Abstract
Intellectual disability (ID) is a common neurodevelopmental disorder exhibiting extreme genetic heterogeneity, and more than 500 genes have been implicated in Mendelian forms of ID. We performed exome sequencing in a large family affected by an autosomal-dominant form of mild syndromic ID with ptosis, growth retardation, and hypotonia, and we identified an inherited 2 bp deletion causing a frameshift in BRPF1 (c.1052_1053del) in five affected family members. BRPF1 encodes a protein modifier of two histone acetyltransferases associated with ID: KAT6A (also known as MOZ or MYST3) and KAT6B (MORF or MYST4). The mRNA transcript was not significantly reduced in affected fibroblasts and most likely produces a truncated protein (p.Val351Glyfs∗8). The protein variant shows an aberrant cellular location, loss of certain protein interactions, and decreased histone H3K23 acetylation. We identified BRPF1 deletions or point mutations in six additional individuals with a similar phenotype. Deletions of the 3p25 region, containing BRPF1 and SETD5, cause a defined ID syndrome where most of the clinical features are attributed to SETD5 deficiency. We compared the clinical symptoms of individuals carrying mutations or small deletions of BRPF1 alone or SETD5 alone with those of individuals with deletions encompassing both BRPF1 and SETD5. We conclude that both genes contribute to the phenotypic severity of 3p25 deletion syndrome but that some specific features, such as ptosis and blepharophimosis, are mostly driven by BRPF1 haploinsufficiency.
Main Text
Intellectual disability (ID) characterizes a group of neurodevelopmental disorders that constitute a major public health, social, and educational problem because of the cumulated frequency and the heavy burden for affected individuals and families. ID is defined by significant limitations in both intellectual functioning and adaptive behavior associated with an intellectual quotient (IQ) below 70, and it affects about 2% of children or young adults. Moderate to severe forms of ID can be caused by chromosomal anomalies, including pathogenic deletions or duplications or single-gene defects with recessive, X-linked, or autosomal-dominant inheritance. More than 500 genes have been implicated in Mendelian forms of ID. Mutations can cause non-syndromic or syndromic ID with other associated clinical features. Additionally, a number of recurrent microdeletions also cause ID.
Terminal 3p and interstitial deletions of the 3p25–p26 region cause 3p deletion syndrome (MIM: 613792), characterized by mild to severe ID, growth retardation, microcephaly, and dysmorphic features, notably ptosis.1 The terminal or interstitial deletions range from large deletions of several megabases to smaller deletions of fewer than 500 kb and do not always overlap, rendering it difficult to identify the genes associated with the phenotype. An increasing number of individuals harboring deletions of this region has advanced the understanding of the critical genes for this 3p25 region. Several individuals with a small 3p25.3 distal deletion present with a non-3p phenotype with ID, epilepsy, poor speech, ataxia, and stereotypic hand movements, and the two genes encoding GABA transporters, SLC6A1 (MIM: 137165) and SLC6A11 (MIM: 607952), were suspected to be involved.2 For the more proximal deletions in 3p25, the most promising gene appears to be SETD5 (MIM: 615743), encoding a putative histone methyltransferase. Indeed, variations in SETD5 in individuals with ID and clinical features consistent with the 3p deletion syndrome have recently been reported.3, 4, 5 However, some clinical features recurrent in 3p25 deletion syndrome, such as ptosis and blepharophimosis, are not consistently observed in individuals with SETD5 mutations.
Here, we investigated the genetic origin of an autosomal-dominant syndromic form of mild ID associated with other features such as growth retardation, ptosis, and relative microcephaly, present in six affected relatives over three generations (Figure 1A). Ethical approval was obtained from the local ethics committees. The proband, III-2, was born at term with intrauterine growth restriction: weight 2,900 g (fifth percentile), height 46 cm (third percentile), and head circumference 32.5 cm (third percentile). Bilateral clubfeet were diagnosed during the pregnancy, and a karyotype was performed but was negative. At birth, edema of the back of the feet was noticed. He was hospitalized at the age of 1 month for the association of hypotonia and eating disorders without weight gain. The clinical examination found dysmorphic features with left ptosis, bilateral epicanthus, anteverted nostrils, a round face, a long philtrum, small and round ears, and unilateral cryptorchidism (Figure 3). Brachymetacarpia and clinodactyly of the toes were also noticed. Echocardiography, renal ultrasound, and cerebral echography found no anomaly. The cerebral computed tomography scan and hearing were normal. Gastroesophageal reflux was diagnosed. His development was significant for growth restriction and development of psychomotor delay. At 4 months old, the proband weighed 4,950 g (−1.5 SDs) and had a length of 54 cm (−3 SDs) and a head circumference of 39.5 cm (−1.5 SDs). At 4 years old, he weighed 14 kg (−1 SD) and had a length of 94 cm (−2 SDs) and a head circumference of 48.5 cm (−2 SDs). The boy sat at 16 months and walked at 30 months of age. He also presented with delayed language, and toilet training was acquired at 4 years of age. He had surgery for his ptosis and for cryptorchidism. His older brother (III-1) presented with no ID, growth disorder, or facial dysmorphism. However, his mother (II-2) presented with mild ID (permitting professional integration), short stature (150 cm), bilateral ptosis, facial dysmorphism similar to that of her son, and brachymetacarpia. Familial history revealed that her mother (deceased) and two of her sisters presented with the same phenotype. The phenotype is more severe for sister II-5, who had surgery twice for her ptosis with limited results and has had limited employment (Figure 3). She also presented with hypothyroidism. The other sister (II-3) also had surgery twice for her ptosis with limited results (Figure 3). She was 153 cm tall. Her daughter (III-4) presented with bilateral ptosis and mild ID with learning difficulties and concentration problems. Secondarily, the mother (II-2) had a new pregnancy: fetal echography showed a suspected anomaly of foot positioning, indicating possible clubfeet. At birth, the baby (III-3) had normal growth parameters: he weighed 3,560 g and had a length of 48 cm and a head circumference of 35 cm. He presented with pes varus, edema of the back of the feet, and the same facial dysmorphism as that of his brother. Progressively, the child presented with growth retardation, relative microcephaly, and developmental delay. At 19 months old, he could not walk. He weighed 8.2 kg (−3 SDs) and had a length of 75 cm (−2 SDs) and a head circumference of 45 cm (−2.5 SDs). His DNA was not available for testing. The child III-4, a cousin of the index individual, was born at term with short stature (47 cm), a normal weight (3,050 g), and a normal head circumference (33 cm). Bilateral ptosis was rapidly diagnosed and surgically repaired. Her motor development was within acceptable limits, given that she could sit at 8 months and walked at 18 months. Later, she presented with delayed language, difficulties at school, and behavioral disorders. Echocardiography, electroencephalogram, cerebral MRI, and a hearing test were normal. Unlike that of her cousins, her growth was normal: at 5.5 years, she weighed 22 kg and had a length of 114 cm and a head circumference of 50 cm. On clinical examination, the child presented with the same familial dysmorphism. Since then, the parents have had another child, who is in good health without developmental delay or facial dysmorphism.
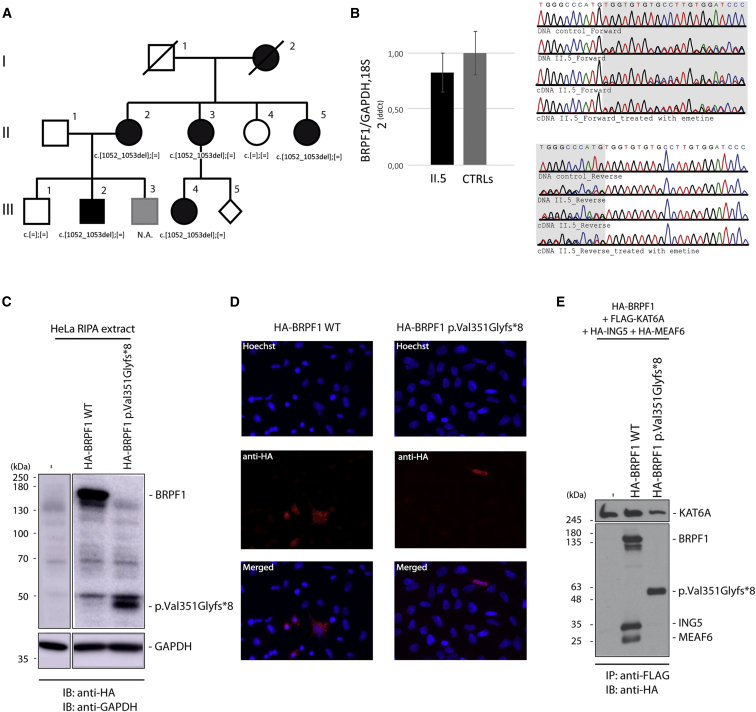
Figure 1.
Identification of a Co-segregating 2 bp Deletion, c.1052_1053del, in BRPF1 in a Family with Three Generations Affected by a Form of Mild ID Associated with Ptosis
(A) Pedigree of family A, which has three affected generations.
(B) The mutation partially escapes NMD. Quantitative real-time PCR was performed on RNA extracted (three extractions per individual) from fibroblasts of individual II-5 and three unrelated control individuals. The expression of BRPF1 in relation to the average of two reference genes, GAPDH and 18S, was calculated by the 2−ΔΔCt method. A t test was performed and showed no significant difference in the BRPF1 mRNA level (error bars indicate the SD of three independent experiments). Sequences of blood DNA and fibroblast cDNA (treated or not with the NMD-blocker emetine) from individual II.5 are shown on the right.
(C) Expression of BRPF1 in HeLa cells. HeLa cells transfected with HA-tagged wild-type or p.Val351Glyfs∗8 BRPF1 cDNA. Cells were harvested 36 hr after transfection. BRPF1 expression was analyzed by SDS-PAGE, and immunoblotting was performed with anti-HA antibody.
(D) HeLa cells were transfected with HA-tagged wild-type or p.Val351Glyfs∗8 BRPF1 cDNA. BRPF1 localization was visualized by immunofluorescence with an anti-HA antibody. Nuclei were colored in blue by Hoechst staining.
(E) HA-tagged wild-type or mutant BRPF1 was transfected along with expression plasmids for FLAG-tagged KAT6A, HA-tagged ING5, and HA-tagged MEAF6 into HEK293 cells. HAT complexes were immunoprecipitated from protein extracts with anti-FLAG antibody to pull down KAT6A, and products of the complex were revealed by western blot using anti-HA antibody.
Figure 3.
Facial Characteristics of the Individuals with BRPF1 Mutations
Pictures of individuals with BRPF1 point mutations and deletions. Common features include a roundish face, blepharophimosis and ptosis, downslanted palpebral fissures, temporal narrowing, and a downturned mouth. Ethical approval was obtained from the local ethics committees. For all individuals included in this figure, families also gave consent for publication of the images.
The most severely affected individual (III-2) underwent multiple genetic tests before we decided to perform whole-exome sequencing (WES). In addition to karyotype, array comparative genomic hybridization, and fragile-X testing, many tests have been conducted, including evaluation of 22q11.2 (MIM: 611867) and 22q13.3 (MIM: 606232) deletion syndromes (by fluorescence in situ hybridization), as well as Prader-Willi (MIM: 176270) (15q11.2–q13 DNA methylation), DM1 myotonic dystrophy (MIM: 160900) (DMPK [MIM: 605377] expansion), Aarskog (MIM: 305400) (FGD1 [MIM: 300546] sequencing), Noonan (MIM: 163950) (PTPN11 [MIM: 176876], SOS1 [MIM: 182530], RAF1 [MIM: 164760], SHOC2 [MIM: 602775] sequencing), and Saethre Chotzen (MIM: 101400) (TWIST1 [MIM: 601622] and FGFR3 [MIM: 134934] sequencing) syndromes.
Given that no pathogenic genetic event could be identified by these genetic investigations, we performed WES for individual III-2, his maternal cousin (III-4), and his maternal aunt (II-5). Libraries and captures from genomic blood DNA were done with the SureSelect XT Human All Exon V5 Kit (Agilent Technologies), and sequencing was performed on a 100 bp paired-end run on the HiSeq 2500 sequencer (Illumina). Reads were aligned and variants were called and annotated as previously described.6, 7 To identify a variant shared by the three affected individuals, we used the family barcode given by the VaRank ranking program.6 Then, we filtered out the frequent mutations by using public databases and a large cohort of ID-affected individuals as previously described.7 Applying these criteria, we identified four candidate variants: one loss-of-function (LoF) and three missense variants in the heterozygous state in all three affected members. The three missense variants, c.650G>A (p.Arg217His) (GenBank: NM_080668.3) in CDCA5 (MIM: 609374), c.143C>T (p.Ser48Leu) (GenBank: NM_005199) in CHRNG (MIM: 100730), and c.1279C>T (p.Pro427Ser) (GenBank: NM_198517) in TBC1D10C (MIM: 610831), were unlikely to be considered pathogenic for the syndromic ID phenotype (Table S1). The unique LoF variant identified was a 2 nt deletion, c.1052_1053del (GenBank: NM_001003694.1) in BRPF1 (MIM: 602410), which encodes bromodomain and PHD finger-containing protein 1. This deletion occurred in a well-conserved region, according to PhastCons and USCS Multiz alignment of 100 vertebrates and orthologs (from Ensembl), and was predicted to cause a frameshift leading to a premature stop codon eight amino acids downstream. Sanger sequencing in available family members confirmed that affected individuals carried deletion c.1052_1053del (Figure 1A), which has been added to ClinVar.
To evaluate whether BRPF1 is tolerant of protein-truncating variants, we looked in the Exome Aggregation Consortium (ExAC) Browser, which contains 60,706 exomes from individuals unaffected by severe pediatric diseases. Here, we found five variants potentially leading to LoF in BRPF1: one nonsense and four splice variants. The nonsense variant is reported in one individual but present in only 21% of reads, suggesting a mosaic status (Table S2). The four splice variants are present in the heterozygous state. One of them is present in several (six) individuals but affects a known processed non-coding transcript (Ensembl: ENST00000469066.1). The three remaining variants are present in one individual each and affect canonical splice sites of exon 8 (324 nucleotides; might create an in-frame deletion of 108 amino acids), exon 9 (285 nucleotides; might create an in-frame deletion of 95 amino acids), or exon 11 (137 nucleotides; might create a frameshift). On the basis of gene length, 36 LoF variants in the BRPF1 coding region could be expected for this gene; however, only five have been reported.8 These data suggest that BRPF1 is an extremely LoF-intolerant gene (probability of LoF intolerance = 1).
To investigate whether the mutant BRPF1 transcript undergoes nonsense-mediated decay (NMD), we obtained dermal fibroblasts from skin biopsy of individual II-5 and three unrelated control individuals. They were expanded as previously described.9 Fibroblast RNA was extracted according to the TRI Reagent protocol (Molecular Research Center), treated with DNaseI (Roche Diagnostic), and reverse transcribed into cDNA with random hexamers and SuperScript II Reverse Transcriptase according to the manufacturer’s recommendation. PCR was performed with specific primers (BRPF1 5′-tgccagaacagcaatgtcatcctc-3′ [forward] and 5′-cgcacaggctccatcttcatgtaa-3′ [reverse]). qPCR were performed in triplicate, and the BRPF1 mRNA level was quantified by the 2−ΔΔCt method with an average of two reference genes, GAPDH and 18S. A parametric Student’s t test was performed to compare the relative BRPF1 expression and revealed a slight but not significant decrease in BRPF1 mRNA levels in individual II-5, suggesting that the mutated transcript partially escapes NMD. cDNA sequencing (GATC) from II-5 revealed the presence of both the wild-type and the mutant transcripts (Figure 1B), but peak heights were lower for the latter. A similar peak height could be restored when fibroblasts from individual II.5 were treated with emetine (100 μg/mL) to block NMD, confirming that the c.1052_1053del BRPF1 transcript undergoes partial NMD.
The deletion leads to a frameshift with the appearance of a premature stop codon: p.Val351Glyfs∗8. The truncated protein is predicted to contain 358 amino acids instead of 1,220 and lacks several essential functional domains, including the second PHD finger domain, the bromodomain, and the PWWP domains, which are involved in histone recognition and binding (Figure 2D). We were not able to detect wild-type BRPF1 by western blot in fibroblasts from control individuals with the anti-BRPF1 antibody (Peregrin N-16, sc-103110, Santa Cruz Biotechnology; PCRB-BRPF1-2A12, DSHB, University of Iowa). To evaluate the protein, we generated N-terminal HA-tagged wild-type and mutant BRPF1. HeLa cells were transfected (Lipofectamine 2000, Invitrogen), and total proteins were extracted after 36 hr. Western blot using anti-HA antibody revealed an ∼50 kDA truncated BRPF1 that accumulated at a lower level than the wild-type, suggesting reduced stability (Figure 1C). Using fluorescence microscopy, we observed that wild-type BRPF1 localized to the cytoplasm with the formation of cytoplasmic puncta, as previously reported (Figure 1D).12 By contrast, the mutant BRPF1 signal was weaker, and the truncated protein appeared to be more uniformly distributed in both the cytoplasm and nucleus.
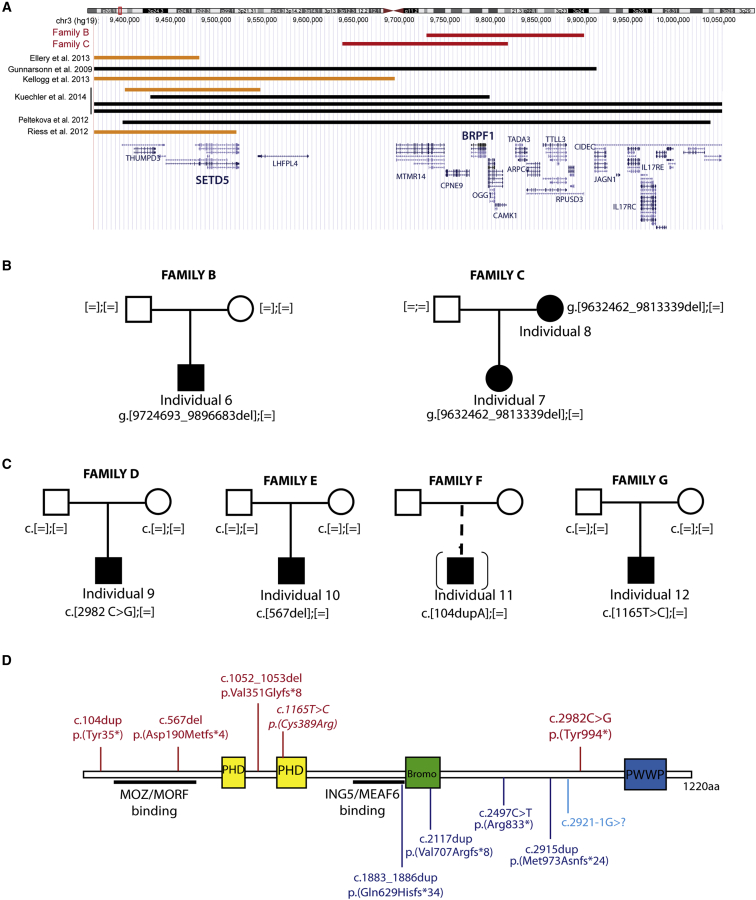
Figure 2.
Mutations in BRPF1 and Deletions in the 3p25 Region
(A) Overview of 3p25 deletions reported in the literature and in DECIPHER (from the UCSC Genome Browser). Black lines indicate a deletion encompassing both SETD5 and BRPF1; orange lines represent the deletion containing SETD5 but not BRPF1; and red lines indicate the two deletions including BRPF1 but not SETD5 reported in DECIPHER.
(B) Pedigree of the two additional families affected by 3p25 deletions encompassing BRPF1 but not SETD5.
(C) Pedigree of four additional families with the BRPF1 pathogenic variants shown in (D).
(D) Top: schematic representation of BRPF1 and localization of the five different LoF and missense mutations. Bottom: the four de novo LoF variants described by the DDD project in individuals with neurodevelopmental conditions10 (in dark blue) and the LoF variant identified in one boy with schizophrenia and mild ID.11 Domains are colored as follows: yellow, PHD finger (PHD) domains; green, bromodomain (Bromo), involved in the recognition of acetylated lysine residues; blue, PWWP nucleosome-binding domain. Regions involved in binding with MOZ, MORF, ING5, and EAF6 are underlined.12
BRPF1 is a chromatin regulator that promotes histone acetylation by bringing different histone acetyltransferases (HATs) of the MYST protein family (HBO1, KAT6A [also known as MOZ], and KAT6B [MORF]) into a complex with other regulator proteins, such as ING5 and MEAF6.12, 13 The truncated protein, p.Val351Glyfs∗8, still contains the KAT6B and KAT6A interaction domains between amino acids 59 and 222.12 A similarly truncated form of BRPF1 (ΔN-term1, truncated after amino acid 354) was still able to bind KAT6A.13 However, the ING5-MEAF6 interaction is mediated by amino acids 540–640,12 suggesting that p.Val351Glyfs∗8 BRPF1 would not be able to bring these two proteins into the HAT complex. To test this, we transfected HA-tagged wild-type and p.Val351Glyfs∗8 BRPF1, along with expression plasmids for FLAG-tagged KAT6A, HA-tagged ING5, and HA-tagged MEAF6, into HEK293 cells. The HAT complexes were immunoprecipitated from protein extracts with anti-FLAG antibody to pull down KAT6A, and products were analyzed by western blot using anti-HA antibody (Figure 1E). We observed that both wild-type and p.Val351Glyfs∗8 BRPF1 were able to bind KAT6A. Whereas the wild-type was able to bind ING5 and MEAF6, the p.Val351Glyfs∗8 variant failed to do so.
To investigate the effect of the BRPF1 mutation on the global acetylation level of histone H3, we extracted histones from the fibroblasts of individual II-5 and three unrelated healthy control individuals. We used 2 μg of histone proteins to detect global histone H3 acetylation with the EpiQuik Global Histone H3 Acetylation Assay Kits (Epigentek) (Figure S1A). No significant difference in H3 acetylation levels was detected. To dissect more specifically the acetylation occurring at the different lysines known to be acetylated by the KAT6A-KAT6B HAT complex,14, 15 we performed western blot analysis on histone extractions with specific anti-H3K9 (ab4441, Abcam), anti-H3K14 (in house), and anti-H3K23 (9674, Cell Signaling) antibodies, and we normalized the intensities obtained to the intensity of global histone H3 (catalog no. 06755, lot 31949, Upstate). No change in acetylation levels was observed for H3K9 or K14 (Figure S1B); however, compared with control individuals, individual II-5 showed a slight but non-significant decrease in the acetylation level of H3K23. Histone H3 acetylation levels were also analyzed in histone extracts obtained from HeLa cells cotransfected with constructs encoding KAT6A, ING5, and MEAF6 with or without wild-type or p.Val351Glyfs∗8 BRPF1. No difference was observed in the ability to stimulate K9 and K14 acetylation between wild-type and mutant BRPF1. However, unlike wild-type BRPF1, the p.Val351Glyfs∗8 variant failed to stimulate K23 acetylation of histone H3 (Figure S1C).
BRPF1-KAT6A-KAT6B complexes are involved in the development of the forebrain and other organs in mice, and complete knockout causes embryonic lethality with vascular defects and abnormal neural tube closure.16 Inactivation in mice and other animal models, including medaka fish, has demonstrated that BRPF1 acts through the regulation of Hox genes to effect skeletal development.13, 17 To determine whether BRPF1 also alters HOX expression in humans, we investigated the expression of human homologs of some Hox genes described as regulated by the murine BRPF1-KAT6A-KAT6B complex in individual II-5 fibroblasts. Results obtained for HOXA7 (MIM: 142950) and HOXC10 (MIM: 605560) were not interpretable as a result of variability in expression among control individuals (data not shown). However, low variability was observed in control individuals for the HOXD8 (MIM: 142985) mRNA level, and we observed that the level of HOXD8 mRNA was significantly higher in individual II-5 than in control individuals (Figure S2).
In order to confirm the association between BRPF1 and ID, we performed data exchange to retrieve additional individuals carrying BRPF1 mutations. We first queried DECIPHER to identify copy-number variants affecting BRPF1 and identified two individuals with 3p25 deletions including BRPF1 but not SETD5 (a gene previously associated with ID) (Figure 2A). Clinical details of these two individuals are compared to the clinical symptoms of the first family (Tables 1 and 2; Table S3). The first individual has a de novo 172 kb deletion encompassing BRPF1 and four other genes (family B individual 6; Figure 2B). The second has a 181 kb deletion including BRPF1 and eight other genes (family C individual 7; Figure 2B; Figure 3); this was inherited from her mildly affected mother (individual 8). Both individuals have mild ID, ptosis or blepharophimosis, and a roundish face, clinical features that overlap those of members of the large family. Next, we used the GeneMatcher exchange database to search for ID-affected individuals with BRPF1 mutations identified by WES analysis (where no other obvious candidate gene was present). We found three nonsense or frameshift variations—c.2982C>G (p. Tyr994∗), c.567delT (p.Asp190Metfs∗24), and c.104dupA (p.Tyr35∗)—and one de novo missense variant, c.1165T>C (p.Cys389Arg) (Figure 2C). Two of the nonsense mutations occurred de novo, and one was from unknown inheritance (in an adopted boy with a family history in his biological family; no DNA was available for testing). The missense variant affects a well-conserved amino acid located in the second PHD domain and is predicted to be pathogenic (by SIFT and PolyPhen-2). These four individuals presented with mild to moderate ID, hand and feet anomalies, and similar facial appearances with the presence of ptosis (Tables 1 and 2; Table S3; Figure 3). In total, all individuals with BRPF1 mutations or deletions have mild or moderate ID. Of the three individuals with moderate ID, two (individuals 10 and 11) carry the earliest truncating mutations, whose protein products would lack at least part of the interaction domain with KAT6A and KAT6B. This truncated protein product might increase the severity of the phenotype, but we cannot exclude other genetic or environmental modifiers in the variable expressivity of this disorder.
Table 1.
Clinical Features of Individuals Carrying BRPF1 Mutations in Families A and B
|
Family A |
Family B |
|||||
|---|---|---|---|---|---|---|
| Individual 1 (III.2) | Individual 2 (II.2) | Individual 3 (II.3) | Individual 4 (III.4) | Individual 5 (II.5) | Individual 6 | |
| Mutation (GenBank: NM_001003694.1)a | c.1052_1053del (p.Val351Glyfs∗8) | c.1052_1053del (p.Val351Glyfs∗8) | c.1052_1053del (p.Val351Glyfs∗8) | c.1052_1053del (p.Val351Glyfs∗8) | c.1052_1053del (p.Val351Glyfs∗8) | deletion of chr3: 9,724,693–9,896,683 |
| Mutation type | intragenic | intragenic | intragenic | intragenic | intragenic | NA, de novo |
| Sex | male | female | female | female | female | male |
| Age of examination | 5 years, 9 months | 32 years | 34 years | 6 years, 10 months | 30 years | 6 years, 6 months |
| Uneventful pregnancy | diagnosis of club feet | NA | NA | yes | NA | no (36.5 WoG) |
| Birth weight | <5th % | NA | NA | normal | NA | 3rd % |
| Birth length | <3rd % | NA | NA | <5th % | NA | NA |
| Birth OFC | <3rd % | NA | NA | <5th % | NA | NA |
| Neonatal hypotonia | yes | NA | NA | no | NA | no |
| Hypotonia | yes | NA | NA | yes | NA | no |
| Small stature | yes (104.5 cm; <3rd %) | yes (150 cm; <3rd %) | yes (153 cm; <3rd %) | no (122 cm) | yes (152 cm; <3rd %) | no (113 cm) |
| Low weight | yes (16 kg; <3rd %) | no (62 kg) | no (67 kg) | no (25 kg; >90th %) | NA | no (21 kg) |
| ID | mild | mild | mild | mild | mild | mild |
| Microcephaly | mild (50 cm; <10th %) | no (54.5 cm) | yes (53 cm; <3rd %) | no (50 cm) | mild (54 cm; <10th %) | mild (50.3 cm; <10th %) |
| Brain anomalies (MRI) | ACC (rostrum) | NA | NA | no | NA | NA |
| Seizures | no | no | no | no | no | no |
| Delay in walking | yes | NA | NA | yes | NA | no |
| Speech delay | yes | NA | NA | yes | NA | mild |
| Behavioral anomalies | no | no | no | hyperactivity | no | hyperactivity, shy, quiet |
| Vision or eye problems | strabismus, amblyopia | refraction problems | refraction problems | refraction problems | strabismus, amblyopia | NA |
| Ptosis and/or blepharophimosis | yes | yes | yes | yes | yes | yes (bilateral) |
| Hand anomalies | BM, BD | BM, BD | BM, BD | BM, BD | BM, BD | bilateral CD of fifth finger |
| Feet anomalies | clinodactyly, club feet | no | NA | no | no | syndactyly of the second and third toes |
Abbreviations are as follows: %, percentile; ACC, agenesis of corpus callosum; BD, brachydactyly; BM, brachymetacarpia; CD, camptodactyly; MRI, magnetic resonance imaging; NA, information not available; OFC, occipital frontal circumference; and WoG, weeks of gestation.
The mutation was absent from all of the available unaffected individuals in family A.
Table 2.
Clinical Features of Individuals Carrying BRPF1 Mutations or Deletions in Families C–G
|
Family C |
Family D |
Family E |
Family F |
Family G |
||
|---|---|---|---|---|---|---|
| Individual 7 | Individual 8 | Individual 9 | Individual 10 | Individual 11 | Individual 12 | |
| Mutation (GenBank: NM_001003694.1) | deletion of chr3: 9,632,462–9,813,339 | c.2982 C>G (p. Tyr994∗) | c.567delT (p.Asp190Metfs∗24) | c.104dupA (p.Tyr35∗) | c.1165T>C (p.Cys389Arg) | |
| Mutation type | NA, inherited from affected mother | intragenic, de novo | intragenic, de novo | intragenic, unknown | intragenic, de novo | |
| Sex | female | female | male | male | male | male |
| Age of examination | 3 years, 8 months | 37 years | 10 years | 3 years | 12 years | 3 years, 9 months |
| Uneventful pregnancy | no (30 WoG) | NA | caesarean (37 WoG) | 33 WoG | NA | yes |
| Birth weight | 2,070 g | NA | normal | normal | NA | normal |
| Birth length | 43 cm | NA | NA | 43.2 cm (normal) | NA | NA |
| Birth OFC | 30 cm | NA | NA | 28cm (<3rd %) | NA | NA |
| Neonatal hypotonia | yes | NA | yes | no | NA | yes |
| Hypotonia | yes | NA | yes | no | NA | yes |
| Small stature | no | NA | 141.5 cm | mild (91.4 cm; <10th %) | yes (<3rd %) | no |
| Low weight | no | NA | no (56.3 kg; >97th %) | no (17.7 kg; >90th %) | NA | no |
| ID | yes | mild | moderate | moderate | moderate | mild |
| Microcephaly | mild (<10th %) | NA | NA | mild (48 cm; <10th %) | yes (<3rd %) | mild (<10th %) |
| Brain anomalies (MRI) | NA | NA | yesa | NA | NA | no |
| Seizures | no | NA | yes | no | yes | no |
| Delay in walking | yes | yes | yes | yes | yes (mild) | yes |
| Speech delay | yes | NA | yes | no | yes (no words at 3 years) | yes (only few words) |
| Behavioral anomalies | impaired social interactions | shyness | NA | yes | hyperactivity, autism | very shy |
| Vision or eye problems | strabismus | NA | strabismus, refraction problems | NA | near sighted | strabismus |
| Ptosis and/or blepharophimosis | yes | NA | yes (bilateral) | yes (bilateral) | yes | yes |
| Hand anomalies | no | NA | CD (left second finger) | bilateral CD of fifth finger | bilateral CD of fifth finger | no |
| Feet anomalies | no | NA | long first toe | no | no | CD |
Abbreviations are as follows: %, percentile; CD, camptodactyly; MRI, magnetic resonance imaging; NA, information not available; OFC, occipital frontal circumference; and WoG, weeks of gestation.
Enlarged perivascular Virchow-Robin spaces.
De novo truncating variants in BRPF1 have also been recently reported in large studies: the Deciphering Developmental Disorders (DDD) study has reported four de novo LoF variations in BRPF1—c.1883_1886dup (p.Gln629Hisfs∗34), c.2117dup (p.Val707Argfs∗8), c.2497C>T (p.Arg833∗), and c.2915dup (p.Met973Asnfs∗24), identified in 4,293 UK individuals with neurodevelopmental disorders.10 A de novo LoF variant was also reported in BRPF1 in one male individual from a schizophrenia cohort.11
3p25 deletion syndrome is characterized by ID, growth retardation, microcephaly, hypotonia, and specific facial dysmorphism. The critical region contains BRPF1 and SETD5, among other genes. Previous work has established that disruption of SETD5 is involved in the cognitive phenotype of this 3p25 syndrome.3, 4, 5 The identification of LoF mutations and deletions of BRPF1 in individuals with ID led us to investigate the contribution of BRPF1 in the 3p25 syndrome. We performed a genotype-phenotype comparison by using those individuals with mutations affecting either BRPF1 (group 1) or SETD5 (group 2) only as well as those with a 3p25 deletion including both SETD5 and BRPF1 (group 3) (Table 3; Table S3). For individuals with BRPF1 disruptions, only one index individual per family was taken into account. Clinical information for individuals with SETD5 disruptions or 3p25 deletions of both BRPF1 and SETD5 was retrieved from the literature.4, 5, 18, 19 We observed that disruption of SETD5 or BRPF1 tends to lead to mild or moderate ID, whereas all of individuals with severe ID have disruptions of both SETD5 and BRPF1. However, the degree of severity was evaluated by different clinical geneticists and lacked IQ testing for the individuals available, which could be biased. We performed a Fischer’s exact test to compare clinical features between these groups. Although the majority of the individuals presented with delay in the acquisition of walking (86%, 83%, and 100% for groups 1, 2, and 3, respectively) and language (86%, 92%, and 100% for groups 1, 2, and 3, respectively), the severity is significantly increased in group 3. All individuals from group 3 acquired walking after 3 years of age (5/5 for group 3 versus 2/19 for groups 1 and 2, p value = 0.0005) and presently have no language (5/5 for group 3 versus 1/19 for groups 1 and 2, p value = 0.0001) (Table 3), suggesting that disruption of both BRPF1 and SETD5 contributes to the phenotype of 3p25 deletion syndrome. Interestingly, both genes encode proteins involved in histone modification and gene regulation, and they might have common targets. SETD5 encodes a methyltransferase involved in the methylation of histones H3 and H4, whereas BRPF1 binds methylated histone H3 and promotes its acetylation.
Table 3.
Dissection of SETD5 and BRPF1 Contributions to Clinical Features of 3p25 Deletion Syndrome
|
Group 1 (BRPF1 Only) |
Group 2 (SETD5 Only) |
Group 3 (Both SETD5 and BRPF1) |
||||
|---|---|---|---|---|---|---|
| Percentage | Number | Percentage | Number | Percentage | Number | |
| ID | 100% | 7/7 | 100% | 14/14 | 100% | 5/5 |
| Mild or moderate ID | 100% | 6/6 | 100% | 6/6 | 40% | 2/5 |
| Severe ID | 0% | 0/6 | 0% | 0/6 | 60% | 3/5a |
| General Characteristics | ||||||
| Uneventful pregnancy (born at term) | 33% | 2/6 | 64% | 9/14 | 40% | 2/5 |
| Low birth parameters | 33% | 2/6 | 8% | 1/13 | 0% | 0/4 |
| Small stature | 43% | 3/7b | 15% | 2/13 | 100% | 4/4a |
| Microcephaly or borderline small head size | 100% | 6/6b,c | 8% | 1/13 | 100% | 4/4 |
| Development | ||||||
| Walking delay | 86% | 6/7 | 83% | 10/12 | 100% | 5/5 |
| Severe walking delay (>3 years) | 0% | 0/7 | 17% | 2/12 | 100% | 5/5a,c |
| Speech delay | 86% | 6/7 | 92% | 12/13 | 100% | 5/5 |
| No speech | 0% | 0/7 | 8% | 1/12 | 100% | 5/5a,c |
| Neurological Features | ||||||
| Seizures | 29% | 2/7 | 21% | 3/14 | 80% | 4/5a |
| Hypotonia | 67% | 4/6 | 67% | 4/6 | 100% | 4/4 |
| Brain anomalies (MRI) | 67% | 2/3 | 0% | 0/4 | 25% | 1/4 |
| Behavioral anomalies | 71% | 5/7 | 77% | 10/13 | 25% | 1/4 |
| Others Features | ||||||
| Strabismus | 80% | 4/5b | 36% | 5/14 | 100% | 4/4 |
| Ptosis and/or blepharophimosis | 100% | 7/7b,c | 7% | 1/14 | 100% | 5/5a |
| Hand anomalies | 71% | 5/7 | 50% | 7/14 | 80% | 4/5 |
| Feet anomalies | 57% | 4/7 | 15% | 2/13 | 40% | 2/5 |
| Congenital heart defect | 0% | 0/7 | 15% | 2/13 | 40% | 2/5 |
Clinical information for individuals with SETD5 point mutations or deletions (group 2) and individuals with large 3p25 deletions encompassing SETD5 and BRPF1 was retrieved from the literature.4, 5, 18, 19 Clinical information for individuals with BRPF1 point mutations or small 3p25 deletions reported in this publication (group 1) was retrieved from physicians attending the families. For the sake of avoiding artifacts, one member per family was considered. A 2 × 2 contingency table was made for analyzing the presence of each clinical sign, and because of the small sample size, a two-tailed Fisher’s exact test was used to calculate the p value to highlight a statistically significant difference between groups.
Clinical feature more prevalent when both genes are deleted (group 3) than when only one gene is deleted (groups 1 and 2) (p value < 0.05).
Clinical feature significantly more associated with BRPF1 disruption, with or without SETD5 (group 1 and 3), than with SETD5 disruption only (group 2) (p value < 0.05).
Significant after Bonferroni correction for multiple testing (p value < 0.0017).
To investigate the contribution of BRPF1 disruption to particular clinical features of the 3p25 microdeletion syndrome, we compared all individuals with disruptions in BRPF1, with or without SETD5 disruptions (group 1 + 3), with those individuals with only SETD5 disruptions (group 2). A significant difference was observed between the two groups for the presence of microcephaly or borderline small head size (10/10 in group 1 + 3 versus 1/13 in group 2, p value < 0.0001) and unilateral or bilateral ptosis and/or blepharophimosis (12/12 in group 1 + 3 versus 1/14 in group 2, p value < 0.0001). These eye and/or eyelid anomalies were present in all individuals carrying a disruption of BRPF1. Other clinical features (small stature and strabismus) were enriched in individuals with BRPF1 disruptions; however, these differences were not significant after Bonferroni correction for multiple testing (threshold p value < 0.0017). Better delineating other clinical features driven by BRPF1 haploinsufficiency will require a larger cohort.
Recently, mutations in KAT6B (MIM: 605880) have been associated with syndromic ID, including Ohdo syndrome (MIM: 603736), genitopatellar syndrome (MIM: 606170), blepharophimosis-ptosis-epicanthus inversus syndrome, and even a Noonan-syndrome-like phenotype.20, 21, 22, 23 Mutations in KAT6A (MIM: 601408) are associated with ID with craniofacial dysmorphism, microcephaly or craniosynostosis, feeding difficulties, cardiac defects, and ocular anomalies (MIM: 616268).24, 25
Zebrafish and mouse models of Brpf1 and BRPF1 disruption, respectively, are reported in the literature.13, 26 Zebrafish mutants show craniofacial defects, with shifts in segmental identities of craniofacial arches, as a result of a progressive loss of anterior Hox gene expression, indicating that Brpf1 plays a role in patterning the vertebrate head by mediating the expression of Hox genes. Mice with homozygous Brpf1 deletion show embryonic lethality with different embryonic defects, including abnormal neural tube closure.16, 26The forebrain-specific deletion of Brpf1 results in early postnatal lethality and growth retardation. Viable mice show neocortical abnormalities, partial agenesis of the corpus callosum, and behavioral anomalies.27 Interestingly, the investigators observed an alteration in the expression of several transcription factors involved in developmental processes and upregulation of Hox gene expression. These data indicate that Brpf1 is involved in forebrain development and acts as both an activator and a repressor of gene expression.
Certain chromatin modifiers that are associated with ID when mutated in the germline are also associated with childhood cancer when mutated at the somatic level, for example, SETBP1 (MIM: 611060) and KMT2A (MIM: 159555). Several somatic mutations affecting different regions of BRPF1 have been reported in childhood leukemia28 and adult medulloblastoma.29
In conclusion, we report here that LoF point mutations and small deletions affecting BRPF1 are responsible for a syndromic form of ID associated with eye and/or eyelid phenotype, i.e., ptosis and/or blepharophimosis. BRPF1 encodes the third member of the HAT KAT6A-KAT6B complex, which is involved in ID when functionally impaired.
We have therefore shown that BRPF1, together with SETD5, contributes to the severity of the 3p25 deletion syndrome phenotype and is responsible for some specific clinical features, such as ptosis and blepharophimosis.
Acknowledgments
The authors thank the families for their participation in this study. The authors also thank Fondation Jerome Lejeune, Fondation Maladies Rares, and Association APLM for financial support. This study was also supported by grant ANR-10-LABX-0030-INRT, a French state fund managed by the Agence Nationale de la Recherche under the frame program Investissements d’Avenir ANR-10-IDEX-0002-02. The authors also thank all members of the Strasbourg Hospital molecular diagnostic lab, the Clinical Genetics Service of Prof. Hélène Dollfus, the Institut Génétique Biologie Moléculaire Cellulaire sequencing platform, and UMR_S 1112 (Bernard Jost, Stéphanie Le Gras, Mathieu Jung, Jean Muller, and Véronique Geoffroy) for their technical and bioinformatics support. We also thank Sylvain Daujat, Robert Schneider, Federica Evangelista, Tiago Baptista, and Lazlo Tora for histone H3 antibodies and technical advice; and Xiang-Jiao Yang for the cDNA of BRPF1, KAT6A, ING5, and MEAF6 expression plasmids and technical help. J.J. is an employee of GeneDx, and A. Pujol is a consultant for GeneDx.
Published: December 8, 2016
Footnotes
Supplemental Data include two figures and three tables and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2016.11.010.
Contributor Information
Jean-Louis Mandel, Email: jlmandel@igbmc.fr.
Amélie Piton, Email: piton@igbmc.fr.
Accession Numbers
The accession number for the data reported in this paper is ClinVar: SCV000328673.1.
Web Resources
ClinVar, http://www.ncbi.nlm.nih.gov/clinvar/
Decipher, https://decipher.sanger.ac.uk/
ExAC Browser, http://exac.broadinstitute.org/
GeneMatcher, https://genematcher.org/
Integrative Genomics Viewer (IGV), http://www.broadinstitute.org/igv/
Mutation Nomenclature, http://www.hgvs.org/mutnomen/recs.html
NHLBI Exome Sequencing Project (ESP) Exome Variant Server, http://evs.gs.washington.edu/EVS/
OMIM, http://www.omim/org/
UCSC Genome Browser, http://genome.ucsc.edu/
Supplemental Data
References
- 1.Narahara K., Kikkawa K., Murakami M., Hiramoto K., Namba H., Tsuji K., Yokoyama Y., Kimoto H. Loss of the 3p25.3 band is critical in the manifestation of del(3p) syndrome: karyotype-phenotype correlation in cases with deficiency of the distal portion of the short arm of chromosome 3. Am. J. Med. Genet. 1990;35:269–273. doi: 10.1002/ajmg.1320350225. [DOI] [PubMed] [Google Scholar]
- 2.Dikow N., Maas B., Karch S., Granzow M., Janssen J.W., Jauch A., Hinderhofer K., Sutter C., Schubert-Bast S., Anderlid B.M. 3p25.3 microdeletion of GABA transporters SLC6A1 and SLC6A11 results in intellectual disability, epilepsy and stereotypic behavior. Am. J. Med. Genet. A. 2014;164A:3061–3068. doi: 10.1002/ajmg.a.36761. [DOI] [PubMed] [Google Scholar]
- 3.Rauch A., Wieczorek D., Graf E., Wieland T., Endele S., Schwarzmayr T., Albrecht B., Bartholdi D., Beygo J., Di Donato N. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet. 2012;380:1674–1682. doi: 10.1016/S0140-6736(12)61480-9. [DOI] [PubMed] [Google Scholar]
- 4.Grozeva D., Carss K., Spasic-Boskovic O., Parker M.J., Archer H., Firth H.V., Park S.M., Canham N., Holder S.E., Wilson M., UK10K Consortium De novo loss-of-function mutations in SETD5, encoding a methyltransferase in a 3p25 microdeletion syndrome critical region, cause intellectual disability. Am. J. Hum. Genet. 2014;94:618–624. doi: 10.1016/j.ajhg.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuechler A., Zink A.M., Wieland T., Lüdecke H.J., Cremer K., Salviati L., Magini P., Najafi K., Zweier C., Czeschik J.C. Loss-of-function variants of SETD5 cause intellectual disability and the core phenotype of microdeletion 3p25.3 syndrome. Eur. J. Hum. Genet. 2015;23:753–760. doi: 10.1038/ejhg.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geoffroy V., Pizot C., Redin C., Piton A., Vasli N., Stoetzel C., Blavier A., Laporte J., Muller J. VaRank: a simple and powerful tool for ranking genetic variants. PeerJ. 2015;3:e796. doi: 10.7717/peerj.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redin C., Gérard B., Lauer J., Herenger Y., Muller J., Quartier A., Masurel-Paulet A., Willems M., Lesca G., El-Chehadeh S. Efficient strategy for the molecular diagnosis of intellectual disability using targeted high-throughput sequencing. J. Med. Genet. 2014;51:724–736. doi: 10.1136/jmedgenet-2014-102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowry W.E., Richter L., Yachechko R., Pyle A.D., Tchieu J., Sridharan R., Clark A.T., Plath K. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc. Natl. Acad. Sci. USA. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McRae J.F., Clayton S., Fitzgerald T.W., Kaplanis J., Prigmore E., Rajan D., Sifrim A., Aitken S., Akawi N., Alvi M. Prevalence, phenotype and architecture of developmental disorders caused by de novo mutation. bioRxiv. 2016 [Google Scholar]
- 11.Xu B., Roos J.L., Dexheimer P., Boone B., Plummer B., Levy S., Gogos J.A., Karayiorgou M. Exome sequencing supports a de novo mutational paradigm for schizophrenia. Nat. Genet. 2011;43:864–868. doi: 10.1038/ng.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ullah M., Pelletier N., Xiao L., Zhao S.P., Wang K., Degerny C., Tahmasebi S., Cayrou C., Doyon Y., Goh S.L. Molecular architecture of quartet MOZ/MORF histone acetyltransferase complexes. Mol. Cell. Biol. 2008;28:6828–6843. doi: 10.1128/MCB.01297-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laue K., Daujat S., Crump J.G., Plaster N., Roehl H.H., Kimmel C.B., Schneider R., Hammerschmidt M., Tübingen 2000 Screen Consortium The multidomain protein Brpf1 binds histones and is required for Hox gene expression and segmental identity. Development. 2008;135:1935–1946. doi: 10.1242/dev.017160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doyon Y., Cayrou C., Ullah M., Landry A.J., Côté V., Selleck W., Lane W.S., Tan S., Yang X.J., Côté J. ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation. Mol. Cell. 2006;21:51–64. doi: 10.1016/j.molcel.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Voss A.K., Collin C., Dixon M.P., Thomas T. Moz and retinoic acid coordinately regulate H3K9 acetylation, Hox gene expression, and segment identity. Dev. Cell. 2009;17:674–686. doi: 10.1016/j.devcel.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 16.You L., Yan K., Zou J., Zhao H., Bertos N.R., Park M., Wang E., Yang X.J. The chromatin regulator Brpf1 regulates embryo development and cell proliferation. J. Biol. Chem. 2015;290:11349–11364. doi: 10.1074/jbc.M115.643189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hibiya K., Katsumoto T., Kondo T., Kitabayashi I., Kudo A. Brpf1, a subunit of the MOZ histone acetyl transferase complex, maintains expression of anterior and posterior Hox genes for proper patterning of craniofacial and caudal skeletons. Dev. Biol. 2009;329:176–190. doi: 10.1016/j.ydbio.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Ellery P.M., Ellis R.J., Holder S.E. Interstitial 3p25 deletion in a patient with features of 3p deletion syndrome: further evidence for the role of SRGAP3 in mental retardation. Clin. Dysmorphol. 2014;23:29–31. doi: 10.1097/MCD.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 19.Pinto D., Delaby E., Merico D., Barbosa M., Merikangas A., Klei L., Thiruvahindrapuram B., Xu X., Ziman R., Wang Z. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am. J. Hum. Genet. 2014;94:677–694. doi: 10.1016/j.ajhg.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campeau P.M., Lu J.T., Dawson B.C., Fokkema I.F., Robertson S.P., Gibbs R.A., Lee B.H. The KAT6B-related disorders genitopatellar syndrome and Ohdo/SBBYS syndrome have distinct clinical features reflecting distinct molecular mechanisms. Hum. Mutat. 2012;33:1520–1525. doi: 10.1002/humu.22141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clayton-Smith J., O’Sullivan J., Daly S., Bhaskar S., Day R., Anderson B., Voss A.K., Thomas T., Biesecker L.G., Smith P. Whole-exome-sequencing identifies mutations in histone acetyltransferase gene KAT6B in individuals with the Say-Barber-Biesecker variant of Ohdo syndrome. Am. J. Hum. Genet. 2011;89:675–681. doi: 10.1016/j.ajhg.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraft M., Cirstea I.C., Voss A.K., Thomas T., Goehring I., Sheikh B.N., Gordon L., Scott H., Smyth G.K., Ahmadian M.R. Disruption of the histone acetyltransferase MYST4 leads to a Noonan syndrome-like phenotype and hyperactivated MAPK signaling in humans and mice. J. Clin. Invest. 2011;121:3479–3491. doi: 10.1172/JCI43428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu H.C., Geiger E.A., Medne L., Zackai E.H., Shaikh T.H. An individual with blepharophimosis-ptosis-epicanthus inversus syndrome (BPES) and additional features expands the phenotype associated with mutations in KAT6B. Am. J. Med. Genet. A. 2014;164A:950–957. doi: 10.1002/ajmg.a.36379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arboleda V.A., Lee H., Dorrani N., Zadeh N., Willis M., Macmurdo C.F., Manning M.A., Kwan A., Hudgins L., Barthelemy F., UCLA Clinical Genomics Center De novo nonsense mutations in KAT6A, a lysine acetyl-transferase gene, cause a syndrome including microcephaly and global developmental delay. Am. J. Hum. Genet. 2015;96:498–506. doi: 10.1016/j.ajhg.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tham E., Lindstrand A., Santani A., Malmgren H., Nesbitt A., Dubbs H.A., Zackai E.H., Parker M.J., Millan F., Rosenbaum K. Dominant mutations in KAT6A cause intellectual disability with recognizable syndromic features. Am. J. Hum. Genet. 2015;96:507–513. doi: 10.1016/j.ajhg.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.You L., Zou J., Zhao H., Bertos N.R., Park M., Wang E., Yang X.J. Deficiency of the chromatin regulator BRPF1 causes abnormal brain development. J. Biol. Chem. 2015;290:7114–7129. doi: 10.1074/jbc.M114.635250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.You L., Yan K., Zou J., Zhao H., Bertos N.R., Park M., Wang E., Yang X.J. The lysine acetyltransferase activator Brpf1 governs dentate gyrus development through neural stem cells and progenitors. PLoS Genet. 2015;11:e1005034. doi: 10.1371/journal.pgen.1005034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huether R., Dong L., Chen X., Wu G., Parker M., Wei L., Ma J., Edmonson M.N., Hedlund E.K., Rusch M.C. The landscape of somatic mutations in epigenetic regulators across 1,000 paediatric cancer genomes. Nat. Commun. 2014;5:3630. doi: 10.1038/ncomms4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kool M., Jones D.T., Jäger N., Northcott P.A., Pugh T.J., Hovestadt V., Piro R.M., Esparza L.A., Markant S.L., Remke M., ICGC PedBrain Tumor Project Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell. 2014;25:393–405. doi: 10.1016/j.ccr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.