Abstract
Herein we present a general protocol for the functionalization of biomolecules with an organotrifluoroborate moiety so that they can be radiolabeled with aqueous 18F fluoride (18F−) and used for positron emission tomography (PET) imaging. Among the β+-emitting radionuclides, fluorine-18 (18F) is the isotope of choice for PET, and it is produced, on-demand, in many hospitals worldwide. Organotrifluoroborates can be 18F-labeled in one step in aqueous conditions via 18F–19F isotope exchange. This protocol features a recently designed ammoniomethyltrifluoroborate, and it describes the following: (i) a synthetic strategy that affords modular synthesis of radiolabeling precursors via a copper-catalyzed ‘click’ reaction; and (ii) a one-step 18F-labeling method that obviates the need for HPLC purification. Within 30 min, 18F-labeled PET imaging probes, such as peptides, can be synthesized in good chemical and radiochemical purity (>98%), satisfactory radiochemical yield of 20–35% (n > 20, non-decay corrected) and high specific activity of 40–111 GBq/µmol (1.1–3.0 Ci/µmol). The entire procedure, including the precursor preparation and 18F radiolabeling, takes 7–10 d.
INTRODUCTION
PET is a fast-expanding, noninvasive imaging technology for visualizing and quantifying the dynamic distribution of target-specific ligands1–4. Peptides, which are a class of relatively large water-soluble molecules with good target selectivity, often demonstrate high-contrast tumor-specific PET images, and thus they are promising imaging agents. A one-step 18F-labeling method, which is sufficiently versatile to afford broad applications for most peptides, has been a longstanding challenge in radiochemistry5,6. To achieve this goal, several approaches have been reported over the past few years that represent substantial advances in the radiolabeling of peptides and small molecules5,7–15. However, to date, most of the reported strategies require anhydrous conditions, which involve an azeotropic ‘drying’ procedure that enhances the reactivity of 18F−, thereby ensuring good radiochemical yields16. Furthermore, to achieve high purity and high specific activity, which defines as GBq/µmol (Ci/µmol) and represents an impartial standard of radiotracer quality, time-consuming HPLC purification is often required17. These laborious procedures, such as azeotropic drying and HPLC purification, often reduce the radiochemical yield, while at the same time they increase the risk of synthetic failure. To solve these problems, a one-step and easy-to-follow protocol is described here for peptide radiolabeling that is based on the use of a recently developed organotrifluoroborate18.
In 2005, the Perrin group published a proof-of-concept study that described the labeling of an aryltrifluoroborate as a radiosynthon that could be 18F labeled in aqueous conditions19. In the following years, this research group obtained promising biological data using this approach to develop PET imaging agents20,21. Despite this initial success, early reports on PET imaging agents obtained via boron-mediated 18F capture suffered from several apparent drawbacks10, namely, (i) insufficient in vivo stability, (ii) limited specific activity and (iii) stepwise and laborious operational procedure.
Over the past few years, most of these disadvantages have been addressed. The first problem was solved by designing and synthesizing a new generation of organotrifluoroborates that are stable in vivo22,23. The second problem was largely solved by exploring 18F radiolabeling at high levels of activity to increase the specific activity of 18F radiotracers up to ~555 GBq/µmol (refs. 7,24), which is orders of magnitude higher than in our previous reports25–27. Nevertheless, to advance this approach to a general and clinician-friendly method, we developed a novel B-18F radiosynthon by screening a series of organotrifluoroborates22. This compound combines high in vivo stability, synthetic and bioconjugation simplicity, and convenient 18F-labeling technology, which is based on 18F-19F isotope exchange (IEX) of the 19F-containing modified biomolecule28, and it represents the core of this protocol and has smoothly solved the third problem22. On the basis of this method, a number of bioligands have been radiolabeled and evaluated, and some of them have demonstrated excellent in vivo performance18,29–31. Particularly, ammoniomethyltrifluoroborate (AMBF3)-TATE (Fig. 1), which is an octreotate derivative, showed excellent somatostatin receptor imaging properties, and the relevant bench-to-bedside translation is now underway29.
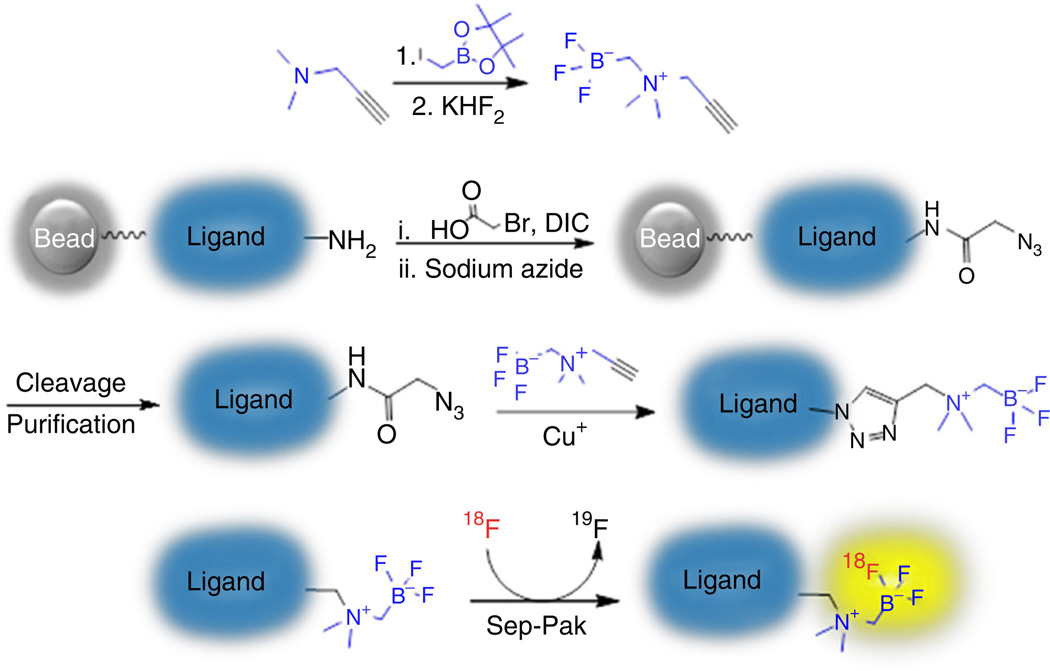
Figure 1.
Outline of the procedure to 18F-label biomolecules with AMBF3.
In contrast to mainstream 18F-labeling methods, which either involve multiple steps or are water sensitive32, this approach has a one-step radiolabeling reaction, and the intermediates are stable in water. For large doses of 18F−, a small quaternary methyl amine (µ-QMA) cartridge can be used (instead of performing azeotropic distillation) to concentrate the radioactivity before radiolabeling. In this approach, Curie levels of 18F− can be directly eluted with <60 µl of saline (recovery rate: >95%, n > 50), and no further concentration is necessary (Step 17A; concentration by azeotropic distillation is still advisable for lower 18F− doses—e.g., 50 mCi doses).
In 18F–19F IEX, the chemical structure of the radiolabeled product is identical to that of the precursor. In this case, a simple Sep-Pak purification affords the tracer in high radiochemical purity, and no HPLC purification is required. Furthermore, because of its operational simplicity, the radiolabeling can be simply performed with remote telemanipulators in a fully shielded hot cell. Thus, Curie levels of radioactivity are routinely used and a >7.4 GBq (>200 mCi) product can be reproducibly obtained at high specific activity. A limitation of this protocol is that when starting with a small amount of 18F− (e.g., 50 mCi) for radiolabeling, as described in Step 17B, an azeotropic-concentrating procedure will be recommended in order to obtain the radiotracer of high specific activity. Overall, this protocol provides researchers a user-friendly tool that can overcome the challenges of preparing 18F-labeled PET tracers.
In summary, this protocol simplifies the radiosynthesis of 18F-labeled biomolecules, an improvement that will potentially promote the development of new PET tracers and accelerate their use from the bench to bedside. Herein, we describe the detailed protocols of (i) µ-QMA cartridge assemblage, (ii) copper-catalyzed radiosynthon conjugation and (iii) 18F–19F IEX.
Experimental design
This protocol describes the synthesis of [18F]BF3-conjugated PET imaging probes. As a representative, [18F]AMBF3-TATE is synthesized and used as an actual example to implement this approach. The procedure is outlined in Figure 1. This protocol is generally suitable for 18F labeling at the N termini of peptides; however, the authors would recommend the readers to implement the PROCEDURE exactly as detailed, so as to acquaint themselves with it, before moving on to using a different fluorinated compound. For very acid-sensitive or fragile peptides, one-pot two-step approaches can be considered33. In addition to radiolabeling peptides, this method can also be used to radiolabel other molecules after minor modification. The precursors will be prepared in similar manners by conjugating alkynyl AMBF3 on biomolecule azide via copper-catalyzed ‘click’ reaction.
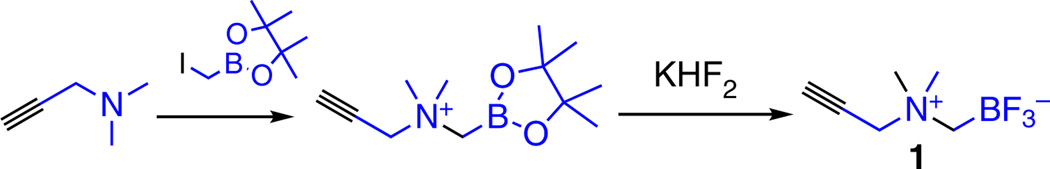
As a general applicable radiosynthon, the preparation of alkynyl AMBF3 (Fig. 2) has been described in detail in this protocol. The peptide azide can be prepared either using solid-phase synthesis (as described in this PROCEDURE) or in liquid phase by directly conjugating azidoacetic acid on an amine-terminated peptide. In addition, there are many publications that describe how to prepare other types of azide compounds, and there are also commercially available azides that could be used.
Figure 2.
Synthetic route of radiosynthon 1.
The crucial step of this protocol is 18F labeling, and the key factor for this step is the reaction pH. On the basis of our previous studies, a pH of 2–2.5 generally gives better radiochemical yield and lower by-product (boronic acid) levels than other conditions34. To maintain the reaction pH within the desired window, a series of buffers were screened and eventually a pyridazine-HCl buffer was chosen for BF3-mediated 18F labeling. A pyridazine-HCl buffer was chosen not only for its ideal pKa but also because of its high solubility in water and low toxicity (LD50 in mice is >2.6 g/kg). The experimental details for the preparation and use of this buffer are described in the Reagent Setup and Step 17 of the PROCEDURE, respectively.
Before radiolabeling, the 18F− starting solution needs to be concentrated. By using an amount of radioactive precursor that affords 1Curie level of radioactivity (~37 GBq), which can be easily obtained in many clinical PET centers, the azeotropic evaporation step can be obviated, as the 18F− can be concentrated by using a µ-QMA cartridge. The details and tips related to the preparation of this cartridge are reported in Step 17A(i–vi) of the PROCEDURE. In this case, noncarrier added 18F− is directly eluted into the reaction vial with ~60 µl of saline solution, and the total reaction volume is ~150 µl. By starting with 100 nmol of precursor, generally 200 mCi (7.4 GBq) of product can be obtained by the end of synthesis (EOS) to give the specific activity of 2.0 Ci/µmol (74 GBq/µmol). However, if you are starting with ~50 mCi of 18F− (~1.85 GBq), which is more suitable for research purpose or preclinical studies, azeotropic evaporation is highly recommended in order to achieve a good radiochemical yield, as well as high specific activity (Step 17B(i–v)). In such cases, <10 nmol of precursor is used for radiolabeling, and consequently the reaction volume is reduced to 15 µl. Therefore, the 18F− solution needs to be concentrated before radiolabeling. The composition of the vial in which the 18F− is dried is crucial, and glass vials should be avoided because of the presence of fluorophilic silicates on the glass surface. After drying (or concentration), the 18F− is resuspended with buffered stock solution to initiate the 18F–19F IEX.
One notable limitation of this protocol might stem from the acidic radiolabeling conditions. Nevertheless, to date, in our hands, these conditions have not been an issue for most bioligands, as they are generally stable in acidic conditions for a short period of time and many of them are even prepared in acidic conditions35,36. For instance, peptides are typically cleaved from the solid base support and purified under the conditions that are substantially more acidic than those used herein (e.g., deprotection in 80% (vol/vol) trifluoroacetic acid (TFA), pH = −2, purification in 0.1–0.5% (vol/vol) TFA, pH = 1–2; refs. 37,38).
MATERIALS
REAGENTS
Synthesis of AMBF3-conjugated biomolecules
N,N-dimethylformamide (DMF; Sigma-Aldrich, cat. no. 270547)
Anhydrous diethyl ether (Sigma-Aldrich, cat. no. 346136)
Iodomethyl-pinacol boronate (Combi-Blocks, cat. no. FM-6291)
Potassium hydrogen fluoride (KHF2; Sigma-Aldrich, cat. no. 239283)
NH4OH (aqueous (aq.), concentrated (conc.), Sigma-Aldrich, cat. no. 320145)
CuSO4·5H2O (Sigma-Aldrich, cat. no. C1297)
Sodium ascorbate (Sigma-Aldrich, cat. no. A7631)
Dichloromethane (Sigma-Aldrich, cat. no. 650463)
Trifluoroacetic acid (TFA; for spectroscopy; Merck Chemicals, cat. no. 108262)
Triisopropylsilane (99%; Sigma-Aldrich, cat. no. 233781)
N,N-Diisopropylethylamine (≥ 98.0%; Fluka, cat. no. 03440)
Acetonitrile anhydrous (ACN; Sigma-Aldrich, cat. no. 271004)
Methanol (Sigma-Aldrich, cat. no. 34860)
DMSO (Sigma-Aldrich, cat. no. D8148)
1 N hydrochloric acid (HCl; Sigma-Aldrich, cat. no. 71763)
Ethanol (Sigma-Aldrich, cat. no. 459844)
Solid-phase peptide synthesis (optional)
Bromoacetyl N-hydroxysuccinimide (NHS) ester (Sigma-Aldrich, cat. no. B8271)
N-methyl-2-pyrrolidone (Sigma-Aldrich, cat. no. 328634)
Sodium azide (Sigma-Aldrich, cat. no. S2002)
Phenol (Sigma-Aldrich, cat. no. P1037)
Piperidine (≥99%, for peptide synthesis; Sigma-Aldrich, cat. no. 104094)
O-(Benzotriazol-1-yl)-N,N,N,N-tetramethyluronium hexafluorophosphate (≥98.0%, Sigma-Aldrich, cat. no. 12804)
Diisopropylcarbodiimide (Sigma-Aldrich, cat. no. 38370)
Fmoc-Thr(tBu)-Wang resin (100–200 mesh; Novabiochem, cat. no. 856017)
Fmoc-Cys(Acm)-OH (Novabiochem, cat. no. 852006)
Fmoc-Thr(tBu)-OH (Novabiochem, cat. no. 852000)
Fmoc-Lys(Boc)-OH (Novabiochem, cat. no. 852012)
Fmoc-D-Trp(Boc)-OH (Novabiochem, cat. no. 852164)
Fmoc-Tyr(tBu)-OH (Novabiochem, cat. no. 852020)
Fmoc-D-Phe-OH (Novabiochem, cat. no. 852148)
Thallium(III) trifluoroacetate (Technical grade; Sigma-Aldrich, cat. no. 150533)
Radiolabeling
Pyridazine (Sigma-Aldrich, cat. no. P57204)
Deionized water (obtained from Milli-Q integral water purification system)
HCl (aq. 37%; Sigma-Aldrich, cat. no. 258148)
Sodium chloride (NaCl; Sigma-Aldrich, cat. no. S9888)
Saline (Thermo Fisher, cat. no. 721016)
Phosphate buffer (Thermo Fisher, cat. no. BP399-500)
Fluorine-18 (1.5 ml, purchased from a supplier or generated by on-site cyclotron from bombardment via the 18O(p,n)18F reaction)39 ! CAUTION Working with radioactive materials needs extra caution. If possible, try to minimize the exposure time to radiation. In addition, to work with radioactivity of more than 200 mCi, all operations should be performed using a telemanipulator in a lead-shielded hot cell.
EQUIPMENT
Synthesis of AMBF3-conjugated biomolecules
Round-bottom flask (Sigma-Aldrich, cat. no. Z723134)
Sand-core funnel (National Institutes of Health (NIH) self-service store or institutional glassware shop)
High-vacuum pump (direct-drive high-vacuum pumps, Welch, cat. no. 8917)
Sep-Pak alumina N light cartridge (Waters, cat. no. WAT023561)
Argon (Robert Oxygen)
Preparation and concentration of 18F−
BD PrecisionGlide needle, 16 gauge × 1 1/2-inch (Med Plus Physical Supplies, cat. no. BD 305198)
µ-QMA cartridge (This product was previously available from ORTG with the item name as 18F− Trap & Release Columns. However, ORTG has been out of business since 2014, and this type of QMA cartridge is currently available from MedChem Imaging)
pH meter (Thermo Fisher, cat. no. S90528)
Wire cutters (Thermo Fisher, cat. no. 17-467-435)
Radiolabeling
Heating block (Thermo Fisher, cat. no. 11-720-10BQ)
Eppendorf tube (Thermo Fisher, cat. no. 05-402-25)
Capintec well counter (CRC-55TW dose calibrator/well counter)
Falcon 15-ml conical centrifuge tubes (polypropylene; Fisher Scientific, cat. no. 14-959-70C)
Precision Seal rubber septa (White, 14/20 joints, Sigma-Aldrich, cat. no. Z553964)
Syringe PP/PE without needle (5 ml; Sigma-Aldrich, cat. no. Z116866)
Syringe PP/PE without needle (3 ml; Sigma-Aldrich, cat. no. Z116858)
Syringe PP/PE without needle (1 ml; Sigma-Aldrich; cat. no. Z230723)
4 × BD PrecisionGlide needles 21 gauge × 2 inch (Med Plus Physical Supplies, cat. no. BD 305129)
Sep-Pak C18 light cartridge (Waters, cat. no. WAT022301)
Analytical HPLC System (Agilent, 1200 series)
Radio-HPLC detector system (Eckert & Ziegler, FC-3200 NaI detector)
REAGENT SETUP
Pyridazine-HCl buffer (1.0 M, pH 2.0–2.5)
Dissolve 720 µl of pyridazine (10 mmol) in 5 ml of DMF and 2.5 ml of deionized water in a 15-ml conical tube. Adjust the pH by using a pH meter; continue adding diluted HCl (aq., 4 M) until the pH is regulated to 2.0. Adjust the final volume to 10 ml with deionized water to obtain a final concentration of the buffer of 1.0 M. This buffer can be stored at 4 °C for up to 1 month. ▲ CRITICAL The pH of this pyridazine buffer may increase after being stored for more than 1 month. If the buffer has been stored for more than 1 week, please make sure to check the buffer pH before radiolabeling. In addition, the buffer should be stored at 4 °C. The color of the buffer may turn light yellow after prolonged storage, but this color change should not affect the yield of radiolabeling.
Aqueous solution of CuSO4·5H2O (1.0 M)
Dissolve CuSO4·5H2O (2.5 g, 10 mmol) with 5 ml of deionized water. Dilute this solution to 10 ml using deionized water to obtain the desired concentration. This solution is stable at room temperature (~20 °C) for at least 3 months.
Aqueous solution of sodium ascorbate (1.0 M)
Dissolve sodium ascorbate (1.98 g, 10 mmol) with 2 ml of deionized water. Dilute this solution to 10 ml using deionized water to obtain the desired concentration. This solution is stable at room temperature (~20 °C) for at least 3 months.
KHF2 aqueous solution (7.5 mM)
Dissolve KHF2 (5.85 mg, 75 µmol) with 1 ml of deionized water. Dilute this solution to 10 ml using deionized water to obtain the desired concentration. This solution is stable at room temperature (~20 °C) for at least 3 months.
NaCl saturated solution
Dissolve NaCl (10 g) with 10 ml of deionized water to obtain a saturated solution. This solution is stable at room temperature (~20 °C) for at least 3 months.
HCl solution (4 M)
Dilute HCl (aq., 37%) with twofold (vol/vol) deionized water to obtain a 4-M HCl aqueous solution. This solution is stable at room temperature (~20 °C) for at least 3 months.
EQUIPMENT SETUP
Self-assembled µ-QMA cartridge manufacture and precondition
Cut a 16-gauge × 1 1/2-inch needle with wire cutters and precisely assemble the µ-QMA cartridge between the two parts of the needle. Seal the joints with Parafilm to ensure no possible leaking. Flush the cartridge with 3 ml of deionized water, 3 ml of brine (NaCl-saturated aqueous solution) and 3 ml of deionized water to convert the carbonate-formed QMA into its chloride form. To better understand how the µ-QMA cartridge is assembled, a descriptive picture is available as referenced18. ▲ CRITICAL A suitable reaction pH (2–2.5) is of paramount importance to give good radiochemical yield for 18F-labeled organotrifluoroborates. To ensure that the final reaction pH is within the correct range, here we highly recommend using a chloride form instead of the regular carbonate form, which gives strongly basic solution (pH = 11) after elution40. The presence of residual carbonate in the QMA may occasionally result in failure of radiosynthesis, and thus it might jeopardize the reproducibility of this radiolabeling protocol. Of note, this self-assembled µ-QMA cartridge can be reused for up to ten cycles of trapping-releasing, and no reconditioning is needed; however, we always recommend flushing the cartridge with 3 ml of deionized water before using it as the 18F− trap.
Sep-Pak C18 light cartridge preconditioning
Precondition this cartridge with 6 ml of ethanol, followed by 6 ml of deionized water.
HPLC system and radioactivity detector
Equip the Agilent 1200 series analytical HPLC system with an Eckert and Ziegler B-FC-3200 NaI detector according to the manufacturer’s instructions.
Analytical HPLC for quality control (method A)
A Phenomenex Jupiter 10-µm C18, 300 Å, 4.6 × 250 mm analytical column is used. The HPLC setup is as follows: solvent A, 0.1% (vol/vol) TFA in water; solvent B, ACN; flow rate, 2 ml/min; and column temperature, 19–21 °C.
| Time (min) | %B |
|---|---|
| 0–2 | 5 |
| 2–7 | 5–20 |
| 7–15 | 20–100 |
| 15–20 | 100–5 |
Semi-prep HPLC method for azide-AMBF3 purification (method B)
An Agilent Eclipse XDB-C18 5 µm 9.2 × 250 mm semi-prep column is used. The setup is as follows: solvent A, 0.1% (vol/vol) TFA in water; solvent B, ACN; flow rate, 4.5 ml/min; and column temperature, 19–21 °C.
| Time (min) | %B |
|---|---|
| 0–2 | 20 |
| 2–20 | 20–35 |
| 20–22 | 35–20 |
Semi-prep HPLC method for 19F-AMBF3-TATE purification (method C)
An Agilent Eclipse XDB-C18 5 µm 9.2 × 250 mm semi-prep column is used. The setup is as follows: solvent A: 0.1% (vol/vol) TFA in water; solvent B: ACN; flow rate: 4.5 ml/min; and column temperature, 19–21 °C.
| Time (min) | %B |
|---|---|
| 0–2 | 5–20 |
| 2–10 | 20–35 |
| 10–17 | 35–40 |
| 17–18 | 40–100 |
| 18–19 | 100–20 |
| 19–22 | 20–5 |
PROCEDURE
Preparation of N-propargyl-N,N-dimethyl-ammoniomethyl-trifluoroborate (1) ● TIMING ~1 d
-
1|
Dissolve 98 µl of N,N-dimethylpropargylamine (1.0 mmol) into 2 ml of anhydrous diethyl ether in a flame-dried round-bottom flask. Next, add 165 µl of iodomethyl-pinacol boronate (0.9 mmol) dropwise into the solution that was previously prepared at room temperature.
-
2|
Continue stirring for 5 min, during which time the solution will become cloudy followed by the formation of a white precipitate, which is the desired N-propargyl-N,N-dimethylammonio-methylboronylpinacolate intermediate product (Fig. 2).
-
3|
Filter the resulting precipitate with a sand-core funnel and wash the solid with diethyl ether; retain the solid.
-
4|
Dry the residue under high vacuum to give a fluffy white powder in 95% yield.
-
5|
Convert 50.0 mg of the N-propargyl-N,N-dimethylammonio-methylboronylpinacolate thus synthesized (142.4 µmol) into the corresponding trifluoroborate (alkynyl-AMBF3, 1) by adding a cocktail of KHF2 (3 M, 300 µl in water), HCl (4 M, 300 µl in water), deionized water (200 µl) and DMF (600 µl).
-
6|
Allow the reaction to proceed at 45 °C for 2 h, and then quench the reaction by adding 10 µl of NH4OH (conc.).
-
7|
Remove the free fluoride by passing the reaction mixture through a silica gel column. Next, further purify the residue by diethyl ether extraction to obtain a white powder as the chemically pure product in 90% yield (21.2 mg).
■ PAUSE POINT N-propargyl-AMBF3 can be stored at −20 °C for up to 2 years.
Synthesis of biomolecule-azide ● TIMING ~1 week
-
8|Synthesize a biomolecule-azide adduct according to option A, if the biomolecule is a peptide, or option B, if the biomolecule is nonpeptidic.
- Synthesis of peptide-azide ● TIMING 4–5 d
- Couple bromoacetyl NHS ester to the N terminus of the peptide sequence by using an excess amount of bromoacetyl NHS ester (20.0 eq.) in N-methyl-2-pyrrolidone at room temperature for 2 h.
-
Displace the terminal bromide of the tethered peptide resulting from implementing Step 8A(ii) with azide by incubating the peptide with an excess amount of sodium azide (27.5 eq.) in DMSO at room temperature overnight.! CAUTION Sodium azide is severely poisonous. Even a minute amount can cause symptoms of toxicity Please wear double gloves and use extra caution in this step.
- Deprotect and simultaneously cleave the peptide from the resin by adding a cocktail of TFA:H2O:triisopropylsilane:phenol at a ratio of 90:2.5:2.5:5 to the peptide-decorated resin and by incubating the mixture for 4 h at room temperature.
- Purify the peptide-azide adduct by HPLC with method B (Equipment Setup) to afford chemically pure peptide-azide in quantities of ~10 mg.
- Preparation of nonpeptide-azide ● TIMING up to ~1 week
- (Optional) Synthesize the azide-conjugated small molecule of choice via a classical SN2 reaction by following published protocols43,44. Alternatively, please note that, because of the rapid development and broad application of copper-catalyzed click reaction (CuAAC), many azide-conjugated functional molecules are readily available from commercial sources.
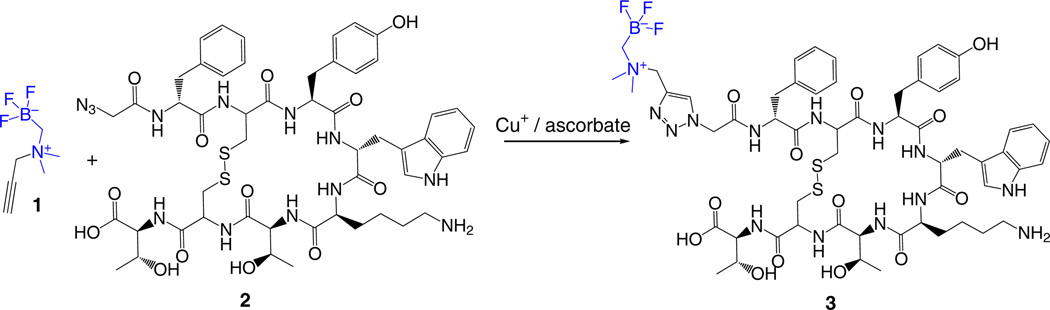
Synthesis of AMBF3-conjugated biomolecule ● TIMING 2 d
-
9|
Mix 5.0 µl of a 1.0-M aqueous solution of CuSO4 with 12.5 µl of a 1.0-M aqueous solution of sodium ascorbate to obtain a dark solution.
-
10|
Immediately quench the solution that was prepared in Step 9 with 50 µl of a 5% (vol/vol) NH4OH in ACN/H2O = 1:1. The resulting mixture should be a greenish, clear solution.
? TROUBLESHOOTING
-
11|
Dissolve 2.0 mg of compound 1 (15 µmol) in 200 µl of ACN/H2O = 1:1.
-
12|
Pool together the two solutions prepared in Steps 10 and 11.
-
13|
Dissolve 4.0 mg of TATE-azide (compound 2, 3.5 µmol) in 200 µl of ACN/H2O = 1:1.
-
14|
Add the TATE-azide solution prepared in Step 13 in portions of 50 µl to the mixture prepared in Step 12. The final reaction mixture (Fig. 3) should be a clear solution without any visible precipitate.
▲ CRITICAL STEP The order in which the reagents are added is essential for this step to be successful.
-
15|
Incubate this reaction mixture at 45 °C for 2 h and directly purify the crude reaction by using the Agilent 1200 HPLC system with method C (see Equipment Setup) to isolate chemically pure AMBF3-TATE (compound 3). Confirm and evaluate the identity and chemical purity of the product with LC-MS.
? TROUBLESHOOTING
-
16|
For convenience and to ensure reproducibility, dilute the purified 19F-AMBF3-TATE in ethanol and store it in aliquots of ~100 nmol in 15 µl for radiolabeling. Please note that these aliquots will be stable in ethanol solution for up to 1 month at room temperature. For longer storage times, the solvent in the aliquots needs to be removed under vacuum.
■ PAUSE POINT The dry AMBF3-conjugated biomolecules should be stored at −20 °C, and it should be used within 6 months.
Figure 3.
Synthesis of 19F-AMBF3-TATE (3) via copper-catalyzed ‘click’ reaction.
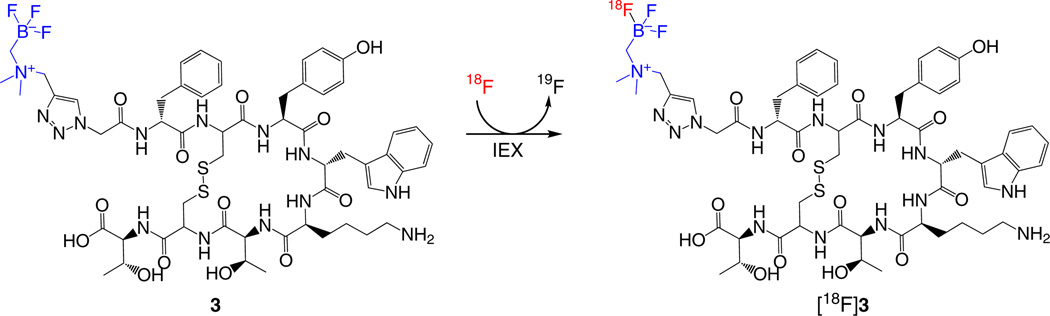
Preparation of the radiosynthesis reaction mixture ● TIMING 30 min
-
17|Prepare a radiolabeling mixture of AMBF3-TATE and 18F (Fig. 4) according to option A, if you are aiming for a 1 Curie level of reactivity of the 18F reactant, or option B, if you are aiming for a level of radioactivity of the 18F reactant lower than 1.85 GBq (50 mCi); see relevant discussion in the INTRODUCTION (Experimental design).
- Preparation of an 18F-labeling mixture with 1 Curie level of radioactivity
-
Resuspend a 100-nmol aliquot of 19F-AMBF3-TATE in a mixture of 15 µl of pyridazine-HCl buffer (pH = 2.0–2.5, 1 M), 15 µl of DMF and 1 µl of a 7.5 mM KHF2 aqueous solution.▲ CRITICAL STEP Check the pH of the previously prepared precursor solution with pH strips (0–6) before proceeding with the next step.? TROUBLESHOOTING
-
Prepare the following materials, and then place them into the hot cell.
Quantity Material 2 0.07 ml of saline in a 1-ml PP/PE syringe without needle 2 2 ml of NH4OH (aq., 5% (vol/vol)) and 0.5 ml air in a 3-ml PP/PE syringe with a 21-gauge × 2-inch needle 3 4 ml of deionized water in a 5-ml PP/PE syringe without needle 2 0.5 ml of 1:1 saline/ethanol in a 1-ml PP/PE syringe without needle 2 2 ml of saline in a 3-ml PP/PE syringe with a 16-gauge × 1½-inch needle 6 1-ml PP/PE syringes equipped with 21-gauge × 2-inch needles 1 Sep-Pak C18 light cartridge ! CAUTION To minimize the radiation dose, properly shield yourself by performing most of the reactions involving radioactivity in a lead-shielded fume hood or a hot cell.▲ CRITICAL STEP Make sure to always have a back-up sample of each reagent, as the hot cell should not be opened once large quantities of radioactivity (i.e., up to 37 GBq) have been transferred into the hot cell. - Preheat the precursor solution prepared in Step 17A(i) in a heating block at 80 °C.
- Close the door of the lead-shielded hot cell, and perform all the following operations with telemanipulators.
- Pass an 18F− solution through the self-assembled µ-QMA cartridge, followed by 5 ml of air.
-
Elute 18F− with ~60 µl of saline in a 1-ml PP/PE syringe into the reaction vial, followed by blowing the µ-QMA cartridge with 0.5 ml of air. The radiolabeling reaction initiates right after eluting 18F− into the reaction vial; no additional mixing procedure is required.▲ CRITICAL STEP Elution must proceed gently and relatively slowly (200 µl/min). With proper operation, >95% radioactivity can be recovered from the µ-QMA cartridge (n = 50).▲ CRITICAL STEP The radiochemical yield of 18F-19F IEX depends greatly on the reaction concentration of AMBF3 conjugates and on the pH of the radiolabeling mixture.
-
- Preparation of a 18F-labeling mixture with <1.85 GBq (50 mCi) radioactivity
- Resuspend 20 nmol of 19F-AMBF3-TATE in a mixture of 5 µl of pyridazine-HCl buffer (pH = 2.0–2.5, 1 M) and 5 µl of DMF.
- Prepare the following list of reagents, and then put them into the hot cell or shielded fume hood.
Quantity Material 2 2 ml NH4OH (aq., 5% (vol/vol)) and 0.5 ml air in a 3-ml PP/PE syringe with a 21-gauge × 2-inch needle 2 0.5 ml of 1:1 saline/ethanol in a 1-ml PP/PE syringe without needle 2 2 ml of saline in a 3-ml PP/PE syringe with a 16-gauge × 1½-inch needle 1 Sep-Pak C18 light cartridge 5 1.5-ml number-labeled Eppendorf tubes - Preheat the reaction vial with the precursor solution in a heating block at 80 °C.
-
Azeotropically concentrate a mixture composed of 200 µl of ACN and 200 µl of aqueous 18F− using a stream of argon at 110 °C for 10 min in a Falcon tube.▲ CRITICAL STEP The 18F− salt solution does not have to be completely dry. Indeed, overdrying this solution may lead to difficulties in suspending 18F−.▲ CRITICAL STEP The composition of the vial in which the 18F− is concentrated is crucial, and glass vials should be avoided, given the presence of fluorophilic silicates on the glass surface.▲ CRITICAL STEP To avoid unwanted evaporation of [18F]HF, we recommend adding 5 µl of 0.1 M NaHCO3 (aq.) to the mixture in Step 17B(iv).
-
Resuspend the 18F− salt with 20 µl of deionized water. Mix the precursor solution prepared in Step 17A(iii) with the 18F− salt solution just prepared in a 1.5-ml Eppendorf tube.▲ CRITICAL STEP Check the pH of the precursor solution with pH strips (0–6) before mixing it with the resuspended 18F− salt solution; the pH of the radiolabeling reaction should be between 2 and 2.5.
Figure 4.
Radiosynthesis of 18F-AMBF3-TATE ([18F]3) via 18F-19F IEX reaction.
18F–19F IEX of 19F-AMBF3-TATE ● TIMING 20 min
-
18|
Incubate the radiolabeling reaction at 80 °C for 12 min.
-
19|
Quench the reaction by adding to it 2 ml of NH4OH (5% (vol/vol) aq.) using a 3-ml PP/PE syringe with a 21-gauge × 2-inch needle. Use the same syringe to load the resulting reaction mixture onto a Sep-Pak C18 light cartridge.
▲ CRITICAL STEP The reaction mixture must be loaded into the Sep-Pak C18 light cartridge gently and slowly (one drop every 2 s). In addition, a 2-inch needle is required for this step, as otherwise the crude reaction would not be completely transferred to the cartridge.
? TROUBLESHOOTING
-
20|
Remove the impurities (i.e., free 18F−) from the reaction mixture by flushing the reaction mixture–loaded C18 light cartridge twice with 4 ml of deionized water each time. The flow rate of this flushing procedure should be one drop every 2 s.
? TROUBLESHOOTING
-
21|
Elute the purified product into a glass vial with 1:1 ethanol/saline (0.5 ml), and measure the radioactivity of product by using a Capintec well counter. The radiochemical yield is determined by dividing the radioactivity of finally purified product by the 18F− being used at the beginning of synthesis.
▲ CRITICAL STEP Do not elute too quickly (the flow rate should be <1 drop every 5 s). In regard to the radiosynthesis, the eluate should be collected in separate fractions every three drops. Measure each fraction using a dose calibrator in the hot cell. The fraction containing the highest concentration of radioactivity should be used for the following studies.
? TROUBLESHOOTING
-
22|
Dilute the solution obtained at the end of Step 21 with 2 ml of isotonic saline, and then transfer 0.1 ml of the solution thus prepared into another glass vial by using a 1-ml PP/PE syringe equipped with a 21-gauge × 2-inch needle.
! CAUTION By starting with 37 GBq of radioactivity (Step 17A(i–vi)), generally >7.4 GBq of 18F-labeled product will be obtained in 2 ml of saline solution. To avoid unnecessary exposure to radiation, always take out the minimum portion of the radioactive product based on the quantities required for subsequent studies (such as imaging or biodistribution experiments).
-
23|
Take the vial with 0.1 ml of the solution out of the hot cell. Dilute the product with an additional 5–10 ml of isotonic saline to obtain a solution that can be readily injected for biological evaluation.
▲ CRITICAL STEP The final concentration of ethanol in the resulting solution should not exceed 10% (vol/vol).
-
24|
Remove a small portion from the solution obtained after implementing Step 23 to determine the radiochemical purity of the product with the analytical HPLC system (method A, see Equipment Setup).
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 1.
TABLE 1.
Troubleshooting table.
| step | problem | possible reason | solution |
|---|---|---|---|
| 10 | Precipitation | Cu+ complex precipitates out because of its poor solubility in aqueous solution |
Continue adding the organic solvent (e.g., MeCN) until the reaction is clear |
| 15 | Defluoridated product | AMBF3 may start losing fluoride when the reaction pH is too basic (pH >12). The defluoridated product, AMB(OH)2-TATE, can be determined by mass spectrum, and it is separable from AMBF3-TATE by using method B (Reagent Setup) |
Adjust the final pH of the click reaction to 7–12 with phosphate buffer |
| 17A(i) | Low efficiency of 18F fluoride elution (<80%) |
Rate of elution is too fast | Elute 18F fluoride slower (one drop for each 10 s) |
| 19 | Low efficiency when ‘trapping’ radiolabeling mixture with C18 cartridge (<10%) |
The product is too polar for C18 purification, or the pH of the reaction mixture is significantly different from the isoelectric point of the peptide |
Adjust the pH value of the crude reaction to the pH that is similar to the isoelectric point of the peptide |
| 20 | Low efficiency when ‘releasing’ radiolabeled product from C18 cartridge (<50%) |
The solubility of the product is not sufficient in ethanol or not enough ethanol is used |
Use more ethanol for elution |
| 21 | Low isolated radiochemical yield (<10%) |
The concentration of the precursor is not sufficient |
The final concentration of the precursor is suggested to be no less than 1 mM. Instead of weighing the HPLC-purified AmBF3 conjugates with a balance, which is not accurate because of contamination (e.g., of TFA salts), we recommend the use of UV absorbance at a certain wavelength (e.g., 277 nm for AMBF3-TATE) to deter- mine the quantity of the AMBF3 conjugates |
| Low isolated radiochemical yield (<10%) |
The pH of the radiolabeling reaction is not suitable |
The final concentration of the pyridazine buffer should be no less than 100 mM |
● TIMING
Steps 1–7, preparation of AMBF3 (1): within 1 d
Step 8, preparation of ‘clickable’ bioligands (e.g., TATE-azide) including HPLC purification: ~1 week
Steps 9–16, synthesis of AMBF3-conjugated bioligands (e.g., 19F-AMBF3-TATE) including HPLC purification and preparation of aliquots: within 2 d
Step 17, equipment setup and precursor preparation for radiolabeling: 30 min
Steps 18–24, 18F-labeling of AMBF3-conjugated bioligands via 18F–19F IEX including Sep-Pak purification: 20 min
ANTICIPATED RESULTS
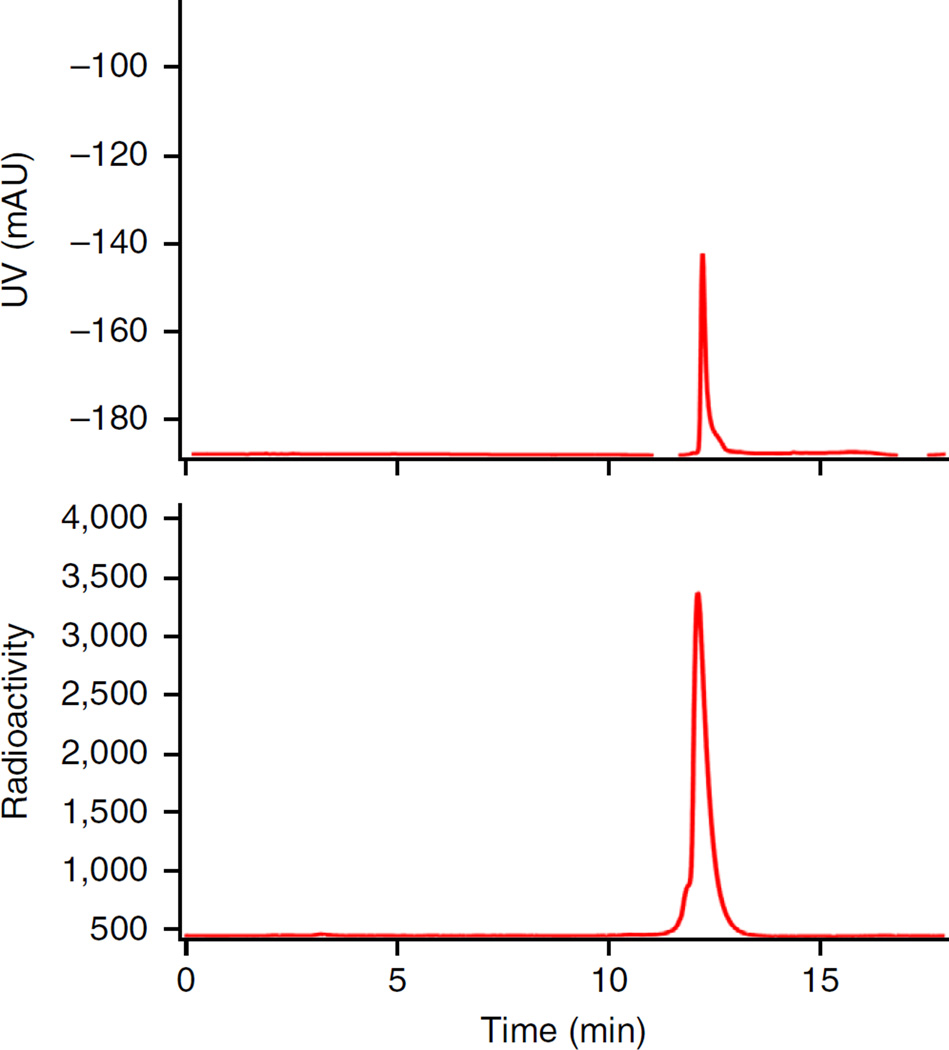
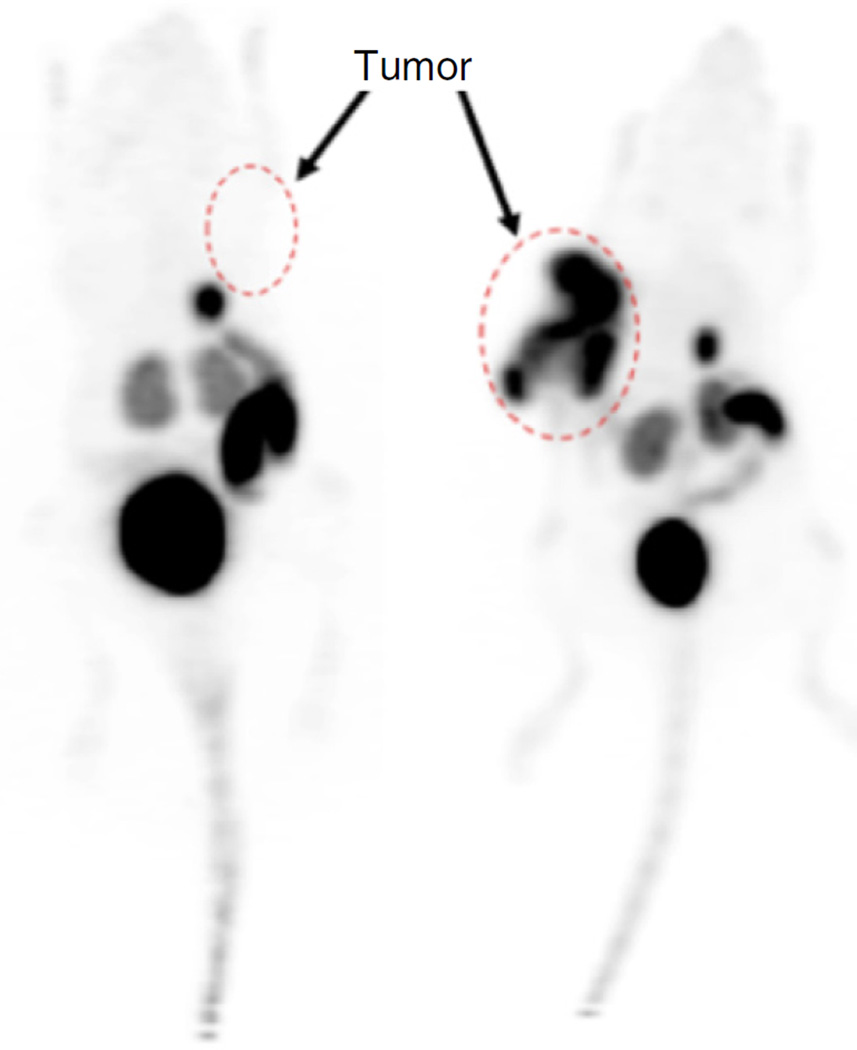
By implementing Step 17A, starting with 29.6–37.0 GBq (800–1,000 mCi) of 18F−, no less than 7.4 GBq (200 mCi) of 18F-AMBF3-TATE should be obtained in 25 min, with a specific activity higher than 74 GBq/µmol (2 Ci/µmol). By implementing Step 17B, starting with 1.48–1.85 GBq (40–50 mCi) of 18F−, 0.56–0.74 GBq (15–20 mCi) of 18F-labeled product should be produced in 45 min, with a specific activity higher than 27.8 GBq/µmol (0.75 Ci/µmol). For both radiolabeling procedures, the radiochemical purity, which is determined by HPLC analysis, should be higher than 98% (Fig. 5), thus eliminating the need for further HPLC purification. If the radiochemical purity of the product is <95%, a second Sep-Pak purification (Steps 19–21) would be recommended to improve the purity. A representative PET imaging of 18F-AMBF3-TATE is shown in Figure 6 here to illustrate the general in vivo performance of AMBF3-TATE.
Figure 5.
HPLC chromatograms of Sep-Pak-purified 18F-AMBF3-TATE. Top, the UV chromatogram measured at 277 nm; bottom, a radiochromatogram. UV, ultraviolet. mAU, one thousandth of an absorbance unit.
Figure 6.
[18F]AMBF3-TATE shows specific uptake in AR42J xenografts (dotted ovals) and low uptake in normal tissues. Maximum intensity projection [18F]AMBF3-TATE PET images of AR42J tumor-bearing mice at 60 min after injection is shown here: blocked (left) and unblocked (right). The tracer specifically accumulated into the tumor, whereas the background radioactivity was rapidly cleared mainly via the kidneys to the bladder. Some gut and gallbladder accumulation occurs because of some hepatobiliary excretion of the tracer.
Analytical data
N-propargyl-N,N-dimethyl-ammoniomethyl-trifluoroborate (1). 1H NMR (300 MHz, CD3CN) δ 4.25 (d, 2H), 3.41 (t, 1H), 3.18 (s, 6H), 2.55 (b, 2H). High-resolution electrospray ionization mass spectrum (HR-ESI-MS) (m/z): 146.10. [M-F]+; calculated for C6H11BF2N, 146.09.
TATE-azide. HR-ESI-MS (m/z): 1,132.39. [M+H]+; calculated for C51H66N13O13S2, 1132.44.
19F-AMBF3-TATE. HR-ESI-MS (m/z): 1,297.49. [M+H]+; calculated for C57H77BF3N14O13S2, 1297.52.
Acknowledgments
This work was supported by the National Sciences and Engineering Research Council, and the Canadian Cancer Society Research Institute, with contributions from the intramural research program at National Institute of Biomedical Imaging and Bioengineering, US National Institutes of Health.
Footnotes
AUTHOR CONTRIBUTIONS Z.L., D.M.P., K.-S.L., F.B. and X.C. conceived and designed this research; Z.L. and M.P. performed the experiments; Z.L., K.-S.L. and F.B. analyzed the data; and Z.L., D.M.P., D.O.K. and X.C. wrote the manuscript.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Tarkin JM, Joshi FR, Rudd JHF. PET imaging of inflammation in atherosclerosis. Nat. Rev. Cardiol. 2014;11:443–457. doi: 10.1038/nrcardio.2014.80. [DOI] [PubMed] [Google Scholar]
- 2.Cai LS, Lu SY, Pike VW. Chemistry with F-18 fluoride ion. Eur. J. Org. Chem. 2008;17:2853–2873. [Google Scholar]
- 3.Tsien RY. Imagining imaging’s future. Nat. Cell Biol. 2003;4:S16–S21. [PubMed] [Google Scholar]
- 4.Garrison JC, et al. In vivo evaluation and small-animal PET/CT of a prostate cancer mouse model using Cu-64 bombesin analogs: Side-by-side comparison of the CB-TE2A and DOTA chelation systems. J. Nucl. Med. 2007;48:1327–1337. doi: 10.2967/jnumed.107.039487. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson O, Kiesewetter DO, Chen X. Fluorine-18 radiochemistry, labeling strategies and synthetic routes. Bioconjug. Chem. 2014;26:1–18. doi: 10.1021/bc500475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brabez N, et al. Synthesis and evaluation of cholecystokinin trimers: a multivalent approach to pancreatic cancer detection and treatment. Biorg. Med. Chem. Lett. 2013;23:2422–2425. doi: 10.1016/j.bmcl.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Z, et al. Kit-like 18F-labeling of RGD-19F-arytrifluroborate in high yield and at extraordinarily high specific activity with preliminary in vivo tumor imaging. Nucl. Med. Biol. 2013;40:841–849. doi: 10.1016/j.nucmedbio.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Wangler C, et al. One-step F-18 labeling of peptides for positron emission tomography imaging using the SiFA methodology. Nat. Protoc. 2012;7:1946–1955. doi: 10.1038/nprot.2012.109. [DOI] [PubMed] [Google Scholar]
- 9.Lee E, et al. A fluoride-derived electrophilic late-stage fluorination reagent for PET imaging. Science. 2011;334:639–642. doi: 10.1126/science.1212625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huiban M, et al. A broadly applicable [18F]trifluoromethylation of aryl and heteroaryl iodides for PET imaging. Nat. Chem. 2013;5:941–944. doi: 10.1038/nchem.1756. [DOI] [PubMed] [Google Scholar]
- 11.McBride WJ, D’Souza CA, Karacay H, Sharkey RM, Goldenberg DM. New lyophilized kit for rapid radiofluorination of peptides. Bioconjug. Chem. 2012;23:538–547. doi: 10.1021/bc200608e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson O, et al. Rapid and simple one-step F-18 labeling of peptides. Bionconj. Chem. 2011;22:422–428. doi: 10.1021/bc100437q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pascali G, et al. Optimization of nucleophilic 18F radiofluorinations using a microfluidic reaction approach. Nat. Protoc. 2014;9:2017–2029. doi: 10.1038/nprot.2014.137. [DOI] [PubMed] [Google Scholar]
- 14.Richarz R, et al. Neither azeotropic drying, nor base nor other additives: a minimalist approach to 18F labeling. Org. Biomol. Chem. 2014;12:8094–8099. doi: 10.1039/c4ob01336k. [DOI] [PubMed] [Google Scholar]
- 15.Glaser M, et al. Three methods for 18F labeling of the HER2-binding affibody molecule ZHER2:2891 including preclinical assessment. J. Nucl. Med. 2013;54:1981–1988. doi: 10.2967/jnumed.113.122465. [DOI] [PubMed] [Google Scholar]
- 16.D’Souza CA, McBride WJ, Sharkey RM, Todaro LJ, Goldenberg DM. High-yielding aqueous F-18 labeling of peptides via (AlF)-F-18 chelation. Bioconjug. Chem. 2011;22:1793–1803. doi: 10.1021/bc200175c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang LX, et al. Comparison study of F-18 FAl-NOTA-PRGD2, F-18 FPPRGD2, and Ga-68 Ga-NOTA-PRGD2 for PET Imaging of U87MG tumors in mice. Bioconjug. Chem. 2011;22:2415–2422. doi: 10.1021/bc200197h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z, et al. An organotrifluoroborate for broadly applicable one-step 18F labeling. Angew. Chem. Int. Ed. Engl. 2014;53:11876–11880. doi: 10.1002/anie.201406258. [DOI] [PubMed] [Google Scholar]
- 19.Ting R, Adam MJ, Ruth TJ, Perrin DM. Arylfluoroborates and alkylfluorosilicates as potential PET imaging agents: high-yielding aqueous biomolecular 18F labeling. J. Am. Chem. Soc. 2005;127:13094–13095. doi: 10.1021/ja053293a. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, et al. Towards kit-like F-18 labeling of marimastat, a noncovalent inhibitor drug for in vivo PET imaging cancer associated matrix metalloproteases. Med Chem Comm. 2011;2:942–949. [Google Scholar]
- 21.Ting R, et al. Towards [18F]-labeled aryltrifluoroborate radiotracers–in vivo PET imaging of stable aryltrifluoroborate clearance in mice. J. Am. Chem. Soc. 2008;130:12045–12055. doi: 10.1021/ja802734t. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, et al. From minutes to years: predicting organotrifluoroborate solvolysis rates. Chem. Eur. J. 2015;21:3924–3928. doi: 10.1002/chem.201405829. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, et al. A new F-18-heteroaryltrifluoroborate radio-prosthetic with greatly enhanced stability that is labelled by F-18–F-19-isotope exchange in good yield at high specific activity. Med Chem Comm. 2014;5:171–179. [Google Scholar]
- 24.Liu ZB, et al. Stoichiometric leverage: Rapid 18F-aryltrifluoroborate radiosynthesis at high specific activity for click conjugation. Angew. Chem. Int. Ed. Engl. 2013;52:2303–2307. doi: 10.1002/anie.201208551. [DOI] [PubMed] [Google Scholar]
- 25.Schirrmacher R, Wangler C, Schirrmacher E. Recent developments and trends in F-18-radiochemistry: syntheses and applications. Mini Rev. Org. Chem. 2007;4:317–329. [Google Scholar]
- 26.Richter S, Wuest F. 18F-labeled peptides: the future is bright. Molecules. 2014;19:20536–20556. doi: 10.3390/molecules191220536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner S, et al. A new 18F-labelled derivative of the MMP inhibitor CGS 27023A for PET: radiosynthesis and initial small-animal PET studies. Appl. Radiat. Isot. 2009;67:606–610. doi: 10.1016/j.apradiso.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Schirrmacher R, et al. F-18-labeling of peptides by means of an organosilicon-based fluoride acceptor. Angew. Chem. Int. Ed. Engl. 2006;45:6047–6050. doi: 10.1002/anie.200600795. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z, et al. Preclinical evaluation of a high-affinity 18F-trifluoroborate octreotate derivative for somatostatin receptor imaging. J. Nucl. Med. 2014;55:1499–1505. doi: 10.2967/jnumed.114.137836. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, et al. Dual mode fluorescent 18F-PET tracers: efficient modular synthesis of rhodamine-[cRGD]2-[18F]-organotrifluoroborate, rapid, and high yielding one-step 18F-labeling at high specific activity, and correlated in vivo pet imaging and ex vivo fluorescence. Bioconjug. Chem. 2014;25:1951–1962. doi: 10.1021/bc5003357. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z, et al. 18F-Trifluoroborate derivatives of [des-Arg10]kallidin for imaging bradykinin B1 receptor expression with positron emission tomography. Mol. Pharm. 2015;12:974–982. doi: 10.1021/acs.molpharmaceut.5b00003. [DOI] [PubMed] [Google Scholar]
- 32.Chin FT, et al. First experience with clinical-grade F-18 FPP(RGD)2: an automated multi-step radiosynthesis for clinical PET studies. Mol. Imaging Biol. 2012;14:88–95. doi: 10.1007/s11307-011-0477-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, et al. Alkyne-18F-ArBF3 for one-pot click 18F-labeling of bombesin for in vivo PET imaging of tumors expressing the GRP-Receptor. Am. J. Nucl. Med. Mol. Imaging. 2013;3:57–70. [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Z, et al. Rapid, one-step, high yielding 18F-labeling of an aryltrifluoroborate bioconjugate by isotope exchange at very high specific activity. J. Labelled Comp. Radiopharm. 2012;14:491–497. [Google Scholar]
- 35.Monti SM, Supuran CT, De Simone G. Carbonic anhydrase IX as a target for designing novel anticancer drugs. Curr. Med. Chem. 2012;19:821–830. doi: 10.2174/092986712799034851. [DOI] [PubMed] [Google Scholar]
- 36.Stillebroer AB, Mulders PFA, Boerman OC, Oyen WJG, Oosterwijk E. Carbonic anhydrase IX in renal cell carcinoma: implications for prognosis, diagnosis, and therapy. Eur. Urol. 2010;58:75–83. doi: 10.1016/j.eururo.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Lam KS, et al. A new type of synthetic peptide library for identifying ligand-binding activity. Nature. 1991;354:82–84. doi: 10.1038/354082a0. [DOI] [PubMed] [Google Scholar]
- 38.Lee S, Xie J, Chen XY. Peptide-based probes for targeted molecular imaging. Biochemistry. 2009;49:1364–1376. doi: 10.1021/bi901135x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kilbourn MR, Hood JT, Welch MJ. A simple 18O water target for 18F production. Appl. Radiat. Isot. 1984;35:599–602. doi: 10.1016/0020-708x(84)90102-9. [DOI] [PubMed] [Google Scholar]
- 40.Wuest F, Berndt M, Bergmann R, van den Hoff J, Pietzsch J. Synthesis and application of 18F FDG-maleimidehexyloxime (18F FDG-MHO): A 18F FDG-based prosthetic group for the chemoselective 18F-labeling of peptides and proteins. Bioconjug. Chem. 2008;19:1202–1210. doi: 10.1021/bc8000112. [DOI] [PubMed] [Google Scholar]
- 41.Lewis JS, Srinivasan A, Schmidt MA, Anderson CJ. In vitro and in vivo evaluation of Cu-64-TETA-Tyr3-octreotate. A new somatostatin analog with improved target tissue uptake. Nucl. Med. Biol. 1999;26:267–273. doi: 10.1016/s0969-8051(98)00105-x. [DOI] [PubMed] [Google Scholar]
- 42.Coin I, Beyermann M, Bienert M. Solid-phase peptide synthesis: from standard procedures to the synthesis of difficult sequences. Nat. Protoc. 2007;2:3247–3256. doi: 10.1038/nprot.2007.454. [DOI] [PubMed] [Google Scholar]
- 43.Bräse S, Gil C, Knepper K, Zimmermann V. Organic azides: an exploding diversity of a unique class of compounds. Angew. Chem. Int. Ed. Engl. 2005;44:5188–5240. doi: 10.1002/anie.200400657. [DOI] [PubMed] [Google Scholar]
- 44.Scriven EFV, Turnbull K. Azides: their preparation and synthetic uses. Chem. Rev. 1988;88:297–368. [Google Scholar]