Abstract
Congenital diaphragmatic hernia (CDH) is one of the most common and lethal congenital anomalies, and significant evidence is available in support of a genetic contribution to its etiology, including single-gene knockout mice associated with diaphragmatic defects, rare monogenetic disorders in humans, familial aggregation, and association of CDH with chromosomal abnormalities. Structural lung defects in the form of lung hypoplasia are almost invariably seen in patients with CDH and frequently in animal models of this condition. Better understanding of the mechanisms of pulmonary defects in CDH has the potential for creating targeted therapies, particularly in postnatal stages, when therapeutics can have maximum clinical impact on the surviving cohorts. Successful treatment of CDH is dependent on the integration of human genomic and genetic data with developmental expression profiling, mouse knockouts, and gene network and pathway modeling, which have generated a large number of candidate genes and pathways for follow-up studies. In particular, defective alveolarization appears to be a common and potentially actionable phenotype in both patients and animal models.
A Multimodal War on Congenital Diaphragmatic Hernia
Congenital diaphragmatic hernia (CDH) is a common and severe birth defect that affects 1:3000 live births.1, 2 It is characterized by defective formation and/or muscularization of the diaphragm, often with displacement of abdominal contents into the chest cavity. CDH is almost invariably accompanied by pulmonary hypoplasia, which is the major cause of morbidity and mortality in patients and requires immediate and complex care.3 The clinical importance of CDH relates to its high frequency among birth defects, its severity, and the challenges related to its treatment, which is intense and expensive, placing a burden on the affected families and the health care system. Approximately 60% of cases are isolated defects that affect otherwise healthy term infants, indicating a good clinical prognosis if lung hypoplasia can be improved.
Significant progress has been made in the care of these patients in the past 2 decades, although mortality rates remain at 30% to 50% worldwide.1 Currently, the main therapeutic goal is to stabilize the cardiac and respiratory systems in infants with CDH, while attempting to minimize iatrogenic (therapy-induced) injury to the delicate pulmonary tissue, before and after surgical repair of the diaphragm. Gentle ventilation techniques, high-frequency ventilation, cardiovascular pharmacologic support, and extracorporeal membrane oxygenation3, 4, 5, 6 have improved survival rates, although these interventions have their own early and long-term complications. Lengthy and expensive stays in neonatal intensive care units are often necessary.7 A significant number of infants still succumb to the pulmonary complications of CDH, with late deaths mostly attributable to pulmonary hypertension and pulmonary insufficiency. Furthermore, many CDH survivors experience adverse long-term outcomes, including residual cardiopulmonary disease, neurodevelopmental defects, sensorineural hearing loss, and feeding difficulties.8, 9, 10
The lungs in infants with CDH have reduced volumes and weight with histologic features of developmental immaturity, manifested by a reduction in the number and size of alveoli. Several mechanisms have been hypothesized to cause CDH-associated lung hypoplasia. Classic teaching indicated that compression of the lung by herniated viscera and the lack of respiratory movements were the root causes of the hypoplasia phenotype; however, other studies suggest that abnormal lung development precedes the herniation of abdominal contents into the thoracic cavity, and it is therefore a primary, not secondary, defect.11, 12, 13 These observations have led to the conclusion that the lung defects are at least partially independent of the diaphragm defect and pose many questions about what links the abnormalities of these two organs at the molecular or mechanistic level.
Whereas others have reviewed the embryology of the diaphragm,14 this review focuses on our current understanding of the mechanisms of pulmonary defects in CDH and on the potential for targeting postnatal stages of lung development, when therapeutics can have maximum clinical impact on the surviving cohorts.
Overview of Diaphragm and Lung Development
The diaphragm forms from multiple tissue sources, including transient embryonic structures of mesenchymal tissue that make up the septum transversum and pleuroperitoneal folds (PPFs). The muscles lying at the periphery of the diaphragm derive from migrating cells delaminating from the somitic dermatomyotome, which also contributes to the skeletal muscle of the limbs and body wall.15, 16, 17, 18 The fundamental role of connective tissue in the PPFs was demonstrated by analysis of conditional Prx1-creTg/+;Gata4Δ/fl mice, with complete Gata4 ablation in the lateral plate mesoderm–derived PPF cells. Abnormalities in the PPF connective tissue result in failure of migration and differentiation of muscle precursor cells, leading to subsequent hernia formation.19 Eighty percent of hernias occur in the left posterolateral diaphragm, begging the questions of why this position is so often targeted for mishap.
Development of the lungs is a complex, multistep process, and perturbation of any of these steps can lead to pulmonary defects (Figure 1). The process initiates with ventral budding from the ventral foregut endoderm. This lung bud then undergoes a process of elongation and branching morphogenesis, which follows a characteristic reproducible pattern based on the Fibonacci sequence, together with subsequent sequential multiplanar rotations that are regulated with a periodicity20 and is regulated by multiple pathways, including fibroblast growth factor, Wnt, sonic hedgehog, transforming growth factor-β, and bone morphogenetic protein.21, 22, 23, 24, 25, 26 Primary buds undergo additional branching to form multiple distal tubules during the pseudoglandular stage, and the tubules elongate during the canalicular phase. In the later stages of lung development, the distal airways form the critical interface for gas exchange. During middle to late gestation, the lungs enter the saccular phase, where the distal airways form rudimentary saccules, and the airway epithelium begins to secrete surfactant.
Figure 1.
Timeline of pulmonary development. Human lung development is divided into prenatal and postnatal phases, separated by the dotted line indicating birth. Developmental stages are plotted against gestational weeks (below) and are divided into the partially overlapping embryonic, pseudoglandular, canalicular, saccular, and alveolar stages. Development of the alveolus continues with increased septation for several years during childhood. A number of CDH-associated genes affect branching morphogenesis in the lungs, but alveolarization and vascular defects are also reported in patients with CDH. The most practical window for therapeutic intervention is indicated by the pink box.
The final stage of lung development, alveolarization, begins before birth and continues through the newborn period and much of childhood in humans, commensurate with overall body growth. During the first phase of alveolarization, known as primary septation, the airway saccules become thinner, bringing the airway epithelium in close contact with the lung vasculature, thereby forming a basic unit for gas exchange. Subsequently, the alveoli mature through the process of secondary septation, in which multiple septa grow inward from the walls of each alveolus. Each septum contains two epithelial layers with a central core that contains a single capillary and mesenchymal components that include myofibroblasts and elastin, as well as other extracellular matrix components. The migration and differentiation of myofibroblasts in the alveolar septa are critical,27 although it remains a poorly understood aspect of lung development, as does the formation of lipofibroblasts.28 The process of secondary septation greatly increases the surface area for gas exchange within the lung, and because it occurs immediately prenatally and continues postnatally, it provides an attractive target for designing therapeutic interventions for patients with CDH and other neonatal lung diseases (Figure 1).
Pulmonary Hypoplasia and Alveolarization Defects in CDH
The eponymous hernia or hole in the diaphragm is accompanied by lung hypoplasia and pulmonary hypertension whose severity determines early mortality or survival. The lungs in patients with CDH appear immature, with thickened alveolar walls and increased interstitial tissue, effectively resulting in reduced alveolar spaces and gas-exchange surface area29 (Figure 2). Incomplete pulmonary mesenchymal thinning attributable to defective apoptosis, rather than true immaturity, has been proposed as a mechanism based on cellular models.13, 30
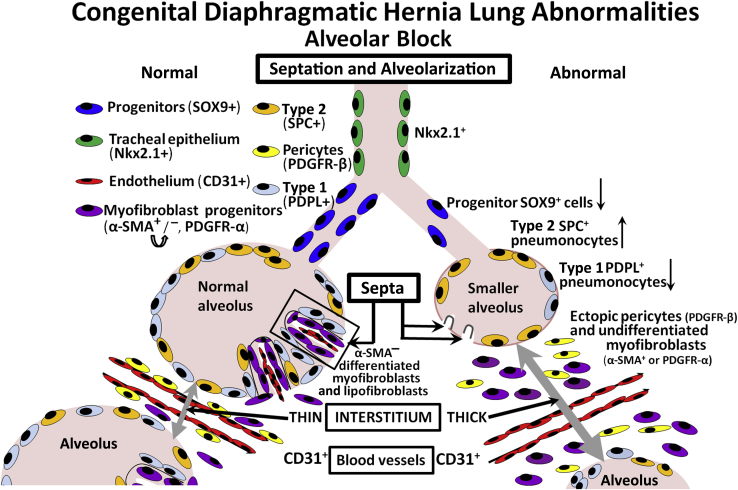
Figure 2.
CDH lung abnormalities. An alveolar block is hypothesized as a late phenotype in CDH, resulting in alveolar simplification. Smaller alveoli, lined by increased type 2 and reduced type 1 pneumocytes, contain stunted septa linked with defective myofibroblast proliferation and migration. Reduced Sox9-positive progenitors indicate a defect in epithelial differentiation. The thickened interstitium suggests a delay in pulmonary maturation or abnormal resorption and results in poor oxygen exchange. α-SMA, α-smooth muscle actin; PDGFR, platelet-derived growth factor receptor; PDPL, podoplanin; SPC, surfactant protein C.
On a finer structural level, the lungs of patients with CDH are characterized by a failure to establish the necessary close association of the air-exposed epithelium to the abnormally differentiated and diminished mesenchymal vessels carrying the blood that must be oxygenated for survival.31, 32 Failure of co-differentiation of the lung epithelium and mesenchymal compartment components can lead to insufficient gas exchange, the final common pathway of lethality in CDH. This can result from mutations in any number of genes, each critical to the linear and parallel morphologic steps necessary to achieve a normally differentiated lung.
Much of our mechanistic understanding of the pulmonary defects in CDH has come from the study of animal models, particularly genetic models with both diaphragm and lung abnormalities (Table 1).33 In addition, surgical models on sheep and rabbits provide valuable information to study the mechanical effects of herniated viscera on the lung and to test interventional strategies, whereas the teratogenic nitrofen model mimics the human condition to a large extent, even though its applications are limited by the fact that nitrofen is not considered a cause of CDH in patients.34 Deficient alveolarization has been observed in CDH, resulting from nitrofen administration, but can be improved by antenatal treatment with corticosteroids.35 Human studies have proven more challenging, likely because of pragmatic clinical difficulties in obtaining appropriate pathology specimens, because most infants with CDH undergo extensive ventilatory interventions that disrupt the natural architecture of the lung. Major bronchial subdivisions are normal in human autopsy specimens of hypoplastic CDH lungs; however, the number of intermediate bronchial branches is often reduced severely in the ipsilateral lung and less so in the contralateral lung, which comparatively maintains the potential of faster growth and complete differentiation during gestation.36, 37 The prevalence of left-sided hernias suggests that left-right asymmetry may also be an important component of the pathophysiology of the hypoplasia.
Table 1.
Mouse Models with Both Diaphragm and Lung Abnormalities
| Symbol | Name | Diaphragmatic phenotype | Lung phenotype |
|---|---|---|---|
| Atp2a1 | ATPase, Ca++ transporting, cardiac muscle, fast twitch 1 | Abnormal diaphragm muscle | Abnormal alveoli (failure to expand, hypercellularity) |
| Ctnnb1 | Catenin (cadherin associated protein), β1 | Diaphragmatic hernia (Wt1-Cre conditional knockout) | Absent lung buds (Shh-Cre conditional knockout) |
| Efemp2 | Epidermal growth factor–containing fibulin-like extracellular matrix protein 2 | Diaphragmatic hernia | Abnormal elastic fibers |
| Eya1 | EYA transcriptional co-activator and phosphatase 1 and 2 | Amuscular diaphragm (double Eya1; Eya2 knockout) | Lung hypoplasia, abnormal epithelium morphologic features |
| Eya2 | EYA transcriptional co-activator and phosphatase 1 and 2 | Amuscular diaphragm (double Eya1; Eya2 knockout) | Lung hypoplasia, abnormal epithelium morphologic features |
| Frem1 | Fras1 related extracellular matrix protein 1 | Diaphragmatic hernia | Fused pulmonary lobes |
| Frem2 | Fras1-related extracellular matrix protein 2 | Diaphragmatic hernia | Fused pulmonary lobes |
| Fuz | Fuzzy planar cell polarity protein | Diaphragmatic hernia | Lung hypoplasia |
| Gata4 | GATA-binding protein 4 | Diaphragmatic hernia | Abnormal saccule morphologic features, abnormal vasculature |
| Gli2, Gli3 | GLI-Kruppel family member 2 and 3 | Diaphragmatic hernia (double Gli2; Gli3 knockout) | Lung hypoplasia, absent right lung accessory lobe, thick mesenchyme |
| Hlx | H2.0-like homeobox | Diaphragmatic hernia | Enlarged lungs with normal structure |
| Igf2 | Insulin-like growth factor 2 | Thin diaphragm muscle (double Igf2; Myod1 knockout) | Abnormal epithelial proliferation/differentiation (organ culture) |
| Kif7 | Kinesin family member 7 | Diaphragmatic hernia, thick diaphragm muscle | Lung hypoplasia |
| Lmnb1 | Lamin B1 | Thin diaphragm muscle, abnormal phrenic nerve | Abnormal alveoli |
| Lmnb2 | Lamin B2 | Thin diaphragm muscle, abnormal phrenic nerve (double Lmnb1; Lmnb2 knockout) | Abnormal alveoli |
| Lox | Lysyl oxidase | Diaphragmatic hernia, thin diaphragm muscle | Lung hypoplasia, abnormal acini, abnormal elastic fibers |
| Met | Met proto-oncogene | Diaphragmatic hernia, thin diaphragm muscle | Abnormal saccule morphologic features (conditional knockout in the respiratory epithelium) |
| Mmp2, Mmp14 | Matrix metallopeptidase 2, and 14 | Thin diaphragm muscle (double Mmp14; Mmp2 knockout) | Lung hypoplasia, abnormal alveoli, dilated alveolar ducts, abnormal elastic fibers |
| Myod1 | Myogenic differentiation 1 | Thin diaphragm muscle (MyoD:mdx) | Pulmonary hypoplasia (MyoD:mdx) |
| Myog | Myogenin | Thin diaphragm muscle | Lung hypoplasia |
| Msc | Musculin | Diaphragmatic hernia (double Msc; Tcf21 knockout) | Lung hypoplasia, abnormal branching, abnormal vasculature |
| Ndst1 | N-deacetylase/N-sulfotransferase (heparan glucosaminyl) 1 | Diaphragmatic hernia, thin diaphragm muscle (conditional knockout) | Lung hypoplasia, thick interalveolar septa |
| Pbx1 | Pre–B-cell leukemia homeobox 1 | Diaphragmatic hernia | Lung hypoplasia |
| Pdgfra | Platelet-derived growth factor receptor, α-polypeptide | Diaphragmatic hernia | Lung hypoplasia, abnormal alveoli, increased cell proliferation |
| Rara, Rarb | Retinoic acid receptor, α and β | Diaphragmatic hernia (double Rara; Rarb knockout) | Lung hypoplasia, abnormal alveoli (double Rara; Rarb knockout) |
| Robo1, Robo 2 | Roundabout guidance receptor 1 | Diaphragmatic hernia (double Robo1; Robo2 knockout) | Abnormal alveoli, thick septa |
| Six1 | Sine oculis homeobox, Drosophila, homolog of, 1 | Amuscular diaphragm (double Six1; Six4 knockout) | Lung hypoplasia |
| Tcf21 | Transcription factor 21 | Diaphragmatic hernia (double Msc; Tcf21 knockout) | Lung hypoplasia, abnormal branching, abnormal vasculature |
| Wdr35 | WD repeat domain 35 | Diaphragmatic hernia | Pulmonary hypoplasia |
| Wt1 | Wilms tumor 1 homolog | Diaphragmatic hernia | Lung hypoplasia |
| Zfpm2 | Zinc finger protein, multitype 2 | Abnormal diaphragm morphologic features | Lung hypoplasia, absent right lung accessory lobe |
The Mouse Genome Database Group 2015.33
Few of the extant alveoli have a normal structure. Pulmonary developmental studies of alveolarization assessed by such measures as mean linear intercepts and septal density38, 39, 40, 41 confirm that patients with CDH never achieve a full number of airway branches or spaces, despite the alveolar multiplication that occurs after birth.42, 43 Vascular growth is even more compromised than alveolar growth. However, because alveolar maturation continues throughout infancy and childhood, there is a long actionable window during which to improve differentiation and pulmonary function in CDH survivors. The question is whether differentiation can be improved postnatally by targeting the developmental pathways found to be involved in the process of alveolarization.
Several homozygous knockout mouse models of CDH result in severe phenotypes with embryonic lethality.44, 45 Many genes involved in the formation of the diaphragm are also likely to play important roles in the early stages of lung development (Table 1). Examples include ZFPM2 (FOG2)46 and members of the fibroblast growth factor signaling pathway, which result in pulmonary hypoplasia attributable to defects in branching morphogenesis.47, 48 The effects of complete ablation of these early-acting genes in mice are especially severe and irreversible.32 However, the pathologic features in humans, who usually have only one nonfunctional copy of the gene, may be less severe and addressable in the immediate postnatal period. An increasing number of mouse models have implicated a role for CDH genes in the later stages of lung development, where early molecular correction or rescue can have potentially maximum clinical benefit and complications of barotrauma (eg, bronchopulmonary dysplasia and pulmonary fibrosis) are minimized or averted. CDH and bronchopulmonary dysplasia share structural and functional abnormalities in alveolar formation and pulmonary vasculature, possibly related to common disturbances in the retinoid acid pathway genes,49 which result in impaired gas exchange.
A complementary set of mammalian genetic, genomic, physiologic, and three-dimensional morphologic approaches have pointed to myofibroblasts and extracellular matrix constituents, such as elastin, as key players in these late-stage CDH-associated pulmonary abnormalities.27, 50 Lipofibroblasts, a lipid-containing population of interstitial fibroblasts first identified during the canalicular phase of lung development, were also reduced in rats treated with the CDH-inducing teratogen nitrofen.51, 52 Lipofibroblast signaling is critical for the completion of alveologenesis, possibly in directing the production of surfactant phospholipids in type 2 pneumocytes.28
Pulmonary neuroendocrine cells (PNECs), detected by acetylcholine esterase staining in earlier studies,53, 54 differentiate from distal progenitors and migrate proximally to form neuroepithelial bodies or clusters. Neuroepithelial bodies were reported to be increased in number and size in neonatal rats with diaphragmatic defects.55 The PNEC-produced factors bombesin and ghrelin, which are likely to play important roles in lung development and regulating vascular tone, are expressed at higher levels in patients with CDH.56 PNEC clusters normally function in vivo as airway sensors for hypoxia, hypercarbia, and other toxic environmental changes. Excess secreted neuropeptides were linked to lung matrix remodeling and alveolar simplification or failure of septation,57 and PNEC factors have been proposed to sensitize the lungs to the effects of retinoic acid by regulating retinoic acid receptors α and γ expression.58
Pulmonary Hypertension in CDH
In addition to the defective gas exchange mechanisms discussed above, another major contributor to the mortality of infants with CDH is pulmonary hypertension, which is characterized by abnormal endothelial and smooth muscle development, associated with failure of the vascular smooth muscle to relax normally after birth.59 The cause of pulmonary hypertension in patients with CDH is multifactorial, with defects that affect lung vessel number and size. Autopsy specimens from patients with CDH reveal that lung vessel density is decreased.59 Also characteristic are the reduced complexity of proximal pulmonary artery branching and the paucity of microvascular or capillary networks, which should occur in parallel to the alveolarization of the distal lung. Diminished lung vessel size is also accompanied in patients by malposition and hypertrophy of vascular smooth muscle, as well as failure of the vascular smooth muscle to respond by relaxation in response to physiologic oxygen or nitrogen levels present at birth, compounded to the reduced response to treatment. It is unclear which of these abnormalities is the major contributing factor to pulmonary hypertension in patients with CDH.
Genomic studies have found little overlap with gene mutations seen in hereditary forms of primary pulmonary hypertension, suggesting that CDH-associated lung hypertension is likely dependent partially on different pathophysiologic mechanisms.60 Pulmonary antihypertensive therapy with inhaled nitric oxide or the use of phosphodiesterase inhibitors is suboptimal in patients with CDH,61 as has been the use of phosphodiesterase inhibitors attempted in clinical practice. In fact, no current pharmacologic regimen improves the pulmonary hypertension associated with CDH.3, 62 Improved understanding of how genetic defects contribute to CDH-associated lung pathologies may lead to the discovery of pathways that affect alveolar septation and pulmonary vascular hemodynamics. This will help generate more accurate models of the pulmonary and vascular pathophysiology associated with CDH and ultimately improve short-term survival and ameliorate long-term morbidity.
Genomic Studies in CDH
Genetic studies on patients with CDH are providing important insight into the molecular mechanisms of both diaphragm and lung development. Several observations point toward genetic causation in CDH.1, 2, 63, 64, 65, 66 Because of the clinical burden of CDH, we and others have invested in genetic studies of rare families with mendelian patterns of inheritance67, 68, 69, 70, 71 and of large patient cohorts.68, 72
Familial studies revealed monogenic causes of CDH, including the critical CDH genes ZFPM2 or FOG2,69, 73 GATA4,71 and GATA6.70 These families are rare, in part because of the early high mortality and reproductive disadvantage in CDH. This is particularly true in complex CDH cases, which make up approximately 40% of all CDH cases. Isolated CDH (60%), without additional birth defects, can be familial, but pedigrees can be difficult to recognize because of reduced penetrance.69, 71 On the other hand, systematic analysis of pedigrees contained in the Utah Population Database revealed distantly related patients with shared genomic regions from several extended families characterized by a high incidence of isolated CDH,74 supporting the notion of heritability in isolated CDH.
CDH with associated lung hypoplasia and pulmonary hypertension is seen in a number of complex monogenic syndromes, including LRP2 (Donnai-Barrow syndrome),75 CHD7 (CHARGE syndrome),76 STRA6 (Matthew-Wood syndrome),77 GPC6 (Simpson-Golabi-Behmel syndrome),78 and NIPBL (Cornelia de Lange syndrome),79 among others.66, 80 The importance of these genes is illustrated by the fact that we identified heterozygous predicted pathogenic variants, possibly risk alleles, in these genes in patients with both isolated and complex CDH.68
Copy number variation studies using array-based comparative genome hybridization have allowed for more detailed delineation of chromosome regions and have provided higher resolution to detect smaller deletions and duplications.81, 82 In addition, recurrent chromosomal anomalies have been described in association with human CDH cases, and these have pointed toward several known and putative CDH genes. Chromosome studies identified several recurrent deletions in patients with CDH, so-called hotspots, including 1q41-42, 8p23.1, and 15q26.83, 84, 85, 86, 87
Recently, next-generation sequencing technology, including whole exome sequencing and whole genome sequencing, allowed detection of single-nucleotide variants; however, the major challenge is how to prioritize the overwhelming number of potentially damaging variants that are detected.68, 72 Bioinformatics can be used to enhance interpretation of individual gene variants by identifying their participation in molecular pathways and by documenting normal gene expression in the developing diaphragms and lungs, first in wild-type mice,88 then in mutant mice, and eventually in diaphragms of patients with CDH for comparison. The underlying hypothesis of these systems biology approaches is that CDH causative genes, however disparate, will fall into a limited number of key developmental pathways,68, 88, 89, 90 as exemplified in a recent study of a cohort of 275 patients with CDH by whole exome sequencing.68
Mouse Models of CDH and Lung Hypoplasia
Because diaphragm and lung development in the mouse closely parallels that of the human, mouse models have played an invaluable role in enhancing our understanding of the genetic mechanisms underlying CDH and CDH-associated lung disease. To date, >70 genes have been found to cause diaphragm defects in existing mouse models, many of which have comorbid lung hypoplasia63, 91 (Table 1). Many of these critical genes, discovered by mouse models, were later found to harbor rare and predicted pathogenic mutations in patients with CDH, thereby implicating these genes in human CDH.68
Unbiased genetics screens can be used to identify further genes that cause CDH and lung defects, for example, through such approaches as the second phase of the Knockout Mouse Project, a multi-institutional initiative to produce a knockout for every known protein-coding gene in the mouse genome.92, 93 Gross phenotyping of pups and adults can be supplemented by high-throughput screening of animal repositories by X-ray microtomography for identifying diaphragm defects in embryonic lethal strains.94
Globally, mouse models have been useful for the characterization of developmental mechanisms (Mouse Genome Informatics, http://www.informatics.jax.org, last accessed February 2016) and provide models for future in vivo rescue experiments after drug discovery screens. CRISPR/Cas9-mediated genome editing has been useful in enhancing the efficiency and specificity of generating targeted mutations in mice.95, 96, 97, 98 When coupled with Cre-directed strategies,27 it has been possible to dissect tissue- and cell-specific roles (eg, epithelial versus mesenchymal) for each gene in the developing lung.
Studies of Alveolar Septation in CDH Models
CDH lungs have aberrant morphologic features characterized by reduced radial alveolar count and thicker alveolar septa. Defective morphogenesis of the distal lung in the form of alveolar simplification is common.99, 100 Late creation of the hernia in surgical models can produce alveolar hypoplasia without obvious branching defects.101
A number of mechanisms have been hypothesized to lead to abnormal septation in these models. Epithelial and endothelial approximation, often referred to as blood-gas barrier, is the functional unit of the alveoli, and collagens together with other extracellular matrix proteins are the major components of the basement membranes that support the architecture and function of the epithelium and endothelium. Reduced elastic fiber deposition may be responsible for the abnormal alveolar walls in patients with CDH,102 more specifically, defects of secondary alveologenesis possibly linked to insufficient fibroblast growth factor-18 expression.103 Reduced tropoelastin and elastin fibers result in stunted secondary septa with immature alveolar myofibroblasts ectopically displaced into the lung interstitium,50, 103 where they disrupt oxygen exchange.27 Because this phenotype mimics the pathologic findings in humans surviving with CDH, it offers a good model in which to test for therapeutic rescue.
Although several techniques have been used to investigate postnatal lung development, few provide the means to study secondary alveolarization directly. In fact, advancements in our understanding of pediatric pulmonary pathophysiology have been hindered by the lack of reproducible in vitro models. To this end, Kelleher and colleagues104 developed an assay based on ex vivo organ culture of P4 murine lungs that overcomes the limitations of substrate diffusion by preinflating the organ with 0.4% low-melt agarose at 20-cm H2O pressure. Organ maturation progresses for up to 4 days in culture, maintaining alveolar architecture, histologic features, and morphometrics (septal density and mean linear intercept). Furthermore, this ex vivo model is amenable to experimental manipulations, which will be useful to validate candidates emerging from drug discovery.
Developmental Expression Profiling
A useful strategy that we and others have used to identify CDH candidate genes is to compare human genetic data against expression profiles of the developing diaphragm, with the assumption that genes expressed at high levels during development are most likely to cause disease. We generated gene expression profiles from laser dissected PPFs, the anlage of the primordial diaphragm, prominent at E11.5 to E12.5 in the mouse embryo. This study identified a group of genes highly expressed in the PPFs but down-regulated in the mature and fully muscularized diaphragm.88 As proof of principle, we validated this approach by detecting the presence of diaphragm and lung defects in the mouse knockout of one of the prioritized gene candidates, Pbx188; furthermore, pathogenic variants in its paralog gene Pbx3 were subsequently identified in exomes of a cohort of 275 patients with CDH while being absent in controls,68 thus adding credence to this integrative approach for identifying novel CDH genes. This developmental data set has also helped in the interpretation of de novo variants from sequencing studies in trios and familial cases.72
Because developmental gene expression profiling in the diaphragm has proven to be a useful tool for identifying CDH genes, the search for candidate genes is being expanded by examining genes expressed in the developing lung,105 with a focus on genes that play a role in the later stages of lung development that may be amenable to therapeutic intervention.104
In addition, RNA sequencing was used to elucidate mRNA and miRNA expression changes in nitrofen-induced rat hypoplastic lungs106 that are evident even before the diaphragmatic defect could be observed. KEGG pathway analysis of significantly differentially expressed transcripts suggested that Wnt, transforming growth factor-β signaling, and retinol metabolism pathways are involved in nitrofen-induced hypoplastic lungs, whereas differentially expressed miRNAs appeared to mediate nitrofen-induced cell cycle arrest and inhibition of proliferation, as well as smooth muscle cell dysfunction.106 Furthermore, miR449a, which functions to control Myc-directed normal lung proliferation, was elevated in mouse and human CDH lungs.107
Drug Discovery and Novel Therapeutic Approaches
Human and animal genetics held the promise of uncovering novel therapeutic targets because current treatment options for infants with CDH are limited to supportive ventilatory care and surgical repair. Attempts to increase pulmonary function with the use of prenatal and perinatal glucocorticoids, surfactants, and a number of pulmonary vasodilators have not been entirely successful.3, 62 In addition, the development of therapeutics for CDH-associated lung hypoplasia and pulmonary hypertension has been hindered by lack of standardization in methods and validated outcomes in preclinical research.108
Recent experimental approaches in nitrofen-treated rats with simvastatin revealed improvement in lung-to-body weight ratios and lung parenchyma structure,109 with effects attributed to changes in the endothelin, nitric oxide, and bone morphogenic protein receptor type 2 signaling pathways, and activation of epithelial apoptosis for tissue remodeling.109
Fetal endotracheal occlusion110, 111 was developed on the premise that increased intrapulmonary pressure would improve lung maturation. In the fetal endotracheal occlusion procedure, an inflatable balloon111, 112, 113 or, more recently, a dissolving gel bolus114 is inserted in the trachea of late-stage fetuses. A fetal endotracheal occlusion study is currently recruiting patients with CDH in the United States (ClinicalTrials.gov identifier: NCT00881660), but the clinical utility of this procedure is still unclear. Stem cell therapy by transplantation of human amniotic fluid stem cells has also been attempted in fetal endotracheal occlusion rabbit models with promising effects on lung-to-body weight ratio, mean terminal bronchiole density, and interstitial thickness.115, 116
Retinoid signaling defects have been associated with diaphragm abnormalities and lung defects.117 Because of its poorly understood therapeutic window, retinoids and their derivatives have been avoided in the treatment of CDH and its related complications. However, improved knowledge of the mechanism of action of retinoic acid in the developing lung, such as through its effect on PNECs,57, 58 may revive its consideration as a treatment option. In fact, the PNEC-secreted hormone ghrelin alleviates the phenotype of pulmonary hypoplasia in a CDH animal model.118
Among molecules currently being tested for their ability to rescue the pulmonary phenotype of CDH is the glucagon-like peptide-1. When administered transplacentally in a surgically induced rabbit model, glucagon-like peptide-1 improved peripheral pulmonary vessel morphologic features without changes in alveolarization. However, its administration was associated with significant maternal and fetal adverse effects.119
Drug discovery should be designed to target the basic developmental processes of alveologenesis in mice with conditional knockouts and directed toward specific cell types in the developing lung as potential therapeutics to rescue animal models in vivo. Similar approaches can be used to uncover pharmacologic agents to correct vascular changes in CDH-associated pulmonary hypertension and in the hyperoxia model of bronchopulmonary dysplasia.49 Molecules that have a successful outcome in vivo could be advanced, in due time, to the design and implementation of clinical trials. To this end, a clinical translational infrastructure already established to study the genetics of CDH, through collaborative efforts among neonatal care centers in the United States and abroad in the form of multicenter consortia, can be used to conduct clinical trials and to carry forward promising candidates as effective therapies for wider use in the clinic.
Acknowledgments
We thank Xin Sun, Ph.D., David J. McCulley, M.D., Wendy Chung, M.D., Yufeng Shen, Ph.D., Maria Loscertales, Ph.D., Carol Bult, Ph.D., and Barbara R. Pober, M.D., for their intellectual input and helpful discussions; the physicians at MassGeneral Hospital for Children and Boston Children's Hospital: Terry L. Buchmiller, Catherine C. Chen, Daniel P. Doody, Dario O. Fauza, Steven J. Fishman, Allan M. Goldstein, Lewis B. Holmes, Tom Jaksic, Russell Jennings, Cassandra M. Kelleher, David Lawlor, Craig W. Lillehei, Peter T. Masiakos, David P. Mooney, Konstantinos Papadakis, Rafael V. Pieretti, Mark Puder, Daniel P. Ryan, Robert C. Shamberger, C. Jason Smithers, Joseph P. Vacanti, Christopher Weldon, and Jill Zalieckas for their care of the patients with CDH in our study; and the Association of Congenital Diaphragmatic Hernia Research, Awareness, and Support and Breath of Hope for their support of patients with CDH.
Footnotes
Lung Ontogeny and Injury Theme Issue
Supported by National Institute of Child Health and Human Development grant P01 HD068250-05 (P.K.D. and M.L.) and National Research Service Award 2T32GM007748-35 (F.A.H.).
This article is part of a review series on lung ontogeny and injury.
Disclosures: None declared.
References
- 1.Pober B.R. Overview of epidemiology, genetics, birth defects, and chromosome abnormalities associated with CDH. Am J Med Genet C Semin Med Genet. 2007;145C:158–171. doi: 10.1002/ajmg.c.30126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pober B.R. Genetic aspects of human congenital diaphragmatic hernia. Clin Genet. 2008;74:1–15. doi: 10.1111/j.1399-0004.2008.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stolar C.J., Dillon P.W. ed 7. Mosby; Philadelphia: 2012. Congenital Diaphragmatic Hernia and Eventration; pp. 809–824. Pediatric Surgery. [Google Scholar]
- 4.Beaumier C.K., Beres A.L., Puligandla P.S., Skarsgard E.D., Canadian Pediatric Surgery Network Clinical characteristics and outcomes of patients with right congenital diaphragmatic hernia: a population-based study. J Pediatr Surg. 2015;50:731–733. doi: 10.1016/j.jpedsurg.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 5.Garcia A.V., Thirumoorthi A.S., Stolar C.J.H. ed 6. Saunders/Elsevier; London, New York: 2014. Extracorporeal membrane oxygenation. Ashcraft's Pediatric Surgery; pp. 83–93. [Google Scholar]
- 6.Snoek K.G., Capolupo I., van Rosmalen J., Hout L.J., Vijfhuize S., Greenough A., Wijnen R.M., Tibboel D., Reiss I.K., CDH Euro Consortium Conventional mechanical ventilation versus high-frequency oscillatory ventilation for congenital diaphragmatic hernia: a randomized clinical trial (The VICI-trial) Ann Surg. 2016;263:867–874. doi: 10.1097/SLA.0000000000001533. [DOI] [PubMed] [Google Scholar]
- 7.Hayward M.J., Kharasch V., Sheils C., Friedman S., Dunleavy M.J., Utter S., Zurakowski D., Jennings R., Wilson J.M. Predicting inadequate long-term lung development in children with congenital diaphragmatic hernia: an analysis of longitudinal changes in ventilation and perfusion. J Pediatr Surg. 2007;42:112–116. doi: 10.1016/j.jpedsurg.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Amoils M., Crisham Janik M., Lustig L.R. Patterns and predictors of sensorineural hearing loss in children with congenital diaphragmatic hernia. JAMA Otolaryngol Head Neck Surg. 2015;141:923–926. doi: 10.1001/jamaoto.2015.1670. [DOI] [PubMed] [Google Scholar]
- 9.Danzer E., Gerdes M., D'Agostino J.A., Bernbaum J., Hoffman C., Herkert L.M., Rintoul N.E., Peranteau W.H., Flake A.W., Adzick N.S., Hedrick H.L. Younger gestational age is associated with increased risk of adverse neurodevelopmental outcome during infancy in congenital diaphragmatic hernia. J Pediatr Surg. 2016;51:1084–1090. doi: 10.1016/j.jpedsurg.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Snoek K.G., Capolupo I., Braguglia A., Aite L., van Rosmalen J., Valfre L., Wijnen R.M., Bagolan P., Tibboel D., IJsselstijn H. Neurodevelopmental outcome in high-risk congenital diaphragmatic hernia patients: an appeal for international standardization. Neonatology. 2016;109:14–21. doi: 10.1159/000438978. [DOI] [PubMed] [Google Scholar]
- 11.Cilley R.E., Zgleszewski S.E., Krummel T.M., Chinoy M.R. Nitrofen dose-dependent gestational day-specific murine lung hypoplasia and left-sided diaphragmatic hernia. Am J Physiol. 1997;272:L362–L371. doi: 10.1152/ajplung.1997.272.2.L362. [DOI] [PubMed] [Google Scholar]
- 12.Jesudason E.C., Connell M.G., Fernig D.G., Lloyd D.A., Losty P.D. Early lung malformations in congenital diaphragmatic hernia. J Pediatr Surg. 2000;35:124–127. doi: 10.1016/s0022-3468(00)80028-7. discussion 8. [DOI] [PubMed] [Google Scholar]
- 13.Keijzer R., Liu J., Deimling J., Tibboel D., Post M. Dual-hit hypothesis explains pulmonary hypoplasia in the nitrofen model of congenital diaphragmatic hernia. Am J Pathol. 2000;156:1299–1306. doi: 10.1016/S0002-9440(10)65000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merrell A.J., Kardon G. Development of the diaphragm – a skeletal muscle essential for mammalian respiration. FEBS J. 2013;280:4026–4035. doi: 10.1111/febs.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ackerman K.G., Greer J.J. Development of the diaphragm and genetic mouse models of diaphragmatic defects. Am J Med Genet C Semin Med Genet. 2007;145C:109–116. doi: 10.1002/ajmg.c.30128. [DOI] [PubMed] [Google Scholar]
- 16.Clugston R.D., Greer J.J. Diaphragm development and congenital diaphragmatic hernia. Semin Pediatr Surg. 2007;16:94–100. doi: 10.1053/j.sempedsurg.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Clugston R.D., Zhang W., Greer J.J. Gene expression in the developing diaphragm: significance for congenital diaphragmatic hernia. Am J Physiol Lung Cell Mol Physiol. 2008;294:L665–L675. doi: 10.1152/ajplung.00027.2008. [DOI] [PubMed] [Google Scholar]
- 18.Clugston R.D., Zhang W., Greer J.J. Early development of the primordial mammalian diaphragm and cellular mechanisms of nitrofen-induced congenital diaphragmatic hernia. Birth Defects Res A Clin Mol Teratol. 2010;88:15–24. doi: 10.1002/bdra.20613. [DOI] [PubMed] [Google Scholar]
- 19.Merrell A.J., Ellis B.J., Fox Z.D., Lawson J.A., Weiss J.A., Kardon G. Muscle connective tissue controls development of the diaphragm and is a source of congenital diaphragmatic hernias. Nat Genet. 2015;47:496–504. doi: 10.1038/ng.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metzger R.J., Klein O.D., Martin G.R., Krasnow M.A. The branching programme of mouse lung development. Nature. 2008;453:745–750. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardoso W.V., Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- 22.El-Bizri N., Wang L., Merklinger S.L., Guignabert C., Desai T., Urashima T., Sheikh A.Y., Knutsen R.H., Mecham R.P., Mishina Y., Rabinovitch M. Smooth muscle protein 22alpha-mediated patchy deletion of Bmpr1a impairs cardiac contractility but protects against pulmonary vascular remodeling. Circ Res. 2008;102:380–388. doi: 10.1161/CIRCRESAHA.107.161059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guzy R.D., Stoilov I., Elton T.J., Mecham R.P., Ornitz D.M. Fibroblast growth factor 2 is required for epithelial recovery, but not for pulmonary fibrosis, in response to bleomycin. Am J Respir Cell Mol Biol. 2015;52:116–128. doi: 10.1165/rcmb.2014-0184OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hogan B.L., Kolodziej P.A. Organogenesis: molecular mechanisms of tubulogenesis. Nat Rev Genet. 2002;3:513–523. doi: 10.1038/nrg840. [DOI] [PubMed] [Google Scholar]
- 25.Ritty T.M., Broekelmann T.J., Werneck C.C., Mecham R.P. Fibrillin-1 and -2 contain heparin-binding sites important for matrix deposition and that support cell attachment. Biochem J. 2003;375:425–432. doi: 10.1042/BJ20030649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sicot F.X., Tsuda T., Markova D., Klement J.F., Arita M., Zhang R.Z., Pan T.C., Mecham R.P., Birk D.E., Chu M.L. Fibulin-2 is dispensable for mouse development and elastic fiber formation. Mol Cell Biol. 2008;28:1061–1067. doi: 10.1128/MCB.01876-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Branchfield K., Li R., Lungova V., Verheyden J.M., McCulley D., Sun X. A three-dimensional study of alveologenesis in mouse lung. Dev Biol. 2016;409:429–441. doi: 10.1016/j.ydbio.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGowan S.E., Torday J.S. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol. 1997;59:43–62. doi: 10.1146/annurev.physiol.59.1.43. [DOI] [PubMed] [Google Scholar]
- 29.Sluiter I., Veenma D., van Loenhout R., Rottier R., de Klein A., Keijzer R., Post M., Tibboel D. Etiological and pathogenic factors in congenital diaphragmatic hernia. Eur J Pediatr Surg. 2012;22:345–354. doi: 10.1055/s-0032-1329409. [DOI] [PubMed] [Google Scholar]
- 30.van Loenhout R.B., Tseu I., Fox E.K., Huang Z., Tibboel D., Post M., Keijzer R. The pulmonary mesenchymal tissue layer is defective in an in vitro recombinant model of nitrofen-induced lung hypoplasia. Am J Pathol. 2012;180:48–60. doi: 10.1016/j.ajpath.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 31.Hislop A., Reid L. Pulmonary arterial development during childhood: branching pattern and structure. Thorax. 1973;28:129–135. doi: 10.1136/thx.28.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reid L.M. Lung growth in health and disease. Br J Dis Chest. 1984;78:113–134. [PubMed] [Google Scholar]
- 33.Eppig J.T., Blake J.A., Bult C.J., Kadin J.A., Richardson J.E., Mouse Genome Database Group The Mouse Genome Database (MGD): facilitating mouse as a model for human biology and disease. Nucleic Acids Res. 2015;43:D726–D736. doi: 10.1093/nar/gku967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Loenhout R.B., Tibboel D., Post M., Keijzer R. Congenital diaphragmatic hernia: comparison of animal models and relevance to the human situation. Neonatology. 2009;96:137–149. doi: 10.1159/000209850. [DOI] [PubMed] [Google Scholar]
- 35.Suen H.C., Bloch K.D., Donahoe P.K. Antenatal glucocorticoid corrects pulmonary immaturity in experimentally induced congenital diaphragmatic hernia in rats. Pediatr Res. 1994;35:523–529. doi: 10.1203/00006450-199405000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Coleman A., Phithakwatchara N., Shaaban A., Keswani S., Kline-Fath B., Kingma P., Haberman B., Lim F.Y. Fetal lung growth represented by longitudinal changes in MRI-derived fetal lung volume parameters predicts survival in isolated left-sided congenital diaphragmatic hernia. Prenat Diagn. 2015;35:160–166. doi: 10.1002/pd.4510. [DOI] [PubMed] [Google Scholar]
- 37.Phithakwatchara N., Coleman A., Peiro J.L., Lee A.E., Keswani S.G., Kline-Fath B., Lim F.Y., Shaaban A.F. Expanded intrathoracic space in fetal cases of isolated congenital diaphragmatic hernia contributes to disparity between percent predicted lung volume and observed to expected total lung volume. Prenat Diagn. 2015;35:154–159. doi: 10.1002/pd.4508. [DOI] [PubMed] [Google Scholar]
- 38.Hsia C.C., Hyde D.M., Ochs M., Weibel E.R., ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tschanz S.A., Burri P.H. A new approach to detect structural differences in lung parenchyma using digital image analysis. Exp Lung Res. 2002;28:457–471. doi: 10.1080/01902140290096719. [DOI] [PubMed] [Google Scholar]
- 40.Weibel E.R. Principles and methods for the morphometric study of the lung and other organs. Lab Invest. 1963;12:131–155. [PubMed] [Google Scholar]
- 41.Weibel E.R. Academic Press; New York, NY: 1963. Morphometry of the Human Lung. [Google Scholar]
- 42.Bachiller P.R., Nakanishi H., Roberts J.D., Jr. Transforming growth factor-beta modulates the expression of nitric oxide signaling enzymes in the injured developing lung and in vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2010;298:L324–L334. doi: 10.1152/ajplung.00181.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakanishi H., Sugiura T., Streisand J.B., Lonning S.M., Roberts J.D., Jr. TGF-beta-neutralizing antibodies improve pulmonary alveologenesis and vasculogenesis in the injured newborn lung. Am J Physiol Lung Cell Mol Physiol. 2007;293:L151–L161. doi: 10.1152/ajplung.00389.2006. [DOI] [PubMed] [Google Scholar]
- 44.Beurskens N., Klaassens M., Rottier R., de Klein A., Tibboel D. Linking animal models to human congenital diaphragmatic hernia. Birth Defects Res A Clin Mol Teratol. 2007;79:565–572. doi: 10.1002/bdra.20370. [DOI] [PubMed] [Google Scholar]
- 45.Chiu P.P. New insights into congenital diaphragmatic hernia - a surgeon's introduction to CDH animal models. Front Pediatr. 2014;2:36. doi: 10.3389/fped.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ackerman K.G., Herron B.J., Vargas S.O., Huang H., Tevosian S.G., Kochilas L., Rao C., Pober B.R., Babiuk R.P., Epstein J.A., Greer J.J., Beier D.R. Fog2 is required for normal diaphragm and lung development in mice and humans. PLoS Genet. 2005;1:58–65. doi: 10.1371/journal.pgen.0010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedmacher F., Doi T., Gosemann J.H., Fujiwara N., Kutasy B., Puri P. Upregulation of fibroblast growth factor receptor 2 and 3 in the late stages of fetal lung development in the nitrofen rat model. Pediatr Surg Int. 2012;28:195–199. doi: 10.1007/s00383-011-2985-2. [DOI] [PubMed] [Google Scholar]
- 48.Friedmacher F., Gosemann J.H., Fujiwara N., Takahashi H., Hofmann A., Puri P. Expression of Sproutys and SPREDs is decreased during lung branching morphogenesis in nitrofen-induced pulmonary hypoplasia. Pediatr Surg Int. 2013;29:1193–1198. doi: 10.1007/s00383-013-3385-6. [DOI] [PubMed] [Google Scholar]
- 49.Silva D.M., Nardiello C., Pozarska A., Morty R.E. Recent advances in the mechanisms of lung alveolarization and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1239–L1272. doi: 10.1152/ajplung.00268.2015. [DOI] [PubMed] [Google Scholar]
- 50.Loscertales M., Nicolaou F., Jeanne M., Longoni M., Gould D.B., Sun Y., Maalouf F.I., Nagy N., Donahoe P.K. Type IV collagen drives alveolar epithelial-endothelial association and the morphogenetic movements of septation. BMC Biol. 2016;14:59. doi: 10.1186/s12915-016-0281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedmacher F., Fujiwara N., Hofmann A.D., Takahashi H., Alvarez L.A., Gosemann J.H., Puri P. Prenatal retinoic acid increases lipofibroblast expression in hypoplastic rat lungs with experimental congenital diaphragmatic hernia. J Pediatr Surg. 2014;49:876–881. doi: 10.1016/j.jpedsurg.2014.01.017. discussion 81. [DOI] [PubMed] [Google Scholar]
- 52.Friedmacher F., Fujiwara N., Hofmann A.D., Takahashi H., Gosemann J.H., Puri P. Evidence for decreased lipofibroblast expression in hypoplastic rat lungs with congenital diaphragmatic hernia. Pediatr Surg Int. 2014;30:1023–1029. doi: 10.1007/s00383-014-3549-z. [DOI] [PubMed] [Google Scholar]
- 53.El-Bermani A.W., Chang T.L. Cobalt iontophoresis of sensory nerves in the rat lung. Am J Anat. 1979;154:277–281. doi: 10.1002/aja.1001540211. [DOI] [PubMed] [Google Scholar]
- 54.Morikawa Y., Donahoe P.K., Hendren W.H. Cholinergic nerve development in fetal lung. Dev Biol. 1978;65:541–546. doi: 10.1016/0012-1606(78)90050-7. [DOI] [PubMed] [Google Scholar]
- 55.IJsselstijn H., Perrin D.G., de Jongste J.C., Cutz E., Tibboel D. Pulmonary neuroendocrine cells in neonatal rats with congenital diaphragmatic hernia. J Pediatr Surg. 1995;30:413–415. doi: 10.1016/0022-3468(95)90044-6. [DOI] [PubMed] [Google Scholar]
- 56.Asabe K., Tsuji K., Handa N., Kajiwara M., Suita S. Immunohistochemical distribution of bombesin-positive pulmonary neuroendocrine cells in a congenital diaphragmatic hernia. Surg Today. 1999;29:407–412. doi: 10.1007/BF02483031. [DOI] [PubMed] [Google Scholar]
- 57.Branchfield K., Nantie L., Verheyden J.M., Sui P., Wienhold M.D., Sun X. Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science. 2016;351:707–710. doi: 10.1126/science.aad7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pereira-Terra P., Moura R.S., Nogueira-Silva C., Correia-Pinto J. Neuroendocrine factors regulate retinoic acid receptors in normal and hypoplastic lung development. J Physiol. 2015;593:3301–3311. doi: 10.1113/JP270477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohseni-Bod H., Bohn D. Pulmonary hypertension in congenital diaphragmatic hernia. Semin Pediatr Surg. 2007;16:126–133. doi: 10.1053/j.sempedsurg.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 60.Best D.H., Austin E.D., Chung W.K., Elliott C.G. Genetics of pulmonary hypertension. Curr Opin Cardiol. 2014;29:520–527. doi: 10.1097/HCO.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 61.Finer N.N., Barrington K.J. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev. 2006:CD000399. doi: 10.1002/14651858.CD000399.pub2. [DOI] [PubMed] [Google Scholar]
- 62.Tsao K.J., Lally K.P. ed 6. Elsevier; London, UK: 2014. Congenital diaphragmatic hernia and eventration. Ashcraft's Pediatric Surgery; pp. 315–340. [Google Scholar]
- 63.Brady P.D., Srisupundit K., Devriendt K., Fryns J.P., Deprest J.A., Vermeesch J.R. Recent developments in the genetic factors underlying congenital diaphragmatic hernia. Fetal Diagn Ther. 2011;29:25–39. doi: 10.1159/000322422. [DOI] [PubMed] [Google Scholar]
- 64.Slavotinek A.M. The genetics of common disorders - congenital diaphragmatic hernia. Eur J Med Genet. 2014;57:418–423. doi: 10.1016/j.ejmg.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 65.Veenma D.C., de Klein A., Tibboel D. Developmental and genetic aspects of congenital diaphragmatic hernia. Pediatr Pulmonol. 2012;47:534–545. doi: 10.1002/ppul.22553. [DOI] [PubMed] [Google Scholar]
- 66.Wynn J., Yu L., Chung W.K. Genetic causes of congenital diaphragmatic hernia. Semin Fetal Neonatal Med. 2014;19:324–330. doi: 10.1016/j.siny.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kantarci S., Ackerman K.G., Russell M.K., Longoni M., Sougnez C., Noonan K.M., Hatchwell E., Zhang X., Pieretti Vanmarcke R., Anyane-Yeboa K., Dickman P., Wilson J., Donahoe P.K., Pober B.R. Characterization of the chromosome 1q41q42.12 region, and the candidate gene DISP1, in patients with CDH. Am J Med Genet A. 2010;152A:2493–2504. doi: 10.1002/ajmg.a.33618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Longoni M., High F.A., Russell M.K., Kashani A., Tracy A.A., Coletti C.M., Hila R., Shamia A., Wells J., Ackerman K.G., Wilson J.M., Bult C.J., Lee C., Lage K., Pober B.R., Donahoe P.K. Molecular pathogenesis of congenital diaphragmatic hernia revealed by exome sequencing, developmental data, and bioinformatics. Proc Natl Acad Sci U S A. 2014;111:12450–12455. doi: 10.1073/pnas.1412509111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Longoni M., Russell M.K., High F.A., Darvishi K., Maalouf F.I., Kashani A., Tracy A.A., Coletti C.M., Loscertales M., Lage K., Ackerman K.G., Woods S.A., Ward-Melver C., Andrews D., Lee C., Pober B.R., Donahoe P.K. Prevalence and penetrance of ZFPM2 mutations and deletions causing congenital diaphragmatic hernia. Clin Genet. 2015;87:362–367. doi: 10.1111/cge.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu L., Bennett J.T., Wynn J., Carvill G.L., Cheung Y.H., Shen Y., Mychaliska G.B., Azarow K.S., Crombleholme T.M., Chung D.H., Potoka D., Warner B.W., Bucher B., Lim F.Y., Pietsch J., Stolar C., Aspelund G., Arkovitz M.S., University of Washington Center for Mendelian Genomics. Mefford H., Chung W.K. Whole exome sequencing identifies de novo mutations in GATA6 associated with congenital diaphragmatic hernia. J Med Genet. 2014;51:197–202. doi: 10.1136/jmedgenet-2013-101989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu L., Wynn J., Cheung Y.H., Shen Y., Mychaliska G.B., Crombleholme T.M., Azarow K.S., Lim F.Y., Chung D.H., Potoka D., Warner B.W., Bucher B., Stolar C., Aspelund G., Arkovitz M.S., Chung W.K. Variants in GATA4 are a rare cause of familial and sporadic congenital diaphragmatic hernia. Hum Genet. 2013;132:285–292. doi: 10.1007/s00439-012-1249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu L., Sawle A.D., Wynn J., Aspelund G., Stolar C.J., Arkovitz M.S., Potoka D., Azarow K.S., Mychaliska G.B., Shen Y., Chung W.K. Increased burden of de novo predicted deleterious variants in complex congenital diaphragmatic hernia. Hum Mol Genet. 2015;24:4764–4773. doi: 10.1093/hmg/ddv196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brady P.D., Van Houdt J., Callewaert B., Deprest J., Devriendt K., Vermeesch J.R. Exome sequencing identifies ZFPM2 as a cause of familial isolated congenital diaphragmatic hernia and possibly cardiovascular malformations. Eur J Med Genet. 2014;57:247–252. doi: 10.1016/j.ejmg.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 74.Arrington C.B., Bleyl S.B., Matsunami N., Bowles N.E., Leppert T.I., Demarest B.L., Osborne K., Yoder B.A., Byrne J.L., Schiffman J.D., Null D.M., DiGeronimo R., Rollins M., Faix R., Comstock J., Camp N.J., Leppert M.F., Yost H.J., Brunelli L. A family-based paradigm to identify candidate chromosomal regions for isolated congenital diaphragmatic hernia. Am J Med Genet A. 2012;158A:3137–3147. doi: 10.1002/ajmg.a.35664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kantarci S., Al-Gazali L., Hill R.S., Donnai D., Black G.C., Bieth E., Chassaing N., Lacombe D., Devriendt K., Teebi A., Loscertales M., Robson C., Liu T., MacLaughlin D.T., Noonan K.M., Russell M.K., Walsh C.A., Donahoe P.K., Pober B.R. Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculo-acoustico-renal syndromes. Nat Genet. 2007;39:957–959. doi: 10.1038/ng2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vissers L.E., van Ravenswaaij C.M., Admiraal R., Hurst J.A., de Vries B.B., Janssen I.M., van der Vliet W.A., Huys E.H., de Jong P.J., Hamel B.C., Schoenmakers E.F., Brunner H.G., Veltman J.A., van Kessel A.G. Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet. 2004;36:955–957. doi: 10.1038/ng1407. [DOI] [PubMed] [Google Scholar]
- 77.Golzio C., Martinovic-Bouriel J., Thomas S., Mougou-Zrelli S., Grattagliano-Bessieres B., Bonniere M., Delahaye S., Munnich A., Encha-Razavi F., Lyonnet S., Vekemans M., Attie-Bitach T., Etchevers H.C. Matthew-Wood syndrome is caused by truncating mutations in the retinol-binding protein receptor gene STRA6. Am J Hum Genet. 2007;80:1179–1187. doi: 10.1086/518177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paine-Saunders S., Viviano B.L., Saunders S. GPC6, a novel member of the glypican gene family, encodes a product structurally related to GPC4 and is colocalized with GPC5 on human chromosome 13. Genomics. 1999;57:455–458. doi: 10.1006/geno.1999.5793. [DOI] [PubMed] [Google Scholar]
- 79.Krantz I.D., McCallum J., DeScipio C., Kaur M., Gillis L.A., Yaeger D., Jukofsky L., Wasserman N., Bottani A., Morris C.A., Nowaczyk M.J., Toriello H., Bamshad M.J., Carey J.C., Rappaport E., Kawauchi S., Lander A.D., Calof A.L., Li H.H., Devoto M., Jackson L.G. Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster Nipped-B. Nat Genet. 2004;36:631–635. doi: 10.1038/ng1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pober BR, Russell MK, Ackerman KG: Congenital diaphragmatic hernia overview. In GeneReviews [Internet]. Copyright University of Washington, Seattle. 1993-2016. Available at http://www.ncbi.nlm.nih.gov/pubmed/20301533. (last revised March 16, 2010). [PubMed]
- 81.Iafrate A.J., Feuk L., Rivera M.N., Listewnik M.L., Donahoe P.K., Qi Y., Scherer S.W., Lee C. Detection of large-scale variation in the human genome. Nat Genet. 2004;36:949–951. doi: 10.1038/ng1416. [DOI] [PubMed] [Google Scholar]
- 82.Sebat J., Lakshmi B., Troge J., Alexander J., Young J., Lundin P., Maner S., Massa H., Walker M., Chi M., Navin N., Lucito R., Healy J., Hicks J., Ye K., Reiner A., Gilliam T.C., Trask B., Patterson N., Zetterberg A., Wigler M. Large-scale copy number polymorphism in the human genome. Science. 2004;305:525–528. doi: 10.1126/science.1098918. [DOI] [PubMed] [Google Scholar]
- 83.Borys D., Taxy J.B. Congenital diaphragmatic hernia and chromosomal anomalies: autopsy study. Pediatr Dev Pathol. 2004;7:35–38. doi: 10.1007/s10024-003-2133-7. [DOI] [PubMed] [Google Scholar]
- 84.Klaassens M., van Dooren M., Eussen H.J., Douben H., den Dekker A.T., Lee C., Donahoe P.K., Galjaard R.J., Goemaere N., de Krijger R.R., Wouters C., Wauters J., Oostra B.A., Tibboel D., de Klein A. Congenital diaphragmatic hernia and chromosome 15q26: determination of a candidate region by use of fluorescent in situ hybridization and array-based comparative genomic hybridization. Am J Hum Genet. 2005;76:877–882. doi: 10.1086/429842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Longoni M., Lage K., Russell M.K., Loscertales M., Abdul-Rahman O.A., Baynam G., Bleyl S.B., Brady P.D., Breckpot J., Chen C.P., Devriendt K., Gillessen-Kaesbach G., Grix A.W., Rope A.F., Shimokawa O., Strauss B., Wieczorek D., Zackai E.H., Coletti C.M., Maalouf F.I., Noonan K.M., Park J.H., Tracy A.A., Lee C., Donahoe P.K., Pober B.R. Congenital diaphragmatic hernia interval on chromosome 8p23.1 characterized by genetics and protein interaction networks. Am J Med Genet A. 2012;158A:3148–3158. doi: 10.1002/ajmg.a.35665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Srisupundit K., Brady P.D., Devriendt K., Fryns J.P., Cruz-Martinez R., Gratacos E., Deprest J.A., Vermeesch J.R. Targeted array comparative genomic hybridisation (array CGH) identifies genomic imbalances associated with isolated congenital diaphragmatic hernia (CDH) Prenat Diagn. 2010;30:1198–1206. doi: 10.1002/pd.2651. [DOI] [PubMed] [Google Scholar]
- 87.Wat M.J., Shchelochkov O.A., Holder A.M., Breman A.M., Dagli A., Bacino C., Scaglia F., Zori R.T., Cheung S.W., Scott D.A., Kang S.H. Chromosome 8p23.1 deletions as a cause of complex congenital heart defects and diaphragmatic hernia. Am J Med Genet A. 2009;149A:1661–1677. doi: 10.1002/ajmg.a.32896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Russell M.K., Longoni M., Wells J., Maalouf F.I., Tracy A.A., Loscertales M., Ackerman K.G., Pober B.R., Lage K., Bult C.J., Donahoe P.K. Congenital diaphragmatic hernia candidate genes derived from embryonic transcriptomes. Proc Natl Acad Sci U S A. 2012;109:2978–2983. doi: 10.1073/pnas.1121621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Glessner J.T., Bick A.G., Ito K., Homsy J.G., Rodriguez-Murillo L., Fromer M., Mazaika E., Vardarajan B., Italia M., Leipzig J., DePalma S.R., Golhar R., Sanders S.J., Yamrom B., Ronemus M., Iossifov I., Willsey A.J., State M.W., Kaltman J.R., White P.S., Shen Y., Warburton D., Brueckner M., Seidman C., Goldmuntz E., Gelb B.D., Lifton R., Seidman J., Hakonarson H., Chung W.K. Increased frequency of de novo copy number variants in congenital heart disease by integrative analysis of single nucleotide polymorphism array and exome sequence data. Circ Res. 2014;115:884–896. doi: 10.1161/CIRCRESAHA.115.304458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Naxerova K., Bult C.J., Peaston A., Fancher K., Knowles B.B., Kasif S., Kohane I.S. Analysis of gene expression in a developmental context emphasizes distinct biological leitmotifs in human cancers. Genome Biol. 2008;9:R108. doi: 10.1186/gb-2008-9-7-r108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wilcox D.T., Irish M.S., Holm B.A., Glick P.L. Animal models in congenital diaphragmatic hernia. Clin Perinatol. 1996;23:813–822. [PubMed] [Google Scholar]
- 92.Bradley A., Anastassiadis K., Ayadi A., Battey J.F., Bell C., Birling M.C. The mammalian gene function resource: the International Knockout Mouse Consortium. Mamm Genome. 2012;23:580–586. doi: 10.1007/s00335-012-9422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.International Mouse Knockout Consortium. Collins F.S., Rossant J., Wurst W. A mouse for all reasons. Cell. 2007;128:9–13. doi: 10.1016/j.cell.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 94.Mallon A.M., Iyer V., Melvin D., Morgan H., Parkinson H., Brown S.D., Flicek P., Skarnes W.C. Accessing data from the International Mouse Phenotyping Consortium: state of the art and future plans. Mamm Genome. 2012;23:641–652. doi: 10.1007/s00335-012-9428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Charpentier E., Doudna J.A. Biotechnology: rewriting a genome. Nature. 2013;495:50–51. doi: 10.1038/495050a. [DOI] [PubMed] [Google Scholar]
- 96.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A., Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iyer V., Shen B., Zhang W., Hodgkins A., Keane T., Huang X., Skarnes W.C. Off-target mutations are rare in Cas9-modified mice. Nat Methods. 2015;12:479. doi: 10.1038/nmeth.3408. [DOI] [PubMed] [Google Scholar]
- 98.Williams A., Henao-Mejia J., Flavell R.A. Editing the mouse genome using the CRISPR-Cas9 system. Cold Spring Harb Protoc. 2016 doi: 10.1101/pdb.top087536. [Epub ahead of print] doi: 10.1101/pdb.top087536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nakamura Y., Harada K., Yamamoto I., Uemura Y., Okamoto K., Fukuda S., Hashimoto T. Human pulmonary hypoplasia: statistical, morphological, morphometric, and biochemical study. Arch Pathol Lab Med. 1992;116:635–642. [PubMed] [Google Scholar]
- 100.Wigglesworth J.S., Desai R., Guerrini P. Fetal lung hypoplasia: biochemical and structural variations and their possible significance. Arch Dis Child. 1981;56:606–615. doi: 10.1136/adc.56.8.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kent G.M., Olley P.M., Creighton R.E., Dobbinson T., Bryan M.H., Symchych P., Zingg W., Cummings J.N. Hemodynamic and pulmonary changes following surgical creation of a diaphragmatic hernia in fetal lambs. Surgery. 1972;72:427–433. [PubMed] [Google Scholar]
- 102.Nakamura Y., Fukuda S., Hashimoto T. Pulmonary elastic fibers in normal human development and in pathological conditions. Pediatr Pathol. 1990;10:689–706. doi: 10.3109/15513819009064705. [DOI] [PubMed] [Google Scholar]
- 103.Boucherat O., Benachi A., Barlier-Mur A.M., Franco-Montoya M.L., Martinovic J., Thebaud B., Chailley-Heu B., Bourbon J.R. Decreased lung fibroblast growth factor 18 and elastin in human congenital diaphragmatic hernia and animal models. Am J Respir Crit Care Med. 2007;175:1066–1077. doi: 10.1164/rccm.200601-050OC. [DOI] [PubMed] [Google Scholar]
- 104.Pieretti A.C., Ahmed A.M., Roberts J.D., Jr., Kelleher C.M. A novel in vitro model to study alveologenesis. Am J Respir Cell Mol Biol. 2014;50:459–469. doi: 10.1165/rcmb.2013-0056OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Beauchemin K.J., Wells J.M., Kho A.T., Philip V.M., Kamir D., Kohane I.S., Graber J.H., Bult C.J. Temporal dynamics of the developing lung transcriptome in three common inbred strains of laboratory mice reveals multiple stages of postnatal alveolar development. Peer J. 2016;4:e2318. doi: 10.7717/peerj.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mahood T.H., Johar D.R., Iwasiow B.M., Xu W., Keijzer R. The transcriptome of nitrofen-induced pulmonary hypoplasia in the rat model of congenital diaphragmatic hernia. Pediatr Res. 2016;79:766–775. doi: 10.1038/pr.2015.277. [DOI] [PubMed] [Google Scholar]
- 107.Sanford E.L., Choy K.W., Donahoe P.K., Tracy A.A., Hila R., Loscertales M., Longoni M. MiR-449a affects epithelial proliferation during the pseudoglandular and canalicular phases of avian and mammal lung development. PLoS One. 2016;11:e0149425. doi: 10.1371/journal.pone.0149425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eastwood M.P., Russo F.M., Toelen J., Deprest J. Medical interventions to reverse pulmonary hypoplasia in the animal model of congenital diaphragmatic hernia: a systematic review. Pediatr Pulmonol. 2015;50:820–838. doi: 10.1002/ppul.23206. [DOI] [PubMed] [Google Scholar]
- 109.Makanga M., Maruyama H., Dewachter C., Da Costa A.M., Hupkens E., de Medina G., Naeije R., Dewachter L. Prevention of pulmonary hypoplasia and pulmonary vascular remodeling by antenatal simvastatin treatment in nitrofen-induced congenital diaphragmatic hernia. Am J Physiol Lung Cell Mol Physiol. 2015;308:L672–L682. doi: 10.1152/ajplung.00345.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Deprest J., Gratacos E., Nicolaides K.H., Group F.T. Fetoscopic tracheal occlusion (FETO) for severe congenital diaphragmatic hernia: evolution of a technique and preliminary results. Ultrasound Obstet Gynecol. 2004;24:121–126. doi: 10.1002/uog.1711. [DOI] [PubMed] [Google Scholar]
- 111.DiFiore J.W., Fauza D.O., Slavin R., Peters C.A., Fackler J.C., Wilson J.M. Experimental fetal tracheal ligation reverses the structural and physiological effects of pulmonary hypoplasia in congenital diaphragmatic hernia. J Pediatr Surg. 1994;29:248–256. doi: 10.1016/0022-3468(94)90328-x. discussion 56–57. [DOI] [PubMed] [Google Scholar]
- 112.Al-Maary J., Eastwood M.P., Russo F.M., Deprest J.A., Keijzer R. Fetal tracheal occlusion for severe pulmonary hypoplasia in isolated congenital diaphragmatic hernia: a systematic review and meta-analysis of survival. Ann Surg. 2016 doi: 10.1097/SLA.0000000000001675. [Epub ahead of print] doi: 10.1097/SLA.0000000000001675. [DOI] [PubMed] [Google Scholar]
- 113.Sananes N., Rodo C., Peiro J.L., Britto I.S., Sangi-Haghpeykar H., Favre R., Joal A., Gaudineau A., Silva M.M., Tannuri U., Zugaib M., Carreras E., Ruano R. Prematurity and fetal lung response after tracheal occlusion in fetuses with severe congenital diaphragmatic hernia. J Matern Fetal Neonatal Med. 2016;29:3030–3034. doi: 10.3109/14767058.2015.1114080. [DOI] [PubMed] [Google Scholar]
- 114.Elattal R., Rich B.S., Harmon C.M., Muensterer O.J. Pulmonary alveolar and vascular morphometry after gel plug occlusion of the trachea in a fetal rabbit model of CDH. Int J Surg. 2013;11:558–561. doi: 10.1016/j.ijsu.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 115.DeKoninck P., Toelen J., Roubliova X., Carter S., Pozzobon M., Russo F.M., Richter J., Vandersloten P.J., Verbeken E., De Coppi P., Deprest J. The use of human amniotic fluid stem cells as an adjunct to promote pulmonary development in a rabbit model for congenital diaphragmatic hernia. Prenat Diagn. 2015;35:833–840. doi: 10.1002/pd.4621. [DOI] [PubMed] [Google Scholar]
- 116.Fauza D.O. Tissue engineering in congenital diaphragmatic hernia. Semin Pediatr Surg. 2014;23:135–140. doi: 10.1053/j.sempedsurg.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 117.Noble B.R., Babiuk R.P., Clugston R.D., Underhill T.M., Sun H., Kawaguchi R., Walfish P.G., Blomhoff R., Gundersen T.E., Greer J.J. Mechanisms of action of the congenital diaphragmatic hernia-inducing teratogen nitrofen. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1079–L1087. doi: 10.1152/ajplung.00286.2007. [DOI] [PubMed] [Google Scholar]
- 118.Santos M., Bastos P., Gonzaga S., Roriz J.M., Baptista M.J., Nogueira-Silva C., Melo-Rocha G., Henriques-Coelho T., Roncon-Albuquerque R., Jr., Leite-Moreira A.F., De Krijger R.R., Tibboel D., Rottier R., Correia-Pinto J. Ghrelin expression in human and rat fetal lungs and the effect of ghrelin administration in nitrofen-induced congenital diaphragmatic hernia. Pediatr Res. 2006;59:531–537. doi: 10.1203/01.pdr.0000202748.66359.a9. [DOI] [PubMed] [Google Scholar]
- 119.Eastwood M.P., Kampmeijer A., Jimenez J., Zia S., Vanbree R., Verbist G., Toelen J., Deprest J.A. The effect of transplacental administration of glucagon-like peptide-1 on fetal lung development in the rabbit model of congenital diaphragmatic hernia. Fetal Diagn Ther. 2016;39:125–133. doi: 10.1159/000436962. [DOI] [PubMed] [Google Scholar]