Abstract
Liver X receptors (LXRs) were identified as receptors that sense oxidized cholesterol derivatives. LXRs are best known for their hepatic functions in regulating cholesterol metabolism and triglyceride synthesis, but whether and how LXRs play a role in the lung diseases is less understood. To study the function of LXRs in acute respiratory distress syndrome (ARDS), we applied the oleic acid (OA) model of ARDS to mice whose LXR was genetically or pharmacologically activated. The VP-LXRα knock-in (LXR-KI) mice, in which a constitutively activated LXRα (VP-LXRα) was inserted into the mouse LXRα locus, were used as the genetic gain-of-function model. We showed that the OA-induced lung damages, including the cytokine levels and total cell numbers and neutrophil numbers in the bronchoalveolar lavage fluid, the wet/dry weight ratio, and morphological abnormalities were reduced in the LXR-KI mice and wild-type mice treated with the LXR agonist GW3965. The pulmonoprotective effect of GW3965 was abolished in the LXR-null mice. Consistent with the pulmonoprotective effect of LXR and the induction of antioxidant enzymes by LXR, the OA-induced suppression of superoxide dismutase and catalase was attenuated in LXR-KI mice and GW3965-treated wild-type mice. Taken together, our results demonstrate that activation of LXRs can alleviate OA-induced ARDS by attenuating the inflammatory response and enhancing antioxidant capacity.
Acute lung injury (ALI), which manifests as bilateral pulmonary infiltrates, noncardiogenic pulmonary edema, respiratory distress, and hypoxemia, is a diffuse inflammatory process in the lung in response to a variety of disease conditions.1, 2 Acute respiratory distress syndrome (ARDS) is a life-threatening condition of ALI. ALI and ARDS were first described by Ashbaugh et al3 in 1967. The prevalence of ARDS is a public health concern.4 The clinical management of ARDS is complex and challenging because the fundamental mechanisms that initiate and propagate the lung injury have not been fully understood.5
The defining histopathological feature of ARDS is diffuse alveolar damage. This is characterized by severe alveolar epithelial and microvascular endothelial destruction and general polymorphonuclear leukocyte infiltration, along with intra-alveolar edema, hemorrhage, and fibrin deposition. Hyaline membranes, which are depositions of cell debris and plasma proteins lining the alveolar wall, can be detected in persistent ARDS.6, 7 Cytokines and proinflammatory mediators in the bronchoalveolar lavage (BAL) fluid or serum are reliable indicators of local or systemic inflammation and activation of polymorphonuclear leukocytes, monocytes, and macrophages.8, 9, 10
Animal models are important in understanding the mechanisms of diseases, and these models are most useful if their characteristics are relevant to humans. The oleic acid (cis-9-octadecenoic acid; OA)–induced lung injury represents a reliable model of ARDS. OA is the most common species of free fatty acid in mammals, representing 60% of the free fatty acid pool. When administered i.v., OA causes direct toxicity to the endothelial cells within 1 minute. Within 30 minutes, OA becomes detectable in the air spaces and induces endothelial necrosis and epithelial injury. It is believed that the OA-induced ARDS exhibits histopathological and physiological features similar to its human counterpart during both the acute and repair phases.11, 12, 13
Liver X receptors (LXRs) α (NR1H3) and β (NR1H2) belong to the nuclear receptor family of transcription factors, which on ligand binding, stimulate the expression of their target genes. LXRs were identified as receptors for the oxidized cholesterol derivatives (oxysterols); therefore, they were also named oxysterol receptors.14 In addition, several synthetic LXR agonists, such as GW3965, have been developed.15 LXRα shows a high expression in the liver, lung, adipose, intestine, and kidney, whereas LXRβ is ubiquitously expressed. LXRs play an important role in cholesterol metabolism and triglyceride synthesis in various tissues. However, whether and how LXRs play a role in the pathophysiology of the lung is less understood. We and others reported that activation of LXR attenuated the lipopolysaccharide-induced acute lung injury.16, 17
Herein, we used the OA-induced ARDS model to study the function of LXRs in lung inflammation and injury. We have uncovered a novel role for LXRs in alleviating the OA-induced ARDS. The pulmonary protective function of LXRs is likely because of the positive regulation of anti-inflammatory and antioxidant genes.
Materials and Methods
Animals
Ten-week-old male and female wild-type (WT), LXR knock-in (LXR-KI), and LXR-DKO mice were used. The production of the LXR-KI mice by homologous recombination has previously been described.16 In brief, the LXR-KI mice were generated by knocking-in the constitutively activated VP-LXRα into the mouse LXRα locus. VP-LXRα was generated by fusing the VP16 activation domain of the herpes simplex virus to the amino terminus of mouse LXRα sequence. The homozygous LXR-KI mice express VP-LXRα, whereas the expression of the endogenous LXRα is disrupted. The LXR-DKO mice, lacking both LXRα and LXRβ, were a gift from Dr. David Mangelsdorf (University of Texas Southwestern Medical Center, Dallas, TX). All mice used are in the C56BL/6 background, except the LXR-DKO mice, which are in the mixed background of C56BL/6 and SvJ129. The animal housing conditions and experimental procedures conformed to institutional regulations and were in accordance with the NIH guidelines on animal care. The institutional animal welfare committee approved all procedures.
Preparation of the OA Solutions
OA purchased from Sigma (St. Louis, MO) was used to prepare the 100 mmol/L Tris-oleate solution, as described.18 In brief, after weighting and water addition, the Tris-powder (Trizma base) was slowly added until the pH reached 10.0. This mixture was sonicated and after a complete oleate solubilization, the pH was carefully adjusted to 7.6 with diluted HCl. The working solution of oleate was prepared by appropriate dilutions of the 100 mmol/L Tris-oleate solution with sterile phosphate-buffered saline (pH 7.4).
OA-Induced Lung Injury and LXR Ligand Treatment
For the OA treatment, mice were subjected to tail vein injection of 0.15 mL/kg OA or vehicle (Tris-HCl) in a total volume of 90 μL, and the mice were sacrificed 2 hours after. The 2-hour time point was chosen because previous studies showed that the concentrations of tumor necrosis factor (TNF)-α and IL-6 in the BAL fluid peaked 1 to 3 hours after the OA application,19 and the tissue injury and an increase in the lung wet/dry weight ratio can also be assessed at this time point.20 When necessary, mice were subjected to a daily gavage of 20 mg/kg of the LXR agonist GW3965 in a total volume of 100 μL 3 days before the OA treatment.
BAL Fluid Collection and Cell Counting
These were performed essentially as described.21 In brief, 2 hours after the OA injection, mice were anesthetized by an i.p. injection of 150 mg/kg ketamine and 10 mg/kg xylazine. A small-caliber tube was inserted into the airway after the trachea was isolated by blunt dissection. The BAL fluids were collected by washing three times with 0.8 mL of sterile phosphate-buffered saline. The recovered BAL fluids were centrifuged at 1400 × g for 10 minutes. The total BAL cells in the BAL fluid pellets were counted using a hemocytometer. The polymorphonuclear neutrophils were selectively identified and counted after staining with the May-Grunewald-Giemsa solution from Sigma. The cell-free supernatants of the BAL fluids were collected and subjected to the measurement of protein concentration using a BCA protein assay kit from Pierce/Thermo Fisher Scientific (Pittsburgh, PA).
Measurement of the Lung W/D Ratio
Mice were sacrificed and lungs were excised 2 hours after the OA treatment. Blood was removed by blotting the lungs with filter papers until dry, and the lungs were then immediately weighed. The lungs were subsequently placed in an oven at 65°C for 72 hours, and the dry weight was recorded. The lung wet/dry weight (W/D) ratio was calculated to assess tissue edema.
Measurement of TNF-α and IL-6 in the BAL Fluid and Serum
These were measured using enzyme-linked immunosorbent assay kits from Pierce/Thermo Fisher Scientific, according to the manufacturer's instructions.
Measurement of SOD and CAT Activities
To measure the superoxide dismutase (SOD) and catalase (CAT) activities, lungs were excised and washed thoroughly with phosphate-buffered saline to remove most of the blood contamination, then stored at −80°C until used. Lungs were homogenized in 50 mmol/L potassium phosphate (pH 7.0) and 1 mmol/L ethylene diamine tetra-acetic acid. The homogenates were clarified by centrifugation at 10,000 × g for 30 minutes at 4°C, and the supernatants were immediately used for the measurements of SOD and CAT activities using assay kits from Cayman Chemical (Ann Arbor, MI). The SOD activity was determined using a nitroblue tetrazolium reduction method for the detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. The absorbance was monitored at 440 to 460 nm. The CATC activity was measured by the rate of decrease in hydrogen peroxide absorbance at 540 nm and defined 1 U as the amount of enzyme that will cause the formation of 1.0 nmol of formaldehyde per minute at 25°C. The CAT activity was expressed as nmol/minute/mL.
Histology and Immunohistochemistry
The lungs were freshly harvested and fixed in 10% neutral-buffered formalin for 24 hours. The tissues were histologically processed, embedded in paraffin, divided into sections (4 μm thick), and stained with hematoxylin and eosin. For immunohistochemistry, the paraffin sections were deparaffinized and rehydrated. The endogenous peroxidase activity was blocked by incubating the sections in 3% H2O2 solution in methanol. Citrate buffer incubation was used to unmask the antigens. The slides were incubated in blocking buffer in a humidified chamber for 1 hour before incubation with the primary anti-myeloperoxidase (MPO) antibody (catalog number AB9353, dilution at 1:25) from Abcam (Cambridge, MA), or the anti–8-hydroxyguanosine antibody (catalog number AB10802, dilution at 1:400) also from Abcam, for 1 hour. The slides were then washed and incubated in biotinylated secondary antibody and conjugates before the color reaction using 3,3′-diaminobenzidine tetrahydrochloride as the substrate. The slides were counterstained by hematoxylin.
Statistical Analysis
The results are expressed as the means ± SD. One-way analysis of variance and Tukey's test were used for statistical analysis using GraphPad Prism software version 6.0 (GraphPad Software, Inc., La Jolla, CA). P < 0.05 was considered statistically significant.
Results
Generation of LXR-KI Mice that Bear the Constitutive Activation of LXRα in the Lung
We have previously reported that both LXRs α and β are abundantly expressed in the mouse lung.16 Immunohistochemical analysis showed that LXRα is expressed in both the type I and type II lung epithelial cells.16 To study the in vivo functions of LXRα, we generated LXRα knock-in mice that express a constitutively activated LXRα (VP-LXRα) in tissues that express the endogenous LXRα, including the lung.16 The VP-LXRα cDNA was generated by fusing the VP16 activation domain of the herpes simplex virus to the amino terminus of mouse LXRα sequence (Figure 1A). To generate the targeting construct, VP-LXRα cDNA was placed in-frame and immediately after the endogenous ATG start codon of the mouse LXRα locus. After the homologous recombination, the sequence spanning part of exon 2, exons 3 to 7, and the introns in between were replaced by VP-LXRα. As such, VP-LXRα will be expressed under the control of the endogenous LXRα promoter, whereas the WT LXRα will be disrupted in the homozygous LXR-KI mice. The expression of VP-LXRα in the LXR-KI mice was confirmed by Northern blot analysis. The expression of VP-LXRα in the LXR-KI mice was detected in the lung, liver, and small intestine, a panel of tissues known to express LXRα (Figure 1B). In contrast, the endogenous LXRα transcript in these three tissues was detected only in the WT but not in the LXR-KI mice. Little expression of VP-LXRα was detected in the brain of the LXR-KI mice, consistent with the known low expression of this LXR isoform in the brain of the WT mice (Figure 1B).
Figure 1.
Generation of liver X receptor (LXR) α knock-in (LXR-KI) mice that bear the constitutive activation of LXRα in the lung. A: Strategy to knock-in VP-LXRα into the mouse LXRα locus. The positions and numbers of the LXRα gene exons are labeled. On gene targeting, part of exon 2, exons 3 to 7, and introns in between of the wild-type (WT) allele are replaced by the VP-LXRα-SV40-Neo cassette. B: The expression of knock-in VP-LXRα and the endogenous LXRα was determined by Northern blot analysis using a LXRα cDNA probe. Ethidium bromide staining of the agarose gel is to show the sample loading.
Activation of LXR Attenuates the OA-Induced Increases in Pulmonary Permeability and Edema
To determine the effect of LXR activation on the OA-induced ARDS, we first treated male WT and LXR-KI mice with OA by tail vein injections. Two hours later, mice were sacrificed and the lung W/D ratio, BAL fluid protein concentrations, and lung histology were evaluated. The lung W/D ratio is widely used to assess pulmonary vascular permeability, which is a marker of damage to the capillary membrane and one of the key features of experimental ARDS.22 Treatment of WT mice with OA resulted in an expected increase in the lung W/D ratio. In a sharp contrast, the OA-induced lung W/D ratio increase in LXR-KI mice was reduced to approximately half of the OA-treated WT mice (Figure 2A). A significantly decreased lung W/D ratio was also observed in male WT mice pretreated with the synthetic LXR agonist GW3965 for 3 days (Figure 2A). However, the protective effect of GW3965 in OA-treated mice was abolished in mice deficient of both LXRα and LXRβ (LXR-DKO) (Figure 2A). A similar pattern of the inhibitory effect of the LXR-KI allele and GW3965 treatment was observed when the protein concentrations were measured in the BAL fluid (Figure 2B). At the histological level, the OA-induced pulmonary interstitial edema and infiltration of cells into the interstitium and alveolar spaces observed in the WT mice was attenuated in the LXR-KI mice and WT, but not LXR DKO, mice treated with GW3965 (Figure 2C). A similar pattern of attenuation of the OA-responsive increase in pulmonary permeability and edema was observed in the female LXR-KI mice (Supplemental Figure S1), suggesting the protective effect of LXR on OA-induced ARDS was not sex specific.
Figure 2.
Activation of liver X receptor (LXR) attenuates the oleic acid (OA)–induced increases in pulmonary permeability and edema. All mice are males. Wild-type (WT), LXR knock-in (LXR-KI), and LXR-DKO [knockout (KO)] mice received a single tail vein injection of vehicle (Veh) or OA. When necessary, WT and LXR-DKO mice were treated with GW3965 (GW; daily i.p. injections at 20 mg/kg) for 7 days before being treated with Veh or OA. Mice were sacrificed 2 hours after the OA treatment and analyzed. A: The wet/dry weight ratio of the lung tissues. B: The protein concentrations in the cell-free bronchoalveolar lavage (BAL) fluid supernatants. C: Hematoxylin and eosin staining of the lung sections. n = 8 (Veh group, A and B); n = 11 (WT + OA group, A and B); n = 4 (other groups, A and B). ∗P < 0.05, ∗∗P < 0.01. Original magnification, ×200 (C).
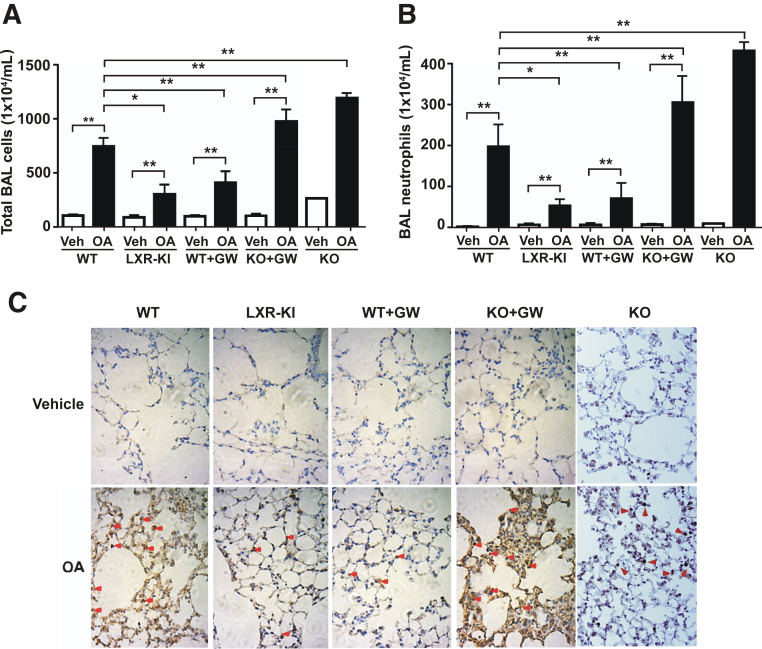
Activation of LXR Attenuates the OA-Induced Infiltration of Neutrophils
Another hallmark of ARDS, including those induced by OA, is the infiltration of neutrophils.13 As expected, treatment of WT male mice with OA resulted in dramatic increases in the total cell numbers (Figure 3A) and neutrophil numbers (Figure 3B) in the BAL fluid. In contrast, the OA-induced total cell and neutrophil number increases in the BAL fluid of the LXR-KI mice were reduced to approximately 50% of the OA-treated WT mice (Figure 3, A and B). The decreased BAL fluid cell and neutrophil numbers were also observed in WT mice treated with GW3965, and the GW3965 effect was abolished in the LXR-DKO mice (Figure 3, A and B). The LXR-DKO mice, in the absence of GW3965 and compared to their WT counterparts, also showed heightened sensitivity to OA (Figure 3, A and B). To further confirm the neutrophil infiltration, we performed immunohistochemical staining of the MPO, a widely used index of polymorphonuclear neutrophil sequestration that reflects the infiltration of lung parenchymal phagocytes.23 Treatment with OA increased the immunostaining of MPO in the lung, and this effect was attenuated in LXR-KI mice and WT mice treated with GW3965 (Figure 3C). Again, the GW3965 effect in reducing the MPO immunostaining was abolished in LXR-DKO mice. The LXR-DKO mice, in the absence of GW3965, also showed increased immunostaining of MPO (Figure 3C). A similar pattern of attenuation of OA-responsive increase in neutrophil infiltration was observed in the female LXR-KI mice (Supplemental Figure S2).
Figure 3.
Activation of liver X receptor (LXR) attenuates the oleic acid (OA)–induced infiltration of neutrophils. Wild-type (WT), LXR knock-in (LXR-KI), and LXR-DKO [knockout (KO)] mice received a single tail vein injection of vehicle (Veh) or OA. When necessary, WT and LXR-DKO mice were treated with GW3965 (GW; daily i.p. injections at 20 mg/kg) for 7 days before being treated with Veh or OA. Mice were sacrificed 2 hours after the OA treatment and analyzed. Last two groups of LXR DKO mice were not treated with GW3965. A: The total bronchoalveolar lavage (BAL) cell numbers were counted using a hemocytometer. B: The polymorphonuclear neutrophils were stained with the May-Grunwald and Giemsa solution, and their cell numbers were counted. C: Immunohistochemical staining of the lung paraffin sections using the anti-myeloperoxidase (MPO) antibody. Arrowheads indicate the positive MPO staining. n = 8 (Veh group, A and B); n = 11 (WT + OA group, A and B); n = 4 (other groups, A and B). ∗P < 0.05, ∗∗P < 0.01. Original magnification, ×400 (C).
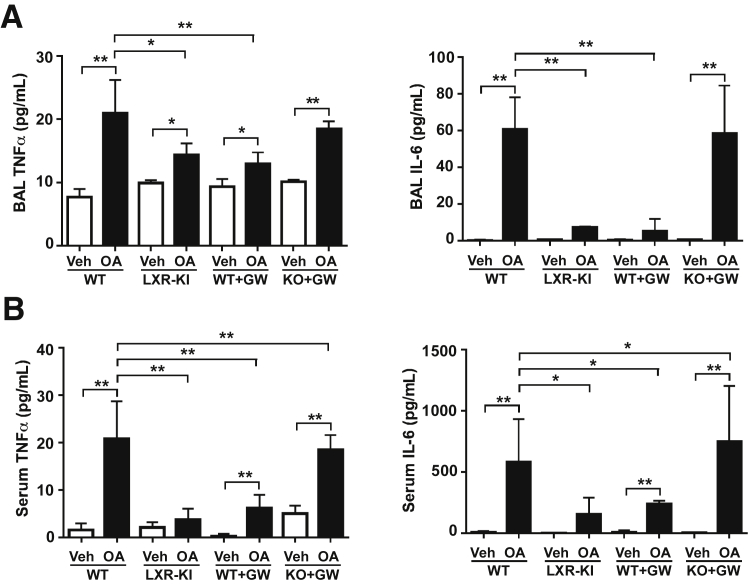
Activation of LXR Attenuates the OA-Induced Local and Systemic Inflammation
A major pathological event associated with ARDS is local and systemic inflammation. The local and systemic inflammation was evaluated by measuring the levels of TNF-α and IL-6 in the BAL fluid and serum, respectively. Indeed, treatment of WT male mice with OA resulted in significantly increased levels of TNF-α and IL-6 in the BAL fluid (Figure 4A) and in the serum (Figure 4B). The OA effect in increasing TNF-α and IL-6 levels in the BAL fluid (Figure 4A) and in the serum (Figure 4B) was substantially attenuated in the LXR-KI mice and the WT mice treated with GW3965. In contrast, the GW3965-treated LXR-DKO mice remained sensitive to OA in inducing the TNF-α and IL-6 levels (Figure 4, A and B). A similar pattern of attenuation of OA-responsive increase in local and systematic inflammation was observed in the female LXR-KI mice (Supplemental Figure S3).
Figure 4.
Activation of liver X receptor (LXR) attenuates the oleic acid (OA)–induced local and systemic inflammation. Wild-type (WT), LXR knock-in (LXR-KI), and LXR-DKO [knockout (KO)] mice received a single tail vein injection of vehicle (Veh) or OA. When necessary, WT and LXR-DKO mice were treated with GW3965 (GW; daily i.p. injections at 20 mg/kg) for 7 days before being treated with Veh or OA. The mice were sacrificed before the bronchoalveolar lavage (BAL) fluid and the blood was collected. A: The concentrations of tumor necrosis factor (TNF)-α and IL-6 in the cell-free BAL fluid supernatants. B: The concentrations of TNF-α and IL-6 in the serum. n = 8 (Veh group); n = 11 (WT + OA group); n = 4 (other groups). ∗P < 0.05, ∗∗P < 0.01.
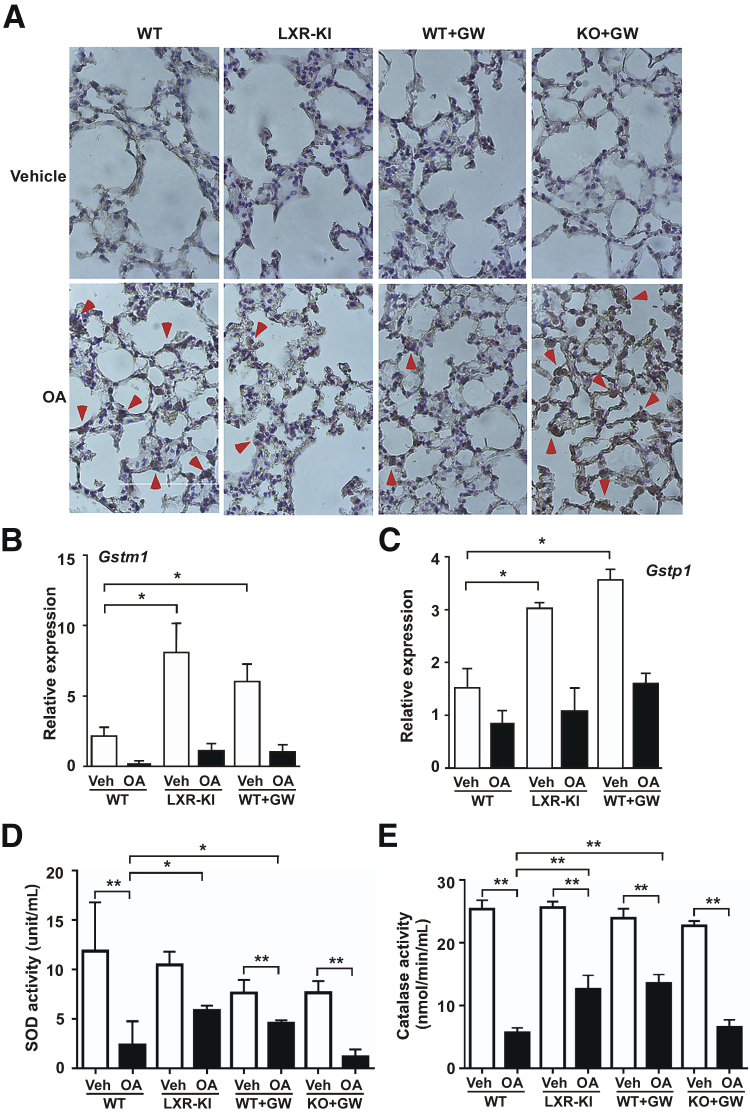
The Protective Effect of LXR Is Associated with Attenuation of OA-Induced Oxidative Stress and Induction of Antioxidant Genes
Oxidative stress plays an important role in the development of OA-induced ARDS.24 Intracellular oxidative stress can lead to the generation of nucleic acid oxidation products, such as the 8-hydroxyguanosine, which has been widely used as a biomarker of oxidative stress.25 Indeed, we showed that treatment with OA increased the immunostaining of 8-hydroxyguanosine in the lung, and this effect was attenuated in LXR-KI mice and WT mice treated with GW3965. The GW3965 effect in reducing the 8-hydroxyguanosine immunostaining was abolished in LXR-DKO mice (Figure 5A). In understanding the protective effect of LXR activation, we found the lung expression of several antioxidant genes, such as the glutathione S-transferase m1 (Gstm1; Figure 5B) and Gstp1 (Figure 5C), was increased in both the LXR-KI mice and the GW3865-treated WT mice in the absence of OA challenge, consistent with our previous report.16 Interestingly, the expression of both Gst isoforms was decreased when mice were treated with OA regardless of the LXR activation, which might be secondary to the tissue damage and responses to the tissue damage. We then measured the activities of SODs and CAT that indicate the overall oxidative stress. SODs and CAT are direct antioxidants. SODs catalyze the dismutation of the superoxide anion to molecular oxygen and hydrogen peroxide.26, 27 CAT is a ubiquitous antioxidant enzyme that is involved in the detoxification of hydrogen peroxide, a reactive oxygen species that causes cellular damages. We found that treatment of male WT mice with OA resulted in significant suppressions of the activities of SODs (Figure 5D) and CAT (Figure 5E) in the lung, and these suppressions were attenuated in the male LXR-KI mice and WT mice treated with GW3965. The effect of GW3965 in attenuating the suppression of SOD and CAT was abolished in the LXR-DKO mice (Figure 5, D and E). A similar pattern of attenuation of suppression of SOD and CAT was observed in the female LXR-KI mice (Supplemental Figure S4).
Figure 5.
The protective effect of liver X receptor (LXR) is associated with attenuation of oleic acid (OA)–induced oxidative stress and induction of antioxidant genes. A: Immunohistochemical staining of the lung paraffin sections using the anti–8-hydroxyguanosine antibody. Wild-type (WT), LXR knock-in (LXR-KI), and LXR-DKO [knockout (KO)] mice received a single tail vein injection of vehicle (Veh) or OA. When necessary, WT and LXR-DKO mice were treated with GW3965 (GW; daily i.p. injections at 20 mg/kg) for 7 days before being treated with Veh or OA. Mice were sacrificed 2 hours after the OA treatment and analyzed. Arrowheads indicate the positive staining. The lung expression of antioxidant genes Gstm1 (B) and Gstp1 (C) in WT and LXR-KI mice and GW3965-treated WT mice, in the absence or presence of OA treatment, was measured by real-time PCR. The activities of superoxide dismutase (SOD; D) and catalase (E) were measured in the lung homogenates. n = 5 (each group, B and C); n = 8 (WT Veh group, D and E); n = 11 (WT + OA group, D and E); n = 4 (other groups, D and E). ∗P < 0.05, ∗∗P < 0.01. Original magnification, ×400 (A).
Discussion
Herein, we demonstrated the preventive effect of LXR on ARDS. Genetic or pharmacological activation of LXR effectively attenuated the OA-induced pulmonary edema, infiltration of neutrophils, production of proinflammatory cytokines, and oxidative stress.
We chose the OA model of ARDS, because the pulmonary injury responses induced by the i.v. administration of OA resemble the clinical ARDS. The OA model also mimics the critical pathogenesis of ALI, especially the marked accumulation of polymorphonuclear neutrophils and the release of inflammatory mediators.13 Moreover, the OA model provokes symptoms similar to those observed in human patients. Indeed, all of the hallmarks of ARDS, including inflammation, neutrophil activation, capillary leakage, tissue edema, and oxidative stress, were presented in the OA-treated WT mice. These pathological features were all significantly attenuated in the LXR-KI mice in which LXRα was genetically activated, or in WT mice whose LXRs were pharmacologically activated by GW3965. The pulmonoprotective effect of GW3965 was abolished in the LXR-null mice, pinpointing that the GW3965 effect was mediated by LXRs. Sex-specific differences in pulmonary morbidity in humans are well documented. The mortality of ARDS was higher in male patients compared to female patients.28 In our model, both the male and female LXR-KI mice were protected from the OA-induced ARDS.
The anti-ARDS effect of LXR may have been contributed to by its positive regulation of antioxidant genes and the consequent relief of oxidative stress. The lung is susceptible to oxidative stresses that are derived from oxygen or inflammatory responses.9 The imbalance of oxidants and antioxidants plays an important role in the initiation and progression of ARDS. Oxidative stress also affects inflammatory responses by altering the activities of SOD, c-Jun N-terminal kinase, and mitogen-activated protein kinase and concentrations of cytokines.29 The expression of several antioxidant genes was induced in the LXR-KI mice and in GW3965-treated WT mice, consistent with our previous report that activation of LXR in mice induces antioxidant genes, leading to protection from lipopolysaccharide-16 and acetaminophen-30 induced lung and liver tissue damages, respectively.
The large numbers of activated neutrophils in the lung in ARDS are generally considered as the major source of reactive oxygen species.13, 31 Given that activation of LXR attenuated the OA-induced infiltration of neutrophils (Figure 3), it is possible that reducing the recruitment of neutrophils to lung by LXR activation may represent another potential mechanism by which LXR relieves ARDS. The inhibition of inflammation and infiltration of neutrophils by LXR has also been reported in experimental intracerebral hemorrhage and spinal cord trauma.32, 33 Future studies are necessary to determine whether LXR activation in neutrophils affects their cytokine production, phagocytic function, and reactive oxygen species generation in OA-induced ARDS model.
In conclusion, we have uncovered a novel function of LXR in preventing ARDS. LXRs have been reported to have multiple potential therapeutic benefits, ranging from anti-atherosclerosis to anti-inflammation,34, 35 macrophage survival and the innate immune response,36, 37 and anti-Alzheimer disease.38 Although avoiding the adverse effect of LXR activation, such as hepatic steatosis,39 remains a challenge, the anti-ARDS activity of LXR further warrants continued exploration of LXRs as therapeutic targets.
Acknowledgments
We thank Dr. David Mangelsdorf (University of Texas Southwestern Medical Center, Dallas, TX) for the liver X receptor DKO mice. We also thank Dr. Haibiao Gong (Fluidigm, San Francisco, CA) for his original creation of the VP-LXR knock-in mice and Dr. Gregg E. Homanics (University of Pittsburgh, Pittsburgh, PA) for his assistance in the creation of the VP-LXR knock-in mice.
Z.Z., D.X., and W.X. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Supported in part by NIH grants ES023438 and DK083952 (W.X.), the National Natural Science Foundation of China Youth Science Fund Project 81402641 (Z.Z.), and the China Scholarship Council Visiting Scholarship 201506275099 (D.X.).
Disclosures: None disclosed.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2016.06.018.
Supplemental Data
Activation of liver X receptor (LXR) attenuates the oleic acid (OA)–induced increases in pulmonary permeability and edema in female mice. All mice are females. Wild-type (WT) and LXR knock-in (LXR-KI) mice received a single tail vein injection of vehicle or OA. Mice were sacrificed 2 hours after the OA treatment and analyzed. A: The wet/dryweight ratio of the lung tissues. B: The protein concentrations in the cell-free bronchoalveolar lavage (BAL) fluid supernatants. C: Hematoxylin and eosin staining of the lung sections. n = 6 (WT + OA group, A and B); n = 4 (other groups, A and B). ∗∗P < 0.01. Original magnification, ×200 (C).
Activation of liver X receptor (LXR) attenuates the oleic acid (OA)–induced infiltration of neutrophils in female mice. Wild-type (WT) and LXR knock-in (LXR-KI) mice received a single tail vein injection of vehicle or OA. Mice were sacrificed 2 hours after the OA treatment and analyzed. A: The total bronchoalveolar lavage (BAL) cell numbers were counted using a hemocytometer. B: The polymorphonuclear neutrophils were stained with the May-Grunwald and Giemsa solution, and their cell numbers were counted. C: Immunohistochemical staining of the lung paraffin sections using the anti-myeloperoxidase (MPO) antibody. Arrowheads indicate the positive MPO staining. n = 6 (WT + OA group, A and B); n = 4 (other groups, A and B). ∗P < 0.05, ∗∗P < 0.01. Original magnification, ×400 (C).
Activation of liver X receptor (LXR) attenuates the oleic acid (OA)–induced local and systematic inflammation in female mice. Wild-type (WT) and LXR knock-in (LXR-KI) mice received a single tail vein injection of vehicle or OA. Mice were sacrificed 2 hours after the OA treatment and analyzed. The mice were sacrificed before the bronchoalveolar lavage (BAL) fluid and the blood was collected. A: The concentrations of tumor necrosis factor (TNF)-α and IL-6 in the cell-free BAL supernatants (A) and in the serum (B). n = 6 (WT + OA group); n = 4 (other groups). ∗P < 0.05, ∗∗P < 0.01.
The protective effect of liver X receptor (LXR) is associated with attenuation of oleic acid (OA)–induced oxidative stress in female mice. Wild-type (WT) and LXR knock-in (LXR-KI) mice received a single tail vein injection of vehicle or OA. Mice were sacrificed 2 hours after the OA treatment and analyzed. The activities of superoxide dismutase (SOD; A) and catalase (B) were measured in the lung homogenates. n = 6 (WT + OA group, A and B); n = 4 (other groups, A and B). ∗P < 0.05, ∗∗P < 0.01.
References
- 1.Dagvadorj J., Shimada K., Chen S., Jones H.D., Tumurkhuu G., Zhang W., Wawrowsky K.A., Crother T.R., Arditi M. Lipopolysaccharide induces alveolar macrophage necrosis via CD14 and the P2X7 receptor leading to interleukin-1α release. Immunity. 2015;42:640–653. doi: 10.1016/j.immuni.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthay M.A., Ware L.B., Zimmerman G.A. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashbaugh D.G., Bigelow D.B., Petty T.L., Levine B.E. Acute respiratory distress in adults. Lancet. 1967;2:319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 4.Gordon D., Rubenfeld G.D., Caldwell E., Peabody E., Weaver J., Martin D.P., Neff M., Stern E.J., Hudson L.D. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 5.Han S., Mallampalli R.K. The acute respiratory distress syndrome: from mechanism to translation. J Immunol. 2015;194:855–860. doi: 10.4049/jimmunol.1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthay M.A., Calfee C.S. Therapeutic value of a lung protective ventilation strategy in acute lung injury. Chest. 2005;128:3089–3091. doi: 10.1378/chest.128.5.3089. [DOI] [PubMed] [Google Scholar]
- 7.Ware L.B., Matthay M.A. Clinical practice: acute pulmonary edema. N Engl J Med. 2005;353:2788–2796. doi: 10.1056/NEJMcp052699. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia M., Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145–156. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 9.Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 10.Lang J.D., McArdle P.J., O'Reilly P.J., Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest. 2002;122:314S–320S. doi: 10.1378/chest.122.6_suppl.314s. [DOI] [PubMed] [Google Scholar]
- 11.Hussain N., Wu F., Zhu L., Thrall R.S., Kresch M.J. Neutrophil apoptosis during the development and resolution of oleic acid-induced acute lung injury in the rat. Am J Respir Cell Mol Biol. 1998;19:867–874. doi: 10.1165/ajrcmb.19.6.3118. [DOI] [PubMed] [Google Scholar]
- 12.Motohiro A., Furukawa T., Yasumoto K., Inokuchi K. Mechanisms involved in acute lung edema induced in dogs by oleic acid. Eur Surg Res. 1986;18:50–57. doi: 10.1159/000128505. [DOI] [PubMed] [Google Scholar]
- 13.Matute-Bello G., Frevert C.W., Martin T.R. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Repa J.J., Mangelsdorf D.J. The liver X receptor gene team: potential new players in atherosclerosis. Nat Med. 2002;8:1243–1248. doi: 10.1038/nm1102-1243. [DOI] [PubMed] [Google Scholar]
- 15.Collins J.L., Fivush A.M., Watson M.A., Galardi C.M., Lewis M.C., Moore L.B., Parks D.J., Wilson J.G., Tippin T.K., Binz J.G., Plunket K.D., Morgan D.G., Beaudet E.J., Whitney K.D., Kliewer S.A., Willson T.M. Identification of a nonsteroidal liver X receptor agonist through parallel array synthesis of tertiary amines. J Med Chem. 2002;45:1963–1966. doi: 10.1021/jm0255116. [DOI] [PubMed] [Google Scholar]
- 16.Gong H., He J., Lee J.H., Mallick E., Gao X., Li S., Homanics G.E., Xie W. Activation of the liver X receptor prevents lipopolysaccharide-induced lung injury. J Biol Chem. 2009;284:30113–30121. doi: 10.1074/jbc.M109.047753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smoak K., Madenspacher J., Jeyaseelan S., Williams B., Dixon D., Poch K.R., Nick J.A., Worthen G.S., Fessler M.B. Effects of liver X receptor agonist treatment on pulmonary inflammation and host defense. J Immunol. 2008;180:3305–3312. doi: 10.4049/jimmunol.180.5.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonçalves-de-Albuquerque C.F., Silva A.R., Burth P., de Moraes I.M., Oliveira F.M., Younes-Ibrahim M., dos Santos Mda C., D'Ávila H., Bozza P.T., Faria Neto H.C., Faria M.V. Oleic acid induces lung injury in mice through activation of the ERK pathway. Mediators Inflamm. 2012;2012:1–11. doi: 10.1155/2012/956509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lesur I., Textoris J., Loriod B., Courbon C., Garcia S., Leone M., Nguyen C. Gene expression profiles characterize inflammation stages in the acute lung injury in mice. PLoS One. 2010;5:e11485. doi: 10.1371/journal.pone.0011485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai J.P., Bao S., Davis I.C., Knoell D.L. Inhibition of the phosphatase PTEN protects mice against oleic acid-induced acute lung injury. Br J Pharmacol. 2009;156:189–200. doi: 10.1111/j.1476-5381.2008.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson R.F., Muggenburg B.A. Use of bronchoalveolar lavage to detect lung injury. Curr Protoc Toxicol. 2004;Chapter 18:Unit18.4. doi: 10.1002/0471140856.tx1804s21. [DOI] [PubMed] [Google Scholar]
- 22.Li J.W., Wu X. Mesenchymal stem cells ameliorate LPS-induced acute lung injury through KGF promoting alveolar fluid clearance of alveolar type II cells. Eur Rev Med Pharmacol Sci. 2015;19:2368–2378. [PubMed] [Google Scholar]
- 23.Bradley P.P., Priebat D.A., Christensen R.D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 24.Chow C.W., Herrera Abreu M.T., Suzuki T., Downey G.P. Oxidative stress and acute lung injury. Am J Respir Cell Mol Biol. 2003;29:427–431. doi: 10.1165/rcmb.F278. [DOI] [PubMed] [Google Scholar]
- 25.Cadet J., Douki T., Ravanat J.L. Oxidatively generated damage to the guanine moiety of DNA: mechanistic aspects and formation in cells. Acc Chem Res. 2008;41:1075–1083. doi: 10.1021/ar700245e. [DOI] [PubMed] [Google Scholar]
- 26.Cho H.Y., Reddy S.P., Kleeberger S.R. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal. 2006;8:76–87. doi: 10.1089/ars.2006.8.76. [DOI] [PubMed] [Google Scholar]
- 27.Ganguly K., Depner M., Fattman C., Bein K., Oury T.D., Wesselkamper S.C., Borchers M.T., Schreiber M., Gao F., von Mutius E., Kabesch M., Leikauf G.D., Schulz H. Superoxide dismutase 3, extracellular (SOD3) variants and lung function. Physiol Genomics. 2009;37:260–267. doi: 10.1152/physiolgenomics.90363.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moss M., Mannino D.M. Race and gender differences in acute respiratory distress syndrome deaths in the United States: an analysis of multiple-cause mortality data (1979–1996) Crit Care Med. 2002;30:1679–1685. doi: 10.1097/00003246-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura T., Nakamura H., Hoshino T., Ueda S., Wada H., Yodoi J. Redox regulation of lung inflammation by thioredoxin. Antioxid Redox Signal. 2005;7:60–71. doi: 10.1089/ars.2005.7.60. [DOI] [PubMed] [Google Scholar]
- 30.Saini S.P., Zhang B., Niu Y., Jiang M., Gao J., Zhai Y., Lee J.H., Uppal H., Tian H., Tortorici M.A., Poloyac S.M., Qin W., Venkataramanan R., Xie W. Activation of LXR increases acetaminophen clearance and prevents its toxicity in mice. Hepatology. 2011;54:2208–2217. doi: 10.1002/hep.24646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams A.E., Chambers R.C. The mercurial nature of neutrophils: still an enigma in ARDS? Am J Physiol Lung Cell Mol Physiol. 2014;306:217–230. doi: 10.1152/ajplung.00311.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paterniti I., Genovese T., Mazzon E., Crisafulli C., Di Paola R., Galuppo M., Bramanti P., Cuzzocrea S. Liver X receptor agonist treatment regulates inflammatory response after spinal cord trauma. J Neurochem. 2010;112:611–624. doi: 10.1111/j.1471-4159.2009.06471.x. [DOI] [PubMed] [Google Scholar]
- 33.Wu C.H., Chen C.C., Lai C.Y., Hung T.H., Lin C.C., Chao M., Chen S.F. Treatment with TO901317, a synthetic liver X receptor agonist, reduces brain damage and attenuates neuroinflammation in experimental intracerebral hemorrhage. J Neuroinflammation. 2016;13:62. doi: 10.1186/s12974-016-0524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castrillo A., Joseph S.B., Marathe C., Mangelsdorf D.J., Tontonoz P. Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages. J Biol Chem. 2003;278:10443–10449. doi: 10.1074/jbc.M213071200. [DOI] [PubMed] [Google Scholar]
- 35.Castrillo A., Joseph S.B., Vaidya S.A., Haberland M., Fogelman A.M., Cheng G., Tontonoz P. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell. 2003;12:805–816. doi: 10.1016/s1097-2765(03)00384-8. [DOI] [PubMed] [Google Scholar]
- 36.Joseph S.B., Bradley M.N., Castrillo A., Bruhn K.W., Mak P.A., Pei L., Hogenesch J., O'Connell R.M., Cheng G., Saez E., Miller J.F., Tontonoz P. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 37.Joseph S.B., Castrillo A., Laffitte B.A., Mangelsdorf D.J., Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 38.Zelcer N., Khanlou N., Clare R., Jiang Q., Reed-Geaghan E.G., Landreth G.E., Vinters H.V., Tontonoz P. Attenuation of neuroinflammation and Alzheimer's disease pathology by liver x receptors. Proc Natl Acad Sci U S A. 2007;104:10601–10606. doi: 10.1073/pnas.0701096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Repa J.J., Liang G., Ou J., Bashmakov Y., Lobaccaro J.M., Shimomura I., Shan B., Brown M.S., Goldstein J.L., Mangelsdorf D.J. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Activation of liver X receptor (LXR) attenuates the oleic acid (OA)–induced increases in pulmonary permeability and edema in female mice. All mice are females. Wild-type (WT) and LXR knock-in (LXR-KI) mice received a single tail vein injection of vehicle or OA. Mice were sacrificed 2 hours after the OA treatment and analyzed. A: The wet/dryweight ratio of the lung tissues. B: The protein concentrations in the cell-free bronchoalveolar lavage (BAL) fluid supernatants. C: Hematoxylin and eosin staining of the lung sections. n = 6 (WT + OA group, A and B); n = 4 (other groups, A and B). ∗∗P < 0.01. Original magnification, ×200 (C).
Activation of liver X receptor (LXR) attenuates the oleic acid (OA)–induced infiltration of neutrophils in female mice. Wild-type (WT) and LXR knock-in (LXR-KI) mice received a single tail vein injection of vehicle or OA. Mice were sacrificed 2 hours after the OA treatment and analyzed. A: The total bronchoalveolar lavage (BAL) cell numbers were counted using a hemocytometer. B: The polymorphonuclear neutrophils were stained with the May-Grunwald and Giemsa solution, and their cell numbers were counted. C: Immunohistochemical staining of the lung paraffin sections using the anti-myeloperoxidase (MPO) antibody. Arrowheads indicate the positive MPO staining. n = 6 (WT + OA group, A and B); n = 4 (other groups, A and B). ∗P < 0.05, ∗∗P < 0.01. Original magnification, ×400 (C).
Activation of liver X receptor (LXR) attenuates the oleic acid (OA)–induced local and systematic inflammation in female mice. Wild-type (WT) and LXR knock-in (LXR-KI) mice received a single tail vein injection of vehicle or OA. Mice were sacrificed 2 hours after the OA treatment and analyzed. The mice were sacrificed before the bronchoalveolar lavage (BAL) fluid and the blood was collected. A: The concentrations of tumor necrosis factor (TNF)-α and IL-6 in the cell-free BAL supernatants (A) and in the serum (B). n = 6 (WT + OA group); n = 4 (other groups). ∗P < 0.05, ∗∗P < 0.01.
The protective effect of liver X receptor (LXR) is associated with attenuation of oleic acid (OA)–induced oxidative stress in female mice. Wild-type (WT) and LXR knock-in (LXR-KI) mice received a single tail vein injection of vehicle or OA. Mice were sacrificed 2 hours after the OA treatment and analyzed. The activities of superoxide dismutase (SOD; A) and catalase (B) were measured in the lung homogenates. n = 6 (WT + OA group, A and B); n = 4 (other groups, A and B). ∗P < 0.05, ∗∗P < 0.01.