Abstract
Background/Objectives:
Epidemiological studies suggest a link between chromium (Cr) status and cardiovascular disease. Increased urinary excretion of Cr was reported in subjects with diabetes compared with non-diabetic controls and those with non-diabetic insulin resistance. Epigenetic alterations have been linked to the presence of Cr, and microRNA (miRNA) expression has been implicated in the pathogenesis of metabolic diseases and cardiovascular diseases (CVDs). We investigated the association between Cr excretion and miRNA expression in leukocytes from obese subjects. We also examined the relationship between altered miRNA expression and selected clinical parameters to further investigate mechanisms linking Cr to metabolic diseases and CVDs.
Subjects/Methods:
We analyzed urinary Cr in 90 Italian subjects using inductively coupled plasma-mass spectrometry. Peripheral blood miRNA levels were screened with TaqMan Low-Density Array Human MicroRNA A. Cr level-associated expression of miRNAs was detected with multivariate regression analyses, and the top 10 candidate miRNAs were selected for validation. We also used multivariate regression analyses to assess possible associations between validated miRNAs and glycated hemoglobin (A1c) and blood pressure (BP). The validated miRNAs were further investigated by functional analysis with Ingenuity Pathway Analysis software.
Results:
Urinary Cr levels (mean: 0.35 μg/l; s.d.=0.24) ranged from 0.05 to 1.27 μg/l. In the screening phase, 43 miRNAs were negatively associated with Cr. Of the top 10 miRNAs selected for validation, nine (miR-451, miR-301, miR-15b, miR-21, miR-26a, miR-362-3p, miR-182, miR-183 and miR-486-3p) were downregulated in association with Cr (P-false discovery rate (FDR)<0.10). miR-451 expression was associated with A1c (β=–0.06; P=0.0416), whereas miR-486-3p expression was associated both with diastolic (β=2.1; P=0.004) and systolic BP (β=3.3; P=0.003).
Conclusions:
These results indicate that miR-451 and miR-486-3p are involved in the link between Cr levels and metabolic diseases and CVDs.
Introduction
Chromium (Cr) is the sixth most abundant element in the earth's crust and seawater, existing in several oxidation states, principally metallic, trivalent and hexavalent Cr. Trivalent Cr, an essential trace element for humans, is a critical cofactor for insulin1 and has a well-documented role in maintaining normal glucose tolerance.2, 3, 4 Dietary Cr is available in drinking water and various foods, including meats, whole-grains, some fruits, vegetables and spices.5 The recommended daily dietary Cr intake for healthy adults is 24–35 μg from age 14 to 50 years and 20–30 μg after 50 years of age.6 Once adsorbed into the blood, Cr is widely distributed, but is most concentrated in the kidneys, muscles and liver.7 Cr effects are dependent upon the form and amount of supplemental Cr Excretion of absorbed Cr occurs primarily via urine.8 Higher than normal urinary Cr excretion has been reported for diabetics9 and non-diabetic insulin-resistant patients.10, 11 Epidemiological studies have suggested that Cr status may be linked with cardiovascular diseases (CVDs).12, 13
Epigenetic alterations, such as DNA methylation, posttranslational histone modifications and alterations of small non-coding RNAs, are linked to the presence of Cr in organisms14 and regulate homeostatic and inducible gene expression.15 MicroRNAs (miRNAs) are non-coding, small, single-stranded RNAs approximately 21–25 nucleotides long that have important regulatory roles in gene expression. They downregulate expression of target genes at the posttranscriptional level by binding to the 3' untranslated regions of target mRNAs.16 Aberrant miRNA expression has been implicated in several metabolic processes, including energy and lipid metabolism, which have been studied in the context of diabetes,17 insulin resistance18 and CVD.19
Obesity, defined as a body mass index (BMI) of 30 kg/m2 or greater, is strongly associated with onset of type 2 diabetes,20, 21 insulin resistance 22 and CVD.23 Therefore, obese individuals may represent a suitable population to investigate the epigenetic mechanisms linking Cr levels with metabolic diseases and CVDs. The glycated hemoglobin (A1c) value is an integrated measure of mean glucose levels over time and is considered the ‘gold standard' for monitoring metabolic control in patients with insulin resistance and diabetes.24 Elevated blood pressure (BP) is a well-established risk factor for CVD; an increase in systolic BP of 1 mm Hg has been estimated to raise the risk of death due to CVD by 2–4%.25
This study aimed to verify a possible association between urinary Cr levels and miRNA expression in leukocytes from obese subjects. In addition, we sought to examine the relationship between altered miRNA expression and two clinical parameters, A1c and diastolic/systolic BP, to further investigate mechanisms linking Cr to metabolic diseases and CVDs.
Materials and methods
Study design
Ninety subjects were recruited from the ‘Center for Obesity and Weight Control' at ‘Ospedale Maggiore Policlinico, Fondazione IRCCS Ca' Granda' in Milan, Italy. The study subjects were a subgroup of the SPHERE (‘Susceptibility to Particle Health Effects, miRNA, and Exosomes') project, funded by ERC-2011-StG (282413) to examine molecular mechanisms underlying the effects of particulate matter and metal exposure on health outcomes. Detailed information on this population has been reported previously.26 Each study participant provided written informed consent in compliance with the ethics committee of the ‘Ospedale Maggiore Policlinico' (approval number 1425) and donated 15 ml of blood and 50 ml of urine. A lifestyle questionnaire collecting information on sociodemographic data, residence, education, smoking history, eating habits, personal and family medical history, medication used in the last year, and physical activity was administered to each participant. The lifestyle questionnaire is a short version of the questionnaire used in the EAGLE study (environment and genetics in lung cancer etiology, a population-based case–control study, http://eagle.cancer.gov) and details about information collected in it can be found in our previous article.26 The questionnaire is checked for completeness at the time of data collection in order to ensure high-quality data.
Urine sampling and Cr analysis
Urine samples were collected in polyethylene tubes and delivered to the laboratory at room temperature within 2 h after collection; 5 ml aliquots were stored at –20 °C until analysis, which occurred within 12 months after collection. Before analysis, samples were thawed at room temperature, then mixed and heated for 30 min at 37 °C to dissolve the sediment. One milliliter of urine was diluted with 4 ml aqueous nitric acid, 0.05% v/v (from ultrapure nitric acid 67%, Merck, Darmstadt, Germany), containing the stock solution IV-ICP-MS-71D with standards (Inorganic Ventures, Christiansburg, VA, USA). All solutions were prepared using Milli-Q ultrapure water (conductivity 0.056 μS/cm) (Merck). Samples were subjected to inductively coupled plasma-mass spectrometry (ICP-MS) with an ICP-MS X Series II machine (Thermo Scientific, Rodano, Italy) using the kinetic energy discrimination mode to reduce interferences, operating with H2 in He (8% v/v) as the collision and reaction gas. The instrument was supplied with standard Ni/Cu cones, a quartz injector (1.5 mm orifice), and a cyclonic spray chamber, and was operated using Ar with a nebulized gas flow of 0.91 l/min, an auxiliary gas flow of 0.6 l/min and a cool gas flow of 13 l/min. Ions m/z 52 and 89 for Cr and yttrium as the analytic and internal standards, respectively, were acquired with a dwell time of 10 ms. Quantification was performed using a calibration curve obtained with aqueous solutions containing Cr in concentrations ranging from 0.05 to 2.5 μg/l. The detection limit of the assay was 0.03 μg/l, with a quantification limit of 0.05 μg/l; the assay accuracy was 97–102% and its precision 2.5–5.9%. Internal quality control was performed using Lyphochek Urine Metals Control, level-1 and level-2 (Bio-Rad Laboratories, Segrate, Italy), and Seronorm Level-1 (Sero AS, Billingstad, Norway).
Blood collection and RNA isolation
Peripheral blood samples were collected in PAXgene Blood RNA tubes, sent immediately to the laboratory and, after 24 h, frozen at –80 °C, according to manufacturer's instructions. PAX tubes were chosen for RNA collection because they contain a solution that inhibits RNA degradation and gene induction as blood is drawn into the tube. Total RNA was extracted from whole blood with MagMAX-96 according to the manufacturer's protocols (Ambion, Austin, TX, USA). RNA was quantified using a ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
Screening of miRNA expression
Reverse transcription (RT) was performed using the TaqMan MicroRNA Reverse Transcription Kit and Megaplex RT Primers A, following the manufacturer's instructions (Life Technologies, Carlsbad, CA, USA). Briefly, 3 μl of RNA (110 ng) from blood were added to 4.5 μl of the RT reaction mixture, which included 0.8 μl Megaplex RT Primer Pools A (10 ×), 0.2 μl dNTPs (100 nM), 1.5 μl MultiScribe Reverse Transcriptase (50 U/μl), 0.8 μl RT Buffer (10 ×), 0.9 μl MgCl2 (25 mM), 0.1 μl RNase inhibitor (20 U/μl) and 0.2 μl nuclease-free water. After incubation on ice for 5 min, RT was performed using a C1000 Thermal Cycler (Bio-Rad, Hercules, CA, USA). Thermal cycler conditions were as follows: 40 cycles of 16 °C for 2 min, 42 °C for 1 min and 50 °C for 1 s, followed by one step of 85 °C for 5 min. The RT reaction was stored at –20 °C and used within 1 month.
For each sample, miRNA profiling was performed with a TaqMan Low-Density Array (TLDA - TaqMan Array Human MicroRNA A Cards Set v2.1; Life Technologies), pre-loaded 384-well microfluidic cards, with 384 spotted assays including 377 human miRNAs selected for their biological functions and relationships, three endogenous small RNA human controls (RNU6, RNU44 and RNU48, the first being in quadruplicate), and a non-human negative control (Ath-miR159a). All reactions were performed as specified in the manufacturers' protocols. Briefly, a total reaction mixture containing RT products and TaqMan Universal PCR Master Mix (Life Technologies) was added to each TLDA line after gentle vortex mixing, and then each card was centrifuged and sealed mechanically with a Life Technologies sealing device. TLDAs were performed with the 7900HT Fast Real-Time PCR System (Life Technologies), and the thermal cycler conditions were as follows: 50 °C for 2 min, 94.5 °C for 10 min, and then 40 cycles of 97 °C for 30 s and 59.7 °C for 1 min. Gene Expression Suite Software (Life Technologies) was used to process miRNA expression data from TLDA, using automatic baseline settings and a threshold of 0.2.
Validation of miRNA expression
Validation of previous findings was performed using the custom TLDA including the following TaqMan miRNA assays: miR-15b, miR-21, miR-26a, miR-101, miR-182, miR-183, miR-301a, miR-362-3p, miR-451, miR-486-3p, RNU6, RNU44 and RNU48. Using the custom RT primer pool, 3 μl of RNA (110 ng) were RT. The 12- μl RT reaction mix included: 6 μl Custom RT primer Pool, 0.3 μl dNTPs (100 nM), 3 μl MultiScribe RT (50 U/μl), 1.5 μl RT Buffer (10 ×), 0.19 μl RNase inhibitor (20 U/μl) and 1.01 μl nuclease-free water. After incubation on ice for 5 min, RT was performed using a C1000 Thermal Cycler (Bio-Rad). Thermal cycler conditions were as follows: one step of 16 °C for 30 min, one step of 42 °C for 30 min, followed by one step of 85 °C for 5 min. The RT reaction was stored at –20 °C and used within one month. All TLDA reactions were performed using 7900HT Fast Real-Time PCR System (Life Technologies), as specified in the protocols of the manufacturer, and each assay was run in triplicate. Furthermore, one no-RT control and one no-template control were carried in the first custom TLDA. The no-RT control experiment was performed with RNase-free water instead of RT to test for the presence of genomic DNA, and the no-template control experiment was performed with RNase-free water instead of RT product to test for primer–dimer formation. As these controls were negative, all samples were analyzed.
miRNA expression data analysis
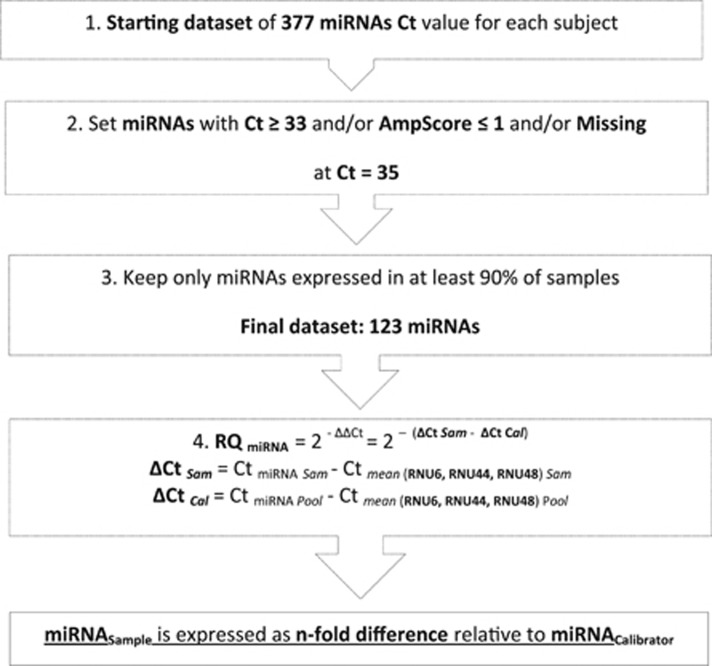
Real-time PCR permitted quantitative measurement, expressed as related cycle threshold (Ct) of miRNA expression.27 Ct is the number of PCR cycles at which the efficiency of the real-time PCR reaction is maximal for each miRNA target. In the screening phase, the starting point was a data set containing, for each subject, the Ct values of 377 miRNAs and an AmpScore value, which is a quality index, ranging from 0 to 2, which indicates amplification curve quality in the linear phase.
We applied an automatic miRNA selection, setting the values of miRNAs with Ct ⩾33 and/or with AmpScore ⩽1, and/or with missing Ct, equal to the detection limit of 35 (Figure 1). To reduce background noise, we excluded miRNAs not expressed in at least 90% of samples (Ct=35), producing a final data set of 123 miRNAs. The mean Ct of the three endogenous small RNA controls was used as an internal control to normalize the amount of individual miRNA in each sample. Relative quantification of miRNA expression was determined by the ΔΔCt method in which expression levels of each normalized miRNA were calculated relative to a calibrator sample that consisted of a pool of 90 samples; it was used as the 1 × sample (or 100%), and the relative value of each miRNA was expressed as an n-fold difference from the calibrator (Figure 1). The same preliminary data cleaning and normalization methods were applied to the subsets of miRNAs selected for validation.
Figure 1.
Data processing workflow. Cal, Calibrator; Ct, related cycle threshold; RQ, relative quantification; Sam, sample.
Statistical analysis
Descriptive statistics were performed on all variables. Normally distributed data are reported as means±s.d. or frequencies, as appropriate. We used linear regression models to test the association between urinary Cr and miRNA expression in both the screening and validation phases. MiRNA expression, quantified by relative quantification, was log transformed to achieve a normal distribution.
Multivariate regression analyses, adjusted for age, sex, BMI, smoking habits and granulocyte percent, were used to evaluate changes in miRNA expression in association with urinary Cr level on the recruitment day. Owing to the high number of comparisons made, we took into account a correction for multiple comparisons based on the false discovery rate (FDR) control. A threshold of 0.10 was applied on FDR P-value significance (P-FDR) to identify the top miRNAs among the 377 screened. The same analyses were repeated in the validation phase on the set of miRNAs selected for confirmation. Multivariate regression models, adjusted for sex, age, BMI, granulocyte percentage and smoking habits, were also applied to assess the possible association between confirmed miRNAs and two selected clinical parameters: A1c and diastolic/systolic BP. Logarithmic transformations were applied, when appropriate, to achieve normal distributions of outcome variables in regression models. We conducted a residual fit analysis to confirm the model fit. All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC, USA).
Prediction of miRNA targets and pathway analysis
Data from the top 10 miRNA were submitted to the IPA Core for Ingenuity Pathway Analysis (Ingenuity Systems, Redwood City, CA, USA) to investigate the signaling and metabolic pathways, molecular networks and biological processes associated with them. Putative miRNA–mRNA relationships were identified with the IPA microRNA Target Filter, based on a knowledge base of prediction with high-confidence and experimentally observed relationships from TargetScan, TarBase, miRecords and Ingenuity Knowledge Base. The resulting target gene lists were analyzed to identify diseases and biological functions associated with miRNA gene sets.
We filtered the results for CVDs, nutritional diseases and metabolic diseases, and the resulting target gene list, in addition to nine miRNAs, was analyzed with IPA core analysis. Functional analysis was carried out to identify canonical pathways and molecular networks that have been associated with the mRNA target gene set. The IPA database considers gene–phenotype associations, molecular interactions, regulatory events and chemical knowledge to provide a global molecular network. Related networks were constructed algorithmically based on connectivity. The statistical significance of each biological function was calculated using Fisher's exact text with alpha set to 0.05.
Results
Characteristics of study participants and urinary Cr assessment
The main characteristics of the study participants are presented in Table 1. Briefly, a majority of the participants were women, and a wide age range (21–75 years) was represented. All subjects had a BMI >25 kg/m2, and 62.2% were obese (BMI >30 kg/m2). We observed a wide range of urinary Cr levels (0.05–1.27 μg/l). In addition, 43.1% of subjects were insulin resistant, and 8.3% of subjects were diabetic. Hypertension was observed in 33.9% of subjects.
Table 1. Study cohort characteristics (N=90).
| Characteristic | NR (proportion) | Mean±s.d. |
|---|---|---|
| Sex | ||
| Men | 15 (16.7%) | |
| Women | 75 (83.3%) | |
| Age, (years) | 51.6±11.9 | |
| BMI, (kg/m2) | 32.9±5.7 | |
| Overweight (25≤BMI<30) | 34 (37.8%) | |
| Class I obesity (30≤BMI<35) | 28 (31.1%) | |
| Class II obesity (35≤BMI<40) | 19 (21.1%) | |
| Class III obesity (BMI≥40) | 9 (10.0%) | |
| Smoke | ||
| Never smoked | 48 (53.3%) | |
| Former smoker | 26 (28.9%) | |
| Current smoker | 16 (17.8%) | |
| Blood count | ||
| White blood cells (%) | 7.0±2.0 | |
| Granulocytes (%) | 61.3±7.6 | |
| Monocytes (%) | 8.4±3.2 | |
| Lymphocytes (%) | 30.4±7.6 | |
| Red blood cells (%) | 4.8±0.5 | |
| A1c (mmol/mol) | 41.6±8.4 | |
| Chromium, (μg/l) | 0.35±0.24 | |
| Diastolic pressure (mmHg) | 79.2±10.2 | |
| Systolic pressure (mmHg) | 124.8±16.5 | |
Abbreviations: A1c, glycated hemoglobin; BMI, body mass index; NR, number.
Continuous variable are expressed as mean±s.d. Discrete variables are expressed as counts (%).
miRNA expression screening and validation in association with urinary Cr
After data cleaning, 123 miRNAs were analyzed, according to the criteria described in the Materials and methods section. In the screening phase, we identified 43 miRNAs that were negatively associated with urinary Cr levels (P<0.05, P-FDR<0.10). Table 2 shows the percentage change of miRNA expression in terms of relative quantification per 1% increment in urinary Cr Ten candidate miRNAs were chosen for validation, on the basis of their significant P-value and P-FDR. Consistent with the screening data, nine miRNAs (miR-451, miR-301, miR-15b, miR-21, miR-26a, miR-362-3p, miR-182, miR-183 and miR-486-3p) were negatively associated with urinary Cr level, with a P-FDR <0.10 (Table 3). The mean coefficient of variability of this study was 0.89%.
Table 2. Percentage change of miRNAs expression in profiling phase.
| miRNA | Δ% |
95% CI |
RawP | P-FDR | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| miR-486-3p | −29.6% | −43.4% | −12.5% | 0.002 | 0.093 |
| miR-21 | −39.1% | −56.4% | −15% | 0.005 | 0.093 |
| miR-362-3p | −43.3% | −61.4% | −16.7% | 0.005 | 0.093 |
| miR-26a | −34.6% | −51.4% | −11.9% | 0.007 | 0.093 |
| miR-15b | −38.7% | −56.6% | −13.5% | 0.007 | 0.093 |
| miR-182 | −51.9% | −71.3% | −19.4% | 0.007 | 0.093 |
| miR-101 | −44.1% | −63% | −15.4% | 0.007 | 0.093 |
| miR-183 | −47.8% | −67.4% | −16.4% | 0.008 | 0.093 |
| miR-301a | −45.3% | −64.8% | −15% | 0.009 | 0.093 |
| miR-451 | −28.4% | −44.1% | −8.3% | 0.010 | 0.093 |
| miR-374a | −42% | −61.3% | −12.9% | 0.011 | 0.093 |
| miR-106b | −32.9% | −50.3% | −9.3% | 0.011 | 0.093 |
| miR-660 | −31.1% | −48.1% | −8.7% | 0.012 | 0.093 |
| miR-598 | −42.8% | −62.7% | −12.2% | 0.013 | 0.093 |
| miR-335 | −34.8% | −53.1% | −9.5% | 0.013 | 0.093 |
| miR-148b | −37.2% | −56% | −10.3% | 0.013 | 0.093 |
| miR-26b | −37.3% | −56.4% | −10% | 0.013 | 0.093 |
| let-7b | −27.9% | −44.2% | −6.8% | 0.015 | 0.093 |
| miR-18a | −38.6% | −58.1% | −9.8% | 0.015 | 0.093 |
| miR-126 | −43.9% | −64.7% | −11% | 0.016 | 0.093 |
| miR-192 | −25.7% | −41.3% | −5.8% | 0.017 | 0.093 |
| miR-22 | −29.1% | −46.3% | −6.4% | 0.018 | 0.093 |
| let-7 g | −35.6% | −54.9% | −8.1% | 0.018 | 0.093 |
| miR-19a | −27.8% | −44.5% | −6% | 0.018 | 0.093 |
| miR-103 | −31.4% | −49.7% | −6.5% | 0.020 | 0.096 |
| miR-142-5p | −32.7% | −51.6% | −6.4% | 0.021 | 0.099 |
| miR-374b | −33.6% | −53.2% | −5.8% | 0.024 | 0.099 |
| miR-106a | −32.1% | −51.4% | −5.3% | 0.025 | 0.099 |
| miR-132 | −29% | −47.1% | −4.6% | 0.026 | 0.099 |
| let-7e | −29.5% | −48% | −4.5% | 0.027 | 0.099 |
| miR-25 | −23.2% | −38.9% | −3.4% | 0.027 | 0.099 |
| miR-17 | −31.7% | −50.9% | −4.9% | 0.027 | 0.099 |
| miR-93 | −30.9% | −50.3% | −4% | 0.031 | 0.099 |
| miR-194 | −25.9% | −43.4% | −3.1% | 0.032 | 0.099 |
| miR-195 | −34.5% | −55.2% | −4.4% | 0.032 | 0.099 |
| miR-532-5p | −25.9% | −43.4% | −3% | 0.033 | 0.099 |
| miR-29c | −33.2% | −53.7% | −3.8% | 0.033 | 0.099 |
| miR-142-3p | −23.8% | −40.3% | −2.6% | 0.033 | 0.099 |
| miR-331-3p | −20.7% | −35.7% | −2.1% | 0.034 | 0.099 |
| miR-130b | −28.3% | −47% | −3% | 0.034 | 0.099 |
| let-7a | −30.5% | −50% | −3.3% | 0.034 | 0.099 |
| miR-340 | −32.9% | −53.3% | −3.6% | 0.034 | 0.099 |
| miR-24 | −23.4% | −39.9% | −2.3% | 0.035 | 0.099 |
Abbreviations: CI, confidence interval; Cr, chromium; FDR, false discovery rate; miRNA, microRNA.
The miRNA percentage change is expressed per 1% increment of Cr exposure. Models were adjusted for sex, age, BMI, granulocyte percentage and smoking habits. All miRNAs, P<0.10. The top 10 miRNAs (bold) were selected for validation.
Table 3. Percentage change of miRNA expression in validation phase.
| miRNA | Δ% | 95% CI | P-value | P-FDR | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| miR-451 | −73.2 | −85.1 | −51.6 | <0.001 | <0.001 |
| miR-301 | −95.3 | −98.9 | −78.8 | 0.000 | 0.001 |
| miR-15b | −83.5 | −94.0 | −54.5 | 0.001 | 0.003 |
| miR-21 | −80.3 | −92.5 | −48.6 | 0.001 | 0.004 |
| miR-26a | −75.7 | −89.7 | −42.6 | 0.002 | 0.004 |
| miR-362-3p | −88.7 | −97.1 | −56.7 | 0.002 | 0.004 |
| miR-182 | −74.0 | −89.5 | −35.5 | 0.005 | 0.007 |
| miR-183 | −77.8 | −94.9 | −4.0 | 0.048 | 0.060 |
| miR-486-3p | −74.8 | −94.1 | 6.8 | 0.065 | 0.072 |
| miR-101 | −30.5 | −69.3 | 57.5 | 0.387 | 0.387 |
Abbreviations: CI, confidence interval; Cr, chromium; FDR, false discovery rate; miRNA, microRNA.
The miRNA percentage change is expressed per 1% increment of Cr exposure in validation. Models were adjusted for sex, age, BMI, granulocyte percentage and smoking habits.
miRNA associations with clinical parameters
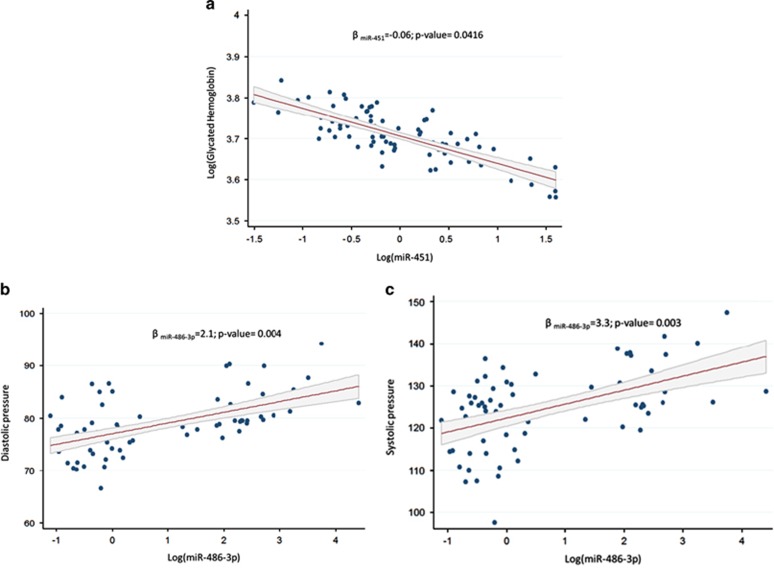
miR-451 expression associated negatively with A1c (Δ%=−0.06% P=0.0416) (Figure 2a), whereas miR-486-3p expression associated positively with both diastolic (Δ%=2.11% P=0.004; Figure 2b) and systolic BP (Δ%=3.34% P=0.003; Figure 2c). None of the other miRNAs were associated with the examined clinical parameters (data not shown).
Figure 2.
Association between log-expression level of (a) miR-451 with log-A1c and of miR-486-3p with diastolic (b) and systolic (c) BP. Multivariate regression models were adjusted for sex, age, BMI, granulocyte percentage and smoking habits.
Target gene identification and functional analysis
We identified 4473 mRNAs that were experimentally observed or highly predicted targets. When the data were filtered for metabolic and signaling pathways only, we found 1570 mRNA targets that mapped to several molecular networks. To investigate if the mRNAs targeted by our nine validated urinary Cr-associated miRNAs (Table 3) were potentially involved in biological processes connected to obesity and related diseases, the list was filtered for cardiovascular, nutritional and metabolic diseases, resulting in 764 genes (Supplementary Table 1). The number of targeted mRNAs for each miRNA was: miR-451=16, miR-301=164, miR-15b=247, miR-21=77, miR-26a=147, miR-362-3p=62, miR-182=185, miR-183=80 and 486-3p=111.
To understand the biological functions of the mRNA targets, we performed IPA's core analysis, and the top canonical pathways were molecular mechanisms of cancer (P=7.96 × 10-61), axonal guidance signaling (P=2.25 × 10-47), protein kinase A signaling (P=8.46 × 10-43), role of NFAT in cardiac hypertrophy (P=1.13 × 10-42) and phosphatase and tensin homolog (PTEN) signaling (P=2.64 × 10-42). The top two networks were enriched for nervous system development (score: 39) and function, tissue morphology, cell death and survival and for cardiovascular system development and function, organismal development and organismal survival (score: 37). We also investigated the diseases possibly associated in our enrichment analysis. Table 4 summarizes the top five diseases, and shows that CVD and metabolic disease are the most significantly involved, with 432 and 391 associated targeted mRNAs, respectively (Supplementary Tables 2 and 3). In the cardiovascular category, the lowest P-value was for vascular disease (4.88 × 10-114) and highest was for congenital heart disease (2.01 × 10-33); in the metabolic category the lowest P-value was for glucose metabolism disorders (9.80 × 10-113) and the highest was for non-insulin-dependent disorders (1.19 × 10-29; Table 4).
Table 4. IPA core analysis: top diseases/disorders with maximum and minimum P-values.
| Disease or disorder | P-value | # mRNA |
|---|---|---|
| CVD | 2.01 × 10-33 - 4.88 × 10-114 | 432 |
| Metabolic disease | 1.19 × 10-29 - 9.80 × 10-113 | 391 |
| Endocrine system disease | 1.19 × 10-29 - 1.89 × 10-83 | 246 |
| Gastrointestinal disease | 1.19 × 10-29 - 1.89 × 10-83 | 630 |
| Inflammatory disease | 1.89 × 10-31 - 1.75 × 10-76 | 315 |
Abbreviation: CVD, cardiovascular disease.
# mRNA, no. individual mRNA species involved in the indicated pathways.
Discussion
To further investigate the mechanisms linking Cr to metabolic and cardiovascular disorders, we analyzed miRNA expression in leukocytes from obese subjects in association with urinary Cr, and examined how altered miRNA expression related to A1c and diastolic/systolic BP. Obese individuals were chosen for this study because of their strong susceptibility to diabetes, insulin resistance and CVD. We identified and validated a distinct signature of nine miRNAs in peripheral blood that associated negatively with urinary Cr and among these only miR-451 was associated with A1c, suggesting a role for miR-451 in the development of diabetes. Meanwhile, miR-486-3p was associated with systolic and diastolic BP, suggesting it may be a factor in CVD risk.
The two most widespread oxidation states of Cr, Cr+3 and Cr+6, have different mobility and bioavailability, resulting in differential penetration of biological membranes, possibly explaining their quite different toxicities. Hexavalent Cr derivatives easily penetrate cell membranes and are easily absorbed from the gastrointestinal tract and lungs, whereas trivalent Cr compounds do not traverse biological membranes well.28 Excretion of Cr occurs primarily via urine and reflects absorption only over the previous 1–2 days. The measurement of urine Cr concentration by ICP-MS is a well-established method in elemental analysis, because of its unique analytical capabilities, and reflects the total Cr level (Cr+6 and Cr3+).29 Only trivalent Cr is an essential mineral; it potentiates insulin effects in normal glucose metabolism. Cr supplementation may, therefore, improve systemic insulin sensitivity.30 People with diabetes may have altered Cr metabolism compared with non-diabetic subjects,31 perhaps because of hyper-absorption and excretion of Cr.32, 33
Epidemiological studies suggest a link between Cr status and CVD.12 Guallar et al.13 found that patients who died from coronary artery disease had significantly lower Cr levels in their aortic tissue than patients who died from accidents. The Cr concentration in biological samples (scalp hair, blood and urine) is inversely associated with risk of myocardial infarction (MI).34
Mounting evidence suggests a crucial role of miRNAs in insulin resistance and β-cell dysfunction, two main features of type 2 diabetes,17 and in the onset and development of CVD.19 The hypothesis that miRNAs have an important role in metabolic diseases and CVDs is strengthened by the observation that many miRNAs perform key roles in critical biological processes that correlate with urinary Cr levels.35 In our study, miR-451 and miR-15b were negatively associated with urinary Cr. In addition, we found a significant association between miR-451 expression and A1c levels, suggesting that miR-451 may be involved in diabetes pathogenesis because greater increases in A1c indicate worse control of blood glucose levels.36 Zhang et al.37 showed that miR-451 regulates p38 mitogen-activated protein kinase signaling and suppresses mesangial hypertrophy in early diabetic nephropathy. MiR-451 and miR-15b, by modulating apoptosis and angiogenesis, are involved in coronary artery disease38, 39 and MI.40, 41
Our data show that miR-486-3p is associated with diastolic and systolic BP regulation, and IPA core analysis identified cardiac hypertrophy and PTEN signaling as canonical pathways interacting with this miRNA. In fact, miR-486-3p has been associated with systemic ventricular contractility42 and is a critical regulator of muscle growth. Small et al.43 showed that miR-486-3p targets PTEN and Foxo1a directly, enhancing the phosphatidylinositide 3-kinase/protein kinase B (PI3K/AKT) signaling in muscle cells. Overexpression of miR-486-3p reduces PTEN and Foxo1a protein expression and enhances PI3K/AKT signaling, eventually leading to muscle hypertrophy. PI3K is a known regulator of skeletal muscle hypertrophy and atrophy.44 The increase of miR-486-3p in the plasma of acute MI patients may reflect an underlying cardiac hypertrophy.45
Our results revealed a Cr-related downregulation in miR-26a and miR-21 expression; some authors have also found that miR-26a was downregulated in the sera of patients with acute MI.46 MiR-21 exhibits increased expression in several forms of CVD47 and is sensitive to high-concentration glucose treatment in macrophages, providing resistance to apoptosis induced by high glucose concentrations via programmed cell death 4 (PDCD4) regulation.48
MiR-182 and miR-183 constitute a well-known miRNA cluster, located on chromosome 7, which is involved in insulin signaling and energy metabolism. Xu and Wong49 provided experimental evidence indicating that this miRNA cluster, miR-183-96-182, targets Irs1, Rasa1 and Grb2, the products of which are all in the insulin signaling pathway. In our obese subjects, two components of this cluster (miR-182 and miR-183) were significantly related to urinary Cr, suggesting their correlation with the insulin pathway.
We also found that miR-301a and miR-362 were negatively associated with urinary Cr, and our IPA core analysis indicated that the PTEN signaling pathway was the top canonical pathway related to expression of these miRNAs. Mir-301a and miR-362 are involved in the NF-kB (nuclear factor kappa light-chain enhancer of B cells) and insulin pathways. MiR-301a, which modulates Kv4.2 (potassium voltage-gated channel subfamily D2) by binding to its 3'-UTR, is downregulated in the hearts of diabetes patients, indicating the direct association of mir-301a with diabetes.50 MiR-301a regulates IL-6-induced insulin resistance by direct regulation of PTEN expression.51
Elevation of miR-451 in red blood cells may affect its quantification in leukocytes,52 but a strength of our study was the use of the PAXgene Blood System, which allows complete red blood cells lysis and loss before miRNA isolation. Another strength of this study was the use of a non-targeted, exploratory approach to select candidate miRNAs. We chose to use a real-time PCR approach for miRNA expression analysis because its provides sufficiently high precision and sensitivity to detect closely related miRNAs, which may differ in sequence by only a single base.53 Furthermore, to limit false positives in the detection of differentially expressed miRNAs, we used restrictive statistical significance cutoffs.
Our population is composed essentially of female gender, but a recent study54 showed that sex seemed to have a weak effect on the miRNA levels. With the aim of taking into account the different distribution of males and females, and different obesity levels in our population we decided to ‘a priori' adjust all models for sex and BMI. Also we tested in all multivariate models the inclusion of the interaction with sex or BMI. In none of our models, the interaction was significant. These results suggest that sex and BMI are not acting as effect modifiers of our analysis.
In our study population, the mean urinary Cr level was higher than that found in the general Italian population55 and was higher than the mean concentration recommended by the Institute of Medicine of the National Research Council (0.22 μg/l). This finding could be explained by the high percentage of people with insulin resistance in the study. We used one of the most robust commercially available ICP-MS instruments, which provided many advantages, including elemental specificity and high measurement sensitivity over a wide linear dynamic range.56 One limitation of our study was the lack of Cr speciation; to achieve Cr speciation, ICP-MS analysis would have to be coupled with high performance liquid chromatography.57 In addition, from the initial list of 43 miRNAs, we chose to validate only 10 based on their P-FDR values; it is possible that we may have excluded some relevant miRNAs involved in Cr metabolism. We did adjust for potential confounding variables in our analysis. Moreover, the relatively small sample size may have limited our ability to detect significant effects, and larger studies are needed to confirm our findings.
Conclusion
Several circulating miRNAs are considered emerging blood biomarkers for many diseases because they are stable molecules, tissue specific, evolutionarily conserved and easy to detect. Changes in miR-451 and miR-486-3p expression, in association with urinary Cr, and their relation with A1c and BP, respectively, are helpful for identifying mechanisms linking Cr with metabolic diseases and CVDs.
Acknowledgments
We thank the Occupational Medicine medical residents for their help in examining and recruiting the study subjects. We are grateful to the nurses of the ‘Medicina del Lavoro' Unit, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Enrico Radice for database development, as well as the volunteers who participated in the study. This work was supported by the EU Programme ‘Ideas' (ERC-2011-StG 282413 to Dr V Bollati).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on European Journal of Clinical Nutrition website (http://www.nature.com/ejcn)
Supplementary Material
References
- Clodfelder BJ, Emamaullee J, Hepburn DD, Chakov NE, Nettles HS, Vincent JB. The trail of chromium(III) in vivo from the blood to the urine: the roles of transferrin and chromodulin. J Biol Inorg Chem 2001; 6: 608–617. [DOI] [PubMed] [Google Scholar]
- Brown RO, Forloines-Lynn S, Cross RE, Heizer WD. Chromium deficiency after long-term total parenteral nutrition. Dig Dis Sci 1986; 31: 661–664. [DOI] [PubMed] [Google Scholar]
- Glinsmann WH, Mertz W. Effect of trivalent chromium on glucose tolerance. Metab Clin Exp 1966; 15: 510–520. [DOI] [PubMed] [Google Scholar]
- Mertz W. Interaction of chromium with insulin: a progress report. Nutr Rev 1998; 56: 174–177. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Bryden NA, Polansky MM. Dietary chromium intake. Freely chosen diets, institutional diet, and individual foods. Biol Trace Elem Res 1992; 32: 117–121. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine FaNB Dietary Reference Intakes for Vitamin A, Vitanmin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academy Press: Washington, DC, 2001. [PubMed] [Google Scholar]
- Hepburn DD, Vincent JB. Tissue and subcellular distribution of chromium picolinate with time after entering the bloodstream. J Inorg Biochem 2003; 94: 86–93. [DOI] [PubMed] [Google Scholar]
- Lukaski HC. Chromium as a supplement. Annu Rev Nutr 1999; 19: 279–302. [DOI] [PubMed] [Google Scholar]
- Morris BW, Kemp GJ, Hardisty CA. Plasma chromium and chromium excretion in diabetes. Clin Chem 1985; 31: 334–335. [PubMed] [Google Scholar]
- Bahijri SM, Alissa EM. Increased insulin resistance is associated with increased urinary excretion of chromium in non-diabetic, normotensive Saudi adults. J Clin Biochem Nutr 2011; 49: 164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA. Chromium and insulin resistance. Nutr Res Rev 2003; 16: 267–275. [DOI] [PubMed] [Google Scholar]
- Alissa EM, Bahjri SM, Ahmed WH, Al-Ama N, Ferns GA. Chromium status and glucose tolerance in Saudi men with and without coronary artery disease. Biol Trace Elem Res 2009; 131: 215–228. [DOI] [PubMed] [Google Scholar]
- Guallar E, Jimenez FJ, van 't Veer P, Bode P, Riemersma RA, Gomez-Aracena J et al. Low toenail chromium concentration and increased risk of nonfatal myocardial infarction. Am J Epidemiol 2005; 162: 157–164. [DOI] [PubMed] [Google Scholar]
- Ray PD, Yosim A, Fry RC. Incorporating epigenetic data into the risk assessment process for the toxic metals arsenic, cadmium, chromium, lead, and mercury: strategies and challenges. Front Genet 2014; 5: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weake VM, Workman JL. Inducible gene expression: diverse regulatory mechanisms. Nat Rev Genet 2010; 11: 426–437. [DOI] [PubMed] [Google Scholar]
- Singh SK, Pal Bhadra M, Girschick HJ, Bhadra U. MicroRNAs—micro in size but macro in function. FEBS J 2008; 275: 4929–4944. [DOI] [PubMed] [Google Scholar]
- Kadamkode V, Banerjee G. Micro RNA: an epigenetic regulator of type 2 diabetes. Microrna 2014; 3: 86–97. [DOI] [PubMed] [Google Scholar]
- Honardoost M, Sarookhani MR, Arefian E, Soleimani M. Insulin resistance associated genes and miRNAs. Appl Biochem Biotechnol 2014; 174: 63–80. [DOI] [PubMed] [Google Scholar]
- Vickers KC, Rye KA, Tabet F. MicroRNAs in the onset and development of cardiovascular disease. Clin Sci (Lond) 2014; 126: 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colagiuri S. Diabesity: therapeutic options. Diabetes Obes Metab 2010; 12: 463–473. [DOI] [PubMed] [Google Scholar]
- Manrique C, Sowers JR. Insulin resistance and skeletal muscle vasculature: significance, assessment and therapeutic modulators. Cardiorenal Med 2014; 4: 244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM. Metabolic complications of obesity. Endocrine 2000; 13: 155–165. [DOI] [PubMed] [Google Scholar]
- Sowers JR. Obesity as a cardiovascular risk factor. Am J Med 2003; 115: 37S–41S. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008; 31: 1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoogen PC, Feskens EJ, Nagelkerke NJ, Menotti A, Nissinen A, Kromhout D. The relation between blood pressure and mortality due to coronary heart disease among men in different parts of the world. Seven Countries Study Research Group. N Engl J Med 2000; 342: 1–8. [DOI] [PubMed] [Google Scholar]
- Bollati V, Iodice S, Favero C, Angelici L, Albetti B, Cacace R et al. Susceptibility to particle health effects, miRNA and exosomes: rationale and study protocol of the SPHERE study. BMC Public Health 2014; 14: 1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- ATSDR. Case Studies in Environmental Medicine (CSEM) Chromium Toxicity. 2008:9-12.
- Sarmiento-Gonzalez A, Marchante-Gayon JM, Tejerina-Lobo JM, Paz-Jimenez J, Sanz-Medel A. High-resolution ICP-MS determination of Ti, V, Cr, Co, Ni, and Mo in human blood and urine of patients implanted with a hip or knee prosthesis. Analytical Bioanalytical Chem 2008; 391: 2583–2589. [DOI] [PubMed] [Google Scholar]
- Hummel M, Standl E, Schnell O. Chromium in metabolic and cardiovascular disease. Hormone Metab Res 2007; 39: 743–751. [DOI] [PubMed] [Google Scholar]
- Basaki M, Saeb M, Nazifi S, Shamsaei HA. Zinc, copper, iron, and chromium concentrations in young patients with type 2 diabetes mellitus. Biol Trace Elem Res 2012; 148: 161–164. [DOI] [PubMed] [Google Scholar]
- Ding W, Chai Z, Duan P, Feng W, Qian Q. Serum and urine chromium concentrations in elderly diabetics. Biol Trace Elem Res 1998; 63: 231–237. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Kozlovsky AS. Chromium intake, absorption and excretion of subjects consuming self-selected diets. Am J Clin Nutr 1985; 41: 1177–1183. [DOI] [PubMed] [Google Scholar]
- Afridi HI, Kazi TG, Kazi N, Kandhro GA, Baig JA, Shah AQ et al. Evaluation of toxic elements in scalp hair samples of myocardial infarction patients at different stages as related to controls. Biol Trace Elem Res 2010; 134: 1–12. [DOI] [PubMed] [Google Scholar]
- Arner P, Kulyte A. MicroRNA regulatory networks in human adipose tissue and obesity. Nat Rev Endocrinol 2015; 11: 276–288. [DOI] [PubMed] [Google Scholar]
- Soliman A, DeSanctis V, Yassin M, Elalaily R, Eldarsy NE. Continuous glucose monitoring system and new era of early diagnosis of diabetes in high risk groups. Indian J Endocrinol Metab 2014; 18: 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Luo X, Ding S, Chen J, Chen T, Chen X et al. MicroRNA-451 regulates p38 MAPK signaling by targeting of Ywhaz and suppresses the mesangial hypertrophy in early diabetic nephropathy. FEBS Lett 2012; 586: 20–26. [DOI] [PubMed] [Google Scholar]
- Zheng X, Chopp M, Lu Y, Buller B, Jiang F. MiR-15b and miR-152 reduce glioma cell invasion and angiogenesis via NRP-2 and MMP-3. Cancer Lett 2013; 329: 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Zhang J, Xu N, Han G, Geng Q, Song J et al. Signature of circulating microRNAs as potential biomarkers in vulnerable coronary artery disease. PloS One 2013; 8: e80738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Yang D, Xie P, Ren G, Sun G, Zeng X et al. MiR-106b and MiR-15b modulate apoptosis and angiogenesis in myocardial infarction. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 2012; 29: 851–862. [DOI] [PubMed] [Google Scholar]
- Bostjancic E, Zidar N, Glavac D. MicroRNA microarray expression profiling in human myocardial infarction. Dis Markers 2009; 27: 255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CT, Ng EK, Chow PC, Kwong A, Cheung YF. Circulating microRNA expression profile and systemic right ventricular function in adults after atrial switch operation for complete transposition of the great arteries. BMC Cardiovasc Disorders 2013; 13: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small EM, O'Rourke JR, Moresi V, Sutherland LB, McAnally J, Gerard RD et al. Regulation of PI3-kinase/Akt signaling by muscle-enriched microRNA-486. Proc Natl Acad Sci USA 2010; 107: 4218–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass DJ. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr Topics Microbiol Immunol 2010; 346: 267–278. [DOI] [PubMed] [Google Scholar]
- Larsson CA, Daka B, Gullberg B, Rastam L, Lindblad U. Clusters of AMI risk factors and their association with left ventricular hypertrophy: a population-based study within the Skaraborg Project, Sweden. Int J Cardiol 2013; 168: 5416–5421. [DOI] [PubMed] [Google Scholar]
- Hsu A, Chen SJ, Chang YS, Chen HC, Chu PH. Systemic approach to identify serum microRNAs as potential biomarkers for acute myocardial infarction. BioMed Res Int 2014; 2014: 418628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Zhang C. MicroRNA-21 in cardiovascular disease. J Cardiovas Transl Res 2010; 3: 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang YY, Fang NN, Wang F, Wang H, Wang ZH, Tang MX et al. MicroRNA-21, induced by high glucose, modulates macrophage apoptosis via programmed cell death 4. Mol Med Rep 2015; 12: 463–469. [DOI] [PubMed] [Google Scholar]
- Xu J, Wong C. A computational screen for mouse signaling pathways targeted by microRNA clusters. RNA 2008; 14: 1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panguluri SK, Tur J, Chapalamadugu KC, Katnik C, Cuevas J, Tipparaju SM. MicroRNA-301a mediated regulation of Kv4.2 in diabetes: identification of key modulators. PloS One 2013; 8: e60545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou L, Wang S, Sui X, Meng X, Shen T, Huang X et al. MiR-301a mediates the effect of IL-6 on the AKT/GSK pathway and hepatic glycogenesis by regulating PTEN expression. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 2015; 35: 1413–1424. [DOI] [PubMed] [Google Scholar]
- Kirschner MB, Kao SC, Edelman JJ, Armstrong NJ, Vallely MP, van Zandwijk N et al. Haemolysis during sample preparation alters microRNA content of plasma. PloS One 2011; 6: e24145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay IM, Arden KE, Nitsche A. Real-time PCR in virology. Nucleic Acids Res 2002; 30: 1292–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meder B, Backes C, Haas J, Leidinger P, Stahler C, Grossmann T et al. Influence of the confounding factors age and sex on microRNA profiles from peripheral blood. Clin Chem 2014; 60: 1200–1208. [DOI] [PubMed] [Google Scholar]
- Aprea MC, Scapellato ML, Bartolucci GB, Apostoli P. [Environmental and biological limit values and reference values]. Giornale Italiano di Medicina del Lavoro ed Ergonomia 2011; 33 (3 Suppl), 430–433. [PubMed] [Google Scholar]
- Tomlinson MJ, Lin L, Caruso JA. Plasma mass spectrometry as a detector for chemical speciation studies. Analyst 1995; 120: 583–589. [DOI] [PubMed] [Google Scholar]
- Ponce de Leon CA, Montes-Bayon M, Caruso JA. Elemental speciation by chromatographic separation with inductively coupled plasma mass spectrometry detection. J Chromatogr A 2002; 974: 1–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.