Abstract
Background
Human skin undergoes distinct changes throughout the aging process, based on both intrinsic and extrinsic factors. In a process called photoaging, UVB irradiation leads to upregulation of matrix metalloproteinase-1, which then causes collagen degradation and premature aging. Mixtures of medicinal plants have traditionally been used as drugs in oriental medicine. Based on the previously reported antioxidant properties of Panax ginseng Meyer and Crataegus pinnatifida, we hypothesized that the mixture of P. ginseng Meyer and C. pinnatifida (GC) would have protective effects against skin aging.
Methods
Anti-aging activity was examined both in human dermal fibroblasts under UVB irradiation by using Western blot analysis and in healthy human skin by examining noninvasive measurements.
Results
In vitro studies showed that GC improved procollagen type I expression and diminished matrix metalloproteinase-1 secretion. Based on noninvasive measurements, skin roughness values, including total roughness (R1), maximum roughness (R2), smoothness depth and average roughness (R3), and global photodamage scores were improved by GC application. Moreover, GC ameliorated the high values of smoothness depth (R4), which means that GC reduced loss of skin moisture.
Conclusion
These results suggest that GC can prevent aging by inhibiting wrinkle formation and increasing moisture in the human skin.
Keywords: antiaging, antiwrinkle, clinical study, Crataegus pinnatifida, Panax ginseng
1. Introduction
Similar to all organ systems, the human skin undergoes physiological changes with advancing age. There are two distinct types of skin aging, intrinsic and extrinsic. Intrinsic aging (chronological aging), which is likely under the control of individual genetics, inevitably occurs as a natural consequence of physiological changes [1]. By contrast, ambient conditions such as exposure to UV radiation, chemical toxins, and smoking lead to extrinsic aging (photoaging) [2]. These two forms of aging are synergistically responsible for different skin changes that can generate lesions, often on the face. The representative clinical sign of both intrinsically and extrinsically aged skin is wrinkles [3]. Fine wrinkles, loss of underlying fat leading to hollowed cheeks and eye sockets, and dry and itchy skin are manifestations of intrinsic aging. The development of permanent facial lines results from the repetitive actions of facial musculature. In particular, eye wrinkles, generally called crow's feet, are formed by contraction of the orbicularis oculi muscle [4]. Deep, coarse wrinkles and roughness are characteristics of photoaging [5].
Collagen alterations are considered to be a primary cause of skin aging and wrinkle formation. In the human dermis, collagen accounts for roughly 90% of the proteins. A subfamily of matrix metalloproteinases (MMPs), MMP-1, initiates the degradation of collagen types I, II, and III in the skin [6]. Type I collagen is synthesized from procollagen type I, a soluble precursor [7]. Exposure to UV irradiation leads to upregulation of MMP-1 production, which disrupts collagen and the other extracellular matrix proteins [8].
Panax ginseng Meyer has been used as an oriental herbal medicine for treatment of various diseases. The biological and pharmacological activities of ginseng, such as antiaging, anti-inflammatory, and antioxidative effects, have been previously investigated [9], [10], [11]. The main components of ginseng are ginsenosides, which are steroidal saponins responsible for the biological activities of ginseng. The major ginsenosides (Rb1, Rb2, Rc, Rd, Re, and Rg1) account for about 80% of ginseng components, and the minor ginsenosides (F1, F2, Rg3, Rh1, Rh2, compound Y, compound Mc, and compound K) are absent or present at low concentrations in ginseng [12]. There are many studies about the pharmacological activities of ginseng extract and ginsenosides with regard to skin aging. The ginsenoside Rb1 exhibits antiaging activities in the skin that result from an increase of type I collagen production and the suppression of UV-induced apoptosis [13], [14]. The topical application of compound K increased the amount of hyaluronan in the skin of hairless mice [15]. The ginsenoside F1 protected human HaCaT keratinocytes against UVB-induced apoptosis [16]. Furthermore, our previous studies showed that enzyme-modified ginseng extract and enzymatically processed Korean Red Ginseng have inhibitory effects against UVB-induced skin aging in human dermal fibroblasts and hairless mice [17], [18].
Traditionally, Crataegus pinnatifida, commonly named Hawthorn, has been used in oriental medicine as a peptic agent and a blood circulation enhancer [19]. Several recent studies have analyzed the dermatological effects of C. pinnatifida. Kao et al [20] reported that polyphenols from fruits of C. pinnatifida have antitransformation and antitumor effects on TPA-induced skin tumors in ICR mice. Huang et al [21] reported that neolignan glycosides from seeds of C. pinnatifida have antioxidant and tyrosinase inhibitory effects. Furthermore, our earlier study demonstrated hair growth activity in C57BL/6 mouse models treated with the fruits of C. pinnatifida [22].
These results have led us to hypothesize that the mixture of Korean P. ginseng and C. pinnatifida (GC) inhibits wrinkle formation in the aging of human skin. To test this hypothesis, markers of skin photoaging, MMP-1 and procollagen type 1, were analyzed using Western blot with normal human dermal fibroblasts (NHDFs). Moreover, a greater understanding of the effect of GC was established by conducting a clinical trial. These results suggest that GC works effectively in cosmetic applications through control of collagen metabolism.
2. Materials and methods
2.1. Part 1. In vitro study
2.1.1. Preparation of the GC mixture
The GC mixture contains a 1:1 ratio of each ingredient and was produced using a patented protocol (Korea Patent, no. 1013848730000; composition comprising extracts of P. ginseng and Crataegis fructus for improving skin beauty). Six-year-old Korean P. ginseng was provided by Dr. Byung-Goo Cho (R&D Headquarters, Korea Ginseng Corporation, Daejeon, Korea) and was extracted using a patent protocol (Korea Patent, No. 1014540660000). Dried C. pinnatifida fruits were purchased from Kumkang Pharm Inc. (Ansung, Korea) and were extracted as previously described. In addition, the compositions of P. ginseng and C. pinnatifida have been analyzed and previously characterized by our research group [17], [22]. Based on the results of our patent, we established an optimal extraction ratio to produce a 1:1 mixture of P. ginseng and C. pinnatifida by weight.
2.1.2. Cell culture
NHDFs (MCTT, Seoul, Korea) were plated in 100-mm tissue culture dishes and maintained in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin–streptomycin (Gibco-BRL, Gaithersburg, MD, USA) at 37°C in a humidified atmosphere containing 5% CO2. All experiments were performed using only those cells between passages 6 and 10.
2.1.3. UV irradiation and sample treatment
UVB irradiation and sample treatment were performed according to the method previously reported by Hwang et al [23]. NHDFs were irradiated with UVB (144 mJ/cm2) using the UVB irradiation machine (Bio-Link BLX-312; Vilber Lourmat GmbH, France). The treatment concentration of the extracts of Korean P. ginseng, C. pinnatifida, and GC was 100 μg/mL. Control cells were subjected to the same culture conditions without UVB exposure. For Western blot analysis, the cells were harvested 48 h after UVB irradiation.
2.1.4. Cell viability
After various treatments, the culture medium was removed and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenultetrazolium bromide (MTT; 0.1 mg/mL) was added, followed by incubation at 37°C for 2 h in a CO2 incubator. After the formazan crystals were completely dissolved by dimethyl sulfoxide, absorbance was measured at 570 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
2.1.5. Western blot analysis
For Western blot analysis, the cells were lysed with lysis buffer [50mM Tris-Cl, pH 8.0, 0.1% sodium dodecyl sulfate (SDS), 150mM NaCl, 1% NP-40, 0.02% sodium azide, 0.5% sodium deoxycholate, 100 μg/mL phenylmethylsulfonyl fluoride, 1 μg/mL aprotinin, and phosphatase inhibitor] and centrifuged at 12,000 g for 7 min. The cells were then homogenized in an equivalent amount of protein, and the protein concentration was measured using Bradford reagent (Bio-Rad, Hercules, CA, USA). Homogenized proteins were electrophoresed on 8% or 10% SDS-polyacrylamide gels (SDS-PAGE) and were transferred from SDS-PAGE to a nitrocellulose membrane (Amersham Pharmacia Biotech, Buckinghamshire, UK). Nonspecific binding was then blocked with 5% nonfat milk in TBST (Tris-buffered saline with Tween 20; 50 mmol/L Tris–HCL, pH 7.5, 150 mmol/L NaCl and 0.1% Tween 20) for 1 h at room temperature, and the primary antibody was applied overnight at 4°C. The membrane was then washed three times with TBST buffer and incubated with secondary antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) for 1 h at room temperature. Finally, the protein level was determined using chemiluminescent detection ECL reagents (Fujifilm, LAS-4000, Tokyo, Japan) and ImageMaster 17 2D Elite software, version 3.1 (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
2.2. Part 2. Clinical trial
2.2.1. Preparation of GC cream
GC and placebo formulations were identical in color, fragrance, and packaging. The composition of formulations is presented in Table S1.
2.2.2. Participants and study design
In this randomized, double-blind study, 21 healthy Korean women aged 30–65 yr presenting with crow's feet wrinkles were enrolled, 20 of whom were recruited as participants. One of the initial 21 participants was excluded from subsequent analysis owing to agreement withdrawal/treatment refusal (n = 1). The participants were clinically diagnosed with a global photo damage score of 2–6 according to the Jung score for photoaging of facial skin. Participants were requested to (1) avoid exposure to sunlight during the trial; (2) refrain from using lotions, creams, or other products on the face; (3) refrain from smoking or drinking alcohol in excess; and (4) refrain from using makeup less than 12 h prior to assessment. Participants were excluded if they reported: (1) use of topical antiaging products in the past 3 mo; (2) use of tretinoin or superficial peels in the past 6 mo; (3) application of steroidal ointment in the previous month; (4) history of fillers, botulinum toxin, medium-depth peel, ablative laser, or surgical lifting in the past 24 mo; (5) pregnancy or breastfeeding; (6) exposure to artificial UV radiation; and (7) allergy to any ingredients in the cream; (h) dermatological problems. All participants provided written informed consent prior to enrolling in the study. After completing the above-mentioned enrollment process, the study coordinator randomly allocated patients into groups based on a computer-generated random number table. Each participant was allocated to one of the following two groups: the LGC group or the RGC group. A random number table was generated by a random number function of the Microsoft Excel application, and was used in this study for the allocation of each participant into one of the groups with allocation ratio of one to one. GC was topically applied to the skin of the left eyelid in the LGC group or the right eyelid in the RGC group. Placebo was topically applied to the skin of the right eyelid in the LGC group of the left eyelid in the RGC group. The participants and investigator were blind to group allocation throughout the study period. The study was conducted in accordance with the guidelines of the Korea Food and Drug Administration. The trial started on August 23, 2012 and was completed on November 17, 2012. The protocol was reviewed and approved by the Ethics Committee (Oriental Hospital of Se-Myung University, Jecheon, South Korea) in August 2011. This study was carried out in accordance with the Declaration of Helsinki (1964), which was subsequently modified in Tokyo (2004) and Seoul (2008).
2.2.3. Blinding
Only the study coordinator knew the group allocation and prepared the creams for both groups accordingly. Both the participants and the investigator were blinded to the information on the group allocation until the end of the study. Allocation concealment was maintained using an opaque case. Group allocation information was opened to the participant at the end of the trial period.
2.2.4. Treatment
All volunteers were instructed on application of the formulation, and crow's feet wrinkles were selected as the testing area. The steps were performed according to the method previously reported by Hwang et al [24]. Active formulation (GC) was applied on the left or right foot manifesting signs of crow's feet, and the placebo (vehicle formulation) was applied on the other foot, twice daily (morning and night) for 12 wks. Prior to application, participants were asked to wash their skin using the same mild facial nickel-tested cleanser. Participants were not allowed to use any other cosmetic products during the treatment period.
2.2.5. Visual assessment
Patients were assessed at the investigating center at baseline [Week (Wk) 0] and at Wk 4, Wk 8, and Wk 12. The primary efficacy endpoint was the investigator's assessment of the global photodamage score (GPDS) at Wk 12. GPDS ranged from 0 to 7 as follows: 0, none; 1, none/mild; 2, mild; 3, mild/moderate; 4, moderate; 5, moderate/severe; 6, severe; and 7, very severe [25].
2.2.6. Photography
Photographs of the patients' faces lateral of the right and left eyes were taken under standardized conditions at Wk 0, Wk 4, Wk 8, and Wk 12. All photographs were taken with participants looking straight ahead under constant lighting conditions using the same camera, camera settings, and camera placement. A Nikon D200 camera (Nikon, Tokyo, Japan) was positioned on an SVK 35D tripod (Slik, Saitama, Japan) to the right of the participant's face and perpendicular to the area to be photographed. Simultaneously, an Olympus twin T28 Macroflash unit (Olympus, Shinjuku, Japan) was positioned with one flash head directly above the lens pointed at the participant and the other directed away. Exposure was controlled by adjusting the lens aperture with the flash set on manual at full output.
2.2.7. Noninvasive measurements of the skin
All measurements were performed under standardized conditions, i.e., room temperature of 22 ± 2°C and a relative humidity level of 50 ± 10%. An acclimatization time of at least 30 min was allowed before measurements were taken. To obtain skin replicas, 1 cm of light-bodied silicone (SilfloR, Flexico, Colchester, UK) was prepared from two components mixed at a 1:1 ratio under decompression (2 drops each, catalyst and thinner). The mixture was then applied to the skin surface. After drying and hardening, the replicas were subjected to further analysis (Fig. 1). Image files were analyzed using the Skin Viscometer SV 600 program (Courage & Khazaka, Cologne, Germany). Arbitrary units (R1–R5) were assigned to each sample based on furrow depth, which was determined from shadow size and brightness due to inflection under illumination. Roughness parameters included R1 (skin roughness), R3 (average roughness), and R5 (arithmetic average roughness). Skin roughness (R1) was defined as the difference between the highest crest and lowest furrow. Maximum roughness (R2) was the largest value recorded. Depth of smoothness (R4) was also recorded.
Fig. 1.
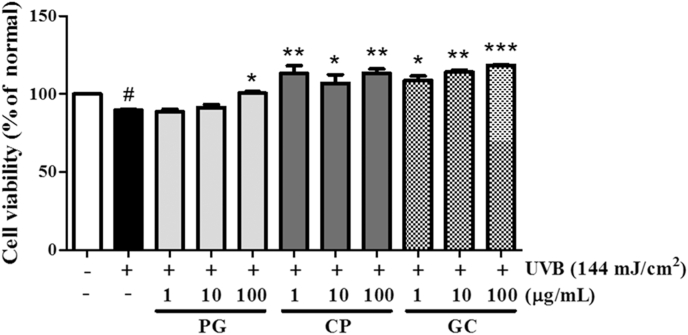
Cell viability of treatment with PG, CP, and GC in UVB-irradiated cultured human dermal fibroblasts. Cells were irradiated with 144 mJ/cm2 UVB and then incubated in the presence of PG, CP, and GC for 48 h. Data are presented as mean ± standard deviation. # and * indicate significant differences (p < 0.05) between UV (−) control and UV (+) control, respectively. #p < 0.05 versus normal control, *p < 0.05, **p < 0.001, ***p < 0.001 versus UVB-irradiated control. CP, Crataegus pinnatifida; GC, mixture of Panax ginseng and Crataegus pinnatifida; PG, Panax ginseng.
2.2.8. Questionnaire study
In clinical research, questionnaires are routinely used to assess the reliability and validity of the study [26]. At the completion of the 12-wk study period, all participants answered the following questions presented in a questionnaire: “Has your skin antiwrinkling improved?”, “Have you noticed an improvement in your skin elasticity?”, “Have you noticed an improvement in your skin moisture?”, and “Have you noticed an improvement in the shining of your skin?” The answer choices were as follows: A, excellent; B, good; C, moderate; D, poor; and F, bad.
2.2.9. Clinical assessment
Assessments were performed at Wk 0, Wk 4, Wk 8, and Wk 12 by the same two professional investigators who were blinded to previous assessments. Right and left eyes were graded separately. Adverse effects included erythema, edema, scaling, itching, stinging, burning, tightness, and prickling, and were graded as none, mild, severe, or very severe. Evaluation was performed in the same location at each visit and with the same lighting.
2.3. Statistical analysis
Statistical analysis was performed for those who completed the trial. SPSS software (version 17.0; SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. Differences between groups were evaluated with Student t test, and differences within groups were analyzed with analysis of covariance. A p value < 0.05 was considered statistically significant. Descriptive statistical analysis was performed using the questionnaire assessment.
3. Results
3.1. Part 1. In vitro study
3.1.1. Effects of GC on cell viability and expression of MMP-1 and procollagen type I in UVB-irradiated NHDFs
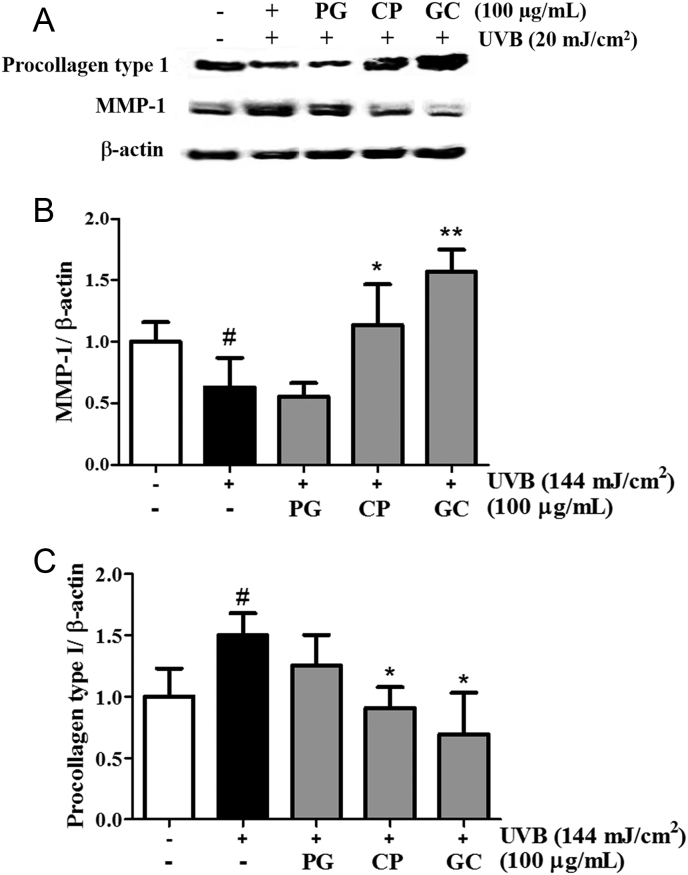
An MTT assay was conducted to measure the effects of P. ginseng, C. pinnatifida, and GC on the cell viability of NHDFs. The cell viability was increased by 112%, 127%, and 132% with 100 μg/mL of P. ginseng, C. pinnatifida, and GC, respectively, compared with that of the UVB-irradiated control (Fig. 1). MMP-1 and procollagen type I protein levels were measured in UVB-irradiated NHDFs, and those treated with P. ginseng, C. pinnatifida, and GC. Western blot data showed that UVB irradiation elevated MMP-1 protein levels and inhibited expression of procollagen type I within 24 h (Fig. 2A). To confirm the comparative effects of P. ginseng, C. pinnatifida, and GC, NHDFs were treated with 100 μg/mL of each substance. The P. ginseng treatment decreased the expression of procollagen type I, whereas C. pinnatifida increased it. As shown in Fig. 2B, GC treatment strongly improved the procollagen type I expression that was reduced by UVB exposure (up to 249%). By contrast, MMP-1 expression decreased by 17% and 40% after treatment with 100 μg/mL of P. ginseng and C. pinnatifida, respectively. Moreover, Western blot data showed that GC-treated cells had the highest reduced UVB-induced protein accumulation of MMP-1 (down to 54%; Fig. 2C). These results suggest that GC may more effectively inhibit photoaging in NHDF cells than either P. ginseng or C. pinnatifida alone.
Fig. 2.
Effects of PG, CP, and GC on (A) the protein expression of MMP-1 and procollagen type 1, and (B,C) results of densitometric analysis in UVB-irradiated cultured human dermal fibroblasts. Cells were irradiated with UVB (144 mJ/cm2) and treated with PG, CP, and GC at 100 μg/mL for 48 h. Western blot analysis was performed on 40 μg of total protein from cell lysates. β-Actin was used as an internal control. The densitometry analysis data are expressed as a percentage relative to the UVB-untreated control. Data are presented as mean ± standard deviation. # and * indicate significant differences (p < 0.05) between the UV (−) control and UV (+) control, respectively. #p < 0.05 versus the normal control; *p < 0.05, **p < 0.01 versus UV-treated control. CP, Crataegus pinnatifida; GC, mixture of Panax ginseng and Crataegus pinnatifida; MMP, matrix metalloproteinase; PG, Panax ginseng.
3.2. Part 2. Clinical trial
3.2.1. Participant demographics
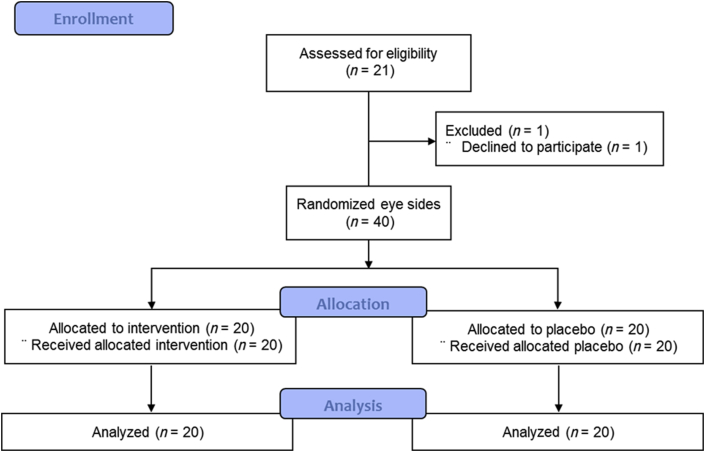
Twenty-one individuals (female) with an average age of 47.00 ± 7.97 yr entered the study (Fig. 3). Owing to failure to adhere to the protocol, one person withdrew. As shown in Table 1, eye wrinkles on the right and left sides of the face were assessed by GPDS prior to treatment with GC. Average GPDS values were 4.29 ± 1.45 and 4.19 ± 1.36 for the right and left sides of the face, respectively. Prior to the topical application of GC and placebo, 57% and 43% of the participants reported dry skin with normal elasticity. Additionally, 62% had thin crow's feet, and 38% had thick, deep crow's feet.
Fig. 3.
Study flowchart of the participants describing trial progress.
Table 1.
GPDS values in Week 0
| GPDS1) | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| Eye side | Right | 9.0 | 29.0 | 14.0 | 24.0 | 19.0 | 5.0 |
| Left | 5.0 | 25.0 | 30.0 | 20.0 | 15.0 | 5.0 | |
Data are presented as %.
Global photodamage score (GPDS): 2, mild; 3, mild/moderate; 4, moderate; 5, moderate/severe; 6, severe; and 7, very severe.
3.2.2. Analysis of GC effects on eye wrinkles in human skin
As shown in Table 2, eye wrinkle comparisons were made by measuring GPDS prior to and after 4 wks, 8 wks, and 12 wks of treatment. Prior to the treatment with GC and placebo, baseline GPDSs were 4.25 ± 1.48 and 4.15 ± 1.40, respectively. At 8 wks of treatment, no difference was observed in the GC group; however, the GPDS value decreased after 12 wks of GC treatment (3.95 ± 1.76). A small change was seen in the placebo group (4.20 ± 1.40 at 4 wks and 8 wks, 4.15 ± 1.46 at 12 wks).
Table 2.
GPDS value changes
| GC |
Placebo |
p1) | p2) | ||
|---|---|---|---|---|---|
| Arbitrary units (average ± SD) | |||||
| GPDS | Baseline | 4.25 ± 1.48 | 4.15 ± 1.39 | ||
| After 4 wks | 4.25 ± 1.48 | 4.20 ± 1.40 | 0.041 | 0.330 | |
| Difference3) | 0.00 ± 0.00 | 0.05 ± 0.22 | 0.050* | ||
| p4) | 0.000 | 0.050* | |||
| After 8 wks | 4.25 ± 1.48 | 4.20 ± 1.40 | 0.041 | 0.330 | |
| Difference5) | 0.00 ± 0.00 | 0.05 ± 0.22 | 0.050* | ||
| p4) | 0.000 | 0.050* | |||
| After 12 wks | 3.95 ± 1.76 | 4.15 ± 1.46 | 0.000 | 0.025* | |
| Difference6) | −0.30 ± 0.47 | 0.00 ± 0.32 | 0.128 | ||
| p4) | 0.105 | 0.073 | |||
*p < 0.05, by Student t test for comparison with placebo group.
GC, mixture of Panax ginseng and Crataegus pinnatifida; GPDS, global photodamage score; SD, standard deviation.
Compared between groups: p value by Student t test.
Compared between groups: p value by analysis of covariance (adjustment with baseline).
After 4 wks – baseline.
Compared within groups: p value by paired t test.
After 8 wks – baseline.
After 12 wks – baseline.
At Wk 0, Wk 4, Wk 8, and Wk 12, comparisons of the assessments at each eye wrinkle site (right and left) were measured using an SV600 visiometer. Prior to the treatment with GC and placebo, values of total skin roughness (R1) were 0.14 ± 0.03 arbitrary unit (AU) and 0.14 ± 0.05 AU, respectively. At Wk 4, Wk 8, and Wk 12 , R1 values for the GC group were significantly decreased by 0.013 ± 0.03 AU, 0.12 ± 0.03 AU, and 0.11 ± 0.03 AU, respectively. Baseline maximum roughness (R2) was 0.09 ± 0.02 AU and 0.08 ± 0.03 AU in the GC and placebo groups, respectively. GC treatment decreased R2 values by 0.08 ± 0.02 AU, 0.07 ± 0.02 AU, and 0.07 ± 0.01 AU at Wk 4, Wk 8, and Wk 12 , respectively. However, no significant difference in R1 or R2 value was found at Wk 4, Wk 8, or Wk 12 in the placebo group. Prior to the treatment with GC and placebo, values of average roughness (R3) were 0.06 ± 0.02 AU and 0.06 ± 0.01 AU, respectively. After 12 wks, R3 values were 0.05 ± 0.01 AU in both groups. After 12 wks, the GC group demonstrated more effectively decreased R1, R2, and R3 roughness values than the placebo group.
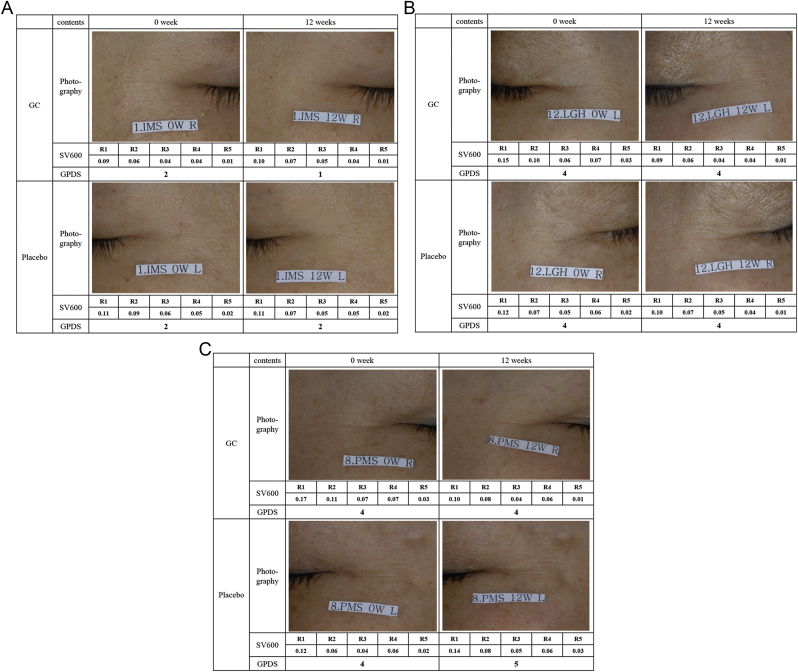
High smoothness depth (R4) values mean that the skin loses moisture, which results in rough surfaces. Baseline R4 values in the GC and placebo groups were 0.07 ± 0.02 AU. At 12 wks, the R4 value was improved only in the GC group (0.06 ± 0.02 AU). GC ameliorated the high values of smoothness depth (R4), which means that GC improved loss of skin moisture. No differences in arithmetic roughness average (R5) value were seen in either group (Table 3). The values of R1, R2, R3, and R4 decreased in a statistically significant manner in the GC group. Photographs of clinical results are shown in Fig. 1, Fig. 2, Fig. 3. Representative pictures of clinical results are shown in Fig. 4. Crow's feet wrinkles in the GC treatment group appeared thinner and dimmer in photographs compared with placebo.
Table 3.
Analysis of skin replicas
| Skin roughness (R1) |
Maximum roughness (R2) |
Average roughness (R3) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GC | Placebo | p1) | p2) | GC | Placebo | p1) | p2) | GC | Placebo | p1) | p2) | |
| Baseline | 0.14 ± 0.03 | 0.14 ± 0.05 | 0.09 ± 0.02 | 0.08 ± 0.03 | 0.06 ± 0.01 | 0.06 ± 0.02 | ||||||
| After 4 wks | 0.13 ± 0.03 | 0.13 ± 0.05 | 0.291 | 0.407 | 0.08 ± 0.02 | 0.08 ± 0.03 | 0.833 | 0.216 | 0.05 ± 0.01 | 0.06 ± 0.02 | 0.968 | 0.478 |
| Difference3) | −0.01 ± 0.04 | 0.00 ± 0.03 | 0.011* | −0.01 ± 0.02 | 0.00 ± 0.02 | 0.005** | 0.00 ± 0.01 | 0.00 ± 0.01 | 0.004** | |||
| p4) | 0.008** | 0.007** | 0.005** | 0.004** | 0.003** | 0.003** | ||||||
| After 8 wks | 0.12 ± 0.03 | 0.12 ± 0.06 | 0.265 | 0.859 | 0.07 ± 0.02 | 0.07 ± 0.04 | 0.272 | 0.579 | 0.05 ± 0.01 | 0.05 ± 0.02 | 0.645 | 0.828 |
| Difference5) | −0.02 ± 0.04 | −0.02 ± 0.04 | 0.011* | −0.01 ± 0.02 | −0.01 ± 0.03 | 0.007** | −0.01 ± 0.01 | −0.01 ± 0.012 | 0.005** | |||
| p4) | 0.007** | 0.009** | 0.004** | 0.006** | 0.003** | 0.003* | ||||||
| After 12 wks | 0.11 ± 0.03 | 0.14 ± 0.06 | 0.693 | 0.015* | 0.07 ± 0.01 | 0.08 ± 0.03 | 0.844 | 0.044* | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.270 | 0.022* |
| Difference6) | −0.03 ± 0.04 | 0.00 ± 0.04 | 0.012* | −0.02 ± 0.02 | 0.00 ± 0.02 | 0.007** | −0.01 ± 0.01 | 0.00 ± 0.02 | 0.004** | |||
| p4) | 0.008* | 0.009** | 0.005** | 0.005** | 0.003** | 0.004** | ||||||
| Smoothness depth (R4) |
Arithmetic average (R5) |
|||||||
|---|---|---|---|---|---|---|---|---|
| GC | Placebo | p1) | p2) | GC | Placebo | p1) | p2) | |
| Baseline | 0.07 ± 0.02 | 0.07 ± 0.02 | 0.02 ± 0.01 | 0.03 ± 0.01 | ||||
| After 4 wks | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.574 | 0.359 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.397 | 0.650 |
| Difference3) | −0.01 ± 0.02 | 0.00 ± 0.02 | 0.006** | 0.00 ± 0.01 | 0.00 ± 0.01 | 0.003** | ||
| p4) | 0.005** | 0.004** | 0.003** | 0.002** | ||||
| After 8 wks | 0.06 ± 0.02 | 0.06 ± 0.03 | 0.279 | 1.000 | 0.02 ± 0.01 | 0.02 ± 0.02 | 0.386 | 0.748* |
| Difference5) | −0.01 ± 0.02 | −0.01 ± 0.02 | 0.006** | 0.00 ± 0.01 | 0.00 ± 0.01 | 0.003** | ||
| p4) | 0.003** | 0.005* | 0.002** | 0.002** | ||||
| After 12 wks | 0.06 ± 0.02 | 0.07 ± 0.03 | 0.955 | 0.034* | 0.02 ± 0.01 | 0.03 ± 0.02 | 0.920 | 0.065* |
| Difference6) | −0.01 ± 0.02 | 0.00 ± 0.02 | 0.007** | −0.00 ± 0.01 | 0.00 ± 0.01 | 0.003** | ||
| p4) | 0.005** | 0.005** | 0.002** | 0.002** | ||||
*p < 0.05, **p < 0.01 by Student t test for comparison with placebo group.
GC, mixture of Panax ginseng and Crataegus pinnatifida.
Compared between groups: p value by Student t test.
Compared between groups: p value by analysis of covariance (adjustment with baseline).
After 8 wks – baseline.
Compared within groups: p value by paired t test.
After 16 wks – baseline.
After 24 wks – baseline.
Fig. 4.
Representative pictures of prior to and after the treatment.
3.2.3. Self-satisfaction assessment after the treatment
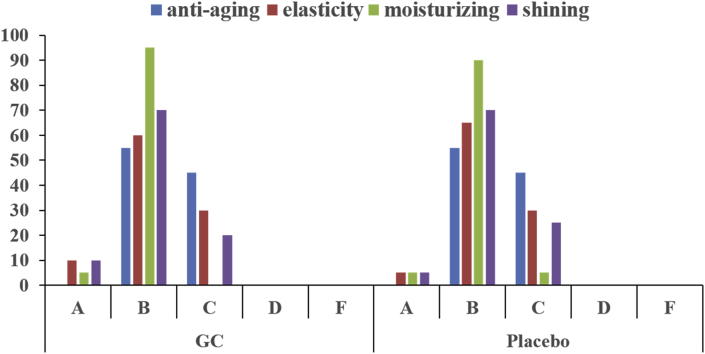
Responses to the questionnaire are summarized in Fig. 5. The respondents also noted that GC was more effective for skin elasticity, moisturizing, and shining than placebo. However, almost all participants reported no difference in antiaging effect between the two products.
Fig. 5.
Questionnaires following a 12-wk treatment with GC and placebo. The answer choices were as follows: A, excellent; B, good; C, moderate; D, poor; and F, bad. GC, mixture of Panax ginseng and Crataegus pinnatifida.
3.2.4. Safety results
No participants reported adverse reactions to treatment, such as erythema, edema, scaling, itching, stinging, burning, tightness, or prickling.
4. Discussion
Previous studies have shown that chemical-based ingredients can cause allergic reactions or irritation. Because of these negative effects, many studies have focused on developing new products using herbal plants or their derived compounds, which are considered to be less toxic and free of adverse effects [27]. Moreover, mixtures of medicinal plants have been used as traditional drugs because compounds in the mixtures have the potential to complement and enhance the effects of one another [28]. Many studies have reported that P. ginseng is a powerful skin antiaging agent [11], [14], [16], [17], [18]. Also, our earlier study demonstrated hair growth induced by the fruits of C. pinnatifida in a C57BL/6 mouse model [22]. Therefore, we investigated GC, the mixture of P. ginseng and C. pinnatifida, in UVB-induced NHDFs and healthy human skin.
The mechanisms of the skin that lead to photoaging are induced mainly by the repetitive absorption of UV radiation, which can generate reactive oxygen species and stimulate the expression of extracellular matrix proteases via AP-1 signaling [29]. AP-1, made of Jun and Fos family proteins, is a transcription factor that inhibits collagen production and upregulates collagen breakdown by activating enzymes called MMPs [30]. Because the skin's natural antioxidant defenses are disrupted by high doses of UV light, reactive oxygen species are considered to be key factors involved in the initiation of the skin aging process [23]. Our previous studies demonstrated that Aloe vera, coriander, azuki beans, and Galla chinensis, all of which have antioxidant activity, have protective effects against UVB-induced skin aging [23], [29], [31], [32]. There have been many studies elucidating the antioxidant activities of P. ginseng and C. pinnatifida. P. ginseng was shown to abrogate oxidative stress in hepatoma cells and brain cells, as well as in human clinical trials [33], [34], [35]. Additional studies have detailed the antioxidant activities of C. pinnatifida in mouse lymphocytes, rat pups with selenite-induced cataract, and in the livers of high-fat fed mice [36], [37], [38]. Therefore, we hypothesized that GC has protective effects against UVB-induced skin aging. Fig. 2 shows that the cells treated with GC exhibited suppressed MMP-1 and increased procollagen type 1 levels under UVB-irradiated conditions, as expected. Additionally, GC promoted proliferation of NHDFs. These effects were weaker when cells were treated with only P. ginseng or C. pinnatifida. However, we did not investigate alternative mechanisms to explain these synergic effects in NHDFs. We will examine the mechanism of GC effects in future studies.
Life expectancy in the United States and other industrialized countries continues to increase and is expected to reach 100 yr by about 2025 [39]. In modern society, there is a strong desire to find methods to maintain a youthful appearance. Because the skin is the most visible indicator of age, reducing the appearance of facial wrinkles is effective in creating a younger visage [40]. In aged skin, reductions in moisture and fat emulsion are observed [41]. Changes in the amino acid composition of aged skin may reduce the amount of cutaneous natural moisturizing factor that supports skin rejuvenation, thereby decreasing its capacity for water binding [42]. Therefore, hydration is an important antiaging factor for the skin. Although our clinical trial was conducted in dry, cold conditions (average humidity, 62.3%; average temperature, 6.9°C; Korea Meteorological Administration), when GC was topically applied at the site of eye wrinkles, skin moisture was improved (R4). Furthermore, the volunteers in the GC group responded in questionnaires that the treatment had a more moisturizing effect than did those in the placebo group. Noninvasive devices permit evaluation of the correlation between hydration and roughness; thus, many investigators use such devices to assess antiwrinkle efficacy [43]. In the present study, we used the SV600 visiometer. Based on this device, the decreased skin roughness values (R1, R2, and R3) observed after GC application suggest that the appearance of wrinkles was more effectively improved compared to the placebo. Moreover, statistical differences in the results were significant. The participants in the GC group showed decreased GPDSs after 12 wks. In photographs, the GC treatment group appeared to have faint and thin crow's feet. However, all antiaging phenomena resulting from GC application could not be explained by our findings. Therefore, we suggest that additional studies be conducted to assess the histological changes and precise mechanisms of GC treatment in the skin.
In conclusion, this study demonstrated that GC can limit the skin aging process. First, GC has a protective effect against UVB-exposed photoaging of skin by regulating procollagen type 1 and MMP-1 expression in NHDFs. Second, after 12 wks, participants in the GC group showed decreased GPDS and skin roughness, a precursor to wrinkle formation. Third, in spite of low humidity in the environment, the moisturizing effects of GC were remarkable. These findings suggest that GC as a natural ingredient may be powerful in regulating skin aging.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
This research was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (iPET, 810006-03-3-SB110), Republic of Korea.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jgr.2016.01.001.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Gilchrest B.A., Yaar M. Ageing and photoaging of the skin: observations at the cellular and molecular level. Br J Dermatol. 1992;127:25–30. doi: 10.1111/j.1365-2133.1992.tb16984.x. [DOI] [PubMed] [Google Scholar]
- 2.Fisher G.J., Wang Z., Datta S.C., Varani J., Kang S., Voorhees J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 3.Rittié L., Fisher G.J. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5 doi: 10.1101/cshperspect.a015370. a015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poljšak B., Dahmane R.G., Godić A. Intrinsic skin aging: the role of oxidative stress. Acta Dermatovenerol Alp Panonica Adriat. 2012;21:33–36. [PubMed] [Google Scholar]
- 5.Fisher G.J., Kang S., Varani J., Bata-Csorgo Z., Wan Y., Datta S., Voorhees J.J. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 6.Quan T., Qin Z., Xia W., Shao Y., Voorhees J.J., Fisher G.J. Matrix-degrading metalloproteinases in photoaging. J Invest Derm Symp P. 2009;14:20–24. doi: 10.1038/jidsymp.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rittié L., Fisher G.J. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002;1:705–720. doi: 10.1016/s1568-1637(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 8.Fisher G.J., Quan T., Purohit T., Shao Y., Cho M.K., He T., Varani J., Kang S., Voorhees J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol. 2009;174:101–114. doi: 10.2353/ajpath.2009.080599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park H.J., Kim D.H., Park S.J., Kim J.M., Ryu J.H. Ginseng in traditional herbal prescriptions. J Ginseng Res. 2012;36:225–241. doi: 10.5142/jgr.2012.36.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong C.E., Lyu S.Y. Anti-inflammatory and antioxidative effects of Korean red ginseng extract in human keratinocytes. Immune Netw. 2011;11:42–49. doi: 10.4110/in.2011.11.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang T.H., Park H.M., Kim Y.B., Kim H., Kim N., Do J.H., Kang C., Cho Y., Kim S.Y. Effects of red ginseng extract on UVB irradiation-induced skin aging in hairless mice. J Ethnopharmacol. 2009;123:446–451. doi: 10.1016/j.jep.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Lü J.M., Yao Q., Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Current Vascular Pharm. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai B.X., Jin S.L., Luo D., Lin X.F., Gao J. Ginsenoside Rb1 suppresses ultraviolet radiation induced apoptosis by inducing DNA repair. Biol Pharm Bull. 2009;32:837–841. doi: 10.1248/bpb.32.837. [DOI] [PubMed] [Google Scholar]
- 14.Kwok H.H., Yue P.Y., Mak N.K., Wong R.N. Ginsenoside Rb1 induces type I collagen expression through peroxisome proliferator-activated receptor-delta. Biochem Pharmacol. 2012;84:532–539. doi: 10.1016/j.bcp.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 15.Kim S., Kang B.Y., Cho S.Y., Sung D.S., Chang H.K., Yeom M.H., Kim D.H., Sim Y.C., Lee Y.S. Compound K induces expression of hyaluronan synthase 2 gene in transformed human keratinocytes and increases hyaluronan in hairless mouse skin. Biochem Biophys Res Commun. 2004;316:348–355. doi: 10.1016/j.bbrc.2004.02.046. [DOI] [PubMed] [Google Scholar]
- 16.Lee E.H., Cho S.Y., Kim S.J., Shin E.S., Chang H.K., Kim D.H., Yeom M.H., Woe K.S., Lee J., Sim Y.C. Ginsenoside F1 protects human HaCaT keratinocytes from ultraviolet-B-induced apoptosis by maintaining constant levels of Bcl-2. J Invest Dermatol. 2003;121:607–613. doi: 10.1046/j.1523-1747.2003.12425.x. [DOI] [PubMed] [Google Scholar]
- 17.Hwang E., Lee T.H., Park S.Y., Yi T.H., Kim S.Y. Enzyme-modified Panax ginseng inhibits UVB-induced skin aging through the regulation of procollagen type I and MMP-1 expression. Food Funct. 2014;5:265–274. doi: 10.1039/c3fo60418g. [DOI] [PubMed] [Google Scholar]
- 18.Hwang E., Sun Z.W., Lee T.H., Shin H.S., Park S.Y., Lee D.G., Cho B.G., Sohn H., Kwon O.W., Kim S.Y. Enzyme-processed Korean Red Ginseng extracts protects against skin damage induced by UVB irradiation in hairless mice. J Ginseng Res. 2013;37:425–434. doi: 10.5142/jgr.2013.37.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang Q., Zuo Z., Harrison F., Chow M.S. Hawthorn. J Clin Pharmacol. 2002;42:605–612. doi: 10.1177/00970002042006003. [DOI] [PubMed] [Google Scholar]
- 20.Kao E.S., Wang C.J., Lin W.L., Chu C.Y., Tseng T.H. Effects of polyphenols derived from fruit of Crataegus pinnatifida on cell transformation, dermal edema and skin tumor formation by phorbol ester application. Food Chem Toxicol. 2007;45:1795–1804. doi: 10.1016/j.fct.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Huang X.X., Liu Q.B., Wu J., Yu L.H., Cong Q., Zhang Y., Lou L.L., LiL Z., Song S.J. Antioxidant and tyrosinase inhibitory effects of neolignan glycosides from Crataegus pinnatifida seeds. Planta Med. 2014;80:1732–1738. doi: 10.1055/s-0034-1383253. [DOI] [PubMed] [Google Scholar]
- 22.Shin H.S., Lee J.M., Park S.Y., Yang J.E., Kim J.H., Yi T.H. Hair growth activity of Crataegus pinnatifida on C57BL/6 mouse model. Phytother Res. 2013;27:1352–1357. doi: 10.1002/ptr.4870. [DOI] [PubMed] [Google Scholar]
- 23.Hwang E., Kim S.H., Lee S., Lee C.H., Do S.G., Kim J., Kim S.Y. A comparative study of baby immature and adult shoots of Aloe vera on UVB-induced skin photoaging in vitro. Phytother Res. 2013;27:1874–1882. doi: 10.1002/ptr.4943. [DOI] [PubMed] [Google Scholar]
- 24.Hwang E., Park S.Y., Jo H., Lee D.G., Kim H.T., Kim Y.M., Yin C.S., Yi T.H. Efficacy and safety of enzyme-modified Panax ginseng for anti-wrinkle therapy in healthy skin: a single-center, randomized, double-blind, placebo-controlled study. Rejuvenation Res. 2015;18:449–457. doi: 10.1089/rej.2015.1660. [DOI] [PubMed] [Google Scholar]
- 25.Chung J.H., Lee S.H., Youn C.S., Park B.J., Kim K.H., Park K.C., Cho K.H., Eun H.C. Cutaneous photodamage in Koreans: influence of sex, sun exposure, smoking, and skin color. Arch Dermatol. 2001;137:1043–1051. [PubMed] [Google Scholar]
- 26.Edwards P. Questionnaires in clinical trials: guidelines for optimal design and administration. Trials. 2010;11:2. doi: 10.1186/1745-6215-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afaq F., Mukhtar H. Photo chemo prevention by botanical antioxidants. Skin Pharmacol Appl. 2002;15:297–306. doi: 10.1159/000064533. [DOI] [PubMed] [Google Scholar]
- 28.Chen Q. Evaluate the effectiveness of the natural cosmetic product compared to chemical-based products. Int J Chem. 2009;1:57–59. [Google Scholar]
- 29.Hwang E., Lee D.G., Park S.H., Oh M.S., Kim S.Y. Coriander leaf extract exerts antioxidant activity and protects against UVB-induced photoaging of skin by regulation of procollagen type I and MMP-1 expression. J Med Food. 2014;17:985–995. doi: 10.1089/jmf.2013.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quan T., He T., Voorhees J.J., Fisher G.J. Ultraviolet irradiation induces Smad7 via induction of transcription factor AP-1 in human skin fibroblasts. J Biol Chem. 2005;280:8079–8085. doi: 10.1074/jbc.M409647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang E., Park S.Y., Lee H.J., Sun Z.W., Lee T.Y., Song H.G., Shin H.S., Yi T.H. Vigna angularis water extracts protect against ultraviolet B-exposed skin aging in vitro and in vivo. J Med Food. 2014;17:1339–1349. doi: 10.1089/jmf.2013.3017. [DOI] [PubMed] [Google Scholar]
- 32.Sun Z.W., Hwang E., Lee H.J., Lee T.Y., Song H.G., Park S.Y., Shin H.S., Lee D.G., Yi T.H. Effects of Galla chinensis extracts on UVB-irradiated MMP-1 production in hairless mice. J Nat Med. 2015;69:22–34. doi: 10.1007/s11418-014-0856-6. [DOI] [PubMed] [Google Scholar]
- 33.Lee M., Sorn S., Baek S., Jang S., Kim S. Antioxidant and apoptotic effects of Korean white ginseng extracted with the same ratio of protopanaxadiol and protopanaxatriol saponins in human hepatoma HepG2 cells. Ann N Y Acad Sci. 2009;1171:217–227. doi: 10.1111/j.1749-6632.2009.04918.x. [DOI] [PubMed] [Google Scholar]
- 34.Tu L.H., Ma J., Liu H.P., Wang R.R., Luo J. The neuroprotective effects of ginsenosides on calcineurin activity and tau phosphorylation in SY5Y cells. Cell Mol Neurobiol. 2009;29:1257–1264. doi: 10.1007/s10571-009-9421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim H.G., Cho J.H., Yoo S.R., Lee J.S., Han J.M., Lee N.H., Ahn Y.C., Son C.G. Antifatigue effects of Panax ginseng C.A. Meyer: a randomised, double-blind, placebo-controlled trial. PLoS One. 2013;8:e61271. doi: 10.1371/journal.pone.0061271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng N., Wang Y., Gao H., Yuan J., Feng F., Cao W., Zheng J. Protective effect of extract of Crataegus pinnatifida pollen on DNA damage response to oxidative stress. Food Chem Toxicol. 2013;59:709–714. doi: 10.1016/j.fct.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Wang T., Zhang P., Zhao C., Zhang Y., Liu H., Hu L., Gao X., Zhang D. Prevention effect in selenite-induced cataract in vivo and antioxidative effects in vitro of Crataegus pinnatifida leaves. Biol Trace Elem Res. 2011;142:106–116. doi: 10.1007/s12011-010-8752-8. [DOI] [PubMed] [Google Scholar]
- 38.Li T.P., Zhu R.G., Dong Y.P., Liu Y.H., Li S.H., Chen G. Effects of pectin pentaoligosaccharide from Hawthorn (Crataegus pinnatifida Bunge. var. Major) on the activity and mRNA levels of enzymes involved in fatty acid oxidation in the liver of mice fed a high-fat diet. J Agric Food Chem. 2013;61:7599–7605. doi: 10.1021/jf400283w. [DOI] [PubMed] [Google Scholar]
- 39.Christensen K., Doblhammer G., Rau R., Vaupel J.W. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–1208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramos-e-Silva M., Carneiro S.C.S. Cosmetics for the elderly. Clin Dermatol. 2001;19:413–423. doi: 10.1016/s0738-081x(01)00181-x. [DOI] [PubMed] [Google Scholar]
- 41.Fiers S.A. Breaking the cycle: the etiology of incontinence dermatitis and evaluating and using skin care products. Ostomy Wound Manag. 1996;42 32−34, 36, 38−40. [PubMed] [Google Scholar]
- 42.Jackson S.M., Williams M.L., Feingold K.R., Elias P.M. Pathobiology of the stratum corneum. West J Med. 1993;158:279–285. [PMC free article] [PubMed] [Google Scholar]
- 43.Pena Ferreira M.R., Costa P.C., Bahia F.M. Efficacy of anti-wrinkle products in skin surface appearance: a comparative study using non-invasive methods. Skin Res Technol. 2010;2:189–194. doi: 10.1111/j.1600-0846.2010.00458.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.