge in the peritoneal cavity, and has demonstrated that antigen-specific splenic responses after such challenges are relatively normal in the absence of DR3, indicating that immune priming is intact. Herein, we show that the DR3/TL1A pathway controls the earliest of immune processes during peritoneal challenge, regulating the production of chemokines that draws proinflammatory leukocytes into sites of inflammation. This effect is dependent on the type of challenge, with SES, but not MCMV, inducing DR3-dependent leukocyte infiltration. There is the possibility that the DR3/TL1A pathway interacts with specific Toll-like receptor signaling pathways as S. epidermidis is recognized through Toll-like receptor 2,52 whereas MCMV triggers Toll-like receptors 3 and 953 and induces neutrophil recruitment through IL-22.50
Before this report, DR3 function has primarily been attributed to the expression of DR3 on multiple cell types of the hematopoietic lineage, ranging from natural killer T cells, CD4+ cells, and CD8+ T and B cells to innate lymphoid cells, macrophages, and neutrophils. This is the first report of significant DR3 expression on a stromal layer in an in vivo setting and in the absence of active immune responses. Previous studies have indicated that DR3 is inducible on stroma by proinflammatory events, as has been described for tubular epithelial cells after ischemic injury41 and possibly in joints undergoing antigen-induced arthritis, although the latter could be attributed to infiltrating cells.51 More intriguingly, stromal DR3 expression seems to be closely regulated in the peritoneal cavity, with the signal disappearing 24 hours after SES challenge. In contrast, although endothelial cells have been reported to release TL1A,7 its major source after SES challenge was resident macrophages, occurring within an hour of stimulation and independent of DR3 expression (Figure 1). This is in agreement with previous reports showing cells of the myeloid lineage respond to bacterial challenges by rapid TL1A release.26, 32
DR3-dependent release of some chemokines has been previously reported, but not on the scale shown herein. Thus, in vitro studies on the myeloid THP-1 cell line have demonstrated IL-8 release requiring TL1A and interferon-γ priming,54 whereas CXCL1 is reduced in the joints of DR3−/− mice undergoing antigen-induced arthritis.51 Both these chemokines, in humans and mice, respectively, are established neutrophil attractants. This report indicates that at least in the murine SES challenge system, the production of CXCL1, but not two other neutrophil chemoattractants (CXCL2/macrophage inflammatory protein-2 and CXCL5/LPS-induced CXC chemokine), is DR3 dependent. It also shows, for the first time, that there is a stromal contribution to this (Figures 3 and 4). Whether this is the dominant DR3-dependent chemokine controlling neutrophil attraction remains an area of further study as there are multiple other chemokine/chemokine receptor interactions capable of this,55 many of which have not been tested in this study.
The stromal release of several other chemokines demonstrated DR3 dependency (namely, CCL2, CCL7, and CXCL13). CCL2 and CXCL13 have primary chemokine receptors, CCR2 and CXCR5, respectively. CCR7 is more pleiotropic, binding CCR1, CCR2, and CCR3, together having the capacity to attract monocytes, eosinophils, dendritic cells, and T and B cells.55 This correlated well with the subsets of leukocytes that showed impaired SES-induced accumulation in the peritoneal cavity of DR3−/− mice. Interestingly, however, there were other chemokines in DR3−/− peritoneal lavage that were reduced in concentration (namely, CCL3, CCL4, and CXCL10). Their ligands include CCR1, CCR5, and CXCR3 found on monocytes, T cells, natural killer cells, and mast cells. We further identified resident macrophages as a DR3-dependent source of CCL3 (Figure 3C). The assumption is that CCL4 and CXCL10 were derived from other as yet unidentified leukocytes as these chemokines either were not produced or did not show DR3-dependent production by resident macrophages, whereas their mRNA levels were unaltered in the DR3−/− mesothelium. Further studies would be required to identify which other cells are producing DR3-dependent CCL4 and CXCL10, and determine the relative contribution of DR3 on stroma versus resident versus infiltrating leukocytes to recruitment in this system. The rapid peak of production for many of the chemokines (<3 hours) (Figure 3) would indicate either a stromal or a resident leukocyte source for these, with the possibility of infiltrating leukocytes contributing to levels of chemokines at later time points. In this context, DR3 expression on innate immune cells is constitutive on natural killer T cells (which have been shown to produce IL-4 and IL-13 in models of allergic lung inflammation),25 or can be induced on macrophages as well as neutrophils.51 The latter can also produce multiple chemokines and is a major infiltrating cell type.56
The leukocyte recruitment function of the DR3/TL1A pathway shows distinct differences to the role that has been characterized for IL-6 using intraperitoneal SES challenge. Although both affect T-cell recruitment, correlating with lower levels of CCL4 and CXCL10, IL-6−/− animals display a reduction in CCL5,57 which was not observed in DR3−/− mice. Furthermore, unlike DR3−/− animals, IL-6−/− mice show normal early neutrophil46 and later B-cell57 infiltration accompanied by unaltered levels of CXCL1 and CXCL13. This indicates that DR3 signaling is distinct and not directly upstream of IL-6. More recently, IL-6 has been shown to be essential for the development of peritoneal membrane fibrosis after multiple acute inflammatory episodes induced by serial applications of SES. This was attributed to IL-6–mediated T helper cell 1 effector commitment.47 Our data indicate that this model is also dependent on DR3, but likely through its general regulation of effector T cells. DR3−/− mice are resistant to the induced increases in peritoneal membrane thickening and collagen deposition. This is consistent with the phenotype of transgenic mice overexpressing TL1A, which spontaneously develop intestinal and colonic fibrosis accompanied by goblet and Paneth cell hyperplasia.22, 23, 58 One significant question is the relative contribution to pathology of DR3's role in leukocyte recruitment versus its function in driving effector cell expansion.22, 26, 27, 29, 33, 34, 59 Although this can be divided into early and late inflammatory processes in the SES challenge model, the two immunological events are not mutually exclusive and could explain some previously observed phenomena, such as the altered localization of T cells and macrophages in DR3−/− mice undergoing ovalbumin-induced lung inflammation.26
It is evident from this work that DR3/TL1A signaling regulates primary inflammatory responses, with potential impact on downstream adjuvant and host defense activities. The understanding of these roles to pathological processes is an area of further research that will be important for developing the DR3/TL1A pathway as a therapeutic target in inflammatory disease, agonism or antagonism of which may prevent disease, depending on the effector T cells being influenced.
Acknowledgments
We thank the Central Biotechnology Service, School of Medicine, Cardiff University, for access to flow cytometers.
W.V.P., G.W.J., J.P.T., and R.K.S. performed experiments; and A.S.W., I.R.H., P.R.T., S.A.J., and E.C.Y.W. contributed through conceptual and experimental design, animal models, and writing of the manuscript.
Footnotes
Supported by a PhD studentship from the I3-IRG, joint funded by the British Medical Research Council (MRC) and the School of Medicine (W.V.P.); Cardiff University award (E.C.Y.W. and S.A.J.); J.P.T. was employed as a post-doctorate researcher on MRC grant G0901119 awarded to E.C.Y.W. and I.R.H.; and Biotechnology and Biological Sciences Research Council/GlaxoSmithKline Collaborative Awards in Science and Engineering studentship BB/H530589/1 (R.K.S.).
W.V.P. and R.K.S. contributed equally to this work.
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2016.07.021.
Supplemental Data
Flow cytometric gating for determining Ki-67+–proliferating cells. Mice were challenged with Staphylococcus epidermidis supernatant, and peritoneal leukocytes were isolated from the cavity by lavage, stained for cell subsets, permeabilized, and proliferation measured using the intracellular marker Ki-67 and DAPI to differentiate binucleate nonproliferating cells. Representative plots of total peritoneal leukocytes show isotype control and Ki-67 staining from DR3+/+ and DR3−/− mice. Early proliferative G1 cells (Ki-67+ DAPIlo), S and G2M phases (Ki-67+ DAPIint/hi), and nonproliferating binucleate cells (Ki-67- DAPIhi) are shown.
Chemokine levels in the naïve peritoneal cavity are not different between DR3+/+ and DR3−/− mice. The peritoneal cavity was lavaged, the cells were spun down, and chemokine concentrations in the supernatant were measured by enzyme-linked immunosorbent assay. Data from DR3+/+ and DR3−/− mice where available, but some values were lower than the manufacturer's detection threshold and therefore excluded from the analysis. No significant differences were detected. n = 3 (DR3+/+ and DR3−/− mice). CCL, chemokine ligand; conc, concentration.
References
- 1.Bodmer J.L., Burns K., Schneider P., Hofmann K., Steiner V., Thome M., Bornand T., Hahne M., Schroter M., Becker K., Wilson A., French L.E., Browning J.L., MacDonald H.R., Tschopp J. TRAMP, a novel apoptosis-mediating receptor with sequence homology to tumor necrosis factor receptor 1 and Fas(Apo-1/CD95) Immunity. 1997;6:79–88. doi: 10.1016/s1074-7613(00)80244-7. [DOI] [PubMed] [Google Scholar]
- 2.Chinnaiyan A.M., O'Rourke K., Yu G.L., Lyons R.H., Garg M., Duan D.R., Xing L., Gentz R., Ni J., Dixit V.M. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science. 1996;274:990–992. doi: 10.1126/science.274.5289.990. [DOI] [PubMed] [Google Scholar]
- 3.Kitson J., Raven T., Jiang Y.P., Goeddel D.V., Giles K.M., Pun K.T., Grinham C.J., Brown R., Farrow S.N. A death-domain-containing receptor that mediates apoptosis. Nature. 1996;384:372–375. doi: 10.1038/384372a0. [DOI] [PubMed] [Google Scholar]
- 4.Marsters S.A., Sheridan J.P., Donahue C.J., Pitti R.M., Gray C.L., Goddard A.D., Bauer K.D., Ashkenazi A. Apo-3, a new member of the tumor necrosis factor receptor family, contains a death domain and activates apoptosis and NF-kappa B. Curr Biol. 1996;6:1669–1676. doi: 10.1016/s0960-9822(02)70791-4. [DOI] [PubMed] [Google Scholar]
- 5.Tan K.B., Harrop J., Reddy M., Young P., Terrett J., Emery J., Moore G., Truneh A. Characterization of a novel TNF-like ligand and recently described TNF ligand and TNF receptor superfamily genes and their constitutive and inducible expression in hematopoietic and non-hematopoietic cells. Gene. 1997;204:35–46. doi: 10.1016/s0378-1119(97)00509-x. [DOI] [PubMed] [Google Scholar]
- 6.Screaton G.R., Xu X.N., Olsen A.L., Cowper A.E., Tan R., McMichael A.J., Bell J.I. LARD: a new lymphoid-specific death domain containing receptor regulated by alternative pre-mRNA splicing. Proc Natl Acad Sci U S A. 1997;94:4615–4619. doi: 10.1073/pnas.94.9.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Migone T.S., Zhang J., Luo X., Zhuang L., Chen C., Hu B., Hong J.S., Perry J.W., Chen S.F., Zhou J.X., Cho Y.H., Ullrich S., Kanakaraj P., Carrell J., Boyd E., Olsen H.S., Hu G., Pukac L., Liu D., Ni J., Kim S., Gentz R., Feng P., Moore P.A., Ruben S.M., Wei P. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002;16:479–492. doi: 10.1016/s1074-7613(02)00283-2. [DOI] [PubMed] [Google Scholar]
- 8.Yamazaki K., McGovern D., Ragoussis J., Paolucci M., Butler H., Jewell D., Cardon L., Takazoe M., Tanaka T., Ichimori T., Saito S., Sekine A., Iida A., Takahashi A., Tsunoda T., Lathrop M., Nakamura Y. Single nucleotide polymorphisms in TNFSF15 confer susceptibility to Crohn's disease. Hum Mol Genet. 2005;14:3499–3506. doi: 10.1093/hmg/ddi379. [DOI] [PubMed] [Google Scholar]
- 9.Jostins L., Ripke S., Weersma R.K., Duerr R.H., McGovern D.P., Hui K.Y. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamazaki K., Umeno J., Takahashi A., Hirano A., Johnson T.A., Kumasaka N., Morizono T., Hosono N., Kawaguchi T., Takazoe M., Yamada T., Suzuki Y., Tanaka H., Motoya S., Hosokawa M., Arimura Y., Shinomura Y., Matsui T., Matsumoto T., Iida M., Tsunoda T., Nakamura Y., Kamatani N., Kubo M. A genome-wide association study identifies 2 susceptibility loci for Crohn's disease in a Japanese population. Gastroenterology. 2013;144:781–788. doi: 10.1053/j.gastro.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Zucchelli M., Camilleri M., Andreasson A.N., Bresso F., Dlugosz A., Halfvarson J., Torkvist L., Schmidt P.T., Karling P., Ohlsson B., Duerr R.H., Simren M., Lindberg G., Agreus L., Carlson P., Zinsmeister A.R., D'Amato M. Association of TNFSF15 polymorphism with irritable bowel syndrome. Gut. 2011;60:1671–1677. doi: 10.1136/gut.2011.241877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zinovieva E., Bourgain C., Kadi A., Letourneur F., Izac B., Said-Nahal R., Lebrun N., Cagnard N., Vigier A., Jacques S., Miceli-Richard C., Garchon H.J., Heath S., Charon C., Bacq D., Boland A., Zelenika D., Chiocchia G., Breban M. Comprehensive linkage and association analyses identify haplotype, near to the TNFSF15 gene, significantly associated with spondyloarthritis. PLoS Genet. 2009;5:e1000528. doi: 10.1371/journal.pgen.1000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura M., Nishida N., Kawashima M., Aiba Y., Tanaka A., Yasunami M. Genome-wide association study identifies TNFSF15 and POU2AF1 as susceptibility loci for primary biliary cirrhosis in the Japanese population. Am J Hum Genet. 2012;91:721–728. doi: 10.1016/j.ajhg.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osawa K., Takami N., Shiozawa K., Hashiramoto A., Shiozawa S. Death receptor 3 (DR3) gene duplication in a chromosome region 1p36.3: gene duplication is more prevalent in rheumatoid arthritis. Genes Immun. 2004;5:439–443. doi: 10.1038/sj.gene.6364097. [DOI] [PubMed] [Google Scholar]
- 15.Zhang F.R., Huang W., Chen S.M., Sun L.D., Liu H., Li Y. Genomewide association study of leprosy. N Engl J Med. 2009;361:2609–2618. doi: 10.1056/NEJMoa0903753. [DOI] [PubMed] [Google Scholar]
- 16.Bamias G., Siakavellas S.I., Stamatelopoulos K.S., Chryssochoou E., Papamichael C., Sfikakis P.P. Circulating levels of TNF-like cytokine 1A (TL1A) and its decoy receptor 3 (DcR3) in rheumatoid arthritis. Clin Immunol. 2008;129:249–255. doi: 10.1016/j.clim.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Bamias G., Kaltsa G., Siakavellas S.I., Papaxoinis K., Zampeli E., Michopoulos S., Zouboulis-Vafiadis I., Ladas S.D. High intestinal and systemic levels of decoy receptor 3 (DcR3) and its ligand TL1A in active ulcerative colitis. Clin Immunol. 2010;137:242–249. doi: 10.1016/j.clim.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Bamias G., Evangelou K., Vergou T., Tsimaratou K., Kaltsa G., Antoniou C., Kotsinas A., Kim S., Gorgoulis V., Stratigos A.J., Sfikakis P.P. Upregulation and nuclear localization of TNF-like cytokine 1A (TL1A) and its receptors DR3 and DcR3 in psoriatic skin lesions. Exp Dermatol. 2011;20:725–731. doi: 10.1111/j.1600-0625.2011.01304.x. [DOI] [PubMed] [Google Scholar]
- 19.Aiba Y., Harada K., Komori A., Ito M., Shimoda S., Nakamura H., Nagaoka S., Abiru S., Migita K., Ishibashi H., Nakanuma Y., Nishida N., Kawashima M., Tokunaga K., Yatsuhashi H., Nakamura M. Systemic and local expression levels of TNF-like ligand 1A and its decoy receptor 3 are increased in primary biliary cirrhosis. Liver Int. 2014;34:679–688. doi: 10.1111/liv.12296. [DOI] [PubMed] [Google Scholar]
- 20.Konsta M., Bamias G., Tektonidou M.G., Christopoulos P., Iliopoulos A., Sfikakis P.P. Increased levels of soluble TNF-like cytokine 1A in ankylosing spondylitis. Rheumatology (Oxford) 2013;52:448–451. doi: 10.1093/rheumatology/kes316. [DOI] [PubMed] [Google Scholar]
- 21.Meylan F., Song Y.J., Fuss I., Villarreal S., Kahle E., Malm I.J., Acharya K., Ramos H.L., Lo L., Mentink-Kane M.M., Wynn T.A., Migone T.S., Strober W., Siegel R.M. The TNF-family cytokine TL1A drives IL-13-dependent small intestinal inflammation. Mucosal Immunol. 2011;4:172–185. doi: 10.1038/mi.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taraban V.Y., Slebioda T.J., Willoughby J.E., Buchan S.L., James S., Sheth B., Smyth N.R., Thomas G.J., Wang E.C., Al-Shamkhani A. Sustained TL1A expression modulates effector and regulatory T-cell responses and drives intestinal goblet cell hyperplasia. Mucosal Immunol. 2011;4:186–196. doi: 10.1038/mi.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shih D.Q., Barrett R., Zhang X., Yeager N., Koon H.W., Phaosawasdi P., Song Y., Ko B., Wong M.H., Michelsen K.S., Martins G., Pothoulakis C., Targan S.R. Constitutive TL1A (TNFSF15) expression on lymphoid or myeloid cells leads to mild intestinal inflammation and fibrosis. PLoS One. 2011;6:e16090. doi: 10.1371/journal.pone.0016090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bamias G., Mishina M., Nyce M., Ross W.G., Kollias G., Rivera-Nieves J., Pizarro T.T., Cominelli F. Role of TL1A and its receptor DR3 in two models of chronic murine ileitis. Proc Natl Acad Sci U S A. 2006;103:8441–8446. doi: 10.1073/pnas.0510903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang L., Adkins B., Deyev V., Podack E.R. Essential role of TNF receptor superfamily 25 (TNFRSF25) in the development of allergic lung inflammation. J Exp Med. 2008;205:1037–1048. doi: 10.1084/jem.20072528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meylan F., Davidson T.S., Kahle E., Kinder M., Acharya K., Jankovic D., Bundoc V., Hodges M., Shevach E.M., Keane-Myers A., Wang E.C., Siegel R.M. The TNF-family receptor DR3 is essential for diverse T cell-mediated inflammatory diseases. Immunity. 2008;29:79–89. doi: 10.1016/j.immuni.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pappu B.P., Borodovsky A., Zheng T.S., Yang X., Wu P., Dong X., Weng S., Browning B., Scott M.L., Ma L., Su L., Tian Q., Schneider P., Flavell R.A., Dong C., Burkly L.C. TL1A-DR3 interaction regulates Th17 cell function and Th17-mediated autoimmune disease. J Exp Med. 2008;205:1049–1062. doi: 10.1084/jem.20071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takedatsu H., Michelsen K.S., Wei B., Landers C.J., Thomas L.S., Dhall D., Braun J., Targan S.R. TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology. 2008;135:552–567. doi: 10.1053/j.gastro.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schreiber T.H., Wolf D., Tsai M.S., Chirinos J., Deyev V.V., Gonzalez L., Malek T.R., Levy R.B., Podack E.R. Therapeutic Treg expansion in mice by TNFRSF25 prevents allergic lung inflammation. J Clin Invest. 2010;120:3629–3640. doi: 10.1172/JCI42933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bull M.J., Williams A.S., Mecklenburgh Z., Calder C.J., Twohig J.P., Elford C., Evans B.A., Rowley T.F., Slebioda T.J., Taraban V.Y., Al-Shamkhani A., Wang E.C. The death receptor 3/TNF-like protein 1A pathway drives adverse bone pathology in inflammatory arthritis. J Exp Med. 2008;205:2457–2464. doi: 10.1084/jem.20072378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calder C.J., Wang E.C. An essential role for death receptor 3 in experimental autoimmune uveoretinitis. Ocul Immunol Inflamm. 2012;20:212–214. doi: 10.3109/09273948.2012.658135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buchan S.L., Taraban V.Y., Slebioda T.J., James S., Cunningham A.F., Al-Shamkhani A. Death receptor 3 is essential for generating optimal protective CD4(+) T-cell immunity against Salmonella. Eur J Immunol. 2012;42:580–588. doi: 10.1002/eji.201041950. [DOI] [PubMed] [Google Scholar]
- 33.Twohig J.P., Marsden M., Cuff S.M., Ferdinand J.R., Gallimore A.M., Perks W.V., Al-Shamkhani A., Humphreys I.R., Wang E.C. The death receptor 3/TL1A pathway is essential for efficient development of antiviral CD4+ and CD8+ T cell immunity. FASEB J. 2012;26:3575–3586. doi: 10.1096/fj.11-200618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meylan F., Hawley E.T., Barron L., Barlow J.L., Penumetcha P., Pelletier M., Sciume G., Richard A.C., Hayes E.T., Gomez-Rodriguez J., Chen X., Paul W.E., Wynn T.A., McKenzie A.N., Siegel R.M. The TNF-family cytokine TL1A promotes allergic immunopathology through group 2 innate lymphoid cells. Mucosal Immunol. 2013;7:958–968. doi: 10.1038/mi.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu X., Pappu R., Ramirez-Carrozzi V., Ota N., Caplazi P., Zhang J., Yan D., Xu M., Lee W.P., Grogan J.L. TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers. Mucosal Immunol. 2013;7:730–740. doi: 10.1038/mi.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones G.W., Stumhofer J.S., Foster T., Twohig J.P., Hertzog P., Topley N., Williams A.S., Hunter C.A., Jenkins B.J., Wang E.C., Jones S.A. Naive and activated T cells display differential responsiveness to TL1A that affects Th17 generation, maintenance, and proliferation. FASEB J. 2011;25:409–419. doi: 10.1096/fj.10-166843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richard A.C., Tan C., Hawley E.T., Gomez-Rodriguez J., Goswami R., Yang X.P., Cruz A.C., Penumetcha P., Hayes E.T., Pelletier M., Gabay O., Walsh M., Ferdinand J.R., Keane-Myers A., Choi Y., O'Shea J.J., Al-Shamkhani A., Kaplan M.H., Gery I., Siegel R.M., Meylan F. The TNF-family ligand TL1A and its receptor DR3 promote T cell-mediated allergic immunopathology by enhancing differentiation and pathogenicity of IL-9-producing T cells. J Immunol. 2015;194:3567–3582. doi: 10.4049/jimmunol.1401220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cavallini C., Lovato O., Bertolaso A., Pacelli L., Zoratti E., Zanolin E., Krampera M., Zamo A., Tecchio C., Cassatella M.A., Pizzolo G., Scupoli M.T. The TNF-family cytokine TL1A inhibits proliferation of human activated B cells. PLoS One. 2013;8:e60136. doi: 10.1371/journal.pone.0060136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLaren J.E., Calder C.J., McSharry B.P., Sexton K., Salter R.C., Singh N.N., Wilkinson G.W., Wang E.C., Ramji D.P. The TNF-like protein 1A-death receptor 3 pathway promotes macrophage foam cell formation in vitro. J Immunol. 2010;184:5827–5834. doi: 10.4049/jimmunol.0903782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Twohig J.P., Roberts M.I., Gavalda N., Rees-Taylor E.L., Giralt A., Adams D., Brooks S.P., Bull M.J., Calder C.J., Cuff S., Yong A.A., Alberch J., Davies A., Dunnett S.B., Tolkovsky A.M., Wang E.C. Age-dependent maintenance of motor control and corticostriatal innervation by death receptor 3. J Neurosci. 2010;30:3782–3792. doi: 10.1523/JNEUROSCI.1928-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Lamki R.S., Wang J., Tolkovsky A.M., Bradley J.A., Griffin J.L., Thiru S., Wang E.C., Bolton E., Min W., Moore P., Pober J.S., Bradley J.R. TL1A both promotes and protects from renal inflammation and injury. J Am Soc Nephrol. 2008;19:953–960. doi: 10.1681/ASN.2007060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Lamki R.S., Lu W., Finlay S., Twohig J.P., Wang E.C., Tolkovsky A.M., Bradley J.R. DR3 signaling protects against cisplatin nephrotoxicity mediated by TNF. Am J Pathol. 2012;180:1454–1464. doi: 10.1016/j.ajpath.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Borysenko C.W., Garcia-Palacios V., Griswold R.D., Li Y., Iyer A.K., Yaroslavskiy B.B., Sharrow A.C., Blair H.C. Death receptor-3 mediates apoptosis in human osteoblasts under narrowly regulated conditions. J Cell Physiol. 2006;209:1021–1028. doi: 10.1002/jcp.20812. [DOI] [PubMed] [Google Scholar]
- 44.Collins F.L., Williams J.O., Bloom A.C., Stone M.D., Choy E., Wang E.C., Williams A.S. Death receptor 3 (TNFRSF25) increases mineral apposition by osteoblasts and region specific new bone formation in the axial skeleton of male DBA/1 mice. J Immunol Res. 2015;2015:901679. doi: 10.1155/2015/901679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McLoughlin R.M., Witowski J., Robson R.L., Wilkinson T.S., Hurst S.M., Williams A.S., Williams J.D., Rose-John S., Jones S.A., Topley N. Interplay between IFN-gamma and IL-6 signaling governs neutrophil trafficking and apoptosis during acute inflammation. J Clin Invest. 2003;112:598–607. doi: 10.1172/JCI17129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hurst S.M., Wilkinson T.S., McLoughlin R.M., Jones S., Horiuchi S., Yamamoto N., Rose-John S., Fuller G.M., Topley N., Jones S.A. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–714. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 47.Fielding C.A., Jones G.W., McLoughlin R.M., McLeod L., Hammond V.J., Uceda J., Williams A.S., Lambie M., Foster T.L., Liao C.T., Rice C.M., Greenhill C.J., Colmont C.S., Hams E., Coles B., Kift-Morgan A., Newton Z., Craig K.J., Williams J.D., Williams G.T., Davies S.J., Humphreys I.R., O'Donnell V.B., Taylor P.R., Jenkins B.J., Topley N., Jones S.A. Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity. 2014;40:40–50. doi: 10.1016/j.immuni.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang E.C., Thern A., Denzel A., Kitson J., Farrow S.N., Owen M.J. DR3 regulates negative selection during thymocyte development. Mol Cell Biol. 2001;21:3451–3461. doi: 10.1128/MCB.21.10.3451-3461.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stacey M.A., Marsden M., Wang E.C., Wilkinson G.W., Humphreys I.R. IL-10 restricts activation-induced death of NK cells during acute murine cytomegalovirus infection. J Immunol. 2011;187:2944–2952. doi: 10.4049/jimmunol.1101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stacey M.A., Marsden M., Pham N.T., Clare S., Dolton G., Stack G., Jones E., Klenerman P., Gallimore A.M., Taylor P.R., Snelgrove R.J., Lawley T.D., Dougan G., Benedict C.A., Jones S.A., Wilkinson G.W., Humphreys I.R. Neutrophils recruited by IL-22 in peripheral tissues function as TRAIL-dependent antiviral effectors against MCMV. Cell Host Microbe. 2014;15:471–483. doi: 10.1016/j.chom.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang E.C., Newton Z., Hayward O.A., Clark S.R., Collins F., Perks W.V., Singh R.K., Twohig J.P., Williams A.S. Regulation of early cartilage destruction in inflammatory arthritis by death receptor 3. Arthritis Rheumatol. 2014;66:2762–2772. doi: 10.1002/art.38770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strunk T., Power Coombs M.R., Currie A.J., Richmond P., Golenbock D.T., Stoler-Barak L., Gallington L.C., Otto M., Burgner D., Levy O. TLR2 mediates recognition of live Staphylococcus epidermidis and clearance of bacteremia. PLoS One. 2010;5:e10111. doi: 10.1371/journal.pone.0010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tabeta K., Georgel P., Janssen E., Du X., Hoebe K., Crozat K., Mudd S., Shamel L., Sovath S., Goode J., Alexopoulou L., Flavell R.A., Beutler B. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang Y.J., Kim W.J., Bae H.U., Kim D.I., Park Y.B., Park J.E., Kwon B.S., Lee W.H. Involvement of TL1A and DR3 in induction of pro-inflammatory cytokines and matrix metalloproteinase-9 in atherogenesis. Cytokine. 2005;29:229–235. doi: 10.1016/j.cyto.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Viola A., Luster A.D. Chemokines and their receptors: drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol. 2008;48:171–197. doi: 10.1146/annurev.pharmtox.48.121806.154841. [DOI] [PubMed] [Google Scholar]
- 56.Tecchio C., Cassatella M.A. Neutrophil-derived chemokines on the road to immunity. Semin Immunol. 2016;28:119–128. doi: 10.1016/j.smim.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McLoughlin R.M., Jenkins B.J., Grail D., Williams A.S., Fielding C.A., Parker C.R., Ernst M., Topley N., Jones S.A. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc Natl Acad Sci U S A. 2005;102:9589–9594. doi: 10.1073/pnas.0501794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meylan F., Richard A.C., Siegel R.M. TL1A and DR3, a TNF family ligand-receptor pair that promotes lymphocyte costimulation, mucosal hyperplasia, and autoimmune inflammation. Immunol Rev. 2011;244:188–196. doi: 10.1111/j.1600-065X.2011.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slebioda T.J., Rowley T.F., Ferdinand J.R., Willoughby J.E., Buchan S.L., Taraban V.Y., Al-Shamkhani A. Triggering of TNFRSF25 promotes CD8 T-cell responses and anti-tumor immunity. Eur J Immunol. 2011;41:2606–2611. doi: 10.1002/eji.201141477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow cytometric gating for determining Ki-67+–proliferating cells. Mice were challenged with Staphylococcus epidermidis supernatant, and peritoneal leukocytes were isolated from the cavity by lavage, stained for cell subsets, permeabilized, and proliferation measured using the intracellular marker Ki-67 and DAPI to differentiate binucleate nonproliferating cells. Representative plots of total peritoneal leukocytes show isotype control and Ki-67 staining from DR3+/+ and DR3−/− mice. Early proliferative G1 cells (Ki-67+ DAPIlo), S and G2M phases (Ki-67+ DAPIint/hi), and nonproliferating binucleate cells (Ki-67- DAPIhi) are shown.
Chemokine levels in the naïve peritoneal cavity are not different between DR3+/+ and DR3−/− mice. The peritoneal cavity was lavaged, the cells were spun down, and chemokine concentrations in the supernatant were measured by enzyme-linked immunosorbent assay. Data from DR3+/+ and DR3−/− mice where available, but some values were lower than the manufacturer's detection threshold and therefore excluded from the analysis. No significant differences were detected. n = 3 (DR3+/+ and DR3−/− mice). CCL, chemokine ligand; conc, concentration.