Abstract
Panax ginseng Meyer, belonging to the genus Panax of the family Araliaceae, is known for its human immune system-related effects, such as immune-boosting effects. Ginseng polysaccharides (GPs) are the responsible ingredient of ginseng in immunomodulation, and are classified as acidic and neutral GPs. Although GPs participate in various immune reactions including the stimulation of immune cells and production of cytokines, the precise function of GPs together with its potential receptor(s) and their signal transduction pathways have remained largely unknown. Animal lectins are carbohydrate-binding proteins that are highly specific for sugar moieties. Among many different biological functions in vivo, animal lectins especially play important roles in the immune system by recognizing carbohydrates that are found exclusively on pathogens or that are inaccessible on host cells. This review summarizes the immunological activities of GPs and the diverse roles of animal lectins in the immune system, suggesting the possibility of animal lectins as the potential receptor candidates of GPs and giving insights into the development of GPs as therapeutic biomaterials for many immunological diseases.
Keywords: animal lectins, ginseng polysaccharides, immune system, Panax ginseng, therapeutic biomaterials
1. Introduction
1.1. Ginseng polysaccharides
1.1.1. Ginseng
Panax ginseng Meyer is a well-known medicinal plant in the world. The ginseng is a deciduous perennial belonging to the family Araliaceae and genus Panax. The genus name of ginseng, Panax, is derived from the Greek pan (all) akos (cure), meaning “cure-all” or “all healing,” which describes the traditional belief that ginseng has properties to heal all aspects of the body. The name ginseng comes from the Chinese words “Jen Sheng,” meaning “man-herb,” because of the humanoid shape of the root or rhizome of the plant, which is the part of the plant most commonly used for extraction [1], [2]. There are about 13 different species of ginseng which have being identified all over the world. Among them, the most commonly used species of ginseng are Asian ginseng (P. ginseng Meyer, Renshen) and American ginseng (Panax quinquefolius L., Xiyangshen) which all belong to the Panax genus of the Araliaceae family [3]. Asian ginseng has been used for thousands of years as a tonic to improve overall health, restore the body to balance, help the body to heal itself, and reduce stress [4], and American ginseng has been used by Native Americans for at least hundreds of years [2], [5]. Ginseng is prepared and used in several ways as fresh ginseng (sliced and eaten, or brewed in a tea), white ginseng (peeled and dried), red ginseng (peeled, steamed, and dried), extract (tincture or boiled extract), powder, tea, tablet, or capsule [1], [2]. It has been reported that ginseng exhibits a wide range of beneficial pharmacological effects including immunomodulation, antitumor, antioxidation, antidepression, hypoglycemic, inhibition of gastric lesions, attenuation of leptin-induced cardiac hypertrophy, heart protection against ischemia and reperfusion injury, prevention of glucose-induced oxidative stress, prevention of diabetic nephropathy, retinopathy, and cardiomyopathy [6], [7], [8], [9], [10]. This broad spectrum of biological activity of ginseng has originated from its multiple bioactive components, namely ginsenosides, polysaccharides (PSs), peptides, polyacetylenic alcohols, and gintonin [11], [12], [13].
1.1.2. The composition of ginseng polysaccharides
Ginsenosides were considered to be responsible for most of ginseng's pharmacological effects. However, recent studies indicate that ginseng polysaccharides (GPs), one of the active components of ginseng [14], also possess a wide range of biological and pharmaceutical activities, including immune-modulation, antitumor, antiadhesive, antioxidant and hypoglycemic activities [8], [15]. Especially, GPs are known for their immunostimulatory effects [10], [16], [17] and a major contributor to the bioactivity of herbal medicines, providing great potential applications in food, pharmaceuticals, and other industries. Therefore, GPs were extensively studied for their constituents and chemical structures. GPs are biopolymers formed from a complex chain of monosaccharides rich in l-arabinose, d-galactose, l-rhamnose, d-galacturonic acid, d-glucuronic acid, and d-galactosyl residue linked together through glycosidic bonds, resulting in complex macromolecular architectures [7], [18], [19]. Their molecular weights range from 3500 Da to 2,000,000 Da [19], which contributes to their diverse physicochemical properties and biological activities [8], [15], [19], [20].
GPs include acidic and neutral PSs. The pharmacological effects of GPs, including immunomodulation, can be attributed to these acidic and neutral PS components [15]. While the acidic GPs contain different amounts of uronic acids and neutral sugars [15], [21], the neutral PSs mainly contain different ratios of neutral sugar residues [3]. So far, the studies about American GPs have mainly been centered on acidic PSs, resulting in relatively limited research that explores neutral PSs. However, researchers also have interest in neutral PSs of American GPs, because neutral PSs are also one of the important active components in the American ginseng roots. The PSs from ginseng roots have many bioactivities, such as immunomodulation, antitumor, and hypoglycemic activities [11], [22], and contain 60% neutral starch-like PSs, 15% arabinogalactans, and 25% pectins [20]. Similarly, the PSs from ginseng leaves are also bioactive, and contain about 70% arabinogalactans and 20% pectins.
1.1.3. The immune functions of GPs
GPs enable enhancement of the production of cytokines and reactive oxygen species by stimulating macrophages [23], [24]. In a recent study, GP was shown to stimulate dendritic cells (DCs) resulting in enhanced production of interferon-γ (IFN-γ) [25]. It was reported that acidic GPs promoted the production of cytotoxic cells against tumors and stimulated macrophages to produce helper types 1 and 2 (Th1 and Th2) cytokines [26], [27]. An acidic GP from P. ginseng has been shown to display immunomodulatory effects either in an immunostimulatory or in an immunosuppressive manner, depending on timing of treatments and disease environments [28]. This acidic GP was also shown to modulate the antioxidant defense systems such as superoxide dismutase and glutathione peroxidase enzymes, probably via inducing regulatory cytokines [15], [29]. Therefore, acidic GPs have been considered as the major bioactive species for immune modulations. Tomoda et al [30] reported that two acidic PSs of P. ginseng enhance the phagocytic activity of macrophages, and Sonoda et al [31] found that an acidic GP of P. ginseng was a potent inducer of interleukin-8 (IL-8) production by human monocytes and THP-1 cells. Shin et al [32] reported that an acidic PS of P. ginseng shows immune modulatory activities via macrophage NO production. Recently, Lemmon et al [29] reported that the immunostimulatory effects of acidic GPs of P. quinquefolius are mediated by PS with a molecular weight higher than 100 kDa. It was reported that acidic GPs promoted the production of cytotoxic cells against tumors and stimulated macrophages to produce helper types 1 and 2 (Th1 and Th2) cytokines [26], [27]. Intravenous pretreatment of GP attenuated the production of serum proinflammatory and antiinflammatory cytokines after septic bacterial infection [33].
In addition, Ginsan, an acidic GP from of P. ginseng, is a well-known medicinal herb and has been shown to have critical effects on immune cells, which shows an immunomodulatory acidic GP from P. ginseng [27]. Kim et al [26] showed that Ginsan induces Th1 cell and macrophage cytokines. Ginsan enhances the production of cytokines and reactive oxygen species by macrophages [24] and stimulates the phagocytic activity of macrophages [23]. Also, Ginsan induces the maturation of DCs [25], profoundly enhancing the production of IL-12, IL-10, and tumor necrosis factor alpha (TNF-α) by DCs and showed that Ginsan may modulate DC function by altering cytokine levels [25]. For neutral GPs, it was reported that neutral GPs of P. ginseng stimulate the proliferation of lymphocytes, increase the cytotoxicity of natural killer cell, enhance the phagocytosis and NO production by macrophages, and increase the level of TNF-α in serum [21], [34]. Due to these results, many scientists have considered both acidic and neutral GPs as stimulators in the immune system. X. Zhang et al and Kim et al reported that both acidic and neutral GPs of P. ginseng (Asian ginseng) may stimulate B cells, T cells and macrophages [20], [35]. In addition, they considered the relation of acidic and neutral GPs as the supporter, in which neutral GPs help the enhancement of immunostimulatory effects of acidic GPs. In fact, W. Ni et al reported that neutral GPs of P. ginseng enhance macrophage production of NO [21].
On the contrary to immunostimulatory effects of GPs, recent studies showed that GPs also suppress the proinflammatory responses. Recently, it was reported that a novel neutral PS (PPQN, 3.1 kDa) was isolated from American ginseng roots and could suppress inflammation by inhibiting the secretion of inflammatory-related mediator nitric oxide (NO) and cytokines (TNF-α, IL-6, and IL-1β) compared to Lipopolysaccharide (LPS) treatment, implicating the therapeutic implications of PPQN in inflammatory-related diseases like tumors, atherosclerosis, and so on [3]. As an example, one study reported that GPs inhibit immunological responses associated with collagen-induced arthritis [36]. Other studies also suggest that CVT-E002, a poly-furanosyl-pyranosyl polysaccharide-rich herbal and unique extract product of the root of American ginseng (P. quinquefolium), suppresses the inflammatory immune responses, reducing the activation of neutrophils [37], inducing the production of IL-2, IFN-γ, TNF-α, and IL-6 in spleen [7], [19], [38], and increasing the proliferation of splenic B lymphocytes, bone marrow, and natural killer cells.
1.1.4. The working mechanisms of GPs in pathogen protection
Intravenous pretreatment of GP attenuated the production of serum proinflammatory and antiinflammatory cytokines after septic bacterial infection [33]. Also, this intravenous pretreatment of GPs in mice enhances macrophage-mediated bactericidal activity by reducing the number of Staphylococcus aureus which is present in the spleen, kidney, and blood and exerts a protective effect against infected septic mice by suppressing early acute inflammation [33], [39]. In addition, recent studies reported that pretreatment with GP suppressed acute inflammatory responses at an early phase resulting in the enhancement of antimicrobial activities and protection of mice from Staphylococcus aureus-induced sepsis as an antiinflammatory function [33], [39]. As an example, CVT-E002 has been shown to be effective for preventing acute respiratory illness caused by influenza and respiratory syncytial virus [7], [40]. Another study revealed that intranasal administration of GPs showed a protective effect on influenza viral infection by lowering the levels of inflammatory cytokines (IL-6) and lung viral titers [28]. Because GPs were reported to significantly increase the viability of peritoneal macrophage cells [8] and ginseng was shown to inhibit degradation of long-lived proteins and to stimulate protein synthesis similar to polypeptide growth factors [41], it was suggested that maintaining the cell viability under the condition of viral infection-induced stress might be an another alternative mechanism for the protective effects of GP.
It was reported that the recognition and binding of plant PSs by Toll-like receptor 4 (TLR4) leads to the recruitment of various cytoplasmic Toll/IL-1 receptor (TIR) domain-containing adaptors such as myeloid differentiation factor 88 (MyD88), TIR domain-containing adaptor protein (TIRAP), and TIR (Toll/interleukin-1 receptor)-domain-containing adapter-inducing interferon-β (TRIF)-related adaptor molecule (TRAM) [42]. It was also shown that the expression of TLRs including TLR2, TLR4, and the adaptor molecule MyD88 is significantly reduced in murine macrophages by GP pretreatment in vitro, which were increased in murine macrophages with the stimulation of S. aureus [33], [39]. On the contrary, Friedl et al [16] and Lemmon et al [29] showed that American GP extracts may mediate the immunostimulatory effect by the inducible nitric oxide synthase (iNOS), mitogen-activated protein kinase (MAPK) kinases such as p38, extracellular signal-regulated kinases 1/2 (ERK1/2), phosphoinositide 3-kinase (PI3K), and nuclear factor kappa B (NF-kB) signaling pathways [16], [29]. Therefore, these results suggest that GPs might be associated with the ability of the extract's PS fractions to bind to TLR4 receptor and upregulate or downregulate TLR4 receptor expression, which triggers or inhibits the intracellular signaling cascades and the production of proinflammatory mediators under basal or LPS endotoxic conditions, respectively.
1.2. Animal lectins
1.2.1. Introduction to animal lectins
Animal lectins are carbohydrate-binding proteins which are highly variable in their amino acid sequences, widely distributed in microorganisms, viruses, animals and higher plants with different functions, structures, tissue localizations, and carbohydrate-binding specificities [43]. Animal lectins were discovered before plant lectins, although many were not recognized as carbohydrate binding proteins for many years after first being reported [44]. Although plant and animal lectins do not have homologous primary structures, they have similar preferential binding to carbohydrates [45]. Animal lectins are neither immune origin nor catalyst, in contrast to antibodies or enzymes, and are able to detect or bind complex carbohydrate structures specifically through the carbohydrate-recognition domain (CRD) [46], [47]. Each animal lectin possess its own CRD which has an identical sequence motif of 115 to 130 amino acid residues and four cysteines that is thoroughly conserved and form two disulfide bonds [47], [48].
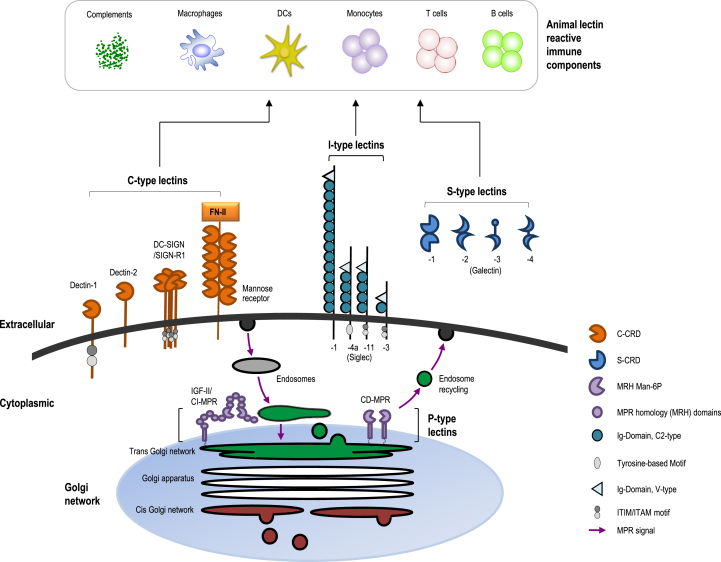
Animal lectin activity is found in association with an astonishing diversity of primary structures [44]. At least 12 structural families are known to exist and bind structures other than carbohydrates via protein-protein, protein-lipid or protein-nucleic acid interactions [44]. Their roles in glycol-recognition systems include complement activation, cell recognition, cell adhesion, cell migration, cell signaling, and morphogenesis. Moreover, animal lectins are able to take part in defense mechanisms, importantly by recognizing a carbohydrate of pathogens [47], [48]. Animal lectins are means of attachment to various cells or viruses via the surface carbohydrate types of the cells to be attached [49]. The function in recognition or cell surface interaction of animal lectins has been implicated in direct first-line defense against pathogens [44]. For example, the mannose specific receptor, presented on the surface of macrophages, can bind to the infectious organisms which expose mannose-containing glycans on their surface, enabling them to ingest and kill the foreign organisms [49]. In addition, animal lectins are involved in cell trafficking, immune regulation, and prevention of autoimmunity [44]. Animal lectins are classified into four families based on structure or function. These are the C-type (calcium-requiring) lectins, P-type (mannose-6-phosphate binding) lectins, S-type (galectins) lectins, and I-type (immunoglobulin-like) lectins (Fig. 1) [47]. Within each family, they have similar sequences and structural properties [46]. Recently, other lectin types have been found, including M-type, L-type, chitinase-like, and F-type lectins. In this review, four traditional families of animal lectins are introduced (Table 1).
Fig. 1.
The structure, cellular distribution, and reactive immune components of animal lectins. Domain structure models, the location of several representative animal lectins, and the immune cells reacting with animal lectins. Carbohydrate-recognition domains (CRDs) of each lectin are depicted in the respective whole structures. S-type lectins are secreted into the extracellular matrix of body fluids. C-type and I-type lectins are localized to the plasma membrane, and P-type lectins are located in luminal compartments of the secretory pathway. CD-MPR, cation-dependent mannose-6-phosphate receptor; CI-MPR, cation-independent mannose-6-phosphate receptor; CRD, carbohydrate-recognition domain; DC, dendritic cell; DC-SIGN, DC-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin; IGF, insulin-like growth factor; MRH Man-6P, mannose 6-phosphate receptor homology; ITIM/ITAM motif, immunoreceptor tyrosine-based inhibition/immunoreceptor tyrosine-based activation motif.
Table 1.
The classification of lectins based on structure of carbohydrate-recognition domain (CRD)
| Lectin | Expressed cell types | Ligands | Location | Role | Example | Species |
||
|---|---|---|---|---|---|---|---|---|
| Human | Mouse | Plant | ||||||
| C-type lectin | DCa, Møb, LCc, Mod, Te | Various (mannose, fucose, β-glucan, glucosamine) | Extracellular, transmembrane | Recognition of pathogens, clearance, endocytosis, adhesion, immune-modulation | MR, DC-SIGN, SIGN-Rs, Dectin, Selectin | Ο | Ο | Ο |
| P-type lectin | Eukaryotic cells | Man-6-phosphate | Secretory pathway, endosome | Endocytosis, intracellular targeting of lysosomal enzymes (lysosome biogenesis) | CD-MPR, IGF-II/CI-MPR | Ο | Ο | Χ |
| S-type lectin | β-galactoside | Secrete (cytoplasm), extracellular | Cell adhesion, growth regulation, cell migration, immune responses | Galectin (1-14) | Ο | Ο | Χ | |
| I-type lectin | Mast, Bf, Mø, Mo | Sialic acid | Membrane | Regulation of myeloid cell inter-action, differentiation of myeloid cells, adhesion, signaling | Siglecs (CD33-related subgroup, sialoadhesin, CD22 and MAG) | Ο | Ο | Χ |
| M-type lectin | Mannose glycan | Endoplasmic reticulum | α-Mannosidase, handling of N-linked glycoproteins | EDEM1, EDEM2 and EDEM3 | Ο | Ο | Ο | |
| L-type lectin | High-mannose Glycan | Intracellular, Golgi, endoplasmic reticulum, ERGICg | Protein sorting, assisting secretion of specific glycoproteins | ERGIC-53, ERGL, VIP36, and VIPL | Ο | Ο | Ο | |
| Chitinase-like lectin | Neutrophil, Mø, epithelial cell | Chito-oligosaccharide | Extracellular | Collagen metabolism | YKL-40, Ym1, Oviduction | Ο | Ο | Ο |
| F-type lectin | Fucose-terminating oligosaccharide | Extracellular | Innate immunity | Fucolectins | Χ | Χ | Χ | |
B, B cell; DCs, dendritic cells; dMø, decidual macrophage; ERGIC, ER-Golgi intermediate compartment; LC, Langerhans cell; Mø, macrophage; Mo, monocyte; T, T cell
1.2.2. C-type lectins
C-type lectins are endocytic receptors which are mostly expressed by macrophages, DCs, and some endothelial cells. C-type lectins require Ca2+ for activity and have common sequence motif of 14 invariable and 18 highly conserved amino acid residues [50], [51]. As they have multi CRDs, C-type lectins are able to recognize a wide range of carbohydrate-based ligands from endogenous molecules to conserved structures found in bacteria, fungi, virus-infected cells, and parasites called pathogen-associated molecular patterns [52]. After recognition, C-type lectins subsequently participate in the uptake for degradation in order to facilitate direct elimination by macrophages or antigen presentation by DCs and macrophages in Major histocompatibility complex (MHC) molecules at the cell surface, resulting in stimulating the adaptive immune system [53], [54]. For example, C-type lectins can recognize diverse bacterial pathogens and induce cytokine production and Th17 responses in antibacterial immunity [55], and are critical in systemic infections with pathogens like Cryptococcus neoformans in antifungal immunity of Th1 effector cells [55]. In addition, they are involved in clearance, homeostasis, and immunomodulation [56]. Many C-type lectins play primary roles in immunity. C-type lectins include Dectins, DC-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin (DC-SIGN), SIGN-R1, mannose receptor (MR), Collectin (MBL, SP-A, SP-D) and selectins (L-, P-, E-) (Table 2).
Table 2.
A summary of the C-type lectin receptors dealt with in this review
| Lectin | Expressed cell types | Ligands | Pathogens | Species |
||
|---|---|---|---|---|---|---|
| Human | Mouse | Plant | ||||
| MR | Møa, DCb subset, Lymphatic and hepatic endothelium | Mannose, Fucose, N-acetyl glucosamine, Sulphated sugar | Yeast, bacteria, HIVn, C. albicans, L. donovani, P. carinii, Klebsiella pneumonia, Trypanosoma cruzi, M. tuberculosis, capsular polysaccharides of S. pneumoniae | Ο | Ο | Ο |
| DC-SIGN | DC, dMøc, aMød | Mannose type CRD, Mannan, ManLam, ICAM-2, ICAM-3 | HIV, Mycobacterium tuberculosis, C. albicans, Leishmania mexicana, A. fumigatus, Helicobacter pylori, Schistosoma mansoni | Ο | Χ | Χ |
| SIGN-R1 | MZ Møe, pMøf | Mannose type CRD, Dextran, ICAM-3 | C. albicans, M. tuberculosis, S. pneumoniae, CPS, HIV, yeast-derived zymosan particles | Χ | Ο | Χ |
| Dectin-1 | DC, LCg, Mø, PMNh | β-glucan, T-cell ligand | C. albicans, Saccharomyces cerevisiae, Coccidioides posadasii, Pneumocystis carinii | Ο | Ο | Χ |
| Dectin-2 | H: Moi, Bj, Activated CD4+ Tk M: Mø, PMN, LC | Mannose type ligands, CD4/CD25+ T-cell ligand | C. Albicans | Ο | Ο | Χ |
| L-selectin | Leukocyte | s6SLex l | Arenavirus, Hantavirus, Coronavirus, Filovirus, Orthomyxovirus | Ο | Ο | Χ |
| P-selectin | Platelets, Endothelium | sLexm, s6SLex | HSVo, VZVp | Ο | Ο | Χ |
| E-selectin | Activated endothelium | sLex, s6SLex | Porphyromonas gingivalis | Ο | Ο | Χ |
aMø, alveolar macrophage; B, B cell; DCs, dendritic cells; dMø, decidual macrophage; HIV, Human immunodeficiency virus; HSV, Herpes simplex virus; LC, Langerhans cell; Mø, macrophage; Mo, monocyte; MZ Mø, marginal zone macrophage; pMø, peritoneal macrophage; PMN, polymorphonuclear cell; s6SLex, sialyl 6-sulpho Lewisx; sLex, sialyl Lewisx; T, T cell; VZV, Varicella zoster virus
1.3. Dectin-1
Dectin-1 specifically binds to β-1,3 and β-1,6 linked glucans of fungi, plant cell walls, and bacteria, including Candida albicans, Saccharomyces cerevisiae, Coccidioides posadasii, and Pneumocystis carinii, but cannot recognize monosaccharides or glucans with other linkages. However, C. neoformans, Histoplasma capsulatum, and Aspergillus fumigatus are not targeted by Dectin-1, in spite of the presence of β-glucans in their cell wall [57], [58]. Dectin-1 plays a primary role in inducing proinflammatory mediators like TNF-α in response to fungal pathogens. Also, Dectin-1 contains an immunotyrosine activation motif within its cytoplasmic tail, helping TLR2 signaling pathway by interaction with the immunotyrosine activation motif of Dectin-1 [57]. Signaling by Dectin-1 regulates various cellular responses including phagocytosis and the production of inflammatory cytokines such as IFNs, IL-23, IL-6, and IL-1 [58]. Dectin-1 expressed on DCs and macrophages can recognize N-glycans of the surface of tumor cells, following nuclear translocation, and the induction of several genes such as Inam, which is known to induce tumor killing by NK cells by hemophilic interactions.
1.4. DC-SIGN
DC-SIGN was originally identified as a receptor for intercellular adhesion molecule-3 (ICAM-3) that induces DC-mediated T-cell proliferation [58]. It was subsequently unveiled to bind ICAM-2 on vascular endothelial cells, regulating DCs migration through interaction of N-linked high mannose structures consisting of from five to nine terminal mannose units [57]. DC-SIGN, which is expressed by DCs, decidual and alveolar macrophages facilitates high-affinity binding to high mannose oligosaccharides through tetramerization [57]. Mannose-dependent interactions demonstrate the ability of DC-SIGN to bind human immunodeficiency virus (HIV) and various pathogens, including Mycobacterium tuberculosis, C. albicans, Leishmania mexicana, A. fumigatus, Helicobacter pylori, and Schistosoma mansoni [58]. For instance, DC-SIGN recognizes mannosylated lipoarabinomannan, which is a mannose-capped glycolipid found in the cell wall of M. tuberculosis. This interaction can induce the secretion of the immunosuppressive cytokine, IL-10, from DCs which expresses DC-SIGN [57]. DC-SIGN expression is mostly induced by IL-4, and is downregulated by IFN-γ, TGFβ, and dexamethasone [58], [59].
1.5. Eight mouse genes homologous to DC-SIGN (SIGN-Related gene)
The murine DC-SIGN homologues were reported to help to identify the roles of DC-SIGN in infection and inflammation and to play important roles in bacterial, fungal, and parasitic infections. There are seven mouse genes in the mouse DC-SIGN locus, containing SIGN-R1-5 and SIGN-R7, 8, and a pseudogene, SIGN-R6 [60], [61]. The mRNA of three SIGN-R genes encode type II transmembrane proteins (SIGN-R1, 325 amino acids; SIGN-R3, 237 amino acids; SIGN-R4, 208 amino acids), but SIGN-R2 gene only encodes a CRD without a cytosolic domain and a transmembrane domain (SIGN-R2, 178 amino acids) [62]. Amino acid sequence similarities between the CRD of human DC-SIGN and the murine homologues are 69% for SIGN-R1, 65% for SIGN-R2, 68% for SIGN-R3, and 70% for SIGN-R4 [62].
SIGN-R1, a murine homologous transmembrane of the DC-SIGN, is expressed by splenic marginal zone macrophages and peritoneal macrophages [53], [57]. Similar to DC-SIGN, SIGN-R1 is essential for the recognition and clearance of Streptococcus pneumoniae-derived capsular PSs. Moreover, it can also recognize C. albicans, M. tuberculosis, S. pneumoniae CPS, HIV, and yeast derived zymosan particles in a mannose inhibitable manner [57]. SIGN-R1 directly binds to C1q and dominantly regulates the immunoglobulin-independent classical complement pathway for C3 deposition of blood borne S. pneumoniae [63]. In SIGN-R1 deficient mice, C3 deposition is abolished and innate resistance against pneumococci is reduced [63], [64]. Also, SIGN-R1 interacts specifically with 2,6 sialylated Fc fragments of immunoglobulins, resulting in the antiinflammatory activity of intravenous immunoglobulin, which has been widely used to treat autoimmune diseases including immune thrombocytopenia, rheumatoid arthritis, and systemic lupus erythematosus [65]. SIGN-R3 makes a unique contribution to the protection of the host against bacterial infection such as M. tuberculosis, but SIGN-R1 and SIGN-R5 do not share this function. Although both SIGN-R1 and SIGN-R3 recognize M. tuberculosis and mycobacterial surface glycoconjugates, only SIGN-R3 which is expressed in lung phagocytes can interact with M. tuberculosis via an intracellular tyrosine-based motif that may induce inflammatory cytokine production in collaboration with TLR2 [60].
1.6. MR and selectins
MR (CD206) is described in myeloid cells and functions as a viral recognition receptor on the cell membrane for yeast, bacteria, HIV, and a wide variety of pathogens, such as C. albicans, Leishmania donovani, P. carinii, Klebsiella pneumonia, Trypanosoma cruzi, M. tuberculosis [66], [67], [68] and capsular PSs of S. pneumoniae through a mannose-type CRD and pathogen-associated high mannose structures [69]. MR which is mainly expressed in immune cells induces uptake and presents mannosylated antigens such as lipoarabinomannan on MHC class II of metallophilic macrophages, resulting in influencing immune responses [70]. For instance, the interaction between MR and hepatitis B virus (HBV) surface antigen (HBsAg) enhances viral uptake by DCs, resulting in the impairment in the function of DCs and the ineffective antiviral response of chronic HBV [71]. The recognition of viral surface glycoproteins by MR is also beneficial to influenza virus [72] and HIV [73] invasion into host cells. In addition, MR is able to mediate the clearance of endogenous inflammatory glycoproteins bearing ligands of the mannose-type CRD [57]. The expression of MR is upregulated by cytokines like IL-4, IL-13, and IL-10, but IFN induces a downregulatory effect to MR [58].
Selectins are cell adhesion molecules and have three groups, including E-selectin (endothelial) and P-selectin (platelet) on endothelium, and L-selectin (leukocyte) on leukocytes [74]. Selectins play roles in leukocyte recruitment from the bloodstream into sites of inflammation [55], [74]. The recruitment of leukocytes proceeds initially by attachment leading to the rolling of leukocytes along endothelial vasculature via selectin-carbohydrate interaction. E-selectin (M.W. 115 kDa) is expressed by endothelial cells after stimulation with activators like TNF-α, IL-1, or bacterial lipopolysaccharide [55]. These cytokines also upregulate the expression of P-selectin (M.W. 140 kDa), which is expressed by endothelial cells and platelets [55]. Also, P-selectin in released to the cell surface from storage vesicles in endothelial cells and platelets minutes after stimulation by a number of activators, such as thrombin or histamine [75]. L-selectin (M.W. 90–110 kDa) is expressed by leukocytes and aids in the homing of leukocytes. L-selectin has high expression on naïve T lymphocytes but, once T lymphocytes are activated, the expression of L-selectin is low or lacking [45]. E-, P-, and L-selectin are composed of an N-terminal C-type lectin CRD, an epidermal growth factor-like subunit, a number of short consensus repeat units, a membrane spanning region, and a C-terminal cytoplasmic tail [76]. There is approximately 72% homology for analogous selectin CRDs across species, and ∼52% homology between different selectins within a species [76].
1.6.1. P-type lectins
P-type lectins are intracellular transmembrane glycoproteins with specificity for mannose-6-phosphate (M6P) to identify and route lysosomal enzymes to the lysosomal compartment (MPR signal) and they have two groups. One is the 43–46 kDa cation-dependent M6P receptor (CD-MPR) which requires Ca2+ for activity and contains single extracellular domain, followed by a single transmembrane domain [47]. The other is the 275–300 kDa insulin-like growth factor II/cation-independent M6P receptor (IGF-II/CI-MPR) which does not require cation for activity and has a large extracellular domain containing two high-affinity binding sites [46]. The CRDs of P-lectins for M6P are located in the extracellular domain of CD-MPR and in extracellular repeats 3 and 9 with high affinity for IGFII/CI-MPR [46]. They are similar both in size and in sequence to the repeating units that consisted of short N-terminal extracellular domain and C-terminal cytoplasmic tail [77]. Their function is the intracellular targeting of lysosomal enzymes (lysosomal hydrolases) in the trans-Golgi network vertebrate animals and delivering them to prelysosomal compartments [78]. Also, the C-terminal cytoplasmic tail of the receptor which targets amino acid sequence plays a role in recognizing signal for transport to the endosomal compartment [47]. IGF-II/CI-MPR has the capacity for endocytosis of ligands from the cell surface and serves to turn over IGF by endocytosis, but not CD-MPR [47], [78].
1.6.2. S-lectins
S-lectins, galectins (from 1 to 14), consist of globular galectin-type CRDs which are specific for β-galactoside ligands and have conserved cysteine residues [51]. They are found predominantly in mammals, but not in plants [46]. S-lectins have a relatively simple structure and share a highly homologous domain named the S-carbohydrate recognition domain (S-CRD) [46]. S-lectins mostly contain multiple sugar-binding sites, as the presence of two type of S-CRD in a single polypeptide or its dimer [51]. The function of S-lectins may be to crosslink N-acetyllactosamine-containing structures found at cell surfaces or in the extracellular matrix [79]. S-Lectins are conserved in unrelated organisms including frogs, birds, and mammals, meaning that β-galactoside binding to lectin may be important biologically [47]. Mammalian S-lectins are conserved in eight residues of the S-CRDs and involved in growth regulation, cell adhesion, cell migration, and immune responses [47]. For example, galectin-1 mediates cell adhesion and apoptosis, and regulates cellular proliferation. Galectin-3 mediates cell adhesion, regulates inflammation, pre-mRNA splicing, and protects against induced apoptosis. Galectin-4 and galectin-6 have a function in cell−cell and cell−extracellular matrix crosslinking and galectin-5 is involved in maturation and erythrocyte adhesion [46], [47].
1.6.3. I-type lectins
I-type lectins are members of the immunoglobulin (Ig) superfamily [46]. They share the structural motif, the Ig fold, with Ig-like domains consisted of similar two planes of β-pleated sheets [80]. The β-sheets are established about 70–110 amino acids, crosslinked by a disulfide bond and contain several hydrogen-bonded antiparallel chains [51]. I-type lectins are classified into two domains according to the number and arrangement of β-strands present in the domains [81]. One is the amino terminal, extracellular domain, which is similar to the variable region (V-type domain) of IgG and is necessary for sialic acid-dependent binding [81]. The other is the constant region (C-type domain) of IgG that has various forms from 1 to 16 [46]. I-type lectins function as not only cell adhesion molecules but also growth factor receptors and extracellular matrix molecules [47]. The major subclasses of I-type lectins are the sialic acid-binding immunoglobulin superfamily lectins (Siglecs) which contain an homologous N-terminal V-type domain with the sialic acid binding site and variable numbers of C-type domains [46]. V-type domain and adjacent C-type domains of Siglecs have conserved cysteine residues, resulting in formation of conventional intrasheet and interdomain disulfide bonds [81]. The C-terminal cytoplasmic tail of most Siglecs has immunoreceptor tyrosine-based motifs in the intracellular domain for signaling events [81]. Sialoadhesin found on the surface of macrophages is a member of the Siglecs family [46]. It contains a V-type domain and 16 C-type domains [51]. The N-terminal V-type domain of sialoadhesin enables binding of sialic acid in the ligands of neutrophils, monocytes, NK cells, B cells, and cytotoxic T cells with sialoadhesin [47].
2. Animal lectins: potential receptors for GPs
The diverse roles of botanical PSs have been reported. The antitumor effect of botanical PSs was first known more than 100 years ago when it was found that PSs could alleviate cancer in cancer patients [82]. For example, lentinan from Lentinus edodes and schizophyllan from Schizophyllum commune have antitumor activities and have been used clinically for cancer therapy [35]. Through several experiments, it was suggested that the antitumor effects of botanical PSs might be due to potentiation of the response of precursor T cells and macrophages to cytokines produced by lymphocytes after specific recognition of tumor cells [83]. Also, botanical PSs of mushroom are known to stimulate natural killer cells, T cells, B cells, and macrophage-dependent immune system responses [84]. In addition, arabinogalactans of botanical PSs possess complement fixation activity and induce chemotaxis of human macrophages, T cells, and NK cells [85].
Although the roles of botanical PSs have been continuously identified, the mechanism of action is not clear yet, because the receptors of botanical PSs remain unknown. However, many scientists have proposed that various pattern recognition receptors (PRRs) might be receptors for botanical PSs. For example, Shao et al [86] suggested that the receptor of PS from the roots of Astragalus membranaceus, a medicinal herb, might be MR, TLR4, β-glucan receptor, etc. Schepetkin and Quinn [87] also introduced PRRs including TLR4, MR, and dectin-1 as potential receptors of PS polymers. Especially, it was reported that specific glycans of botanical PSs act as immune stimulating agents and effective T cell immune adjuvants [88], [89]. Therefore, it was suggested that animal lectins are strong candidates for receptors of botanical PSs among various PRRs, because animal lectins are specialized in recognizing various PSs [90].
With extensive research for GPs, the roles and chemical composition of GPs have gradually been discovered. For instance, the GPs of P. ginseng, the most common ginseng, are composed of sugars including mannose of 1.2∼2.8% by weight, glucose of 64.1∼82.8% by weight, galactose of 10.0∼26.6% by weight, and arabinose of 0.6∼5.4% by weight [91]. In addition, CVT-E002, an aqueous extract of the roots of North American ginseng, is composed of 80% poly-furanosyl-pyranosyl-saccharides including rhamnose, glucose, galacturonic acid, galactose, and arabinose [7]. Therefore, some of the animal lectins might recognize these glycan structures of GPs, because there are many animal lectins to recognize glycan structures of galactose, glucose, rhamnose, and mannose [47], [48], [49], [51], [57], [58], [69], [92]. This could be speculated from the extensive research of specific receptors for botanical PSs in the above section. In particular, clustering of animal lectins might enhance the recognition of GPs, since clustering of simple binding sites in oligomers of the animal lectin polypeptides dramatically increases the affinity for diverse PS structures [93]. For example, Byeon et al [95] expected dectin-1 as a receptor of Red ginseng acidic PS, which are known to interact with PS fractions such as β-glucan and zymosan [94], [95].
3. Concluding remarks
GPs which are isolated from a variety of traditional medicinal ginsengs involved in various innate immune responses, such as the production of cytokines and maturation of DCs in vivo and in vitro, show potential to be immunomodulators with wide applications [23], [24], [25]. In addition, most of them are relatively nontoxic and do not cause significant side effects, which are major problems of immunomodulatory bacterial PSs and synthetic compounds. Thus, GPs are becoming ideal candidates for therapeutics for collagen-induced arthritis, inflammation, and inflammatory-related diseases like tumors, atherosclerosis, and so on [3], [27], [28], [32], [36]. Although the roles of GPs are being continuously identified, the detailed mechanisms of actions are not clear yet, because the receptors of GPs remain largely unknown. Therefore, it is tempting to speculate that animal lectins could be strong candidates of receptors for GPs. To prove this possibility, extensive researches for the specific structures of GPs and the interaction between GPs and animal lectins is required in the near future. By unraveling receptors of GPs in vivo, it is possible to specifically understand the detailed mechanism for the immunological activities of GPs in the immune system, giving insights into the development of GPs as therapeutic biomaterials for many immunological diseases.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This paper was supported by Konkuk University in 2012.
References
- 1.Hofseth L.J., Wargovich M.J. Inflammation, cancer, and targets of ginseng. J Nutr. 2007;137:183S–185S. doi: 10.1093/jn/137.1.183S. [DOI] [PubMed] [Google Scholar]
- 2.Angelova N., Kong H.W., van der Heijden R., Yang S.Y., Choi Y.H., Kim H.K., Wang M., Hankemeier T., van der Greef J., Xu G. Recent methodology in the phytochemical analysis of ginseng. Phytochem Anal. 2008;19:2–16. doi: 10.1002/pca.1049. [DOI] [PubMed] [Google Scholar]
- 3.Wang L., Yu X., Yang X., Li Y., Yao Y., Lui E.M., Ren G. Structural and anti-inflammatory characterization of a novel neutral polysaccharide from North American ginseng (Panax quinquefolius) Int J Biol Macromol. 2014;74C:12–17. doi: 10.1016/j.ijbiomac.2014.10.062. [DOI] [PubMed] [Google Scholar]
- 4.Ni Xiu-zhen, Wang Bing-qing, Zhi Yuan, Wei Ning-ning, Zhang Xu, Li Shan-shan, Tai Gui-hua, Zhou Yi-fa, Zhao Ji-ming. Total fractionation and analysis of polysaccharides from leaves of Panax ginseng C.A. Meyer. Chem Res Chniese Universities. 2010;26:230–234. [Google Scholar]
- 5.Borchers A.T., Keen C.L., Stern J.S., Gershwin M.E. Inflammation and native American medicine: the role of botanicals. Am J Clin Nutr. 2000;72:339–347. doi: 10.1093/ajcn/72.2.339. [DOI] [PubMed] [Google Scholar]
- 6.Lee D.C., Yang C.L., Chik S.C., Li J.C., Rong J.H., Chan G.C., Lau A.S. Bioactivity-guided identification and cell signaling technology to delineate the immunomodulatory effects of Panax ginseng on human promonocytic U937 cells. J Transl Med. 2009;7:34. doi: 10.1186/1479-5876-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biondo P.D., Goruk S., Ruth M.R., Ruth M.R., O'Connell E., Field C.J. Effect of CVT-E002 (COLD-fX) versus a ginsenoside extract on systemic and gut-associated immune function. Int Immunopharmacol. 2008;8:1134–1142. doi: 10.1016/j.intimp.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Lim T.S., Na K., Choi E.M., Chung J.Y., Hwang J.K. Immunomodulating activities of polysaccharides isolated from Panax ginseng. J Med Food. 2004;7:1–6. doi: 10.1089/109662004322984626. [DOI] [PubMed] [Google Scholar]
- 9.Sun X.B., Matsumoto T., Kiyohara H., Hirano M., Yamada H. Cytoprotective activity of pectic polysaccharides from the root of Panax ginseng. J Ethnopharmacol. 1991;31:101–107. doi: 10.1016/0378-8741(91)90148-7. [DOI] [PubMed] [Google Scholar]
- 10.Assinewe V.A., Amason J.T., Aubry A., Mullin J., Lemaire I. Extractable polysaccharides of Panax quinquefolius L. (North American ginseng) root stimulate TNFalpha production by alveolar macrophages. Phytomedicine. 2002;9:398–404. doi: 10.1078/09447110260571625. [DOI] [PubMed] [Google Scholar]
- 11.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 12.Kitts D., Hu C. Efficacy and safety of ginseng. Public Health Nutr. 2000;3:473–485. doi: 10.1017/s1368980000000550. [DOI] [PubMed] [Google Scholar]
- 13.Hwang S.H., Shin T.J., Choi S.H., Cho H.J., Lee B.H., Pyo M.K., Lee J.H., Kang J., Kim H.J., Park C.W. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates G protein-coupled lysophosphatidic acid receptors with high affinity. Mol Cells. 2012;33:151–162. doi: 10.1007/s10059-012-2216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yun T.-K. Panax ginseng–a non-organ-specific cancer preventive? Lancet Oncol. 2001;2:49–55. doi: 10.1016/S1470-2045(00)00196-0. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y. Structure and biological activities of the polysaccharides from the leaves, roots and fruits of Panax ginseng C.A. Meyer: an overview. Carbohydr Polym. 2011;85:490–499. [Google Scholar]
- 16.Friedl R., Moeslinger T., Kopp B., Spieckermann P.G. Stimulation of nitric oxide synthesis by the aqueous extract of Panax ginseng root in RAW 264.7 cells. Br J Pharmacol. 2001;134:1663–1670. doi: 10.1038/sj.bjp.0704425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi H.S., Kim K.H., Sohn E., Park J.D., Kim B.O., Moon E.Y., Rhee D.K., Pyo S. Red ginseng acidic polysaccharide (RGAP) in combination with IFN-gamma results in enhanced macrophage function through activation of the NF-kappaB pathway. Biosci Biotechnol Biochem. 2008;72:1817–1825. doi: 10.1271/bbb.80085. [DOI] [PubMed] [Google Scholar]
- 18.Srivastava R. Bioactive polysaccharides from plants. Phytochemistry. 1989;28:2877–2883. [Google Scholar]
- 19.Wang M., Guilbert L.J., Li J., Wu Y., Pang P., Basu T.K., Shan J.J. A proprietary extract from North American ginseng (Panax quinquefolium) enhances IL-2 and IFN-gamma productions in murine spleen cells induced by Con-A. Int Immunopharmacol. 2004;4:311–315. doi: 10.1016/j.intimp.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Xu Zhang, Li Yu, Hongtao Bi, Xianhua Li, Weihua Ni, Han Han, Nan Li, Bingqing Wang, Yifa Zhou, Guihua Tai. Total fractionation and characterization of the water-soluble polysaccharides isolated from Panax ginseng CA Meyer. Carbohydr Polym. 2009;77:544–552. [Google Scholar]
- 21.Ni W., Zhang X., Wang B., Chen Y., Han H., Fan Y., Zhou Y., Tai G. Antitumor activities and immunomodulatory effects of ginseng neutral polysaccharides in combination with 5-fluorouracil. J Med Food. 2010;13:270–277. doi: 10.1089/jmf.2009.1119. [DOI] [PubMed] [Google Scholar]
- 22.Lee J.H., Shim J.S., Lee J.S., Kim M.K., Chung M.S., Kim K.H. Pectin-like acidic polysaccharide from Panax ginseng with selective antiadhesive activity against pathogenic bacteria. Carbohydr Res. 2006;341:1154–1163. doi: 10.1016/j.carres.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 23.Song J.Y., Han S.K., Son E.H., Pyo S.N., Yun Y.S., Yi S.Y. Induction of secretory and tumoricidal activities in peritoneal macrophages by ginsan. Int Immunopharmacol. 2002;2:857–865. doi: 10.1016/s1567-5769(01)00211-9. [DOI] [PubMed] [Google Scholar]
- 24.Shin J.Y., Song J.Y., Yun Y.S., Yang H.O., Rhee D.K., Pyo S. Immunostimulating effects of acidic polysaccharides extract of Panax ginseng on macrophage function. Immunopharmacol Immunotoxicol. 2002;24:469–482. doi: 10.1081/iph-120014730. [DOI] [PubMed] [Google Scholar]
- 25.Kim M.H., Byon Y.Y., Ko E.J., Song J.Y., Yun Y.S., Shin T., Joo H.G. Immunomodulatory activity of ginsan, a polysaccharide of Panax ginseng, on dendritic cells. Korean J Physiol Pharmacol. 2009;13:169–173. doi: 10.4196/kjpp.2009.13.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim K.H., Lee Y.S., Jung I.S., Park S.Y., Chung H.Y., Lee I.R., Yun Y.S. Acidic polysaccharide from Panax ginseng, ginsan, induces Th1 cell and macrophage cytokines and generates LAK cells in synergy with rIL-2. Planta Med. 1998;64:110–115. doi: 10.1055/s-2006-957385. [DOI] [PubMed] [Google Scholar]
- 27.Lee Y.S., Chung I.S., Lee I.R., Kim K.H., Hong W.S., Yun Y.S. Activation of multiple effector pathways of immune system by the antineoplastic immunostimulator acidic polysaccharide ginsan isolated from Panax ginseng. Anticancer Res. 1997;17:323–331. [PubMed] [Google Scholar]
- 28.Yoo D.G., Kim M.C., Park M.K., Park K.M., Quan F.S., Song J.M., Wee J.J., Wang B.Z., Cho Y.K., Compans R.W. Protective effect of ginseng polysaccharides on influenza viral infection. PLoS One. 2012;7:e33678. doi: 10.1371/journal.pone.0033678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemmon H.R., Sham J., Chau L.A., Madrenas J. High molecular weight polysaccharides are key immunomodulators in North American ginseng extracts: characterization of the ginseng genetic signature in primary human immune cells. J Ethnopharmacol. 2012;142:1–13. doi: 10.1016/j.jep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Tomoda M., Hirabayashi K., Shimizu N., Gonda R., Ohara N., Takada K. Characterization of two novel polysaccharides having immunological activities from the root of Panax ginseng. Biol Pharm Bull. 1993;16:1087–1090. doi: 10.1248/bpb.16.1087. [DOI] [PubMed] [Google Scholar]
- 31.Sonoda Y., Kasahara T., Mukaida N., Shimizu N., Tomoda M., Takeda T. Stimulation of interleukin-8 production by acidic polysaccharides from the root of Panax ginseng. Immunopharmacology. 1998;38:287–294. doi: 10.1016/s0162-3109(97)00091-x. [DOI] [PubMed] [Google Scholar]
- 32.Shin H.J., Kim Y.S., Kwak Y.S., Song Y.B., Kim Y.S., Park J.D. Enhancement of antitumor effects of paclitaxel (taxol) in combination with red ginseng acidic polysaccharide (RGAP) Planta Med. 2004;70:1033–1038. doi: 10.1055/s-2004-832643. [DOI] [PubMed] [Google Scholar]
- 33.Ahn J.Y., Choi I.S., Shim J.Y., Yun E.K., Yun Y.S., Jeong G., Song J.Y. The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals. Eur J Immunol. 2006;36:37–45. doi: 10.1002/eji.200535138. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen V., Tang J., Oroudjev E., Lee C.J., Marasigan C., Wilson L., Ayoub G. Cytotoxic effects of bilberry extract on MCF7-GFP-tubulin breast cancer cells. J Med Food. 2010;13:278–285. doi: 10.1089/jmf.2009.0053. [DOI] [PubMed] [Google Scholar]
- 35.Kim Y.S., Kang K.S., Kim S.I. Study on antitumor and immunomodulating activities of polysaccharide fractions from Panax ginseng: Comparison of effects of neutral and acidic polysaccharide fraction. Arch Pharm Res. 1990;13:330–337. [Google Scholar]
- 36.Zhao H.Y., Zhang W.D., Xiao C., Lu C., Xu S.H., He X.J., Li X.B., Chen S.L., Yang D.J., Chan A.S.C. Effect of ginseng polysaccharide on TNF-alpha and IFN-gamma produced by enteric mucosal lymphocytes in collagen induced arthritic rats. J Med Plant Res. 2011;5:1536–1542. [Google Scholar]
- 37.Pillai R., Lacy P. Inhibition of neutrophil respiratory burst and degranulation responses by CVT-E002, the main active ingredient in COLD-FX. Allergy Asthma Clin Immunol. 2011;7:A31. [Google Scholar]
- 38.Wang M., Guilbert L.J., Ling L., Li J., Wu Y., Xu S., Pang P., Shan J.J. Immunomodulating activity of CVT-E002, a proprietary extract from North American ginseng (Panax quinquefolium) J Pharm Pharmacol. 2001;53:1515–1523. doi: 10.1211/0022357011777882. [DOI] [PubMed] [Google Scholar]
- 39.Ahn J.Y., Song J.Y., Yun Y.S., Jeong G., Choi I.S. Protection of Staphylococcus aureus-infected septic mice by suppression of early acute inflammation and enhanced antimicrobial activity by ginsan. FEMS Immunol Med Microbiol. 2006;46:187–197. doi: 10.1111/j.1574-695X.2005.00021.x. [DOI] [PubMed] [Google Scholar]
- 40.McElhaney J.E., Gravenstein S., Cole S.K., Davidson E., O'Neill D., Petitjean S., Rumble B., Shan J.J. A placebo-controlled trial of a proprietary extract of North American ginseng (CVT-E002) to prevent acute respiratory illness in institutionalized older adults. J Am Geriatr Soc. 2004;52:13–19. doi: 10.1111/j.1532-5415.2004.52004.x. [DOI] [PubMed] [Google Scholar]
- 41.Lu Z.Q., Dice J.F. Ginseng extract inhibits protein degradation and stimulates protein synthesis in human fibroblasts. Biochem Biophys Res Commun. 1985;126:636–640. doi: 10.1016/0006-291x(85)90653-9. [DOI] [PubMed] [Google Scholar]
- 42.Kumar H., Kawai T., Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 43.Etzler M.E. The lectins. Academic Press; San Diego: 2012. Distribution and function to plant lectins; pp. 371–436. [Google Scholar]
- 44.Kilpatrick D.C. Animal lectins: a historical introduction and overview. Biochim Biophys Acta. 2002;1572:187–197. doi: 10.1016/s0304-4165(02)00308-2. [DOI] [PubMed] [Google Scholar]
- 45.Ghazarian H., Idoni B., Oppenheimer S.B. A glycobiology review: carbohydrates, lectins and implications in cancer therapeutics. Acta Histochem. 2011;113:236–247. doi: 10.1016/j.acthis.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharon H., La N. Lectins: carbohydrate-specific proteins that mediate cellular recognition. Chem Rev. 1998;98:637–674. doi: 10.1021/cr940413g. [DOI] [PubMed] [Google Scholar]
- 47.Anderson K., David Evers, Rice Kevin G. Structure and function of mammalian carbohydrate-lectin interactions. Glycoscience. 2008:2445–2482. [Google Scholar]
- 48.Matsumoto J., Nakamoto C., Fujiwara S., Yubisui T., Kawamura K. A novel C-type lectin regulating cell growth, cell adhesion and cell differentiation of the multipotent epithelium in budding tunicates. Development. 2001;128:3339–3347. doi: 10.1242/dev.128.17.3339. [DOI] [PubMed] [Google Scholar]
- 49.Singh H. Insight of lectins–A review. Int J Sci Eng Res. 2012;3:1–9. [Google Scholar]
- 50.Drickamer K., Taylor M.E. Biology of animal lectins. Annu Rev Cell Biol. 1993;9:237–264. doi: 10.1146/annurev.cb.09.110193.001321. [DOI] [PubMed] [Google Scholar]
- 51.Gabius H.J. Animal lectins. Eur J Biochem. 1997;243:543–576. doi: 10.1111/j.1432-1033.1997.t01-1-00543.x. [DOI] [PubMed] [Google Scholar]
- 52.Graham L.M. The C-type lectin receptor CLECSF8 (CLEC4D) is expressed by myeloid cells and triggers cellular activation through syk kinase. J Biol Chem. 2012;287:25964–25974. doi: 10.1074/jbc.M112.384164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cambi A. How C-type lectins detect pathogens. Cell Microbiol. 2005;7:481–488. doi: 10.1111/j.1462-5822.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 54.Figdor A., Ca C.G. Dual function of C-type lectin-like receptors in the immune system. Curr Opin Cell Biol. 2003;15:539–546. doi: 10.1016/j.ceb.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 55.Dambuza I.M., Brown G.D. C-type lectins in immunity: recent developments. Curr Opin Immunol. 2015;32:21–27. doi: 10.1016/j.coi.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–930. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- 57.McGreal E.P., Martinez-Pomares L., Gordon S. Divergent roles for C-type lectins expressed by cells of the innate immune system. Mol Immunol. 2004;41:1109–1121. doi: 10.1016/j.molimm.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 58.Kerrigan A.M., Brown G.D. C-type lectins and phagocytosis. Immunobiology. 2009;214:562–575. doi: 10.1016/j.imbio.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Relloso M., Puig-Kröger A., Pello O.M., Rodríguez-Fernández J.L., de la Rosa G., Longo N., Navarro J., Muñoz-Fernández M.A., Sánchez-Mateos P., Corbí A.L. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-beta, and anti-inflammatory agents. J Immunol. 2002;168:2634–2643. doi: 10.4049/jimmunol.168.6.2634. [DOI] [PubMed] [Google Scholar]
- 60.Zhang F., Ren S., Zuo Y. DC-SIGN, DC-SIGNR and LSECtin: C-type lectins for infection. Int Rev Immunol. 2014;33:54–66. doi: 10.3109/08830185.2013.834897. [DOI] [PubMed] [Google Scholar]
- 61.Powlesland A.S., Ward E.M., Sadhu S.K., Guo Y., Taylor M.E., Drickamer K. Widely divergent biochemical properties of the complete set of mouse DC-SIGN-related proteins. J Biol Chem. 2006;281:20440–20449. doi: 10.1074/jbc.M601925200. [DOI] [PubMed] [Google Scholar]
- 62.Park C.G., Takahara K., Umemoto E., Yashima Y., Matsubara K., Matsuda Y., Clausen B.E., Inaba K., Steinman R.M. Five mouse homologues of the human dendritic cell C-type lectin, DC-SIGN. Int Immunol. 2001;13:1283–1290. doi: 10.1093/intimm/13.10.1283. [DOI] [PubMed] [Google Scholar]
- 63.Kang Y.S., Do Y., Lee H.K., Park S.H., Cheong C., Lynch R.M., Loeffler J.M., Steinman R.M., Park C.G. A dominant complement fixation pathway for pneumococcal polysaccharides initiated by SIGN-R1 interacting with C1q. Cell. 2006;125:47–58. doi: 10.1016/j.cell.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 64.Lanoue A., Clatworthy M.R., Smith P., Green S., Townsend M.J., Jolin H.E., Smith K.G., Fallon P.G., McKenzie A.N. SIGN-R1 contributes to protection against lethal pneumococcal infection in mice. J Exp Med. 2004;200:1383–1393. doi: 10.1084/jem.20040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anthony R.M., Wermeling F., Karlsson M.C., Ravetch J.V. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci U S A. 2008;105:19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chakraborty R., Chakraborty P., Basu M.K. Macrophage mannosyl fucosyl receptor: its role in invasion of virulent and avirulent L. donovani promastigotes. Biosci Rep. 1998;18:129–142. doi: 10.1023/a:1020192512001. [DOI] [PubMed] [Google Scholar]
- 67.Kabha K., Nissimov L., Athamna A., Keisari Y., Parolis H., Parolis L.A., Grue R.M., Schlepper-Schafer J., Ezekowitz A.R., Ohman D.E. Relationships among capsular structure, phagocytosis, and mouse virulence in Klebsiella pneumoniae. Infect Immun. 1995;63:847–852. doi: 10.1128/iai.63.3.847-852.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kahn S. Trypanosoma cruzi amastigote adhesion to macrophages is facilitated by the mannose receptor. J Exp Med. 1995;182:1243–1258. doi: 10.1084/jem.182.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zamze S., Martinez-Pomares L., Jones H., Taylor P.R., Stillion R.J., Gordon S. Recognition of bacterial capsular polysaccharides and lipopolysaccharides by the macrophage mannose receptor. J Biol Chem. 2002;277:41613–41623. doi: 10.1074/jbc.M207057200. [DOI] [PubMed] [Google Scholar]
- 70.McGreal E.P., Miller J.L., Gordon S. Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr Opin Immunol. 2005;17:18–24. doi: 10.1016/j.coi.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.den Brouw M.L.O., Binda R.S., Geijtenbeek T.B.H., Janssen H.L., Woltman A.M. The mannose receptor acts as hepatitis B virus surface antigen receptor mediating interaction with intrahepatic dendritic cells. Virology. 2009;393:84–90. doi: 10.1016/j.virol.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 72.Reading P.C., Miller J.L., Anders E.M. Involvement of the mannose receptor in infection of macrophages by influenza virus. J Virol. 2000;74:5190–5197. doi: 10.1128/jvi.74.11.5190-5197.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lai J., Bernhard O.K., Turville S.G., Harman A.N., Wilkinson J., Cunningham A.L. Oligomerization of the macrophage mannose receptor enhances gp120-mediated binding of HIV-1. J Biol Chem. 2009;284:11027–11038. doi: 10.1074/jbc.M809698200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kogelberg H., Feizi T. New structural insights into lectin-type proteins of the immune system. Curr Opin Struct Biol. 2001;11:635–643. doi: 10.1016/s0959-440x(00)00259-1. [DOI] [PubMed] [Google Scholar]
- 75.Springer T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 76.Kansas G.S. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 77.Pohlmann R., Boeker M.W., von Figura K. The two mannose 6-phosphate receptors transport distinct complements of lysosomal proteins. J Biol Chem. 1995;270:27311–27318. doi: 10.1074/jbc.270.45.27311. [DOI] [PubMed] [Google Scholar]
- 78.Arason G.J. Lectins as defence molecules in vertebrates and invertebrates. Fish Shellfish Immunol. 1966;6:277–289. [Google Scholar]
- 79.Dodd R.B., Drickamer K. Lectin-like proteins in model organisms: implications for evolution of carbohydrate-binding activity. Glycobiology. 2001;11:71R–79R. doi: 10.1093/glycob/11.5.71r. [DOI] [PubMed] [Google Scholar]
- 80.Williams A.F. A year in the life of the immunoglobulin superfamily. Immunol Today. 1987;8:298–303. doi: 10.1016/0167-5699(87)90016-8. [DOI] [PubMed] [Google Scholar]
- 81.Angata T., Brinkman-Van der Linden E. I-type lectins. Biochim Biophys Acta. 2002;1572:294–316. doi: 10.1016/s0304-4165(02)00316-1. [DOI] [PubMed] [Google Scholar]
- 82.Nauts H.C., Swift W.E., Coley B.L. The treatment of malignant tumors by bacterial toxins as developed by the late William B. Coley, M.D., reviewed in the light of modern research. Cancer Res. 1946;6:205–216. [PubMed] [Google Scholar]
- 83.Hamuro J., Chihara G., Lentinan . a T-cell oriented immunopotentiator: its experimental and clinical applications and possible mechanism of immune modulation. In: Fenichel R.L., Chirigos M.A., editors. Immune modulation agents and their mechanisms. Marcel Dekker; New York: 1984. p. 409. [Google Scholar]
- 84.Wasser S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002;60:258–274. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- 85.Nergard C.S., Matsumoto T., Inngjerdingen M., Inngjerdingen K., Hokputsa S., Harding S.E., Michaelsen T.E., Diallo D., Kiyohara H., Paulsen B.S. Structural and immunological studies of a pectin and a pectic arabinogalactan from Vernonia kotschyana Sch. Bip. ex Walp. (Asteraceae) Carbohydr Res. 2005;340:115–130. doi: 10.1016/j.carres.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 86.Shao B.M., Xu W., Dai H., Tu P., Li Z., Gao X.M. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem Biophys Res Commun. 2004;320:1103–1111. doi: 10.1016/j.bbrc.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 87.Schepetkin I.A., Quinn M.T. Botanical polysaccharides: macrophage immunomodulation and therapeutic potential. Int Immunopharmacol. 2006;6:317–333. doi: 10.1016/j.intimp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 88.El Enshasy H.A., Hatti-Kaul R. Mushroom immunomodulators: unique molecules with unlimited applications. Trends Biotechnol. 2013;31:668–677. doi: 10.1016/j.tibtech.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 89.Ramberg J.E., Nelson E.D., Sinnott R.A. Immunomodulatory dietary polysaccharides: a systematic review of the literature. Nutr J. 2010;9:54. doi: 10.1186/1475-2891-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chang W.T., Lai T.H., Chyan Y.J., Yin S.Y., Chen Y.H., Wei W.C., Yang N.S. Specific medicinal plant polysaccharides effectively enhance the potency of a DC-based vaccine against mouse mammary tumor metastasis. PLoS One. 2015;10:e0122374. doi: 10.1371/journal.pone.0122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yeon-Sook Yun, Jie-Young Song, In-Sung Jung, investors; Korea Institute of Radiological & Medical Sciences, assignee. Composition comprising polysaccharide extracted from Panax ginseng preventing and treating liver diseases. US patent 20110046086. February 24, 2011.
- 92.Vasta G.R., Ahmed H., Bianchet M.A., Fernández-Robledo J.A., Amzel L.M. Diversity in recognition of glycans by F-type lectins and galectins: molecular, structural, and biophysical aspects. Ann N Y Acad Sci. 2012;1253:E14–E26. doi: 10.1111/j.1749-6632.2012.06698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weis W.I., Drickamer K. Structural basis of lectin-carbohydrate recognition. Annu Rev Biochem. 1996;65:441–473. doi: 10.1146/annurev.bi.65.070196.002301. [DOI] [PubMed] [Google Scholar]
- 94.Yadav M., Schorey J.S. The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood. 2006;108:3168–3175. doi: 10.1182/blood-2006-05-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Byeon S.E., Lee J., Kim J.H., Yang S., Kwak Y.S., Kim S.Y., Choung E.S., Rhee M.H., Cho J.Y. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean red ginseng. Mediators Inflamm. 2012;2012:732860. doi: 10.1155/2012/732860. [DOI] [PMC free article] [PubMed] [Google Scholar]