Abstract
Neurodegenerative diseases affect not only the life quality of aging populations, but also their life spans. All forms of neurodegenerative diseases have a massive impact on the elderly. The major threat of these brain diseases includes progressive loss of memory, Alzheimer's disease (AD), impairments in the movement, Parkinson's disease (PD), and the inability to walk, talk, and think, Huntington's disease (HD). Oxidative stress and mitochondrial dysfunction are highlighted as a central feature of brain degenerative diseases. Oxidative stress, a condition that occurs due to imbalance in oxidant and antioxidant status, has been known to play a vital role in the pathophysiology of neurodegenerative diseases including AD, PD, and HD. A large number of studies have utilized oxidative stress biomarkers to investigate the severity of these neurodegenerative diseases and medications are available, but these only treat the symptoms. In traditional medicine, a large number of medicinal plants have been used to treat the symptoms of these neurodegenerative diseases. Extensive studies scientifically validated the beneficial effect of natural products against neurodegenerative diseases using suitable animal models. This short review focuses the role of oxidative stress in the pathogenesis of AD, PD, and HD and the protective efficacy of natural products against these diseases.
1. Introduction
Neurodegenerative diseases were believed to be incurable and debilitating conditions, which primarily affected the neurons in the human brain resulting in the loss of nerve structure and function and ultimately leading to the death of nerve cells [1]. The major characteristic features of neurodegenerative diseases include ataxias (impairment in movement) and dementia (decline in memory). The three main types of neurodegenerative diseases that affect the life quality and life span of the elderly include Alzheimer's disease (AD), Parkinson's disease (PD), and Huntington's disease (HD) [2, 3].
2. Alzheimer's Disease
Alzheimer's disease (AD) is one of the most common neurodegenerative disorders affecting the elderly population worldwide [4]. The specific pathological lesions that were noticed in AD include deposition of amyloid beta protein, neuronal and/or synaptic loss, and brain atrophy in specific brain areas [5]. Both the neocortex and hippocampus are affected and brain plaques and tangles are the major features of AD. AD symptoms usually start with mild confusion and amnesia and end with a dramatic personality change. AD destroys memory and other important mental functions. Other signs of AD include finding the right words, vision/spatial issues, and impaired reasoning or judgment [6]. Worldwide, around 16 million peoples are affected by AD and over four million Americans are currently affected, a figure that may rise further due to the increase in the life span [7]. This age-related, progressive, neurodegenerative disorder is the fourth leading cause of death in developed nations and accounts for 70% of dementia in the elderly population [8]. It has been suggested the incidence of AD could double every five years beyond the age of 65 [9]. Currently available medications only treat the symptoms of neurodegenerative diseases.
3. Oxidative Stress and Alzheimer's Disease
The etiology of AD is multifactorial. Both genetic and environmental factors are regarded as a risk factor of AD [9, 10]. Free radicals are chemical species with an unpaired electron and are formed during both physiological and pathological processes. Although reactive oxygen species (ROS) play a pivotal role in several cellular and signaling pathways at physiological concentrations (cell cycle regulation, phagocytosis, and enzyme activation), excessive generation of ROS leads to several harmful effects including DNA, lipid, and protein damage [11–14]. ROS are, however, scavenged by defence mechanisms, known as enzymatic and nonenzymatic antioxidants. An imbalance in this oxidant-antioxidant status could determine the extent of cell damage. Oxidative damage due to ROS has been implicated in the pathogenesis of neurodegenerative diseases, cancer, diabetes, and aging [15].
Mitochondrial dysfunction and enhanced apoptosis accompanied by a poor antioxidant status are the mechanisms for AD pathogenesis. Extensive studies pointed out the role of superoxide anion, hydroxyl radical, hydrogen peroxide, and nitric oxide in the oxidative stress mediated neurodegeneration in AD [16, 17]. Microglia activation due to neuronal lesions generates excessive superoxide radicals [18]. Higher metabolic demand and the postmitotic nature of glial cells and neurons make them more susceptible to oxidative stress. The low rate of brain regeneration and insufficient antioxidant potential in the brain further favors oxidative stress [19]. Mitochondrial autophagy serves as a major source of ROS production [20].
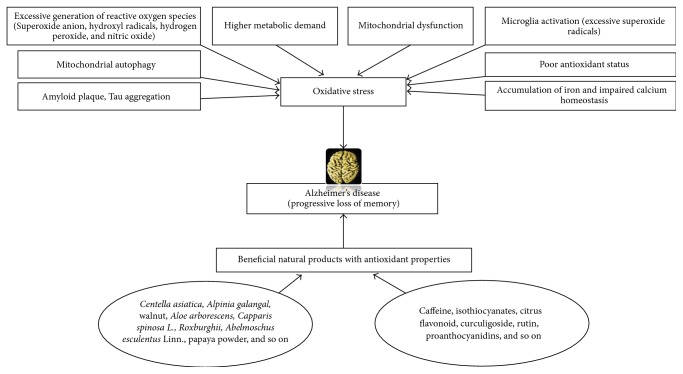
Aβ(1-42) has been recognized as a key factor in the neurodegeneration in AD patients and it mediates its harmful effect via inducing oxidative stress in the brain [21–23]. A positive association has been shown between the amyloid plaque and the lipid peroxidation markers such as 4-hydroxynonenal and malondialdehyde [24]. Elevated lipid peroxidation and insufficient enzymatic and nonenzymatic antioxidants were shown in the peripheral tissues of AD patients [25, 26]. A large number of studies have shown an elevated level of lipid peroxidation marker in the brain of AD patients, especially in the region of the temporal lobe [27–29]. An increased level of 4-hydroxynonenal, the byproduct of oxidative stress, has been reported as well [30, 31]. Iron-induced oxidative stress, as evidenced by iron accumulation in the brain of AD, is responsible for neurodegeneration in patients diagnosed with AD [32]. Profound studies explored iron accumulation in the brain of AD patients and found that, as a transition metal, it is capable of generating hydroxyl radical through the Fenton reaction [33, 34]. β-Amyloid could elevate oxidative stress mainly by binding with iron [35]. The neuronal death occurs due to reactive oxygen species mediated changes in the neuronal lipid molecules, which includes alterations in the membrane, fluidity, rigidity, permeability, and transport [36]. It has been noticed that the entorhinal cortex and CAI region of the hippocampus are the two major susceptible cerebral regions to oxidative stress [37]. Mitochondrial damage in AD could lead to excessive generation of ROS and lowered ATP production [38, 39]. Vitamin E, the major lipid-soluble nonenzymatic antioxidant, inhibits oxidative damage induced by Aβ(1-42) [40]. Diminished levels of reduced glutathione in astrocytes have been reported [41]. Melo et al. [42] suggested that addition of antioxidants inhibited the activity of acetylcholine esterase in the neuronal culture. Also, superoxide dismutase activity was shown to have increased in the CAI regions of hippocampus and amygdale [43]. The causes of oxidative stress in AD are given in Figure 1.
Figure 1.
The causes of oxidative stress in Alzheimer's disease.
4. Natural Products and Alzheimer's Disease
Medicinal plants serve as a good source for the treatment of several illnesses, including neurodegenerative diseases, diabetes mellitus, and cancer [44, 45]. A large number of therapeutic medicines recommended worldwide for several diseases have been identified from medicinal plants. Indian traditional medicine has recommended several medicinal plants for the treatment of neurodegenerative diseases. In traditional medicine, several plants have been used to treat the symptoms of neurodegenerative diseases. A large number of studies scientifically validated the beneficial effects of natural products in the treatment of AD using suitable animal models [46, 47].
Veerendra Kumar and Gupta [48] explored the neuroprotective effect of aqueous extract of Centella asiatica in a streptozotocin model of AD in rats. They suggested that Centella asiatica reduced the oxidative stress as well. Dhanasekaran et al. [49] pointed out the neuroprotective role of Centella asiatica in B6C3-Tg(APPswe, PSEN1dE9)85 Dbo/J (PSAPP) mice. They concluded that the antioxidant role of Centella asiatica modulated the amyloid pathology in PSAPP mice. Clementi et al. [50] suggested that Aloe arborescens exerted a significant neuroprotective effect in IMR-32 cells via reducing the oxidative stress in the cells. Gong et al. [51] suggested the lotus seed pod Proanthocyanidins was a promising candidate for the treatment of AD as it exhibited a significant protective effect against cognitive impairment and brain aging induced by D-galactose. Turgut et al. [52] proposed oxidative stress reduction as a major mechanism for the neuroprotective effect of Capparis spinosa L. in D-galactose-induced cognitive impairment. Yu et al. [53] demonstrated the neuroprotective role of rutin against amylin-induced neurocytotoxicity in neuronal cells and concluded that the antioxidant property of rutin might have played a role in the protection of neuronal cells. Mairuae et al. [54] showed the in vitro neuroprotective effect of okra in SH-SY5Y cells and suggested that the antioxidant effect of okra was responsible for the protective role. Uddin et al. [55] pointed out that the potent phenolic antioxidants present in the Vanda roxburghii could be responsible for the inhibition of the activation of acetylcholinesterase and butyrylcholinesterase. Barbagallo et al. [56] suggested that fermented papaya powder counteracted the excessive generation of reactive oxygen species in patients diagnosed with AD. Lu et al. [57] explored the protective role of Rhubarb extract against irradiation-induced apoptotic neuronal cell death and excessive ROS generation.
Giacoppo et al. [58] revealed the neuroprotective effect of isothiocyanates by highlighting their antioxidant potential as a major mechanism. Zhao et al. [59] demonstrated the neuroprotective effect of curculigoside on memory impairment in APP/PSI mutated transgenic mice. They suggested that its antioxidant character played a major role. Muthaiyah et al. [60] reported that walnut extract has the ability to counteract amyloid beta peptide-induced oxidative stress in PC12 cells. Hartman et al. [61] pointed out that the antioxidant polyphenolic substances of pomegranate juice reduced amyloid load and improved behavior in an AD mouse. Subash et al. [62] suggested that dietary supplementation of dates and figs improved cognitive and behavioral deficit via maintaining oxidant-antioxidant balances in APPsw/Tg 2576 transgenic AD mice. Nakajima et al. [63] suggested that nobiletin significantly reduced oxidative stress and improved the cognitive impairment in a 3XTg-AD mouse model. Sun et al. [64] proposed antioxidant potential of saponin as one of the mechanisms involved in neuroprotection. Prasanthi et al. [65] showed that caffeine reduced the oxidative stress and improved the cognitive deficits induced by cholesterol-enriched diet in rabbit hippocampus. Boyd-Kimball et al. [66] reported that glutathione upregulation protected neuron against oxidative stress and neurotoxicity induced by A(1-42) in the AD affected brain. Hanish Singh et al. [67] reported that ethanolic extract of Alpinia galangal improved the antioxidant status and inhibited the acetylcholine esterase activity in AD mice. Our research group from Oman reported the beneficial effects of natural products including pomegranate and figs on AD transgenic mice models [62, 68–77].
5. Parkinson's Disease
Parkinson's disease (PD), the most common neurodegenerative disease of the elderly, is characterized by progressive loss of muscle control. Premature death often results due to complications such as movement impairment-related injuries or pneumonia [78, 79]. PD is predominant at the 6th decade of life and men are 1.5 to 2 times more likely to contract the disease than women [80]. Head trauma, illness, or exposure to environmental toxins is identified as a risk factor. This neurodegenerative disorder is characterized by tremor, rigidity, bradykinesia, and impairment in balance [81]. PD also causes cognitive, psychiatric, autonomic, and sensory disturbances. Cognitive impairments are common in a large fraction of patients with PD at initial diagnosis and afflict a majority of patients as the disease progresses. The secondary manifestation includes anxiety, insecurity, stress, confusion, memory loss, constipation, depression, difficulty in swallowing and excessive salivation, diminished sense of smell, increased sweating, erectile dysfunction, skin problems, and a monotone voice [82, 83].
The pathology of PD is characterized by the gradual and selective loss of dopaminergic neurons in the substantia nigra pars compacta. Imbalance in dopamine metabolism due to oxidative stress has been recognised as a contributor to this disease [84]. The major pathological findings include the presence of Lewy bodies in the substantia nigra and loss of nerve cells in the portions of its ventral tier [85]. The treatment modality for PD involves either enhancing the activities of dopaminergic neuron activity or inhibiting the cholinergic effects to the stratum. While there is no cure for PD, medications provide dramatic relief from the symptoms. Recent advancement in medical and surgical treatment options has enormously improved the quality and length of life for patients with PD [86]. Worldwide, it is the second most common neurological disease and affects around 1.5 million Americans [87]. It has been pointed out that PD may double over the next 25 years in the United States and more than double in the developing nations of Asia and South America [88]. Research has indicated that 80% of the untreated PD patients die within 10 to 14 years after the onset of the disease [89].
6. Oxidative Stress and Parkinson's Disease
The brain utilizes around 20% of the basal oxygen from the total oxygen supplied to the human body. ROS mediated oxidative DNA damage is one of the prominent features in PD [90]. Several studies have reported impaired respiratory chain and somatic mitochondrial DNA mutations in the brain of patients with PD, which suggests the extensive role of oxidative metabolism in PD [91]. Enhanced dopamine metabolism in the brain of patients with PD could account for the accumulation of toxic radicals such as hydroxyl in the brain [92]. Iron accumulation in the neurons in the redox active form plays a crucial role in pathogenesis of this disease [93]. Accumulation of iron has been reported in the substantia nigra in patients diagnosed with PD, which suggests the critical role of iron-induced lipid peroxidation in pathogenesis of PD [94–96]. The accumulation of lipid peroxidation byproducts has been reported in the serum and cerebral spinal fluid of patients with PD [97] while an increase in malondialdehyde and hydroperoxides has been reported in the substantia nigra of patients diagnosed with PD [98, 99].
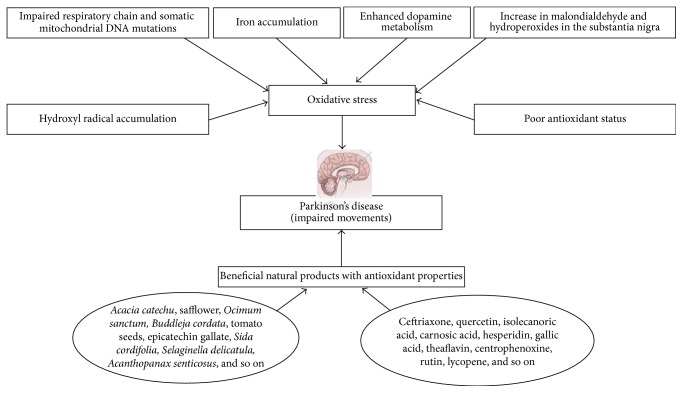
Elevated levels of malondialdehyde, thiobarbituric acid reactive substance, and 4-hydroxy-2,3-nonenal have been reported in the substantia nigra and stratum of PD brains [100, 101]. A twofold increase in protein oxidation has been shown in the substantia nigra of PD patients compared to healthy subjects [102]. Accumulation of hydroxyl radical due to lowered glutathione content in the brain has been reported in PD patients [103]. Lowered activities of antioxidant enzymes and nonenzymatic antioxidants could be responsible for the progression of PD [104, 105]. Reduced glutathione and increased oxidized glutathione levels have been reported in PD patients while lowered glutathione content in the substantia nigra, due to neuronal loss, has been reported in patients with PD [106–109]. Decreased activity of glutathione peroxidase and a decline in glutathione content have been reported in the brain of PD patients and reduced glutathione content was found to be decreased in both human and experimental models of PD [110–112]. Lowered GSH content was reported in the substantia nigra and corpus striatum of PD patients [113]. The causes of oxidative stress in Parkinson's disease are given in Figure 2.
Figure 2.
The causes of oxidative stress in Parkinson's disease.
7. Natural Products and Parkinson's Disease
Extensive studies scientifically explored the protective effect of natural products against Parkinson's disease using suitable animal models. Weng et al. [114] reported that ceftriaxone prevented the loss of neuronal activity and decreased the neurogenesis in the brain of PD rats. Sharma et al. [115] suggested that administration of quercetin attenuated the neuronal death and reduced the oxidative stress in aluminium-induced neurodegeneration in the rat hippocampus. Saha et al. [116] explored the antineurogenic and antioxidant potential of Acacia catechu leaf extract using in vitro studies. Ren et al. [117] reported that safflower flavonoid extract could be used as the herbal therapy for PD treatment. De Pedro et al. [118] explored the in vitro protective effect of isolecanoric acid against the PD development. Wu et al. [119] investigated the neuroprotective effect of carnosic acid against 6-hydroxydopamine induced neurotoxicity. They concluded that the antioxidant and antiapoptotic potential of carnosic acid could play a protective role in the prevention of neurodegeneration. Siddique et al. [120] demonstrated the neuroprotective effect of Ocimum sanctum leaf extract in the transgenic Drosophila model of PD. Antunes et al. [121] suggested that hesperidin attenuated 6-hydroxydopamine induced oxidative stress in aged mice. Pérez-Barrón et al. [122] explored the antioxidant and neuroprotective effect of Buddleja cordata methanolic extract in the 1-methyl-4-phenylpyridinium induced PD rat model. Beppe et al. [123] suggested that the aqueous extract of Albizia adianthifolia leaves possesses antioxidant potential, which was responsible for the memory-enhancing activities in the rodent model of PD.
Gokul and Muralidhara [124] reported that tomato seeds alleviated motor abnormality, oxidative impairments, and neurotoxicity in a chronic ROT model of neurotoxicity in mice. Siddique et al. [125] reported that epicatechin gallate dietary supplementation reduced the oxidative stress and apoptosis in the brain of transgenic Drosophila model of PD. Khurana and Gajbhiye [126] showed the ameliorative effect of Sida cordifolia against rotenone-induced oxidative stress and neurochemical and behavioral alterations in a rat model of PD. Chandran and Muralidhara [127] showed the neuroprotective effect of aqueous extract of Selaginella delicatula in a chronic ROT exposure model of neurotoxicity in mice. They suggested that the neuroprotective property of Selaginella delicatula is largely attributed to the antioxidant properties. Prakash et al. [128] demonstrated the neuroprotective role of Withania somnifera root extract in parkinsonism mice. They suggested that Withania somnifera extract improved the behavioral, anatomical, and biochemical deformities. Mansouri et al. [129] suggested that the neuroprotective effect of oral gallic acid is due to the enhancement of cerebral antioxidant defense against oxidative stress induced by 6-hydroxydopamine in rats. Shalavadi et al. [130] suggested that the neuroprotective effect of the methanolic extract of Stereospermum suaveolens DC could be attributed to its antioxidant potential in 6-OHDA induced PD rats. Liu et al. [131] explored the neuroprotective effect of Acanthopanax senticosus in PD. Anandhan et al. [132] suggested that the neuroprotective effect of theaflavin may be due to its antioxidative and antiapoptotic activities in chronic MPTP/probenecid induced PD. Some of our research group members reported the beneficial effects of natural products on PD animals [133–137].
Ahmad et al. [138] pointed out that the antioxidant efficacy of sesame seed oil is responsible for the neuroprotective effect in 6-hydroxydopamine induced neurotoxicity in mice. Martins et al. [139] demonstrated the protective effect of Melissa officinalis in manganese-induced oxidative stress in chronically exposed mice. They concluded that the antioxidant potential of this plant is responsible for the neuroprotective effect. Hritcu et al. [140] pointed out that the methanolic extract of Hibiscus asper leaves exerted neuroprotective activity through antioxidant and antiapoptotic activities in PD model. Ranpariya et al. [141] suggested that the antioxidant potential of Matricaria recutita could be largely attributed to its neuroprotective activity against fluoride-induced stress in rats. Wang et al. [142] suggested that the free radical scavenging activity of resveratrol protected the abnormal rotational behavior and the loss and apoptosis of nigral cells in Parkinsonian rats. Verma and Nehru [143] demonstrated the antioxidant effect of centrophenoxine against rotenone-induced oxidative stress in PD rodent. Kaur et al. [144] demonstrated the beneficial effect of lycopene in rotenone-induced model of PD. They suggested that the therapeutic potential of lycopene is attributed to its antioxidant efficacy. Khan et al. [145] pointed out that rutin can protect dopaminergic neurons from oxidative stress in a PD rat. Essa et al. [146] suggested that walnut partially reversed MPTP-induced neurodegeneration in a mouse model of PD. They suggested that the antioxidant role of walnut might have played a neuroprotective role. Jahromi et al. [147] suggested that the antioxidants present in the Decalepis hamiltonii roots attenuated neuromotor deficits in transgenic Drosophila model of PD.
Tseng et al. [148] showed the protective effect of Liuwei Dihuang in Parkinson's toxin-induced dopaminergic neurodegeneration. Guo et al. [149] suggested that tetramethylpyrazine nitrone rescued dopaminergic neurons by reducing ROS and increasing cellular antioxidative defense capability in the animal models of PD. Sudati et al. [150] concluded that Valeriana officinalis improved the antioxidant defence mechanism in the rotenone-induced toxicity in Drosophila melanogaster. Pasban-Aliabadi et al. [151] suggested that the protective effect of olive (Olea europaea L.) leaf extract in the 6-hydroxydopamine-induced PC12 cell apoptosis is due to their antioxidative and antiapoptotic properties. Kim et al. [152] explored the neuroprotective role of Rhus verniciflua in rotenone model of PD via its antioxidant efficacy. Li and Pu [153] reported that kaempferol inhibited MPTP induced oxidative stress in the mouse model of PD. Liang et al. [154] pointed out that tenuigenin exhibited potent neuroprotective effect through antioxidant potential in a SH-SY5Y cell model with 6-OHDA-induced injury. Hu et al. [155] showed that the ginseng attenuated (MPP(+)) induced cytotoxicity in SH-SY5Y cells through its antioxidant potential. Choi et al. [156] suggested that Polygalae Radix, through its antioxidant and antiapoptotic efficacy, inhibited the neuronal death in PD models. Sengupta et al. [157] reported that the hydroxyl scavenging potential of Hyoscyamus niger seeds is responsible for its neuroprotective effect.
An et al. [158] reported that Acanthopanacis senticosus prevented the MPP+ induced damage in PC12 cells by reducing the levels of MDA, which suggested its antioxidant potential. Kim et al. [159] pointed out that Chunghyuldan exhibited neuroprotective effect against ROS-mediated neuronal cell death in PD model. Lee et al. [160] suggested that Cyperi rhizome exhibited the neuroprotective effects through antioxidant and antiapoptotic activities in an in vitro PD model. Shu et al. [161] suggested that the neuroprotective effect of Chuanxiong Chatiao may be associated with its potent antioxidant efficacy in MPTP-induced Parkinson's mice. Shim et al. [162] suggested that Uncaria rhynchophylla exhibited neuroprotective effect through antioxidative and antiapoptotic activities in PD models. Sankar et al. [163] suggested that Withania somnifera root extract exhibited potent neuroprotective effect by mitigating MPTP-induced oxidative stress in PD mice. Ahmad et al. [164] showed the neuroprotective effect of Delphinium denudatum via its antioxidant property in PD rats. Ahmad et al. [165] reported that Nardostachys jatamansi attenuated 6-hydroxydopamine-induced parkinsonism in rats via antilipid peroxidative potential. Zhang et al. [166] explored the neuroprotective effect of Forsythia suspensa with antioxidant property in an experimental model of rotenone-induced neurotoxicity. Lu et al. [167] suggested that resveratrol showed a neuroprotective effect in MPTP-induced parkinsonism through free radical scavenging potential. A large number of experimental studies on neurodegenerative diseases highlighted curcumin as a potent neuroprotective agent [168]. Braidy et al. [137] explored the neuroprotective effect of pomegranate extract in MPTP induced oxidative stress in human primary neurons.
8. Huntington's Disease
Huntington's disease (HD) is a devastating familial and inherited disease characterized by the progressive loss of brain and muscle function. It occurs due to the genetically programmed degeneration of neurons, which causes uncontrolled movements, loss of intellectual abilities, and emotional disturbances. HD is caused by a CAG trinucleotide expansion in exon 1 of the Huntingtin (HTT) gene, which is located on chromosome 4 (4p63) [169]. Healthy individuals have 6–35 CAG repeats, and affected individuals have more than 36 repeats. The accumulation of mutant Huntingtin proteins contains a long polyglutamine region which causes neuronal death and the degeneration of neuronal networks within the brain. The pathological changes in the cerebral cortex and striatum elicit the development of chorea and cognitive impairments and lead to premature death. There is a 50% chance that children will inherit HD from HD affected parents. Men and women are equally affected by HD which appears during 4th to 5th decade of life. The symptoms usually appear between the ages of 35 and 55. However, the age of onset and its progression varies from person to person [170]. The clinical course of HD typically progresses over 10 to 20 years from a presymptomatic state to complete disability and death. The early symptoms includes tumbling, lack of focus, concentration and movement problems, clumsiness, lapses in short-term memory, and depression. As the disease progresses, difficulty in speech, weight loss, feeding problems, swallowing difficulties, uncontrollable movements of the face, and itching and stumbling are the major symptoms. It has been estimated that around 6000 and 30,000 people are affected by HD in UK and USA, respectively [171].
9. Oxidative Stress and Huntington's Disease
The exact cause of neuronal death in HD is unknown. However, oxidative stress may play an important role. The two major factors that make the brain more prone to oxidative damage are higher lipid concentrations and high energy requirement [172]. Compelling data supports a critical role for oxidative stress in the pathogenesis of HD, a disorder caused by polyglutamine expansion in Huntingtin (Htt). mHTT proteins serve as the source of reactive oxygen species (ROS), due to a significant amount of oxidized proteins in partially purified mHTT aggregates [173]. Though oxidative damage is not much reported in the early stages of HD, it is proposed as one of the major mechanisms in HD as it progresses [174].
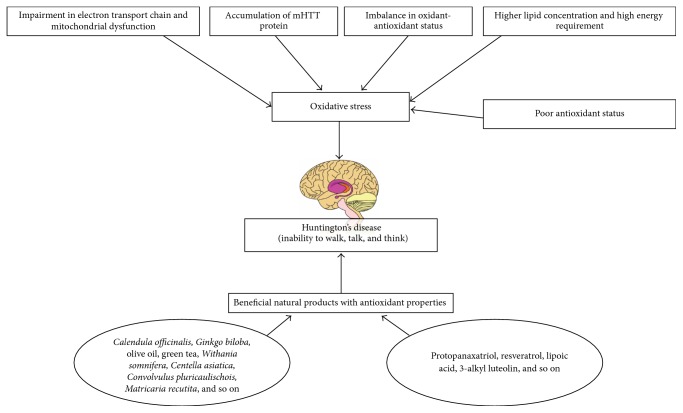
Elevated oxidative stress plays a critical role in the late stage of HD pathogenesis. Impairment in the electron transport chain and mitochondrial dysfunction are the major mechanisms involved in the ROS mediated etiopathogenesis of HD [175, 176]. Dysfunction in the oxidative phosphorylation components has been documented in the brain tissues of HD patients [177]. HD patients showed an increased level of oxidative stress markers accompanied by a decrease in antioxidant status compared to healthy subjects [178]. ROS mediated oxidative damage to mitochondria has been postulated as a reasonable mechanism for the defect in glucose metabolism in the brain tissue of symptomatic HD patients [179]. A positive correlation between plasma lipid peroxidation byproduct and the severity of disease in patients with HD has been shown [180]. Enhanced lipid peroxidation has been reported in patients with severe symptoms of HD [178, 181]. An increase in the plasma lipid peroxidation accompanied by reduced glutathione content has been reported in HD patients [182]. The extensive oxidative DNA damage has been reported in a HD mouse model [183, 184]. Enhanced oxidative stress and a decline in nonenzymatic antioxidants have been reported in the peripheral blood of HD patients [185]. Stoy et al. [186] reported that abnormal tryptophan metabolism with enhanced oxidative stress could be responsible for brain dysfunction in HD. Duran et al. [187] reported that symptomatic HD patients are more prone to oxidative stress than asymptomatic HD patients. The causes of oxidative stress in HD are given in Figure 3.
Figure 3.
The causes of oxidative stress in Huntington's disease.
10. Natural Products and Huntington's Disease
Researches utilized suitable experimental models to scientifically validate the protective efficacy of natural products against HD. Oliveira et al. [188] suggested that the protective effect of luteolin derivatives on Huntington's mouse striatal cells is due to its antioxidant potential. Shivasharan et al. [189] showed the protective efficacy of Calendula officinalis flowers in 3-nitropropionic acid-induced HD in rats. They concluded that the anti-inflammatory and antioxidant potential of Calendula officinalis might have played a neuroprotective role. Mahdy et al. [190] explored the beneficial effect of Ginkgo biloba extract on 3-nitropropionic acid-induced neurobehavioral changes and striatal lesions. They concluded that the antioxidant and antiapoptotic potential of Ginkgo biloba extract might be responsible for the neuroprotective role. Tasset et al. [191] reported that olive oil reduced oxidative damage in 3-nitropropionic acid-induced HD in rats. They concluded that extravirgin olive oil and hydroxytyrosol served as a powerful brain antioxidant. Sagredo et al. [192] provided preclinical evidence for the neuroprotective effect of phytocannabinoid-based medicines in HD. Gao et al. [193] investigated the neuroprotective effect of protopanaxatriol against 3-nitropropionic acid-induced oxidative stress in experimental HD. Túnez et al. [194] showed the protective effect of melatonin in 3-nitropropionic acid-induced oxidative stress in synaptosomes in rat with HD. They concluded that melatonin modified the neural response to 3-nitropropionic acid with the antioxidative mechanism.
Rocha-González et al. [195] reported the neuroprotective role of resveratrol against HD. Andreassen et al. [196] suggested that lipoic acid, as an antioxidant, has the potential to improve the survival of transgenic mouse models of HD. Ehrnhoefer et al. [197] pointed out that green tea (-)-epigallocatechin gallate prevented the early events of HD pathogenesis such as Huntington's misfolding. Denny Joseph and Muralidhara [198] suggested that fish oil in combination with quercetin provided better neuroprotection against 3-nitropropionic acid-induced HD. Fu et al. [199] suggested that trans-(−)-ε-Viniferin could be considered as a promising candidate to treat HD, since it increased mitochondrial sirtuin 3 (SIRT3) and activated the AMP-activated protein kinase. Huang et al. [200] explored the neuroprotective role of N(6)-(4-hydroxybenzyl) adenine riboside against experimental HD. Ranpariya et al. [201] showed the neuroprotective effect of German chamomile against aluminium fluoride-induced oxidative stress in rats. P. Kumar and A. Kumar [202] explored the neuroprotective effect of Withania somnifera root extract against 3-nitropropionic acid-induced HD. They suggested that neuroprotective actions of Withania somnifera are mediated via its antioxidant activity. Shinomol and Muralidhara [203] reported that the prophylactic neuroprotective property of Centella asiatica could be related to the enhancement of GSH, thiols, and antioxidant machinery in the brain regions of 3-nitropropionic acid-induced HD prepubertal mice. Kaur et al. [204] suggested that Convolvulus pluricaulis exhibited a potent neuroprotective effect by accelerating the brain antioxidant defence mechanisms in 3-nitropropionic acid treated rats. Al-Sabahi et al. [205] reported the benefit of pomegranate seed oil on 3-NP induced HD.
11. Conclusion
Neurodegenerative diseases impose a significant health burden not only to the affected patients, but also to their families and society. The incidences of these life threatening disorders are rapidly increasing in aged populations worldwide. Although several mechanisms have been postulated for the pathogenesis of neurodegenerative diseases, oxidative stress and mitochondrial dysfunctions are pointed out as a major mechanism. At present, medications are only available to treat the symptoms of neurodegenerative diseases. Several in vivo and in vitro studies have documented the protective role of various natural products or synthetic entities in the prevention of neurodegenerative diseases. However, the solution for these neurodegenerative diseases has not yet been found. Thus, researches are warranted to investigate the nontoxic active constituents found in natural resources which could correct the biochemical, metabolic, and behavioral abnormalities that occur in neurodegenerative diseases.
12. Opinion of the Authors
This review highlights the crucial role of oxidative stress in the pathogenesis of various neurodegenerative diseases. Based on the literature researched for this paper, it is clear that oxidative stress mediates its adverse effects either directly, causing neuronal damage, or by inducing the harmful effects of neurotoxicants. This review also explores the beneficial effects of various natural products against neurodegenerative diseases.
While many reports have focused on the role of protective efficacy of natural products against oxidative stress-induced neurodegenerative diseases, as yet, there have been no effective treatment solutions reported for these diseases. This indicates that the antioxidants alone are not sufficient to treat neurodegenerative diseases. Thus, intense research should be undertaken to investigate, or identify, the novel compounds that could be used to counteract the oxidative stress pathogenesis and for a better therapeutic agent for the treatment of neurodegenerative diseases.
13. Literature Search Strategy
For this study, an intense literature search on neurodegenerative diseases (AD, PD, and HD) was mainly done through PubMed articles published from 1982 to 2016. The articles were then scrutinized and the most relevant selected to write this review. We have also referred to previous review articles on neurodegenerative diseases and the references cited were also considered. The key words used to search the relevant articles included neurodegenerative diseases, Alzheimer's disease, Parkinson's disease, Huntington's disease, Reactive Oxygen Species, antioxidants, medicinal plants, and so forth.
Competing Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Uttara B., Singh A. V., Zamboni P., Mahajan R. T. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Current Neuropharmacology. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossor M. N., Fox N. C., Mummery C. J., Schott J. M., Warren J. D. The diagnosis of young-onset dementia. The Lancet Neurology. 2010;9(8):793–806. doi: 10.1016/S1474-4422(10)70159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin M. T., Beal M. F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 4.Butterfield D. A., Boyd-Kimball D. Amyloid β-peptide(1-42) contributes to the oxidative stress and neurodegeneration found in Alzheimer disease brain. Brain Pathology. 2004;14(4):426–432. doi: 10.1111/j.1750-3639.2004.tb00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemere C. A., Masliah E. Can Alzheimer disease be prevented by amyloid-β immunotherapy? Nature Reviews Neurology. 2010;6(2):108–119. doi: 10.1038/nrneurol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosconi L., Pupi A., De Leon M. J. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer's disease. Annals of the New York Academy of Sciences. 2008;1147:180–195. doi: 10.1196/annals.1427.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferri C. P., Prince M., Brayne C., et al. Global prevalence of dementia: a Delphi consensus study. The Lancet. 2005;366(9503):2112–2117. doi: 10.1016/s0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Praticò D. Evidence of oxidative stress in Alzheimer's disease brain and antioxidant therapy: lights and shadows. Annals of the New York Academy of Sciences. 2008;1147:70–78. doi: 10.1196/annals.1427.010. [DOI] [PubMed] [Google Scholar]
- 9.Joseph F. P., Darrell Jennings C., Richard J. K., et al. Association of HFE mutations with neurodegeneration and oxidative stress in Alzheimer's disease and correlation with APOE. American Journal of Medical Genetics. 2003;119:48–53. doi: 10.1002/ajmg.b.10069. [DOI] [PubMed] [Google Scholar]
- 10.Bird T. D. Genetic aspects of Alzheimer disease. Genetics in Medicine. 2008;10(4):231–239. doi: 10.1097/gim.0b013e31816b64dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verbon E. H., Post J. A., Boonstra J. The influence of reactive oxygen species on cell cycle progression in mammalian cells. Gene. 2012;511(1):1–6. doi: 10.1016/j.gene.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 12.Son Y., Kim S., Chung H.-T., Pae H.-O. Reactive oxygen species in the activation of MAP kinases. Methods in Enzymology. 2013;528:27–48. doi: 10.1016/B978-0-12-405881-1.00002-1. [DOI] [PubMed] [Google Scholar]
- 13.Knock G. A., Ward J. P. T. Redox regulation of protein kinases as a modulator of vascular function. Antioxidants and Redox Signaling. 2011;15(6):1531–1547. doi: 10.1089/ars.2010.3614. [DOI] [PubMed] [Google Scholar]
- 14.Lo Conte M., Carroll K. S. The redox biochemistry of protein sulfenylation and sulfinylation. The Journal of Biological Chemistry. 2013;288(37):26480–26488. doi: 10.1074/jbc.r113.467738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X., Guo C., Kong J. Oxidative stress in neurodegenerative diseases. Neural Regeneration Research. 2012;7(5):376–385. doi: 10.3969/j.issn.1673-5374.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Z., Wei M., Morgan T. E., et al. Peroxynitrite mediates neurotoxicity of amyloid beta-peptide1-42- and lipopolysaccharide-activated microglia. The Journal of Neuroscience. 2002;22(9):3484–3492. doi: 10.1523/JNEUROSCI.22-09-03484.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Dyke K. The possible role of peroxynitrite in Alzheimer's disease: a simple hypothesis that could be tested more thoroughly. Medical Hypotheses. 1997;48(5):375–380. doi: 10.1016/s0306-9877(97)90031-1. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima K., Kohsaka S. Microglia: activation and their significance in the central nervous system. Journal of Biochemistry. 2001;130(2):169–175. doi: 10.1093/oxfordjournals.jbchem.a002969. [DOI] [PubMed] [Google Scholar]
- 19.Friedman J. Why is the nervous system vulnerable to oxidative stress? In: Gadoth N., Göbel H. H., editors. Oxidative Stress in Applied Basic Research and Clinical Practice. Totowa, NJ, USA: Humana Press; 2011. pp. 19–27. [DOI] [Google Scholar]
- 20.Munkácsy E., Rea S. L. The paradox of mitochondrial dysfunction and extended longevity. Experimental Gerontology. 2014;56:221–233. doi: 10.1016/j.exger.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yatin S. M., Yatin M., Aulick T., Ain K. B., Butterfield D. A. Alzheimer's amyloid β-peptide associated free radicals increase rat embryonic neuronal polyamine uptake and ornithine decarboxylase activity: protective effect of vitamin E. Neuroscience Letters. 1999;263(1):17–20. doi: 10.1016/s0304-3940(99)00101-9. [DOI] [PubMed] [Google Scholar]
- 22.Butterfield D. A., Lauderback C. M. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress. Free Radical Biology and Medicine. 2002;32(11):1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 23.Drake J., Link C. D., Butterfield D. A. Oxidative stress precedes fibrillar deposition of Alzheimer's disease amyloid β-peptide (1–42) in a transgenic Caenorhabditis elegans model. Neurobiology of Aging. 2003;24(3):415–420. doi: 10.1016/s0197-4580(02)00225-7. [DOI] [PubMed] [Google Scholar]
- 24.Massaad C. A. Neuronal and vascular oxidative stress in Alzheimer's disease. Current Neuropharmacology. 2011;9(4):662–673. doi: 10.2174/157015911798376244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baldeiras I., Santana I., Proença M. T., et al. Peripheral oxidative damage in mild cognitive impairment and mild Alzheimer's disease. Journal of Alzheimer's Disease. 2008;15(1):117–128. doi: 10.3233/jad-2008-15110. [DOI] [PubMed] [Google Scholar]
- 26.Greilberger J., Koidl C., Greilberger M., et al. Malondialdehyde, carbonyl proteins and albumin-disulphide as useful oxidative markers in mild cognitive impairment and Alzheimer's disease. Free Radical Research. 2008;42(7):633–638. doi: 10.1080/10715760802255764. [DOI] [PubMed] [Google Scholar]
- 27.Palmer A. M., Burns M. A. Selective increase in lipid peroxidation in the inferior temporal cortex in Alzheimer's disease. Brain Research. 1994;645(1-2):338–342. doi: 10.1016/0006-8993(94)91670-5. [DOI] [PubMed] [Google Scholar]
- 28.Lovell M. A., Ehmann W. D., Butler S. M., Markesbery W. R. Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer's disease. Neurology. 1995;45(8):1594–1601. doi: 10.1212/WNL.45.8.1594. [DOI] [PubMed] [Google Scholar]
- 29.Marcus D. L., Thomas C., Rodriguez C., et al. Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer's disease. Experimental Neurology. 1998;150(1):40–44. doi: 10.1006/exnr.1997.6750. [DOI] [PubMed] [Google Scholar]
- 30.Gabbita S. P., Lovell M. A., Markesbery W. R. Increased nuclear DNA oxidation in the brain in Alzheimer' s disease. Journal of Neurochemistry. 1998;71(5):2034–2040. doi: 10.1046/j.1471-4159.1998.71052034.x. [DOI] [PubMed] [Google Scholar]
- 31.Hensley K., Hall N., Subramaniam R., et al. Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. Journal of Neurochemistry. 1995;65(5):2146–2156. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- 32.Smith M. A., Harris P. L. R., Sayre L. M., Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(18):9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lovell M. A., Robertson J. D., Teesdale W. J., Campbell J. L., Markesbery W. R. Copper, iron and zinc in Alzheimer's disease senile plaques. Journal of the Neurological Sciences. 1998;158(1):47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 34.Casadesus G., Smith M. A., Zhu X., et al. Alzheimer disease: evidence for a central pathogenic role of iron-mediated reactive oxygen species. Journal of Alzheimer's Disease. 2004;6(2):165–169. doi: 10.3233/jad-2004-6208. [DOI] [PubMed] [Google Scholar]
- 35.Rottkamp C. A., Raina A. K., Zhu X., et al. Redox-active iron mediates amyloid-β toxicity. Free Radical Biology and Medicine. 2001;30(4):447–450. doi: 10.1016/S0891-5849(00)00494-9. [DOI] [PubMed] [Google Scholar]
- 36.Rowan M. J., Klyubin I., Wang Q., Anwyl R. Mechanisms of the inhibitory effects of amyloid β-protein on synaptic plasticity. Experimental Gerontology. 2004;39(11-12):1661–1667. doi: 10.1016/j.exger.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 37.Karelson E., Bogdanovic N., Garlind A., et al. The cerebrocortical areas in normal brain aging and in Alzheimer's disease: noticeable differences in the lipid peroxidation level and in antioxidant defense. Neurochemical Research. 2001;26(4):353–361. doi: 10.1023/a:1010942929678. [DOI] [PubMed] [Google Scholar]
- 38.Castellani R., Hirai K., Aliev G., et al. Role of mitochondrial dysfunction in Alzheimer's disease. Journal of Neuroscience Research. 2002;70(3):357–360. doi: 10.1002/jnr.10389. [DOI] [PubMed] [Google Scholar]
- 39.Gibson G. E., Sheu K.-F. R., Blass J. P. Abnormalities of mitochondrial enzymes in Alzheimer disease. Journal of Neural Transmission. 1998;105(8-9):855–870. doi: 10.1007/s007020050099. [DOI] [PubMed] [Google Scholar]
- 40.Yatin S. M., Varadarajan S., Butterfield D. A. Vitamin E prevents Alzheimer's amyloid β-peptide (1-42)-induced neuronal protein oxidation and reactive oxygen species production. Journal of Alzheimer's Disease. 2000;2(2):123–131. doi: 10.3233/jad-2000-2212. [DOI] [PubMed] [Google Scholar]
- 41.Abramov A. Y., Canevari L., Duchen M. R. Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity. Journal of Neuroscience. 2003;23(12):5088–5095. doi: 10.1523/JNEUROSCI.23-12-05088.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melo J. B., Agostinho P., Oliveira C. R. Involvement of oxidative stress in the enhancement of acetylcholinesterase activity induced by amyloid beta-peptide. Neuroscience Research. 2003;45(1):117–127. doi: 10.1016/S0168-0102(02)00201-8. [DOI] [PubMed] [Google Scholar]
- 43.Rahman K. Studies on free radicals, antioxidants, and co-factors. Clinical Interventions in Aging. 2007;2(2):219–236. [PMC free article] [PubMed] [Google Scholar]
- 44.Singhal A., Bangar O., Naithani V. Medicinal plants with a potential to treat Alzheimer and associated symptoms. International Journal of Nutrition, Pharmacology, Neurological Diseases. 2012;2(2):84–91. doi: 10.4103/2231-0738.95927. [DOI] [Google Scholar]
- 45.Upadhyay P., Panjwani D., Yadav A. K. Neuropathology staging and treatment strategies of Alzheimer's disease: an update. International Journal of Nutrition, Pharmacology, Neurological Diseases. 2014;4(1):28–42. doi: 10.4103/2231-0738.124612. [DOI] [Google Scholar]
- 46.Kim G. H., Kim J. E., Rhie S. J., Yoon S. The role of oxidative stress in neurodegenerative diseases. Experimental Neurobiology. 2015;24(4):325–340. doi: 10.5607/en.2015.24.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ven Murthy M. R., Ranjekar P. K., Ramassamy C., Deshpande M. Scientific basis for the use of Indian ayurvedic medicinal plants in the treatment of neurodegenerative disorders: 1. Ashwagandha. Central Nervous System Agents in Medicinal Chemistry. 2010;10(3):238–246. doi: 10.2174/1871524911006030238. [DOI] [PubMed] [Google Scholar]
- 48.Veerendra Kumar M. H., Gupta Y. K. Effect of Centella asiatica on cognition and oxidative stress in an intracerebroventricular streptozotocin model of Alzheimer's disease in rats. Clinical and Experimental Pharmacology and Physiology. 2003;30(5-6):336–342. doi: 10.1046/j.1440-1681.2003.03842.x. [DOI] [PubMed] [Google Scholar]
- 49.Dhanasekaran M., Holcomb L. A., Hitt A. R., et al. Centella asiatica extract selectively decreases amyloid β levels in hippocampus of alzheimer's disease animal model. Phytotherapy Research. 2009;23(1):14–19. doi: 10.1002/ptr.2405. [DOI] [PubMed] [Google Scholar]
- 50.Clementi M. E., Tringali G., Triggiani D., Giardina B. Aloe arborescens extract protects IMR-32 Cells against Alzheimer amyloid beta peptide via inhibition of radical peroxide production. Natural Product Communications. 2015;10(11):1993–1995. [PubMed] [Google Scholar]
- 51.Gong Y.-S., Guo J., Hu K., et al. Ameliorative effect of lotus seedpod proanthocyanidins on cognitive impairment and brain aging induced by D-galactose. Experimental Gerontology. 2016;74:21–28. doi: 10.1016/j.exger.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 52.Turgut N. H., Kara H., Arslanbaş E., Mert D. G., Tepe B., Güngör H. Effect of Capparis spinosa L. On cognitive impairment induced by D-galactose in mice via inhibition of oxidative stress. Turkish Journal of Medical Sciences. 2015;45(5):1127–1136. doi: 10.3906/sag-1405-95. [DOI] [PubMed] [Google Scholar]
- 53.Yu X.-L., Li Y.-N., Zhang H., et al. Rutin inhibits amylin-induced neurocytotoxicity and oxidative stress. Food and Function. 2015;6(10):3296–3306. doi: 10.1039/c5fo00500k. [DOI] [PubMed] [Google Scholar]
- 54.Mairuae N., Connor J. R., Lee S. Y., Cheepsunthorn P., Tongjaroenbuangam W. The effects of okra (Abelmoschus esculentus Linn.) on the cellular events associated with Alzheimer's disease in a stably expressed HFE neuroblastoma SH-SY5Y cell line. Neuroscience Letters. 2015;31(603):6–11. doi: 10.1016/j.neulet.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 55.Uddin M. N., Afrin R., Uddin M. J., et al. Vanda roxburghii chloroform extract as a potential source of polyphenols with antioxidant and cholinesterase inhibitory activities: identification of a strong phenolic antioxidant. BMC Complementary and Alternative Medicine. 2015;15(1, article 195) doi: 10.1186/s12906-015-0728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barbagallo M., Marotta F., Dominguez L. J. Oxidative stress in patients with Alzheimer's disease: effect of extracts of fermented papaya powder. Mediators of Inflammation. 2015;2015:6. doi: 10.1155/2015/624801.624801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu K., Zhang C., Wu W., Zhou M., Tang Y., Peng Y. Rhubarb extract has a protective role against radiation-induced brain injury and neuronal cell apoptosis. Molecular Medicine Reports. 2015;12(2):2689–2694. doi: 10.3892/mmr.2015.3693. [DOI] [PubMed] [Google Scholar]
- 58.Giacoppo S., Galuppo M., Montaut S., et al. An overview on neuroprotective effects of isothiocyanates for the treatment of neurodegenerative diseases. Fitoterapia. 2015;106:12–21. doi: 10.1016/j.fitote.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Zhao L., Liu S., Wang Y., et al. Effects of curculigoside on memory impairment and bone loss via anti-oxidative character in APP/PS1 mutated transgenic mice. PLoS ONE. 2015;10(7) doi: 10.1371/journal.pone.0133289.e0133289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muthaiyah B., Essa M. M., Chauhan V., Chauhan A. Protective effects of walnut extract against amyloid beta peptide-induced cell death and oxidative stress in PC12 cells. Neurochemical Research. 2011;36(11):2096–2103. doi: 10.1007/s11064-011-0533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hartman R. E., Shah A., Fagan A. M., et al. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer's disease. Neurobiology of Disease. 2006;24(3):506–515. doi: 10.1016/j.nbd.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 62.Subash S., Essa M. M., Al-Asmi A., Al-Adawi S., Vaishnav R., Guillemin G. J. Effect of dietary supplementation of dates in Alzheimer’s disease APPsw/2576 transgenic mice on oxidative stress and antioxidant status. Nutritional Neuroscience. 2015;18(6):281–288. doi: 10.1179/1476830514Y.0000000134. [DOI] [PubMed] [Google Scholar]
- 63.Nakajima A., Aoyama Y., Shin E.-J., et al. Nobiletin, a citrus flavonoid, improves cognitive impairment and reduces soluble Aβ levels in a triple transgenic mouse model of Alzheimer's disease (3XTg-AD) Behavioural Brain Research. 2015;289:69–77. doi: 10.1016/j.bbr.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 64.Sun A., Xu X., Lin J., Cui X., Xu R. Neuroprotection by saponins. Phytotherapy Research. 2015;29(2):187–200. doi: 10.1002/ptr.5246. [DOI] [PubMed] [Google Scholar]
- 65.Prasanthi J. R. P., Dasari B., Marwarha G., et al. Caffeine protects against oxidative stress and Alzheimer's disease-like pathology in rabbit hippocampus induced by cholesterol-enriched diet. Free Radical Biology and Medicine. 2010;49(7):1212–1220. doi: 10.1016/j.freeradbiomed.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boyd-Kimball D., Sultana R., Mohmmad Abdul H., Butterfield D. A. γ-glutamylcysteine ethyl ester-induced up-regulation of glutathione protects neurons against Aβ(1-42)-mediated oxidative stress and neurotoxicity: implications for Alzheimer's disease. Journal of Neuroscience Research. 2005;79(5):700–706. doi: 10.1002/jnr.20394. [DOI] [PubMed] [Google Scholar]
- 67.Hanish Singh J. C., Alagarsamy V., Sathesh Kumar S., Narsimha Reddy Y. Neurotransmitter metabolic enzymes and antioxidant status on Alzheimer's disease induced mice treated with Alpinia galanga (L.) Willd. Phytotherapy Research. 2011;25(7):1061–1067. doi: 10.1002/ptr.3364. [DOI] [PubMed] [Google Scholar]
- 68.Akbar M., Song B.-J., Essa M. M., Khan M. A. Pomegranate: an ideal fruit for human health. International Journal of Nutrition, Pharmacology, Neurological Diseases. 2015;5(4):141–143. doi: 10.4103/2231-0738.167506. [DOI] [Google Scholar]
- 69.Subash S., Essa M. M., Braidy N., et al. Diet rich in date palm fruits improves memory, learning and reduces beta amyloid in transgenic mouse model of Alzheimer's disease. Journal of Ayurveda and Integrative Medicine. 2015;6(2):111–120. doi: 10.4103/0975-9476.159073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Subash S., Braidy N., Essa M. M., et al. Long-term (15 mo) dietary supplementation with pomegranates from Oman attenuates cognitive and behavioral deficits in a transgenic mice model of Alzheimer's disease. Nutrition. 2015;31(1):223–229. doi: 10.1016/j.nut.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 71.Subash S., Essa M., Al-Asmi A., et al. Pomegranate from oman alleviates the brain oxidative damage in transgenic mouse model of alzheimer′s disease. Journal of Traditional and Complementary Medicine. 2014;4(4):232–238. doi: 10.4103/2225-4110.139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Subash S., Essa M. M., Al-Adawi S., Memon M. A., Manivasagam T., Akbar M. Neuroprotective effects of berry fruits on neurodegenerative diseases. Neural Regeneration Research. 2014;9(16):1557–1566. doi: 10.4103/1673-5374.139483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Subash S., Essa M. M., Al-Asmi A., Al-Adawi S., Vaishnav R. Chronic dietary supplementation of 4% figs on the modification of oxidative stress in Alzheimer's disease transgenic mouse model. BioMed Research International. 2014;2014:8. doi: 10.1155/2014/546357.546357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Subash S., Essa M. M., Braidy N., et al. Consumption of fig fruits grown in Oman can improve memory, anxiety, and learning skills in a transgenic mice model of Alzheimer's disease. Nutritional Neuroscience. 2016;19(10):475–483. doi: 10.1179/1476830514y.0000000131. [DOI] [PubMed] [Google Scholar]
- 75.Essa M. M., Subash S., Dhanalakshmi C., et al. Dietary supplementation of walnut partially reverses 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced neurodegeneration in a mouse model of Parkinson’s disease. Neurochemical Research. 2015;40(6):1283–1293. doi: 10.1007/s11064-015-1593-2. [DOI] [PubMed] [Google Scholar]
- 76.Essa M. M., Subash S., Akbar M., Al-Adawi S., Guillemin G. J. Long-Term dietary supplementation of pomegranates, figs and dates alleviate neuroinflammation in a transgenic mouse model of alzheimer's disease. PLOS ONE. 2015;25(10) doi: 10.1371/journal.pone.0120964.e0120964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muthaiyah B., Essa M. M., Lee M., Chauhan V., Kaur K., Chauhan A. Dietary supplementation of walnuts improves memory deficits and learning skills in transgenic mouse model of Alzheimer's disease. Journal of Alzheimer's Disease. 2014;42(4):1397–1405. doi: 10.3233/jad-140675. [DOI] [PubMed] [Google Scholar]
- 78.Goldenberg M. M. Medical management of Parkinson's disease. P and T. 2008;33(10):590–606. [PMC free article] [PubMed] [Google Scholar]
- 79.DeMaagd G., Philip A. Parkinson's disease and its management: part 1: disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. Pharmacy and Therapeutics. 2015;40(8):504–532. [PMC free article] [PubMed] [Google Scholar]
- 80.Massano J., Bhatia K. P. Clinical approach to Parkinson's disease: features, diagnosis, and principles of management. Cold Spring Harbor Perspectives in Medicine. 2012;2(6) doi: 10.1101/cshperspect.a008870.a008870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leverenz J. B., Quinn J. F., Zabetian C., Zhang J., Montine K. S., Montine T. J. Cognitive impairment and dementia in patients with Parkinson disease. Current Topics in Medicinal Chemistry. 2009;9(10):903–912. doi: 10.2174/156802609789378218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Savitt J. M., Dawson V. L., Dawson T. M. Diagnosis and treatment of Parkinson disease: molecules to medicine. Journal of Clinical Investigation. 2006;116(7):1744–1754. doi: 10.1172/jci29178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parker W. D., Jr., Parks J. K., Swerdlow R. H. Complex I deficiency in Parkinson's disease frontal cortex. Brain Research. 2008;1189:215–218. doi: 10.1016/j.brainres.2007.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alexander G. E. Biology of Parkinson's disease: pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues in Clinical Neuroscience. 2004;6(3):259–280. doi: 10.31887/DCNS.2004.6.3/galexander. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sarkar S., Raymick J., Imam S. Neuroprotective and therapeutic strategies against Parkinson’s disease: recent perspectives. International Journal of Molecular Sciences. 2016;17(6, article no. 904) doi: 10.3390/ijms17060904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Albin R. L. Parkinson's disease: background, diagnosis, and initial management. Clinics in Geriatric Medicine. 2006;22(4):735–751. doi: 10.1016/j.cger.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 87.Dorsey E. R., Constantinescu R., Thompson J. P., et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 88.Hardy J. Genetic analysis of pathways to parkinson disease. Neuron. 2010;68(2):201–206. doi: 10.1016/j.neuron.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Friedlich A. L., Smith M. A., Zhu X., et al. Oxidative stress in Parkinson's disease. The Open Pathology Journal. 2009;3(1):38–42. doi: 10.2174/1874375700903010038. [DOI] [Google Scholar]
- 90.Cadet J. L., Brannock C. Invited review Free radicals and the pathobiology of brain dopamine systems. Neurochemistry International. 1998;32(2):117–131. doi: 10.1016/s0197-0186(97)00031-4. [DOI] [PubMed] [Google Scholar]
- 91.Zecca L., Shima T., Stroppolo A., et al. Interaction of neuromelanin and iron in substantia nigra and other areas of human brain. Neuroscience. 1996;73(2):407–415. doi: 10.1016/0306-4522(96)00047-4. [DOI] [PubMed] [Google Scholar]
- 92.Gerlach M., Double K. L., Ben-Shachar D., Zecca L., Youdim M. B. H., Riederer P. Neuromelanin and its interaction with iron as a potential risk factor for dopaminergic neurodegeneration underlying Parkinson's disease. Neurotoxicity Research. 2003;5(1-2):35–43. doi: 10.1007/BF03033371. [DOI] [PubMed] [Google Scholar]
- 93.Gorell J. M., Ordidge R. J., Brown G. G., Deniau J.-C., Buderer N. M., Helpern J. A. Increased iron-related MRI contrast in the substantia nigra in Parkinson's disease. Neurology. 1995;45(6):1138–1143. doi: 10.1212/WNL.45.6.1138. [DOI] [PubMed] [Google Scholar]
- 94.Friedlich A. L., Beal M. F. Prospects for redox-based therapy in neurodegenerative diseases. Neurotoxicity Research. 2000;2(2-3):229–237. doi: 10.1007/BF03033796. [DOI] [PubMed] [Google Scholar]
- 95.Atasoy H. T., Nuyan O., Tunc T., Yorubulut M., Unal A. E., Inan L. E. T2-weighted MRI in Parkinson's disease; substantia nigra pars compacta hypointensity correlates with the clinical scores. Neurology India. 2004;52(3):332–337. [PubMed] [Google Scholar]
- 96.Kikuchi A., Takeda A., Onodera H., et al. Systemic increase of oxidative nucleic acid damage in Parkinson's disease and multiple system atrophy. Neurobiology of Disease. 2002;9(2):244–248. doi: 10.1006/nbdi.2002.0466. [DOI] [PubMed] [Google Scholar]
- 97.Faucheux B. A., Martin M.-E., Beaumont C., Hauw J.-J., Agid Y., Hirsch E. C. Neuromelanin associated redox-active iron is increased in the substantia nigra of patients with Parkinson's disease. Journal of Neurochemistry. 2003;86(5):1142–1148. doi: 10.1046/j.1471-4159.2003.01923.x. [DOI] [PubMed] [Google Scholar]
- 98.Dexter D. T., Carter C. J., Wells F. R., et al. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. Journal of Neurochemistry. 1989;52(2):381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 99.Dexter D. T., Wells F. R., Lees A. J., et al. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson's disease. Journal of Neurochemistry. 1989;52(6):1830–1836. doi: 10.1111/j.1471-4159.1989.tb07264.x. [DOI] [PubMed] [Google Scholar]
- 100.Yoritaka A., Hattori N., Uchida K., Tanaka M., Stadtman E. R., Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(7):2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Floor E., Wetzel M. G. Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. Journal of Neurochemistry. 1998;70(1):268–275. doi: 10.1046/j.1471-4159.1998.70010268.x. [DOI] [PubMed] [Google Scholar]
- 102.Ihara Y., Chuda D., Kuroda S., Hayabara T. Hydroxyl radical and superoxide dismutase in blood of patients with Parkinson's disease: relationship to clinical data. Journal of the Neurological Sciences. 1999;170(2):90–95. doi: 10.1016/s0022-510x(99)00192-6. [DOI] [PubMed] [Google Scholar]
- 103.Abraham S., Soundararajan C. C., Vivekanandhan S., Behari M. Erythrocyte antioxidant enzymes in Parkinson's disease. Indian Journal of Medical Research. 2005;121(2):111–115. [PubMed] [Google Scholar]
- 104.Chen C.-M., Liu J.-L., Wu Y.-R., et al. Increased oxidative damage in peripheral blood correlates with severity of Parkinson's disease. Neurobiology of Disease. 2009;33(3):429–435. doi: 10.1016/j.nbd.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 105.Dickson D. W. Linking selective vulnerability to cell death mechanisms in Parkinson's disease. The American Journal of Pathology. 2007;170(1):16–19. doi: 10.2353/ajpath.2007.061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Perry T. L., Godin D. V., Hansen S. Parkinson's disease: a disorder due to nigral glutathione deficiency? Neuroscience Letters. 1982;33(3):305–310. doi: 10.1016/0304-3940(82)90390-1. [DOI] [PubMed] [Google Scholar]
- 107.Sofic E., Lange K. W., Jellinger K., Riederer P. Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson's disease. Neuroscience Letters. 1992;142(2):128–130. doi: 10.1016/0304-3940(92)90355-B. [DOI] [PubMed] [Google Scholar]
- 108.Dias V., Junn E., Mouradian M. M. The role of oxidative stress in parkinson's disease. Journal of Parkinson's Disease. 2013;3(4):461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Perry T. L., Yong V. W. Idiopathic Parkinson's disease, progressive supranuclear palsy and glutathione metabolism in the substantia nigra of patients. Neuroscience Letters. 1986;67(3):269–274. doi: 10.1016/0304-3940(86)90320-4. [DOI] [PubMed] [Google Scholar]
- 110.Surendran S., Rajasankar S. Parkinson's disease: oxidative stress and therapeutic approaches. Neurological Sciences. 2010;31(5):531–540. doi: 10.1007/s10072-010-0245-1. [DOI] [PubMed] [Google Scholar]
- 111.Beal M. F. Experimental models of Parkinson's disease. Nature Reviews Neuroscience. 2001;2(5):325–332. doi: 10.1038/35072550. [DOI] [PubMed] [Google Scholar]
- 112.Todd B. S., Ranjita B., Amy K. S., et al. An in vitro model of Parkinson's disease: linking mitochondrial impairment to altered-synuclein metabolism and oxidative damage. The Journal of Neuroscience. 2002;22(16):7006–7015. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pearce R. K. B., Owen A., Daniel S., Jenner P., Marsden C. D. Alterations in the distribution of glutathione in the substantia nigra in Parkinson's disease. Journal of Neural Transmission. 1997;104(6-7):661–677. doi: 10.1007/BF01291884. [DOI] [PubMed] [Google Scholar]
- 114.Weng J., Tikhonova M. A., Chen J., et al. Ceftriaxone prevents the neurodegeneration and decreased neurogenesis seen in a Parkinson’s disease rat model: an immunohistochemical and MRI study. Behavioural Brain Research. 2016;305:126–139. doi: 10.1016/j.bbr.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 115.Sharma D., Wani W., Sunkaria A., et al. Quercetin attenuates neuronal death against aluminum-induced neurodegeneration in the rat hippocampus. Neuroscience. 2016;324:163–176. doi: 10.1016/j.neuroscience.2016.02.055. [DOI] [PubMed] [Google Scholar]
- 116.Saha M. R., Dey P., Begum S., et al. Effect of Acacia catechu (L.f.) willd. on oxidative stress with possible implications in alleviating selected cognitive disorders. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150574.e0150574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ren R., Shi C., Cao J., et al. Neuroprotective effects of a standardized flavonoid extract of safflower against neurotoxin-induced cellular and animal models of Parkinson's disease. Scientific Reports. 2016;6 doi: 10.1038/srep22135.22135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.De Pedro N., Cantizani J., Ortiz-López F. J., et al. Protective effects of isolecanoric acid on neurodegenerative in vitro models. Neuropharmacology. 2016;101:538–548. doi: 10.1016/j.neuropharm.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 119.Wu C.-R., Tsai C.-W., Chang S.-W., Lin C.-Y., Huang L.-C., Tsai C.-W. Carnosic acid protects against 6-hydroxydopamine-induced neurotoxicity in in vivo and in vitro model of Parkinson's disease: involvement of antioxidative enzymes induction. Chemico-Biological Interactions. 2015;225:40–46. doi: 10.1016/j.cbi.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 120.Siddique Y. H., Faisal M., Naz F., Jyoti S., Rahul Role of Ocimum sanctum leaf extract on dietary supplementation in the transgenic Drosophila model of Parkinson's disease. Chinese Journal of Natural Medicines. 2014;12(10):777–781. doi: 10.1016/S1875-5364(14)60118-7. [DOI] [PubMed] [Google Scholar]
- 121.Antunes M. S., Goes A. T. R., Boeira S. P., Prigol M., Jesse C. R. Protective effect of hesperidin in a model of Parkinson's disease induced by 6-hydroxydopamine in aged mice. Nutrition. 2014;30(11-12):1415–1422. doi: 10.1016/j.nut.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 122.Pérez-Barrón G., Ávila-Acevedo J. G., García-Bores A. M., et al. Neuroprotective effect of Buddleja cordata methanolic extract in the 1-methyl-4-phenylpyridinium Parkinson's disease rat model. Journal of Natural Medicines. 2015;69(1):86–93. doi: 10.1007/s11418-014-0866-4. [DOI] [PubMed] [Google Scholar]
- 123.Beppe G. J., Dongmo A. B., Foyet H. S., et al. Memory-enhancing activities of the aqueous extract of Albizia adianthifolia leaves in the 6-hydroxydopamine-lesion rodent model of Parkinson's disease. BMC Complementary and Alternative Medicine. 2014;14, article 142 doi: 10.1186/1472-6882-14-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gokul K., Muralidhara Oral supplements of aqueous extract of tomato seeds alleviate motor abnormality, oxidative impairments and neurotoxicity induced by rotenone in mice: relevance to Parkinson's disease. Neurochemical Research. 2014;39(7):1382–1394. doi: 10.1007/s11064-014-1323-1. [DOI] [PubMed] [Google Scholar]
- 125.Siddique Y. H., Jyoti S., Naz F. Effect of epicatechin gallate dietary supplementation on transgenic drosophila model of parkinson's disease. Journal of Dietary Supplements. 2014;11(2):121–130. doi: 10.3109/19390211.2013.859207. [DOI] [PubMed] [Google Scholar]
- 126.Khurana N., Gajbhiye A. Ameliorative effect of Sida cordifolia in rotenone induced oxidative stress model of Parkinson's disease. NeuroToxicology. 2013;39:57–64. doi: 10.1016/j.neuro.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 127.Chandran G., Muralidhara M. Neuroprotective effect of aqueous extract of selaginella delicatula as evidenced by abrogation of rotenone-induced motor deficits, oxidative dysfunctions, and neurotoxicity in mice. Cellular and Molecular Neurobiology. 2013;33(7):929–942. doi: 10.1007/s10571-013-9959-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Prakash J., Yadav S. K., Chouhan S., Singh S. P. Neuroprotective role of withania somnifera root extract in maneb-paraquat induced mouse model of parkinsonism. Neurochemical Research. 2013;38(5):972–980. doi: 10.1007/s11064-013-1005-4. [DOI] [PubMed] [Google Scholar]
- 129.Mansouri M. T., Farbood Y., Sameri M. J., Sarkaki A., Naghizadeh B., Rafeirad M. Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food Chemistry. 2013;138(2-3):1028–1033. doi: 10.1016/j.foodchem.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 130.Shalavadi M. H., Chandrashekhar V. M., Avinash S. P., Sowmya C., Ramkishan A. Neuroprotective activity of Stereospermum suaveolens DC against 6-OHDA induced Parkinson's disease model. Indian Journal of Pharmacology. 2012;44(6):737–743. doi: 10.4103/0253-7613.103275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu S.-M., Li X.-Z., Huo Y., Lu F. Protective effect of extract of Acanthopanax senticosus harms on dopaminergic neurons in Parkinson's disease mice. Phytomedicine. 2012;19(7):631–638. doi: 10.1016/j.phymed.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 132.Anandhan A., Tamilselvam K., Radhiga T., Rao S., Essa M. M., Manivasagam T. Theaflavin, a black tea polyphenol, protects nigral dopaminergic neurons against chronic MPTP/probenecid induced Parkinson's disease. Brain Research. 2012;1433:104–113. doi: 10.1016/j.brainres.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 133.Nataraj J., Manivasagam T., Thenmozhi A. J., Essa M. M. Lutein protects dopaminergic neurons against MPTP-induced apoptotic death and motor dysfunction by ameliorating mitochondrial disruption and oxidative stress. Nutritional Neuroscience. 2016;19(6):237–246. doi: 10.1179/1476830515y.0000000010. [DOI] [PubMed] [Google Scholar]
- 134.Nataraj J., Manivasagam T., Justin Thenmozhi A., Essa M. M. Neuroprotective effect of asiatic acid on rotenone-induced mitochondrial dysfunction and oxidative stress-mediated apoptosis in differentiated SH-SYS5Y cells. Nutritional Neuroscience. 2016 doi: 10.1080/1028415x.2015.1135559. [DOI] [PubMed] [Google Scholar]
- 135.Dhanalakshmi C., Manivasagam T., Nataraj J., Justin Thenmozhi A., Essa M. M. Neurosupportive role of vanillin, a natural phenolic compound, on rotenone induced neurotoxicity in SH-SY5Y neuroblastoma cells. Evidence-Based Complementary and Alternative Medicine. 2015;2015:11. doi: 10.1155/2015/626028.626028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tamilselvam K., Braidy N., Manivasagam T., et al. Neuroprotective effects of hesperidin, a plant flavanone, on rotenone-induced oxidative stress and apoptosis in a cellular model for Parkinson's disease. Oxidative Medicine and Cellular Longevity. 2013;2013:11. doi: 10.1155/2013/102741.102741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Braidy N., Selvaraju S., Essa M. M., et al. Neuroprotective effects of a variety of pomegranate juice extracts against MPTP-induced cytotoxicity and oxidative stress in human primary neurons. Oxidative Medicine and Cellular Longevity. 2013;2013:12. doi: 10.1155/2013/685909.685909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ahmad S., Khan M. B., Hoda M. N., et al. Neuroprotective effect of sesame seed oil in 6-hydroxydopamine induced neurotoxicity in mice model: cellular, biochemical and neurochemical evidence. Neurochemical Research. 2012;37(3):516–526. doi: 10.1007/s11064-011-0638-4. [DOI] [PubMed] [Google Scholar]
- 139.Martins E. N., Pessano N. T. C., Leal L., et al. Protective effect of Melissa officinalis aqueous extract against Mn-induced oxidative stress in chronically exposed mice. Brain Research Bulletin. 2012;87(1):74–79. doi: 10.1016/j.brainresbull.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 140.Hritcu L., Foyet H. S., Stefan M., Mihasan M., Asongalem A. E., Kamtchouing P. Neuroprotective effect of the methanolic extract of Hibiscus asper leaves in 6-hydroxydopamine-lesioned rat model of Parkinson's disease. Journal of Ethnopharmacology. 2011;137(1):585–591. doi: 10.1016/j.jep.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 141.Ranpariya V. L., Parmar S. K., Sheth N. R., Chandrashekhar V. M. Neuroprotective activity of Matricaria recutita against fluoride-induced stress in rats. Pharmaceutical Biology. 2011;49(7):696–701. doi: 10.3109/13880209.2010.540249. [DOI] [PubMed] [Google Scholar]
- 142.Wang Y., Xu H., Fu Q., Ma R., Xiang J. Protective effect of resveratrol derived from Polygonum cuspidatum and its liposomal form on nigral cells in Parkinsonian rats. Journal of the Neurological Sciences. 2011;304(1-2):29–34. doi: 10.1016/j.jns.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 143.Verma R., Nehru B. Effect of centrophenoxine against rotenone-induced oxidative stress in an animal model of Parkinson's disease. Neurochemistry International. 2009;55(6):369–375. doi: 10.1016/j.neuint.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 144.Kaur H., Chauhan S., Sandhir R. Protective effect of lycopene on oxidative stress and cognitive decline in rotenone induced model of Parkinson's disease. Neurochemical Research. 2011;36(8):1435–1443. doi: 10.1007/s11064-011-0469-3. [DOI] [PubMed] [Google Scholar]
- 145.Khan M. M., Raza S. S., Javed H., et al. Rutin protects dopaminergic neurons from oxidative stress in an animal model of Parkinson's disease. Neurotoxicity Research. 2012;22(1):1–15. doi: 10.1007/s12640-011-9295-2. [DOI] [PubMed] [Google Scholar]
- 146.Essa M. M., Subash S., Dhanalakshmi C., et al. Dietary supplementation of walnut partially reverses 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced neurodegeneration in a mouse model of Parkinson's disease. Neurochemical Research. 2015;40(6):1283–1293. doi: 10.1007/s11064-015-1593-2. [DOI] [PubMed] [Google Scholar]
- 147.Jahromi S. R., Haddadi M., Shivanandappa T., Ramesh S. R. Modulatory effect of Decalepis hamiltonii on ethanol-induced toxicity in transgenic Drosophila model of Parkinson's disease. Neurochemistry International. 2015;80:1–6. doi: 10.1016/j.neuint.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 148.Tseng Y.-T., Chang F.-R., Lo Y.-C. The Chinese herbal formula Liuwei dihuang protects dopaminergic neurons against Parkinson's toxin through enhancing antioxidative defense and preventing apoptotic death. Phytomedicine. 2014;21(5):724–733. doi: 10.1016/j.phymed.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 149.Guo B., Xu D., Duan H., et al. Therapeutic effects of multifunctional tetramethylpyrazine nitrone on models of Parkinson's disease in vitro and in vivo. Biological and Pharmaceutical Bulletin. 2014;37(2):274–285. doi: 10.1248/bpb.b13-00743. [DOI] [PubMed] [Google Scholar]
- 150.Sudati J. H., Vieira F. A., Pavin S. S., et al. Valeriana officinalis attenuates the rotenone-induced toxicity in drosophila melanogaster . NeuroToxicology. 2013;37:118–126. doi: 10.1016/j.neuro.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 151.Pasban-Aliabadi H., Esmaeili-Mahani S., Sheibani V., Abbasnejad M., Mehdizadeh A., Yaghoobi M. M. Inhibition of 6-hydroxydopamine-induced PC12 cell apoptosis by olive (Olea europaea L.) leaf extract is performed by its main component oleuropein. Rejuvenation Research. 2013;16(2):134–142. doi: 10.1089/rej.2012.1384. [DOI] [PubMed] [Google Scholar]
- 152.Kim K.-H., Song K., Yoon S.-H., Shehzad O., Kim Y.-S., Son J. H. Rescue of PINK1 protein null-specific mitochondrial complex IV deficits by ginsenoside Re activation of nitric oxide signaling. The Journal of Biological Chemistry. 2012;287(53):44109–44120. doi: 10.1074/jbc.m112.408146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li S., Pu X.-P. Neuroprotective effect of kaempferol against a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson's disease. Biological and Pharmaceutical Bulletin. 2011;34(8):1291–1296. doi: 10.1248/bpb.34.1291. [DOI] [PubMed] [Google Scholar]
- 154.Liang Z., Shi F., Wang Y., et al. Neuroprotective effects of tenuigenin in a SH-SY5Y cell model with 6-OHDA-induced injury. Neuroscience Letters. 2011;497(2):104–109. doi: 10.1016/j.neulet.2011.04.041. [DOI] [PubMed] [Google Scholar]
- 155.Hu S., Han R., Mak S., Han Y. Protection against 1-methyl-4-phenylpyridinium ion (MPP+)-induced apoptosis by water extract of ginseng (Panax ginseng C.A. Meyer) in SH-SY5Y cells. Journal of Ethnopharmacology. 2011;135(1):34–42. doi: 10.1016/j.jep.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 156.Choi J. G., Kim H. G., Kim M. C., et al. Polygalae radix inhibits toxin-induced neuronal death in the Parkinson's disease models. Journal of Ethnopharmacology. 2011;134(2):414–421. doi: 10.1016/j.jep.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 157.Sengupta T., Vinayagam J., Nagashayana N., Gowda B., Jaisankar P., Mohanakumar K. P. Antiparkinsonian effects of aqueous methanolic extract of Hyoscyamus niger seeds result from its monoamine oxidase inhibitory and hydroxyl radical scavenging potency. Neurochemical Research. 2011;36(1):177–186. doi: 10.1007/s11064-010-0289-x. [DOI] [PubMed] [Google Scholar]
- 158.An L., Liu S., Dong Y., Tang B., Dong W. Protective effect of effective part of Acanthopanacis senticosus on damage of PC12 cells induced by MPP+ Zhongguo Zhongyao Zazhi. 2010;35(15):2021–2026. doi: 10.4268/cjcmm20101524. [DOI] [PubMed] [Google Scholar]
- 159.Kim H. G., Ju M. S., Kim D.-H., et al. Protective effects of Chunghyuldan against ROS-mediated neuronal cell death in models of Parkinson's disease. Basic & Clinical Pharmacology & Toxicology. 2010;107(6):958–964. doi: 10.1111/j.1742-7843.2010.00612.x. [DOI] [PubMed] [Google Scholar]
- 160.Lee C.-H., Hwang D.-S., Kim H. G., et al. Protective effect of Cyperi Rhizoma against 6-hydroxydopamine-induced neuronal damage. Journal of Medicinal Food. 2010;13(3):564–571. doi: 10.1089/jmf.2009.1252. [DOI] [PubMed] [Google Scholar]
- 161.Shu D., He J., Chen J. Neuroprotective effects and mechanisms of Chuanxiong Chatiao pulvis against MPTP-induced dopaminergic neurotoxicity in mice model of parkinson's disease. Zhongguo Zhongyao Zazhi. 2009;34(19):2494–2497. [PubMed] [Google Scholar]
- 162.Shim J. S., Kim H. G., Ju M. S., Choi J. G., Jeong S. Y., Oh M. S. Effects of the hook of Uncaria rhynchophylla on neurotoxicity in the 6-hydroxydopamine model of Parkinson's disease. Journal of Ethnopharmacology. 2009;126(2):361–365. doi: 10.1016/j.jep.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 163.Sankar S. R., Manivasagam T., Krishnamurti A., Ramanathan M. The neuroprotective effect of Withania somnifera root extract in MPTP-intoxicated mice: an analysis of behavioral and biochemical varibles. Cellular and Molecular Biology Letters. 2007;12(4):473–481. doi: 10.2478/s11658-007-0015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ahmad M., Yousuf S., Khan M. B., et al. Protective effects of ethanolic extract of Delphinium denudatum in a rat model of Parkinson's disease. Human and Experimental Toxicology. 2006;25(7):361–368. doi: 10.1191/0960327106ht635oa. [DOI] [PubMed] [Google Scholar]
- 165.Ahmad M., Yousuf S., Khan M. B., et al. Attenuation by Nardostachys jatamansi of 6-hydroxydopamine-induced parkinsonism in rats: behavioral, neurochemical, and immunohistochemical studies. Pharmacology Biochemistry and Behavior. 2006;83(1):150–160. doi: 10.1016/j.pbb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 166.Zhang S., Shao S.-Y., Song X.-Y., et al. Protective effects of Forsythia suspense extract with antioxidant and anti-inflammatory properties in a model of rotenone induced neurotoxicity. NeuroToxicology. 2016;52:72–83. doi: 10.1016/j.neuro.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 167.Lu K.-T., Ko M.-C., Chen B.-Y., et al. Neuroprotective effects of resveratrol on MPTP-induced neuron loss mediated by free radical scavenging. Journal of Agricultural and Food Chemistry. 2008;56(16):6910–6913. doi: 10.1021/jf8007212. [DOI] [PubMed] [Google Scholar]
- 168.Mythri R. B., Srinivas Bharath M. M. Curcumin: a potential neuroprotective agent in Parkinson's disease. Current Pharmaceutical Design. 2012;18(1):91–99. doi: 10.2174/138161212798918995. [DOI] [PubMed] [Google Scholar]
- 169.Szlachcic W. J., Switonski P. M., Krzyzosiak W. J., Figlerowicz M., Figiel M. Huntington disease iPSCs show early molecular changes in intracellular signaling, the expression of oxidative stress proteins and the p53 pathway. DMM Disease Models and Mechanisms. 2015;8(9):1047–1057. doi: 10.1242/dmm.019406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li X., Valencia A., Sapp E., et al. Aberrant Rab11-dependent trafficking of the neuronal glutamate transporter EAAC1 causes oxidative stress and cell death in Huntington's disease. Journal of Neuroscience. 2010;30(13):4552–4561. doi: 10.1523/jneurosci.5865-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sari Y. Huntington's disease: from mutant huntingtin protein to neurotrophic factor therapy. International Journal of Biomedical Science. 2011;7(2):89–100. [PMC free article] [PubMed] [Google Scholar]
- 172.Walker F. O. Huntington's disease. Lancet. 2007;369(9557):218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- 173.Rotblat B., Southwell A. L., Ehrnhoefer D. E., et al. HACE1 reduces oxidative stress and mutant Huntingtin toxicity by promoting the NRF2 response. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(8):3032–3037. doi: 10.1073/pnas.1314421111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Johri A., Beal M. F. Antioxidants in Huntington's disease. Biochimica et Biophysica Acta. 2012;1822(5):664–674. doi: 10.1016/j.bbadis.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Trushina E., McMurray C. T. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience. 2007;145(4):1233–1248. doi: 10.1016/j.neuroscience.2006.10.056. [DOI] [PubMed] [Google Scholar]
- 176.Sayre L. M., Perry G., Smith M. A. Oxidative stress and neurotoxicity. Chemical Research in Toxicology. 2008;21(1):172–188. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- 177.Lee D.-H., Gold R., Linker R. A. Mechanisms of oxidative damage in multiple sclerosis and neurodegenerative diseases: therapeutic modulation via fumaric acid esters. International Journal of Molecular Sciences. 2012;13(9):11783–11803. doi: 10.3390/ijms130911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chen C.-M., Wu Y.-R., Cheng M.-L., et al. Increased oxidative damage and mitochondrial abnormalities in the peripheral blood of Huntington's disease patients. Biochemical and Biophysical Research Communications. 2007;359(2):335–340. doi: 10.1016/j.bbrc.2007.05.093. [DOI] [PubMed] [Google Scholar]
- 179.Choudhary S., Kumar P., Malik J. Plants and phytochemicals for Huntington′s disease. Pharmacognosy Reviews. 2013;7(14):81–91. doi: 10.4103/0973-7847.120505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gu M., Gash M. T., Mann V. M., Javoy-Agid F., Cooper J. M., Schapira A. H. V. Mitochondrial defect in Huntington's disease caudate nucleus. Annals of Neurology. 1996;39(3):385–389. doi: 10.1002/ana.410390317. [DOI] [PubMed] [Google Scholar]
- 181.Túnez I., Sánchez-López F., Agüera E., Fernández-Bolaños R., Sánchez F. M., Tasset-Cuevas I. Important role of oxidative stress biomarkers in Huntington's disease. Journal of Medicinal Chemistry. 2011;54(15):5602–5606. doi: 10.1021/jm200605a. [DOI] [PubMed] [Google Scholar]
- 182.Klepac N., Relja M., Klepac R., Hećimović S., Babić T., Trkulja V. Oxidative stress parameters in plasma of Huntington's disease patients, asymptomatic Huntington's disease gene carriers and healthy subjects: a cross-sectional study. Journal of Neurology. 2007;254(12):1676–1683. doi: 10.1007/s00415-007-0611-y. [DOI] [PubMed] [Google Scholar]
- 183.Goula A.-V., Berquist B. R., Wilson D. M., III, Wheeler V. C., Trottier Y., Merienne K. Stoichiometry of base excision repair proteins correlates with increased somatic CAG instability in striatum over cerebellum in Huntington's disease transgenic mice. PLoS Genetics. 2009;5(12) doi: 10.1371/journal.pgen.1000749.e1000749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Browne S. E., Bowling A. C., MacGarvey U., et al. Oxidative damage and metabolic dysfunction in huntington's disease: selective vulnerability of the basal ganglia. Annals of Neurology. 1997;41(5):646–653. doi: 10.1002/ana.410410514. [DOI] [PubMed] [Google Scholar]
- 185.Ribeiro M., Rosenstock T. R., Cunha-Oliveira T., Ferreira I. L., Oliveira C. R., Rego A. C. Glutathione redox cycle dysregulation in Huntington's disease knock-in striatal cells. Free Radical Biology and Medicine. 2012;53(10):1857–1867. doi: 10.1016/j.freeradbiomed.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 186.Stoy N., Mackay G. M., Forrest C. M., et al. Tryptophan metabolism and oxidative stress in patients with Huntington's disease. Journal of Neurochemistry. 2005;93(3):611–623. doi: 10.1111/j.1471-4159.2005.03070.x. [DOI] [PubMed] [Google Scholar]
- 187.Duran R., Barrero F. J., Morales B., Luna J. D., Ramirez M., Vives F. Oxidative stress and plasma aminopeptidase activity in Huntington's disease. Journal of Neural Transmission. 2010;117(3):325–332. doi: 10.1007/s00702-009-0364-0. [DOI] [PubMed] [Google Scholar]
- 188.Oliveira A. M., Cardoso S. M., Ribeiro M., Seixas R. S. G. R., Silva A. M. S., Rego A. C. Protective effects of 3-alkyl luteolin derivatives are mediated by Nrf2 transcriptional activity and decreased oxidative stress in Huntington's disease mouse striatal cells. Neurochemistry International. 2015;91:1–12. doi: 10.1016/j.neuint.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 189.Shivasharan B. D., Nagakannan P., Thippeswamy B. S., Veerapur V. P., Bansal P., Unnikrishnan M. K. Protective effect of Calendula officinalis Linn. flowers against 3-nitropropionic acid induced experimental Huntington's disease in rats. Drug and Chemical Toxicology. 2013;36(4):466–473. doi: 10.3109/01480545.2013.776583. [DOI] [PubMed] [Google Scholar]
- 190.Mahdy H. M., Tadros M. G., Mohamed M. R., Karim A. M., Khalifa A. E. The effect of Ginkgo biloba extract on 3-nitropropionic acid-induced neurotoxicity in rats. Neurochemistry International. 2011;59(6):770–778. doi: 10.1016/j.neuint.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 191.Tasset I., Pontes A. J., Hinojosa A. J., de la Torre R., Túnez I. Olive oil reduces oxidative damage in a 3-nitropropionic acid-induced huntington's disease-like rat model. Nutritional Neuroscience. 2011;14(3):106–111. doi: 10.1179/1476830511Y.0000000005. [DOI] [PubMed] [Google Scholar]
- 192.Sagredo O., Pazos M. R., Satta V., Ramos J. A., Pertwee R. G., Fernández-Ruiz J. Neuroprotective effects of phytocannabinoid-based medicines in experimental models of Huntington's disease. Journal of Neuroscience Research. 2011;89(9):1509–1518. doi: 10.1002/jnr.22682. [DOI] [PubMed] [Google Scholar]
- 193.Gao Y., Chu S.-F., Li J.-P., et al. Protopanaxtriol protects against 3-nitropropionic acid-induced oxidative stress in a rat model of Huntington's disease. Acta Pharmacologica Sinica. 2015;36(3):311–322. doi: 10.1038/aps.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Túnez I., Montilla P., Del Carmen Muñoz M., Feijóo M., Salcedo M. Protective effect of melatonin on 3-nitropropionic acid-induced oxidative stress in synaptosomes in an animal model of Huntington's disease. Journal of Pineal Research. 2004;37(4):252–256. doi: 10.1111/j.1600-079x.2004.00163.x. [DOI] [PubMed] [Google Scholar]
- 195.Rocha-González H. I., Ambriz-Tututi M., Granados-Soto V. Resveratrol: a natural compound with pharmacological potential in neurodegenerative diseases. CNS Neuroscience and Therapeutics. 2008;14(3):234–247. doi: 10.1111/j.1755-5949.2008.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Andreassen O. A., Ferrante R. J., Dedeoglu A., Beal M. F. Lipoic acid improves survival in transgenic mouse models of Huntington's disease. NeuroReport. 2001;12(15):3371–3373. doi: 10.1097/00001756-200110290-00044. [DOI] [PubMed] [Google Scholar]
- 197.Ehrnhoefer D. E., Duennwald M., Markovic P., et al. Green tea (-)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington's disease models. Human Molecular Genetics. 2006;15(18):2743–2751. doi: 10.1093/hmg/ddl210. [DOI] [PubMed] [Google Scholar]
- 198.Denny Joseph K. M., Muralidhara Enhanced neuroprotective effect of fish oil in combination with quercetin against 3-nitropropionic acid induced oxidative stress in rat brain. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;40(1):83–92. doi: 10.1016/j.pnpbp.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 199.Fu J., Jin J., Cichewicz R. H., et al. Trans-(-)-ε-viniferin increases mitochondrial sirtuin 3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of huntington disease. Journal of Biological Chemistry. 2012;287(29):24460–24472. doi: 10.1074/jbc.m112.382226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Huang N., Lin J., Lin J., et al. A new drug design targeting the adenosinergic system for Huntington's disease. PLoS ONE. 2011;6(6) doi: 10.1371/journal.pone.0020934.20934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ranpariya V. L., Parmar S. K., Sheth N. R., Chandrashekhar V. M. Neuroprotective activity of Matricaria recutita against fluoride-induced stress in rats. Pharmaceutical Biology. 2011;49(7):696–701. doi: 10.3109/13880209.2010.540249. [DOI] [PubMed] [Google Scholar]
- 202.Kumar P., Kumar A. Possible neuroprotective effect of withania somnifera root extract against 3-Nitropropionic acid-induced behavioral, biochemical, and mitochondrial dysfunction in an animal model of huntington's disease. Journal of Medicinal Food. 2009;12(3):591–600. doi: 10.1089/jmf.2008.0028. [DOI] [PubMed] [Google Scholar]
- 203.Shinomol G. K., Muralidhara Prophylactic neuroprotective property of Centella asiatica against 3-nitropropionic acid induced oxidative stress and mitochondrial dysfunctions in brain regions of prepubertal mice. NeuroToxicology. 2008;29(6):948–957. doi: 10.1016/j.neuro.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 204.Kaur M., Prakash A., Kalia A. N. Neuroprotective potential of antioxidant potent fractions from Convolvulus pluricaulis Chois. in 3-nitropropionic acid challenged rats. Nutritional Neuroscience. 2016;19(2):70–78. doi: 10.1179/1476830515y.0000000022. [DOI] [PubMed] [Google Scholar]
- 205.Al-Sabahi B. N., Fatope M. O., Essa M. M., et al. Pomegranate seed oil: effect on 3-nitropropionic acid-induced neurotoxicity in PC12 cells and elucidation of unsaturated fatty acids composition. Nutritional Neuroscience. 2017;20(1):40–48. doi: 10.1179/1476830514y.0000000155. [DOI] [PubMed] [Google Scholar]