Abstract
Background
20(S)-Protopanaxadiol 20-O-D-glucopyranoside, also called compound K (CK), exerts antidiabetic effects that are mediated by insulin secretion through adenosine triphosphate (ATP)-sensitive potassium (KATP) channels in pancreatic β-cells. However, the antidiabetic effects of CK may be limited because of its low bioavailability.
Methods
In this study, we aimed to enhance the antidiabetic activity and lower the toxicity of CK by including it with β-cyclodextrin (CD) (CD-CK), and to determine whether the CD-CK compound enhanced pancreatic islet recovery, compared to CK alone, in an alloxan-induced diabetic zebrafish model. Furthermore, we confirmed the toxicity of CD-CK relative to CK alone by morphological changes, mitochondrial damage, and TdT-UTP nick end labeling (TUNEL) assays, and determined the ratio between the toxic and therapeutic dose for both compounds to verify the relative safety of CK and CD-CK.
Results
The CD-CK conjugate (EC50 = 2.158μM) enhanced the recovery of pancreatic islets, compared to CK alone (EC50 = 7.221μM), as assessed in alloxan-induced diabetic zebrafish larvae. In addition, CD-CK (LC50 = 20.68μM) was less toxic than CK alone (LC50 = 14.24μM). The therapeutic index of CK and CD-CK was 1.98 and 9.58, respectively.
Conclusion
The CD-CK inclusion complex enhanced the recovery of damaged pancreatic islets in diabetic zebrafish. The CD-CK inclusion complex has potential as an effective antidiabetic efficacy with lower toxicity.
Keywords: β-cyclodextrin, adenosine triphosphate-sensitive potassium blocker, compound K, pancreatic islet, zebrafish larva
1. Introduction
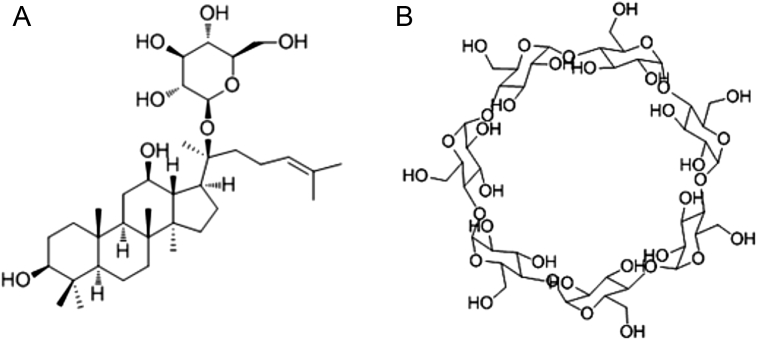
20(S)-Protopanaxadiol 20-O-D-glucopyranoside, also called compound K (CK, Fig. 1A), is a minor ginsenoside of Panax ginseng [1]. Compund K is the main metabolite obtained through biotransformation by human intestinal bacteria after the oral administration of protopanaxadiol (PPD)-ginsenosides [2], [3], [4]; therefore, CK is manufactured by the biodeglycosylation of PPD-ginsenosides [5]. Compound K has anticancer [6], [7], [8], anti-inflammatory [9], [10], [11], [12], and antidiabetic activity related to insulin secretion through adenosine triphosphate (ATP)-sensitive potassium (KATP) channels [13]. Compound K has low solubility in water; therefore, these bioactivity effects may be limited and many investigators have focused on increasing its solubility. For example, CK including in glycol chitosan increased its solubility and antitumor activity [14]. In addition, CK has been included in β-cyclodextrin (CD, Fig. 1B) to improve its bioavailability [15]. β-Cyclodextrin is a natural cyclic oligosaccharide formed from the enzymatic degradation of starch by bacteria; it has a hydrophilic outer surface and a hydrophobic central cavity that can be used to solubilize compounds with less polarity [16]. Therefore, CD is widely used to improve the dissolution of less polar compounds and drugs [17], [18]. In addition, CD is nontoxic and has good stability [19], [20], [21]. A systematic assessment of CK toxicity has not been reported; however, CK is cytotoxic against tumor cells, and subchronic toxicity studies [22], [23] suggest it may be hepatotoxic. In this study, we aimed to enhance the antidiabetic activity and lower the toxicity of CK by including it with CD (CD-CK), and to determine whether the CD-CK compound enhances pancreatic islet recovery, compared to CK alone, in an alloxan-induced diabetic zebrafish model. To investigate this mechanism, we used glimepiride (GLM) and diazoxide, which affect the KATP channels. Furthermore, we confirmed the toxicity of CD-CK relative to CK alone and determined the ratio between the toxic and therapeutic dose for both compounds to verify the relative safety of CK and CD-CK.
Fig. 1.
The molecular structure of the compounds used in the study. (A) 20(S)-protopanaxadiol 20-O-D-glucopyranoside (i.e., compound K). (B) β-cyclodextrin.
2. Materials and methods
2.1. Reagents and equipment
β-Cyclodextrin was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan) and 20(S)-protonaxadiol 20-O-D-glucopyranoside (compound K) was obtained from Climax Biotech Co., Ltd. (Chengdu, China). Alloxan monohydrate and sea salts were obtained from Sigma Chemical Co. (St. Louis, MO, USA), and 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) was purchased from Invitrogen Co., Ltd. (Grand Island, NY, USA). Glimepiride and diazoxide were purchased from Cayman Chemical Co. (Ann Arbor, MI, USA) and Santa Cruz Biotechnology Inc. (Dallas, TX, USA), respectively. Fluorescence microscopy was conducted using an Olympus 1X70 microscope (Olympus Co., Ltd., Tokyo, Japan). Focus Lite (Focus Co, Daejeon, Korea) and Image J (National Institutes of Health, Bethesda, MD, USA) software were used for image analysis.
2.2. β-Cyclodextrin-compound K (CD-CK) inclusion complex
Compound K (CK) (1.3 mg) was added to 2 mL of 10 mM CD in water solution and stirred for 20 h. After equilibrium, the sample was filtered through a 0.45-μm membrane filter. Compound K at 1mM in dimethyl sulfoxide (DMSO) was prepared as the reference sample.
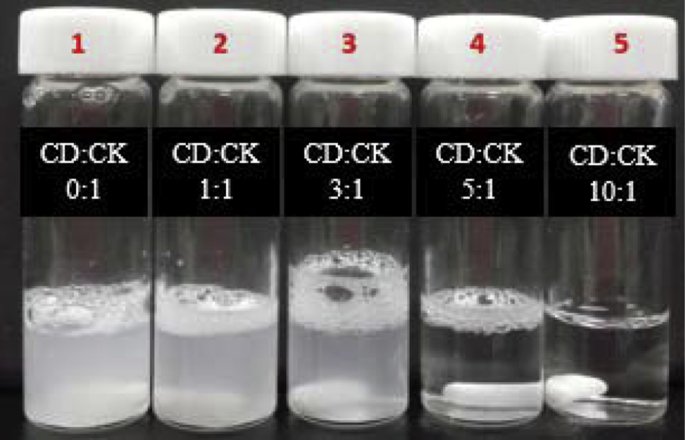
2.3. Turbidity study
Turbidity changes, based on the molar ratio of CD to CK (i.e., 0:1, 1:1, 3:1, 5:1, 10:1), were determined by photography after 20 h of stirring at room temperature.
2.4. Zebrafish maintenance and egg collection
Adult zebrafish were maintained in a zebrafish S-type housing system [1500 mm (width) × 400 mm (depth) × 2050 mm (height); Genomic Design Bioengineering Co., Daejeon, Korea]. Two pairs of zebrafish were placed in a spawning box overnight. The next day, the zebrafish initiated spawning after a 30-min light period. Zebrafish eggs were collected 3 h postfertilization (hpf) and incubated in Petri dishes in a 0.03% sea salt solution. Embryos were maintained in a 14-h light:10-h dark photoperiod in an incubator at 28.5°C. Zebrafish were cared for in accordance with standard zebrafish protocols approved by the Animal Care and Use Committee of Kyung Hee University (Seoul, Korea).
2.5. Compound K and CD-CK efficacy on alloxan-induced pancreatic islet damage zebrafish larvae
We evaluated CK and CD-CK using zebrafish larvae. Wild-type zebrafish larvae 5 d postfertilization (dpf) were placed into a 96-well plate. The larvae were exposed to 25μM 2-NBDG for 12 h and rinsed three times using 0.03% sea salt solution. To induce pancreatic islet damage, we used alloxan at a concentration of 100μM. After 6 h of alloxan treatment, the larvae were stained for 1 h with 25μM 2-NBDG. The larvae were mounted in 96-well plates, and observed using fluorescence microscopy. To determine the efficacy of CK, CD-CK, and GLM as a positive control, zebrafish were treated with 5μM CK, CD-CK, or GLM for 1 h, after which the larvae were restained for 1 h with 25μM 2-NBDG and observed under fluorescence microscopy. All captured images were analyzed for pancreatic islet size and histogram using Focus Lite and Image J Software. Before and after treatment, we analyzed pancreatic islet size via images. All values are expressed as the size change value, based on the following equation: size change value = post-treatment pancreatic islet size – pretreatment pancreatic islet size.
2.6. The 50% effective concentration
Zebrafish were treated with nine different concentrations (i.e., 0.05μM, 0.1μM, 0.5μM, 1.0μM, 2.5μM, 5.0μM, 7.5μM, 10.0μM, and 15.0μM) of CK alone or CD-CK. The 50% effective concentration (EC50) values were calculated by nonlinear regression using GraphPad Prism version 5.01 software (Graph Pad Software, San Diego, CA, USA).
2.7. Action of diazoxide on alloxan-induced pancreatic islet-damaged zebrafish larvae
Wild-type zebrafish larvae 5 dpf were divided into the following seven groups: normal, 100μM alloxan, 25μM diazoxide as a negative control, 5μM CK, 5μM CK + 25μM diazoxide, 5μM CD-CK, and 5μM CD-CK + 25μM diazoxide. Zebrafish larvae were treated with 25μM 2-NBDG for 12 h. To damage the pancreatic islets, zebrafish larvae were treated with 100μM alloxan and stained with 25μM 2-NBDG for 1 h. Fluorescence microscopy images were then obtained. After first capture, zebrafish larvae were treated with CK and CD-CK with diazoxide. Second images were captured by fluorescence microscopy. All images were analyzed using Focus Lite and Image J software.
2.8. Identification of the development of toxicity related to CK and CD-CK
Twenty zebrafish embryos were chosen to undergo treatment for toxic testing. Embryos were placed in six-well plates, which were incubated under temperature control at 28.5°C and a 14-h light:10-h dark photoperiod. Sixteen treatments were used: control, CD, CD-CK, and GLM at 5.0μM, 10.0μM, 15.0μM, 20.0μM and 25.0μM concentrations, respectively. The embryos were observed under microscopy at 2 d post-treatment (dpt) and dead embryos were recorded. We evaluated the survival rate, heartbeat, and body length.
2.9. Efficacy of CK and CD-CK in expressing enhanced green fluorescent protein fused to the mitochondrial localization sequence of zebrafish COXVIII
Enhanced green fluorescent protein (EGFP) fused to the mitochondrial localization sequence (MLS) of zebrafish COXVIII (MLS-EGFP) zebrafish larvae were obtained from Genomic Design Bioengineering Co. (Daejeon, Korea). The MLS-EGFP zebrafish larvae at 3 dpf were treated with CK and CD-CK at a 20μM concentration. After 48 h treatment, MLS-EGFP zebrafish larvae were observed under fluorescent microscopy and all images were analyzed using Focus Lite and Image J software.
2.10. TdT-UTP nick end labeling assay
Wild-type zebrafish larvae at 3 dpf were treated with 20μM CK or CD-CK. After 48 h treatment, zebrafish larvae were fixed in fresh 4% paraformaldehyde in phosphate-buffered saline (PBS) with 0.05% Tween 20 (PBST) overnight at 4°C, dehydrated with methanol, stored overnight at −20°C, and rehydrated using decreasing concentrations of methanol in PBST. A TdT-UTP nick end labeling (TUNEL) assay was performed using the DeadEnd Fluorometric TUNEL System (Promega Ltd., Madison, WI, USA). Fluorescent TUNEL-positive cells were detected and counted under a fluorescent microscope.
2.11. The 50% lethal concentration values of CD and CD-CK
Zebrafish were treated with six different concentrations (i.e., 5μM, 10μM, 15μM, 20μM, 25μM, and 30μM) of CD or CD-CK. The 50% lethal concentration (LC50) values were calculated by nonlinear regression using GraphPad Prism version 5.01 software.
2.12. Therapeutic index
The therapeutic index (TI) (also called the therapeutic window or safety margin) is the ratio between the toxic dose and the therapeutic dose of a drug. It is used to measure the relative safety of the drug for a particular treatment. We calculated the TI by the following equation: TI = LC50/EC50.
2.13. Statistical analysis
Statistical analysis was performed using GraphPad Prism software (version 5.01). The data are expressed as the mean ± standard error of mean and significance was determined using repeated one-way analysis of variance (ANOVA) followed by Tukey's test. The probability level for statistical significance was p < 0.05.
3. Results
3.1. Turbidity study
To evaluate the turbidity of CK by CD, we confirmed that the vials (1–5) had a molar ratio of CD to CK at 0:1, 1:1, 3:1, 5:1, 10:1, respectively. Bubbles were observed in vials 1–4 and the vials contained free saponin in solution. Vial 5 contained a clear solution without any bubbles and had a complete CD-CK inclusion complex (Fig. 2).
Fig. 2.
Turbidity changes in accordance with the concentration of β-cyclodextrin (CD) to compound K (CK).
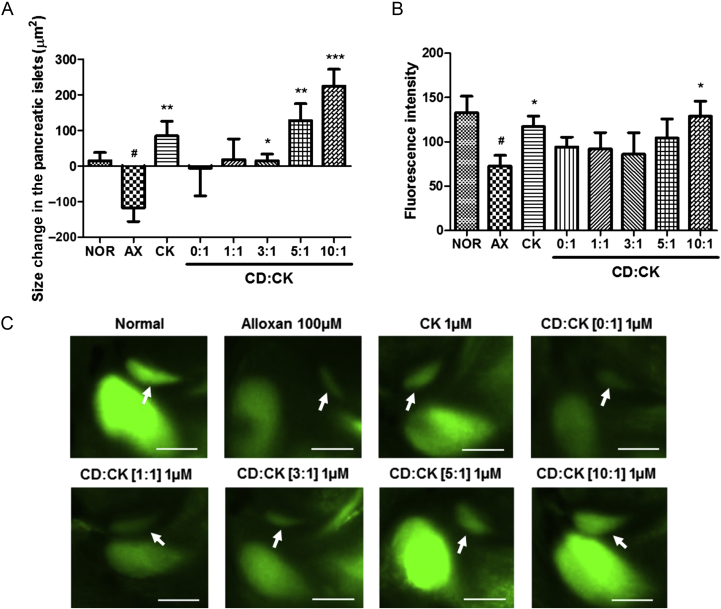
3.2. Optimal CD-CK inclusion complex
To evaluate the bioavailability of CD-CK, damaged zebrafish pancreatic islets were treated with different molar ratios of CD to CK (0:1, 1:1, 3:1, 5:1, and 10:1) and CK in DMSO as the control. Pancreatic islet size in the alloxan-treated group was significantly decreased by 8.81-fold (p = 0.0112), compared to the normal group. The CK-treated group had a 1.72-fold increase in the islet size (p = 0.0045), compared to the alloxan-treated group. Pancreatic islet size in the CD:CK [0:1]- and [1:1]-treated groups was increased by 0.95-fold (p = 0.2700) and 1.16-fold (p = 0.0735), respectively, compared to the alloxan-treated group. In addition, the CD:CK [3:1]-, [5:1]-, and [10:1]-treated groups had a significant increase in the pancreatic size of 1.19-fold (p = 0.0303), 2.09-fold (p = 0.0002), and 2.92-fold (p = 0.0112), respectively (Figs. 3A and 3C).
Fig. 3.
The effects of various ratios of β-cyclodextrin included in compound K (CD-CK) in alloxan-induced pancreatic islet-damaged zebrafish. (A) The size change in the pancreatic islets in each group. (B) Fluorescence intensity analysis of the zebrafish pancreatic islets. (C) Representative final pancreatic islet images of the normal (NOR), alloxan (AX), compound K (CK), β-cyclodextrin (CD):CK [0:1], CD:CK [1:1], CD:CK [3:1], CD:CK [5:1] and CD:CK [10:1] groups. Pancreatic islet stained with 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose. The white arrow indicates the location of pancreatic islet. Scale bar = 100 μm. #p < 0.05; compared to the normal group. * p < 0.05. ** p < 0.01, and *** p < 0.001, compared to the alloxan group.
To investigate the histogram of pancreatic islets, we used Image J software. The histogram is a count of pixel values that indicate fluorescence intensity (green color) ranging from level 0 to level 255. The green level in the alloxan-treated group (the mean count of pixels = 72, p = 0.0162) was significantly decreased, compared with that of the normal group (the mean count of pixels = 132). The CK-treated (the mean count of pixels = 117, p = 0.0213) and CD:CK [10:1]-treated groups (the mean count of pixels = 128, p = 0.0183) were significantly increased, compared to the alloxan-treated group (Figs. 3B and 3C).
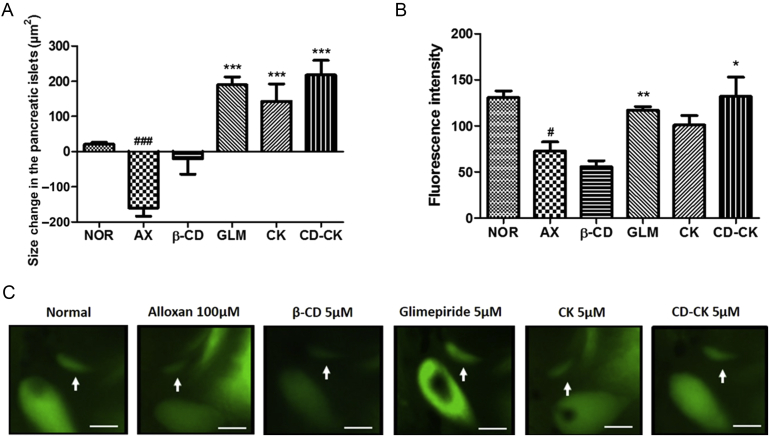
3.3. The effect CK and CD-CK on alloxan-induced pancreatic islet-damaged zebrafish larvae
To evaluate the efficacy of CK and CD-CK, we investigated the pancreatic islets after alloxan treatment with GLM, CD, CK and CD-CK at 5μM. As the positive control, GLM promoted insulin secretion by closing K+ATP channels [24]. The pancreatic islet size in the alloxan-treated group was significantly decreased by 8.54-fold (p < 0.0001), compared to the normal group. The GLM-treated group had a 2.19-fold increase in the islet size (p < 0.0001), compared to the alloxan-treated group. The CD-treated group had a 0.87-fold increase in the pancreatic size (p = 0.0121), compared to the alloxan-treated group. In addition, the CK-treated and CD-CK-treated groups had a 1.89-fold (p < 0.0001) and 2.36-fold (p < 0.0001) increase, respectively, in the pancreatic size, which was significant (Fig. 4A).
Fig. 4.
The effects of 5μM compound K (CK) and β-cyclodextrin included in compound K (CD-CK) in alloxan-induced pancreatic islet-damaged zebrafish. (A) The size change in the pancreatic islets in each group. (B) Fluorescence intensity analysis in the pancreatic islets of zebrafish. (C) Representative final pancreatic islet images of the normal (NOR), alloxan (AX), β-cyclodextrin (β-CD), glimepiride (GLM), CK, and CD-CK groups. The pancreatic islets are stained with 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose. The white arrow indicates the location of pancreatic islet. Scale bar = 100 μm. #p < 0.05 and ###p < 0.001, compared to the normal group. * p < 0.05, ** p < 0.01, and *** p < 0.001, compared to the alloxan group.
Moreover, we measured fluorescence intensity under these same treatments. The green level in the alloxan-treated group (the mean count of pixels = 72, p = 0.0014) was significantly decreased, compared to the normal group (the mean count of pixels = 130). The GLM-treated group was significantly increased (the mean count of pixels = 117, p = 0.0037), compared to the alloxan-treated group. The CK-treated group was increased (the mean count of pixels = 100, p = 0.0818), compared to the alloxan-treated group. The CD-CK-treated group was significantly increased (the mean count of pixels = 132, p = 0.0292), compared to the alloxan-treated group (Fig. 4B). The CD-CK-treated group had a similar recovery pattern as that of the GLM-treated group.
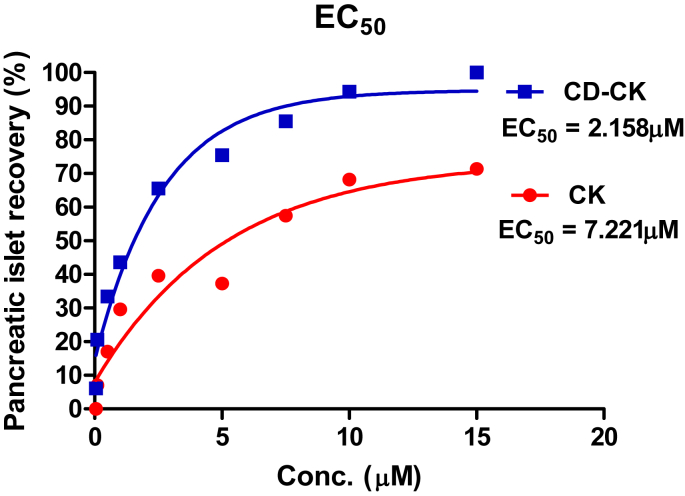
3.4. The EC50 values of CK and CD-CK
To evaluate the EC50, we generated a dose-effect curve using zebrafish treated with CK and CD-CK at nine different CK and CD-CK concentrations. The EC50 values of CK and CD-CK were calculated at 7.221μM and 2.158μM, respectively (Fig. 5).
Fig. 5.
The dose-effect curves of compound K (CK) and β-cyclodextrin included in compound K (CD-CK). The EC50 of CK was 7.221μM, and the EC50 of CD-CK was 2.158μM. Conc., concentration; EC50, 50% effective concentration.
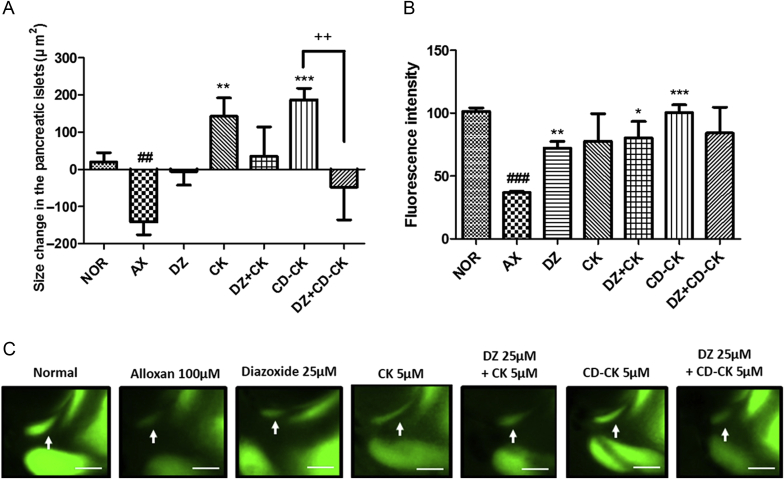
3.5. Action of diazoxide on alloxan-induced pancreatic islet-damaged zebrafish larvae
To evaluate the involvement of CK and CD-CK in pancreatic β-cell KATP channels, we investigated pancreatic islet size after diazoxide treatment. Diazoxide inhibits insulin secretion as a KATP channel opener in the prediabetic and diabetic state [25]. During diazoxide treatment, the pancreatic islet size of the CK with the diazoxide-treated group was decreased by 0.76-fold (p = 0.2437) compared to the CK-treated group. Furthermore, the CD-CK with diazoxide-treated group was significantly decreased by 1.25-fold (p = 0.0099), compared to the CD-CK-treated group (Fig. 6A). However, the fluorescence intensity of the pancreatic islets did not show different changes during diazoxide treatment (Fig. 6B).
Fig. 6.
The effects of β-cyclodextrin included in compound K (CD-CK) and CK (compound K) with diazoxide (DZ) in alloxan-induced pancreatic islet-damaged zebrafish. (A) The size change in the pancreatic islets in each group. (B) Fluorescence intensity analysis in the pancreatic islets of the zebrafish. (C) Representative final pancreatic islet images of the normal (NOR), alloxan (AX), DZ, CK, CK with DZ, CD-CK, and CD-CK with DZ groups. Pancreatic islets are stained with 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose. The white arrow indicates the location of pancreatic islet. Scale bar = 100 μm. ##p < 0.01, ###p < 0.001 compared to normal. * p < 0.05, ** p < 0.01, *** p < 0.001 compared to alloxan. ++ p < 0.01.
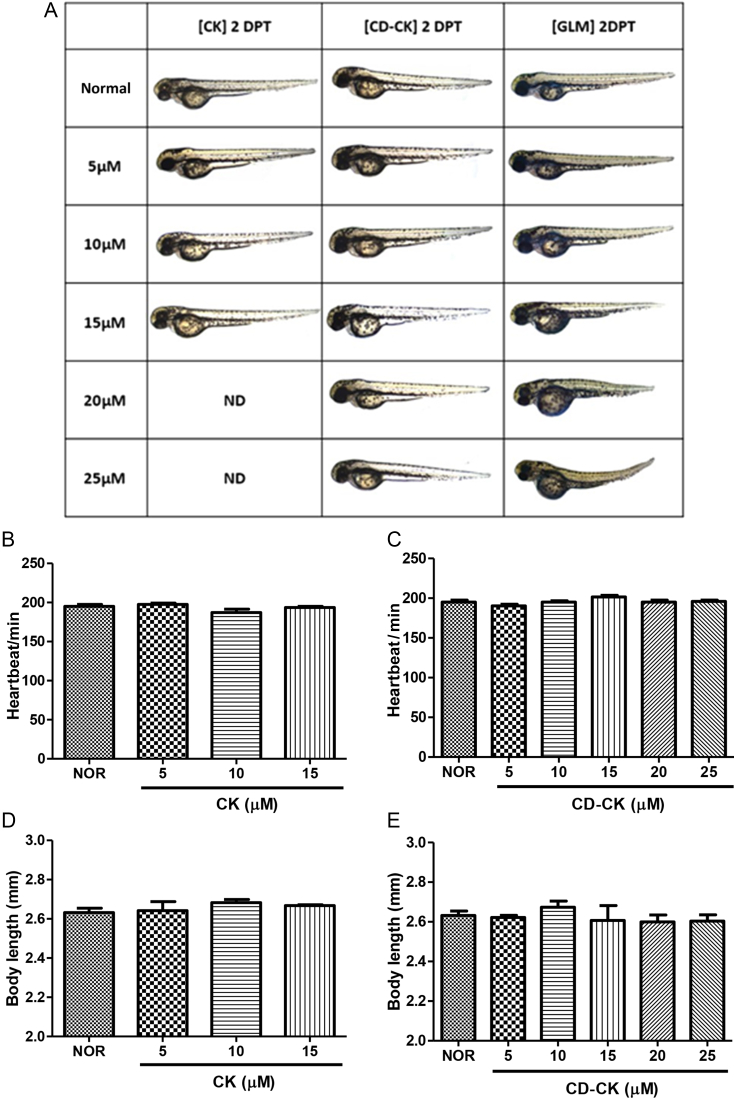
3.6. Toxicity test
We investigated morphological changes, heartbeat, and body length of the zebrafish embryos to evaluate the toxicity of CK and CD-CK. We did not observe morphological changes during CD-CK-treatment. However, 20.0μM CK-treated zebrafish had a mortality rate of 100% (Fig. 7A). The GLM-treated zebrafish had bent spines and yolk sac edema at a 20.0μM concentration. Furthermore, zebrafish treated with 25.0μM GLM did not develop eyes or heads, and also developed bent spines. At 2 dpt, there was no significant difference between CK- and CD-CK-treated zebrafish in the heartbeat and body length, compared to the normal group (Figs. 7B–7E).
Fig. 7.
The toxicity of compound K (CK), β-cyclodextrin included in compound K (CD-CK), and glimepiride (GLM), based on zebrafish embryo testing. (A) Comparison of the toxicity results of CK, CD-CK, and GLM on zebrafish embryonic development. The data are 2 d post-treatment (dpt) statistics. (B) The heartbeat/min in zebrafish treated with CK at 2 dpt. (C) The heartbeat/min in zebrafish treated with CD-CK at 2 dpt. (D) The body length of zebrafish treated with CK at 2 dpt. (E) The body length of zebrafish treated with CD-CK at 2 dpt. ND, not determined.
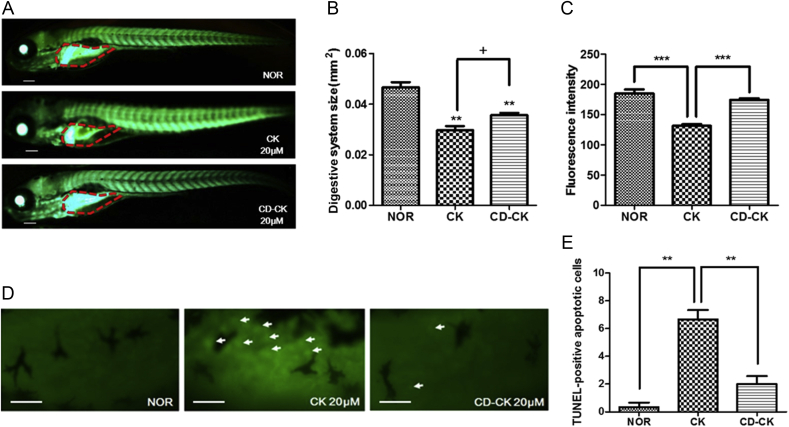
3.7. Toxicity of CK and CD-CK in MLS-EGFP
To evaluate mitochondrial changes due to CK and CD-CK, MLS-EGFP zebrafish larvae were treated with 20μM CK and 20μM CD-CK. The digestive system size of the CK-treated group was significantly decreased by 36.43% (p = 0.0029), compared to the normal group. The CD-CK-treated group was significantly decreased by 23.57% (p = 0.0076), compared to the normal group. There was also a significant size difference in the digestive system between the CK and CD-CK-treated groups, whereby the CK-treated group size was decreased by 16.82% (p = 0.0335) relative to the CD-CK-treated group (Figs. 8A and 8B).
Fig. 8.
The toxicity of compound K (CK) and β-cyclodextrin included in compound K (CD-CK) in enhanced green fluorescent protein (EGFP) expression fused to the mitochondrial localization sequence (MLS) of zebrafish COXVIII (MLS-EGFP) and TUNEL assays of CK- and CD-CK-treated digestive systems in zebrafish larvae. (A) Representative whole zebrafish images. The red dotted line indicates the digestive system. Scale bar = 100 μm. (B) Size of the digestive system analysis. (C) The fluorescence intensity analysis. (D) The white arrow indicates the location of apoptosis in the digestive system. Scale bar = 50 μm. (E) Quantification of TUNEL-positive apoptosis cells. TUNEL, TdT-UTP nick end labeling. ** p < 0.01 compared to the normal (NOR) group. *** p < 0.001. + p < 0.05.
The digestive system of the CK-treated group (mean number of pixels = 131; p = 0.0018 and p = 0.0002, respectively) had significantly decreased fluorescence intensity, compared to the normal group (the mean count of pixels = 184) and the CD-CK-treated group (the mean count of pixels = 174) (Figs. 8A and 8C). These results indicate that CK decreased the expression of mitochondria in the digestive system of treated zebrafish. In addition, CD-CK suppressed the mitochondrial damage incurred by CK alone.
3.8. The TUNEL assays of CK- and CD-CK-treated digestive systems in zebrafish larvae
We examined apoptosis of the digestive system using the TUNEL assay. The TUNEL staining of the digestive system showed apoptotic cells in the CK- and CD-CK-treated zebrafish larvae. There were differences in the TUNEL-positive apoptotic cells. The CK-treated zebrafish larvae had an increased number of TUNEL-positive cells, compared to the CD-CK-treated zebrafish larvae (Figs. 8D and 8E).
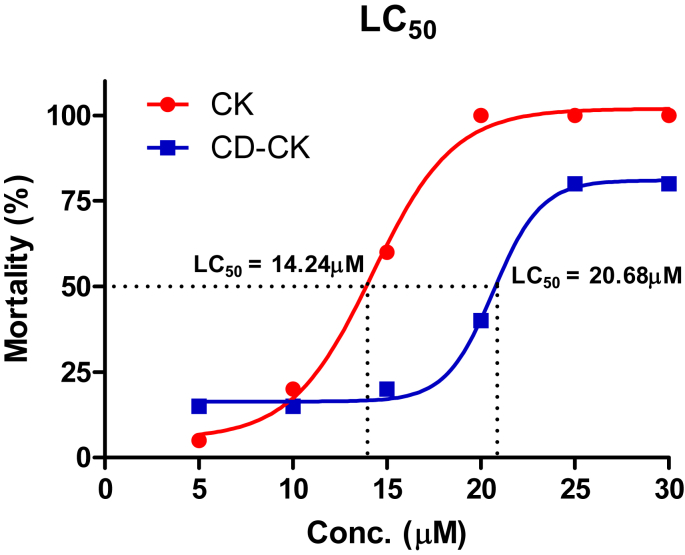
3.9. The LC50 values of CK and CD-CK
To evaluate LC50, we investigated mortality related to CK and CD-CK in zebrafish. Zebrafish were treated with CK and CD-CK at six different concentrations. The LC50 values of CK and CD-CK were calculated at 14.24μM and 20.68μM, respectively (Fig. 9).
Fig. 9.
The LC50 of zebrafish embryos exposed to compound K (CK) and β-cyclodextrin included in compound K (CD-CK) for 48 h. The LC50 of CK was 14.24μM. The LC50 of CD-CK was 20.68μM. Conc., concentration; EC50, 50% effective concentration; LC50, 50% lethal concentration.
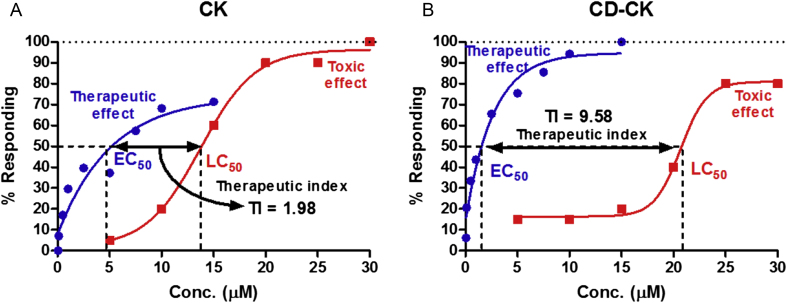
3.10. Therapeutic index
The TI of CK and CD-CK were calculated by LC50/EC50. The TI is an index of drug safety: safer drugs have a higher TI. The TI of CK and CD-CK were calculated at 1.98 and 9.58, respectively (Fig. 10).
Fig. 10.
The therapeutic index (TI) of compound K (CK) and β-cyclodextrin conjugated with compound K (CD-CK). The TI of CK and CD-CK was 1.98 and 9.58, respectively. Conc., concentration; EC50, 50% effective concentration.
4. Discussion
In our study, CD included in CK (CD-CK) enhanced bioavailability through recovery of pancreatic islet damage in an alloxan-induced diabetes zebrafish model. Alloxan causes pancreatic β-cell necrosis, which decreases β-cell mass, blocks insulin secretion, and thereby induces diabetes [26], [27], [28]. We previously reported a decreased size in the pancreatic islets and β-cells in alloxan-induced diabetic zebrafish, which verified zebrafish as a diabetes model [29]. We therefore used alloxan-induced diabetes zebrafish and treated them with GLM, CD, CD-CK, and CK. Glimepiride was the positive control because it promotes insulin secretion by closing K+ATP channels and permits calcium (Ca2+) inflow [24]. These processes involve glucose utilization at the cellular level and regulate blood glucose level in various tissues [30]. Compound K shows antidiabetic activity by insulin secretion in β-cells, which acts on the K+ATP channels and improves insulin sensitivity [13]. The CK-treated group showed recovery of damaged pancreatic islets and the CD-CK-treated group exhibited a significantly higher recovery, compared to the CK-treated group, and improves the antidiabetic effect of CK. This finding is consistent with previous CK treatment results in type 2 diabetic mice and MIN6 β-cells [31].
Based on these results, we investigated the pathway of insulin secretion by modulating KATP channels. Closure of the KATP channels leads to membrane depolarization, the opening of voltage-gated Ca2+ channels, and Ca2+ inflow and increase, which result in insulin secretion [32], [33]. Many diabetic models use diazoxide to open KATP channels, which inhibits insulin secretion in alloxan-induced diabetic mice and zebrafish [29], [34]. Compound K increases insulin secretion by the KATP channel pathway in HIT-T15 cells, which have decreased insulin secretion with cotreatment with diazoxide [13]. Therefore, we investigated how CD-CK enhanced insulin secretion in diabetic zebrafish using diazoxide and CD-CK with diazoxide cotreated zebrafish exhibiting significantly decreased pancreatic islet size, compared to CD-CK-treated zebrafish. These results suggest that CD-CK may stimulate insulin secretion by closing KATP channels in pancreatic β-cells.
To verify the improved bioavailability of CD-CK, we investigated the EC50 in the recovery effect on alloxan-related pancreatic islet damage because CD-included compounds remove toxicity and increase the rate of compound absorption [19], [21]. The EC50 value of CD-CK was 2.158μM, which showed that CD-CK has a recovery effect on damaged pancreatic islets at lower concentrations, compared to CK alone (EC50 value 7.221μM). In addition, the heartbeat and body length of CD-CK- and CK-treated zebrafish indicated no toxicity. We also calculated the LC50 by the zebrafish survival rate. Our results demonstrated that CD-CK had better antidiabetic effects and lower toxicity, compared to CK, with LC50 values 20.68μM and 14.24μM, respectively.
Systematic toxicity of CK has not been reported; however, in a subchronic toxicity study [22], CK showed possible hepatotoxicity. In addition, CK cytotoxicity against tumor cells has been reported [23]. The relationship between the EC50 and LC50 can be calculated as the TI, which was used in quantitative comparisons of drugs as the ratio of LC50 to EC50 [35]. The larger the TI, the safer the drug. However, if the TI is small, the drug must be dosed carefully and the patient receiving the drug should be monitored closely for any signs of drug toxicity [35]. In this study, we demonstrated that CD-CK is a safer drug, relative to CK, through the TI.
The mechanism for CK toxicity is by mitochondrial damage. Mitochondria are very sensitive to endogenous and exogenous environmental stresses such as toxicants, iatrogenic medications, immune activation, and metabolic disorders [36]. For CD-CK, the expression of mitochondria was not changed, compared to the control, but the CK-treated zebrafish showed decreased mitochondrial expression. Therefore, CK toxicity may be related to mitochondrial damage. In addition, the TUNEL assays showed increased apoptosis in the digestive system of CK-treated zebrafish. Such aberrant apoptosis may contribute to the consequent morphologic anomalies and damage of mitochondria in CK-treated animals.
In conclusion, CD-CK inclusion complexes exhibit improved solubility. We determined enhanced antidiabetic efficacy and lowered toxicity of CD-CK relative to CK treatment alone.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
This research was supported by Basic Science Research program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (Seoul, Korea; grant number, NRF-2015R1D1A1A09060469). This work was supported by a grant from Kyung Hee University (Seoul, Korea) in 2015 (Grant No. KHU-20150739).
Contributor Information
Tae Woo Kim, Email: tw1275@khu.ac.kr.
Tong Ho Kang, Email: panjae@khu.ac.kr.
References
- 1.Liu C.Y., Zhou R.X., Sun C.K., Jin Y.H., Yu H.S., Zhang T.Y., Xu L.Q., Jin F.X. Preparation of minor ginsenosides C-Mc, CY, F2, and CK from American ginseng PPD-ginsenoside using the special ginsenosidase type-I from Aspergillus Niger g.848. J Ginseng Res. 2015;39:221–229. doi: 10.1016/j.jgr.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae E.A., Choo M.K., Park E.K., Park S.Y., Shin H.Y., Kim D.H. Metabolism of ginsenoside R (c) by human intestinal bacteria and its related antiallergic activity. Biol Pharm Bull. 2002;25:743–747. doi: 10.1248/bpb.25.743. [DOI] [PubMed] [Google Scholar]
- 3.Yan X., Fan Y., Wei W., Wang P., Liu Q., Wei Y., Zhang L., Zhou G., Yue J., Zhou Z. Production of bioactive ginsenoside compound K in metabolically engineered yeast. Cell Res. 2014;24:770–773. doi: 10.1038/cr.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu L., Jin Y., Yin C., Bai L. Co-transformation of Panax major ginsenosides Rb1 and Rg1 to minor ginsenosides C–K and F1 by Cladosporium cladosporioides. J Ind Microbiol Biotechnol. 2012;39:521–527. doi: 10.1007/s10295-011-1058-9. [DOI] [PubMed] [Google Scholar]
- 5.Zhou W., Yan Q., Li J.Y., Zhang X.C., Zhou P. Biotransformation of Panax notoginseng saponins into ginsenoside compound K production by Paecilomyces bainier sp 229. J Appl Microbiol. 2008;104:699–706. doi: 10.1111/j.1365-2672.2007.03586.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim D.Y., Park M.W., Yuan H.D., Lee H.J., Kim S.H., Chung S.H. Compound K induces apoptosis via CAMK-IV/AMPK pathways in HT-29 colon cancer cells. J Agric Food Chem. 2009;57:10573–10578. doi: 10.1021/jf902700h. [DOI] [PubMed] [Google Scholar]
- 7.Lee I.K., Kang K.A., Lim C.M., Kim K.C., Kim H.S., Kim D.H., Kim B.J., Chang W.Y., Choi J.H., Hyun J.W. Compound K, a metabolite of ginseng saponin, induces mitochondria-dependent and caspase-dependent apoptosis via the generation of reactive oxygen species in human colon cancer cells. Int J Mol Sci. 2010;11(12):4916–4931. doi: 10.3390/ijms11124916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim A.D., Kang K.A., Kim H.S., Kim D.H., Choi Y.H., Lee S.J., Hyun J.W. A ginseng metabolite, compound K, induces autophagy and apoptosis via generation of reactive oxygen species and activation of JNK in human colon cancer cells. Cell Death Dis. 2013;4:e750. doi: 10.1038/cddis.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joh E.H., Lee I.A., Jung I.H., Kim D.H. Ginsenoside Rb1 and its metabolite compound K inhibit IRAK–1 activation—the key step of inflammation. Biochem Pharmacol. 2011;82:278–286. doi: 10.1016/j.bcp.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Chen W., Wang J., Luo Y., Wang T., Li X., Li A., Li J., Liu B. Ginsenoside Rb1 and compound K improve insulin signaling and inhibit ER stress-associated NLRP3 inflammasome activation in adipose tissue. J Ginseng Res. 2015;40:351–358. doi: 10.1016/j.jgr.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hossen M.J., Hong Y.D., Baek K.S., Yoo S., Hong Y.H., Kim J.H., Lee J.O., Kim D., Park J., Cho J.Y. In vitro antioxidative and anti-inflammatory effects of the compound K-rich fraction BIOGF1K, prepared from Panax ginseng. J Ginseng Res. 2017;41:43–51. doi: 10.1016/j.jgr.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim D.Y., Ro J.Y., Lee C.H. 20 (S)-Protopanaxatriol inhibits release of inflammatory mediators in immunoglobulin E-mediated mast cell activation. J Ginseng Res. 2015;39:189–198. doi: 10.1016/j.jgr.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han G.C., Ko S.K., Sung J.H., Chung S.H. Compound K enhances insulin secretion with beneficial metabolic effects in db/db mice. J Agric Food Chem. 2007;55:10641–10648. doi: 10.1021/jf0722598. [DOI] [PubMed] [Google Scholar]
- 14.Mathiyalagan R., Subramaniyam S., Kim Y.J., Kim Y.C., Yang D.C. Ginsenoside compound K-bearing glycol chitosan conjugates: synthesis, physicochemical characterization, and in vitro biological studies. Carbohydr Polym. 2014;112:359–366. doi: 10.1016/j.carbpol.2014.05.098. [DOI] [PubMed] [Google Scholar]
- 15.Lee P.S., Han J.Y., Song T.W., Sung J.H., Kwon O.S., Song S., Chung Y.B. Physicochemical characteristics and bioavailability of a novel intestinal metabolite of ginseng saponin (IH901) complexed with β-cyclodextrin. Int J Pharm. 2006;316:29–36. doi: 10.1016/j.ijpharm.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 16.Wang X., Brusseau M.L. Solubilization of some low-polarity organic compounds by hydroxypropyl-.beta.-cyclodextrin. Environ Sci Technol. 1993;27:2821–2825. [Google Scholar]
- 17.Pitha J., Pitha J. Amorphous water-soluble derivatives of cyclodextrins: nontoxic dissolution enhancing excipients. J Pharm Sci. 1985;74:987–990. doi: 10.1002/jps.2600740916. [DOI] [PubMed] [Google Scholar]
- 18.Samperio C., Boyer R., Eigel W.N., III, Holland K.W., McKinney J.S., O'Keefe S.F., Smith R., Marcy J.E. Enhancement of plant essential oils' aqueous solubility and stability using alpha and beta cyclodextrin. J Agric Food Chem. 2010;58:12950–12956. doi: 10.1021/jf103275a. [DOI] [PubMed] [Google Scholar]
- 19.Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem Rev. 1998;98:1743–1754. doi: 10.1021/cr970022c. [DOI] [PubMed] [Google Scholar]
- 20.Irie T., Uekama K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J Pharm Sci. 1997;86:147–162. doi: 10.1021/js960213f. [DOI] [PubMed] [Google Scholar]
- 21.Loftsson T., Jarho P., Masson M., Järvinen T. Cyclodextrins in drug delivery. Expert Opin Drug Deliv. 2005;2:335–351. doi: 10.1517/17425247.2.1.335. [DOI] [PubMed] [Google Scholar]
- 22.Gao Y.L., Liu Z.F., Li C.M., Shen J.Y., Yin H.X., Li G.S. Subchronic toxicity studies with ginsenoside compound K delivered to dogs via intravenous administration. Food Chem Toxicol. 2011;49:1857–1862. doi: 10.1016/j.fct.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Shin J.E., Park E.K., Kim E.J., Hong Y.H., Lee K.T., Kim D.H. Cytotoxicity of compound K (IH–901) and ginsenoside Rh2, main biotransformants of ginseng saponins by bifidobacteria, against some tumor cells. J Ginseng Res. 2003;27:129–134. [Google Scholar]
- 24.Klepzig H., Kober G., Matter C., Luus H., Schneider H., Boedeker K.H., Kiowski W., Amann K.W., Gruber D., Harris S. Sulfonylureas and ischaemic preconditioning; a double-blind, placebo-controlled evaluation of glimepiride and glibenclamide. Eur Heart J. 1999;20:439–446. doi: 10.1053/euhj.1998.1242. [DOI] [PubMed] [Google Scholar]
- 25.Huang Q., Bu S., Yu Y., Guo Z., Ghatnekar G., Bu M., Yang L., Lu B., Feng Z., Lin S. Diazoxide prevents diabetes through inhibiting pancreatic β-cells from apoptosis via Bcl–2/Bax rate and p38-β mitogen-activated protein kinase. J Endocrinol. 2007;148:81–91. doi: 10.1210/en.2006-0738. [DOI] [PubMed] [Google Scholar]
- 26.Desgraz R., Bonal C., Herrera P.L. β-cell regeneration: the pancreatic intrinsic faculty. Trends Endocrinol Metab. 2011;22:34–43. doi: 10.1016/j.tem.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Dunn J.S., McLetchie N.G.B. Experimental alloxan diabetes in the rat. Lancet. 1943;242:384–387. [Google Scholar]
- 28.Soto C., Del Razo L.M., Neri L. Alloxan decreases intracellular potassium content of the isolated frog skin epithelium. Comp Biochem Physiol C Toxicol Pharmacol. 2001;130:19–27. doi: 10.1016/s1532-0456(01)00213-7. [DOI] [PubMed] [Google Scholar]
- 29.Nam Y.H., Hong B.N., Rodriguez I., Ji M.G., Kim K., Kim U.J., Kang T.H. Synergistic potentials of coffee on injured pancreatic islets and insulin action via KATP channel-blocking in zebrafish. J Agric Food Chem. 2015;63:5612–5621. doi: 10.1021/acs.jafc.5b00027. [DOI] [PubMed] [Google Scholar]
- 30.Sane R.T., Menon S.N., Inamdar S., Mote M., Gundi G. Simultaneous determination of pioglitazone and glimepiride by high-performance liquid chromatography. Chromatographia. 2004;59(7–8):451–453. [Google Scholar]
- 31.Guan F.Y., Gu J., Li W., Zhang M., Ji Y., Li J., Chen L., Hatch G.M. Compound K protects pancreatic islet cells against apoptosis through inhibition of the AMPK/JNK pathway in type 2 diabetic mice and in MIN6 β-cells. Life Sci. 2014;107:42–49. doi: 10.1016/j.lfs.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 32.Ashcroft F.M. ATP-sensitive K+ channels and disease: from molecule to malady. Am J Physiol Endocrinol Metab. 2007;293:E880–E889. doi: 10.1152/ajpendo.00348.2007. [DOI] [PubMed] [Google Scholar]
- 33.Henquin J.C. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- 34.Tabachnick I.I.A., Gulbenkian A., Seidman F. The effect of a benzothiadiazine, diazoxide, on carbohydrate metabolism. Diabetes. 1964;13:408–418. doi: 10.2337/diab.13.4.408. [DOI] [PubMed] [Google Scholar]
- 35.Muller P.Y., Milton M.N. The determination and interpretation of the therapeutic index in drug development. Nat Rev Drug Discov. 2012;11:751–761. doi: 10.1038/nrd3801. [DOI] [PubMed] [Google Scholar]
- 36.Gorrindo P., Williams K.C., Lee E.B., Walker L.S., McGrew S.G., Levitt P. Gastrointestinal dysfunction in autism: parental report, clinical evaluation, and associated factors. Autism Res. 2012;5:101–108. doi: 10.1002/aur.237. [DOI] [PMC free article] [PubMed] [Google Scholar]