Abstract
Background
American ginseng (Panax quinquefolius L.) and Asian ginseng (Panax ginseng Meyer) products, such as slices, have a similar appearance, but they have significantly different prices, leading to widespread adulteration in the commercial market. Their aroma characteristics are attracting increasing attention and are supposed to be effective and nondestructive markers to determine adulteration.
Methods
The aroma characteristics of American and Asian ginseng were investigated using gas chromatography–mass spectrometry(GC-MS) and an electronic nose (E-nose). Their volatile organic compounds were separated, classified, compared, and analyzed with different pattern recognition.
Results
The E-nose showed a good performance in grouping with a principle component analysis explaining 94.45% of variance. A total of 69 aroma components were identified by GC-MS, with 35.6% common components and 64.6% special ingredients between the two ginsengs. It was observed that the components and the number of terpenes and alcohols were markedly different, indicating possible reasons for their difference. The results of pattern recognition confirmed that the E-nose processing result is similar to that of GC-MS. The interrelation between aroma constituents and sensors indicated that special sensors were highly related to some terpenes and alcohols. Accordingly, the contents of selected constituents were accurately predicted by corresponding sensors with most R2 reaching 90%.
Conclusion
Combined with advanced chemometrics, the E-nose is capable of discriminating between American and Asian ginseng in both qualitative and quantitative angles, presenting an accurate, rapid, and nondestructive reference approach.
Keywords: aroma characteristics, electronic nose, GC-MS, Panax ginseng, Panax quinquefolius
1. Introduction
The Asian ginseng root (Panax ginseng Meyer), particularly in Korea, China, and Japan, has been used as an important herbal medicine for thousands of years. In recent years, American ginseng (Panax quinquefolius L.), mainly cultivated in America and Canada, has also been well known in Asian countries. A wide range of their therapeutic functions in antistress, health promotion, maintaining and enhancing central and immune systems, preventing certain chronic diseases, as well as aging deterrent properties have been reported [1], [2], [3], but American ginseng seems to be more effective in cardiovascular disease treatment and acute postprandial glycemic inhibition [4], [5]. This explains why American ginseng roots are usually five to 10 times more expensive than Asian ginseng roots in the herbal market [6].
Owing to the similar appearance of the American and Asian ginseng roots, most commercial products are processed in various shapes [7], and the adulteration of American ginseng products with Asian ginseng is commonly seen. Thus, it is becoming difficult to distinguish various processed products by using traditional sensory evaluation, especially for customers without professional knowledge. Typical chemical and analytical methods, such as high-performance liquid chromatography and gas chromatography-mass spectrometry (GC-MS), are based on individual chemical constituents rather than comprehensive information [8], [9]. Furthermore, they are destructive to samples, which is obviously not cost-effective for precious ginseng products. Therefore, new nondestructive analytical methods based on significant features, including aroma information, have attracted considerable attention.
Aroma, an important profile in sensory judgment, has been traditionally used in differentiating ginseng species. The variety in aroma has been further demonstrated by using advanced chemical analytical techniques, for example, GC-MS. Shellie et al [6] analyzed Asian and American ginseng aroma using comprehensive two-dimensional GC and GC GC-quadrupole MS. Changes in aroma characteristics during the preparation of red ginseng have also been investigated with GC-MS [10]. Although GC-MS can discriminate between the chemical components of aroma and quantify the target volatile constituents, this approach is expensive and time-consuming.
The electronic nose (E-nose), an artificial olfactory system mimicking the human nose, has been developed and widely used in the food industry [11], [12], the environment [13], and medicines [14], [15]. As the E-nose is designed to mimic a human nose, it can partially provide results similar to that of the human nose; moreover, it can provide half-quantity outputs when used as a quantitative tool similar to GC-MS [16], [17]. In other words, it combines the advantage of sensory evaluation and GC-MS; more importantly, the application of E-nose is nondestructive [18]. Li et al [19] explored a rapid way to discriminate between Chinese red ginseng and Korean ginseng using only an E-nose, but few studies have specifically focused on the aroma information of ginsengs with the E-nose technology. Furthermore, only a few studies have focused on determining the differences between American ginseng and Asian ginseng by their aroma characteristics with a rapid and accurate method.
This study was carried out (1) to investigate the aroma fingerprint characteristics of American ginseng and Asian ginseng from both comprehensive and individual volatile components with E-nose and GC-MS data, and then selecting critical constituents for determining American ginseng from Asian ginseng; (2) to study the quantitative profiles and to build content prediction models for index components by comparing and relating E-nose and GC-MS data; and (3) to build a simple and easily understood prediction model to distinguish American ginseng from Asian ginseng. The purpose of the current study is to provide references for a fast, easy-to-operate, accurate, and nondestructive method for distinguishing different ginseng species.
2. Materials and methods
2.1. Ginseng materials
In the Chinese ginseng market, Asian ginseng is usually grown in Changbai Mountain (Fu Song County, Jilin Province, China), which is the major planting base of Asian ginseng in China. Thus, Asian ginseng growing in Fu Song was chosen as the typical Asian ginseng. Although American ginseng is usually imported from Canada and America, those originating from the former are much more popular in China; therefore, American ginseng grown in Toronto, Canada, was chosen as the typical American ginseng.
Asian ginseng samples (P. ginseng Meyer, individual roots, 6 years cultivation age) in this study were collected in Fu Song County, Jilin, China, in Autumn 2012. American ginseng samples (P. quinquefolius L., individual roots, 6 years old cultivation age) were purchased from Ontario Ginseng Growers Association, Toronto, Canada, in the same year. Prior to the experiments, the collected ginseng samples were identified by the China Food and Drug Administration. To minimize the variability caused by processing, all ginseng root samples were washed, preprocessed, and dried at 45°C for 6 h in the same oven, respectively. During E-nose measurements and GC-MS identification, experiment conditions were optimized first, then ginseng samples were detected under the optimized condition.
2.2. Preparation of volatile oil and ginsenoside
For volatile oil and ginsenoside preparation, ginseng roots were first sliced into pieces. The slices of American and Asian ginseng were milled into a powder and sieved with 40 meshes; then 4 × 80 g of ginseng powder was extracted using a Soxhlet Extractor (SER148/6; VELP, Usmate Velate, Italy) in ethyl ether for 5 h, and were evaporated (105–115°C) after collection. The ethyl ether extract was extracted with 500 mL of water with a steam distillation method for 10 h, which was developed by the Chinese Pharmacopoeia [20]. The yield rate was calculated with the following equation:
| (1) |
where W is the yield rate, is the volatile oil weight, and m2 denotes ginseng weight. The same recovered volatile oil was diluted and used in further GC-MS determination.
Ginsenoside was prepared according to the procedure described in our previous study [21], using an electronic tongue.
2.3. E-Nose
This study used an E-nose system (FOX 4000; Alpha MOS, Toulouse, France) equipped with 18 metal oxide gas sensors (MOS sensors) based on different sensing materials. Gas sensors were located in three temperature-controlled chambers with high temperature and zero humidity, and a purified air generator was used to provide carrier gas for cleaning sensors. According to the E-nose manufacturer and engineers, the sensor array comprised three types of sensors: LY2-type, T-type, and P-type. Table 1 shows the detailed characteristics of these three types of sensors. For T-type and P-type sensors, the working condition is approximately 300°C to 350°C, but for the LY2-type sensor, the working condition is approximately 400°C. Sensor response is recorded by Alpha Soft11.0 software (Alpha MOS).
Table 1.
Summaries for different types of sensors and their application in odor detection
| Gas description | Sensors |
Applications | |||
|---|---|---|---|---|---|
| P-type | T-type | LY2-type | |||
| Flammable gases | Hydrocarbons | P10/1 | LY2/gCT LY2/GH |
Cooking, roasting; Dairy products; Vegetables; Petro-chemistry |
|
| Methane | P10/2 | ||||
| Propane | LY2/gTCl | ||||
| Hydrogen | |||||
| Organic compounds | Aldehydes | LY2/LG | Rancidity odor; Alcohol beverages; Perfumes; Fermentation; Herbs; |
||
| Solvents | P30/1 | T30/1 | |||
| Alcohol | P30/2-PA/2 | TA/2 | LY2/AA | ||
| Aromatic compounds | P40/1-P40/2 | T40/1-T40/2 T70/2 |
LY2/G–LY2/GH | ||
In order to reflect the same sample condition used in GC-MS detection, for E-nose detection, the ginseng samples were also sliced in to pieces, grouped in two different sealed bags, and then stored in a vacuum dryer.
When detected, sensor resistance was measured for 120 s at the rate of one acquisition every 1 s. Data were recorded with Alpha Soft11.0 software (Alpha MOS). The sampling conditions (quantity, volume, temperature, and head space generating time) were optimized previously in order to improve sensor performance. Details of the experiments were designed and carried out as follows. At first, different quantities (1 g, 3 g, and 5 g) were measured. Then, the effect on the volume of vials (50 mL, 250 mL, and 500 mL) was observed. Third, different temperatures (25°C, 40°C, 60°C, and 90°C) and headspace generated times (0.5 h, 1 h, 1.5 h, and 2 h) were considered, respectively. Next, an optimal condition would be compared and adopted based on their principal component analysis (PCA) results. The PCA result shows that the ginseng sample of 3 g kept in a sealed 250-mL bottle for 1 h at 60°C proved to be the optimal working condition, which is in a good agreement with our previous study [22].
The impact of temperature and humidity to sensor response was investigated to determine better working conditions. Asian ginseng samples of 3 g kept in a sealed 250-mL bottle for 1 h at 10°C, 20°C, 30°C, 40°C, 50°C, 60°C, 70°C, and 80°C, were measured separately using an E-nose (FOX 4000). Similarly, 3-kg Asian ginseng samples kept in 250-mL bottles for 1 h at 60°C and at different humidity levels [10, 20, 30, 40, 50 and 60 RH(%)] were measured separately using an E-nose (FOX 4000). The sensors' response was compared in the function of different temperature and humidity, and an optimal condition of temperature and humidity was chosen as the final detection condition. For a clear presentation and explanation, six gas sensors (LY2/G, P30/1, LY2/AA, T30/1, T70/2, P10/1) were selected from three groups of sensors to represent the original 18 sensors.
Following the E-nose system directions, a 2-mL headspace was drawn off and injected into the E-nose, and each sensor response was measured at a 1-s interval every 4 min. For each ginseng (6-year-old American ginseng and Asian ginseng), 20 parallels of individual ginseng root samples with similar shapes and with the same mass were prepared for E-nose detection under the same procedure (total, 20 × 2 = 40 samples).
2.4. GC-MS
Prior to detection, volatile oils of American and Asian ginseng were diluted 10 times, 100 times, and 1,000 times with ethyl ether separately in order to obtain an effective GC-MS spectrogram, and the second one (1:100 dilution, v/v) was determined to be the best. Ginseng volatile oil compounds were identified by gas chromatography (GC) coupled with a mass spectrometer selective detector (MSD) using an Agilent 6890 N Network for GC (Agilent Technologies, Palo Alto, CA, USA) and an Agilent 5975 Network detector for MSD (Agilent Technologies). The GC–MSD system was also equipped with NIST (NIST 11.0; National Institute of Standards and Technology, Gaithersburg, MD, USA) and Wiley (Wiley, Chichester, West Sussex, UK) library search data system. After optimizing detection conditions, the selected GC and MSD parameters and conditions were as follows.
-
(1)
GC conditions: HP-5 Methyl Siloxane chromatographic column (250 μm × 30 m, 0.25 μm). Helium was used as a carrier gas; the injection port temperature was made in the splitless mode, and the injection port temperature was at 250°C. The oven temperature was programmed as follows: initial temperature 50°C, hold for 3 min, ramp to 120°C at 20°C/min, and then ramp to 250°C at 5°C/min, hold for 10 min, and finally ramp to 270°C at 20°C/min and kept for 2 min. The system was returned to the original condition followed by reequilibration, and also an autosampler program was used. The flow program was as follows: rate, 1.00 mL/min; injection volume, 2 μL; gasification chamber temperature, 280°C.
-
(2)
MS conditions: EI source, electron energy of 70 eV, ionization temperature 230°C, interface temperature at 280°C, and temperature quadrupole 150°C, solvent delayed for 3 min, multiplier voltage of 1964.7 V, quantity scanning range was from 30 amu to 500 amu. Ginseng volatile oil was processed for five replicate samples, and each sample was measured in triplicate by GC-MS within the same condition. The average value with the relative standard deviation was computed, and the repeatability of the GC-MS method was performed with 6-year-old ginseng samples.
2.5. Data analysis
For GC-MS qualitative and quantitative analysis, identification of the volatile components was carried out by comparing the mass spectra with the NIST mass-spectral library (NIST 11.0, National Institute of Standards and Technology, Gaithersburg, MD, USA) and Wiley library search data system, retention index (RI), and comparison of previous reports [19] and published index data (www.flavornet.org). The RIs were calculated from all volatile constituents using a homologous series of n-alkanes (C6–C28) (Sigma-Aldrich, Shanghai Trading Co., Ltd., Shanghai, China). Each sample was analyzed in triplicate, and the mean values with relative standard deviation (mean ± standard deviation, %) are reported. The relative contents of the volatile constituents were calculated using the area normalized method.
PCA was introduced for qualitative and half-quantitative analysis based on both E-nose sensor data and GC-MS component values. The partial least squares (PLS) method was applied to build appropriate models and to predict the contents of aroma ingredients.
A bioplot was used to illustrate the PCA and PLS results, which are the combination of the score and loading plot, revealing distribution of samples but also pointing out which factors contributed most to the separation [23]. A bioplot of PCA was introduced to display which sensors for E-nose or volatile constituents for GC-MS focused on ginseng separation. A bioplot of PLS was used to reveal the correlation among the ginseng samples, E-nose gas sensors, and the chemical volatile compounds. It also revealed correlation among dependents, independents, and factors. PCA and PLS were performed by Unscrambler Software, version 10.3 (CAMO ASA, Trondheim, Norway).
3. Results and discussion
3.1. Determination of American and Asian ginseng using E-nose
This section discusses the comprehensive aroma fingerprint of American ginseng and Asian ginseng characterized by E-nose sensors and the determination of two types of ginsengs with different pattern recognition.
3.1.1. Temperature and humidity effect on sensor response
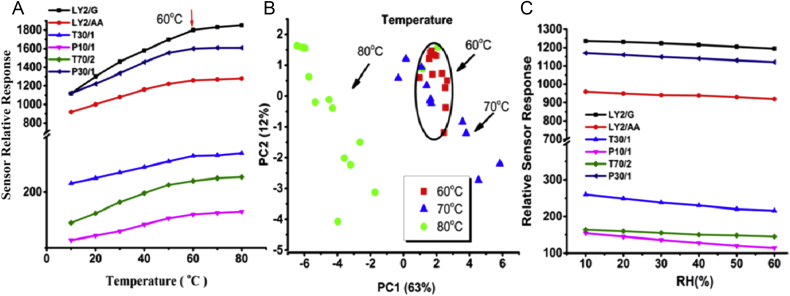
It is reported that the sampling temperature and humidity exert an effect on sensor sensitivity, which further affects the sensor response and measurement. Therefore, to obtain an optimal experimental condition in sampling, the effect of temperature and humidity on sensor response was considered. As described in Methods and materials section, ginseng samples in sealed bottles at different temperatures (10°C, 20°C, 30°C, 40°C, 50°C, 60°C, 70°C, and 80°C) were detected, and sensor responses were measured. The result is shown in Fig. 1A. It was observed that temperatures ranging from approximately 60°C to 80°C indicated better response. Temperatures below 60°C led to lower sensor responses, which might be attributed to the fact that higher temperatures help ginseng samples to generate more volatile organic components. But with increasing temperatures from 60°C to 80°C, sensor responses were not significantly increased. It is probably because a relative saturated volatilization has been reached, but only a slight amount of growth can be found. To determine the optimal temperature, a PCA among samples at 50°C, 60°C, 70°C, and 80°C was carried out, and the results are shown in Fig. 1B. It was found that 60°C was a better temperature for sampling with better grouping. Therefore, in this work, 60°C was chosen as the optimal temperature. For humidity impact, as shown in Fig. 1C, only a slight change was observed. This is probably attributable to the following reasons. First, there are three gas chambers in this E-nose system (FOX 4000). Each chamber is independent and consists of six MOS sensors including T-type, P-type, or LY2-type. In order to access the effective working condition of the MOS sensor, each chamber is carried out under 300–400°C at zero humidity. When the headspace gases flow through these chambers, where the temperature is approximately 300–400°C, the tested headspace gases will proceed to gasification immediately, which to a certain extent reduces the impact of humidity. Therefore, humidity has only a slight influence on sensor response. Thus, it can be concluded that temperature has a greater effect on sensor response, with 60°C being the optimal condition, whereas humidity's influence can be ignored. The sampling experiments were all carried out under these optimal conditions, which involved 3-g ginseng samples (Asian and American ginseng) being kept in sealed 250-mL bottles for 1 h at 60°C.
Fig. 1.
Effect of temperature and humidity on sensor response, and principal component analysis (PCA). (A) Influence of temperature. (B) PCA among ginseng samples at 60°C, 70°C, and 80°C. (C) Influence of humidity on sensor response.
3.1.2. Sensor response
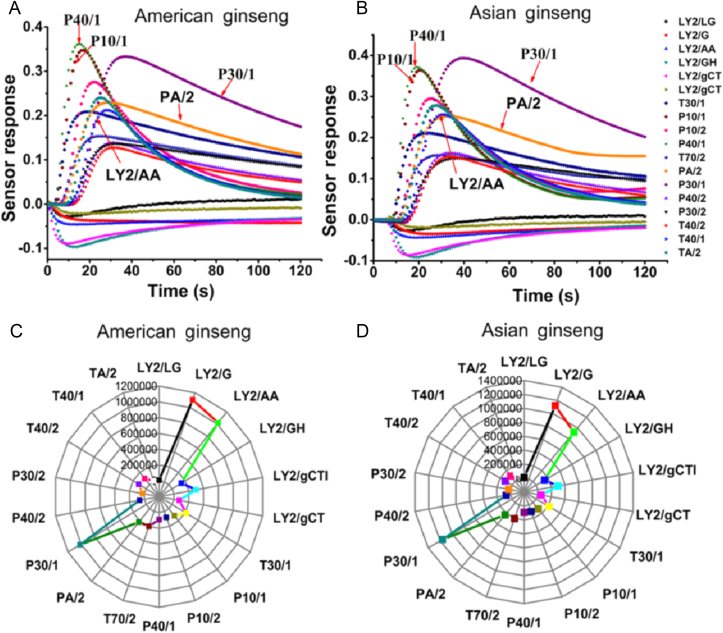
Sensor responses of both American ginseng and Asian ginseng are shown in Fig. 2. As indicated in Figs. 2A and B, sensor behavior was stable and credible. It appeared that different sensors have similar sensor behaviors, but sensors LY2/AA, LY2/gCT, LY2/gCTL, P40/1, P30/1, and P10/1 show obvious differences. For instance, the response of P40/1 and P10/1 was higher than that of P30/1 in American ginseng detection, but a contrasting sensor performance was observed in Asian ginseng, demonstrating that aroma varied between the two species. For a clearer explanation, their radar charts are also exhibited in Figs. 2C and D. As shown in Figs. 2C and D, the responses of sensors LY2/AA and P30/1 are confirmed again to be clearly different between the two ginseng samples, which could be used as eigenvalues in further data processing. Therefore, the changes in sensor behaviors guaranteed the rationality of data, and they could be considered aroma markers.
Fig. 2.
A typical sensor response of American ginseng and Asian ginseng. (A) Sensor response of American ginseng. (B) Sensor response of Asian ginseng. (C, D) Related radar chart of sensor response.
3.1.3. Bioplot of PCA
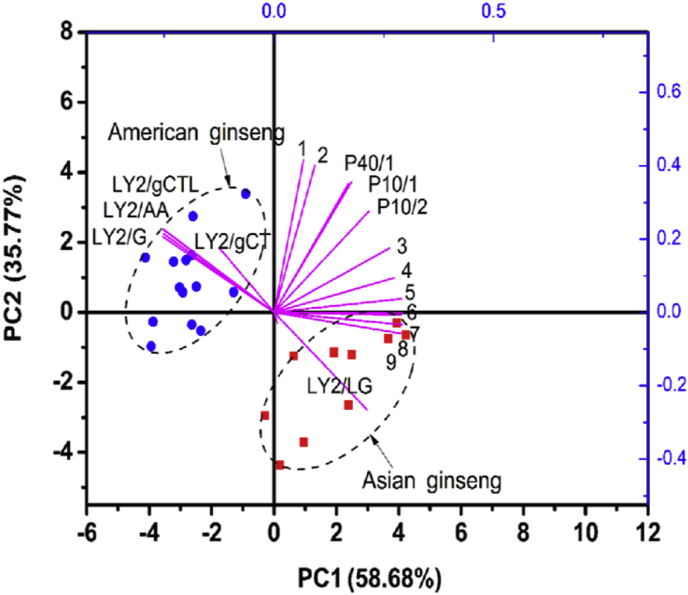
To determine the differences between American and Asian ginseng, PCA was conducted as shown in Fig. 3. The first two PCs accounted for 94.54% of the total variance. As indicated, two species of ginsengs were separated and respectively located in the positive and negative axes along with PC1 (58.68%). It was noted that sensors P40/1, P10/1, and P10/2 weighed more when separating both the PC1 and PC2 axes, whereas sensors LY2/G, LY2/AA, LY2/gCTL, and LY2/LG made a significant contribution in PC1 classification, as their projections on the PC1 axis were relatively longer than those of other sensors. Considering sensor characteristics, it was found that most LY2-type sensors are sensitive to terpenes, and T-type sensors are sensitive to food and fruit aroma; therefore, it could be assumed that terpenes and fruit flavor-like components probably relate to classification.
Fig. 3.
Bioplot of principal component analysis (PCA) based on the E-nose data. In this plot, a total of 16 copies of the data were selected for PCA with the first four data ignored. LY2/gCTL, LY2/AA, LY2/G, and LY2/LG are LY2-type sensors; P10/1, P10/2 and P40/1 are P-type sensors.
3.2. Determination of American and Asian ginseng using GC-MS
This section focuses on the individual aroma components identified by GC-MS. The contents, species, and number of each ginseng were calculated and compared to the study of common and special aroma information between American and Asian ginseng. Pattern recognitions were also used to illustrate the differences on the basis of individual aroma fingerprints.
3.2.1. Repeatability of GC-MS
The repeatability of the GC-MS conditions was assessed in preexperiments using five parallel measurements of Asian ginseng. The relative standard deviation values were less than 2.0%, which indicated good repeatability of the selected methods.
3.2.2. Identification and comparison of volatile compounds between American and Asian ginseng
To find out whether terpenes or other aromatic compounds were the key factors that differentiated American ginseng from Asian ginseng and to study the critical internal volatile constituents, the identified aroma constituents and their relative contents (%) and RI values are summarized in Table 2. A total of 52 and 55 constituents of American and Asian ginseng comprising 91.4% and 95.7%, respectively, of the total volatile constituents were identified.
Table 2.
Identified volatile compounds of American and Asian ginseng by GC-MS
| Peak | RI | Constituents | Identification1) | Formula | Relative content (%), mean ± SD2) |
Selected3) | |
|---|---|---|---|---|---|---|---|
| American ginseng | Asian ginseng | ||||||
| Terpenes | |||||||
| Common terpenes | |||||||
| 1 | 1,390 | β-Panasinsene* | MS, RI | C15H24 | 0.24 ± 0.05 | 3.7 ± 1.38 | 1 |
| 2 | 1,416 | α-Gurjunene* | MS, RI | C15H24 | 0.12 ± 0.06 | 10.36 ± 2.13 | 2 |
| 3 | 1,432 | β-Selinene* | MS, RI | C15H24 | 0.13 ± 0.03 | 0.18 ± 0.12 | |
| 4 | 1,458 | (+)-Aromadendrene* | MS, RI | C15H24 | 0.13 ± 0.02 | 0.53 ± 0.17 | 3 |
| 5 | 1,361 | (Z)-β-Farnesene* | MS, RI | C15H24 | 13.14 ± 2.17 | 3.62 ± 1.09 | 4 |
| 6 | 1,474 | (−)-Aromadendrene* | MS, RI | C15H24 | 0.84 ± 0.10 | 1.44 ± 0.82 | 5 |
| 7 | 1,459 | γ-Elemene* | MS, RI | C15H24 | 0.17 ± 0.04 | 8.13 ± 1.84 | 6 |
| 8 | 1,435 | γ-Gurjunene* | MS, RI | C15H24 | 0.29 ± 0.11 | 0.77 + 2.13 | 7 |
| 9 | 1,469 | β-Patchoulene* | MS, RI | C15H24 | 0.19 ± 0.05 | 0.04 ± 0.19 | |
| Special terpenes in American ginseng | |||||||
| 10 | 1,367 | α-Copaene* | MS, RI | C15H24 | 0.11 ± 0.05 | nd | 8 |
| 11 | 1,435 | α-Bergamotene* | MS, RI | C15H24 | 0.11 ± 0.03 | nd | 9 |
| 12 | 1,533 | α-Curcumene* | MS, RI | C15H24 | 0.16 ± 0.07 | nd | 10 |
| 13 | 1,568 | β-Sesquiphellandrene* | MS, RI | C15H24 | 2.55 ± 0.37 | nd | 11 |
| 14 | 1,854 | α-Calacorene* | MS, RI | C15H24 | 0.09 ± 0.03 | nd | 12 |
| 15 | 1,511 | α-Farnesene* | MS, RI | C15H24 | 0.08 ± 0.05 | nd | 13 |
| 16 | 1,477 | γ-Muurolene* | MS, RI | C15H24 | 0.54 ± 0.13 | nd | 14 |
| 17 | 1,458 | Cyclooctene,4-methylene-6-(1-propenylidene)*- | MS, RI | C12H16 | 0.27 ± 0.04 | nd | 15 |
| Special terpenes in Asian ginseng | |||||||
| 18 | 1,342 | γ-Pyronene* | MS, RI | C10H16 | nd | 0.62 ± 0.29 | 16 |
| 19 | 1,449 | g-Selinene* | MS, RI | C15H24 | nd | 0.48 ± 0.02 | 17 |
| 20 | 1,479 | β-Caryophyllene* | MS, RI | C15H24 | nd | 1.4 ± 0.24 | 18 |
| 21 | 1,486 | β-Neoclovene* | MS, RI | C15H24 | nd | 0.71 ± 0.33 | 19 |
| 22 | 1,449 | (−)-α-Selinene* | MS, RI | C15H24 | nd | 0.71 ± 0.05 | 20 |
| 23 | 1,743 | Viridiflorene* | MS, RI | C15H24 | nd | 4.36 ± 0.95 | 21 |
| Alcohol | |||||||
| Common alcohol | |||||||
| 24 | 1,541 | E-nerolidol | MS, RI | C15H26O | 0.41 ± 0.22 | 0.22 ± 0.73 | |
| 25 | 1,634 | Ledol | MS, RI | C15H26O | 0.19 ± 0.07 | 0.4 ± 0.07 | |
| 26 | 1,590 | Espatulenol* | MS, RI | C15H24O | 0.17 ± 0.05 | 3.41 ± 1.07 | 22 |
| Special alcohol in American ginseng | |||||||
| 27 | 1,589 | Guaiol* | MS, RI | C15H26O | 0.27 ± 0.15 | nd | 23 |
| 28 | 1,593 | Hinesol* | MS, RI | C15H26O | 0.28 ± 0.13 | nd | 24 |
| Special alcohol in Asian ginseng | |||||||
| 29 | 1,568 | Nerolidol* | MS, RI | C15H26O | nd | 0.27 ± 0.11 | 25 |
| 30 | 1,763 | (+)-Viridiflorol* | MS, RI | C15H260 | nd | 0.29 ± 0.045 | 26 |
| Acid | |||||||
| Common acid | |||||||
| 31 | 1,634 | n-Hexadecanoic acid | MS, RI | C16H32O2 | 3.12 ± 10.55 | 1.43 ± 0.81 | |
| 32 | 2,093 | 9,12-Octadecadienoic acid (Z,Z)– | MS, RI | C18H32O2 | 0.04 ± 0.03 | 3.22 ± 0.53 | |
| Special acid in Asian ginseng | |||||||
| 33 | 1,256 | Octanoic acid | MS, RI | C8H16O2 | nd | 0.06 ± 0.02 | |
| 34 | 2,186 | Lauric acid | MS, RI | C12H24O2 | nd | 0.25 ± 0.15 | |
| 35 | 2,094 | Octadecanoic acid | MS, RI | C18H36O2 | nd | 1.16 ± 0.48 | |
| Ester | |||||||
| Common ester | |||||||
| 36 | 1,927 | Hexadecanoic acid, methyl ester | MS, RI | C17H34O2 | 0.43 ± 0.07 | 0.5 ± 0.1 | |
| 37 | 2,063 | Linoleic acid ethyl ester | MS, RI | C20H36O2 | 0.99 ± 0.15 | 0.13 ± 0.05 | |
| Special ester in American ginseng | |||||||
| 38 | 1,914 | Phthalic acid, butyl isohexyl ester | MS, RI | C22H34O2 | 0.59 ± 0.10 | nd | |
| 39 | 1,965 | Ethyl oleate | MS, RI | C20H38O2 | 0.48 ± 0.13 | nd | |
| 40 | 1,982 | Hexadecanoic acid, ethyl ester | MS, RI | C18H36O2 | 1.12 ± 0.57 | nd | |
| Special Ester in Asian ginseng | |||||||
| 41 | 2,102 | 10,13-Octadecadienoic acid, methylester | MS, RI | C19H3402 | nd | 0.24 ± 0.02 | |
| 42 | 2,164 | Linoleic acid ethyl ester | MS, RI | C20H36O2 | nd | 0.24 ± 0.03 | |
| Aldehydes | |||||||
| Special aldehydes in American ginseng | |||||||
| 44 | 1,009 | Octanal* | MS, RI | C8H16O | 0.08 | nd | 27 |
| Special aldehydes in Asian ginseng | |||||||
| 45 | 2,054 | 9,17-Octadecadienal, (Z)*– | MS, RI | C18H32O | nd | 0.62 ± 0.23 | 28 |
| Aromatics | |||||||
| Common aromatics | |||||||
| 46 | 1,465 | (+)-Calarene* | MS, RI | C15H24 | 1.26 ± 0.55 | 4.26 ± 1.27 | 29 |
| Special in Asian ginseng | |||||||
| 47 | 1,531 | Naphthalene, 1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-,(1S-cis)*– | MS, RI | C15H24 | nd | 0.29 ± 0.12 | 30 |
| Others | |||||||
| 48 | 1,209 | Dodecane | MS, RI | C12H26 | nd | 0.08 ± 0.03 | |
| 49 | 1,287 | Tridecane | MS, RI | C13H28 | 0.05 ± 0.03 | 0.1 ± 0.05 | |
| 50 | 1,396 | Tetradecane | MS, RI | C14H30 | 0.43 ± 0.17 | nd | |
| 51 | 1,423 | Cyclotetradecane | MS, RI | C14H28 | nd | 0.1 ± 0.05 | |
| 52 | 1,371 | Dodecane, 2,6,10-trimethyl– | MS, RI | C15H32 | 0.08 ± 0.03 | nd | |
| 53 | 1,507 | Pentadecane | MS, RI | C15H32 | 0.79 ± 0.29 | nd | |
| 54 | 1,514 | Dodecane, 2,6,11-trimethyl– | MS, RI | C15H32 | nd | 0.08 ± 0.03 | |
| 55 | 1,589 | Hexadecane | MS, RI | C16H34 | 0.4 ± 0.16 | nd | |
| 56 | 1,712 | Heptadecane | MS, RI | C17H36 | 0.2 ± 0.05 | 0.19 ± 0.06 | |
| 57 | 1,802 | Octadecane | MS, RI | C18H38 | 0.23 ± 0.10 | 0.18 ± 0.07 | |
| 58 | 1,863 | Heptadecane, 2-methyl– | MS, RI | C18H38 | nd | 0.08 ± 0.04 | |
| 59 | 1,787 | Pentadecane, 2,6,10,14-tetramethyl | MS, RI | C19H40 | 0.16 ± 0.06 | 0.17 ± 0.03 | |
| 60 | 1,910 | Nonadecane | MS, RI | C19H40 | 0.21 ± 0.06 | nd | |
| 61 | 1,862 | Hexadecane, 2,6,10,14-tetramethyl– | MS, RI | C20H42 | 0.2 ± 0.03 | 0.36 ± 0.16 | |
| 62 | 2,041 | Eicosane | MS, RI | C20H42 | 0.2 ± 0.05 | 0.31 ± 0.04 | |
| 63 | 2,007 | Cyclotetradecane, 1,7,11-trimethyl-4-(1-methylethyl)- | MS, RI | C20H40 | nd | 0.2 ± 0.02 | |
| 64 | 2,103 | Heneicosane | MS, RI | C21H44 | 0.27 + 0.09 | 0.18 ± 0.08 | |
| 65 | 2,210 | Docosane | MS, RI | C22H44 | nd | 0.94 ± 0.07 | |
| 66 | 2,304 | Tricosane | MS, RI | C23H48 | nd | 0.4 ± 0.09 | |
| 67 | 2,411 | Tetracosane | MS, RI | C24H50 | nd | 0.41 ± 0.17 | |
| 68 | 2,508 | Pentacosane | MS, RI | C25H52 | nd | 0.74 ± 0.26 | |
| 69 | 2,632 | Hexacosane | MS, RI | C26H54 | 0.17 ± 0.04 | 0.86 ± 0.39 | |
GC-MS, gas chromatography-mass spectrometry; NIST, National Institute of Standards and Technology; nd, no data; RI, retention index; SD, standard deviation.
Method of identification: MS, mass spectrum comparison using Wiley and NIST libraries, RI in agreement with literature value.
Five replicated sample were prepared for each species of ginseng sample and each sample were measured in triple; therefore, for each kind of ginseng, a total of 15 tests were carried out. So, the calculation of the selected volatile compounds concentration was based on these recorded data within the same experimental condition.
Selected: the selected 30 constituents according to the four criteria described in Section 3.2.3.
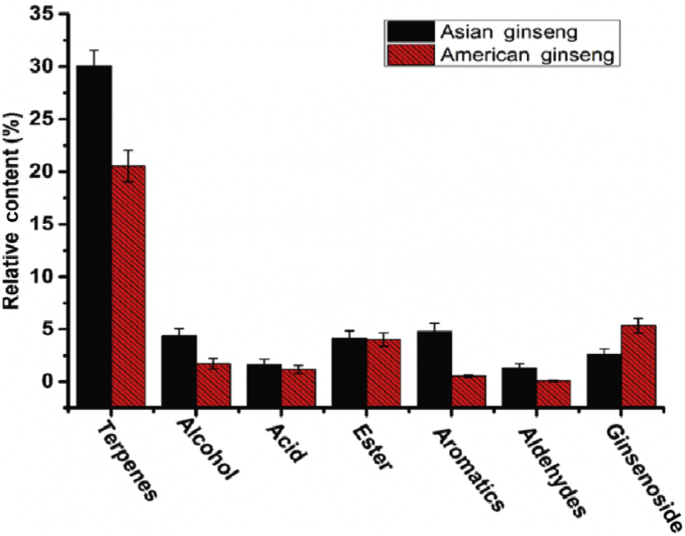
As listed in Table 2, the different chemical classes (terpenes, alcohol, acid, ester, aldehydes, and alkane) between the two ginsengs were calculated and compared, and the result is shown in Fig. 4. It was noteworthy that terpenes were the compounds with the highest content in both American ginseng (19.12%) and Asian ginseng (30.05%). In Asian ginseng, the following volatile compounds were found: aromatics (4.79%), alcohol (4.37%), ester (4.175), aldehydes (1.65%), and acid (1.33%). In American ginseng, the following result was noted: ester (4.03%), alcohol (1.72%), acid (1.16%), aromatics (0.54%), and aldehydes (0.08%). It should also be noted that the contents of the above six classes of chemical compounds in Asian ginseng were higher than those in American ginseng, especially for terpenes, revealing that Asian ginseng might contain more herb flavor. To confirm the difference between the two species, the ginsenosides, which are considered anticancer bioactive compounds, were introduced. Fig. 4 illustrates that American ginseng contains twice the amount of ginsenoside found in Asian ginseng, agreeing with previous reports [24]. This result demonstrates the designed ginseng selection to be reasonable and effective, and also explains why American ginseng carries a much more expensive price.
Fig. 4.
Selected compounds of different chemical classes between American ginseng and Asian Ginseng. Calculation of each class of both American and Asian ginseng was performed five times according to the data described in Sections 2.2, 2.4.
The results in Table 2 show that both American and Asian ginseng are rich in sesquiterpenes with nine compounds overlapping (a total of 17 and 18 constituents have been identified as terpenes in Asian and American ginseng, respectively). Among the common compounds, most of their relative contents varied greatly. In detail, β-panasinsene (which specifically exists in ginsengs) in Asian ginseng (3.7%) was almost 19 times higher than that in American ginseng (0.24%); Asian ginseng contained three times more α-gurjunene (3.6%), which contains balsamic flavor, than American ginseng (1.2%); alloaromadendrene, processing wood flavor, was 1.44% in Asian ginseng but 0.84% in American ginseng; γ-gurjunene and calarene in Asian ginseng were also two to three times greater than in American ginseng; however, β-farnesene, which has a specially sweet flavor, was 3.6 times higher in American ginseng (13.14%) than in Asian ginseng (3.62%). Additionally, special volatile constituents were only found in different ginsengs. As indicated in Table 2, β-sesquiphellandrene, γ-muurolene, and α-farnesene (which process wood and sweet flavors) were only detected in American ginseng, whereas γ-selinene and viridiflorene, emitting herb and wood flavors, were only identified in Asian ginseng. In conclusion, sesquiterpenes in Asian ginseng had higher content and processed more herb and wood flavors, whereas sesquiterpenes in American ginseng showed sweet flavors, which could be used for distinguishing between these two species of ginseng. Although both of them had common flavors, Asian ginseng seemed to have a stronger intensity.
For alcohol, the relative content of Asian ginseng (4.27%) was higher than that of American ginseng (1.72%). Three compounds were identified as common compositions: E-nerolidol, ledol, and espatulenol. Among these compounds, nerolidol, processing wood, flower, and wax flavors, was measured as 0.41% in American ginseng versus 0.22% in Asian ginseng, indicating that American ginseng produced more flower flavors. For individual compounds, American ginseng had guaiol (0.27%, wood and balsamic flavor) and viridiflorol (0.28%, green, sweet), whereas Asian ginseng contained nerolidol (0.27%, wood, flower, wax) and hinesol (0.29%). Both shared individual alcohol compositions, confirming that American ginseng produced more green, sweet, and balsamic flavor compared with Asian ginseng.
For aromatics, 1H-cyclopropa[a]naphthalene, and 1a,2,3,5,6,7,7a,7b-octahydro-1,1,7,7a-tetramethyl–processing wood fragrance was the only overlapping constituent in both ginsengs, but the amount in American ginseng was almost 10 times less than that in Asian ginseng. Octanal, which generates a stronger fruit flavor, was only detected in American ginseng. Information on aromatics and aldehydes between the two ginsengs matched the previous assumption. For acid and ester, the two ginsengs seemed to have similar relative contents, which might play a minor contribution in discriminating between ginseng species.
As previous discussion has shown, both ginsengs had overlapping constituents presenting their aroma similarity; however, the obvious variations in contents of common compounds and different species in specific compounds account for their differences and provide possible markers for distinguishing between the two. This might be associated with the various behaviors of E-nose sensor performance. In other words, different aroma constituents with different concentrations resulted in different sensor responses, showing a potential relationship between individual aroma compounds and fragrance fingerprint.
3.2.3. PCA based on GC-MS data
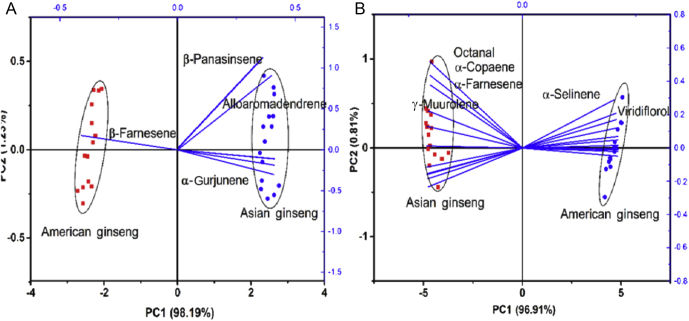
To determine which and how aroma volatile compounds play a critical role in differentiating American ginseng from Asian ginseng, 30 possible aroma constituents were selected (marked with star superscript, see Table 2). The criteria for selecting compounds were as follows: (1) alkane compounds were ignored, because they were reported to have less aromatic information [25], [26]; (2) according to previous reports [10], [27], the target volatile components were limited in terpenes, alcohols, acid, ester, and aldehydes; (3) for overlapping constituents between the two ginsengs, the meaningful ones were chosen, whose amounts varied greatly—for example, α-gurjunene in terpenes was selected because its content was significantly different in the two ginsengs; and (4) all the specific components were chosen because they were only detected in one ginseng and were considered important to differentiating between these two. A total of 30 aroma constituents (18 terpenes, eight alcohols, three aromatics, and one aldehyde) were chosen. A PCA was performed based on the overlapping and special components, respectively, and their bioplot is shown in Fig. 5. In both plots, American and Asian ginseng were scattered significantly with the first two PCs, explaining 99.42% of the whole variance based on common components (Fig. 5A) and 97.72% of variation based on specific components (Fig. 5B), both of which performed a little better than that based on the E-nose data. It appears that GC-MS might provide more effective determination information than the E-nose data, but they were similar in grouping samples. In Fig. 5A, β-panasinsene and alloaromadendrene, located in the right upper quadrant showing a higher weight, appeared to be the most effective components in classification. Then, β-farnesene and α-gurjunene were followed by (located in the second and fourth quadrants, respectively), and showed great projection at the first PCs. Similarly, in Fig. 5B, most of the components made a contribution to separating the two species, but a few of them also contributed to differentiating the variation in one species. These components will be focused on in a further study, since more of an aroma fingerprint can be extracted. In detail, the constituents octanal, α-copaene, α-farnesene, and γ-muurolene, distributed in the negative part of the plot, were the first four highest volatile compounds differentiating American ginseng from Asian ginseng; meanwhile, α-selinene and viridiflorol were the first two highest constituents distinguishing American ginseng from Asian ginseng. Furthermore, these 10 selected components were mainly terpenes and alcohol, which highly agreed with the previous analysis (Section 3.2.2). In other words, the internal relationship between effective volatile constituents and sensors should be investigated.
Fig. 5.
Bioplot of principal component analysis (PCA) based on 30 selected aroma constituents. (A) Based on the shared compounds among American ginseng and Asian ginseng. (B) Based on the special compounds among American ginseng and Asian ginseng. In this PCA, 15 copies of data, obtained accorded to the designed procedures (Section 2.4), were used.
3.3. Comparison and correlation between E-nose and GC-MS
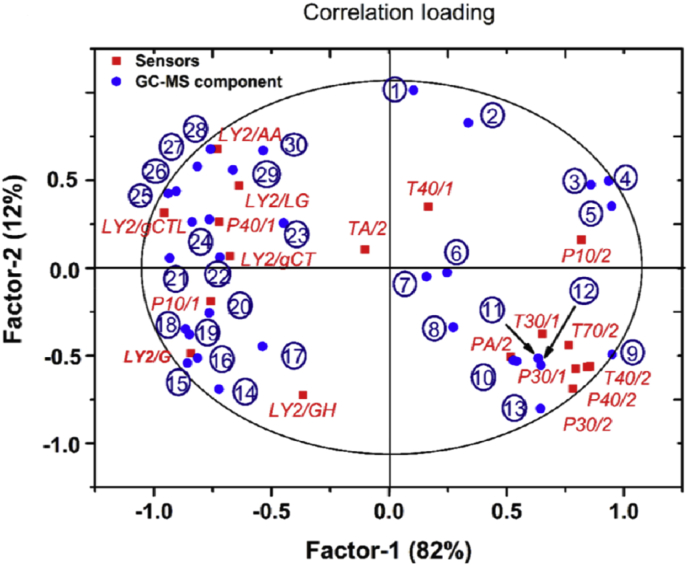
It is necessary to investigate the correlation between E-nose and GC-MS. A bioplot of the PLS was performed between the 30 selected chemical constituents and 18 sensors, and their relationship is presented in Fig. 6.
Fig. 6.
Bioplot for the correlation between volatile constituents and E-nose sensors. The compounds are 30 selected characteristic constituents, labeled from one to 30. These compounds are as follows: (1) ethyl oleate; (2) naphthalene, 1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl); (3) α-selinene; (4) phthalic acid, butyl isohexyl ester; (5) 10,13-octadecadienoic acid, methyl ester; (6) linoleic acid methyl ester; (7) cyclooctene, 4-methylene-6-(1-propenylidene); (8) nerolidol; (9) calarene; (10) viridiflorol; (11) 9,17-octadecadienal; (12) β-neoclovene ; (13) γ-muurolene; (14) espatulenol; (15) γ-pyronene; (16) α-gurjunene; (17) β-caryophyllene; (18) β-sesquiphellandrene; (19) alloaromadendrene; (20) α-bergamotene; (21) α-copaene; (22) hinesol; (23) α-curcumene; (24) α-calacorene; (25) guaiol; (26) γ-gurjunene; (27) octanal; (28) β-farnesene; (29) β-panasinsene; (30) γ-selinene. The 18 E-nose sensors are P10/1, P10/2, P30/1, P40/1, P40/2, P30/1, PA/2, T70/2, T40/2, TA/2, T40/1, LY2/AA, LY2/LG, LY2/GCTL, LY2/GCT, LY2/G, and LY2/GH.
As shown, sensors LY2/AA, LY2/LG, LY2/gCTL, LY2/gCT, LY2/G, P40/1, and P10/1 were all located in the left part of the coordinate system, which were far from the origin and surrounding the terpenes and alcohols. As the shorter the distance between samples in the bioplot, the higher the correlation, the selected seven sensors (sensors LY2/AA, LY2/LG, LY2/gCTL, LY2/gCT, LY2/G, P40/1, and P10/1) and nearby chemical components were considered to be highly related. For example, LY2/AA and LY2/gCTL are greatly associated with guaiol, γ-gurjunene, octanal, and γ-farnesene. This result was highly associated with both PCA based on E-nose and PCA based on GC-MS (Fig. 5). Because these sensors overlapped those sensors contributing most in separating the ginsengs in Fig. 3, some of the mentioned terpenes and alcohols were also the most critical compounds in distinguishing between the ginsengs in Fig. 5 (e.g., octanal, β-farnesene, β-panasinsene, and guaiol). Therefore, it may be concluded that some of the terpenes and alcohols interacted with the seven LY2-type and P-type sensors, thereby producing different sensor behaviors and leading to successful ginseng differentiation. Furthermore, it can be construed that the concentration of critical components may be predicted by highly related sensors.
3.4. Relative contents prediction by E-nose sensor in PLS
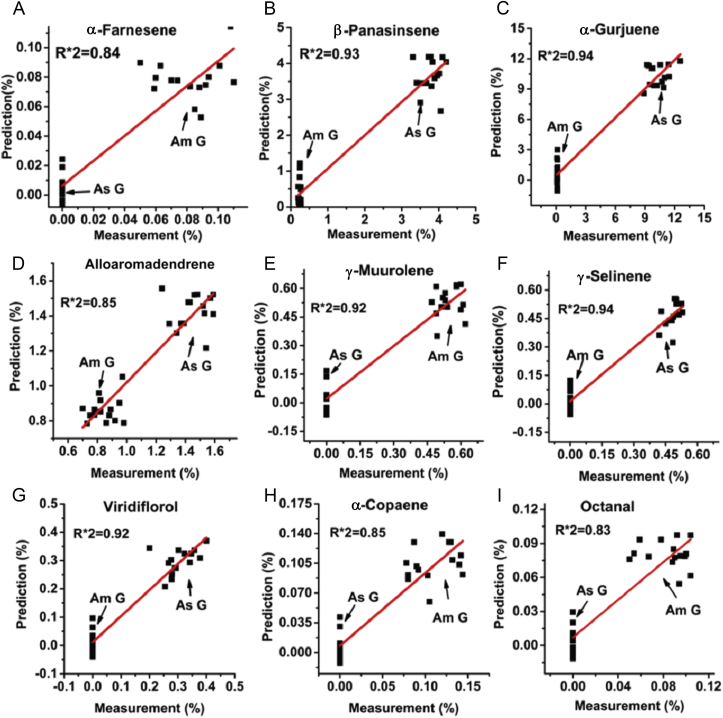
On the basis of correlation analysis between selected MOS sensors and crucial volatile compounds, seven MOS sensors (LY2/AA, LY2/LG, LY2/gCTL, LY2/gCT, LY2/G, P40/1, and P10/1) and nine volatile constituents (β-panasinsene, α-gurjunene, β-farnesene, and alloaromadendrene were from the shared compounds; octanal, α-copaene, γ-muurolene, and α-farnesene were from American ginseng; and viridiflorol was from Asian ginseng) were selected for qualitative analysis by using PLS. Seven sensors were used as independent variables, and the relative contents of nine volatile constituents were used as dependent variables. A total of 30 samples (15 American ginseng sample data, 15 Asian ginseng sample data) were used for PLS training, and the determination coefficient (R2) was compared to evaluate the prediction. As shown in Fig. 7, most of the contents of the selected compounds could be accurately predicted with R2 being more than 85%; only the R2 values of octanal and α-farnesene were a little less than 85%, but approximately reached 85%. These results are also confirmed in Table 3, which shows the Root Mean Square of Calibration (RMSEC) and Root Mean Square Error of Cross Validation (RMSECV) values of the PLS models. It can be concluded that the quantitative characteristics of volatile chemical constituents can be predicted and explained by using E-nose detection. Considering that the application of GC-MS is expensive, complex, and time-consuming, whereas the E-nose has been demonstrated to be fast, nondestructive, and accurate, the partial replacement of GC-MS application with the E-nose would make a difference in ginseng determination.
Fig. 7.
The fitting plot of measured contents and predicted contents of selected constituents (α-farnesene, β-panasinsene, α-gurjunene, alloaromadendrene, γ-muurolene, γ-selinene, viridiflorol, α-copaene, and octanal). As G, Asian ginseng; Am G, American ginseng.
Table 3.
R2, RMSEC, and RMSECV value of selected PLS model
| R2 | RMSEC | RMSECV | |
|---|---|---|---|
| α-Farnesene | 0.84 | 0.610 | 1.353 |
| β-Panasinsene | 0.93 | 0.227 | 0.447 |
| α-Gurjunene | 0.94 | 0.242 | 0.358 |
| Alloaromadendrene | 0.85 | 0.349 | 0.833 |
| γ-Muurolene | 0.92 | 0.280 | 0.279 |
| γ-Selinene | 0.94 | 0.245 | 0.388 |
| Viridiflorol | 0.92 | 0.377 | 0.408 |
| α-Copaene | 0.85 | 0.558 | 1.127 |
| Octanal | 0.83 | 0.729 | 1.226 |
PLS, partial least squares; RMSEC, Root Mean Square of Calibration; RMSECV, Root Mean Square Error of Cross Validation.
4. Conclusion
In this work, the aroma characteristics of American ginseng and Asian ginseng were investigated from the viewpoints of comprehensive odor and individual fragrance components by using E-nose and GC-MS. The E-nose showed a good performance in classifying the two ginseng species with LY- and T-type sensors changing considerably and with the PCA explaining 94% of the variance. After further study of the chemical components, we found that the differences between American and Asian ginseng aroma were possibly caused by the 69 aroma constituents of both ginsengs, which were identified by GC-MS. Comparing the detailed profiles of each component, terpenes and alcohol were the main components used to distinguish American ginseng from Asian ginseng. After comparing the qualitative analysis of the E-nose and GC-MS performance, it was found that the PCA results based on GC-MS data performed a little better than those based on the E-nose, but they almost reached the same level and significantly differentiated the ginseng samples. The interrelation between aroma constituents and E-nose sensors indicated that different sensors were highly related to most different aroma compounds. In other words, this implies that these terpenes and alcohols might induce the behavior of some specific sensors to change considerably and thus enable the E-nose to differentiate between American and Asian ginseng successfully. Based on the correlation, the contents of nine aroma ingredients were selected to be predicted by E-nose sensors by using PLS, and a satisfied quantitative prediction was presented. Combined with the advantage of the E-nose and its good performance in this study, it appears that the E-nose is capable of providing an easily operated, accurate, and nondestructive approach for determining ginseng species and can predict the contents of critical aroma components.
Conflicts of interest
All authors of this research paper have directly participated in the planning, execution, or analysis of the study. All authors of this paper have read and approved the final version submitted. The contents of this manuscript have not been copyrighted or published previously. The contents of this manuscript are not under consideration for publication elsewhere. The contents of this manuscript will not be copyrighted, submitted, or published elsewhere while acceptance by the manuscript is under consideration. There are no directly related manuscripts or abstracts, published or unpublished, by any author(s) of this paper.
Acknowledgments
The authors appreciate the technical support from Professor Shahe Liu and the experiments support from Ms Qiao.
References
- 1.Kitts D.D., Hu C. Efficacy and safety of ginseng. Public Health Nutr. 2000;3:437–485. doi: 10.1017/s1368980000000550. [DOI] [PubMed] [Google Scholar]
- 2.Oliver Chen C.Y., Ribaya-Mercado J.D., McKay D.L., Croom E., Blumberg J.B. Differential antioxidant and quinone reductase inducing activity of American, Asian, and Siberian ginseng. Food Chem. 2010;119:445–451. [Google Scholar]
- 3.Kaneko H., Nakanishi K. Proof of the mysterious efficacy of ginseng: basic and clinical trials: clinical effects of medical ginseng, Korean Red Ginseng: specifically, its anti-stress action for prevention of disease. J Pharmacol Sci. 2004;95:158–162. doi: 10.1254/jphs.fmj04001x5. [DOI] [PubMed] [Google Scholar]
- 4.Shao Z.H., Xie J.T., Vanden Hoek T.L., Mehendale S., Aung H., Li C.Q., Qin Y., Schumacker P.T., Becker L.B., Yuan C.S. Antioxidant effects of American ginseng berry extract in cardiomyocytes exposed to acute oxidant stress. Biochim Biophys Acta. 2004;1670:165–171. doi: 10.1016/j.bbagen.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Sievenpiper J.L., Arnason J.T., Leiter L.A., Vuksan V. Decreasing, null and increasing effects of eight popular types of ginseng on acute postprandial glycemic indices in healthy humans: the role of ginsenosides. J Am Coll Nutr. 2004;23:248–258. doi: 10.1080/07315724.2004.10719368. [DOI] [PubMed] [Google Scholar]
- 6.Shellie R.A., Marriott P.J., Huie C.W. Comprehensive two-dimensional gas chromatography (GC×GC) and GC×GC-quadrupole MS analysis of Asian and American ginseng. J Sep Sci. 2003;26:1185–1192. [Google Scholar]
- 7.Li T.S.C. Asian and American ginseng — a review. HortTechnology. 1995;5:27–34. [Google Scholar]
- 8.Brown P.N. Determination of ginsenoside content in Asian and North American ginseng raw materials and finished products by high-performance liquid chromatography: single-laboratory validation. J AOAC Int. 2011;94:1391–1399. doi: 10.5740/jaoacint.9-543. [DOI] [PubMed] [Google Scholar]
- 9.Harnly J., Chen P., Harrington P.D.B. Probability of identification: adulteration of American ginseng with Asian ginseng. J AOAC Int. 2013;96:1258–1265. doi: 10.5740/jaoacint.13-290. [DOI] [PubMed] [Google Scholar]
- 10.Lee S.K., Kim J.H., Sohn H.J., Wang J.W. Changes in aroma characteristics during the preparation of red ginseng estimated by electronic nose, sensory evaluation and gas chromatography/mass spectrometry. Sens Actuators B Chem. 2005;106:7–12. [Google Scholar]
- 11.Stitzel S.E., Aernecke M.J., Walt D.R. Artificial noses. Annu Rev Biomed Eng. 2011;13:1–25. doi: 10.1146/annurev-bioeng-071910-124633. [DOI] [PubMed] [Google Scholar]
- 12.Peris M., Escuder-Gilabert L. A 21st century technique for food control: electronic nose. Anal Chim Acta. 2009;638:1–15. doi: 10.1016/j.aca.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Kuske M., Romain A.C., Nicolas J. Microbial volatile organic compounds as indicators of fungi. Can an electronic nose detect fungi in indoor environments? Build Environ. 2005;40:824–831. [Google Scholar]
- 14.Daniels R. Electronic noses and tongues in development and quality control: Part 1. Pharm Ind. 2005;67:945–949. [Google Scholar]
- 15.Daniels R. Electronic noses and tongues in development and quality control: Part 2. Pharm Ind. 2005;67:1096–1100. [Google Scholar]
- 16.Zhang H.X., Balban M., Portier K., Sims C.A. Quantification of spice mixture compositions by electronic nose: Part 2. Comparison with GC and sensory methods. J Food Sci. 2005;70:E259–E264. [Google Scholar]
- 17.Benedetti S., Drusch S., Mannino S. Monitoring of autoxidation in LCPUFA-enriched lipid microparticles by electronic nose and SPME-GCMS. Talanta. 2009;78:1266–1271. doi: 10.1016/j.talanta.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 18.Cevoli C., Cerretani L., Gori A., Caboni M.F., Gallina Toschi T., Fabbri A. Classification of Pecorino cheeses using electronic nose combined with artificial neural network and comparison with GC-MS analysis of volatile compounds. Food Chem. 2011;129:1315–1319. doi: 10.1016/j.foodchem.2011.05.126. [DOI] [PubMed] [Google Scholar]
- 19.Li S., Li X., Wang G.L., Nie L.X., Yang Y.J., Wu H.Z., Wei F., Zhang J., Tian J.G., Lin R.C.J. Rapid discrimination of Chinese red ginseng and Korean ginseng using an electronic nose coupled with chemometrics. Pharmaceut Biomed. 2012;70:605–608. doi: 10.1016/j.jpba.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Pharmacopoeia of the People's Republic of China (2010). The measurement of volatile oil, Appendix X D in Chinese Pharmacopoeia (1), 2010. p. 63.
- 21.Cui S.Q., Wang J., Geng L.H., Wei Z.B., Tian X.J. Determination of ginseng with different ages using a taste-sensing system. Sensor Mater. 2013;25:241–255. [Google Scholar]
- 22.Cui S.Q., Wang J., Yang L.C., Wu J.F., Wang X.L. Qualitative and quantitative analysis on aroma characteristics of ginseng at different ages using E-nose and GC-MS combined with chemometrics. J Pharm Biomed Anal. 2015;102:64–77. doi: 10.1016/j.jpba.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 23.Bro R., Smilde A.K. Principal component analysis. Anal Method. 2014;6:2812–2831. [Google Scholar]
- 24.Qi L.W., Wang C.Z., Yuan C.S. Ginsenosides from American ginseng: chemical and pharmacological diversity. Phytochemistry. 2011;72:689–699. doi: 10.1016/j.phytochem.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toldra F. Dry-cured meat products. Food and Nutrition Press; Trumbull, CT: 2002. Flavor development; p. 157. [Google Scholar]
- 26.Toldra F. Meat, poultry and seafood — biochemical of fermented meat. In: Simpson B.K., Nollet L.M.L., Toldra F., Benjakul S., Paliyath G., Hui Y.H., editors. Food biochemistry and food processing. 2nd ed. John Wiley & Sons; Ames, IA: 2012. p. 341. [Google Scholar]
- 27.Iwabuchi H., Yoshikura M., Kamisako W. Studies on the sesquiterpenoids of Panax ginseng C.A. Meyer (3) Chem Pharm Bull. 1989;37:509–513. doi: 10.1248/cpb.36.2447. [DOI] [PubMed] [Google Scholar]