Abstract
Background
Korean Red Ginseng extract (KRG, Panax ginseng Meyer) and its constituents have been used for treating diabetes. However, in diet-induced obese mice, it is unclear whether KRG can enhance the glucose-lowering action of rosiglitazone (ROSI), a peroxisome proliferator-activated receptor gamma synthetic activator.
Methods
Oral glucose tolerance tests (oGTTs) were performed after 4 days of treatment with a vehicle (CON), KRG [500 mg/kg body weight (b.w.)], ROSI (3.75 mg/kg b.w, 7.5 mg/kg b.w, and 15 mg/kg b.w.), or ROSI and KRG (RK) in obese mice on a high-fat diet. Adipose tissue morphology, crown-like structures (CLSs), and inflammation were compared by hematoxylin-eosin staining or quantitative reverse transcription polymerase chain reaction.
Results
The area under the glucose curve (AUC) was significantly lower in the RK group (15 mg/kg b.w. and 500 mg/kg b.w. for ROSI and KRG, respectively) than in the CON group. There was no significant difference in the AUC between the CON and the other groups. Furthermore, the AUC was significantly lower in the RK group than in the ROSI group. The expression of the Ccl2 gene and the number of CLSs were significantly reduced in the RK group than in the CON group.
Conclusion
Our results show a potential enhancement of ROSI-induced improvement of glucose regulation by the combined treatment with KRG.
Keywords: adipose tissue, glucose regulation, inflammation, Korean Red Ginseng, rosiglitazone
1. Introduction
Diabetes mellitus is a chronic disease that causes serious comorbidities such as neuropathy, nephropathy, and retinopathy. The global prevalence of diabetes mellitus was estimated at 7.7% in 2010 and continues to increase, especially in low or middle-income countries, and it is often attributed to a westernized lifestyle and increasing obesity [1]. Because of the seriousness of this surge in diabetes, there are intensive efforts to develop novel potent antidiabetic agents.
Rosiglitazone (ROSI), a synthetic peroxisome proliferator-activated receptor gamma (PPARγ) agonist, has been used as an insulin sensitizer for several years. Multiple molecular, tissue-dependent mechanisms are involved in the insulin-sensitizing action of ROSI. For example, the activation of PPARγ increases lipid storage, adipogenesis, lipid metabolism, glucose homeostasis, and anti-inflammatory processes in adipose tissue. It also reduces gluconeogenesis in the liver and enhances glucose uptake in skeletal muscle [2], [3], [4]. It was recently found that the release of certain regulatory factors in adipose tissue such as adiponectin and fibroblast growth factor 1 and 21 increased after ROSI treatment and that these factors have an important role in mediating ROSI's insulin-sensitizing effects [2], [3]. However, ROSI causes adverse effects such as weight gain and fluid retention, and it increases the risk of congestive heart failure and myocardial infarction, based on meta-analyses of clinical studies [5], [6], [7]. This limits the use of ROSI in the United States and Europe. Further investigation is required before ROSI can be applied clinically.
Korean Red Ginseng (KRG, Panax ginseng Meyer) is a traditionally prescribed herb in Asian countries such as China, Korea, and Japan. It has a variety of beneficial actions such as enhancing brain function, analgesia, and anticancer and anti-inflammatory effects [8], [9]. This pleiotropic trait has often caused KRG to be called an adaptogen [10]. KRG and some of its constituents such as Re, Rb1, and Rb2 reportedly have an antidiabetic action in in vitro, animal, and clinical studies [11], [12]. The molecular mechanisms underlying KRG's actions have recently been actively investigated; adenosine monophosphate-activated protein kinase (AMPK) in skeletal muscle has been proposed as a potential target for the KRG-induced antidiabetic action [13], [14].
In this study, we aimed to determine whether KRG has beneficial effects on the ROSI-induced changes in glucose regulation in obese mice fed a high-fat diet (HFD). Furthermore, we sought to investigate the underlying mechanism of the improvement of glucose regulation by analyzing the changes in adipose tissue morphology and inflammation and by comparing the expression of key energy sensors, including AMP-activated protein kinase (AMPK) and Protein kinase B (Akt), in skeletal muscle and the liver in obese mice treated with ROSI and KRG.
2. Materials and methods
2.1. Animals
Seventy-two male C57BL/6 mice (8 weeks old, 21–23 g) were purchased from Orient Co., Ltd. (Seoul, Korea). The mice were individually housed in standard mouse cages under a 12-hour light/dark cycle (lights on at 06:00 AM) and maintained under environmentally controlled conditions (22°C, 50% relative humidity). The mice had access to food and water ad libitum, except during the overnight fast required for the oral glucose tolerance tests (oGTTs), when only water was freely available. All mice became obese on a HFD containing 45% fat, 20% protein, and 35% carbohydrates (4.73 kcal/g, D12451; Research Diets, New Brunswick, NJ, USA), which they were fed for 12 weeks before the experiments started. Body weight and food intake were monitored weekly before the experiments and during the washout periods, and monitored daily during the experiments. At the end of the experiment, the mice were sacrificed after a 15-hour fast and their blood and tissues were collected and stored at −80°C. All efforts were made to minimize the number of animals used and animal suffering. All experimental procedures followed the guidelines on the ethical use of animals, after all animal protocols were approved by the Institutional Animal Care and Use Committee of Korea University (Seoul, Korea).
2.2. KRG extract
KRG was manufactured by the Korea Ginseng Corporation (Seoul, Korea) from the roots of a 6-year-old red ginseng (Panax ginseng Meyer) harvested in the Republic of Korea. KRG was produced by steaming fresh ginseng at 90–100°C for 3 hours and then drying it at 50–80°C. The KRG water extract was subsequently prepared by extracting at 85–90°C during 8 hours of circulating in hot water. This procedure was repeated three times. The water content of the pooled extract was 36% of the total weight. KRG was analyzed by high-performance liquid chromatography. KRG contained the major ginsenosides Rg1 (2.40 mg/g), Rb1(8.28 mg/g), Rg1 + Rb1 (10.67 mg/g), Rg3s (1.12 mg/g), Re (2.48 mg/g), Rc (3.32 mg/g), Rb2 (2.98 mg/g), Rd (0.88 mg/g), Rf (1.19 mg/g), Rh1 (0.77 mg/g), Rg2s (1.02 mg/g), and other minor ginsenosides.
2.3. Experimental designs
To determine the subthreshold dose of KRG that affects glucose regulation, we performed oGTTs with KRG [0 mg/kg body weight (b.w.), 125 mg/kg b.w., 250 mg/kg b.w., and 500 mg/kg b.w.] in obese mice that were fed a HFD for 5 weeks and 11 weeks. Unlike previous studies [15], [16], [17], we did not observe any significant difference in glucose tolerance between the groups at the KRG doses tested. Therefore, we used 500 mg/kg b.w. of KRG as the subthreshold dose for KRG in this study. The obese mice were divided into four groups: (1) vehicle-treated [i.e., control (CON)]; (2) ROSI-treated (3.75 mg/kg b.w., 7.5 mg/kg b.w., or 15 mg/kg b.w.); (3) KRG-treated (500 mg/kg b.w.); and (4) ROSI combined with KRG-treated (RK; 500 mg/kg b.w. KRG combined with 3.75 mg/kg b.w. ROSI, 7.5 mg/kg b.w. ROSI, or 15 mg/kg b.w. ROSI) group. During the experiments, the mice received the vehicle, ROSI, KRG, or RK daily for 4 days via oral administration. Oral glucose tolerance tests were performed after an overnight fast on Day 4 of the treatments. Each experiment was followed by a 3-week washout period.
2.4. Combined oral administration of ROSI and KRG
The vehicle solution for ROSI was prepared by dissolving 0.25 g of methyl cellulose (Sigma-Aldrich, St. Louis, MO, USA) in 50-mL deionized water. The ROSI solution was prepared by suspending ROSI (Cayman Chemicals, Ann Arbor, MI, USA) in the vehicle solution, which was stirred until the time of administration. The KRG solution was prepared by dissolving KRG in vehicle (0.9% saline). The amount of KRG extract was calculated after excluding the water content. The ROSI vehicle (0.5% methyl cellulose) or ROSI solution was administered 1 hour before the administration of the KRG vehicle (0.9% saline) or the KRG solution, which were administered before the beginning of the 12-hour dark cycle. All solutions were prepared daily and administered via gastric gavage.
2.5. Oral glucose tolerance tests
After an overnight fast, we performed oGTTs in the obese mice on a HFD in accordance with the aforementioned experimental design. A glucose solution (1.5 g/kg b.w.) was orally administered to the mice after a 2-hour acclimation period to the experimental conditions. Glucose levels were determined by tail vein blood samples using a glucose analyzer (Roche Diagnostics, Mannheim, Germany) at 0 minutes, 15 minutes, 30 minutes, 45 minutes, 60 minutes, and 120 minutes after the glucose load.
2.6. Measurement of serum insulin levels
Blood samples were collected from the tail vein at 0 minutes and 15 minutes during the oGTTs after the glucose load. Serum insulin levels were subsequently determined using the mouse Ultra Sensitive Insulin ELISA Kit (Crystal Chem, Chicago, IL, USA).
2.7. Quantitative real-time polymerase chain reaction analysis
Obese mice were sacrificed during the light phase after a 15-hour fast started on the last day of the treatments. The epididymal fat was quickly removed, frozen in 3-methyl butane on dry ice, and stored at −80°C. After the tissue samples were homogenized in TRIZOL reagent (Life Technologies, Carlsbad, CA, USA) and processed in accordance with the manufacturer's instructions, the total RNA was isolated. cDNA was synthesized using iScript reverse transcription supermix (Bio-Rad, Hercules, CA, USA).
For the quantitative real-time polymerase chain reaction (RT-qPCR), we used the TaqMan RT-PCR Ready-Mix Kit (PE Applied Biosystems, Foster City, CA, USA). Gene expression was measured using an ABI7500 device (Applied Biosystems, Piscataway, NJ, USA). Forty PCR cycles were performed using a two-step amplification (95°C for 10 seconds, annealing temperature at 60°C for 30 seconds) using the 7500 software (v2.0.6, Applied Biosystems, Piscataway). Ribosomal protein L32 was used as an endogenous control. Mouse primers used are listed in Table S1.
To normalize the data, the ΔCT (Cycle threshold) was calculated for each sample by subtracting the CT of L32 from the CT of the gene of interest. For relative quantization, the ΔCT was averaged for the defined control group and was then subtracted from the ΔCT of each experimental sample to generate the ΔΔCT. The ΔΔCT was used to calculate the approximate fold difference, based on the instructions of Applied Biosystem [18].
2.8. Measurement of hepatic and skeletal glycogen
The levels of hepatic and skeletal muscle glycogen were measured according to the acid-hydrolysis method [19]. In brief, after the mice were sacrificed, their liver was isolated and stored at −80°C until use. A piece of liver (approximately 10–20 mg) was transferred into tubes containing 0.5 mL of a 2.0M hydrochloride solution at 100°C. After the tubes were tightly sealed, the samples were completely hydrolyzed in the same solution at 100°C for 1 hour. The hydrolysis products were neutralized with 0.5 mL of a 2.0M sodium hydroxide solution and their glucose concentration was determined using the manufacturer's instructions (GAHK-20; Sigma-Aldrich, St. Louis, MO, USA). In brief, the supernatant was placed in a microtube containing the enzymatic reagent solution (1.5mM nicotinamide adenine dinucleotide, 1mM adenosine triphosphate, 20 U hexokinase, 20 U glucose-6-phosphate dehydrogenase, and 20-mL deionized water). The tube contents were incubated for 15 minutes at room temperature. The absorbance of the samples was determined using a spectrophotometer (SpectraMax Plus384; Molecular Devices, Sunnyvale, CA, USA). The glucose levels (μmole/g) were adjusted for total volume and tissue weight.
2.9. Adipose tissue morphometry
For histological analysis, the epididymal fat was fixed for 18 hours in an alcoholic zinc formalin solution at room temperature. It was then dehydrated by exposure to increasing ethanol concentrations (70%, 80%, 90%, 95%, and 100%), and embedded in paraffin. Tissue sections (5 μm) were cut using a microtome (HM 505 E microtome; Microm Laborgeräte, Walldorf, Germany). After deparaffinization, the sections were stained with Harris' hematoxylin and eosin. To determine the adipocyte size, the cross-sectional area of adipocytes was measured on sections of epididymal fat at a 100× magnification by image processing with customized software written in Labview 9.0 Student Edition (National Instruments, Austin, TX, USA) [18]. Approximately 1,200 adipocytes were analyzed per mouse. The distribution of adipocyte size was determined by the relative frequencies of adipocytes having a specific size within set intervals. The number of crown-like structures (CLSs) was counted on the same image by four investigators in a double-blind manner. The density of the CLSs was calculated by dividing the CLSs by the number of adipocytes on the same image.
2.10. Western blotting
To investigate the involvement of key energy sensors related to glucose regulation (e.g., AMPK and Akt) the mice were sacrificed after a 15-hour fast on the last day of the treatments. The phosphorylation of AMPK and Akt was measured as previously described [20]. The liver and skeletal muscle were quickly dissected and the tissue was homogenized and lysed with radioimmunoprecipitation assay buffer (1.25% Triton X-100, 0.1% sodium dodecyl sulfate, 50mM Tris-HCl, 150mM sodium chloride, 5mM EDTA, pH 7.6) containing a mixture of phosphatase inhibitors, sodium fluoride (Sigma-Aldrich, St. Louis, MO, USA), and sodium pyrophosphate decahydrate (Sigma-Aldrich). Protein (20 μg) was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and electrophoretically transferred to polyvinylidene difluoride membranes. The membranes were blocked in Tris-buffered saline (TBS; 10mM Tris, 150mM sodium chloride, pH 8.0) with 5% skim milk or 1% bovine serum albumin (Sigma-Aldrich). It was then incubated overnight at 4°C with a primary antibody against phospho-AMPK-alpha (Thr172; 1:1,000), AMPK-alpha (1:2,000), phospho-Akt (1:1,000), Akt (1:2,000), or beta-actin (1:4,000). All primary antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). After washing in TBS containing 0.05% Tween-20 (TBST), the membranes were incubated with a horse radish peroxidase-conjugated secondary antibody (1:2,000, Cell Signaling Technology) for 1 hour at room temperature. After further washing in TBST, the proteins were visualized using an ECL kit (Thermo Scientific, Rockford, IL, USA). The intensity of the bands was analyzed using the ImageJ software (National Institutes of Health, Bethesda, MD, USA) and normalized to the intensity of an internal control.
2.11. Statistical analysis
The results are displayed as the mean ± the standard error of the mean. All data from the animal studies were analyzed by two-way repeated measures analysis of variance (ANOVA), followed by Bonferroni's multiple comparisons test; one-way ANOVA followed by a Fisher's least significant difference test; or a two-tailed Student t test (GraphPad Prism 5; GraphPad Software Inc., San Diego, CA, USA). For gene expression and western blotting, a two-tailed Student t test was performed. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. The effects of ROSI combined with KRG on body weight and food intake in diet-induced obese mice
To investigate the effects of ROSI and KRG on body weight and food intake in obese mice fed a HFD, we compared weight gain and cumulative food intake for 4 days in the three different RK groups (500 mg/kg b.w. KRG combined with 3.75 mg/kg b.w. ROSI, 7.5 mg/kg b.w. ROSI, or 15 mg/kg b.w. ROSI). No significant difference in weight gain and cumulative food intake was observed between the groups (Fig. 1).
Fig. 1.
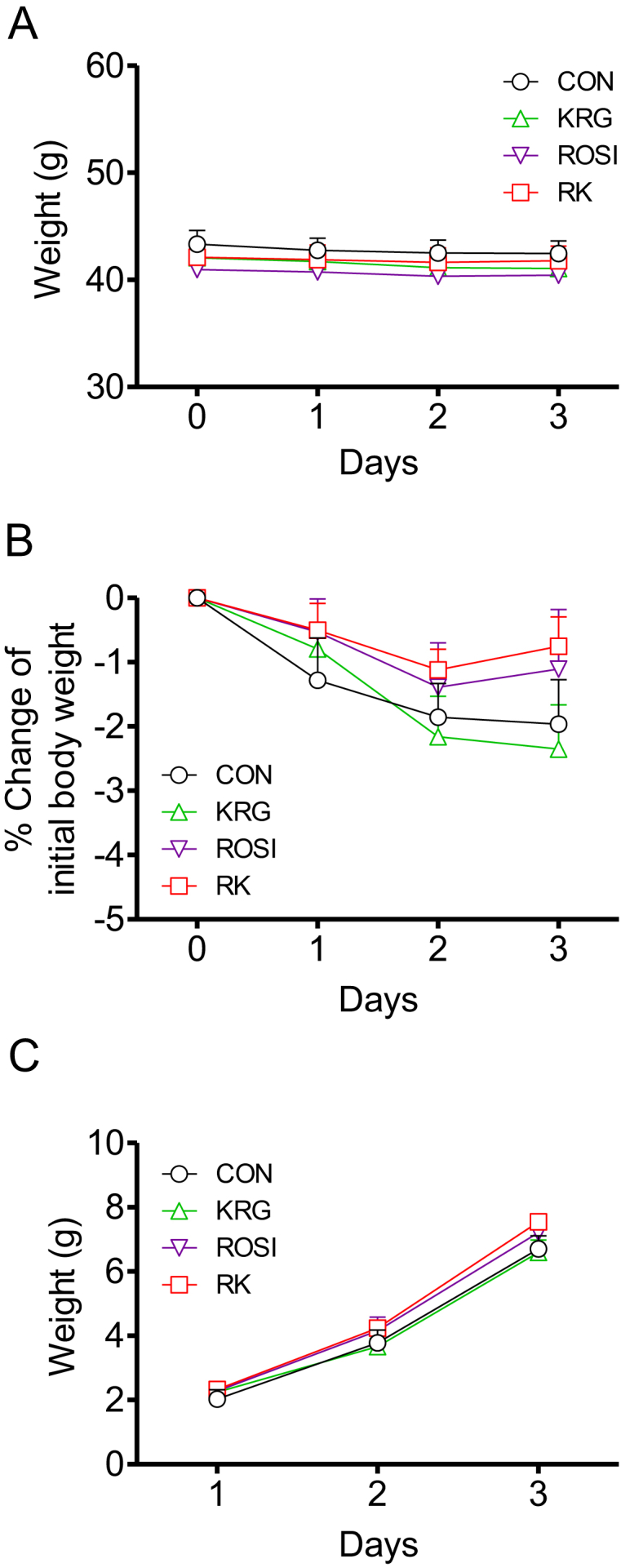
The effects of rosiglitazone combined with Korean Red Ginseng on body weight and food intake in diet-induced obese mice. (A) Body weight change during 4 days of treatment. (B) Percentage change in body weight during 4 days of treatment. (C) Cumulative food intake during 4 days of treatment. Data are presented as mean ± the standard error of the mean. CON, study group treated with 0.5% methyl cellulose and 0.9% saline; KRG, study group treated with 500 mg/kg body weight (b.w.) of Korean Red Ginseng; RK, study group treated with 500 mg/kg b.w. KRG combined with 15 mg/kg b.w. rosiglitazone; ROSI, study group treated with 15 mg/kg b.w. of rosiglitazone.
3.2. The effects of ROSI combined with KRG on glucose tolerance and serum insulin in diet-induced obese mice
To determine the effect of ROSI and KRG on glucose regulation in obese mice on a HFD, we compared the compounds' ability to reduce glucose by performing oGTTs at the three ROSI doses (3.75 mg/kg b.w., 7.5 mg/kg b.w., or 15 mg/kg b.w.) after 4 days of treatment.
Time and treatment showed a significant correlation with the change in glucose levels after the glucose challenge between the treatment groups, as determined by two-way repeated measures ANOVA (F 15, 170 = 3.998 for 15 mg/kg b.w. ROSI; p < 0.05; Fig. 2A). One-way ANOVA revealed a significant difference in the area under the glucose curve (AUC) between the groups at the dose of 15 mg/kg b.w. ROSI. The AUC was significantly lower in the RK group than in the ROSI group (p < 0.05; Fig. 2B). In the oGTT at the dose of 3.75 mg/kg b.w. ROSI and 7.5 mg/kg b.w. ROSI, similar trends were consistently observed. These results indicated that KRG enhanced the glucose-lowering action of ROSI in obese mice fed a HFD.
Fig. 2.
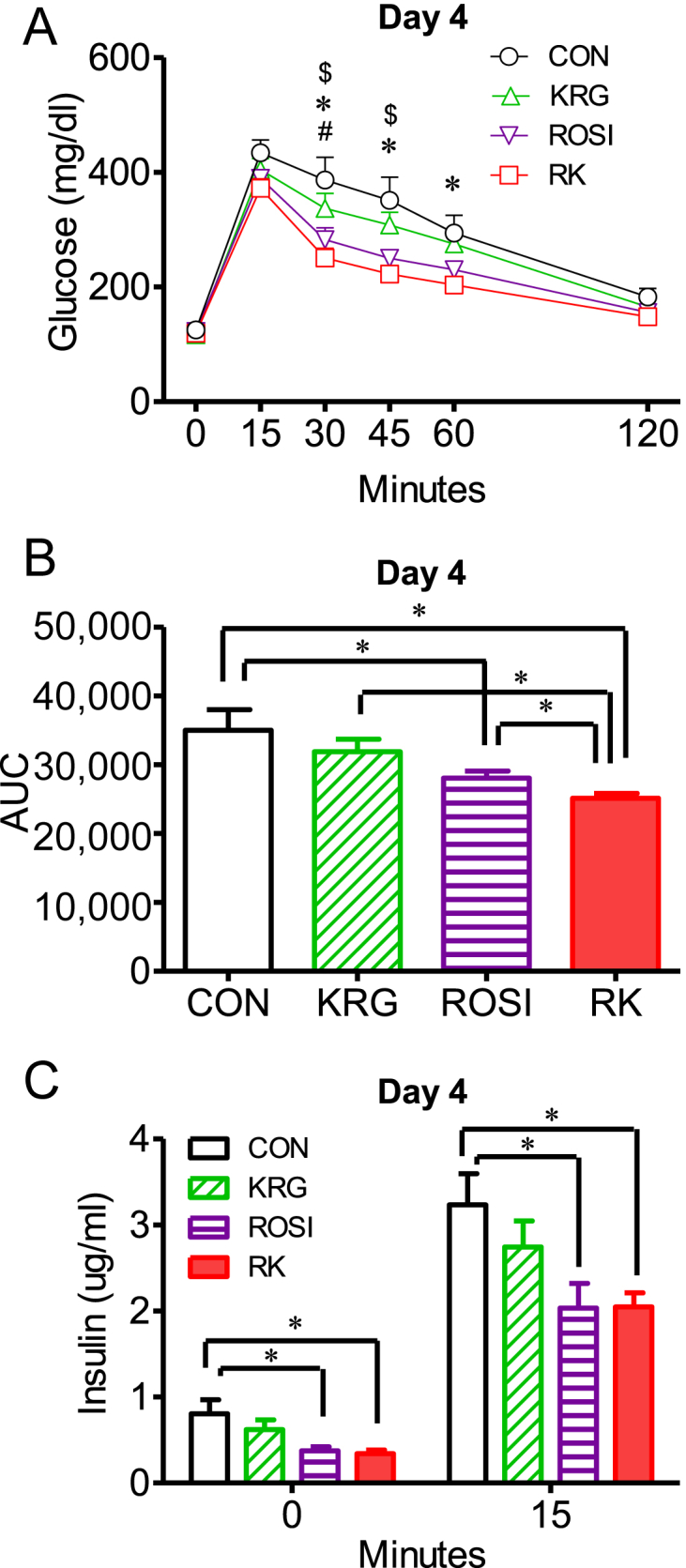
The effect of rosiglitazone combined with Korean Red Ginseng on glucose tolerance and serum insulin in diet-induced obese mice. (A) Oral glucose tolerance tests (oGTTs) were performed after 4 days of treatment with ROSI and KRG in obese mice fed a high-fat diet. (B) Comparison of the area under the glucose curve (AUC). (C) Serum insulin levels at 0 minutes and 15 minutes after the glucose load during the oGTTs. Data are presented as the mean ± the standard error of the mean. $p < 0.05, CON versus ROSI. & p < 0.05, ROSI versus KRG. # p < 0.05, KRG versus RK. * p < 0.05, CON versus RK. ∗ p < 0.05; AUC, area under the glucose curve. CON, study group treated with 0.5% methyl cellulose and 0.9% saline; KRG, study group treated with 500 mg/kg body weight (b.w.) of Korean Red Ginseng; RK, study group treated with 500 mg/kg b.w. KRG combined with 15 mg/kg b.w. rosiglitazone; ROSI, study group treated with 15 mg/kg b.w. of rosiglitazone.
To determine the responsiveness of insulin secretion to the action of ROSI and KRG, we compared the serum insulin levels between the groups at 0 minutes and 15 minutes after the glucose load. The basal insulin levels were significantly lower in the ROSI and RK groups, compared to the levels in the CON group, as measured by one-way ANOVA (F 3, 34 = 4.290 for 15 mg/kg b.w. ROSI; CON vs. ROSI or RK; p < 0.05; Fig. 2C). However, no significant difference in the insulin levels was observed between the ROSI and the RK groups. The insulin levels at 15 minutes after the glucose load were significantly lower in the ROSI and the RK groups, compared to the levels in the CON group, as determined by two-way repeated measures ANOVA, followed by the Bonferroni multiple comparisons test (F 3, 34 = 2.270 for 15 mg/kg b.w. ROSI; CON vs. ROSI or RK; p < 0.05; Fig. 2C). These results indicated that the demand for insulin in regulating serum glucose levels was decreased in the ROSI and RK groups, compared to the CON group.
3.3. The effects of ROSI combined with KRG on hepatic and skeletal muscle glycogen content in diet-induced obese mice
The glycogen levels were significantly lower in the liver of the mice in the RK group that had fasted for 15 hours, compared to the levels in the liver of the CON animals (p < 0.05; Fig. 3A). However, no significant difference in glycogen levels existed in the skeletal muscle between the groups (Fig. 3B).
Fig. 3.
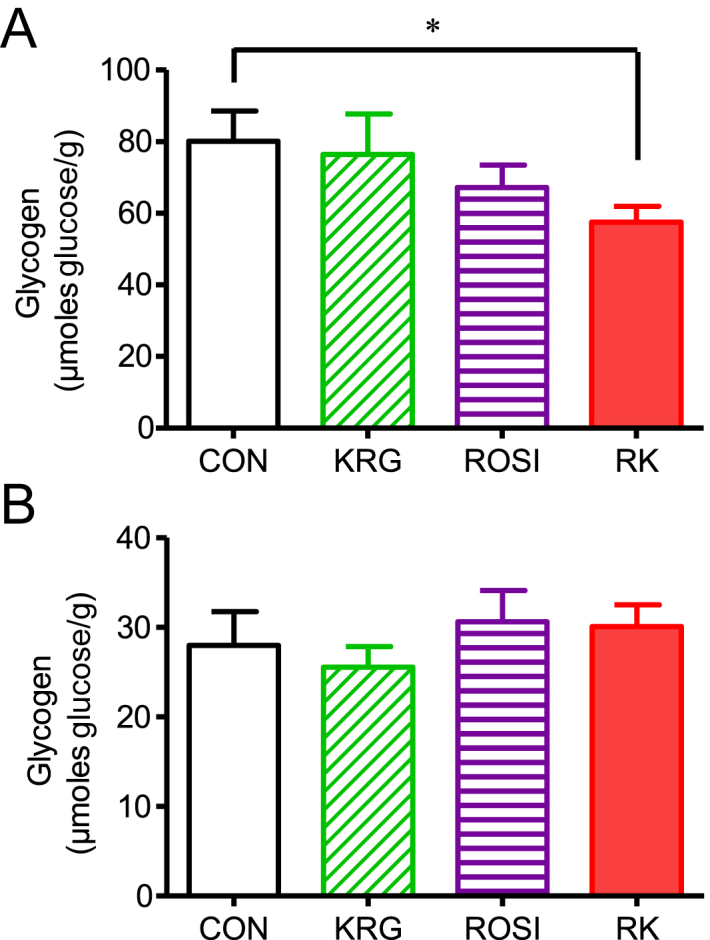
Effects of rosiglitazone combined with Korean Red Ginseng in diet-induced obese mice. (A) Effect on hepatic glycogen content. (B) Effect on skeletal muscle glycogen content. Data are presented as the mean ± the standard error of the mean. * p < 0.05. CON, study group treated with 0.5% methyl cellulose and 0.9% saline; KRG, study group treated with 500 mg/kg body weight (b.w.) of Korean Red Ginseng; RK, study group treated with 500 mg/kg b.w. KRG combined with 3.75 mg/kg b.w. rosiglitazone; ROSI, study group treated with 3.75 mg/kg b.w. of rosiglitazone.
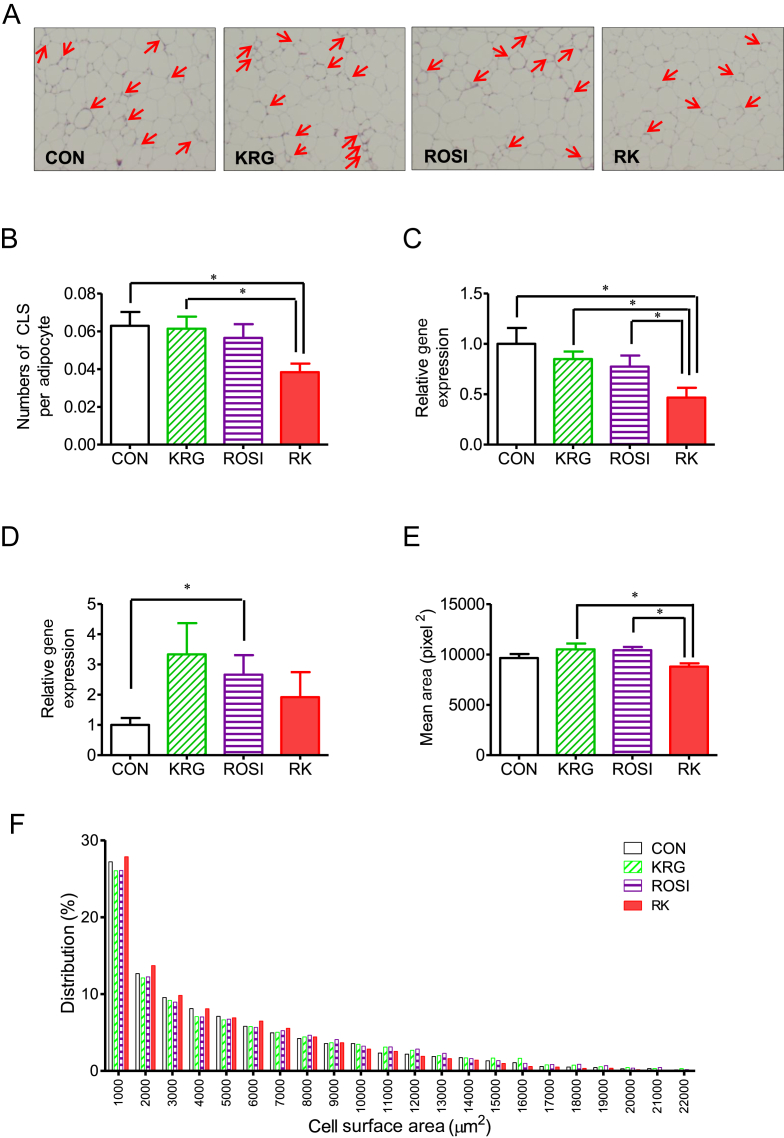
3.4. The effects of ROSI combined with KRG on adipose tissue morphology and CLSs in diet-induced obese mice
To determine the effect of ROSI and KRG on adipose tissue morphology, we analyzed adipocyte size and measured the number of CLSs in obese mice on a HFD. After 4 days of treatment, the number of CLSs was significantly different between the groups as measured by one-way ANOVA (F 3, 28 = 3.019; p < 0.05) and was significantly lower in the RK group than in the CON and KRG groups (p < 0.05; Fig. 3, Fig. 4B). The cross-sectional area of adipocytes was significantly different between the groups, as shown by one-way ANOVA (F 3, 28 = 3.709; p < 0.05), and was significantly lower in the RK group than in the ROSI and the KRG groups (p < 0.05; Figs. 4A, 4E, 4F).
Fig. 4.
The effect of rosiglitazone combined with Korean red ginseng on adipose tissue morphology and crown-like structures (CLSs) in diet-induced obese mice. (A) Representative figures of epididymal fat after 4 days of treatment (red arrows indicate CLSs of macrophages. (B) Comparison of CLSs per field. (C) Ccl2 gene expression in adipose tissue. (D) Arg1 gene expression in adipose tissue. (E) Comparison of the mean surface area of adipocytes. (F) The distribution of adipocyte size. Data are presented as the mean ± the standard error of the mean. * p < 0.05. Ccl2 (MCP-1), chemokine (C–C motif) ligand 2; CON, study group treated with 0.5% methyl cellulose and 0.9% saline; KRG, study group treated with 500 mg/kg b.w. of Korean Red Ginseng; RK, study group treated with 500 mg/kg b.w. KRG combined with 3.75 mg/kg b.w. rosiglitazone; ROSI, study group treated with 3.75 mg/kg b.w. of rosiglitazone.
3.5. The effects of ROSI combined with KRG on adipose tissue inflammation in diet-induced obese mice
To determine whether ROSI and KRG affected adipose tissue inflammation, we compared the expression of genes related to Type M1 and M2 macrophages including Cd68, Emr1, Ccl2, Ccr2, Itgax, Il6, Arg1, Jag1, and Chil3.
Unlike the histological findings, the expression of Emr1 and Cd68 was not significantly different between the treatment groups. However, the expression of Ccl2 was significantly lower in the RK group compared with that in the other groups (p < 0.05 for all groups; Fig. 4C). By contrast, the expression of Arg1 was significantly increased in the ROSI group, compared to its expression in the CON group (p < 0.05; Fig. 4D), whereas the expression of the other genes related to Type M1 and M2 macrophages did not significantly differ between the groups.
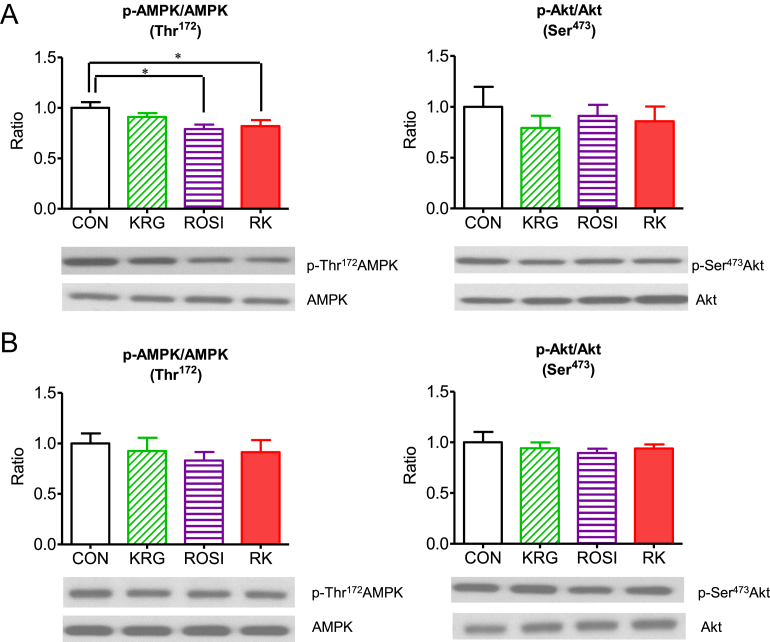
3.6. The effects of ROSI combined with KRG on the phosphorylation of AMP-activated protein kinase and Akt in the liver and skeletal muscle of diet-induced obese mice
The level of AMPK phosphorylation at Thr172 was significantly lower in the liver of the mice in the ROSI and the RK groups compared to the liver of the animals in the CON group (p < 0.05; Fig. 5A and 5B); however, no significant difference in phosphorylation at Thr172 was observed in skeletal muscle. However, neither ROSI and KRG nor their combination changed Akt phosphorylation in the liver and skeletal muscle of the obese mice (Fig. 5A and 5B).
Fig. 5.
The effect of rosiglitazone combined with Korean Red Ginseng on the phosphorylation of adenosine monophosphate-activated protein kinase (AMPK) and Akt in diet-induced obese mice. (A) The effect in the liver. (B) The effect in the skeletal muscle. Data are presented as the mean ± the standard error of the mean. * p < 0.05. CON, study group treated with 0.5% methyl cellulose and 0.9% saline; KRG, study group treated with 500 mg/kg body weight (b.w.) of Korean Red Ginseng; RK, study group treated with 500 mg/kg b.w. KRG combined with 3.75 mg/kg b.w. rosiglitazone; ROSI, study group treated with 3.75 mg/kg b.w. of rosiglitazone.
4. Discussion
The aim of this study was to determine whether KRG could enhance the antidiabetic action of ROSI in a diet-induced obesity animal model. We showed that short-term treatment with KRG increased the ROSI-induced improvement of glucose regulation in HFD-fed obese mice. This effect was reflected by a lower level of hepatic glycogen in the fasted obese mice treated with the combination of ROSI and KRG (i.e., the RK group) relative to the other groups. In addition, we found that the number of macrophages and the level of Ccl2 gene expression were significantly lower in adipose tissue of the RK group than in the other groups, which indicated reduced capability of macrophage recruitment. Furthermore, the adipocyte size was significantly smaller in the RK group than in the ROSI group and KRG group. However, the activation of AMPK and Akt in the liver and skeletal muscle that we observed may not have contributed to the enhancement of the glucose-lowering action in the RK group.
In this study, we aimed to determine whether the coadministration of KRG enhances this ROSI-induced improvement of glucose regulation in obese mice on a HFD, which are glucose-intolerant. To accomplish this, we administered KRG at a dose that did not improve glucose regulation. At the highest dose of ROSI we tested (30 mg/kg b.w. ROSI; data not shown), the glucose tolerance of both groups was better than that of the CON group, whereas no such difference existed between the ROSI and RK groups. This indicates that the effect of KRG on glucose regulation was not sufficiently robust to exceed the potent ability of ROSI (30 mg/kg b.w.) to improve glucose regulation. However, KRG enhanced the ROSI-induced improvement in glucose regulation at the lower doses of ROSI used in this study. We also found that the hepatic glycogen content was lower only in the fasted RK group relative to that in the CON group. Because of the absence of increased food intake in both groups owing to fasting, the similarity in the basal glucose level between the RK group and the other groups may be attributable to an increased consumption of stored glycogen in the liver (Fig. 2, Fig. 3). Therefore, the finding of a lower glycogen content in the RK group may support the hypothesis of an enhancing ability of KRG on the glucose-lowering action of ROSI. These results imply that KRG could be used as an adjuvant in the treatment of diabetics with ROSI.
It has been hypothesized that low-grade adipose tissue inflammation is strongly associated with the development of obesity and insulin resistance, and that M1 macrophages and proinflammatory cytokines have an important role in insulin resistance in obese mice [21], [22], [23]. Therefore, we investigated the change in the inflammatory status of adipose tissue after the short-term treatment with RK. We found a reduced number of macrophages and Ccl2 expression in adipose tissue of the RK group compared to the expression in the other groups after this short-term RK treatment (Fig. 4). Ccl2, a marker of M1 macrophages, has a critical role in macrophage infiltration into adipose tissue and insulin resistance in obesity [24]. The lower Ccl2 expression in the RK group may indicate that the number of M1 macrophages was indeed reduced in adipose tissue, partially underlying the enhancing effect of KRG on the glucose-lowering action of ROSI.
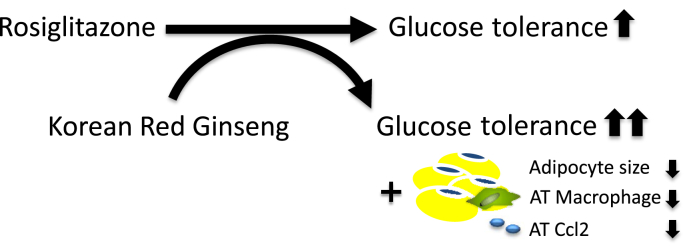
In Korea, China, and Japan, KRG has traditionally been widely used to treat inflammatory diseases such as chronic gastritis and colitis [25]. Several lines of evidence on the ability of KRG to ameliorate inflammation have recently been provided at the molecular level [26], [27]. For example, in human gastric epithelial cells infected with Helicobacter pylori, red ginseng extract suppressed the induction of Ccl2 expression by inhibiting nicotinamide adenine dinucleotide phosphate oxidase and Jak2/Stat3 activities [27]. In RAW264.7 cells treated with lipopolysaccharide, KRG water extract reduced inflammatory responses such as the gene expression of interferon-beta, cyclooxygenase-2, and inducible nitric oxide synthase [26]. In human keratinocytes stimulated by lipopolysaccharides, KRG decreased the secretion of tumor necrosis factor-alpha and interleukin-8 [28]. Because of the evidence of the anti-inflammatory action of KRG, we hypothesized that KRG per se may reduce the increased inflammatory status in the adipose tissue of obese mice. We did not observe a change in adipose tissue inflammation in obese mice after the short-term treatment with KRG at the dose we used. However, when obese mice were coadministered ROSI and KRG, the number of macrophage clusters along with Ccl2 gene expression was significantly lower than in the other groups. The underlying mechanisms remain to be determined; however, the rapid reduction in these macrophage clusters and Ccl2 gene expression may contribute to the enhancement of improved glucose regulation in the RK group by reducing the proinflammatory status of the adipose tissue of the obese mice (Fig. 6).
Fig. 6.
Potential mechanism underlying the beneficial action of the combined treatment with rosiglitazone (ROSI) and Korean Red Ginseng (KRG) on glucose regulation. KRG may enhance the glucose-lowering action of ROSI by reducing adipocyte size and macrophage recruitment in the adipose tissue of obese mice on a high-fat diet. AT, adipose tissue.
It is interesting that we observed a significant decrease in adipocyte size in the RK group, compared to the adipocyte size in the ROSI group. Because of the increased number of smaller adipocytes after ROSI treatment, which was reported in a previous study [29], the smaller adipocyte size we observed in the RK group relative to that in the ROSI group may partly explain the enhancing ability of KRG in the antidiabetic action of ROSI.
To further investigate the mechanisms underlying the beneficial action of the combined treatment with ROSI and KRG in glucose regulation, we focused on the change in the activation of AMPK and Akt in skeletal muscle. Long-term administration of KRG improved glucose tolerance by stimulating fatty acid oxidation through the activation of AMPK and the phosphorylation of acetyl–coenzyme A carboxylase in skeletal muscle of Otsuka Long–Evans Tokushima Fatty rats [14], [17]. In addition, the long-term treatment with KRG increased insulin sensitivity via activating IR/IRS-1/Akt/Glut4 signaling in skeletal muscle of diet-induced obese rats [17]. Because of the critical role of AMPK and Akt on improving glucose regulation induced by KRG, we hypothesized that the activation of AMPK or Akt in skeletal muscle may have a key role in the enhanced action of KRG in the ROSI-induced improvement of glucose regulation. However, we did not observe any significant change in AMPK and Akt activities in skeletal muscle after 4 days of treatment with RK and ROSI, compared to the CON treatment. Therefore, this implies that other mechanisms may be involved in the improved glucose regulation by AMPK and Akt after the short-term treatment with RK.
5. Conclusion
In summary, this study provides evidence of the enhancement of the action of ROSI on glucose regulation by coadministering KRG. This effect may be attributable to the rapid inhibition of macrophage recruitment, accompanied by lower Ccl2 gene expression and smaller adipocytes (Fig. 6). This study raises the potential for applying ROSI in combination with KRG as an adjuvant. Based on these results, further studies are required for the development of a clinical application of this combination drug therapy in diabetic patients. Therefore, we propose that further detailed investigations would enable KRG to be a useful choice with ROSI for treating diabetes.
Conflicts of interest
The authors declare that no conflicts of interest exist.
Acknowledgements
This research was supported by 2012 grant from the Korean Society of Ginseng, Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (Seoul, Korea; grant number, NRF-2014R1A1A1A05002310), and a Korea University Grant (Seoul, Korea). We would also like to thank Hyung-Ha Lee and the Institute of Biomedical Science and Food Safety at the Korea University Food Safety Hall (Seoul, Korea) for technical support.
Mi-Jeong Oh and Dong-Hoon Kim designed the study and wrote the manuscript. Mi-Jeong Oh, Hyun-Ju Kim, Mun-Gyu Song, Eun-Young Park, and Na-Hee Ha conducted the experiments. Sang-Hyun Choi, Boe-Gwun Chun, and Dong-Hoon Kim edited the manuscript. All authors have approved the final manuscript.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jgr.2015.12.011.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Whiting D.R., Guariguata L., Weil C., Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Cariou B., Charbonnel B., Staels B. Thiazolidinediones and PPARγ agonists: time for a reassessment. Trends Endocrinol Metab. 2012;23:205–215. doi: 10.1016/j.tem.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Ahmadian M., Suh J.M., Hah N., Liddle C., Atkins A.R., Downes M., Evans R.M. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tontonoz P., Spiegelman B.M. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 5.Kung J., Henry R.R. Thiazolidinedione safety. Expert Opin Drug Saf. 2012;11:565–579. doi: 10.1517/14740338.2012.691963. [DOI] [PubMed] [Google Scholar]
- 6.Graham D.J., Ouellet-Hellstrom R., MaCurdy T.E., Ali F., Sholley C., Worrall C., Kelman J.A. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304:411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 7.Nissen S.E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 8.Park H.J., Kim D.H., Park S.J., Kim J.M., Ryu J.H. Ginseng in traditional herbal prescriptions. J Ginseng Res. 2012;36:225–241. doi: 10.5142/jgr.2012.36.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baeg I.H., So S.H. The world ginseng market and the ginseng (Korea) J Ginseng Res. 2013;37:1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi J., Kim T.H., Choi T.Y., Lee M.S. Ginseng for health care: a systematic review of randomized controlled trials in Korean literature. PLoS One. 2013;8:e59978. doi: 10.1371/journal.pone.0059978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan H.D., Kim J.T., Kim S.H., Chung S.H. Ginseng and diabetes: the evidences from in vitro, animal and human studies. J Ginseng Res. 2012;36:27–39. doi: 10.5142/jgr.2012.36.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bang H., Kwak J.H., Ahn H.Y., Shin D.Y., Lee J.H. Korean red ginseng improves glucose control in subjects with impaired fasting glucose, impaired glucose tolerance, or newly diagnosed type 2 diabetes mellitus. J Med Food. 2014;17:128–134. doi: 10.1089/jmf.2013.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong K.J., Kim G.W., Chung S.H. AMP-activated protein kinase: an emerging target for ginseng. J Ginseng Res. 2014;38:83–88. doi: 10.1016/j.jgr.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee H.J., Lee Y.H., Park S.K., Kang E.S., Kim H.J., Lee Y.C., Choi C.S., Park S.E., Ahn C.W., Cha B.S. Korean red ginseng (Panax ginseng) improves insulin sensitivity and attenuates the development of diabetes in Otsuka Long–Evans Tokushima fatty rats. Metabolism. 2009;58:1170–1177. doi: 10.1016/j.metabol.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Lee H., Park D., Yoon M. Korean red ginseng (Panax ginseng) prevents obesity by inhibiting angiogenesis in high fat diet-induced obese C57BL/6J mice. Food Chem Toxicol. 2013;53:402–408. doi: 10.1016/j.fct.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 16.Song Y.B., An Y.R., Kim S.J., Park H.W., Jung J.W., Kyung J.S., Hwang S.Y., Kim Y.S. Lipid metabolic effect of Korean red ginseng extract in mice fed on a high-fat diet. J Sci Food Agric. 2012;92:388–396. doi: 10.1002/jsfa.4589. [DOI] [PubMed] [Google Scholar]
- 17.Lee S.H., Lee H.J., Lee Y.H., Lee B.W., Cha B.S., Kang E.S., Ahn C.W., Park J.S., Kim H.J., Lee E.Y. Korean red ginseng (Panax ginseng) improves insulin sensitivity in high fat fed Sprague–Dawley rats. Phytother Res. 2012;26:142–147. doi: 10.1002/ptr.3610. [DOI] [PubMed] [Google Scholar]
- 18.Kim D.H., Gutierrez-Aguilar R., Kim H.J., Woods S.C., Seeley R.J. Increased adipose tissue hypoxia and capacity for angiogenesis and inflammation in young diet-sensitive C57 mice compared with diet-resistant FVB mice. Int J Obes (Lond) 2013;37:853–860. doi: 10.1038/ijo.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Passonneau J.V., Lauderdale V.R. A comparison of three methods of glycogen measurement in tissues. Anal Biochem. 1974;60:405–412. doi: 10.1016/0003-2697(74)90248-6. [DOI] [PubMed] [Google Scholar]
- 20.Kim H.J., Park E.Y., Oh M.J., Park S.S., Shin K.H., Choi S.H., Chun B.G., Kim D.H. Central administration of metformin into the third ventricle of C57BL/6 mice decreases meal size and number and activates hypothalamic S6 kinase. Am J Physiol Regul Integr Comp Physiol. 2013;305:R499–R505. doi: 10.1152/ajpregu.00099.2013. [DOI] [PubMed] [Google Scholar]
- 21.Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu H., Barnes G.T., Yang Q., Tan G., Yang D., Chou C.J., Sole J., Nichols A., Ross J.S., Tartaglia L.A. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanda H., Tateya S., Tamori Y., Kotani K., Hiasa K., Kitazawa R., Kitazawa S., Miyachi H., Maeda S., Egashira K. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung J.Y., Lee J.H., Chung S.H. The influence of Donguibogam during the middle Joseon era based on clinical records on low back pain in Seungjeongwon ilgi. Uisahak. 2011;20:1–28. [in Korean] [PubMed] [Google Scholar]
- 26.Yang Y., Yang W.S., Yu T., Sung G.H., Park K.W., Yoon K., Son Y.J., Hwang H., Kwak Y.S., Lee C.M. ATF-2/CREB/IRF-3-targeted anti-inflammatory activity of Korean red ginseng water extract. J Ethnopharmacol. 2014;154(1):218–228. doi: 10.1016/j.jep.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Cho S.O., Lim J.W., Kim H. Red ginseng extract inhibits the expression of MCP-1 and iNOS in Helicobacter pylori-infected gastric epithelial cells by suppressing the activation of NADPH oxidase and Jak2/Stat3. J Ethnopharmacol. 2013;150:761–764. doi: 10.1016/j.jep.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Hong C.E., Lyu S.Y. Anti-inflammatory and anti-oxidative effects of Korean red ginseng extract in human keratinocytes. Immune Netw. 2011;11:42–49. doi: 10.4110/in.2011.11.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okuno A., Tamemoto H., Tobe K., Ueki K., Mori Y., Iwamoto K., Umesono K., Akanuma Y., Fujiwara T., Horikoshi H. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest. 1998;101:1354–1361. doi: 10.1172/JCI1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.