Abstract
Background
Various Panax ginseng cultivars exhibit a range of diversity for morphological and physiological traits. However, there are few studies on diversity of metabolic profiles and genetic background to understand the complex metabolic pathway in ginseng.
Methods
To understand the complex metabolic pathway and related genes in ginseng, we tried to conduct integrated analysis of primary metabolite profiles and related gene expression using five ginseng cultivars showing different morphology. We investigated primary metabolite profiles via gas chromatography–mass spectrometry (GC-MS) and analyzed transcriptomes by Illumina sequencing using adventitious roots grown under the same conditions to elucidate the differences in metabolism underlying such genetic diversity.
Results
GC-MS analysis revealed that primary metabolite profiling allowed us to classify the five cultivars into three independent groups and the grouping was also explained by eight major primary metabolites as biomarkers. We selected three cultivars (Chunpoong, Cheongsun, and Sunhyang) to represent each group and analyzed their transcriptomes. We inspected 100 unigenes involved in seven primary metabolite biosynthesis pathways and found that 21 unigenes encoding 15 enzymes were differentially expressed among the three cultivars. Integrated analysis of transcriptomes and metabolomes revealed that the ginseng cultivars differ in primary metabolites as well as in the putative genes involved in the complex process of primary metabolic pathways.
Conclusion
Our data derived from this integrated analysis provide insights into the underlying complexity of genes and metabolites that co-regulate flux through these pathways in ginseng.
Keywords: adventitious root, metabolome, Panax ginseng, primary metabolic pathways, transcriptome
1. Introduction
Panax ginseng Meyer, is a traditional herb grown in Korea and China that is widely used as a source of herbal medicines due to its tonic, stimulant, restorative, and anti-aging properties [1]. Recent pharmacological studies have contributed to our understanding of the medicinal efficacy of ginseng, revealing its pain-relieving, anti-carcinogen, anti-diabetes, antihypertension, and immunization-enhancing effects [2]. P. ginseng (commonly known as Korean ginseng) is widely cultivated and represents one of most valuable medicinal crops in Korea. Korean ginseng includes a number of cultivars derived from inbred lines developed by pedigree selection from three local landraces, Jakyung, Chungkyung, and Hwangsook [3].
Different ginseng cultivars have different phenotypes [4], genetic polymorphisms [3], [5], [6], and ginsenoside contents [7], [8], [9]. Many previous studies have revealed genetic and metabolic diversities based on DNA polymorphisms and metabolite profiles. Genetic diversity among cultivars has been revealed by simple sequence repeat [3], single nucleotide polymorphism [5], and sequence characterized amplified region marker [6] analyses based on DNA polymorphisms. The metabolic diversity of ginseng cultivars has been investigated via HPLC [7], Fourier transform infrared [8], and nuclear magnetic resonance [9] analyses. However, to date, only a few studies have been performed on the diversity of primary metabolites in ginseng cultivars [9].
Recently, integrated transcriptome and metabolome analysis has been introduced as a new approach to systemically elucidate the mechanisms that regulate metabolic networks in plants [10], [11], [12]. First, genes regulating metabolic pathways and relevant metabolites are extensively analyzed by high-throughput sequencing and non-targeted metabolome analysis, respectively. The resulting profiles are then comparatively analyzed to elucidate gene–metabolite correlations and to identify the functions of unknown genes. The coordinated regulation of genes and metabolites involved in phenylpropanoid metabolism has been explored using this approach in Medicago truncatula [13], Populus x canescens [14], and Arabidopsis thaliana [15]. Moreover, to analyze alkaloid and triterpene metabolism, comparative analyses of transcriptomes and metabolomes have been carried out in Papaver somniferum [16], M. truncatula [17], and Catharanthus roseus [18]. A similar approach has also been applied to Solanum tuberosum using gas chromatography–mass spectrometry (GC-MS); a major platform for primary metabolite analysis [19].
In this study, we performed integrated analysis of the transcriptomes and metabolomes via GC-MS and Illumina platform next-generation sequencing technology in adventitious roots from several ginseng cultivars. We compared the data derived from the same tissues to obtain an integrated understanding of primary metabolic pathways and related genes. The results of this study increase our understanding of the complexity of metabolite biosynthesis and will enhance efforts to identify functional genes in each step of primary metabolite biosynthesis in ginseng.
2. Materials and methods
2.1. Chemicals and reagents
Growth hormones and media required for adventitious root culture were purchased from Duchefa (Haarlem, The Netherlands). All authentic compounds, alkane mixtures, and solvents for GC-MS analysis were obtained from Sigma–Aldrich (St. Louis, MO, USA) and J.T. Baker (Phillipsburg, NJ, USA). All reference chemicals were dissolved in 100% methanol.
2.2. Plant materials
Adventitious roots from five P. ginseng cultivars [Chunpoong (CP), Cheongsun (CS), Sunhyang (SH), Goopong (GO), and Sunun (SU)] were used in this study. Adventitious roots were induced and cultivated in bioreactors as previously described [20]. Adventitious roots maintained in bioreactors were transferred into 250-mL flasks containing 100 mL Schenk and Hildebrandt liquid medium [21] supplemented with 3 mg/L indole-3-butyric acid (IBA) and 5% sucrose and cultured at 25°C on a rotary shaker under dark conditions. After 1 month, the adventitious roots were collected and used for GC-MS and transcriptome analysis.
2.3. Sample preparation for GC-MS analysis
Analysis of primary metabolites using GC-MS was carried out as follows. Freeze-dried adventitious roots from five ginseng cultivars were pulverized and extracted with 100% methanol under sonication for 30 min at room temperature (10 mg/mL methanol). The extracts were centrifuged at 13,500 g for 5 min at room temperature, filtered through a 0.5-μm polytetrafluoroethylene syringe filter (Toyo Roshi Kaisha, Tokyo, Japan), and mixed with 40 μL 1,000 ppm C17:0 fatty acid as an internal standard. The extracts were completely dried under N2 purge. The residues were resuspended in 100 μL 20 mg/mL methoxyamine hydrochloride in pyridine at 37°C for 90 min and then trimethylsilylated with 100 μL N,O-bis (trimethylsilyl) trifluoroacetamide at 37°C for 30 min. The prepared solutions were transferred to vials, which were capped immediately. All samples collected under the same conditions were analyzed with five individual replicates.
2.4. GC-MS conditions
GC-MS analysis was conducted on a GCMS 2010 plus (Shimadzu, Tokyo, Japan). A DB-5 capillary column (30 m × 0.25 mm, 0.25 μm thickness; Agilent, Santa Clara, CA, USA) was used with helium at a constant flow rate of 1 mL/min. The oven temperature conditions were as follows: 100°C for 2 min, ramping up to 300°C at a rate of 5°C/min, and holding at 300°C for 10 min. The sample (1 μL) was injected in split mode (50: 1). The ionization energy was 70 eV in electron impact mode. The transfer line and ion source temperatures were set at 300°C and 250°C, respectively. Mass spectra were obtained at 3.06 scans/s with a mass range of 50–500 m/z.
2.5. GC-MS data processing and statistical analysis
All raw data analyzed by GC-MS were converted into cdf format and exported to MZmine software version 2.10 (http://mzmine.sourceforge.net/). The raw data were deconvoluted using a Savitzky–Golay filter and aligned through the RANdom SAmple Consensus algorithm. The aligned data were exported to a CSV-formatted bucket table, followed by normalization based on the area of the internal standard. Statistical analysis of the data was carried out using SIMCA-P+ version 12.0 (Umetrics, Umeå, Sweden) to compare metabolite variations among the five ginseng cultivars. The measured abundances of all variables were scaled to unit variance (UV scaling), to calculated base weight as 1/standard deviation, for a selection of all samples. The resulting data were subjected to supervised partial least square discriminant analysis (PLS-DA), and a permutation test was carried out 200 times to validate the PLS-DA model. Metabolites that were significantly different (p < 0.05) and had high variable importance for projection values (VIP value > 1.0) were regarded as biomarkers for determining metabolic differences among ginseng cultivars.
2.6. RNA isolation and Illumina sequencing
Total RNA from the adventitious roots of two ginseng cultivars (CS and SH) was isolated using a Plant RNeasy Mini Kit (Qiagen, Hilden, Germany). The concentration and quality of the extracted RNA were checked by ND-1000 (NanoDrop Technologies Inc., Wilmington, DE, USA) and electrophoresis using a 1% formaldehyde–agarose gel. Total RNA fragments 300 bp in length from adventitious roots of CS and SH were used to construct cDNA libraries. Paired-end sequencing of fragments 150 bp in size was performed using the Illumina sequencing platform (NextSeq 500; Lab Genomics Co., Pankyo, Korea). The raw RNA-seq data from CS and SH were deposited in the NCBI Sequence Read Archive (SRA, http://www.ncbi.nlm.nih.gov/Traces/sra) under accession numbers SRR1688723 and SRR1688724, respectively. The raw RNA-seq data from CP were obtained from our previous report [20] (Accession number: SRR619718). Adapter sequences and low-quality bases were filtered using NGS QC Toolkit [22]. Adventitious roots of CS and SH collected under the same conditions were analyzed with three individual replicates.
2.7. Identification of candidate genes involved in primary metabolism and digital expression analysis
The sequences of genes involved in primary metabolite biosynthesis pathways in ginseng were retrieved from the CP root transcriptome through comparisons with homologous sequences in the NCBI and TAIR databases using TBLASTN [23]. Genes with e values < 10−5 and coverage above 80 were selected as candidate genes. The expression levels of the candidate genes in adventitious roots of CP, CS and SH were determined using fragments per kilobase of exon per million fragments (FPKM) values calculated from RNA-seq data via Expectation Maximization [24]. Analysis of variance (p < 0.05) and false discovery rate (q < 0.05) tests were performed using the R program (version 3.1.0, www.R-project.org) to select genes exhibiting significantly different expression patterns among the three cultivars. Mean values of FPKM for each gene were transformed to log2-fold changes and visualized using the R (version 3.1.0) heatmap package.
3. Results and discussion
3.1. Primary metabolite profiles of the adventitious roots of five ginseng cultivars
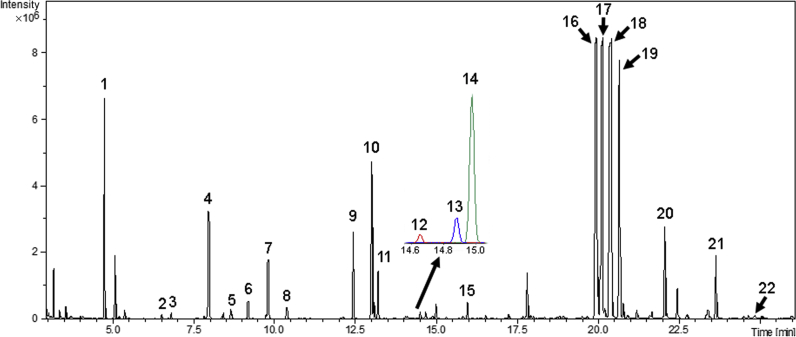
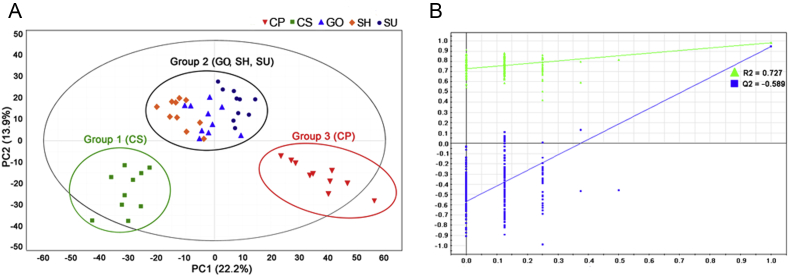
We performed primary metabolite profiling using adventitious roots derived from five officially registered inbred ginseng cultivars that were selected among 10 cultivars based on normal adventitious root growth in our preliminary survey (Fig. 1). Previous studies demonstrated that ginseng cultivars have different phenotypes, ginsenoside types, and contents [7]. However, there have been no in-depth studies on the variation in primary metabolism responsible for the diversities among the cultivars. Therefore, we aimed to investigate metabolic diversities among the cultivars and to identify the genes corresponding with the identified metabolic differences using integrative analysis of transcriptome and metabolome, focused on primary metabolism. Peaks obtained by GC-MS were subjected to multivariate analysis to examine the differences in metabolite composition among ginseng cultivars (Fig. 2). Based on PLS-DA analysis, which was validated by 200 random permutation tests (R2 = 0.727 and Q2 = −0.589), the five ginseng cultivars were separated into three groups: Group 1 consisted of CS; Group 2 consisted of GO, SH and SU; and Group 3 consisted of CP (Fig. 3). These results indicate that the metabolite compositions of GO, SH, and SU are similar, while those of CS and CP are distinct. SH, SU, and GO were bred from Jakyung, while CS and CP were bred from Chungkyung, through pure-line selection [3]. This breeding pedigree reflects the grouping results of our PLS-DA analysis, and the phylogenetic relationships of nine Korea ginseng cultivars revealed by analysis with Simple sequence repeats (SSR)-based markers [3] is also consistent with the clustering based on PLS-DA analysis. We identified some metabolites from the GC-MS analysis by comparing their MS spectra with those available in the National Institute of Standards and Technology (NIST) library (www.nist.gov) or with authentic reference standards. A total of 20 compounds were identified in the five ginseng cultivars (Table 1). Among these, eight compounds were selected as biomarker compounds, as they enabled discrimination among each of the five ginseng cultivars; these markers include l-alanine, malonate, l-valine, phosphoric acid, propanoic acid, l-serine, dl-malate, and l-glutamine.
Fig. 1.
Adventitious roots from five Panax ginseng cultivars maintained in bioreactors (CS, Cheogsun; SU, Sunun; SH, Sunhyang; CP, Chunpoong; GO, Gopoong). Adventitious roots from each cultivar were cultured in Schenk and Hildebrandt medium including 5 mg/L IBA for 1 year with subculturing every 4 weeks. The adventitious roots, which were maintained under dark conditions, were transferred to flasks and collected after 1 month for GC-MS and transcriptome analysis. IBA, indole-3-butyric acid; GC-MS, gas chromatography–mass spectrometry.
Fig. 2.
Representative GC-MS chromatogram of adventitious roots from five Panax ginseng cultivars. Extracts from the adventitious roots of five ginseng cultivars were subjected to GC-MS analysis. Independent analyses were performed five times. The numbers in the peaks indicate assigned compounds using NIST or authentic standards, and numbering follows the system used in Table 1. GC-MS, gas chromatography–mass spectrometry; NIST, National Institute of Standards and Technology.
Fig. 3.
PLS-DA loading plot of adventitious roots from five Panax ginseng cultivars obtained by GC-MS. (A) PLS-DA loading plot and (B) validation of PLS-DA loading plots with 200 permutation tests (CS, Cheogsun, square; SU, Sunun, circle; SH, Sunhyang, diamond; CP, Chunpoong, inverted triangle; GO, Gopoong, regular triangle). The profiles of primary metabolites from the cultivars were discriminated according to selected variable importance for projection lists. GC-MS, gas chromatography–mass spectrometry; PLS-DA, partial least square discriminant analysis.
Table 1.
Assignment of metabolites detected by gas chromatography-mass spectrometry
| No. | Class | Retention time (min) | Compound |
|---|---|---|---|
| 1 | Amino acids | 4.78 | l-Alanineabd |
| 2 | 6.84 | l-Valineabd | |
| 3 | 8.67 | Glycineab | |
| 4 | 9.82 | l-Serineabd | |
| 5 | 10.4 | l-Threonineab | |
| 6 | 13.01 | l-Prolineab | |
| 7 | 13.07 | l-Aspartic acidab | |
| 8 | 14.87 | Ornithineab | |
| 9 | 14.97 | l-Glutamineabd | |
| 10 | 15.94 | l-Asparagineab | |
| 11 | Carboxylic acids | 6.54 | Malonateabd |
| 12 | 9.21 | Propanoic acidabd | |
| 13 | 12.44 | dl-Malateabd | |
| 14 | 14.65 | Pentanedioic acidab | |
| 15 | Fatty acids | 22.07 | Hexadecanoic acidab |
| 16 | 23.58 | Heptadecanoic acidab | |
| 17 | 24.58 | 12-Octadecadienoic acidab | |
| 18 | Inorganic acids | 7.99 | Phosphoric acidabd |
| 19 | Monosaccharides | 19.9 | d-Fructosec |
| 20 | 20.63 | d-Glucosec |
VIP = variable importance for projection.
Metabolites were derivatized with trimethylsilyl.
Annotation of metabolites identified by NIST Mass Spectral Library.
Annotation of metabolites identified by mass spectrum of authentic compound.
Metabolites identified with VIP list with cutoff values of VIP > 1.0 and p < 0.05.
Metabolite profiling through GC-MS analysis has been widely used as strategy for detecting polar (organic acids, amino acids, sugars, and sugar alcohols) and nonpolar (fatty acids and sterols) compounds representing primary metabolites [25]. GC-MS analysis, with the high accuracy of MS and excellent reproducibility of retention time, has been used in many studies to discriminate among species [26], [27] or cultivars [28], [29]. GC-MS analysis has also been used to detect differences in volatile compounds among P. ginseng, Panax notoginseng and Panax quinquefolius [30]. Although metabolite accumulation patterns are diverse among accessions in same species [28], [29], no previous report has described the intra-species diversity of metabolites within P. ginseng. Our data demonstrate that there is much diversity in the contents and composition of primary metabolites in P. ginseng (Table 2). The levels of compounds in the amino acid and carboxylic acid biosynthesis pathways were higher in CS and SH than in CP. The levels of phosphoric acid (in the inorganic acid category) as well as glucose and fructose (monosaccharides) were lower in CS and SH than in CP.
Table 2.
Relative abundance of primary metabolites in CS, SH and CP
| Class | Fold change (compared to CP) |
||
|---|---|---|---|
| CS | SH | CP | |
| Amino acids | 1.32a | 1.39a | 1b |
| Carboxylic acids | 2.00a | 0.96b | 1b |
| Fatty acids | 1.07 | 0.99 | 1 |
| Inorganic acids | 0.64a | 0.55a | 1b |
| Monosaccharides | 0.91ab | 0.56a | 1b |
Different letters in each column indicate significant differences based on least significant difference test (p < 0.05).
CP = Chunpoong; CS = Cheongsun; SH = Sunhyang.
3.2. Transcriptome analysis and isolation of candidate genes involved in primary metabolite biosynthesis pathways
Ginsenosides, which are representative secondary metabolites in P. ginseng, are biosynthesized via the triterpenoid biosynthesis pathway. Acetyl coenzyme A (acetyl-CoA), which is one of the precursors for entry into the triterpenoid biosynthesis pathway, undergoes multiple chemical changes, resulting in the production of diverse ginsenosides. Previous studies have investigated some of the putative genes associated with the ginsenoside biosynthesis pathway via transcriptome analysis [20], [31] and have characterized their functions [32], [33], [34]. However, genes involved in primary metabolite biosynthesis, which is an upstream reaction of ginsenoside biosynthesis, have not been reported in ginseng.
Our PLS-DA analysis demonstrated differential accumulation of primary metabolites among ginseng cultivars (Fig. 3). Seven pathways were identified in the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.genome.jp/kegg/), including the glycolysis pathway (ko00010), valine, leucine, and isoleucine biosynthesis pathway (ko00290), glycine, serine, and threonine biosynthesis pathway (ko00260), citrate cycle [tricarboxylic acid (TCA) cycle] (ko00020), fructose and mannose biosynthesis pathway (ko00051), and arginine and proline pathway (ko00330) (Table 3). These pathways are responsible for the biosynthesis of primary metabolites. We retrieved information about 30 genes involved in the seven primary metabolite biosynthesis pathways in other species from public databases (NCBI and TAIR database) and identified 100 unigenes in the ginseng unigene sets that were assembled from 12 root transcriptomes of the CP cultivar (Table 3) [23].
Table 3.
Enzymes in seven pathways involved in primary metabolite biosynthesis
| Pathway | EC number | Enzyme | Queries | Number of genes from Panax ginseng |
|---|---|---|---|---|
| Glycolysis pathway ko00010 | EC 3.1.3.11 | Fructose-1,6-bisphosphatase | AT3G54050.1a | 3 |
| EC 2.7.1.11 | 6-Phosphofructokinase | AT2G22480.1a | 4 | |
| EC 4.1.2.13 | Fructose-bisphosphate aldolase | AT2G22480.1 a | 3 | |
| EC 1.2.1.12 | Glyceraldehyde-3-phosphate dehydrogenase | AK243763.1b | 1 | |
| EC 2.7.2.3 | Phosphoglycerate kinase | AT1G56190.1 a | 1 | |
| EC 4.2.1.11 | Phosphopyruvate hydratase | AT2G29560.1 a | 4 | |
| EC 2.7.1.40 | Pyruvate kinase | AT5G52920.1 a | 6 | |
| Alanine, aspartate, and glutamate metabolism ko00250 |
EC 2.6.1.2 | Alanine transaminase | AT1G23310.1 a | 4 |
| EC 2.6.1.44 | Alanine–glyoxylate aminotransferase | AT3G08860.1 a | 4 | |
| EC 2.6.1.1 | Aspartate transaminase | AT1G62960.1 a | 2 | |
| EC 1.2.1.24 | Succinate–semialdehyde dehydrogenase | AT1G79440.1 a | 11 | |
| EC 2.6.1.19 | 4-Aminobutyrate-2-oxoglutarate transaminase | AT3G22200.1 a | 4 | |
| EC 6.3.1.2 | Glutamine synthase | AT5G16570.1 a | 2 | |
| Valine, leucine and isoleucine biosynthesis ko00290 | EC 2.2.1.6 | Acetolactate synthase | AT3G48560.1 a | 3 |
| EC 1.1.1.86 | Ketol-acid reductoisomerase | XM_002525069.1c | 1 | |
| Glycine, serine and threonine metabolism ko00260 | EC 4.3.1.17 | l-Serine ammonia-lyase | AT1G21540.1 a | 6 |
| EC 2.1.2.1 | Serine hydroxymethyl transferase | AT4G13890.1 a | 1 | |
| EC 4.1.2.5 | l-Threonine aldolase | AT3G04520.1 a | 1 | |
| EC 2.7.1.39 | Homoserine kinase | XM_002517652.1c | 2 | |
| EC 4.2.3.1 | Threonine synthase | XM_002521317.1c | 2 | |
| Citrate cycle (TCA cycle) ko00020 |
EC 1.2.4.1 | Pyruvate dehydrogenase | XM_002523354.1c | 2 |
| EC 1.8.1.4 | Dihydrolipoamide dehydrogenase | NM_001247841.1d | 2 | |
| EC 1.1.1.37 | Cytosolic malate dehydrogenase | NM_001247223.1d | 1 | |
| Fructose and mannose metabolism ko00051 |
EC 5.3.1.5 | Xylose isomerase | XM_002532363.1c | 1 |
| EC 1.1.1.14 | Sorbitol dehydrogenase | XM_002510809.1c | 6 | |
| EC 1.1.1.21 | Aldo-keto reductase | XM_002529821.1c | 16 | |
| Arginine and proline metabolism ko00330 |
EC 6.3.4.5 | Argininosuccinate synthase | AT4G24830.1 a | 1 |
| EC 3.5.3.1 | Arginase | AT4G08870.1 a | 1 | |
| EC 2.6.1.13 | Ornithine aminotransferase | AT5G46180 a | 4 | |
| EC 1.5.1.2 | Pyrroline-5-carboxylate reductase | AT5G14800.1 a | 1 |
Enzymes derived from Arabidopsis thaliana.
Enzymes derived from Glycine max.
Enzymes derived from Ricinus communis.
Enzymes derived from Solanum lycopersicum.
To identify genes involved in the seven primary metabolite biosynthesis pathways, we selected three representative cultivars from each group (Group 1, CS; Group 2, SH; and Group 3, CP) and performed transcriptome analysis using the Illumina platform. In CS and SH, 45,840,306 and 50,899,396 reads were generated, respectively. After trimming, 41,530,292 and 45,930,374 high-quality reads were obtained, respectively (Table 4). The RNA-seq data from CP were obtained from a previous study [20]. We evaluated the expression levels of the 100 unigenes related to primary metabolite pathways using the transcriptome data from the three cultivars. Digital gene expression profiling based on FPKM values revealed 21 unigenes (encoding 15 enzymes) with statistically significant differential gene expression between two cultivars, CS and SH (Table 5).
Table 4.
Summary of transcriptome data from adventitious roots of three Panax ginseng cultivars
| Adventitious root sample | Raw data |
QC filtered data |
||
|---|---|---|---|---|
| Total no. of reads | Length (bp) | Total no. of reads | Length (bp) | |
| CS, replicate 1 | 16,731,664 | 2,321,621,040 | 15,291,398 | 2,115,447,628 |
| CS, replicate 2 | 14,306,820 | 1,977,938,763 | 12,831,592 | 1,766,225,247 |
| CS, replicate 3 | 14,801,822 | 2,056,937,756 | 13,407,302 | 1,857,154,376 |
| SH, replicate 1 | 17,526,160 | 2,446,652,196 | 15,710,392 | 2,185,318,113 |
| SH, replicate 2 | 16,947,000 | 2,354,285,233 | 15,409,700 | 2,134,138,490 |
| SH, replicate 3 | 16,426,236 | 2,281,803,413 | 14,810,282 | 2,050,279,385 |
| CPa | 90,242,024 | 9,114,444,424 | 85,335,736 | 8,441,707,472 |
| Total | 186,981,726 | 22,553,682,825 | 172,796,402 | 20,550,270,711 |
CP = Chunpoong; CS = Cheongsun; SH = Sunhyang.
RNA-seq data from CP were obtained from a previous study [20].
Table 5.
List of candidate genes with differential expression in three Panax ginseng cultivars
| Enzyme | No. | ID of candidate genes from P. ginsenga | FPKM |
||
|---|---|---|---|---|---|
| CS | SH | CP | |||
| Glycolysis pathway | |||||
| Fructose-bisphosphate aldolase (EC 4.1.2.13) | 1 | Pg_Root115165_c0_seq8 b | 3.51 ± 0.33 | 0.95 ± 0.67 | 6.39 |
| 2 | Pg_Root111379_c0_seq1 b | 619.89 ± 174.76 | 1133.21 ± 50.76 | 710.45 | |
| Phosphopyruvate hydratase (EC 4.2.1.11) | 3 | Pg_Root114308_c0_seq1 | 71.14 ± 7.63 | 73.18 ± 7.43 | 26.6 |
| 4 | Pg_Root123973_c0_seq21 | 84.72 ± 15.64 | 162.85 ± 35.61 | 25.55 | |
| Alanine, aspartate, and glutamate metabolism | |||||
| Alanine–glyoxylate aminotransferase (EC 2.6.1.44) | 5 | Pg_Root127493_c1_seq3 | 106.5 ± 12.43 | 30.82 ± 2.77 | 11.53 |
| Aspartate transaminase (EC 2.6.1.1) | 6 | Pg_Root118729_c0_seq1 | 0.92 ± 1.3 | 0.52 ± 0.74 | 7.83 |
| Succinate-semialdehyde dehydrogenase (EC 1.2.1.24) | 7 | Pg_Root111075_c0_seq7 | 31.23 ± 12.41 | 6.67 ± 5.33 | 68.34 |
| 8 | Pg_Root110835_c0_seq14 | 1.26 ± 0.92 | 6.77 ± 1.74 | 5.83 | |
| Glutamine synthase (EC 6.3.1.2) | 9 | Pg_Root126266_c2_seq14 | 11.24 ± 2.42 | 15.24 ± 0.98 | 1.96 |
| 10 | Pg_Root126266_c2_seq17 | 7.03 ± 3.66 | 5.73 ± 1.15 | 23.05 | |
| Valine, Leucine, and isoleucine biosynthesis | |||||
| Ketol-acid reductoisomerase (EC 1.1.1.86) | 11 | Pg_Root123419_c1_seq1 | 327.57 ± 32.2 | 450.81 ± 55.83 | 205.72 |
| Glycine, serine, and threonine metabolism | |||||
| l-Threonine aldolase (EC 4.1.2.5) | 12 | Pg_Root127874_c0_seq2 | 12.68 ± 3.8 | 25.55 ± 1.98 | 20.04 |
| Citrate cycle (TCA cycle) | |||||
| Pyruvate dehydrogenase (EC 1.2.4.1) | 13 | Pg_Root120502_c0_seq11 | 67.61 ± 12.48 | 32.67 ± 2.83 | 61.48 |
| 14 | Pg_Root120502_c0_seq16 b | 19.06 ± 1.1 | 14.24 ± 4.82 | 47.2 | |
| Cytosolic malate dehydrogenase (EC 1.1.1.37) | 15 | Pg_Root118760_c1_seq1 | 4.9 ± 0.54 | 0.78 ± 1.11 | 1.8 |
| Fructose and mannose metabolism | |||||
| Xylose isomerase (EC 5.3.1.5) | 16 | Pg_Root123272_c0_seq20 b | 51.98 ± 10.23 | 42.88 ± 6.29 | 110.22 |
| Sorbitol dehydrogenase (EC 1.1.1.14) | 17 | Pg_Root111773_c0_seq1 b | 118.64 ± 7.39 | 39.05 ± 10.19 | 34.76 |
| 18 | Pg_Root104286_c0_seq1 b | 617.39 ± 34.81 | 355.12 ± 39.18 | 232.37 | |
| Aldo-keto reductase (EC 1.1.1.21) | 19 | Pg_Root118679_c0_seq18 | 4.13 ± 5.84 | 0 | 27.6 |
| Arginine and proline metabolism | |||||
| Argininosuccinate synthase (EC 6.3.4.5) | 20 | Pg_Root123415_c0_seq10 b | 6.95 ± 4.89 | 9.23 ± 3.61 | 55.98 |
| Pyrroline-5-carboxylate reductase (EC 1.5.1.2) | 21 | Pg_Root121216_c0_seq15 | 3.37 ± 2.67 | 12.48 ± 1.84 | 14.32 |
CP = Chunpoong; CS = Cheongsun; FPKM = fragments per kilobase of exon per million fragments; SH = Sunhyang.
Unigenes had significantly different FPKM values among CS, SH and CP according to analysis of variance test (p < 0.05).
Unigenes with cutoff value q < 0.05.
3.3. Integrated analysis of gene expression and metabolome data
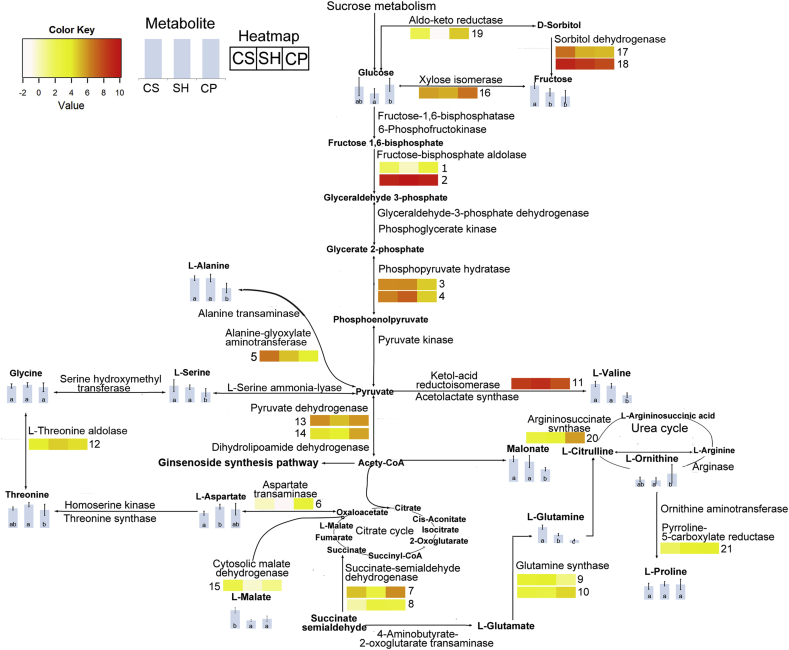
Integrated analysis of transcriptome and metabolome data is a useful technique for discovering genes that are potentially involved in metabolic networks. We therefore examined the correlation of various gene expression and metabolome datasets to elucidate regulatory steps in primary metabolite biosynthesis pathways (Fig. 4).
Fig. 4.
Comparative analysis of primary metabolites and genes involved in their biosynthesis pathways in three ginseng cultivars, Cheongsun (CS), Sunhyang (SH), and Chunpoong (CP). The contents in adventitious roots from three ginseng cultivars of compounds detected by GC-MS analysis are represented in vertical bar graphs. Genes found in the CP root transcriptome are shown on the map. Genes with significantly different expression levels among the three cultivars are represented in the heatmaps. The expression levels of unigenes putatively encoding the enzymes were determined using FPKM values, and the mean FPKM values of significantly differently expressed genes (p < 0.05) were transformed using log2 and represented on the heatmaps using a white-to-red gradient. The numbering in the heatmap follows the system used in Table 5. FPKM, fragments per kilobase of exon per million fragments; GC-MS, gas chromatography–mass spectrometry.
3.4. Fructose and mannose metabolism and the glycolysis pathway
Sugars are important carbon sources that serve as energy sources for plant growth and development [35]. Among sugars, we detected glucose and fructose in three ginseng cultivars. The fructose content was higher in CS than in the other cultivars. The expression levels of Pg_Root111773_c0_seq1 (No. 17) and Pg_Root104286_c0_seq1 (No. 18), encoding sorbitol dehydrogenase (EC 1.1.1.14), which catalyzes the conversion of sorbitol and fructose, were also higher in CS than in the other cultivars. The levels of another sugar, glucose, were highest in CP, followed by CS and SH. One unigene, Pg_Root123272_c0_seq20 (No. 16), encoding xylose isomerase (EC 5.3.1.5), which converts fructose to glucose, was identified in the CP root transcriptome. Pg_Root123272_c0_seq20 (No. 16) was highly expressed in CP, which coincided with the high glucose content in this cultivar. Glucose subsequently enters the glycolysis pathway and is converted into pyruvate via several enzymatic steps [36].
Seven enzymes in the glycolysis pathway were also identified in the CP root transcriptome (Table 3). In an early step of the glycolytic pathway (Fig. 4), transcripts of the unigenes encoding fructose-1,6-bisphosphatase (EC 3.1.3.11) and 6-phosphofructokinase (EC 2.7.1.11) were detected at similar levels in all three cultivars. However, the three cultivars showed differential expression of unigenes encoding other enzymes, including Pg_Root115165_c0_seq8(No. 1) and Pg_Root111379_c0_seq1 (No. 2), encoding fructose-bisphosphate aldolase (EC 4.1.2.13), which catalyzes the biosynthesis of fructose 1,6-bisphosphate into glyceraldehyde 3-phosphate; and Pg_Root114308_c0_seq1 (No. 3) and Pg_Root123973_c0_seq21 (No. 4), encoding phosphopyruvate hydratase (EC 4.2.1.11), which catalyze the interconversion of glycerate 2-phosphate to phosphoenolpyruvate.
3.5. Alanine, aspartate, and glutamate metabolism
Pyruvate, which is produced via the glycolysis pathway, serves as a carbon substrate for the biosynthesis of a wide range of compounds [37]. Alanine, valine, and serine (derived from pyruvate) were also detected in our GC-MS analysis. The relative concentration of alanine was higher in CS and SH than in CP. The transcript levels of Pg_Root127493_c1_seq3 (No. 5), encoding alanine–glyoxylate aminotransferase (EC 2.6.1.44), were highest in the CS cultivar. A previous study demonstrated that overexpression of the gene encoding alanine–glyoxylate aminotransferase increases the nitrogen uptake ability of Oryza sativa [38]. Therefore, the rapid growth rates of CS adventitious roots [20] might result from increased expression of alanine–glyoxylate aminotransferase. However, the relatively high concentration of alanine in SH did not correlate with the expression pattern of alanine–glyoxylate aminotransferase, suggesting that other factors led to an increase in alanine content in SH.
Four unigenes encoding two enzymes in the glutamine biosynthesis pathway, that is, succinate–semialdehyde dehydrogenase (EC 1.2.1.24, which converts succinate to succinate semialdehyde) and glutamine synthase (EC 6.3.1.2, which directly converts l-glutamate to l-glutamine), were differentially expressed among the three ginseng cultivars. Glutamine levels were highest in CS, followed by SH and CP. However, the gene expression patterns were not well correlated with the accumulation patterns of glutamine. Two unigenes, Pg_Root111075_c0_seq7 (No. 7) and Pg_Root110835_c0_seq14 (No. 8), both encoding succinate–semialdehyde dehydrogenase (EC 1.2.1.24), were identified in the CP root transcriptome. Pg_Root111075_c0_seq7 (No. 7) was highly expressed in CP, whereas Pg_Root110835_c0_seq14 (No. 8) was highly expressed in SH and CP. Moreover, Pg_Root126266_c2_seq14 (No. 9) and Pg_Root126266_c2_seq17 (No. 10), encoding glutamine synthase (EC 6.3.1.2), were highly expressed in SH and CP, respectively. This result suggested that various enzymes might be associated with glutamine synthesis.
3.6. Valine, leucine, and isoleucine biosynthesis
Unigenes encoding acetolactate synthase (EC 2.2.1.6) and ketol-acid reductoisomerase (EC 1.1.1.86), which function in the valine biosynthesis pathway, were isolated in the CP root transcriptome. Among these, Pg_Root123419_c1_seq1 (No. 11), encoding ketol-acid reductoisomerase (EC 1.1.1.86), which catalyzes the conversion of acetolactate to 2,3-dihydroxy-3-methylbutanoate, exhibited significant differential expression among the cultivars. The valine levels were higher in CS and SH than in CP, but the expression of Pg_Root123419_c1_seq1 (No. 11) was highest in SH. The accumulation patterns of valine in the cultivars did not correlate with the expression of the unigenes involved in its biosynthesis, indicating that expression of other genes involved in valine biosynthesis pathway might be more increased in CS than other cultivars.
3.7. Glycine, serine, and threonine metabolism
Serine, glycine, threonine, and aspartate were monitored in adventitious roots from the three ginseng cultivars through GC-MS analysis. The contents of serine, threonine, and aspartate were significantly different among the three cultivars. CP had the lowest levels of serine. However, unigenes encoding l-serine ammonialyase (EC 4.3.1.17, which converts pyruvate into serine) and serine hydroxymethyltransferase (EC 2.1.2.1, which catalyzes the conversion of serine to glycine) exhibited similar expression patterns among cultivars.
According to the KEGG database, five enzymes regulate the formation of threonine from aspartate. Aspartate kinase (EC 2.7.2.4), aspartate–semialdehyde dehydrogenase (EC 1.2.1.11), and homoserine dehydrogenase (EC 1.1.1.3) catalyze the conversion of aspartate to homoserine, followed by the formation of threonine by homoserinekinase (EC 2.7.1.39) and threonine synthase (EC 4.2.3.1). l-threonine aldolase (EC 4.1.2.5) is required for the conversion of threonine to glycine. We identified unigenes putatively encoding three of these enzymes, that is, l-threonine aldolase (EC 4.1.2.5), homoserinekinase (EC 2.7.1.39), and threonine synthase (EC 4.2.3.1), in the CP root transcriptome. High levels of threonine and aspartate were detected in SH, while the levels of homoserinekinase (EC 2.7.1.39) and threonine synthase (EC 4.2.3.1), which regulate the final step in threonine biosynthesis, were similar among the three cultivars. However, the transcript levels of Pg_Root127874_c0_seq2 (No. 12), encoding l-threonine aldolase (EC 4.1.2.5), were highest in SH, as were threonine levels, indicating that threonine aldolase (EC 4.1.2.5) might partially account for the increased threonine biosynthesis observed in SH.
3.8. Arginine and proline metabolism
We detected ornithine and proline in the adventitious roots of the three ginseng cultivars. According to the KEGG database, three enzymes regulate the biosynthesis pathway from citrulline to ornithine. Among these, Pg_Root123415_c0_seq10 (No. 20), encoding argininosuccinate synthase (EC 6.3.4.5), was identified in the CP root transcriptome. The expression level of Pg_Root123415_c0_seq10 (No. 20) was highest in CP, as was the relative content of ornithine. We also detected a unigene that functions in the biosynthesis pathway from ornithine to proline, namely Pg_Root121216_c0_seq15 (No. 21). This unigene encodes pyrroline-5-carboxylate reductase (EC 1.5.1.2), which catalyzes the oxidation of l-glutamate-5-semialdehyde to proline. The expression levels of this unigene were significantly different among cultivars, while the proline contents were similar among the three ginseng cultivars.
3.9. TCA cycle
We identified three enzymes involved in the citrate cycle in the P. ginseng cultivars, including cytosolic malate dehydrogenase (EC 1.1.1.37), which converts oxaloacetate to malate as well as pyruvate dehydrogenase (EC 1.2.4.1) and dihydrolipoamide dehydrogenase (EC 1.8.1.4), where the activities of both enzymes together convert pyruvate to acetyl-CoA. For acetyl-CoA to enter the ginsenoside biosynthesis pathway, two acetyl-CoAs must be condensed to acetoacetyl-CoA by acetyl-CoA acetyltransferase (EC 2.3.1.9). Pg_Root120502_c0_seq11 (No. 13) and Pg_Root120502_c0_seq16 (No. 14), encoding pyruvate dehydrogenase (EC 1.2.4.1), were expressed at low levels in SH and CS, respectively, suggesting that the different expression levels of these genes might lead to distinct ginsenoside accumulation patterns among cultivars. Malate levels were highest in CS, and the expression of Pg_Root118760_c1_seq1 (No. 15), encoding cytosolic malate dehydrogenase (EC 1.1.1.37), was also highest in CS. The levels of malonate were higher in CS and SH than in CP; however, we failed to detect genes encoding enzymes involved in this pathway in the P. ginseng transcriptomes.
More than 20 ginseng cultivars have been officially registered with the Korea Seed and Variety Service and cultivated in Korea. Each cultivar has unique genetic and morphological diversity. In this study, we tried to understand the diversity of primary metabolites and the expression levels of genes related to the biosynthesis of these metabolites among Korean ginseng cultivars. The results demonstrated that the ginseng cultivars could be discriminated based on the variation of primary metabolite profiles and classified with eight primary metabolites as biomarkers. We also report expression level differences for candidate genes in the related biosynthetic pathways among ginseng cultivars. Our data demonstrate the value of an integrated approach of transcriptome and metabolome analysis to promote our understanding of complex metabolic pathways of plants.
Conflicts of interest
The authors confirm that they have no conflicts of interest.
Acknowledgments
This research was carried out with the support of “Next-Generation BioGreen21 Program for Agriculture & Technology Development (Project No. PJ01103001)” Rural Development Administration, Republic of Korea.
Contributor Information
Sung Won Kwon, Email: swkwon@snu.ac.kr.
Tae-Jin Yang, Email: tjyang@snu.ac.kr.
References
- 1.Vogler B.K., Pittler M.H., Erns T.E. The efficacy of ginseng. A systematic review of randomised clinical trials. Eur J Clin Pharmacol. 1999;55:567–575. doi: 10.1007/s002280050674. [DOI] [PubMed] [Google Scholar]
- 2.Choi K.T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim N.H., Choi H.I., Ahn I.O., Yang T.J. EST-SSR marker sets for practical authentication of all nine registered ginseng cultivars in Korea. J Ginseng Res. 2012;36:298–307. doi: 10.5142/jgr.2012.36.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon W.S., Lee M.G., Lee J.H. Characteristics of flowering and fruiting in new varieties and lines of Panax ginseng CA Meyer. J Ginseng Res. 2001;25:41–44. [Google Scholar]
- 5.Sun H., Wang H.T., Kwon W.S., Kim Y.J., In J.G., Yang D.C. A simple and rapid technique for the authentication of the ginseng cultivar, Yunpoong, using an SNP marker in a large sample of ginseng leaves. Gene. 2011;487:75–79. doi: 10.1016/j.gene.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Lee J.W., Kim Y.C., Jo I.H., Seo A.Y., Lee J.H., Kim O.T., Hyun D.Y., Cha S.W., Bang K.H., Cho J.H. Development of an ISSR-derived SCAR marker in Korean ginseng cultivars (Panax ginseng CA Meyer) J Ginseng Res. 2011;35:52–59. [Google Scholar]
- 7.Ahn I.O., Lee S.S., Lee J.H., Lee M.J., Jo B.G. Comparison of ginsenoside contents and pattern similarity between root parts of new cultivars in Panax ginseng CA Meyer. J Ginseng Res. 2008;32:15–18. [Google Scholar]
- 8.Kwon Y.K., Ahn M.S., Park J.S., Liu J.R., In D.S., Min B.W., Kim S.W. Discrimination of cultivation ages and cultivars of ginseng leaves using Fourier transform infrared spectroscopy combined with multivariate analysis. J Ginseng Res. 2014;38:52–58. doi: 10.1016/j.jgr.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee E.J., Shaykhutdinov R., Weljie A.M., Vogel H.J., Facchini P.J., Park S.U., Kim Y.K., Yang T.J. Quality assessment of ginseng by 1H NMR metabolite fingerprinting and profiling analysis. J Agri Food Chem. 2009;57:7513–7522. doi: 10.1021/jf901675y. [DOI] [PubMed] [Google Scholar]
- 10.Goossens A., Rischer H. Implementation of functional genomics for gene discovery in alkaloid producing plants. Phytochem Rev. 2007;6:35–49. [Google Scholar]
- 11.Oksman-Caldentey K.M., Saito K. Integrating genomics and metabolomics for engineering plant metabolic pathways. Curr Opin Biotech. 2005;16:174–179. doi: 10.1016/j.copbio.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Fukushima A., Kanaya S., Nishida K. Integrated network analysis and effective tools in plant systems biology. Front Plant Sci. 2014;5:1–9. doi: 10.3389/fpls.2014.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farag M.A., Huhman D.V., Dixon R.A., Sumner L.W. Metabolomics reveals novel pathways and differential mechanistic and elicitor-specific responses in phenylpropanoid and isoflavonoid biosynthesis in Medicago truncatula cell cultures. Plant Physiol. 2008;146:387–402. doi: 10.1104/pp.107.108431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behnke K., Kaiser A., Zimmer I., Brüggemann N., Janz D., Polle A., Hampp R., Hänsch R., Popko J., Schmitt-Kopplin RNAi-mediated suppression of isoprene emission in poplar transiently impacts phenolic metabolism under high temperature and high light intensities: a transcriptomic and metabolomic analysis. Plant Mol Biol. 2010;74:61–75. doi: 10.1007/s11103-010-9654-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tohge T., Nishiyama Y., Hirai M.Y., Yano M., Nakajima J.I., Awazuhara M., Inoue E., Takahashi H., Goodenowe D.B., Kitayama Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over-expressing an MYB transcription factor. Plant J. 2005;42:218–235. doi: 10.1111/j.1365-313X.2005.02371.x. [DOI] [PubMed] [Google Scholar]
- 16.Zulak K.G., Cornish A., Daskalchuk T.E., Deyholos M.K., Goodenowe D.B., Gordon P.M., Klassen D., Pelcher L.E., Sensen C.W., Facchini P.J. Gene transcript and metabolite profiling of elicitor-induced opium poppy cell cultures reveals the coordinate regulation of primary and secondary metabolism. Planta. 2007;225:1085–1106. doi: 10.1007/s00425-006-0419-5. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki H., Achnine L., Xu R., Matsuda S.P.T., Dixon R.A. A genomics approach to the early stages of triterpene saponin biosynthesis in Medicago truncatula. Plant J. 2002;32:1033–1048. doi: 10.1046/j.1365-313x.2002.01497.x. [DOI] [PubMed] [Google Scholar]
- 18.Rischer H., Oresic M., Seppanen-Laakso T., Katajamaa M., Lammertyn F., Ardiles-Diaz W., Van Montagu M.C.E., Inze D., Oksman-Caldentey K.M., Goossens A. Gene-to-metabolite networks for terpenoid indole alkaloid biosynthesis in Catharanthus roseus cells. Proc Natl Acad Sci. 2006;103:5614–5619. doi: 10.1073/pnas.0601027103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urbanczyk-Wochniak E., Baxter C., Kolbe A., Kopka J., Sweetlove L.J., Fernie A.R. Profiling of diurnal patterns of metabolite and transcript abundance in potato (Solanum tuberosum) leaves. Planta. 2005;221:891–903. doi: 10.1007/s00425-005-1483-y. [DOI] [PubMed] [Google Scholar]
- 20.Jayakodi M., Lee S.C., Park H.S., Jang W., Lee Y.S., Choi B.S., Nah G.J., Kim D.S., Natesan S., Sun C. Transcriptome profiling and comparative analysis of Panax ginseng adventitious roots. J Gingseng Res. 2014;38:278–288. doi: 10.1016/j.jgr.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schenk R.U., Hildebrandt A. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot. 1972;50:199–204. [Google Scholar]
- 22.Patel R.K., Jain M. NGS QC Toolkit: a toolkit for quality control of next generation sequencing data. PloS ONE. 2012;7:e30619. doi: 10.1371/journal.pone.0030619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayakodi M., Lee S.C., Lee Y.S., Park H.S., Kim N.H., Jang W., Lee H.O., Joh H.J., Yang T.J. Comprehensive transcriptome analysis of Panax ginseng root tissues identifies tissue-specific and year dependent genes. BMC Plant Biol. 2015;15:1–12. doi: 10.1186/s12870-015-0527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B., Dewey C.N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:1–16. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee D.K., Yoon M.H., Kang Y.P., Yu J., Park J.H., Lee J., Sung W.K. Comparison of primary and secondary metabolites for suitability to discriminate the origins of Schisandra chinensis by GC/MS and LC/MS. Food Chem. 2013;141:3931–3937. doi: 10.1016/j.foodchem.2013.06.064. [DOI] [PubMed] [Google Scholar]
- 26.Lisko J.G., Stanfill S.B., Duncan B.W., Watson C.H. Application of GC-MS/MS for the analysis of tobacco alkaloids in cigarette filler and various tobacco species. Anal Chem. 2013;85:3380–3384. doi: 10.1021/ac400077e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francisco C.S., Messiano G.B., Lopes L.M., Tininis A.G., de Oliveira J.E., Capellari L., Jr. Classification of Aristolochia species based on GC–MS and chemometric analyses of essential oils. Phytochemistry. 2008;69:168–175. doi: 10.1016/j.phytochem.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Degenkolbe T., Do P.T., Kopka J., Zuther E., Hincha D.K., Köhl K.I. Identification of drought tolerance markers in a diverse population of rice cultivars by expression and metabolite profiling. PLoS ONE. 2013;8:e63637. doi: 10.1371/journal.pone.0063637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du X., Rouseff R. Aroma active volatiles in four southern highbush blueberry cultivars determined by GC-Olfactometry and GC-MS. J Agric Food Chem. 2014;62:4537–4543. doi: 10.1021/jf500315t. [DOI] [PubMed] [Google Scholar]
- 30.Cho I.H., Lee H.J., Kim Y.S. Differences in the volatile compositions of ginseng species (Panax sp.) J Agr Food Chem. 2012;60:7616–7622. doi: 10.1021/jf301835v. [DOI] [PubMed] [Google Scholar]
- 31.Chen S., Luo H., Li Y., Sun Y., Wu Q., Niu Y., Song J., Lv A., Zhu Y., Sun C. 454 EST analysis detects genes putatively involved in ginsenoside biosynthesis in Panax ginseng. Plant Cell Rep. 2011;30:1593–1601. doi: 10.1007/s00299-011-1070-6. [DOI] [PubMed] [Google Scholar]
- 32.Tansakul P., Shibuya M., Kushiro T., Ebizuka Y. Dammarenediol-II synthase, the first dedicated enzyme for ginsenoside biosynthesis in Panax ginseng. FEBS Lett. 2006;580:5143–5149. doi: 10.1016/j.febslet.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 33.Lee M.H., Jeong J.H., Seo J.W., Shin C.G., Kim Y.S., In J.G., Yang D.C., Yi J.S., Choi Y.E. Enhanced triterpene and phytosterol biosynthesis in Panax ginseng overexpressing squalene synthase gene. Plant Cell Physiol. 2004;45:976–984. doi: 10.1093/pcp/pch126. [DOI] [PubMed] [Google Scholar]
- 34.Han J.Y., Hwang H.S., Choi S.W., Kim H.J., Choi Y.E. Cytochrome P450 CYP716A53v2 catalyzes the formation of protopanaxatriol from protopanaxadiol during ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol. 2012;53:1535–1545. doi: 10.1093/pcp/pcs106. [DOI] [PubMed] [Google Scholar]
- 35.Huston M., Smith T. Plant succession: life history and competition. Am Nat. 1987;130:168–198. [Google Scholar]
- 36.Causey T., Shanmugam K., Yomano L., Ingram L. Engineering Escherichia coli for efficient conversion of glucose to pyruvate. Proc Natl Acad Sci U S A. 2004;101:2235–2240. doi: 10.1073/pnas.0308171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tcherkez G., Mahé A., Boex-Fontvieille E., Gout E., Guérard F., Bligny R. Experimental evidence of phosphoenolpyruvate resynthesis from pyruvate in illuminated leaves. Plant Physiol. 2011;157:86–95. doi: 10.1104/pp.111.180711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shrawat A.K., Carroll R.T., DePauw M., Taylor G.J., Good A.G. Genetic engineering of improved nitrogen use efficiency in rice by the tissue-specific expression of alanine aminotransferase. Plant Biotechnol J. 2008;6:722–732. doi: 10.1111/j.1467-7652.2008.00351.x. [DOI] [PubMed] [Google Scholar]