Abstract
Tissue injury triggers the activation and differentiation of multiple cell types to minimize damage and initiate repair processes. In systemic sclerosis, these repair processes appear to run unchecked, leading to aberrant remodeling and fibrosis of the skin and multiple internal organs, yet the fundamental pathological defect remains unknown. We describe herein a transition wherein the abundant CD34+ dermal fibroblasts present in healthy human skin disappear in the skin of systemic sclerosis patients, and CD34−, podoplanin+, and CD90+ fibroblasts appear. This transition is limited to the upper dermis in several inflammatory skin diseases, yet in systemic sclerosis, it can occur in all regions of the dermis. In vitro, primary dermal fibroblasts readily express podoplanin in response to the inflammatory stimuli tumor necrosis factor and IL-1β. Furthermore, we show that on acute skin injury in both human and murine settings, this transition occurs quickly, consistent with a response to inflammatory signaling. Transitioned fibroblasts partially resemble the cells that form the reticular networks in organized lymphoid tissues, potentially linking two areas of fibroblast research. These results allow for the visualization and quantification of a basic stage of fibroblast differentiation in inflammatory and fibrotic diseases in the skin.
Systemic sclerosis (SSc) is a connective tissue disease characterized by immune dysregulation, vasculopathy, and fibrosis in multiple organ systems.1 Chronic tissue injury as in cases of unresolved infection, inflammatory disease, or cancer can often lead to fibrosis.2 In SSc, these remodeling processes appear to proceed unchecked over years, yet the fundamental driver of the pathology remains unknown. Considerable attention has focused on transforming growth factor (TGF)-β–triggered conversion of mesenchymal lineage cells to α-smooth muscle actin (SMA)–positive myofibroblasts as the central event in fibrosis.3, 4 Recently, elegant fate-mapping strategies have been used in the mouse to define the origin of these myofibroblasts and potentially the fibrotic process.5, 6, 7 Indeed, a separate fibroblast lineage with enhanced fibrogenic potential was revealed using these methods.8 Despite these advances, the etiology of SSc remains poorly understood and patients lack effective treatments. We were intrigued by the rapid progress in the understanding of fibroblast specialization in the context of lymphoid organs. In this setting, reticular stromal networks are not mere matrix scaffolds, but play active roles in orchestrating lymphocyte interactions and in the formation and wrapping of specialized collagen fibrils, called conduits.9, 10, 11 The responsive or reactive lymph node represents an unusual case of reversible, physiological tissue remodeling in what is effectively an inflammatory microenvironment.12, 13, 14 With chronic lymph node reactivity, such as in HIV or Yersinia infection, forms of Hodgkin lymphoma and IgG4-related disease, fibrosis can ensue.15, 16, 17, 18 Therefore, we questioned whether similar processes were in play in the skin of SSc patients.
There is considerable heterogeneity in fibroblast populations and phenotypic information on the various differentiation states remains imprecise.19, 20 The skin is richly populated throughout the dermis by CD34-expressing fibroblasts with long dendritic processes.21 Electron microscopy reveals the presence of an intricate network of fibroblasts within the dermis, perhaps even interlinked by intercellular stromal connections.21 Dermal fibroblast nomenclature is not standardized and includes such terms as telocytes, fibrocytes, and dendrocytes. Herein, we will use the newly proposed term CD34+ stromal fibroblastic cell or CD34+ SFC.22 Curiously, it has been known for >20 years that this expression of dermal stromal CD34 is lost in SSc.23, 24, 25, 26, 27, 28, 29 This change is not limited to SSc skin, as it is seen in the scarring after skin lesion excision, dialysis-induced peritoneal fibrosis, and keloids.30, 31, 32 A fundamental question is whether these cells simply degenerate and disappear or are they activated and have changed their phenotype. In this work, we show that CD34+ SFC convert or are replaced by podoplanin+ (Pdpn) and CD90+ fibroblasts in SSc and this process can occur quickly in response to normal skin injury.
Materials and Methods
Patient Specimens
The Boston University Medical Center Institutional Review Board approved procedures and analyses, and informed consent was obtained from all patients and healthy subjects. Skin biopsy specimens were provided by SScores, the NIH Scleroderma Core Center at Boston University Rheumatology. The skin biopsy specimens for histology were taken from the dorsal mid-forearm region of SSc patients, including 48 diffuse cutaneous SSc (dcSSc), 2 undifferentiated, 24 limited cutaneous SSc, and 11 healthy controls. The characteristics of these subjects are outlined in Supplemental Table S1. Additional biopsy specimens (n) from affected skin were obtained from cutaneous (discoid) lupus erythematosus (eight, aged 26 to 71 years, female 100%), psoriasis (six, aged 43 to 77 years, female 33%), subacute (five, aged 45 to 74 years, female 60%), and chronic (twelve, aged 19 to 73 years, female 42%) spongiotic dermatitis and lesional tissue from dermatofibroma (eight, aged 43 to 60 years, female 75%), dermatofibrosarcoma protuberans (six, aged 49 to 68 years, female 50%), atypical fibroxanthomas (six, aged 47 to 95 years, female 20%), and neurofibroma (eight, aged 41 to 78 years, female 75%) patients. The human skin scars were obtained after re-excision of the margins from previously excised skin lesions after varying intervals. Scar tissues were examined from five different patients with recent excisions (early scar, 7 to 8 days) and eight patients with longer intervals (late scars, 28 to 31 days). The Boston University Medical Center Dermatopathology Section provided tonsil and skin samples.
Microarray data from skin biopsy specimens were available for 35 dcSSc and seven healthy controls, as previously described.33 Two separate data sets were obtained using the same U133A 2.0 chip (Affymetrix, Santa Clara, CA).33, 34 These data were normalized for batch differences using the ComBat algorithm (SVA 3.18 package; Bioconductor http://www.bioconductor.org/packages/release/BiocViews.html#_Software).35 Determination of genes whose expression correlated with either the Pdpn/CD34, CD90/CD34 RNA ratios or modified Rodnan skin score (MRSS) was performed using R (3.2.1) on the combined data from 35 dcSSc and seven controls. Paired biopsy specimens were obtained from the same forearm region from a subset of patients (30 dcSSc) for both histological and RNA analyses. In addition, histological analysis was conducted on multiple (two to three) biopsy specimens obtained over a 1-year spread from three of the dcSSc patients and, similarly, transcriptome data were available from two to three biopsy specimens from four of the dcSSc patients.
Immunohistochemistry
A double-staining protocol was used on paraffin-embedded slides after dewaxing and heat antigen retrieval in Tris/EDTA pH 9.0 for 20 minutes, except in the case of Figure 1, no heat retrieval was performed. Blocking was achieved using 3% H2O2 followed by BloxAll (Vector Labs, Burlingame, CA) or 2% horse serum. Appropriate Vector ImmPress Polymers (mouse, rat, and rabbit) were used to detect primary antibodies, followed by development with either Vector AMEC [red, horseradish peroxidase (HRP)], HighDef Blue (Enzo, HRP), or Vector Blue (alkaline phosphatase). Vector ABC was used for detection of biotinylated primary antibodies. For immunofluorescence, the same procedures were used, only the detection of HRP-ImmPress polymers was performed sequentially using Alexa-Tyramide reagents (Thermo Fisher Scientific, Waltham, MA): Alexa Fluor 488 and Alexa Fluor 594 tyramide. Biotinylated anti–α-SMA was detected using Vector-ABC-HRP, a Biotin-XX–tyramide step followed by Streptavidin-Alexa Fluor 405 or Vector-ABC-HRP was followed with CF405S Tyramide (Biotium, Fremont, CA). Each labeling step was followed by HRP quenching with 3% H2O2 before the next stain. Typically, primary antibodies were retitrated for use with tyramide amplification. Triple-stained immunofluorescence protocols for transitional cells included sequential layers of mouse anti-CD34, rabbit anti-CD90, or mouse anti-Pdpn, rabbit anti-CD34 followed by biotinylated mouse anti-CD31. Costaining for procollagen used mouse anti-procollagen I, rabbit anti-CD90 (or rabbit anti-Pdpn). Immunofluorescence slides were imaged using an Olympus FlouView FV10i confocal microscope (Olympus, Waltham, MA). Antibodies were as follows: mouse anti-human CD31 (JC70A; Dako, Carpinteria, CA), biotinylated mouse anti-human CD31 monoclonal antibody (mAb; C31.3+JC/70A; Novus Biologics, Littleton, CO), mouse anti-human CD34 mAb (QBEnd10; Dako), rabbit anti-human or mouse CD34 mAb (EP373Y; Abcam, Cambridge, MA), rabbit anti-human CD90 mAb (EPR3132; Abcam), mouse anti-human Pdpn mAb (D2-40, Dako), rabbit anti-human Pdpn mAb (128994, Abcam), biotin-hamster anti-mouse Pdpn mAb (8.1.1; eBioscience, San Diego, CA), rabbit anti–nerve growth factor receptor mAb (EP1039Y, Abcam), biotin and nonbiotinylated mouse anti–α-SMA mAb (1A4/asm-1, Dako and Thermo Scientific), and mouse anti-procollagen I mAb (clone SP1.D8, Developmental Studies Hybridoma Bank, Iowa City, IA).
Figure 1.
CD34+ stromal fibroblast cells are oriented parallel to the collagen fibers in normal skin forming an intricate network. A: Low magnification image of normal skin stained for CD34 (blue) and CD90 (brown). CD34 stains the stromal fibroblasts as well as endothelial cells, whereas CD90 marks predominantly perivascular adventitial cells and structures within the hair follicle. Two dermal regions are magnified (solid boxed area is shown in B and the dashed boxed area is shown in C) and highlighted by phase contrast to show many fine CD34+ processes captured in cross section (arrowheads in B) when fibers were similarly transected. Long extended CD34+ fibroblast-like cells are observed running parallel to the fibers (arrowheads in C). Epidermis is to the right. Scale bars: 200 μm (A); 25 μm (B and C).
Mouse Skin Wounds
For skin wound experiments, C57.B6 mice were anesthetized and the back was shaved. A mid-dorsal 1-cm incision cut was made through the skin with a scalpel and the wound was sealed with Liquid Bandaid. Mice were euthanized, and the cut skin was taken on days 0, 1, and 4 after the initial incision. Skin sections were formalin-fixed and paraffin-embedded for histology. The Boston University Institutional Animal Care and Use Committee approved the mouse procedures.
Dermal Fibroblast Cultures
Primary human dermal fibroblasts from skin biopsy specimens from healthy donors or SSc patients as well as human foreskin fibroblasts were derived as previously described and used before passage 6 to 7.36 Various agents were added in fresh media with 10% fetal bovine serum with 20 ng/mL tumor necrosis factor (TNF), 20 ng/mL IL-1β, 20 ng/mL IL-6 (R&D Systems, Minneapolis, MN), 100 ng/mL LTβ receptor (LTβR) agonist BS1, 10 ng/mL platelet-derived growth factor (PDGF) AA and PDGFBB (Preprotech, Rocky Hill, NJ), 500 U/mL interferon-α4 (PBL Interferon Source, Piscataway, NJ) or 5 ng/mL human TGF-β (R&D Systems). BS1 is a bispecific antibody recognizing two non–cross-blocking epitopes on the receptor, thereby enabling extensive oligomerization of the receptor on the surface. As such, it is an effective mimic of the normal cell surface ligand and its properties have been described.37 BS1 was a gift from Biogen (Cambridge, MA). RNA was isolated from these cultures after 24 hours and subjected to either quantitative PCR using TaqMan primers or a custom human gene specific 78 gene Nanostring array effectively as described.38 For immunohistological analysis, cells were grown on Nunc glass chamber slides, stimulated as above, fixed with formaldehyde, and permeabilized with 100% methanol. Cells were stained sequentially with anti-Pdpn (D2-40), Alexa 594–labeled goat anti-mouse IgG, quenching with mouse IgG, biotinylated anti–α-SMA (1A4, Thermo Fisher), and streptavidin-Alexa 488, followed by counterstaining with DAPI and imaging on the Olympus confocal.
Results
Loss of CD34+ Dermal Fibroblasts Is Accompanied by the Appearance of CD34−/Podoplanin+/CD90+ Reticular Cells in SSc
In normal skin, reticular cells expressing CD34 form a dense network within the dermis. Figure 1 shows at relatively high resolution, long fibroblast-like cells running parallel to the fibers; however, when a fiber was transected, the CD34 staining was punctate in appearance. This staining pattern reveals a dense and intricate web of fibroblastic processes consistent with the electron microscopy–based description of a network of CD34+ SFC with thin, long processes.21 In dcSSc skin, cells positive for Pdpn and CD90 (alias Thy1) replaced to varying extents the dense network of CD34+ SFC in normal healthy skin (Figure 2A). Fibroblastic Pdpn staining was weaker than the normally intense lymphatic staining yet was readily visible using the standard D2-40 mAb. Loss of lymphatic staining occurs in dcSSc skin and, although not documented herein, we also noted the loss of lymphatic capillaries in the upper dermis.39, 40, 41, 42 Some biopsy specimens displayed a partial conversion that was observed in either the papillary or reticular dermis, and an example of such a partial transition in the reticular dermis is seen in Figure 2A. Other patient biopsy specimens completely lacked CD34+ SFC and transitioned cells dominated both dermal layers (ie, a pan-dermal transition). Thus, there was considerable patient-to-patient heterogeneity with the range of patterns varying between conversion in only one layer (only Pdpn or CD90 or both), a pan-dermal transitioned state and a presumably more final state with few cells of any phenotype. When examined, biopsy specimens obtained 6 to 12 months apart were similar in histological status (not shown). Biopsy specimens from patients with limited cutaneous SSc (n = 24) from clinically unaffected forearms largely resembled normal skin in terms of this transition.
Figure 2.
A transition of CD34+ dermal fibroblasts to a Pdpn and CD90+ state occurs in the dermis of SSc patients. A: Examples are shown of normal skin and skin from SSc patients with either a partial or complete transition (top panels). Boxed area is magnified in bottom panels. Pdpn expression is seen in transitioned fibroblasts as well as lymphatic vessels, keratinocytes, hair follicles, and sebaceous glands. CD90 expression is observed in vascular adventitia and transitioned fibroblasts. B: Immunofluorescence imaging showing a subset of transitioning cells in SSc skin displaying both CD34 and Pdpn and/or CD90. Pdpn and CD90 costaining was performed on a different section compared to the CD34 panels. Scale bars: 0.5 mm (A, top panels); 100 μm (A, bottom panels); 50 μm (B). A, adventitia; L, lymphatic.
Pdpn is a common marker for lymphatic endothelium, but it also defines the specialized reticular stroma in lymphoid organs in mice and human.43, 44, 45, 46 In addition, Pdpn is expressed in normal sebaceous glands, hair follicle roots, activated keratinocytes, some carcinoma cells, and non-transformed cancer-associated fibroblasts.43, 47, 48 Enhanced Pdpn expression in keratinocytes was frequently observed in SSc, indicating some level of epidermal perturbation. CD90 is normally found on neuronal cells, mesenchymal stem cells, pericytes, perivascular adventitial fibroblasts, and a relatively rare subset of human T lymphocytes.49, 50, 51 Using immunofluorescence microscopy and a SSc biopsy with a partial transition, we found a subset of dermal cells that were positive for CD34 along with Pdpn and/or CD90, and these cells were not CD31+ endothelial cells (Figure 2B and Supplemental Figure S1). One interpretation of this staining is that these are CD34+ SFC caught in the process of conversion. Many stromal cells displayed both Pdpn and CD90 (Figure 2B). However, fibroblasts positive for just Pdpn or CD90 were readily observed, consistent with phenotypic heterogeneity. CD34+ SFCs, as well as the transitioned cells, did not stain for nerve growth factor receptor (CD271), although some fibroblast-like cells in the perivascular adventitial layer were positive (data not shown).
To determine whether the Pdpn or CD90+ cells in SSc skin were actually fibroblasts, we costained sections with anti-procollagen I. Procollagen was found in a roughly Golgi-like perinuclear localization and where cell bodies were seen, CD34+ SFC, Pdpn+, and CD90+ cells displayed procollagen staining (Supplemental Figure S2). Given the convoluted network-like nature of these staining patterns, not all Pdpn or CD90+ processes could be exactly linked to a particular cell body. In addition, we costained the cells with vimentin (Supplemental Figure S3). CD34+ SFC-like cells in fibrous tumors have a vimentin cytoskeleton as typical of fibroblasts.22 Likewise, the Pdpn+ or CD90+ cells also costained with vimentin, indicating that all three cell types have a cytoskeleton consistent with a fibroblastic lineage. Murine CD90+ fibroblasts were previously shown to be vimentin positive.52 These observations indicate that the Pdpn and CD90+ stromal cells appearing in SSc skin are fibroblasts.
Relationship Between Transitioned Fibroblasts and Myofibroblasts
As this transition appeared to represent a new differentiation event or an activation state of normal dermal fibroblasts, we questioned whether these cells are simply the classic α-SMA+ myofibroblasts. α-SMA+ myofibroblasts are observed in many SSc skin biopsy specimens in regions with thickened parallel collagen bundles deep in the dermis.53, 54 More important, CD90+ or Pdpn+ cells were readily observed in sections lacking α-SMA+ myofibroblasts, indicating that this differentiation pathway to the Pdpn/CD90+ state can occur independently from the conversion to myofibroblast (Figure 3 and Supplemental Figure S4). In SSc biopsy specimens with pronounced fibrosis, conventional α-SMA+ myofibroblasts were readily observed and α-SMA colocalized with either or both CD90 and Pdpn+ cells (Figure 3). Myofibroblasts expressing either CD90 or Pdpn have been observed in several settings, although CD90 interactions are reported to block fibrosis.52, 55, 56 These α-SMA+ costaining patterns were often complex, yet consistent with a narrow focal plane through contorted, twisting cells (Supplemental Figure S5). Myofibroblasts run parallel to the fibers and like with CD34+ SFC, the staining patterns are additionally dictated by the plane-of-section of the fiber bundles. Separate α-SMA+ and Pdpn/CD90+ cells could be entwined; however, the similar patterns of vimentin costaining with CD34, Pdpn, and CD90 favor the simple interpretation. Similar complex patterns of CD90 and α-SMA costaining were seen previously in myofibroblasts in mouse sclerotic skin.52 α-SMA+ myofibroblasts without any Pdpn or CD90 display appeared to be rare, but this plane-of-section complexity prevented an absolute determination. Therefore, the transition defines phenotypic changes that may occur either in parallel with or as an intermediate in the progression to a myofibroblast.
Figure 3.
Immunofluorescence analysis of the relationship between CD34, α-smooth muscle actin (α-SMA), Pdpn, and CD90 expression in SSc. Sections from dcSSc skin from a patient with extensive deep fibrosis were stained with either Pdpn/α-SMA or CD90/α-SMA and counterstained with DAPI. A: Entire biopsy specimen is shown with just the α-SMA (red) and phase contrast channels. B: Example of the papillary dermis showing few Pdpn+ transitioned fibroblasts, yet there is extensive CD90 expression. Within this region, there was a Pdpn+ α-SMA+ lymphatic (collecting duct), but no α-SMA+ myofibroblasts. C: In the fibrotic region, there is a range of Pdpn expression on α-SMA+ myofibroblasts; however, most myofibroblasts express CD90. Scale bar = 50 μm (B and C, large panels).
Transition Occurs in Both Mouse and Human Skin Wounds
We postulated that this phenotypic conversion is a basic form of fibroblast differentiation in response to injury. Human skin undergoing repair and scar formation after an excisional biopsy exhibited the transition with more Pdpn or CD90 being observed in both early (7 to 8 days) and late (28 to 31 days) phase scar samples (Figure 4A). Mouse skin also has CD34+ SFC in the dermis and, similar to the human setting, introduction of an incisional wound led to the appearance of Pdpn+ cells (Figure 4B). By day 1 postincision, cells displaying both CD34 and Pdpn were observed (Figure 4C) and, therefore, this rapid and extensive conversion in response to injury is consistent with fibroblast differentiation and less likely to result from replacement by newly immigrating cells or rapid expansion of local progenitor cells. Thus, the transition can be a fast response to skin injury in both human and rodent skin.
Figure 4.
Analysis of the fibroblast conversion in human and mouse skin wounds. A: Transitioning fibroblasts in re-excised human skin 8 days (early scar) and 29 days (late scar) after the initial biopsy. Normal in this case was a region of skin with unperturbed architecture considerably distal to the initial lesion. B: Similar analysis of mouse skin after a skin incisional wound showing the rapid appearance of Pdpn+ transitioned cells. At day 0, the network of CD34+ SFC-like fibroblasts can be seen in the dermis in the layer containing the hair follicles. C: Immunofluorescence analysis of CD34 and Pdpn showing co-expression in samples from B. Scale bars: 200 μm (A); 100 μm (B); 40 μm (C). E, epidermis; F, hair follicle; L, lymphatic; V, blood vessel.
Human Dermal Fibroblasts Express Podoplanin in Response to Inflammatory Stimuli
To determine whether dermal fibroblasts have the capacity to display Pdpn or CD90, we examined various signaling systems for the ability to induce these markers in cultured primary dermal fibroblasts from normal adults. Exposure of cells to TNF or IL-1β led to a robust induction of Pdpn RNA, but did not appreciably affect CD90 or CD34 RNA (Figure 5A). Dermal fibroblasts derived from SSc patients and normal foreskin fibroblasts behaved similarly. Interferon-α, IL-6, PDGFAA, PDGFBB, TGF-β, and retinoic acid did not increase Pdpn or CD90 expression in contrast to the ability of TGF-β and STAT3 activation to induce Pdpn in cultured epithelial cells.48 Cultured fibroblasts derived from murine lymphoid tissue up-regulated Pdpn after a combination of canonical and alternative NF-κB activation, and we paralleled this format with the human dermal fibroblasts.57 Alternate NF-κB signaling was triggered with an agonist antibody to the LTβR and weakly induced Pdpn RNA expression, yet the combination with TNF was most effective. Immunohistological analysis of stimulated fibroblasts showed that stimuli that increased Pdpn RNA also increased protein expression (Figure 5B). Although the TNF or IL-1β induction of Pdpn RNA was maximal at day 1, robust protein expression required 3 to 5 days, as was the case with the cultured lymphoid reticular cells and in vivo in reactive lymph nodes.12, 57 CD34 RNA levels were low in these cultured primary fibroblasts and therefore we do not believe there were any major changes in CD34 expression with the various stimuli (Figure 4A). The typical relative RNA abundance of these genes based on quantitative PCR was 18S, 1; CD34, 1 × 10−6; Pdpn, 3 × 10−5; CD90, 1 × 10−3. Thus, the cultured cells have low CD34 coupled with high-level CD90 expression. We examined whether TGF-β signaling could induce Pdpn expression in parallel with myofibroblast formation. Pdpn RNA was not induced by TGF-β treatment, yet expression of Serpine1, a known TGF-β–induced gene, was elevated (Figure 5C). Likewise, TGF-β–treated dermal fibroblasts did not increase Pdpn expression; however, organized α-SMA+ fibers were readily seen (Figure 5D). In a culture of dermal fibroblasts derived from a dcSSc patient, some α-SMA+ as well as Pdpn+ cells were observed without stimulation, as could be expected with their increased presence in SSc skin. These data show that human dermal fibroblasts can express Pdpn in an inflammatory environment, and this event is independent of the TGF-β–induced myofibroblast transition.
Figure 5.
Inflammatory stimuli induce Pdpn expression in cultured dermal fibroblasts. A: RNA levels of Pdpn, but not CD34 or CD90, are induced by inflammatory stimuli in primary adult human dermal fibroblasts. Representative experiments with cells from normal and SSc skin after culturing for 24 hours with tumor necrosis factor (TNF), IL-1β, BS1 anti-LTβ receptor agonist antibody, and the combination of TNF and BS1. Bars show the range of values from biological replicates. B: Immunofluorescence imaging analysis of the induction of Pdpn protein and α-smooth muscle actin (α-SMA) expression in normal human dermal fibroblasts after 5 days of exposure to media alone, TNF, or IL-1β. C: Induction of α-SMA but not Pdpn expression after culturing for 5 days in transforming growth factor (TGF)-β. Cells from this dcSSc patient when cultured in control media retain a low percentage of α-SMA+ and Pdpn+ cells and in this regard, this field is illustrative, but overrepresents the density of α-SMA+ cells. These α-SMA+ or Pdpn+ cells are not normally seen in unstimulated cultured normal dermal fibroblasts. D: RNA analysis of TGF-β–treated cells (24-hour exposure, normal dermal fibroblasts) showing robust induction of TGF-β–induced Serpine1, a slight increase in α-SMA, yet no increase in Pdpn or CD90 RNA. Scale bar = 100 μm (B and D).
A More Spatially Limited Transition Occurs in Other Inflammatory Skin Diseases
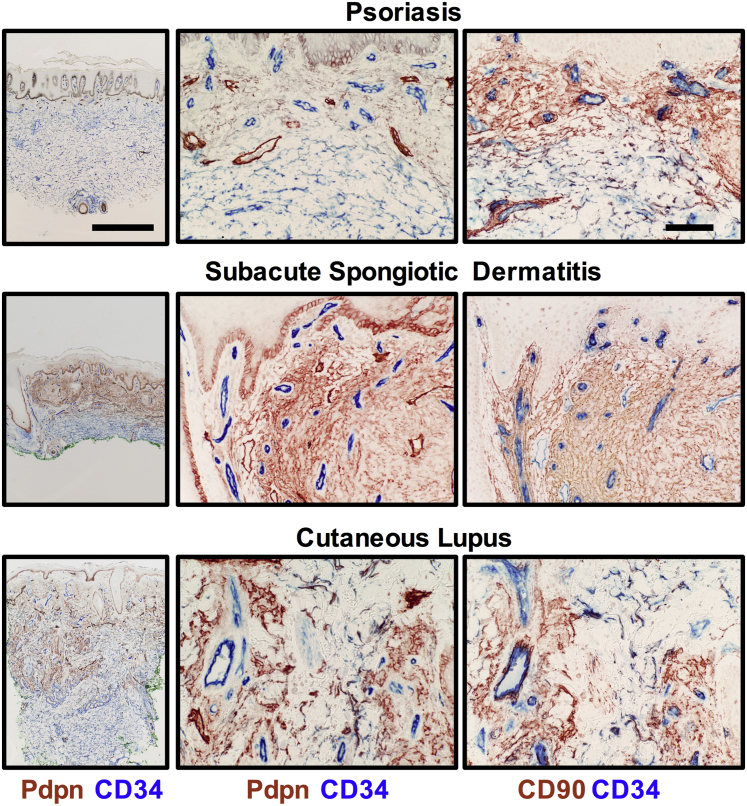
The transition was observed in the inflamed skin from patients with psoriasis, subacute and chronic spongiotic dermatitis, and cutaneous (discoid) lupus erythematous. In general, the transition was localized to the inflamed papillary dermal layer (Figure 6). In this survey, SSc was unique, as the transition in some patients could be found in the deep dermis or throughout the entire dermis. Our observations also include phenotypic changes in the fibroblasts in the vascular adventitial layer. Notably in SSc, the perivascular adventitial fibroblasts exhibited increased CD90, but not Pdpn, staining. Consistent with vascular disruption, this compartment was often expanded in SSc skin.
Figure 6.
The fibroblast transition in inflammatory human skin diseases. Dual staining showing the CD34 to Pdpn/CD90 transition in various diseases (low and high magnification of the Pdpn/CD34 staining). Transitioned fibroblasts are found primarily in the upper dermis with relatively normal CD34+ fibroblastic cells being seen in the deeper regions of the dermis in each disease. Scale bars: 1 mm (left column); 100 μm (middle and right columns).
Relationship Between the Transition and Markers of SSc Disease
We compared the histological features of the fibroblast transition in SSc skin with the levels of CD34, Pdpn, and CD90 RNA using adjacent paired skin biopsy specimens from 30 SSc patients. Given the loss of CD34 and gain of CD90 or Pdpn protein expression in SSc, we speculated that the ratio of Pdpn/CD34 or CD90/CD34 RNA may track with the histological transition. The extent of histological conversion was graded as low (no or little), medium (partial), or high (extensive to complete transition). Histological conversion correlated with higher ratios of Pdpn/CD34 and CD90/CD34 RNA (Figure 7A). The CD90/CD34 RNA ratio correlated well with the degree of dermal thickening, as defined by the standard MRSS, a subjective scoring system for skin thickness (Figure 7B). The Pdpn/CD34 ratio increased with increasing disease; however, the correlation was not strong in patients with lower MRSS values. If Pdpn expression is decreasing because of loss of lymphatics coupled with a gain of Pdpn expression with the transition, then the net effect of disease severity on the Pdpn/CD34 ratio could be more variable. In contrast, CD90 expression increased in all settings (ie, in both transitioning fibroblasts and in perivascular adventitial cells).
Figure 7.
Relationship between the histology transition, biopsy RNA levels, and skin disease score. A: Relationship between the level of histological conversion from CD34 to Pdpn+ scored as low to high and the ratio of CD34/Pdpn RNA as determined in adjacent skin biopsy specimens (significance one-way analysis of variance). B: Relationship between the modified Rodnan skin score (MRSS) measure of SSc disease and the RNA ratio of either Pdpn or CD90 to CD34 (Spearman correlation analysis). C: Heat map showing genes correlating with the Pdpn/CD34 ratio. Genes with a fdr q < 0.05 are shown, and the subjects are ordered from right to left with increasing Pdpn/CD34 ratio. Normal subjects are marked with a black box. ∗P < 0.05, ∗∗P < 0.01.
The lack of precise correlation between the two ratios, the heterogeneity in the immunohistochemical patterns, and the apparently differing requirements for RNA induction in cultured fibroblasts suggested that Pdpn and CD90 may be reporting on different aspects of the biology. Historically, elements of inflammation, proliferation, and fibrosis have been defined by transcriptional profiling of SSc skin.58, 59 To explore this question, we identified those genes from the total skin biopsy microarray analysis that correlated with the Pdpn/CD34 and CD90/CD34 RNA ratios as well as MRSS values. Using a cutoff of q < 0.05, we found 49 genes associated with the Pdpn/CD34 ratio, 530 with CD90/CD34, and 437 with MRSS (Figure 4 and Supplemental Table S2). Figure 7C shows a heat map for association with the Pdpn/CD34 ratio, indicating there is a molecular signature of this transition. Further heat maps for CD90/CD34 and MRSS can be seen in Supplemental Figures S6 and S7. Given the good correlation of the CD90/CD34 ratio with MRSS, it was not surprising that THBS1, a previously defined marker of fibrosis, correlated well with both MRSS and CD90/CD34 ratio, but not with Pdpn/CD34. In general, the spectrum of genes associating with the Pdpn/CD34 ratio was rather distinct from those in the MRSS and CD90/CD34 data sets. In all three analyses, macrophage genes (eg, AIF1, CD163, or MRC1) did not reach the cutoff. This analysis is consistent with the concept that the Pdpn/CD34 transition reports potentially on a novel aspect of the pathology.
Lymphoid Reticular Cells Are Phenotypically Similar to Transitioned Dermal Fibroblasts
In light of our original premise that the lymphoid stromal networks may provide novel insight into fibroblast differentiation in nonlymphoid tissues, we compared the expression patterns of CD34, Pdpn, and CD90 in the human tonsil. Some fibroblastic reticular cells in the T-cell regions of the human tonsil express both Pdpn and CD90, yet lack CD34, thus resembling the phenotype of the dermal transitioned fibroblasts (Figure 8 and Supplemental Table S3). A subset of Pdpn/CD90 double-positive reticular cells resembling murine marginal reticular cells was described in the human lymph node.60 Notably, we observed in the submucosal region of the tonsil, CD34+ SFC-like cells resembling those in the skin. In addition, CD34/Pdpn double-positive stromal cells could be seen bridging into the lymphoid regions (Supplemental Figure S8). The second major reticular network in lymphoid tissues scaffolds the B-cell follicles and germinal center reactions and is composed of follicular dendritic cells, which are also of fibroblastic lineage. These cells are CD34 negative and Pdpn bright, but lacked CD90 expression. Thus, it is clear that there is heterogeneity in terms of CD90 and Pdpn expression in these specialized lymphoid reticula. From these observations, we conclude that lymphoid reticular networks share some phenotypic components with transitioned dermal fibroblasts.
Figure 8.
Fibroblastic reticular cells in the human tonsil are Pdpn+, CD90+ and lack CD34. Left panels: low magnification images of immunofluorescence staining of reticular networks in the human tonsil. Boxed areas are magnified in the right panels. A: The tonsil is attached to a CD34+ layer potentially resembling the CD34+ SFC in the dermis. The CD90/CD34 pairing reveals some vascular heterogeneity (B), and CD90+ fibroblastic reticular cells. C: Follicular dendritic cell networks within the follicles display Pdpn but not CD90. In contrast, some of the fibroblastic reticular cells outside the follicles express both Pdpn and CD90 (yellow-orange cells, C). Scale bars: 200 μm (A–C, left panels); 50 μm (A–C, right panels). F, follicle; H, high endothelial venule; L, lymphatic; P, possible CD90+ pericyte layer.
Transition in Dermally Derived Tumors
Mesenchyme-derived skin tumors could stem from transformative events occurring at different points along their differentiation paths. We examined four such tumors, dermatofibroma (DF), dermatofibrosarcoma protuberans (DFSP), atypical fibroxanthoma (AFX), and neurofibroma for CD34, Pdpn, and CD90 expression (Supplemental Table S4 and Supplemental Figure S9). Previously, it was known that CD34 expression was a hallmark of a DFSP, whereas DF and AFX were typically CD34 negative.61 In addition, DF and AFX but not DFSP are Pdpn positive.62, 63, 64 These patterns suggested that DF and AFX are composed of cells that resemble transitioned fibroblasts. Costaining for CD34/Pdpn or CD34/CD90 showed that the spindle-like cells in DF and AFX were CD34 negative, as expected, but displayed both Pdpn and CD90. Both DFSP and neurofibroma tumor cells resemble the dermal CD34+ SFC; however, neurofibromas contained some interspersed CD90+ transitioned cells. It was not within the scope of this work to determine whether these tumors were phenotypically heterogeneous or variably infiltrated (eg, in DF, AFX, or neurofibromas), with a non-transformed cancer-associated fibroblast (ie, a reactive stroma resembling the transitioned fibroblast). Nonetheless, we interpret the observations on these mesenchymal and neural tumors or potentially attendant reactive stroma as consistent with the basic notion of a fibroblast conversion.
Discussion
In addition to the actual fibrosis, the extensive loss of CD34 expression in the dermal stromal cells remains one of the most dramatic histological observations in SSc. This disappearance could result from a phenotypic transition of existing dermal fibroblasts to a new state or, alternatively, cell degeneration followed by de novo replacement with fibroblasts lacking CD34 expression. Replacement cells could derive from local or immigrating progenitor cells. It is likely that in more advanced stages of the disease, there is even a general loss of cellularity. Degenerating stromal cells have been observed in SSc; however, they have not been phenotypically identified.28 We propose herein that normal CD34+ SFCs convert to CD34−, Pdpn, or CD90+ fibroblasts, and this transition at least partially accounts for the loss of CD34 expression.
In support of this hypothesis, first, we found stromal cells in SSc skin that appeared to be caught in transition (ie, displaying Pdpn/CD90 along with CD34). Second, this transition occurs early after an incisional wound in the mouse, indicative of a quick phenotypic conversion rather than de novo immigration and replacement. At day 1 after incision, the presence of these cells precedes the appearance of myofibroblasts that occurs later in the granulation phase of wound repair.65 Third, cultured primary dermal fibroblasts convert to a Pdpn+ state when exposed to the inflammatory triggers TNF or IL-1β, proving that fibroblasts have the capacity at least in vitro to transition. Given the extended nature of the disease process, we cannot exclude that cellular degeneration is also occurring followed by de novo replacement with new fibroblasts having the transitioned phenotype. Recapitulation of the entire transition in vitro, including loss of CD34 and up-regulation of CD90, would help solidify this hypothesis and awaits further advances in dermal fibroblast culturing. Limitations in this study include the difficulty of using cross-sectional data to define events in a disease process progressing over several years. Interpretation is further obfuscated by variability in the extent and nature of the transition in SSc skin biopsy specimens because of either intrinsic disease heterogeneity or imprecise staging. Technically, there is the difficulty of assessing molecular phenotypes in thin sections where the cells have long thin processes and highly contorted shapes. Whole tissue imaging methods should prove useful here.
Beyond the well-documented loss of CD34 expression in SSc, various aspects of this change have been noted previously. In the skin, normal dermal fibroblasts are CD90− (except for perivascular adventitial cells), yet in SSc CD90+ fibroblast-like cells were observed, including α-SMA+ CD90+ cells, and similar changes were observed in a murine fibrosis model.28, 52, 54 Likewise, the reciprocal nature of CD34 and Pdpn expression forms one basis for distinguishing the types of mesenchymal skin tumors. Our observations point toward the existence of at least five phenotypes of dermal fibroblasts (ie, conventional CD34+ SFC, CD34− Pdpn+, CD34− CD90+, CD34− Pdpn+ CD90+, and α-SMA+ myofibroblasts with CD90 and/or Pdpn expression). Thus, this fibroblastic transition in SSc skin is consistent with prior observations in multiple settings.
The CD34+ SFC cells have been proposed to serve a sentinel function to initiate repair.22 Peduto et al66 analyzed the appearance of Pdpn+ stromal cells after tissue insult in mice. In that study, Pdpn+ stromal cells were derived from local cells (ie, resident fibroblasts, pericytes, or progenitors), and not from blood-borne cells or epithelial-to-mesenchymal transitioning. As the conversion occurs in systemic sclerosis, in multiple inflammatory skin diseases, and in skin injury, our data are consistent with the proposal by Peduto et al66 that this transition represents a common fibroblast response to inflammation correlating with a shift to a tissue repair program. Indeed, a generic transition model is supported by the observation that CD34+ SFC cells were lost not only in the skin, but also in the gastric lining, myocardium, and lungs of SSc patients as well as in human skin wounds, peritoneal fibrosis, and keloids.29, 30, 31, 32 Synovial fibroblasts in the joints of rheumatoid arthritis patients also display Pdpn, suggesting that this transition accompanies inflammation.67, 68
Herein, we demonstrate that fibroblasts in vitro can transition in response to inflammatory stimuli that have NF-κB activation as a common element and, likewise, as noted in an acute mouse ear model of inflammation, Toll-like receptor and interferon signaling pathways did not contribute to Pdpn induction.66 Pdpn is constitutively expressed in lymphoid reticular stroma; however, when the lymph node becomes reactive or effectively inflamed, Pdpn levels increase even further.12, 14 While pursuing the notion of parallels between dermal fibrotic events and the reticular networks in lymphoid tissues, we were interested in the role of alternate NF-κB signaling and LTβR involvement. The LTβR system is crucial for the ontogeny of reticular networks in mouse lymphoid organs and has been linked to asthmatic airway remodeling.66, 69, 70, 71 Mouse fibroblastic reticular cells (FRCs) in lymph nodes make and wrap a collagen fibril, generating the conduit unit.11 Robust induction of Pdpn expression and matrix deposition in FRCs occurred in vitro with dual TNF receptor and LTβR activation.57 In parallel with the murine FRC study, in dermal fibroblasts, LTβR activation was not a strong inducer of Pdpn, yet combined TNF and LTβR agonism was the most effective trigger we observed.
The triggers for decreased CD34 and increased CD90 expression remain unclear. A complication in replicating these differentiation events in vitro is that primary dermal fibroblasts transition to a CD34 low, CD90 RNA high state in cell culture. Loss of CD34 was reported on adaptation of fibroblasts into tissue culture and a similar event occurs in endothelial cells.22, 72, 73, 74, 75 Given these changes in phenotypic expression and the observation that exposure of dermal fibroblasts to serum leads to a rapid transition with many elements of a wound repair response, it is clear that dermal fibroblasts are reprogramed on culturing.76 Despite these gaps in understanding, our in vitro work and the in vivo expression patterns suggest that different programs control Pdpn and CD90.
These three heavily glycosylated surface molecules still lack precisely defined biological roles. Pdpn can partner with CD9, CD44, and galectin-8 and displays sialylated sugars that bind to the C-type lectin-2 on dendritic cells and platelets.43 More important, in the lymph node environment, Pdpn promotes actinomyosin contractility in fibroblastic reticular cells via the RhoA/C-ROCK signaling pathway.13, 77 Shifts in fibroblast contractility dependent on Pdpn binding status may be especially relevant in the fibrotic setting given the attention being given to the role of matrix stiffness and mechanotransduction in fibroblasts.78 CD90 has been linked to efficient wound closure.79 A more detailed comprehension of these molecular functions should improve the understanding of both physiological and pathological skin remodeling and repair.
By comparing the reticular networks found in lymphoid tissues, we have uncovered parallels in perturbed skin. FRCs are found in inflammatory diseases in organized tertiary lymphoid tissues where there is a substantial lymphocytic infiltrate (eg, the salivary gland in Sjogren syndrome and the liver in primary biliary cirrhosis).44 In contrast, the SSc skin fibroblast transition usually appears in lieu of major classic lymphocytic infiltrates. We postulate that the skin fibroblast transition in SSc mirrors partially the differentiation events leading to the mature lymphoid reticular networks. Further work will be needed to ascertain the extent of any functional similarity. A dendritic cell-LTβR-Pdpn axis was recently shown to be crucial for FRC specialization and maintenance of immune responses in reactive lymph nodes.14 Whether such an axis is operational in SSc or systemic lupus erythematosus skin is an interesting question.
These data suggest that changes in fibroblast status can at least partially account for the dramatic loss of CD34+ SFCs observed in SSc. SSc is commonly portrayed as a triad of autoimmunity, vasculopathy, and fibrosis, with the fibrotic component dominating the life-threatening pathology. Uncovering these fibroblast changes may provide new tools to study earlier events in SSc before the major fibrotic process. It is possible that further insight will result from linking the rapid advances in understanding the lymphoid networks as well as the stromal reaction in tumors. By defining this simple transition, it is possible that disease heterogeneity within SSc can be probed and patients more accurately staged (ie, progressive or quiescent, early or late). In addition, this transition could provide a new metric for interventional clinical studies.
Acknowledgments
We thank Salma Goummih and Christina Lisk for assistance, Romy Christmann for valuable discussion, Jessica Zeimek, Eric Stratton, Sarah McLaughlin, and Robert Simms for the SSc skin biopsy repository, and Biogen for the gift of the BS1 LTβ receptor bispecific antibody.
Footnotes
Supported by NIH Clinical & Translational Science Institute grant UL1-TR000157; National Institute of Arthritis and Musculoskeletal and Skin Diseases grants 5P30AR061271, 1P50AR060780, and 2R01AR051089 (R.L.); and NIH grant 5T32A1007309-27 (A.M.S.B.).
B.N. and L.M.R. contributed equally to this work.
Disclosures: BS1 antibody was provided by Biogen.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2016.06.020.
Supplemental Data
Supplemental Figure S1.
CD34+ and CD90/Pdpn+ cells are distinct from CD31 endothelial cells. A–C: Diffuse cutaneous SSc skin biopsy specimens stained with triple combinations of CD31 [biotinylated cocktail of murine monoclonal antibodies (mAbs) C31.3 and JC/70A, Novus Biologicals], Pdpn (D2-40), rabbit anti-CD90 mAb, rabbit anti-CD34 mAb, and mouse anti-CD34 mAb (Qbend10). Staining without the CD31 overlay (left panels) and with only the CD31 layer (right panels). Double-positive Pdpn/CD34, CD90/CD34, and CD90/Pdpn cells can be seen (arrowheads) that are not CD31+ endothelial cells. Scale bar = 50 μm (A–C).
Supplemental Figure S2.
Procollagen expression by CD34+, CD90+, and Pdpn+ dermal fibroblastic-like cells. Skin biopsy specimens were stained with DAPI and procollagen [mouse monoclonal antibody (mAb)] along with rabbit anti-CD34, rabbit anti-CD90, or rabbit anti-Pdpn mAb (Abcam 128994). The overlap between procollagen and CD34 is yellow. Procollagen staining is generally perinuclear, with CD34, Pdpn, and CD90 expression extending further away from the nucleus. A: Typical CD34+ SFCs that express procollagen in the skin of a SSc patient lacking obvious disease in the upper dermis. B and C: Procollagen positive fibroblasts costaining with either Pdpn or CD90 in the skin of a SSc with a partial loss of CD34+ fibroblasts (not shown). Arrowheads indicate fibroblastic cells costaining with procollagen and CD34, CD90, or Pdpn. With the limited perinuclear localization of the procollagen staining yet more widespread expression of CD34, CD90, and Pdpn on the 3D contorted fibroblast, precise colocalization of procollagen with every fibroblast marker is not expected in these sections (3 μm thick). Scale bars: 400 μm (A–C, left panels); 50 μm (A–C, right panels).
Supplemental Figure S3.
CD34+, CD90+, and Pdpn+ dermal fibroblasts express vimentin. Skin biopsy specimens were stained with DAPI and vimentin, along with CD34, CD90, or Pdpn. The overlap between vimentin and CD34 is in yellow and imaged with a confocal microscope. Faint blue staining is autofluorescence from elastin fibers. A: Typical CD34+ SFCs in the papillary dermis of a normal subject. Arrowheads in A and B show likely vimentin+ monocytes lacking Pdpn or CD34 expression. B and C: Costaining of vimentin and Pdpn or CD90 in a transitioned region from a diffuse cutaneous SSc patient biopsy. Scale bar = 50 μm (A–C).
Supplemental Figure S4.
Pdpn+ CD90+ double-positive cells in SSc skin. Low-resolution immunofluorescence images of skin biopsy specimens stained for α-smooth muscle actin (α-SMA; blue), Pdpn (green), and CD90 (red). A: Normal skin (epidermis is in lower right corner) with a relative lack of Pdpn or CD90+ dermal fibroblasts. In contrast, in B (diffuse cutaneous SSc skin, upper dermis) and C (diffuse cutaneous SSc fibrotic region in the deep reticular dermis), there is an abundance of cells expressing these markers, including many that are double positive. A: CD90 also stains perivascular adventitial cells, and strong Pdpn staining identifies lymphatic vessels. α-SMA+ vascular smooth muscle is visible in all three panels, but in C, large numbers of α-SMA+ myofibroblasts are present as well as two glandular structures. B lacks myofibroblasts. B and C are from different patients. Scale bar = 200 μm (A–C).
Supplemental Figure S5.
CD90 and Pdpn+ expression in myofibroblasts. A diffuse cutaneous SSc patient skin biopsy was triple stained with α-smooth muscle actin (α-SMA; biotinylated 1A4), CD90 (rabbit pAb), and Pdpn mouse (D2-40), and the overlap is defined here in pseudo colors (white = overlap). Because of the similarity with the intracellular vimentin staining, it is believed that these complex staining patterns are due to the contorted nature of these myofibroblasts with intracellular α-SMA. A: A CD90+ and α-SMA+ myofibroblast (MF). CD90+ adventitia-like cells are often observed around a Pdpn+ lymphatic (L). Arrowheads mark a Pdpn−, CD90+ fibroblast. B: (From same biopsy) shows another myofibroblast expressing α-SMA+, Pdpn+, and CD90+. Scale bar = 20 μm (A and B).
Supplemental Figure S6.
Genes whose RNA expression correlates with modified Rodnan skin score (MRSS). Genes whose expression correlates with MRSS with P < 0.01 were clustered in an unsupervised manner and then reordered from right to left with increasing MRSS (upper gray bar). Analysis included 35 dcSSc and seven healthy controls and normal subjects are indicated with a black box. Genes and r values are listed in Supplemental Table S3.
Supplemental Figure S7.
Genes whose RNA expression correlates with the CD90/CD34 RNA ratio. Top panel: Genes whose expression correlated with the CD90/CD34 ratio with P < 0.01 were clustered in an unsupervised manner and then reordered from right to left with increasing modified Rodnan skin score (MRSS; upper gray bar). Analysis includes 35 diffuse cutaneous SSc and seven healthy controls and these controls are indicated with a black box. Genes and r values are listed in Supplemental Table S3. Bottom panel: defines the extent of overlapping genes with inclusion of genes whose expression correlated with the Pdpn/CD34, CD90/CD34 RNA ratios or MRSS with P < 0.05.
Supplemental Figure S8.
Podoplanin+/CD34− fibroblastic reticular cells (FRCs) in lymph nodes. Top row shows FRC networks in the human tonsil are CD34− and Pdpn+ (D2-40 mAb). Middle and lower rows show a layer of CD34+ reticular cells at the edge and base of the tonsil and these cells phenotypically resemble CD34+ SFC. Some cells appear to be in transition displaying both CD34 and Pdpn. In the top and middle panels, double-positive lymphatic-like vessels are seen and previously Lyve1+/CD34+ lymphatic vessels have been observed in tumors. Scale bars: 200 μm (left panels); 50 μm (right panels, showing boxed area). A, adipose; F, follicle; HEV, high endothelial venule; L, CD34+, Pdpn+ lymphatic vessel.
Supplemental Figure S9.
CD34, podoplanin, and CD90+ cells in various human dermal fibroblastic tumors. Low and high magnification (boxed areas) views of representative tumors stained for podoplanin (D2-40, red) and CD34 (blue) or CD90 (red) and CD34 (blue). Normal: This image is from a normal appearing region of skin adjacent to a dermatofibroma (DF) tumor (not visible in low magnification image). Pdpn+ lymphatics are visible. DF: Mixed CD34 and Pdpn+ stroma cells are present, although all cells now display CD90. Dermatofibrosarcoma protuberans (DFSP): Tumor is CD34+ and Pdpn−, some reactive CD90+ stroma appears to be present. Neurofibroma (NF): Presumably CD34-, Pdpn− tumor is interlaced within a normal appearing CD34+ stroma. The tumor is likely to be weakly CD90+. Atypical fibroxanthoma (AFX): Entire tumor is CD34−, Pdpn+, CD90+. Scale bars: 200 μm (main images); 50 μm (boxed areas).
References
- 1.Allanore Y., Simms R., Distler O., Trojanowska M., Pope J., Denton C.P., Varga J. Systemic sclerosis. Nat Rev Dis Primers. 2015;1:15002. doi: 10.1038/nrdp.2015.2. [DOI] [PubMed] [Google Scholar]
- 2.Wynn T.A., Ramalingam T.R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbane A.J., Denton C.P., Holmes A.M. Scleroderma pathogenesis: a pivotal role for fibroblasts as effector cells. Arthritis Res Ther. 2013;15:215. doi: 10.1186/ar4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lafyatis R. Transforming growth factor beta-at the centre of systemic sclerosis. Nat Rev Rheumatol. 2014;10:706–719. doi: 10.1038/nrrheum.2014.137. [DOI] [PubMed] [Google Scholar]
- 5.LeBleu V.S., Taduri G., O'Connell J., Teng Y., Cooke V.G., Woda C., Sugimoto H., Kalluri R. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–1053. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramann R., Schneider R.K., DiRocco D.P., Machado F., Fleig S., Bondzie P.A., Henderson J.M., Ebert B.L., Humphreys B.D. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16:51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marangoni R.G., Korman B.D., Wei J., Wood T.A., Graham L.V., Whitfield M.L., Scherer P.E., Tourtellotte W.G., Varga J. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol. 2015;67:1062–1073. doi: 10.1002/art.38990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rinkevich Y., Walmsley G.G., Hu M.S., Maan Z.N., Newman A.M., Drukker M., Januszyk M., Krampitz G.W., Gurtner G.C., Lorenz H.P., Weissman I.L., Longaker M.T. Skin fibrosis: identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science. 2015;348:2151. doi: 10.1126/science.aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roozendaal R., Mebius R.E. Stromal cell-immune cell interactions. Annu Rev Immunol. 2011;29:23–43. doi: 10.1146/annurev-immunol-031210-101357. [DOI] [PubMed] [Google Scholar]
- 10.Siegert S., Luther S.A. Positive and negative regulation of T cell responses by fibroblastic reticular cells within paracortical regions of lymph nodes. Front Immunol. 2012;3:285. doi: 10.3389/fimmu.2012.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malhotra D., Fletcher A.L., Turley S.J. Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity. Immunol Rev. 2013;251:160–176. doi: 10.1111/imr.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang C.Y., Vogt T.K., Favre S., Scarpellino L., Huang H.Y., Tacchini-Cottier F., Luther S.A. Trapping of naive lymphocytes triggers rapid growth and remodeling of the fibroblast network in reactive murine lymph nodes. Proc Natl Acad Sci U S A. 2014;111:E109–E118. doi: 10.1073/pnas.1312585111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acton S.E., Farrugia A.J., Astarita J.L., Mourao-Sa D., Jenkins R.P., Nye E., Hooper S., van Blijswijk J., Rogers N.C., Snelgrove K.J., Rosewell I., Moita L.F., Stamp G., Turley S.J., Sahai E., Reis e Sousa C. Dendritic cells control fibroblastic reticular network tension and lymph node expansion. Nature. 2014;514:498–502. doi: 10.1038/nature13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar V., Dasoveanu D.C., Chyou S., Tzeng T.C., Rozo C., Liang Y., Stohl W., Fu Y.X., Ruddle N.H., Lu T.T. A dendritic-cell-stromal axis maintains immune responses in lymph nodes. Immunity. 2015;42:719–730. doi: 10.1016/j.immuni.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng M., Haase A.T., Schacker T.W. Lymphoid tissue structure and HIV-1 infection: life or death for T cells. Trends Immunol. 2012;33:306–314. doi: 10.1016/j.it.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Grimm K.E., Barry T.S., Chizhevsky V., Hii A., Weiss L.M., Siddiqi I.N., Brynes R.K., O'Malley D.P. Histopathological findings in 29 lymph node biopsies with increased IgG4 plasma cells. Mod Pathol. 2012;25:480–491. doi: 10.1038/modpathol.2011.177. [DOI] [PubMed] [Google Scholar]
- 17.Tataroglu C., Sarioglu S., Kargi A., Ozkal S., Aydin O. Fibrosis in Hodgkin and non-Hodgkin lymphomas. Pathol Res Pract. 2007;203:725–730. doi: 10.1016/j.prp.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Fonseca D.M., Hand T.W., Han S.J., Gerner M.Y., Glatman Zaretsky A., Byrd A.L., Harrison O.J., Ortiz A.M., Quinones M., Trinchieri G., Brenchley J.M., Brodsky I.E., Germain R.N., Randolph G.J., Belkaid Y. Microbiota-dependent sequelae of acute infection compromise tissue-specific immunity. Cell. 2015;163:354–366. doi: 10.1016/j.cell.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Driskell R.R., Watt F.M. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol. 2015;25:92–99. doi: 10.1016/j.tcb.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Abraham D.J., Eckes B., Rajkumar V., Krieg T. New developments in fibroblast and myofibroblast biology: implications for fibrosis and scleroderma. Curr Rheumatol Rep. 2007;9:136–143. doi: 10.1007/s11926-007-0008-z. [DOI] [PubMed] [Google Scholar]
- 21.Rusu M.C., Mirancea N., Manoiu V.S., Valcu M., Nicolescu M.I., Paduraru D. Skin telocytes. Ann Anat. 2012;194:359–367. doi: 10.1016/j.aanat.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Diaz-Flores L., Gutierrez R., Garcia M.P., Saez F.J., Diaz-Flores L., Jr., Valladares F., Madrid J.F. CD34+ stromal cells/fibroblasts/fibrocytes/telocytes as a tissue reserve and a principal source of mesenchymal cells: location, morphology, function and role in pathology. Histol Histopathol. 2014;29:831–870. doi: 10.14670/HH-29.831. [DOI] [PubMed] [Google Scholar]
- 23.Aiba S., Tabata N., Ohtani H., Tagami H. CD34+ spindle-shaped cells selectively disappear from the skin lesion of scleroderma. Arch Dermatol. 1994;130:593–597. [PubMed] [Google Scholar]
- 24.Skobieranda K., Helm K.F. Decreased expression of the human progenitor cell antigen (CD34) in morphea. Am J Dermatopathol. 1995;17:471–475. doi: 10.1097/00000372-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Gilmour T.K., Wilkinson B., Breit S.N., Kossard S. Analysis of dendritic cell populations using a revised histological staging of morphoea. Br J Dermatol. 2000;143:1183–1192. doi: 10.1046/j.1365-2133.2000.03886.x. [DOI] [PubMed] [Google Scholar]
- 26.Walters R., Pulitzer M., Kamino H. Elastic fiber pattern in scleroderma/morphea. J Cutan Pathol. 2009;36:952–957. doi: 10.1111/j.1600-0560.2009.01201.x. [DOI] [PubMed] [Google Scholar]
- 27.de-Sa-Earp A.P., do Nascimento A.P., Carneiro S.C., Porto L.C., Monte-Alto-Costa A. Dermal dendritic cell population and blood vessels are diminished in the skin of systemic sclerosis patients: relationship with fibrosis degree and disease duration. Am J Dermatopathol. 2013;35:438–444. doi: 10.1097/DAD.0b013e3182712d1a. [DOI] [PubMed] [Google Scholar]
- 28.Manetti M., Guiducci S., Ruffo M., Rosa I., Faussone-Pellegrini M.S., Matucci-Cerinic M., Ibba-Manneschi L. Evidence for progressive reduction and loss of telocytes in the dermal cellular network of systemic sclerosis. J Cell Mol Med. 2013;17:482–496. doi: 10.1111/jcmm.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manetti M., Rosa I., Messerini L., Guiducci S., Matucci-Cerinic M., Ibba-Manneschi L. A loss of telocytes accompanies fibrosis of multiple organs in systemic sclerosis. J Cell Mol Med. 2014;18:253–262. doi: 10.1111/jcmm.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aiba S., Tagami H. Inverse correlation between CD34 expression and proline-4-hydroxylase immunoreactivity on spindle cells noted in hypertrophic scars and keloids. J Cutan Pathol. 1997;24:65–69. doi: 10.1111/j.1600-0560.1997.tb01098.x. [DOI] [PubMed] [Google Scholar]
- 31.Jimenez-Heffernan J.A., Aguilera A., Aroeira L.S., Lara-Pezzi E., Bajo M.A., del Peso G., Ramirez M., Gamallo C., Sanchez-Tomero J.A., Alvarez V., Lopez-Cabrera M., Selgas R. Immunohistochemical characterization of fibroblast subpopulations in normal peritoneal tissue and in peritoneal dialysis-induced fibrosis. Virchows Arch. 2004;444:247–256. doi: 10.1007/s00428-003-0963-3. [DOI] [PubMed] [Google Scholar]
- 32.Erdag G., Qureshi H.S., Patterson J.W., Wick M.R. CD34-positive dendritic cells disappear from scars but are increased in pericicatricial tissue. J Cutan Pathol. 2008;35:752–756. doi: 10.1111/j.1600-0560.2007.00895.x. [DOI] [PubMed] [Google Scholar]
- 33.Rice L.M., Ziemek J., Stratton E.A., McLaughlin S.R., Padilla C.M., Mathes A.L., Christmann R.B., Stifano G., Browning J.L., Whitfield M.L., Spiera R.F., Gordon J.K., Simms R.W., Zhang Y., Lafyatis R. A longitudinal biomarker for the extent of skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 2015;67:3004–3015. doi: 10.1002/art.39287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice L.M., Padilla C.M., McLaughlin S.R., Mathes A., Ziemek J., Goummih S., Nakerakanti S., York M., Farina G., Whitfield M.L., Spiera R.F., Christmann R.B., Gordon J.K., Weinberg J., Simms R.W., Lafyatis R. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest. 2015;125:2795–2807. doi: 10.1172/JCI77958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson W.E., Li C., Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 36.Farina G.A., York M.R., Di Marzio M., Collins C.A., Meller S., Homey B., Rifkin I.R., Marshak-Rothstein A., Radstake T.R., Lafyatis R. Poly(I: C) drives type I IFN- and TGFbeta-mediated inflammation and dermal fibrosis simulating altered gene expression in systemic sclerosis. J Invest Dermatol. 2010;130:2583–2593. doi: 10.1038/jid.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu X., Zimmerman M.A., Bardhan K., Yang D., Waller J.L., Liles G.B., Lee J.R., Pollock R., Lev D., Ware C.F., Garber E., Bailly V., Browning J.L., Liu K. Lymphotoxin beta receptor mediates caspase-dependent tumor cell apoptosis in vitro and tumor suppression in vivo despite induction of NF-kappaB activation. Carcinogenesis. 2013;34:1105–1114. doi: 10.1093/carcin/bgt014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stifano G., Affandi A.J., Mathes A.L., Rice L.M., Nakerakanti S., Nazari B., Lee J., Christmann R.B., Lafyatis R. Chronic Toll-like receptor 4 stimulation in skin induces inflammation, macrophage activation, transforming growth factor beta signature gene expression, and fibrosis. Arthritis Res Ther. 2014;16:R136. doi: 10.1186/ar4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akhmetshina A., Beer J., Zwerina K., Englbrecht M., Palumbo K., Dees C., Reich N., Zwerina J., Szucs G., Gusinde J., Nevskaya T., Distler O., Kerjaschki D., Schett G., Distler J.H. Decreased lymphatic vessel counts in patients with systemic sclerosis: association with fingertip ulcers. Arthritis Rheum. 2010;62:1513–1522. doi: 10.1002/art.27406. [DOI] [PubMed] [Google Scholar]
- 40.Honda N., Jinnin M., Kajihara I., Makino T., Fukushima S., Ihn H. Impaired lymphangiogenesis due to excess vascular endothelial growth factor-D/Flt-4 signalling in the skin of patients with systemic sclerosis. Br J Dermatol. 2010;163:776–780. doi: 10.1111/j.1365-2133.2010.09853.x. [DOI] [PubMed] [Google Scholar]
- 41.Manetti M., Milia A.F., Guiducci S., Romano E., Matucci-Cerinic M., Ibba-Manneschi L. Progressive loss of lymphatic vessels in skin of patients with systemic sclerosis. J Rheumatol. 2011;38:297–301. doi: 10.3899/jrheum.100767. [DOI] [PubMed] [Google Scholar]
- 42.Rossi A., Sozio F., Sestini P., Renzoni E.A., Khan K., Denton C.P., Abraham D.J., Weber E. Lymphatic and blood vessels in scleroderma skin, a morphometric analysis. Hum Pathol. 2010;41:366–374. doi: 10.1016/j.humpath.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Astarita J.L., Acton S.E., Turley S.J. Podoplanin: emerging functions in development, the immune system, and cancer. Front Immunol. 2012;3:283. doi: 10.3389/fimmu.2012.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Link A., Hardie D.L., Favre S., Britschgi M.R., Adams D.H., Sixt M., Cyster J.G., Buckley C.D., Luther S.A. Association of T-zone reticular networks and conduits with ectopic lymphoid tissues in mice and humans. Am J Pathol. 2011;178:1662–1675. doi: 10.1016/j.ajpath.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marsee D.K., Pinkus G.S., Hornick J.L. Podoplanin (D2-40) is a highly effective marker of follicular dendritic cells. Appl Immunohistochem Mol Morphol. 2009;17:102–107. doi: 10.1097/PAI.0b013e318183a8e2. [DOI] [PubMed] [Google Scholar]
- 46.Ordonez N.G. Value of podoplanin as an immunohistochemical marker in tumor diagnosis: a review and update. Appl Immunohistochem Mol Morphol. 2014;22:331–347. doi: 10.1097/PAI.0b013e31828a83c5. [DOI] [PubMed] [Google Scholar]
- 47.Gomaa A.H., Yaar M., Bhawan J. Cutaneous immunoreactivity of D2-40 antibody beyond the lymphatics. Am J Dermatopathol. 2007;29:18–21. doi: 10.1097/01.dad.0000249885.11195.15. [DOI] [PubMed] [Google Scholar]
- 48.Honma M., Minami-Hori M., Takahashi H., Iizuka H. Podoplanin expression in wound and hyperproliferative psoriatic epidermis: regulation by TGF-beta and STAT-3 activating cytokines, IFN-gamma, IL-6, and IL-22. J Dermatol Sci. 2012;65:134–140. doi: 10.1016/j.jdermsci.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 49.Gerlach J.C., Over P., Turner M.E., Thompson R.L., Foka H.G., Chen W.C., Peault B., Gridelli B., Schmelzer E. Perivascular mesenchymal progenitors in human fetal and adult liver. Stem Cells Dev. 2012;21:3258–3269. doi: 10.1089/scd.2012.0296. [DOI] [PubMed] [Google Scholar]
- 50.Guillot-Delost M., Le Gouvello S., Mesel-Lemoine M., Cherai M., Baillou C., Simon A., Levy Y., Weiss L., Louafi S., Chaput N., Berrehar F., Kerbrat S., Klatzmann D., Lemoine F.M. Human CD90 identifies Th17/Tc17 T cell subsets that are depleted in HIV-infected patients. J Immunol. 2012;188:981–991. doi: 10.4049/jimmunol.1101592. [DOI] [PubMed] [Google Scholar]
- 51.Corselli M., Chen C.W., Sun B., Yap S., Rubin J.P., Peault B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012;21:1299–1308. doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Juniantito V., Izawa T., Yuasa T., Ichikawa C., Tanaka M., Kuwamura M., Yamate J. Immunophenotypical analysis of myofibroblasts and mesenchymal cells in the bleomycin-induced rat scleroderma, with particular reference to their origin. Exp Toxicol Pathol. 2013;65:567–577. doi: 10.1016/j.etp.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Kissin E.Y., Merkel P.A., Lafyatis R. Myofibroblasts and hyalinized collagen as markers of skin disease in systemic sclerosis. Arthritis Rheum. 2006;54:3655–3660. doi: 10.1002/art.22186. [DOI] [PubMed] [Google Scholar]
- 54.Rajkumar V.S., Howell K., Csiszar K., Denton C.P., Black C.M., Abraham D.J. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res Ther. 2005;7:R1113–R1123. doi: 10.1186/ar1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatakeyama K., Kaneko M.K., Kato Y., Ishikawa T., Nishihira K., Tsujimoto Y., Shibata Y., Ozaki Y., Asada Y. Podoplanin expression in advanced atherosclerotic lesions of human aortas. Thromb Res. 2012;129:e70–e76. doi: 10.1016/j.thromres.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Zhou Y., Hagood J.S., Lu B., Merryman W.D., Murphy-Ullrich J.E. Thy-1-integrin alphav beta5 interactions inhibit lung fibroblast contraction-induced latent transforming growth factor-beta1 activation and myofibroblast differentiation. J Biol Chem. 2010;285:22382–22393. doi: 10.1074/jbc.M110.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katakai T., Hara T., Sugai M., Gonda H., Shimizu A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J Exp Med. 2004;200:783–795. doi: 10.1084/jem.20040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milano A., Pendergrass S.A., Sargent J.L., George L.K., McCalmont T.H., Connolly M.K., Whitfield M.L. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS One. 2008;3:e2696. doi: 10.1371/journal.pone.0002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Christmann R.B., Mathes A., Affandi A.J., Padilla C., Nazari B., Bujor A.M., Stifano G., Lafyatis R. Thymic stromal lymphopoietin is up-regulated in the skin of patients with systemic sclerosis and induces profibrotic genes and intracellular signaling that overlap with those induced by interleukin-13 and transforming growth factor beta. Arthritis Rheum. 2013;65:1335–1346. doi: 10.1002/art.37859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park S.M., Angel C.E., McIntosh J.D., Brooks A.E., Middleditch M., Chen C.J., Ruggiero K., Cebon J., Rod Dunbar P. Sphingosine-1-phosphate lyase is expressed by CD68+ cells on the parenchymal side of marginal reticular cells in human lymph nodes. Eur J Immunol. 2014;44:2425–2436. doi: 10.1002/eji.201344158. [DOI] [PubMed] [Google Scholar]
- 61.Thum C., Husain E.A., Mulholland K., Hornick J.L., Brenn T. Atypical fibroxanthoma with pseudoangiomatous features: a histological and immunohistochemical mimic of cutaneous angiosarcoma. Ann Diagn Pathol. 2013;17:502–507. doi: 10.1016/j.anndiagpath.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 62.Tardio J.C. CD34-reactive tumors of the skin: an updated review of an ever-growing list of lesions. J Cutan Pathol. 2009;36:89–102. doi: 10.1111/j.1600-0560.2008.01212.x. [DOI] [PubMed] [Google Scholar]
- 63.Bandarchi B., Ma L., Marginean C., Hafezi S., Zubovits J., Rasty G. D2-40, a novel immunohistochemical marker in differentiating dermatofibroma from dermatofibrosarcoma protuberans. Mod Pathol. 2010;23:434–438. doi: 10.1038/modpathol.2009.176. [DOI] [PubMed] [Google Scholar]
- 64.Buonaccorsi J.N., Plaza J.A. Role of CD10, wide-spectrum keratin, p63, and podoplanin in the distinction of epithelioid and spindle cell tumors of the skin: an immunohistochemical study of 81 cases. Am J Dermatopathol. 2012;34:404–411. doi: 10.1097/DAD.0b013e318236b17f. [DOI] [PubMed] [Google Scholar]
- 65.Darby I., Skalli O., Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63:21–29. [PubMed] [Google Scholar]
- 66.Peduto L., Dulauroy S., Lochner M., Spath G.F., Morales M.A., Cumano A., Eberl G. Inflammation recapitulates the ontogeny of lymphoid stromal cells. J Immunol. 2009;182:5789–5799. doi: 10.4049/jimmunol.0803974. [DOI] [PubMed] [Google Scholar]
- 67.Ekwall A.K., Eisler T., Anderberg C., Jin C., Karlsson N., Brisslert M., Bokarewa M.I. The tumour-associated glycoprotein podoplanin is expressed in fibroblast-like synoviocytes of the hyperplastic synovial lining layer in rheumatoid arthritis. Arthritis Res Ther. 2011;13:R40. doi: 10.1186/ar3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takakubo Y., Oki H., Naganuma Y., Saski K., Sasaki A., Tamaki Y., Suran Y., Konta T., Takagi M. Distribution of podoplanin in synovial tissues in rheumatoid arthritis patients using biologic or conventional disease-modifying anti-rheumatic drugs. Curr Rheumatol Rev. 2016 doi: 10.2174/1573397112666160331143607. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 69.Lu T.T., Browning J.L. Role of the lymphotoxin/LIGHT system in the development and maintenance of reticular networks and vasculature in lymphoid tissues. Front Immunol. 2014;5:47. doi: 10.3389/fimmu.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Remouchamps C., Boutaffala L., Ganeff C., Dejardin E. Biology and signal transduction pathways of the Lymphotoxin-alphabeta/LTbetaR system. Cytokine Growth Factor Rev. 2011;22:301–310. doi: 10.1016/j.cytogfr.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 71.Doherty T.A., Soroosh P., Khorram N., Fukuyama S., Rosenthal P., Cho J.Y., Norris P.S., Choi H., Scheu S., Pfeffer K., Zuraw B.L., Ware C.F., Broide D.H., Croft M. The tumor necrosis factor family member LIGHT is a target for asthmatic airway remodeling. Nat Med. 2011;17:596–603. doi: 10.1038/nm.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Delia D., Lampugnani M.G., Resnati M., Dejana E., Aiello A., Fontanella E., Soligo D., Pierotti M.A., Greaves M.F. CD34 expression is regulated reciprocally with adhesion molecules in vascular endothelial cells in vitro. Blood. 1993;81:1001–1008. [PubMed] [Google Scholar]
- 73.Boquest A.C., Shahdadfar A., Brinchmann J.E., Collas P. Isolation of stromal stem cells from human adipose tissue. Methods Mol Biol. 2006;325:35–46. doi: 10.1385/1-59745-005-7:35. [DOI] [PubMed] [Google Scholar]
- 74.Janson D.G., Saintigny G., van Adrichem A., Mahe C., El Ghalbzouri A. Different gene expression patterns in human papillary and reticular fibroblasts. J Invest Dermatol. 2012;132:2565–2572. doi: 10.1038/jid.2012.192. [DOI] [PubMed] [Google Scholar]
- 75.Walmsley G.G., Rinkevich Y., Hu M.S., Montoro D.T., Lo D.D., McArdle A., Maan Z.N., Morrison S.D., Duscher D., Whittam A.J., Wong V.W., Weissman I.L., Gurtner G.C., Longaker M.T. Live fibroblast harvest reveals surface marker shift in vitro. Tissue Eng Part C Methods. 2015;21:314–321. doi: 10.1089/ten.tec.2014.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iyer V.R., Eisen M.B., Ross D.T., Schuler G., Moore T., Lee J.C., Trent J.M., Staudt L.M., Hudson J., Jr., Boguski M.S., Lashkari D., Shalon D., Botstein D., Brown P.O. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- 77.Astarita J.L., Cremasco V., Fu J., Darnell M.C., Peck J.R., Nieves-Bonilla J.M., Song K., Kondo Y., Woodruff M.C., Gogineni A., Onder L., Ludewig B., Weimer R.M., Carroll M.C., Mooney D.J., Xia L., Turley S.J. The CLEC-2-podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture. Nat Immunol. 2015;16:75–84. doi: 10.1038/ni.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ho Y.Y., Lagares D., Tager A.M., Kapoor M. Fibrosis: a lethal component of systemic sclerosis. Nat Rev Rheumatol. 2014;10:390–402. doi: 10.1038/nrrheum.2014.53. [DOI] [PubMed] [Google Scholar]
- 79.Lee M.J., Shin J.O., Jung H.S. Thy-1 knockdown retards wound repair in mouse skin. J Dermatol Sci. 2013;69:95–104. doi: 10.1016/j.jdermsci.2012.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.