Abstract
A2A adenosine receptors (A2ARs) are endogenous inhibitor of inflammation. Macrophages that are key effectors of kidney disease progression express A2ARs. We investigated the role of A2ARs in kidney inflammation in a macrophage-mediated anti–glomerular basement membrane reactive serum-induced immune nephritis in A2AR-deficient mice. Sub-threshold doses of glomerular basement membrane–reactive serum induced more severe and prolonged kidney damage with higher levels of proinflammatory cytokines and greater accumulation of inflammatory cells in A2AR−/− mice than wild-type (WT) mice. To investigate the role of macrophage A2AR in progressive kidney injury, glomerulonephritis was induced in CD11b-DTR transgenic mice. Macrophages were selectively depleted in the established phase of the disease and reconstituted with macrophages from WT or A2AR-deficient mice and then treated with an A2AR agonist. In mice receiving WT macrophages and treated with an A2AR agonist, the glomerular cellularity, crescent formation, sclerotic glomeruli, and tubulointerstitial injury were significantly reduced compared with the control group. In contrast, in mice reconstituted with A2AR-deficient macrophages and treated with an A2AR agonist, the kidney injury was more severe with increased deposition of collagen I, III, and IV. These findings suggest that disruption of the protective A2AR amplifies inflammation to accelerate glomerular damage and endogenous macrophage A2ARs are essential to protect from progressive kidney fibrosis.
Despite an alarming increase in the number of patients with chronic kidney disease (CKD), current treatment options remain limited and unchanged from the last century, with a general failure in arresting or reversing kidney injury and its cardiovascular complications.1
Inflammation has an important role in the development and progression of most CKD. In its end stage, the diseased kidney is characterized histologically by chronic inflammation and fibrosis. However, understanding and preventing inflammation and the progressive fibrosis of CKD remain unsolved challenges. Time-course studies have shown an association between macrophage accumulation and development of kidney injury. Macrophages are linked with the irreversible scarring that leads to end-stage renal disease.2, 3 The ability of macrophages to secrete a wide range of factors that cause tissue injury has led to the proposal that they participate in mediating kidney injury, rather than simply being a response to tissue damage. Consistent with this hypothesis, the depletion of macrophages reduces glomerular injury and proteinuria in several experimental models of kidney injury.4
Regulation of inflammation is accomplished by diverse endogenous mechanisms, including adenosine.5, 6, 7, 8, 9 Genetic and pharmacological evidence support a significant, nonredundant role for both endogenous adenosine and their receptors (A2ARs) in protecting tissue from acute inflammatory damage in different models of inflammatory injury and systemic inflammation. The mechanism involves down-regulation of activated immune cells in vivo.7 A2AR is found on most bone marrow–derived cells and not only attenuates inflammation and protects from tissue damage, but is also involved in tissue repair. Moreover, wound healing is accelerated by A2AR agonists.10, 11, 12
Expression and activation of A2AR on macrophages can inhibit production of IL-12, tumor necrosis factor-α, and nitric oxide, and enhance the secretion of IL-10 in response to lipopolysaccharide.13 A2AR agonists protect kidneys from ischemia-reperfusion and attenuate inflammation and injury in diabetic nephropathy.14, 15
Crescentic glomerulonephritis is a life-threatening disease in which glomerular inflammation progresses rapidly into CKD.
We have previously reported that macrophages infiltrating nephritic glomeruli express A2AR, and stimulation of A2AR with a specific agonist attenuates kidney injury.16, 17 We report herein that lack of A2AR amplifies inflammation to accelerate glomerular damage, and it is the activation of macrophage A2AR that is responsible for protecting the kidney from inflammation and tissue injury.
Materials and Methods
Glomerular Basement Membrane–Reactive NTS and NTS Nephritis
Male and female A2AR−/− (8 weeks old, kindly provided by Dr. Jiang-Fan Chen, Boston University, Boston, MA) and age- and sex-matched littermate wild-type (WT) controls were used. The primary animal model used was a type of nephrotoxic serum (NTS) nephritis in which mice are preimmunized with rabbit IgG 5 days before i.v. injection of sub-threshold doses of NTS (15 μL/20 g body weight). Preparation of NTS has been described.17, 18 This model results in subclinical disease, but is ideal for models in which endogenous anti-inflammatory mechanisms are blocked, as the disease then becomes manifest. Mice were euthanized at day 14 after the injection of NTS to collect kidney tissue and blood. In other groups of mice, the full (optimal) amount of NTS (30 μL/20 g body weight) was administered and mice were euthanized at day 8. At this time, pronounced glomerular injury and marked tubulointerstital abnormalities are observed.19, 20 Serum creatinine was determined by liquid chromatography–tandem mass spectrometry.21 All experiments were performed under protocols approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine (Houston, TX) and the University of Colorado Denver (Aurora).
mRNA Expression of Cytokines/Chemokines
Kidney total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA). Five micrograms of total RNA from each sample was used for RNase protection assay. mCK2b, mCK3b, and mCK5c multiprobe template sets (BD Biosciences, San Jose, CA) were used to investigate cytokine and chemokine expression. Riboprobes for osteopontin-1 (OPN), 316 bp (nucleotide 331 to 647; http://www.ncbi.nlm.nih.gov/nuccore/AF515708; accession number AF515708), and thrombospondin-1 (TSP-1), 416 bp (nucleotide 1430 to 1846; http://www.ncbi.nlm.nih.gov/nuccore/M87276; accession number M87276), were generated from kidney by RT-PCR. RNase protection assay was performed using the Torrey Pines Biolabs kit (East Orange, NJ), as described.16, 22, 23, 24, 25 Phosphoimage quantitation of blots was performed using the PhosphorImager SI scanning instrument and ImageQuaNT software version 5.1 (Molecular Dynamics, Sunnyvale, CA). L-32 was used as a housekeeping gene. Final values were expressed as a ratio of counts per minute for the specific mRNA/L-32 mRNA to ensure a constant quantity of RNA in each sample.
Histopathology
Kidney samples fixed in formalin and methanol-Carnoy fixative solution were embedded in paraffin. Sections (3 to 4 μm thick) were stained with periodic acid-Schiff reagent. Infiltrating macrophages and T cells were immunohistochemically stained with monoclonal antibody anti-CD68 (AbD Serotec, Raleigh, NC) and the pan-T-cell marker CD3 (BD Biosciences, San Jose, CA), respectively, using frozen sections.26, 27 We selected CD68 antibody to detect macrophages, because CD68 is expressed by all macrophages that accumulate in nephritic glomeruli. In contrast, CD11b and F4/80 appear to be expressed by a subset of glomerular macrophages.28 Bound antibodies were detected using a horseradish peroxidase–based detection system. Positively stained cells per 60 glomeruli were counted and expressed per glomerular section. Interstitial cell counts were determined in 10 high-power fields (×400). All quantitative morphological analyses were performed in a blinded manner (L.D.T.).
Immunohistochemistry of Collagen
Paraffin sections of methanol-Carnoy fixed tissue were stained for collagens I, III and IV, with specific antibodies (Southern Biotech, Birmingham, AL). The secondary antibodies consisted of peroxidase-coupled rabbit anti-goat IgG (Dako North America Inc., Carpinteria, CA). Histological morphometry for collagens was performed using a ScanScope digital scanner (Aperio Technologies, Inc., Vista, CA), and results were expressed as means ± SEM % area.
Isolation of BMDMs
Bone marrow–derived macrophages (BMDMs) were isolated and cultured as described.29, 30 In brief, bone marrow cells were harvested from femurs and tibias of A2AR-deficient mice or WT mice. Cells were incubated in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 1% penicillin/streptomycin, and 15% L929 cell-conditioned medium as a source of macrophage colony-stimulating factor at 37°C. After 18 hours, nonadherent cells (containing BMDM) were transferred to a new dish and incubated for an additional 7 days. More than 90% of the cells were CD68 positive macrophages, as determined by flow cytometry.
Macrophage Labeling and Adoptive Transfer to CD11b-DTR Mice
For in vivo tracking, macrophages were labeled with PKH-26GL (Sigma, St. Louis, MO), according to the manufacturer's instructions, and harvested into serum-free medium immediately before injection.
CD11b-DTR mice (stock 006000; The Jackson Laboratory, Bar Harbor, ME) are transgenic for human diphtheria toxin (DT) receptor (DTR) under the control of the CD11b promoter. Administration of nanogram doses of DT results in rapid and marked macrophage ablation in different tissues, including kidney.4, 31, 32, 33, 34, 35, 36 NTS nephritis was induced in CD11b-DTR male mice and macrophages were depleted at day 5 by 20 ng/g DT (i.p.). Twenty-four hours later, when there is almost complete absence of macrophages, one million labeled BMDMs (the number of monocytes in one blood volume37, 38) from WT or A2AR−/− mice were transferred into CD11b-DTR mice by tail vain injection (CD11b-DTR mice and A2AR−/− mice are in a C57BL/6 background). Mice received a second dose of DT at day 7 after induction of the NTS nephritis to maintain a depleted endogenous macrophage population, as described. Mice were euthanized at day 8.4 Mice without NTS nephritis received labeled BMDMs and were euthanized 48 hours later.
Frozen sections from kidney, spleen, and lung were stained with Alexa Fluor 488 phalloidin (Life Technologies, Grand Island, NY) and visualized under fluorescence microscopy.
Conditional Ablation of Macrophages and Reconstitution with WT or A2AR-Deficient Macrophages
NTS nephritis was induced in CD11b-DTR transgenic mice, and macrophages were depleted and reconstituted with WT or A2AR-deficient macrophages, as described above. Mice received an A2AR agonist, CGS21680 (2 mg/kg twice a day i.p.7), the same day of macrophage transfer until they were euthanized (day 8 after injection of NTS). In addition, a group of mice reconstituted with A2AR-deficient macrophages was not treated with the A2AR agonist to further investigate the role of macrophage A2AR in kidney injury in NTS nephritis. CD11b-DTR transgenic mice depleted of macrophages and reconstituted with WT macrophages without receiving treatment were used as a control. Kidneys were collected for histological examination, as described above.
Circulating Antibody and Glomerular IgG Deposition
Mouse anti-rabbit IgG titers were measured by enzyme-linked immunosorbent assay using sera collected at the time mice were euthanized as described.16, 20, 39 Bound mouse IgG was detected using peroxidase-conjugated anti-mouse IgG (Dako, Carpinteria, CA) at 1:1000 dilution and absorbance reading at 450 nm. Normal sera served as a negative control. IgG deposition was determined in kidney frozen sections using fluorescein isothiocyanate–labeled anti-rabbit IgG (Dako) or anti-mouse IgG (Dako), as described.16, 20, 22, 40, 41 Immunofluorescence images were analyzed by ImageJ software version 1.48v (NIH, Bethesda, MD), as described.20
Statistical Analysis
Mice sample size was determined using SD values and power analyses of our previous studies on the NTS nephritis.20 Statistical analyses were performed using the one-way analysis of variance with multiple pairwise comparisons with the Bonferroni adjustment for multiple hypothesis testing. Normality and homogeneity of variance were confirmed using the Kolmogorov-Smirnov analysis. Analysis of variance was used to compare all treatments and specific pairwise comparisons as stated in the experiments. Student's t-test (Mann-Whitney U test) was used to compare mean values between two experimental groups. Data are reported as means ± SEM. Values of P < 0.05 were considered statistically significant.
Results
Absence of A2AR Increases and Prolongs Inflammation and Kidney Damage in NTS Nephritis
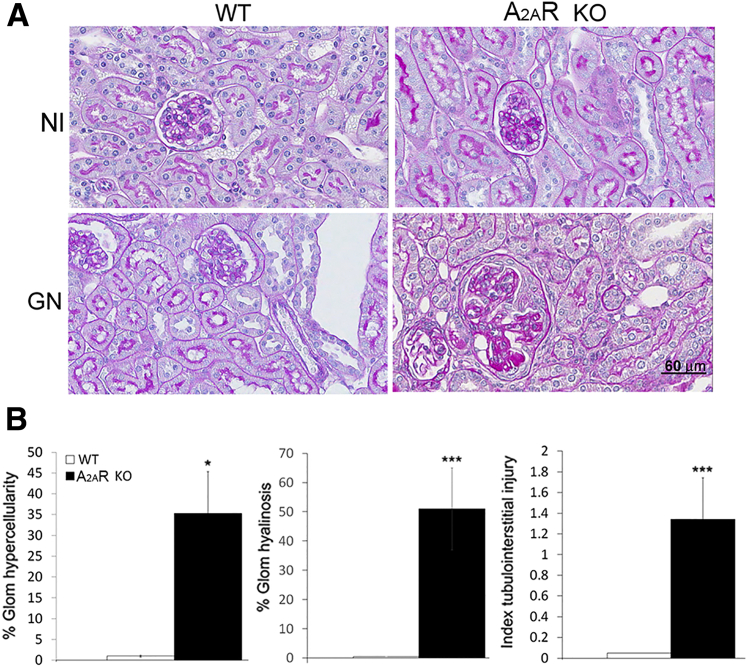
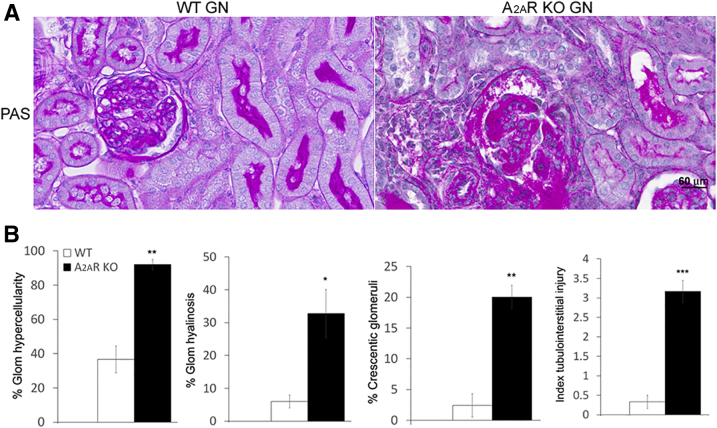
A2AR−/− mice developed normally and showed no gross anatomical defects, as reported.42 In addition, there were no histological differences in glomeruli and tubulointerstitium between normal control A2AR−/− and WT mice (Figure 1). To investigate whether these animals were more prone to kidney injury, the suboptimal (subclinical) model of NTS nephritis was induced in both WT and A2AR−/− mice. A2AR−/− mice showed severe glomerular cell proliferation and glomerular hyalinosis in 35% and 51% of the glomeruli, respectively, at day 14 after the induction of NTS nephritis. Crescent formation was observed in 40% of the glomeruli. In contrast, WT mice receiving suboptimal NTS started recovering from the disease at this time and only 0.2% of glomeruli demonstrated glomerular proliferation, whereas glomerular hyalinosis and crescent formation were not observed. In addition, the tubulointerstitial injury (TIN) (index, 1.34 ± 0.4) was marked in A2AR−/− mice, whereas no TIN injury was observed in WT mice receiving suboptimal NTS (Figure 1). Normal kidney function was maintained in WT mice with suboptimal NTS (Nl versus WT, 0.70 ± 0.02 versus 0.85 ± 0.05 μg/mL); however, serum creatinine levels were increased in A2AR−/− mice with NTS nephritis compared to normal controls (0.70 ± 0.02 versus 1.1491 ± 0.054 μg/mL; P < 0.01) and WT mice with NTS nephritis (0.85 ± 0.05 versus 1.1491 ± 0.054 μg/mL; P < 0.05) (Supplemental Figure S1).
Figure 1.
Sub-threshold doses of nephrotoxic serum (NTS) induce more severe and prolonged kidney injury in A2AR−/− mice. A: Periodic acid-Schiff (PAS) staining of kidney sections of wild-type (WT) and A2AR-deficient mice with and without glomerulonephritis (GN). Nephritic kidneys from mice lacking A2AR have more severe lesion compared with WT mice. B: Quantitation of kidney injury in WT and A2AR−/− mice with NTS nephritis. Each data point represents sections sampled from six (WT) or seven (A2AR−/−) mice. Data are expressed as means ± SEM (B). ∗P < 0.05, ∗∗∗P < 0.001. Scale bar = 60 μm (A). Original magnification, ×400 (A). Glom, glomerular; KO, knockout; Nl, normal.
Severity of NTS Nephritis in A2AR−/− Mice Is Associated with Increased Infiltration of Inflammatory Cells
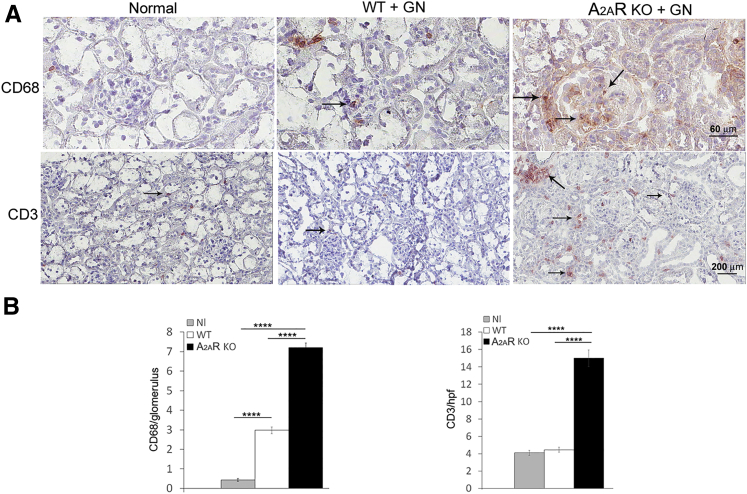
Because macrophages are implicated in causing kidney injury, we examined infiltration of macrophages into the kidney in A2AR−/− mice and WT mice. There was higher accumulation of macrophages in glomeruli of A2AR−/− mice compared with WT mice (P < 0.0001) (Figure 2). In addition, although there was no difference between WT and A2AR−/− mice in T cells infiltrating glomeruli, increased recruitment of T cells to kidney interstitium was observed in A2AR−/− mice (Figure 2).
Figure 2.
Glomerular macrophages and kidney interstitium T-cell infiltration increases in A2AR−/− mice. A: Immunohistochemistry stained for CD68+ monocytes/macrophages (arrows) and CD3 (arrows) of kidney sections of wild-type (WT) and A2AR-deficient mice. B: Quantitation of CD68+ and CD3 cell infiltration from WT and A2AR−/− mice with nephrotoxic serum nephritis. Each data point represents sections sampled from six (WT) or seven (A2AR−/−) mice. Data are expressed as means ± SEM (B). ∗∗∗∗P < 0.0001. Scale bars: 60 μm (A, CD68); 200 μm (A, CD3). Original magnifications: ×400 (A, CD68); ×200 (A, CD3). GN, glomerulonephritis; hpf, high-power field; KO, knockout; Nl, normal.
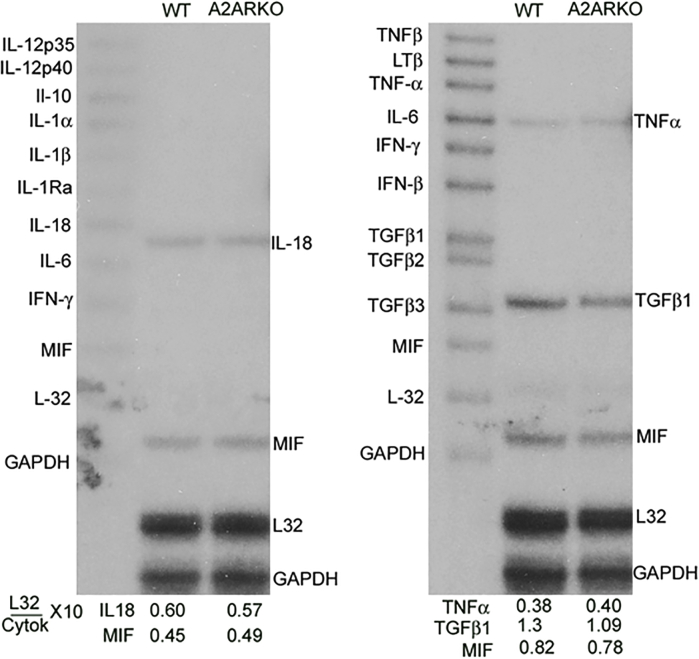
Cytokine Expression Is Induced in Kidneys from A2AR−/− Mice with NTS Nephritis
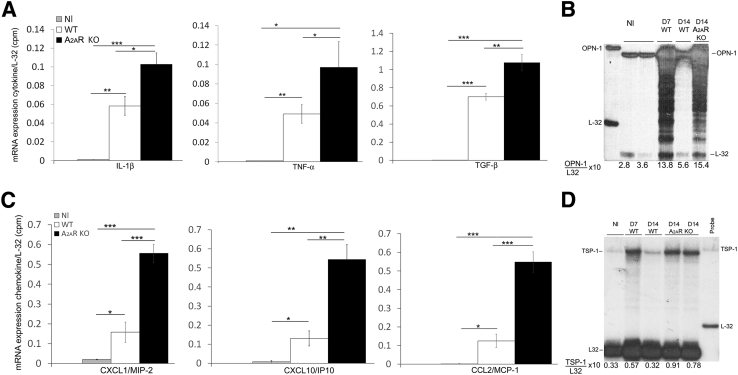
Increased expression of proinflammatory cytokines is observed in macrophages from A2AR−/− mice.43 Consequently, an absence of A2AR could increase inflammatory damage and macrophage infiltration through increased expression of cytokines/chemokines. In nephritic kidneys from A2AR−/− mice, higher expression of proinflammatory cytokines IL-1β and tumor necrosis factor-α mRNA was observed compared with WT mice. Enhanced expression of the profibrotic cytokine transforming growth factor-β was present in mice lacking A2AR. In addition, CXCL1/macrophage inflammatory protein-2, CXCL10/interferon-γ-inducible protein-10, and chemokine ligand 2/monocyte chemoattractant protein-1 were induced in nephritic kidneys from A2AR−/− mice compared with WT mice (Figure 3, A and C).
Figure 3.
Analysis of proinflammatory and profibrotic molecules in nephrotoxic serum (NTS) nephritis. A and C: Higher expression of cytokines/chemokines is induced in kidneys from A2AR-deficient mice compared with wild-type (WT) mice. Densitometric analysis of blots from RNase protection assay of cytokines and chemokines expressed in the kidneys of A2AR−/− mice and WT mice with NTS nephritis. The data are presented as a ratio of the counts per minute for the specific mRNA/L-32 mRNA to ensure a constant quantity of RNA in each sample. Results were sampled from six (WT) or seven (A2AR−/−) mice per group. B and D: Robust expression of osteopontin-1 (OPN-1) and thrombospondin-1 (TSP-1) in nephritic kidneys from A2AR−/− mice. Representative figure of RNase protection assay of OPN-1 and TSP-1 from three separated assays. Probes contain polylinker regions and are longer than the protected bands. Mouse ribosomal L-32 gene was used as a housekeeping gene. The data are presented as a ratio of the counts per minute for the specific mRNA/L-32 mRNA to ensure a constant quantity of RNA in each sample. Data are expressed as means ± SEM (A and C). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. CCL2, chemokine ligand 2; KO, knockout; MCP-1, monocyte chemoattractant protein-1; Nl, normal; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α.
Robust Expression of Profibrotic Molecules in the Kidney from A2AR Knockout Mice with NTS Nephritis
TSP-1 (an activator of transforming growth factor-β and anti-angiogenic factor) and OPN-1 (a profibrotic molecule) are increased in glomerulonephritis (GN). A2AR agonist treatment prevented the induction of TSP-1 and significantly reduced the expression of OPN-1 in nephritic kidneys.16 Accordingly, we determined if absence of A2AR affects the expression of these profibrotic molecules. In WT mice with GN, OPN-1 and TSP-1 increased at day 7 and returned to basal levels at day 14. In contrast, in mice lacking A2AR, nephritic kidneys had sustained increases of both OPN-1 and TSP-1 through day 14 (Figure 3, B and D).
Enhanced Collagen Deposition in Kidneys from A2AR−/− Mice with NTS Nephritis
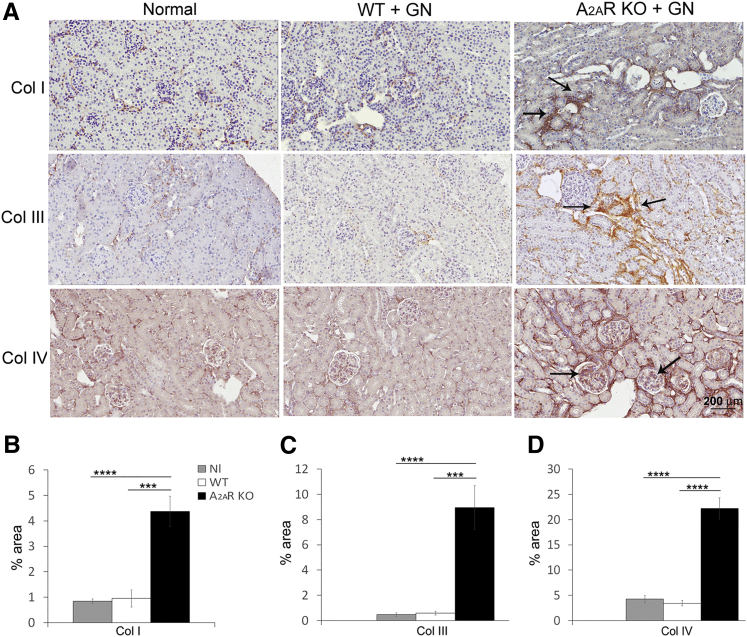
In A2AR−/− mice, increased deposition of collagen I and collagen III was observed. In contrast, collagen I and collagen III expression in WT mice was no different compared to normal kidneys. Collagen IV, an important component of glomerular extracellular matrix, was enhanced in the glomeruli of A2AR−/− mice; however, collagen IV expression in WT mice was not different when compared with normal kidney (Figure 4).
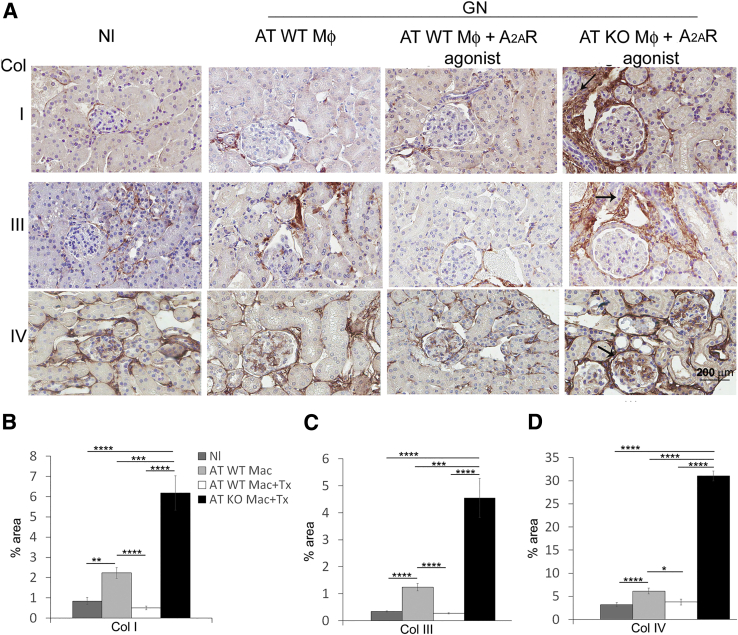
Figure 4.
Collagen (Col) deposition increases in the kidney from A2AR−/− mice. Collagen I and collagen III expression (arrows) increase in the interstitium (A–C), and collagen IV deposition (arrows) enhances in the glomeruli from nephritic kidneys (A and D) in A2AR−/− mice. Collagen staining was used. Results are expressed as means ± SEM (B–D). ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001. Scale bar = 200 μm (A). Original magnification, ×200 (A). GN, glomerulonephritis; KO, knockout; Nl, normal; WT, wild type.
Lack of A2AR Does Not Affect Humoral Immune Response or Glomerular IgG Deposition
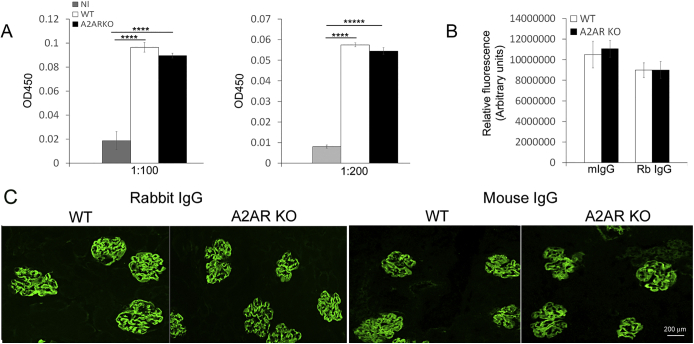
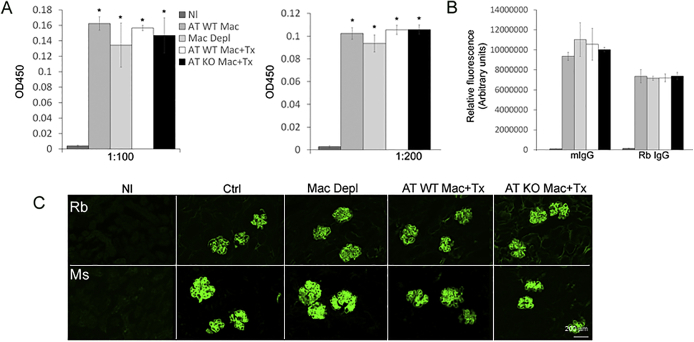
To analyze systemic humoral response in WT and A2AR−/− mice with NTS nephritis, we assessed antigen-specific total mouse anti-rabbit IgG in serum by specific enzyme-linked immunosorbent assay. Total mouse anti-rabbit globulin IgG levels were similar in both groups (Supplemental Figure S2A). In addition, rabbit IgG and mouse IgG glomerular deposits were not different between WT and A2AR−/− mice (Supplemental Figure S2,B and C).
Severe Kidney Injury in Mice Deficient in A2AR Using Optimal Dose of NTS
We investigated if higher amount of NTS (full, optimal dose) could induce more severe damage in A2AR−/− mice compared to WT mice. In WT mice, an optimal dose of NTS led to marked glomerular hypercellularity, glomerular hyalinosis, crescent formation, and TIN injury. However, the glomerular and TIN damage was more prominent in mice deficient in A2AR (Figure 5).
Figure 5.
Severe kidney injury in A2AR−/− mice with higher (optimal/full) dose of nephrotoxic serum (NTS). A: Wild-type (WT) mice receiving optimal dose of NTS display pronounced kidney damage; glomeruli (Glom) are hypercellular with hyalinosis and crescent formation, and kidneys have marked tubulointersitial injury. However, in A2AR−/− mice, the glomerular and tubuloinerstitial injury is more prominent. B: Quantitation of kidney injury in WT and A2AR−/− mice with NTS nephritis. Periodic acid-Schiff (PAS) staining was used. Data are expressed as means ± SEM (B). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Scale bar = 60 μm (A). Original magnification, ×400 (A). GN, glomerulonephritis; KO, knockout.
Kidney Macrophage Depletion and Reconstitution
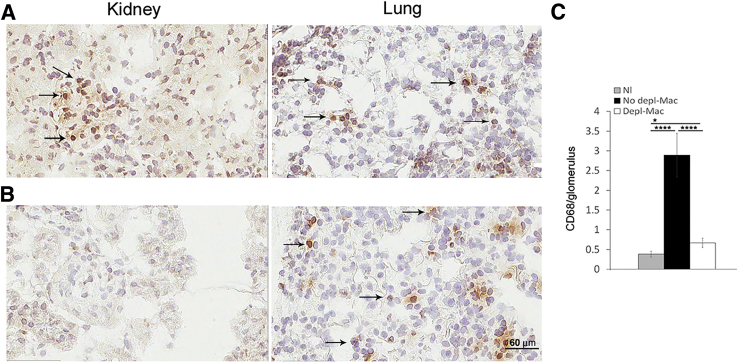
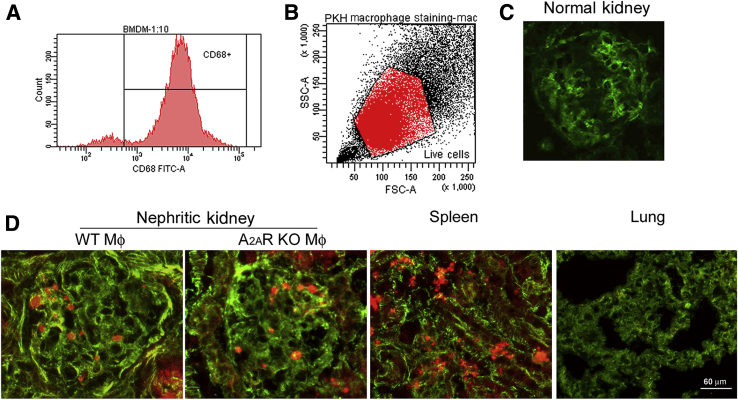
Macrophages infiltrating nephritic glomeruli express A2AR.17 To identify if the lack of macrophage A2ARs is responsible for the deleterious effect in NTS nephritis in A2AR−/− mice, we used a conditional macrophage ablation and adoptive transfer approach. Administration of 20 ng/g DT to CD11b-DTR transgenic (Tg) mice with NTS nephritis at day 5 and day 7 after induction of the disease was highly effective at ablating kidney macrophages but not alveolar macrophages (CD11b−/CD11c+/high) at the time mice were euthanized (day 8 after NTS injection), as described (Figure 6).4, 32, 44, 45 Injection of DT resulted in approximately 80% reduction in glomeruli macrophages, as reported.4, 35, 37 In contrast, in CD11b-DTR mice with NTS nephritis receiving vehicle control, glomerular macrophage infiltration was not affected (Figure 6). We next tracked glomerular accumulation of transferred BMDMs isolated from A2AR−/− mice or WT mice (the fraction of CD68-positive cells was >90%; Figure 7A) by labeling them with PKH-26GL (94.3% viable labeled cells were obtained; Figure 7B). One million macrophages were injected on day 6 after induction of NTS nephritis in CD11b-DTR mice ablated of macrophages by injecting DT at days 5 and 7, as described above. Mice were euthanized at day 8 after induction of the disease, and recruited macrophages were detected in the kidney. A similar number of macrophages from A2AR−/− mice and WT mice were observed into nephritic glomeruli (4.3 ± 0.5214 and 4.6 ± 0.53, respectively); however, there was no macrophage infiltration in CD11b-DTR mice without GN, as described (Figure 7, C and D).29, 46 Labeled macrophages were also distributed to spleen but not to the lung, as reported (Figure 7D).47
Figure 6.
Diphtheria toxin (DT) depletes glomerular macrophages in nephritic kidneys but not in the lungs. Immunohistochemistry stained for CD68+ monocytes/macrophages of kidney and lung sections at day 8 after the induction of GN. A: In CD11b-DTR transgenic (Tg) mice with GN receiving vehicle control, glomeruli macrophage infiltration (arrows) is not affected. B: In contrast, macrophages (Mac) are depleted (Depl) in CD11b-DTR Tg mice with GN that received two doses of DT starting at day 5. C: Quantitation of glomeruli CD68. Data are expressed as means ± SEM (C). ∗P < 0.05, ∗∗∗∗P < 0.0001. Scale bar = 60 μm (A and B). Original magnification, ×400 (A and B). GN, glomerulonephritis; Nl, normal.
Figure 7.
Transferred macrophages (Mφ) migrate to nephritic kidney. A: Purity of isolated bone marrow–derived macrophages (BMDMs). Representative flow cytometry analysis of CD68-positive cell population. B: Fluorescence-activated cell sorter analysis of BMDMs stained with PKH-26GL. C: Representative fluorescence micrograph showing no macrophage infiltration of normal glomeruli. D: Representative fluorescence micrographs of BMDM-PKH-26GL stained. Similar number of wild-type (WT) and A2AR−/− macrophages migrate to nephritic glomeruli. Macrophages also migrate to the spleen but not to the lung. Scale bar = 60 μm (D). Original magnification, ×400 (C and D). FSC, forward scatter; KO, knockout; SSC, side scatter.
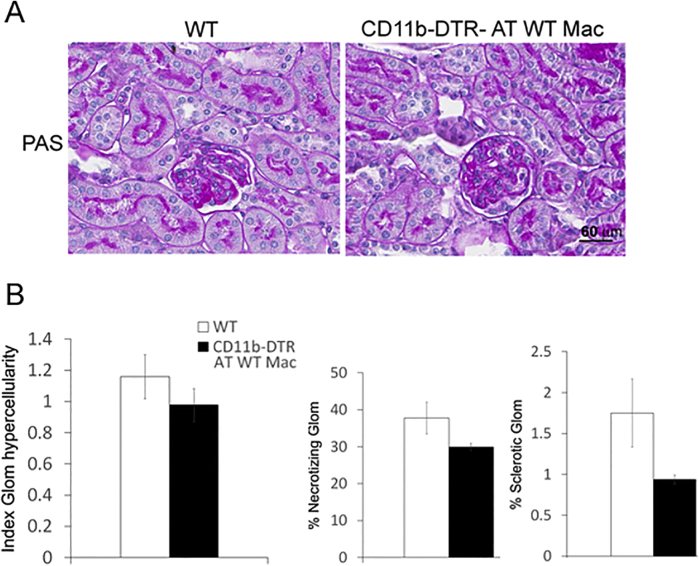
The course of NTS nephritis was similar in CD11b-DTR-Tg mice receiving WT macrophages compared with WT mice. Although the kidney injury had a tendency to be more severe in WT mice, the difference was not statistically significant, notwithstanding there was also more variation in kidney lesions among WT mice (Supplemental Figure S3).
To determine whether BMDMs from A2AR−/− mice express more cytokines at basal level compared to WT, mRNA expression of several cytokines was analyzed by RNase protection assay. There was no difference in the mRNA expression of proinflammatory cytokines between unstimulated BMDMs from WT and A2AR−/− mice (Supplemental Figure S4).
Macrophage Depletion and Reconstitution during Established Nephritis: Lack of Macrophage A2AR Increases Kidney Injury
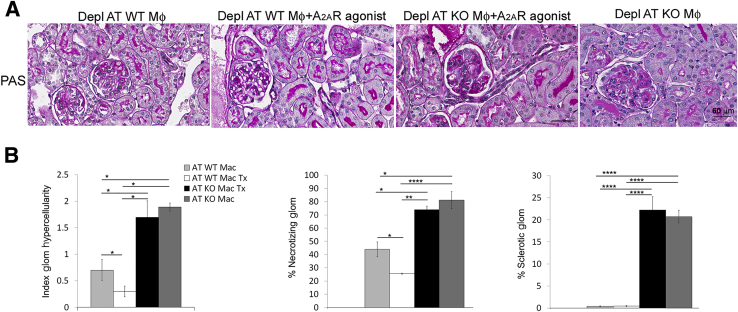
To determine the role of A2AR expressing macrophages in kidney injury in GN, endogenous macrophages were removed with DT in CD11b-DTR Tg mice with NTS nephritis at day 5 after induction of the diseases, as described above. Adoptive transfer of macrophages from A2AR−/− mice or WT mice was performed at day 6, and mice received treatment with the A2AR agonist CGS21680 (2 mg/kg twice a day7) the same day until mice were euthanized at day 8. In addition, a group of mice transferred with A2AR−/− macrophages were not treated with the A2AR agonist. CD11b-DTR Tg mice with GN receiving WT macrophages but not treated with A2AR agonist were used as control.
At day 8 after induction of GN, glomerular hypercellularity, necrotizing lesion, and sclerotic glomeruli were increased in mice reconstituted with A2AR−/− macrophages and treated with A2AR agonist (AT KO Mac Tx) compared with mice receiving WT macrophages without treatment (AT WT Mac) (Figure 8, A and B) and mice reconstituted with WT macrophages and treated with A2AR agonist. In contrast, adoptive transfer of WT macrophage plus treatment with A2AR agonist (AT WT Mac Tx) significantly reduced glomerular hypercellularity and necrotizing lesion; sclerotic glomeruli were not observed (Figure 8, A and B). No significant difference was observed between mice reconstituted with A2AR−/− macrophages treated with the agonist and mice transferred with A2AR−/− macrophages but not treated with the A2AR agonist CGS21680 (Figure 8, A and B).
Figure 8.
Lack of macrophage A2AR increases kidney injury in nephrotoxic serum (NTS) nephritis. A: Periodic acid-Schiff staining of kidney sections of nephritic kidneys from mice that were depleted (Depl) of macrophages (Mφ) and received: adoptive transfer of wild-type (WT) macrophages (Depl AT WT Mφ, AT WT Mac); adoptive transfer of WT Mφ and treated with A2AR agonist (Depl AT WT Mφ + A2AR agonist, AT WT Mac Tx); adoptive transfer of Mφ deficient in A2AR and treated with A2AR agonist (Depl AT KO Mφ + A2AR agonist, AT KO Mac Tx); and adoptive transfer of A2AR−/− macrophages without treatment with A2AR agonist (Depl AT KO Mφ, AT KO Mac). Sections were sampled on day 8 after NTS injection. B: Quantitation of kidney injury. Data are expressed as means ± SEM (B). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗∗P < 0.0001. Scale bar = 60 μm (A). Original magnification, ×400 (A). Glom, glomerular.
Increased Kidney Fibrosis in Absence of Macrophage A2AR
Pathological deposition of collagen I, III, and IV was significantly increased in mice with adoptive transfer of A2AR−/− macrophages and treated with A2AR agonist (Figure 9). In contrast, pharmacological activation of A2AR in mice receiving WT macrophages significantly reduced collagen I, III, and IV accumulation compared with untreated GN mice (Figure 9).
Figure 9.
Absence of macrophage A2AR enhances collagen (Col) deposition. Collagen I and collagen III expression (arrows) increases in the interstitium (A–C), and collagen IV deposition (arrows) enhances in the glomeruli (A and D) from nephritic kidneys in mice receiving adoptive transfer of A2AR−/− macrophages (AT KO Mac + Tx). Results are expressed as means ± SEM (B–D). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001. Scale bar = 200 μm (A). Original magnification, ×200 (A). GN, glomerulonephritis; KO, knockout; Nl, normal; WT, wild type.
Kidney Function Is Deteriorated in Mice Reconstituted with A2AR−/− Macrophages
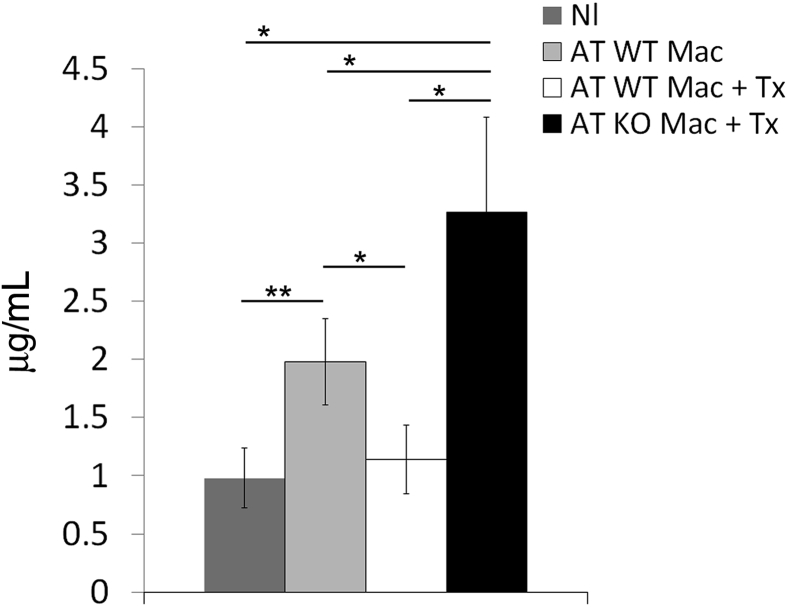
As a result of the severity of kidney injury in mice receiving A2AR−/− macrophages (AT KO mac Tx), serum creatinine was significantly higher in AT KO Mac Tx mice compared with control mice (AT WT mice, P < 0.05) (Figure 10). In contrast, serum creatinine was not affected in mice receiving WT macrophages treated with A2AR agonist (Figure 10).
Figure 10.
Kidney function decreases in mice lacking macrophage A2AR. Serum creatinine was increased in mice with adoptive transfer of A2AR−/− macrophages (AT KO Mac + Tx). Results are expressed as means ± SEM. ∗P < 0.05, ∗∗P < 0.01.
In CD11b-DTR-Tg Mice, Antigen-Specific Humoral Immune Response or Glomerular IgG Deposition Is Not Affected
To analyze if the systemic humoral immune response could be affected by DT or adoptive transfer of macrophages, we measured antigen-specific total mouse anti-rabbit IgG serum by enzyme-linked immunosorbent assay. Total mouse anti-rabbit globulin IgG levels were similar between WT mice and mice with NTS nephritis without depletion of macrophages and mice with GN and macrophage depletion and reconstitution with WT macrophages or A2AR−/− macrophages (Supplemental Figure S5A). In addition, rabbit IgG and mouse IgG glomerular deposition were not different among the groups (Supplemental Figure S5,B and C).
Discussion
In WT mice, low doses of NTS caused minimal kidney injury with recovery of the disease by day 14. However, in mice deficient in A2AR, sub-threshold doses of NTS induced extensive and prolonged kidney injury with enhanced inflammatory response. Administration of higher amount of NTS (optimal dose) also led to a more prominent glomerular and TIN injury in A2AR−/− deficient mice. In addition, macrophage depletion and reconstitution with WT or A2AR−/− macrophages plus treatment with an A2AR agonist, during the established phase of the disease, showed that mice receiving A2AR−/− macrophages had increased kidney injury; in contrast, kidney damage was significantly attenuated in mice reconstituted with WT macrophages. These data indicate that A2A adenosine receptors play a critical role in regulating inflammatory response in NTS nephritis, disruption of the endogenous protective A2AR amplifies inflammation to accelerate kidney damage, and endogenous macrophage A2ARs are essential to protect from progressive kidney injury.
Increasing evidence shows a key role for inflammation in the induction and progression of GN and other kidney diseases.19, 48, 49 NTS nephritis is a rapidly progressive glomerular disease with a poor prognosis. Macrophages are known to have a key role in the induction and development of rapidly progressive glomerular disease; the magnitude of proteinuria and percentage of crescentic glomeruli are related with the number of macrophages infiltrating the glomerulus.2
A2ARs modulate the activity of inflammatory cells and may block acute inflammatory damage in different models of inflammatory injury. Herein, we show that an absence of A2AR in GN using sub-threshold doses of NTS, increased kidney injury, inflammatory cytokines, macrophages, and deposition of collagens. In addition, an increased expression of profibrotic molecules, transforming growth factor-β1, TSP-1, and OPN-1 was observed. In contrast, WT mice given suboptimal NTS were recovering from the glomerular injury at the time mice were euthanized (day 14 after induction of the disease). The amplification of inflammation in A2AR−/− mice could be attributed to lack of regulation of macrophage activation. A2AR down-regulates classic macrophage activation, reducing production of proinflammatory cytokines such as tumor necrosis factor-α, and increasing expression of the anti-inflammatory cytokine IL-10.50 In addition, we reported that A2AR inactivation with an A2AR antagonist promotes M1 phenotype of macrophage infiltrating nephritic glomeruli.16 Uncontrolled or persistent inflammation leads to progressive fibrosis.51 Consequently, kidney fibrosis in nephritic kidneys from A2AR-deficient mice could be explained, at least in part, by the severe and persistent inflammatory reaction. Macrophages express and produce fibrogenic cytokines; nephritic macrophages express transforming growth factor-β, TSP-1, and OPN-1, molecules contributing to kidney fibrosis, and are decreased in nephritic kidneys by treatment with an A2AR agonist.16 Absence of macrophage A2AR could be responsible for the increased expression of these profibrotic molecules in nephritic kidneys. More important, an infiltration of T cells in kidney interstitium was also observed in A2AR−/− mice. Infiltrating T cells also play a key role in the interstitial fibrosis and progression of kidney injury.52
Previously, we found that macrophages infiltrating nephritic glomeruli express A2AR and pharmacological activation of A2AR with a specific agonist attenuated kidney injury.17 Interestingly, macrophage-derived chemokines were reduced in response to A2AR agonist, and chemokines not expressed in macrophages did not respond to A2AR activation, suggesting activated A2AR expressed in macrophages inhibits immune-associated inflammation.17 To determine the protective contribution of activated A2AR expressed in macrophages in progressive kidney injury, we adoptively transferred A2AR-deficient macrophages or WT macrophages into CD11b DTR-Tg mice (previously depleted of macrophages) once the disease was already established. In addition, mice were treated with an A2AR agonist. In mice receiving WT macrophages and treated with the A2AR agonist, kidney injury was significantly attenuated. However, in mice reconstituted with A2AR−/− macrophages, kidney damage was worse. These data demonstrate that endogenous macrophage A2ARs are essential to protect from the progression of kidney injury in GN. The main role of macrophage A2ARs to prevent progressive kidney fibrosis is supported by the finding that there was no difference in kidney injury between mice reconstituted with A2AR−/− macrophages treated with the agonist and mice transferred with A2AR−/− macrophages but not treated with an A2AR agonist.
More important, transfected BMDMs from WT and A2AR−/− mice did not show any difference in the basal expression of cytokines. A2AR−/− macrophages have been previously reported to express an increase of proinflammatory cytokines at transcriptional level after induction of acute inflammation by treating A2AR−/− mice with Toll-like receptor activating agents or oligodeoxyribonucleotides.43 Consequently, after A2AR−/− BMDMs migrate to the nephritic kidney, they become exposed to an inflammatory milieu that can induce them to produce more proinflammatory mediators (cytokines/chemokines) compared to WT BMDMs. In addition, the lack of A2AR will prevent BMDMs from being inactivated by the A2AR agonist and BMDMs can continue producing more cytokines/chemokines to amplify inflammation. In contrast, WT BMDMs will be inactivated by the A2AR agonist, hence preventing them to produce more proinflammatory mediators. These events can explain in part the worse kidney injury observed in mice receiving A2AR−/− BMDMs.
Previous studies have reported an increase of kidney damage in A2AR-deficient mice in diabetic nephropathy, ischemia-reperfusion, and unilateral ureteral obstruction, supporting the role of A2ARs as natural brakes of inflammation.7, 14, 15, 53, 54
Although protection of kidney ischemia-reperfusion by activation of A2AR was independent of macrophage A2AR, we demonstrated herein that in mice reconstituted with macrophages lacking A2AR, the disease was worsened, even though they were also treated with an A2AR agonist.55 These studies not only emphasize the main role of macrophages in the pathogenesis of GN but also the crucial role of macrophage A2AR as a physiological feedback mechanism to limit the inflammatory response and kidney injury in NTS nephritis. The discrepancy in the protective mechanism between the model of ischemia-reperfusion and NTS nephritis could be explained in part because NTS nephritis is an auto-immune model.
Podocytes also express A2AR, and these cells play a role in the pathogenesis of NTS nephritis; our studies do not exclude the possibility that podocyte A2AR may also protect the mice from kidney damage. However, the increased kidney injury observed in absence of macrophage A2ARs documents an important role of macrophage A2AR activation in mediating kidney protection in this model of GN.56
The rapid beneficial effects observed with A2AR agonist treatment in mice receiving WT macrophages are similar to those observed in our previous study in which pharmacological activation of A2AR in established GN decreased proteinuria 24 hours after its administration.17
In summary, our study demonstrates that endogenous macrophage A2ARs are essential to protect animals from progressive kidney injury in a model of nephrotoxic nephritis. A2AR agonists may represent a therapeutic option to treat GN and potentially other inflammatory kidney diseases. More important, targeting a natural inhibitor of inflammation may provide an excellent therapeutic option with fewer adverse effects than those observed with non-specific immunosuppressive agents (eg, steroids and cytotoxic agents) that has been the mainstay of treatment for the past 50 years.
Acknowledgments
This article is a tribute to Lili Feng. She was a brilliant mentor who cared deeply for her mentees. She had the kindness of heart that every individual should have who strives for the greatest success in the practice of science.
We thank Dr. Richard Johnson for helpful comments and discussions, and Dr. Jiang-Fan Chen (Boston University, Boston, MA) for A2AR−/− mice.
Footnotes
Supported in part by a Norman S. Coplon grant and NIH grant DK082509 (G.E.G.).
Disclosures: None declared.
Current address of G.E.G., Division of Renal Diseases and Hypertension, Department of Medicine, University of Colorado Denver, Aurora, CO.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2016.06.017.
Supplemental Data
Supplemental Figure S1.

Kidney function is reduced in mice lacking A2AR. Serum creatinine levels are not affected in wild-type (WT) mice but increase in A2AR−/− mice. Results are expressed as means ± SEM. ∗P < 0.05, ∗∗P < 0.01. KO, knockout; Nl, normal.
Supplemental Figure S2.
Systemic humoral immune response and IgG deposition are not affected in the absence of A2AR. A: Circulating titers of mouse anti-rabbit total IgG measured at day 14 after nephritis induction. B: Immunofluorescence image analysis of rabbit IgG (Rb IgG) and mouse IgG (mIgG) deposition in wild-type (WT) mice and A2AR−/− mice by ImageJ software version 1.48v (NIH, Bethesda, MD). Immunofluorescence is expressed as arbitrary units. C: Immunofluorescence staining of rabbit IgG and mouse IgG. Results are expressed as means ± SEM (A). n = 4 for each group (A). ∗∗∗∗P < 0.0001 versus normal (Nl). Scale bar = 200 μm (C). Original magnification, ×200 (C). KO, knockout.
Supplemental Figure S3.
The course of nephrotoxic serum (NTS) nephritis is similar in wild-type (WT) mice and CD11b-DTR transgenic (Tg) mice. A: Periodic acid-Schiff (PAS) staining of kidney sections of WT and CD11b-DTR Tg mice with glomerulonephritis (GN). Nephritic kidneys from WT mice and CD11b-DTR Tg mice show similar kidney injury. B: Quantitation of kidney injury in WT and CD11b-DTR Tg mice with NTS nephritis. PAS staining was used. Scale bar = 60 μm (A). Original magnification, ×400 (A). Glom, glomerular.
Supplemental Figure S4.
Bone marrow–derived macrophages (BMDMs) from wild-type (WT) mice and A2AR−/− express similar levels of cytokines at basal level. RNase protection assay of cytokines expressed in BMDMs. Probes contain polylinker regions and are longer than the protected bands. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and L-32 were used as housekeeping genes. Densitometric analysis of blots from RNase protection assay of cytokines is presented as a ratio of the counts per minute for the specific mRNA/L-32 mRNA to ensure a constant quantity of RNA in each sample. Representative figure from BMDMs isolated from three different WT mice and A2AR−/− mice. IFN-γ, interferon-γ; MIF, macrophage migration inhibition factor; TGF, transforming growth factor; TNF, tumor necrosis factor.
Supplemental Figure S5.
Humoral immune response (A) and glomerular IgG deposition (B and C) are not affected by diphtheria toxin (DT) or adoptive transfer of wild-type (WT) or A2AR knockout (KO) macrophages. Normal (Nl) versus Ctrl (anti–glomerular basement membrane glomerulonephritis), depleted macrophages with DT (Mac Depl), adoptive transfer of WT macrophages plus treated with A2AR agonist (AT WT Mac + Tx), and adoptive transfer of A2AR deficient macrophages plus treated with A2AR agonist (AT KO Mac + Tx). Immunofluorescence is expressed as arbitrary units. ∗P < 0.05. Scale bar = 200 μm (C). Original magnification, ×200 (C). Ms, mouse; Rb, rabbit.
References
- 1.Meguid E., Nahas A., Bello A.K. Chronic kidney disease: the global challenge. Lancet. 2005;365:331–340. doi: 10.1016/S0140-6736(05)17789-7. [DOI] [PubMed] [Google Scholar]
- 2.Isome M., Fujinaka H., Adhikary L.P., Kovalenko P., El-Shemi A.G., Yoshida Y., Yaoita E., Takeishi T., Takeya M., Naito M., Suzuki H., Yamamoto T. Important role for macrophages in induction of crescentic anti-GBM glomerulonephritis in WKY rats. Nephrol Dial Transplant. 2004;19:2997–3004. doi: 10.1093/ndt/gfh558. [DOI] [PubMed] [Google Scholar]
- 3.Nikolic-Paterson D.J., Lan H.Y., Hill P.A., Atkins R.C. Macrophages in renal injury. Kidney Int Suppl. 1994;45:S79–S82. [PubMed] [Google Scholar]
- 4.Duffield J.S., Tipping P.G., Kipari T., Cailhier J.F., Clay S., Lang R., Bonventre J.V., Hughes J. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol. 2005;167:1207–1219. doi: 10.1016/S0002-9440(10)61209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borovikova L.V., Ivanova S., Zhang M., Yang H., Botchkina G.I., Watkins L.R., Wang H., Abumrad N., Eaton J.W., Tracey K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 6.Kledal T.N., Rosenkilde M.M., Coulin F., Simmons G., Johnsen A.H., Alouani S., Power C.A., Luttichau H.R., Gerstoft J., Clapham P.R., Clark-Lewis I., Wells T.N., Schwartz T.W. A broad-spectrum chemokine antagonist encoded by Kaposi's sarcoma-associated herpesvirus. Science. 1997;277:1656–1659. doi: 10.1126/science.277.5332.1656. [DOI] [PubMed] [Google Scholar]
- 7.Ohta A., Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 8.Chen J.F., Eltzschig H.K., Fredholm B.B. Adenosine receptors as drug targets: what are the challenges? Nat Rev Drug Discov. 2013;12:265–286. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karmouty-Quintana H., Xia Y., Blackburn M.R. Adenosine signaling during acute and chronic disease states. J Mol Med (Berl) 2013;91:173–181. doi: 10.1007/s00109-013-0997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montesinos M.C., Gadangi P., Longaker M., Sung J., Levine J., Nilsen D., Reibman J., Li M., Jiang C.K., Hirschhorn R., Recht P.A., Ostad E., Levin R.I., Cronstein B.N. Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J Exp Med. 1997;186:1615–1620. doi: 10.1084/jem.186.9.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montesinos M.C., Shaw J.P., Yee H., Shamamian P., Cronstein B.N. Adenosine A(2A) receptor activation promotes wound neovascularization by stimulating angiogenesis and vasculogenesis. Am J Pathol. 2004;164:1887–1892. doi: 10.1016/S0002-9440(10)63749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montesinos M.C., Desai-Merchant A., Cronstein B.N. Promotion of wound healing by an agonist of adenosine A receptor is dependent on tissue plasminogen activator. Inflammation. 2015;38:2036–2041. doi: 10.1007/s10753-015-0184-3. [DOI] [PubMed] [Google Scholar]
- 13.Hasko G., Szabo C., Nemeth Z.H., Kvetan V., Pastores S.M., Vizi E.S. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol. 1996;157:4634–4640. [PubMed] [Google Scholar]
- 14.Awad A.S., Huang L., Ye H., Duong E.T., Bolton W.K., Linden J., Okusa M.D. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:F828–F837. doi: 10.1152/ajprenal.00310.2005. [DOI] [PubMed] [Google Scholar]
- 15.Day Y.J., Huang L., McDuffie M.J., Rosin D.L., Ye H., Chen J.F., Schwarzschild M.A., Fink J.S., Linden J., Okusa M.D. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia G.E., Truong L.D., Chen J.F., Johnson R.J., Feng L. Adenosine A(2A) receptor activation prevents progressive kidney fibrosis in a model of immune-associated chronic inflammation. Kidney Int. 2011;80:378–388. doi: 10.1038/ki.2011.101. [DOI] [PubMed] [Google Scholar]
- 17.Garcia G.E., Truong L.D., Li P., Zhang P., Du J., Chen J.F., Feng L. Adenosine A2A receptor activation and macrophage-mediated experimental glomerulonephritis. FASEB J. 2008;22:445–454. doi: 10.1096/fj.07-8430com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng L., Garcia G.E., Yang Y., Xia Y., Gabbai F.B., Peterson O.W., Abraham J.A., Blantz R.C., Wilson C.B. Heparin-binding EGF-like growth factor contributes to reduced glomerular filtration rate during glomerulonephritis in rats. J Clin Invest. 2000;105:341–350. doi: 10.1172/JCI2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lloyd C.M., Minto A.W., Dorf M.E., Proudfoot A., Wells T.N., Salant D.J., Gutierrez-Ramos J.C. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med. 1997;185:1371–1380. doi: 10.1084/jem.185.7.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Truong L.D., Trostel J., Garcia G.E. Absence of nicotinic acetylcholine receptor alpha7 subunit amplifies inflammation and accelerates onset of fibrosis: an inflammatory kidney model. FASEB J. 2015;29:3558–3570. doi: 10.1096/fj.14-262493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi N., Boysen G., Li F., Li Y., Swenberg J.A. Tandem mass spectrometry measurements of creatinine in mouse plasma and urine for determining glomerular filtration rate. Kidney Int. 2007;71:266–271. doi: 10.1038/sj.ki.5002033. [DOI] [PubMed] [Google Scholar]
- 22.Feng L., Xia Y., Yoshimura T., Wilson C.B. Modulation of neutrophil influx in glomerulonephritis in the rat with anti-macrophage inflammatory protein-2 (MIP-2) antibody. J Clin Invest. 1995;95:1009–1017. doi: 10.1172/JCI117745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia G.E., Xia Y., Chen S., Wang Y., Ye R.D., Harrison J.K., Bacon K.B., Zerwes H.G., Feng L. NF-kappaB-dependent fractalkine induction in rat aortic endothelial cells stimulated by IL-1beta, TNF-alpha, and LPS. J Leukoc Biol. 2000;67:577–584. doi: 10.1002/jlb.67.4.577. [DOI] [PubMed] [Google Scholar]
- 24.Garcia G.E., Xia Y., Harrison J., Wilson C.B., Johnson R.J., Bacon K.B., Feng L. Mononuclear cell-infiltrate inhibition by blocking macrophage-derived chemokine results in attenuation of developing crescentic glomerulonephritis. Am J Pathol. 2003;162:1061–1073. doi: 10.1016/S0002-9440(10)63903-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia Y., Pauza M.E., Feng L., Lo D. RelB regulation of chemokine expression modulates local inflammation. Am J Pathol. 1997;151:375–387. [PMC free article] [PubMed] [Google Scholar]
- 26.Chen S., Bacon K.B., Li L., Garcia G.E., Xia Y., Lo D., Thompson D.A., Siani M.A., Yamamoto T., Harrison J.K., Feng L. In vivo inhibition of CC and CX3C chemokine-induced leukocyte infiltration and attenuation of glomerulonephritis in Wistar-Kyoto (WKY) rats by vMIP-II. J Exp Med. 1998;188:193–198. doi: 10.1084/jem.188.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikezumi Y., Hurst L.A., Masaki T., Atkins R.C., Nikolic-Paterson D.J. Adoptive transfer studies demonstrate that macrophages can induce proteinuria and mesangial cell proliferation. Kidney Int. 2003;63:83–95. doi: 10.1046/j.1523-1755.2003.00717.x. [DOI] [PubMed] [Google Scholar]
- 28.Masaki T., Chow F., Nikolic-Paterson D.J., Atkins R.C., Tesch G.H. Heterogeneity of antigen expression explains controversy over glomerular macrophage accumulation in mouse glomerulonephritis. Nephrol Dial Transplant. 2003;18:178–181. doi: 10.1093/ndt/18.1.178. [DOI] [PubMed] [Google Scholar]
- 29.Nishida M., Okumura Y., Fujimoto S., Shiraishi I., Itoi T., Hamaoka K. Adoptive transfer of macrophages ameliorates renal fibrosis in mice. Biochem Biophys Res Commun. 2005;332:11–16. doi: 10.1016/j.bbrc.2005.04.083. [DOI] [PubMed] [Google Scholar]
- 30.Lee S., Huen S., Nishio H., Nishio S., Lee H.K., Choi B.S., Ruhrberg C., Cantley L.G. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold L., Henry A., Poron F., Baba-Amer Y., van Rooijen N., Plonquet A., Gherardi R.K., Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cailhier J.F., Partolina M., Vuthoori S., Wu S., Ko K., Watson S., Savill J., Hughes J., Lang R.A. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J Immunol. 2005;174:2336–2342. doi: 10.4049/jimmunol.174.4.2336. [DOI] [PubMed] [Google Scholar]
- 33.Duffield J.S., Forbes S.J., Constandinou C.M., Clay S., Partolina M., Vuthoori S., Wu S., Lang R., Iredale J.P. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heidt T., Courties G., Dutta P., Sager H.B., Sebas M., Iwamoto Y., Sun Y., Da Silva N., Panizzi P., van der Laan A.M., Swirski F.K., Weissleder R., Nahrendorf M. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res. 2014;115:284–295. doi: 10.1161/CIRCRESAHA.115.303567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin S.L., Li B., Rao S., Yeo E.J., Hudson T.E., Nowlin B.T., Pei H., Chen L., Zheng J.J., Carroll T.J., Pollard J.W., McMahon A.P., Lang R.A., Duffield J.S. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A. 2010;107:4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stoneman V., Braganza D., Figg N., Mercer J., Lang R., Goddard M., Bennett M. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res. 2007;100:884–893. doi: 10.1161/01.RES.0000260802.75766.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin S.L., Castano A.P., Nowlin B.T., Lupher M.L., Jr., Duffield J.S. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183:6733–6743. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 38.Sunderkotter C., Nikolic T., Dillon M.J., Van Rooijen N., Stehling M., Drevets D.A., Leenen P.J. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 39.Panzer U., Steinmetz O.M., Paust H.J., Meyer-Schwesinger C., Peters A., Turner J.E., Zahner G., Heymann F., Kurts C., Hopfer H., Helmchen U., Haag F., Schneider A., Stahl R.A. Chemokine receptor CXCR3 mediates T cell recruitment and tissue injury in nephrotoxic nephritis in mice. J Am Soc Nephrol. 2007;18:2071–2084. doi: 10.1681/ASN.2006111237. [DOI] [PubMed] [Google Scholar]
- 40.Feng L., Chen S., Garcia G.E., Xia Y., Siani M.A., Botti P., Wilson C.B., Harrison J.K., Bacon K.B. Prevention of crescentic glomerulonephritis by immunoneutralization of the fractalkine receptor CX3CR1 rapid communication. Kidney Int. 1999;56:612–620. doi: 10.1046/j.1523-1755.1999.00604.x. [DOI] [PubMed] [Google Scholar]
- 41.Gessi S., Varani K., Merighi S., Ongini E., Borea P.A. A(2A) adenosine receptors in human peripheral blood cells. Br J Pharmacol. 2000;129:2–11. doi: 10.1038/sj.bjp.0703045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J.F., Huang Z., Ma J., Zhu J., Moratalla R., Standaert D., Moskowitz M.A., Fink J.S., Schwarzschild M.A. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lukashev D., Ohta A., Apasov S., Chen J.F., Sitkovsky M. Cutting edge: physiologic attenuation of proinflammatory transcription by the Gs protein-coupled A2A adenosine receptor in vivo. J Immunol. 2004;173:21–24. doi: 10.4049/jimmunol.173.1.21. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez-Juarrero M., Shim T.S., Kipnis A., Junqueira-Kipnis A.P., Orme I.M. Dynamics of macrophage cell populations during murine pulmonary tuberculosis. J Immunol. 2003;171:3128–3135. doi: 10.4049/jimmunol.171.6.3128. [DOI] [PubMed] [Google Scholar]
- 45.Landsman L., Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J Immunol. 2007;179:3488–3494. doi: 10.4049/jimmunol.179.6.3488. [DOI] [PubMed] [Google Scholar]
- 46.Kluth D.C., Erwig L.P., Pearce W.P., Rees A.J. Gene transfer into inflamed glomeruli using macrophages transfected with adenovirus. Gene Ther. 2000;7:263–270. doi: 10.1038/sj.gt.3301060. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y., Wang Y.P., Zheng G., Lee V.W., Ouyang L., Chang D.H., Mahajan D., Coombs J., Wang Y.M., Alexander S.I., Harris D.C. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int. 2007;72:290–299. doi: 10.1038/sj.ki.5002275. [DOI] [PubMed] [Google Scholar]
- 48.Atkins R.C., Holdsworth S.R., Glasgow E.F., Matthews F.E. The macrophagen in human rapidly progressive glomerulonephritis. Lancet. 1976;1:830–832. doi: 10.1016/s0140-6736(76)90480-3. [DOI] [PubMed] [Google Scholar]
- 49.Schreiner G.F., Cotran R.S., Pardo V., Unanue E.R. A mononuclear cell component in experimental immunological glomerulonephritis. J Exp Med. 1978;147:369–384. doi: 10.1084/jem.147.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hasko G., Pacher P. Regulation of macrophage function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32:865–869. doi: 10.1161/ATVBAHA.111.226852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee S.B., Kalluri R. Mechanistic connection between inflammation and fibrosis. Kidney Int Suppl. 2010;78:S22–S26. doi: 10.1038/ki.2010.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kurts C., Panzer U., Anders H.J., Rees A.J. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13:738–753. doi: 10.1038/nri3523. [DOI] [PubMed] [Google Scholar]
- 53.Sitkovsky M.V. Use of the A(2A) adenosine receptor as a physiological immunosuppressor and to engineer inflammation in vivo. Biochem Pharmacol. 2003;65:493–501. doi: 10.1016/s0006-2952(02)01548-4. [DOI] [PubMed] [Google Scholar]
- 54.Xiao H., Shen H.Y., Liu W., Xiong R.P., Li P., Meng G., Yang N., Chen X., Si L.Y., Zhou Y.G. Adenosine A2A receptor: a target for regulating renal interstitial fibrosis in obstructive nephropathy. PLoS One. 2013;8:e60173. doi: 10.1371/journal.pone.0060173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Day Y.J., Huang L., Ye H., Linden J., Okusa M.D. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am J Physiol Renal Physiol. 2005;288:F722–F731. doi: 10.1152/ajprenal.00378.2004. [DOI] [PubMed] [Google Scholar]
- 56.Awad A.S., Rouse M., Liu L., Vergis A.L., Rosin D.L., Linden J., Sedor J.R., Okusa M.D. Activation of adenosine 2A receptors preserves structure and function of podocytes. J Am Soc Nephrol. 2008;19:59–68. doi: 10.1681/ASN.2007030276. [DOI] [PMC free article] [PubMed] [Google Scholar]