Abstract
BACKGROUND
Improved pain control after cesarean delivery remains a challenging objective. Poorly treated acute pain following delivery is associated with an increased risk of chronic pain and depression. This study was conducted to determine whether the addition of systemic acetaminophen and an increased dose of intrathecal morphine would further reduce acute pain. The primary outcome was pain intensity with movement at 24 hours postoperatively. Secondary measures included persistent pain and depression at 8 weeks.
METHODS
Seventy-four parturients scheduled for elective cesarean delivery under spinal anesthesia that were predicted to be above the 80th percentile for evoked pain intensity based on a 3-item preoperative screening questionnaire were enrolled. Patients in the intervention group received 300 mcg spinal morphine and 1 gram acetaminophen every 6 hours for 24 hours postoperatively. Patients in the control group received 150 mcg spinal morphine and placebo tablets. All patients received scheduled ibuprofen by mouth and IV morphine patient-controlled analgesia. At 24 hours, patients rated their pain intensity with movement, at rest, on average, and worst score using a visual analog scale for pain (100-mm unmarked line). The presence of persistent pain and depression was assessed at 8 weeks using the Edinburgh postpartum depression survey.
RESULTS
Providing a higher dose of spinal morphine combined with systemic acetaminophen to patients predicted to be at high risk for severe post–cesarean delivery pain significantly reduced evoked pain scores with movement at 24 hours (mean ± SD: 46 ± 25 mm in control group versus 31 ±17 mm in intervention group, P = 0.009; 95% confidence interval for the difference between means: 4 mm, 26 mm). There was no difference in the incidence of persistent pain (13% (4/30) in control group versus 10% (3/30) in intervention group, P > 0.99), or depression at 8 weeks postoperatively (10% (3/30) in control group versus 13% (4/30) in intervention group, P > 0.99).
CONCLUSIONS
Adding a higher dose of intrathecal morphine and oral acetaminophen to a multimodal pain regimen in patients predicted to be at risk for high acute postpartum pain after cesarean delivery results in a significant reduction of acute postoperative pain scores at 24 hours.
The wide interindividual variability in post–cesarean delivery pain poses a significant clinical challenge and is a source of patient morbidity in obstetrics. Inadequately treated postoperative pain can cause numerous undesirable physiologic and psychological consequences in this population, 1–3 and severe acute pain following delivery is predictive of persistent pain, and independently, of depression at 2 months postpartum.2 Since the number of cesarean deliveries exceeds 1.4 million annually in the United States, improving post–cesarean delivery pain remains an important health and socioeconomic objective. Numerous systemic and neuraxial medications have been used to treat acute post–cesarean delivery pain with varied success, but also with accompanied side effects. In addition, although the dose response for analgesia from intrathecal morphine after cesarean delivery has been described, there is little guidance as to whether and how this can be titrated for individual patient needs.
Identifying patients preoperatively who are at high risk for severe pain after cesarean delivery may allow more effective tailoring of postoperative pain regimens to their analgesic requirements.4,5 We previously showed that a simple, 3-question preoperative survey could predict the intensity of pain with movement (evoked pain) on the first day following cesarean delivery with modest efficiency.6 We used the same 3-question survey in this study to identify women predicted to have postoperative evoked pain scores above the 80th percentile and who may benefit from an improved multimodal analgesic regimen.
Multimodal analgesia is commonly applied following nonobstetric surgery, but many of these approaches have minimal efficacy after cesarean section (e.g., transversus abdominis plane block) or their neonatal safety has been inadequately studied in the acute postpartum period (e.g., gabapentin). For this reason, many women in the United States after cesarean delivery receive a mixture of systemic nonsteroidal anti-inflammatory drugs, opioids, and a single dose of intrathecal morphine. The goal of this study was to test whether addition of systemic acetaminophen and an increased dose of intrathecal morphine would reduce pain in an enriched population of women expected to experience severe post–cesarean delivery pain. The primary outcome was pain intensity with movement at 24 hours postoperatively. Secondary measures included persistent pain and depression at 8 weeks postpartum.
A final goal of this study was to explore a hypothesis regarding the use of rescue patient-controlled analgesia (PCA) drug after surgery. Recently, the strength of behavioral drive and fun-seeking, as measured by the Behavioral Appetitive Scale (BAS), was found to be significantly associated with the degree of placebo analgesia and brain activation in human volunteers.7,8 Given the anticipatory nature of the PCA paradigm, we tested whether there was also a positive correlation between this scale and rescue PCA drug consumption.
METHODS
With approval from institutional review boards at Wake Forest School of Medicine and at Forsyth Medical Center in Winston-Salem, NC, written informed consent was obtained from 74 parturients scheduled for elective cesarean delivery under spinal anesthesia (Clinicaltrials.gov NCT01298778). Patients with allergies to study medications, those with hepatic disease, those with contraindications to neuraxial anesthesia, and those weighing more than 300 pounds were excluded. The parturients eligible for the study were American Society of Anesthesiologists physical class 1 or 2 with an uncomplicated singleton pregnancy undergoing elective cesarean delivery and were predicted to be above the 80th percentile for evoked pain intensity at 24 hours after cesarean delivery. All patients routinely completed a 3-item preoperative screening questionnaire on admission as part of our standard care, and women with a calculated overall screening score > 53.5 (predicted to be above the 80th percentile for postoperative evoked pain intensity) were eligible for inclusion when research personnel (principal investigators, clinical fellows, and/or research coordinator) were available to enroll them.6
After written informed consent, patients completed the BAS and the Edinburgh depression inventory questionnaires preoperatively.6,9 Routine demographic and medical and obstetric history were collected, as well as a detailed history of pain during pregnancy or preexisting chronic pain, including diagnosis, location, and intensity of the pain. A computer-generated randomization sequence was created for 2 study groups. Subjects excluded after randomization were subsequently replaced with new subjects at the end of the study to obtain 60 evaluable subjects. A numbered, white, sealed envelope with group allocation was opened by the anesthesiologist preparing the spinal mixture for injection. All patients received spinal surgical anesthesia for cesarean delivery with 12 mg hyperbaric bupivacaine with 15–20 mcg fentanyl. Patients were then randomized to receive standard (control group) or additional analgesic treatment (intervention group). Patients in the intervention group received 300 mcg spinal preservative-free morphine and 1 gram acetaminophen by mouth (first dose administered in the postanesthesia care unit) every 6 hours for the first 24 hours postoperatively. Patients in the control group received 150 mcg spinal preservative-free morphine and a placebo tablet by mouth (first dose administered in the postanesthesia care unit) every 6 hours for the first 24 hours postoperatively. The acetaminophen and placebo tablets were identical in appearance and were dispensed by the hospital pharmacy based on the patient’s randomization number. In addition, all patients postoperatively received ibuprofen 800 mg by mouth every 6 hours (first dose administered on admission to the postpartum unit) and IV PCA with morphine as needed (3 mg initial bolus, no basal rate, 1 mg bolus with an 8-minute lockout, and 8 mg maximum total per hour which was started in the postanesthesia care unit). The anesthesiologist preparing the spinal anesthetic mixture was not blinded to group, but the patient and the investigators taking experimental measures and other health care providers, including the postanesthesia care unit and postpartum nurses who administered supplemental analgesic drugs, were blinded to group assignment. The patients were monitored and managed as per our usual intraoperative and postoperative management. Patients were able to receive additional IV fentanyl, IV morphine, or oral oxycodone tablets in the postanesthesia care unit for uncontrolled pain at the nurse’s discretion according to our routine postanesthesia care unit orders.
Breakthrough pain, pruritus, and nausea or vomiting was treated at patient request according to our routine practice and protocol. Breakthrough pain, defined as a reported pain score ≥4 on a 0–10 verbal pain scale despite IV morphine PCA, was treated on the postpartum unit with oral oxycodone or additional IV morphine. Pruritus was treated with IV nalbuphine or diphenhydramine. Nausea or vomiting was treated with IV ondansetron, metoclopramide, or promethazine. The number of treatment interventions for side effects or breakthrough pain was recorded for the first 24 hours postoperatively. In addition, the patient’s vital signs, sedation level, and respiratory rate were monitored every 1 to 2 hours for the first 24 hours with a standard institutional protocol that applies to all patients receiving intrathecal morphine.
On the first postoperative day, the patient was methodically asked by a research nurse, blinded to the group assignment, to rate their current pain intensity at rest, the worst pain during the past 24 hours, the average pain during the past 24 hours, and the pain intensity upon sitting up in bedside (evoked pain with movement), using a visual analog scale (VAS) of 0 mm (no pain) to 100 mm (worst possible pain). Patients were contacted 8 weeks after their surgery by telephone to assess for the presence and location of persistent pain and to complete the Edinburgh postpartum depression inventory (EPDI). A score > 12 on the EPDI was used to identify women with postpartum depression.9 The primary outcome measure of the study was VAS pain scores with movement at 24 hours. Secondary outcome measures included VAS of the other pain scores (average 24 hour pain score, worst pain score, and pain score at rest), 24 hour IV morphine PCA use, total oral and/or IV opioid use, incidence of nausea and pruritus requiring treatment, and the incidence of persistent pain and/or depression at 8 weeks postpartum.
Statistical analyses were conducted using SigmaStat version 3.0 for Windows (SPSS Inc., Chicago, IL, and then acquired by IBM in 2009). A priori sample size analysis, using 2-tailed Student t-test with power = 0.8 and α = 0.05 estimated a sample size of 30 per group was adequate to show a clinically relevant 33% reduction between groups from an estimated 70 ± 31 mm VAS for pain intensity with movement at 24 hours, obtained from the interim data of a previous study on patients at risk for having severe post–cesarean delivery pain.6 Descriptive statistics were calculated for all variables and compared between groups, such that mean ± SD were used for normally distributed variables; median (interquartile range) for data that were not normally distributed or for data with outliers or ordinal data; and number (percentage) for categorical data. Lilliefors test was used to test for normality of the residuals of the primary end point. Unpaired 2-tailed Student t-tests were used for comparing parametric data between groups, and Mann-Whitney rank sum test for nonparametric data. χ2 Analysis and Fisher exact test were applied for comparing incidences and proportions between groups. If over 20% of the expected values in contingency table are less than 5 with the χ2 analysis, Fisher exact test was applied instead because of the corresponding χ2 would be inaccurate. The 95% confidence interval for the mean difference in the primary outcome measure between groups was calculated with the method that assumes homogeneity of variance, normality of population distribution, and independent sampling of each value. Pearson correlation was used to analyze the correlation between individual trait scores on the BAS survey and morphine consumption (IV morphine PCA or total morphine consumption). For all analyses, P was set at 0.05 for statistical significance.
RESULTS
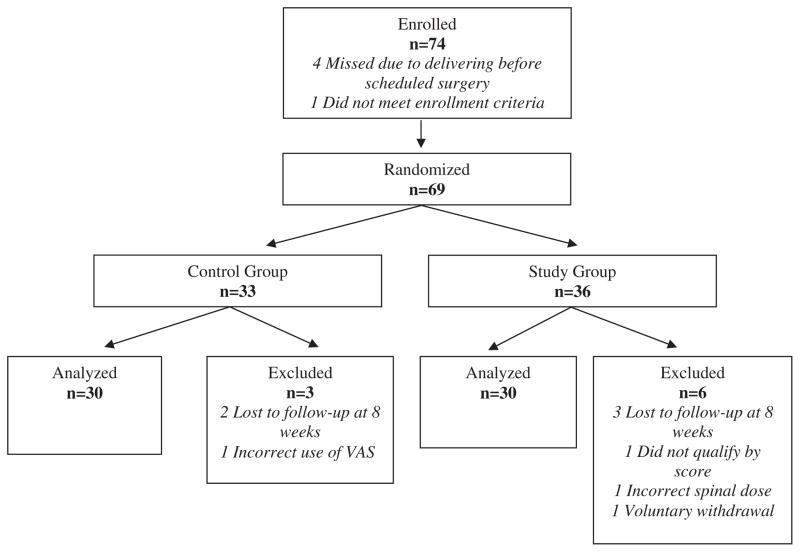
Seventy-four women were enrolled; 69 women were randomized, and 60 completed the study (Fig. 1). Nine patients were excluded after randomization, as noted in Figure 1, and were subsequently replaced for a total of 60 completed patients for analysis. Maternal demographic variables were similar between the 2 groups (Table 1). The overall preoperative screening scores were similar between groups, although patients randomized to the intervention group had higher responses to the expected pain screening question than patients in the control group (P = 0.002) (Table 1). On the basis of the previous study6 (when 150 micrograms of intrathecal morphine was used as post–cesarean delivery analgesia without using acetaminophen or IV morphine PCA), the predicted post–cesarean delivery pain scores for all patients above the 80th percentile for acute postoperative pain were 73 ± 12 mm and 67 ± 9 mm in the intervention and control groups of this study, respectively. Evoked pain 24 hours after surgery for the entire study population was 38 ± 23 mm and was significantly lower in the intervention group than in the control group (46 ± 25 mm in the control group versus 31 ± 17 mm in the intervention group, P = 0.009; 95% CI for the difference between the means: 4 mm, 26 mm). Median VAS pain scores at rest and on average pain scores over 24 hours were also significantly lower in the intervention group, although the groups did not differ significantly in worst pain score (Table 2). We excluded from the final results the 5 patients who did not complete the 8-week follow-up, but analysis including their VAS pain scores at 24 hours in these patients did not alter the conclusions of the statistical analysis (46 ± 25 mm in the control group versus 30 ± 17 mm in the intervention group, P = 0.003; 95% CI for the difference between the means: 6 mm, 27 mm). The incidence of pruritus and/or nausea requiring treatment 24 hours after surgery was similar between groups (Table 2). Absolute risk increase of side effects for the intervention compared with the control group for overall pruritus was 20% (95% CI of the difference: −5%, 42%) and for overall emetic symptoms was 7% (95% CI of the difference: −18%, 30%). No patient in the study had a respiratory rate < 12 per minute in the first 24 hours after surgery.
Figure 1.
Enrollment flow chart of randomized patients.
Table 1.
Demographic Variables
| 150 mcg PF-morphine + placebo (control group, n = 30) | 300 mcg PF-morphine + acetaminophen (intervention group, n = 30) | P value | |
|---|---|---|---|
| Age (years) | 30 ± 5 | 28 ± 5 | 0.22 |
| Height (cm) | 163 ± 6 | 164 ± 6 | 0.29 |
| Weight (kg) | 88 ±17 | 92 ± 19 | 0.33 |
| American Society of Anesthesiologists classification | 2 (1–2) | 2 (1–2) | 0.31 |
| Parity | 1 (1–2) | 1 (1–2) | 0.44 |
| Previous cesarean delivery | 27 (90%) | 24 (80%) | 0.16 |
| Fetal gestational age (weeks) | 39 ± 0.3 | 39 ± 0.7 | 0.48 |
| Q1 score (anxious, 0–100 scale) | 75 (50–90) | 72 (60–90) | 0.93 |
| Q2 score (expect to hurt, 0–100 scale) | 75 (50–75) | 85 (70–90) | 0.002 |
| Q3 score (pain medications, 0–5 scale) | 3 (3–4) | 3 (3–4) | 0.30 |
| Preoperative overall calculated score | 69 (61–76) | 75 (64–81) | 0.07 |
Age, height, weight, and fetal gestational age are expressed as mean ± SD; previous cesarean delivery is in n (%), and American Society of Anesthesiologists classification, parity, Q1 score, Q2 score, Q3 score, and Preoperative overall calculated score are median (interquartile range).6
Table 2.
Visual Analog Pain Score Assessment and Side Effects for the First 24 Hours After Cesarean Delivery
| Visual analog pain score (0–100 mm)a | 150 mcg PF-morphine + placebo (control group, n = 30) | 300 mcg PF-morphine + acetaminophen (intervention group, n = 30) | P value |
|---|---|---|---|
| Movement at 24 hours,b mean ± SD (mm) | 46 ± 25 | 31 ± 17 | 0.009 |
| Resting at 24 hours, median [IQR] (mm) | 19 (0–33) | 4 (0–10) | 0.011 |
| Average over 24 hours, mean ± SD (mm) | 37 ± 19 | 23 ± 14 | 0.002 |
| Worst over 24 hours, median [IQR] (mm) | 69 (49–77) | 62 (42–70) | 0.15 |
| Side effects | |||
| Percentage of pruritus requiring treatment | Overall: 50% | Overall: 70% | 0.11 |
| Postanesthesia care unit: 33% | Postanesthesia care unit: 47% | 0.35 | |
| Postpartum unit: 40% | Postpartum unit: 57% | 0.17 | |
| Percentage of nausea requiring treatment | Overall: 53% | Overall: 60% | 0.60 |
| Postanesthesia care unit: 33% | Postanesthesia care unit: 23% | 0.33 | |
| Postpartum unit: 37% | Postpartum unit: 50% | 0.33 | |
Mean ± SD were used for normally distributed variables; median (interquartile range) were used for data that were not normally distributed or for data with outliers or ordinal data; and number (percentage) were used for categorical data.
IQR = interquartile range.
Visual analog scale of pain using a 100 mm unmarked line.
Primary outcome; data are expressed as mean ± SD or median (interquartile range).
Median [IQR] total IV morphine PCA use did not differ between the control (15 [11–32] mg) and intervention groups (15 [11–29] mg; P = 0.92). Similarly, the total 24 hour opioid use, including fentanyl and oxycodone converted to morphine equivalents,a did not differ between groups (21 [11–37] mg and 15 [13–33] mg in the control and intervention groups, respectively; P = 0.72). Of note, only 1 patient in the intervention group and none in the control group did not use IV PCA to supplement their post–cesarean delivery analgesia, whereas 4 patients in each group also requested supplement oral analgesic while in the postpartum unit for breakthrough pain. Individual trait scores for fun-seeking and behavioral drive, as measured by subgroups of questions on the BAS, were similar between groups (Table 3). Pearson moment correlation test showed that there was no significant association between these subgroup scores and 24 hour IV morphine PCA use (data not shown, R2 = 0.02).
Table 3.
Behavioral Appetitive Scalea and Morphine Patient-Controlled Analgesia (PCA) Usage
| 150 mcg PF-morphine + placebo (control group) | 300 mcg PF-morphine + acetaminophen (intervention group) | P value | |
|---|---|---|---|
| Fun seeking | 11 ± 2 | 11 ± 2 | 0.80 |
| Behavioral drive | 11 ± 3 | 11 ± 2 | >0.99 |
| Total IV morphine PCA use (mg) | 15 (11–32) | 15 (11–29) | 0.92 |
Behavioral Appetitive Scale responses were reverse-scored. Data are expressed as mean ± SD or median (interquartile range).
The incidence of persistent pain 8 weeks postpartum was 13% (4/30) in the control group and 10% (3/30) in the intervention group (P > 0.99). Three patients in the intervention group acknowledged having chronic pain before pregnancy, but these patients differed from the patients that identified having persistent pain at 8 weeks. One patient had chronic back pain of unknown duration, 1 patient had chronic hip pain from a prior pregnancy, and 1 patient had constant chronic right shoulder pain of unknown duration. All 3 patients used acetaminophen for treatment of these pains. None of the patients with preexisting chronic pain prior to pregnancy required opioid medications, and they were not regularly seen by a physician for their pain. In the intervention group, 2 patients developed a chronic headache and 1 patient complained of pain at the surgical site at 8 weeks postoperatively. In the control group, 3 patients complained of lower back pain and 1 patient complained of pain in her lower back and at the surgical site at 8 weeks postoperatively. Two patients, both from the control group, were clinically diagnosed with postpartum depression by a physician and started taking antidepressant medications prior to the 8-week follow-up telephone interview. Therefore, we identified these patients as having postpartum depression, even though their scores on the Edinburgh postpartum depression survey did not reflect their clinical state. The incidence of postpartum depression at 8 weeks, as identified by either a formal clinical diagnosis or a score > 12 on the EPDI, was 10% (3/30) in the control group and 13% (4/30) in the intervention group (P > 0.99). Two patients, one from each group, were identified as having both persistent pain and postpartum depression during their 8-week follow-up telephone interview.
DISCUSSION
The results of our study show that providing a higher dose of spinal morphine combined with systemic acetaminophen and IV PCA to patients predicted to be at high risk for severe post–cesarean delivery pain significantly reduces acute pain scores at 24 hours. Our primary outcome was VAS pain scores with movement at 24 hours, but we also demonstrated a significant improvement in pain scores in the study group at rest and on average over 24 hours. The overall worst pain score over 24 hours did not differ between the 2 groups, which was likely attributable to severely painful acute events such as postsurgical abdominal uterine massage and checks. We were unable to demonstrate a significant difference in the incidence of persistent pain or postpartum depression at 8 weeks, however.
In comparison to the previous study in which the 3-item predictive screening questionnaire was derived and validated, 6 the 24-hour evoked pain VAS score with movement for parturients above the 80th percentile of predicted pain intensity was lower in both the control and intervention groups. This is most likely due to the difference in postoperative analgesic regimen between studies in which the intervention group of this study received a higher dose of spinal morphine and oral acetaminophen, and both groups in this study also received IV morphine PCA in addition to the usual low-dose spinal morphine administered in the previous validation study. The addition of IV PCA in this study was originally intended to allow more precise measure of supplement opioid need, as well as for our secondary measure in exploring rescue PCA use after surgery and its association with behavior scores. We did not anticipate in our study design the potential significant effect of supplement IV PCA on overall pain score reduction. Comparing pain scores from this study with the previous study is not appropriate because the analgesic regimens differed. There may have been a placebo response in the control group from participating in a pain study and/or the addition of a supplemental IV morphine PCA. However, despite the lower 24-hour evoked VAS score in this study, the 3-item screening questionnaire allowed us to appropriately select and enroll an enriched population of patients at high risk for postoperative pain after cesarean delivery. Furthermore, this study also demonstrated the addition of acetaminophen and higher dose of spinal morphine reduced the post–cesarean delivery pain scores in this selected population.
Among the 3 questions in the predictive screening questionnaire, the scores for the second question that asked patients how much they expected to hurt after surgery were statistically different between the control and intervention group. Patients randomized to the intervention group had significantly higher scores than patients randomized to the control group, which may be a result of our overall small sample size. Although this is unlikely to bias our study results, the fact that patients in the intervention group expected higher post–cesarean delivery pain but had significantly lower evoked pain scores at 24 hours further supports the efficacy of our improved multimodal regimen.
Multiple studies have demonstrated that a multimodal analgesic regimen is effective in reducing postoperative pain.10–12 Optimal doses of these analgesics improve pain without increasing side effects. The higher dose of spinal morphine chosen in this study and the additional doses of acetaminophen are considered to be within safe limits from previous studies and have a long history of efficacy and safety.12–15 However, as a precaution, we excluded patients with signs or symptoms or risk of liver diseases and proscribed the delivery of other acetaminophen-containing drugs during the 24-hour study period to avoid exceeding the 4 g/24 h limit. The effectiveness of other agents such as oral gabapentin, used as a component of multimodal postoperative analgesic for acute and chronic pain in obstetric populations, is inconclusive and its use may result in significant sedation.11,16 Furthermore, the short- and long-term neonatal effects from gabapentin require further investigation before its routine use. Transverses abdominis plane block also does not further improve analgesia over intrathecal morphine.17 A continuous epidural opioid infusion, in addition to more frequent assessment and treatment, may potentially be beneficial to further improve postoperative analgesia.
Changing the dose of spinal morphine and adding scheduled oral acetaminophen in the intervention group was a practical way to reduce acute pain scores. In comparison to the control group that received our hospital’s routine analgesic regimen after cesarean delivery, a significant difference in acute pain scores with movement at 24 hours was shown without a significant increase in IV morphine PCA usage. Thus, in select patients predicted to be above the 80th percentile for evoked pain intensity at 24 hours after cesarean delivery, the addition of both of these analgesics as a routine intervention may be very useful.
In addition, although the study group used a higher dose of spinal morphine, there was no significant difference in nausea or pruritus requiring treatment in the first 24 hours between the 2 groups. The upper limit of the confidence interval of the difference between groups for emetic symptoms (30%) and pruritus (42%), however, could represent a clinically significant difference. Thus, our sample size was not large enough to detect a potentially significant increase in side effects in the high-dose morphine group. Several previous studies demonstrated an increase in side effects as the dose of spinal morphine was increased, and we did find a nonsignificant increase in the overall incidence of nausea and pruritus of 7% and 20%, respectively, in the intervention group.18,19 However, all of the patients were able to receive appropriate medications to treat the aforementioned side effects if they so desired. Future studies should evaluate patient satisfaction scores in the intervention group to determine the overall side effects versus analgesic benefits ratio.
Since the incidence of persistent pain is low in the postpartum population, our study was too small to find a difference in outcome. Our previous study found a 9.8% incidence of persistent pain 8 weeks after childbirth.2 This correlates with our current study that found a 13% and 10% incidence in the control and intervention groups, respectively. Even though we were able to improve acute pain in the first 24 hours after delivery in the intervention group, there are likely additional factors that contribute to the development of chronic pain after childbirth, including psychologic traits (anxiety, depression, catastrophizing) and degree of surgical trauma.
The overall incidence of postpartum depression, 10% in the control group and 13% in the intervention group, correlates with previous studies, which demonstrated an 11% incidence of depression at 8 weeks.2 Of note, 2 of the patients, 1 from each study arm, had both persistent pain and depression at 8 weeks. Patients also completed the EPDI preoperatively as a practice measure before completing the survey at 8 weeks. Although the EPDI has not been validated in the antenatal period, the incidence of depression, defined as a score > 12,9 was 13% in both the control and intervention groups (P = 1.0). Interestingly, the 4 patients in each group who met depression criteria using the EPDI in the preoperative setting did not score > 12 on the EPDI at 8 weeks.
Previous studies have shown a relationship between personality traits and dopaminergic neurotransmission that correlates with the placebo analgesic response in human volunteers.7 We therefore wanted to determine whether these dopamine-related traits affected opioid derived analgesia based on the motivational drive to obtain a reward. Since there is an anticipatory element to the morphine PCA, we wanted to see whether patients would be motivated to use the PCA to obtain opioid-mediated pain relief. Individual trait scores for behavioral drive and fun seeking, as measured by the BAS survey, did not correlate with total 24-hour IV morphine PCA use or number of IV PCA attempts. However, there was minimal variance in the BAS scores and in the IV morphine PCA scores, diminishing our ability to assess a correlation.
In summary, we show that using a multimodal postoperative pain regimen in patients predicted to be at risk for high acute postpartum pain after cesarean delivery results in a reduction of acute postoperative pain scores. We were unable to demonstrate a reduction in the incidence of persistent pain or depression at 8 weeks postpartum, although much larger studies in a broader population range are needed. Instead of implementing a universal postpartum pain protocol for women undergoing a cesarean delivery, preoperative identification of parturients at high risk for severe postpartum pain could lead to improved analgesia and overall patient satisfaction. Future studies should also address identifying patients likely to have low intensity of post–cesarean delivery pain and administering a tailored lower analgesic dose to reduce side effects from postoperative analgesia.
Acknowledgments
Funding: This study was supported by NIH GM048085 (Eisenach).
Footnotes
ACP Observer. Dosing and conversion chart for opioid analgesics. Available at: http://www.acpinternist.org/archives/2004/12/pain/clinical.htm. Accessed October 2013.
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
DISCLOSURES
Name: Jessica L. Booth, MD.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Attestation: Jessica L. Booth has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Lynnette C. Harris, BSN, CCRC.
Contribution: This author helped design the study, conduct the study, and analyze the data.
Attestation: Lynnette C. Harris has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: James C. Eisenach, MD.
Contribution: This author helped design the study, conduct the study, and analyze the data.
Attestation: James C. Eisenach has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Peter H. Pan, MD.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Attestation: Peter H. Pan has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
This manuscript was handled by: Cynthia A. Wong, MD.
References
- 1.Nikolajsen L, Sørensen HC, Jensen TS, Kehlet H. Chronic pain following Caesarean section. Acta Anaesthesiol Scand. 2004;48:111–6. doi: 10.1111/j.1399-6576.2004.00271.x. [DOI] [PubMed] [Google Scholar]
- 2.Eisenach JC, Pan PH, Smiley R, Lavand’homme P, Landau R, Houle TT. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140:87–94. doi: 10.1016/j.pain.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kainu JP, Sarvela J, Tiippana E, Halmesmäki E, Korttila KT. Persistent pain after caesarean section and vaginal birth: a cohort study. Int J Obstet Anesth. 2010;19:4–9. doi: 10.1016/j.ijoa.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Pan PH, Coghill R, Houle TT, Seid MH, Lindel WM, Parker RL, Washburn SA, Harris L, Eisenach JC. Multifactorial preoperative predictors for postcesarean section pain and analgesic requirement. Anesthesiology. 2006;104:417–25. doi: 10.1097/00000542-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Granot M, Lowenstein L, Yarnitsky D, Tamir A, Zimmer EZ. Postcesarean section pain prediction by preoperative experimental pain assessment. Anesthesiology. 2003;98:1422–6. doi: 10.1097/00000542-200306000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Pan PH, Tonidandel AM, Aschenbrenner CA, Houle TT, Harris LC, Eisenach JC. Predicting acute pain after cesarean delivery using three simple questions. Anesthesiology. 2013;118:1170–9. doi: 10.1097/ALN.0b013e31828e156f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. J Pers Soc Psychol. 1994;67:319–33. [Google Scholar]
- 8.Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC. The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. J Neurosci. 2009;29:4882–7. doi: 10.1523/JNEUROSCI.5634-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 10.Gadsden J, Hart S, Santos AC. Post-cesarean delivery analgesia. Anesth Analg. 2005;101:S62–9. doi: 10.1213/01.ANE.0000177100.08599.C8. [DOI] [PubMed] [Google Scholar]
- 11.Moore A, Costello J, Wieczorek P, Shah V, Taddio A, Carvalho JC. Gabapentin improves postcesarean delivery pain management: a randomized, placebo-controlled trial. Anesth Analg. 2011;112:167–73. doi: 10.1213/ANE.0b013e3181fdf5ee. [DOI] [PubMed] [Google Scholar]
- 12.Toms L, McQuay HJ, Derry S, Moore RA. Single dose oral paracetamol (acetaminophen) for postoperative pain in adults. Cochrane Database Syst Rev. 2008;4:CD004602. doi: 10.1002/14651858.CD004602.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swart M, Sewell J, Thomas D. Intrathecal morphine for caesarean section: an assessment of pain relief, satisfaction and side-effects. Anaesthesia. 1997;52:373–7. doi: 10.1111/j.1365-2044.1997.az0083c.x. [DOI] [PubMed] [Google Scholar]
- 14.Sarvela J, Halonen P, Soikkeli A, Korttila K. A double-blinded, randomized comparison of intrathecal and epidural morphine for elective cesarean delivery. Anesth Analg. 2002;95:436–40. doi: 10.1097/00000539-200208000-00037. [DOI] [PubMed] [Google Scholar]
- 15.Abouleish E, Rawal N, Tobon-Randall B, Rivera-Weiss M, Meyer B, Wu A, Rashad MN. A clinical and laboratory study to compare the addition of 0.2 mg of morphine, 0. 2 mg of epinephrine, or their combination to hyperbaric bupivacaine for spinal anesthesia in cesarean section. Anesth Analg. 1993;77:457–62. doi: 10.1213/00000539-199309000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Short J, Downey K, Bernstein P, Shah V, Carvalho JC. A single preoperative dose of gabapentin does not improve postcesarean delivery pain management: a randomized, double-blind, placebo-controlled dose-finding trial. Anesth Analg. 2012;115:1336–42. doi: 10.1213/ANE.0b013e31826ac3b9. [DOI] [PubMed] [Google Scholar]
- 17.McMorrow RC, Ni Mhuircheartaigh RJ, Ahmed KA, Aslani A, Ng SC, Conrick-Martin I, Dowling JJ, Gaffney A, Loughrey JP, McCaul CL. Comparison of transversus abdominis plane block vs spinal morphine for pain relief after Caesarean section. Br J Anaesth. 2011;106:706–12. doi: 10.1093/bja/aer061. [DOI] [PubMed] [Google Scholar]
- 18.Yang T, Breen TW, Archer D, Fick G. Comparison of 0.25 mg and 0. 1 mg intrathecal morphine for analgesia after Cesarean section. Can J Anaesth. 1999;46:856–60. doi: 10.1007/BF03012975. [DOI] [PubMed] [Google Scholar]
- 19.Palmer CM, Emerson S, Volgoropolous D, Alves D. Dose-response relationship of intrathecal morphine for postcesarean analgesia. Anesthesiology. 1999;90:437–44. doi: 10.1097/00000542-199902000-00018. [DOI] [PubMed] [Google Scholar]