Abstract
Herpesvirus saimiri is an oncogenic herpesvirus that induces rapidly progressing lymphomas in New World primates. Using retrovirus vectors for gene transfer, specific open reading frames of H. saimiri were tested for their ability to transform rodent cells in culture. One open reading frame, designated STP-C488 (for saimiri-transformation-associated protein of the subgroup C strain 488), phenotypically transformed Rat-1 cells, resulting in formation of foci, growth at reduced serum concentration, and growth to higher cell densities. Cells transformed by STP-C488 formed invasive tumors in nude mice. The STP-A11 reading frame of strain 11 (subgroup A) was much less potent in its transforming ability than STP-C488. These results demonstrate the oncogene nature of these two open reading frames and provide a means for studying their transforming functions independent of the rest of the H. saimiri genome.
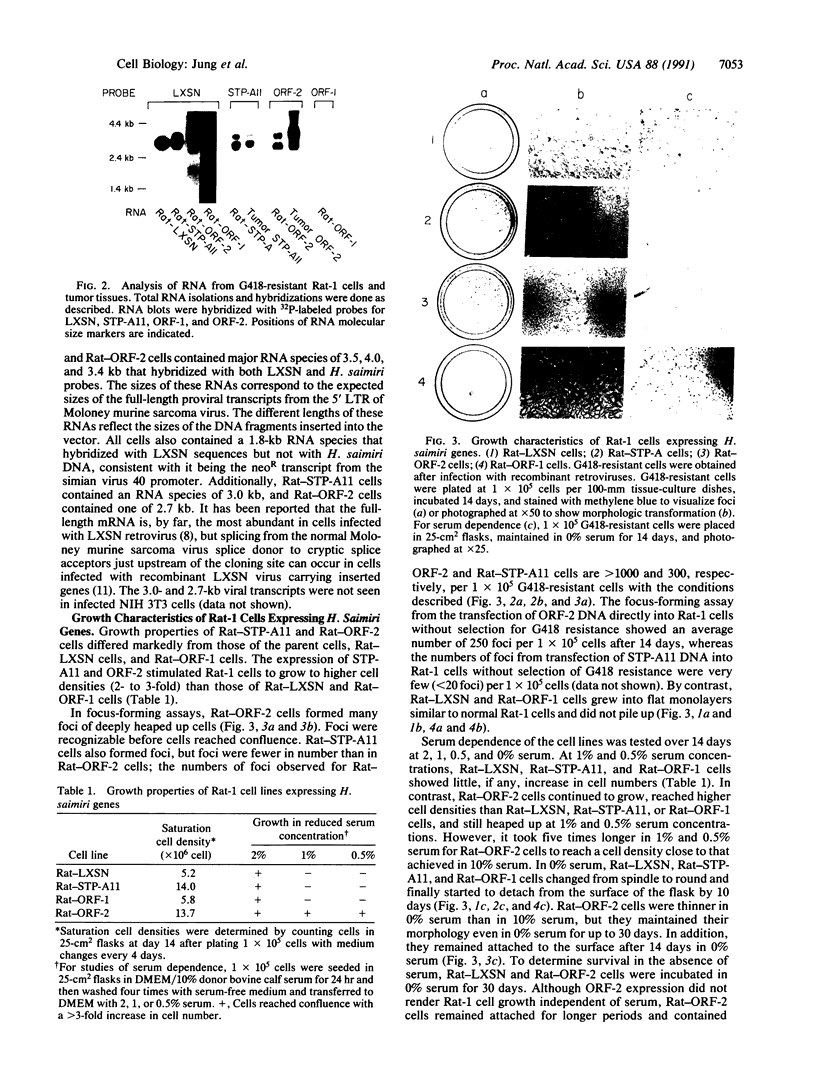
Full text
PDF

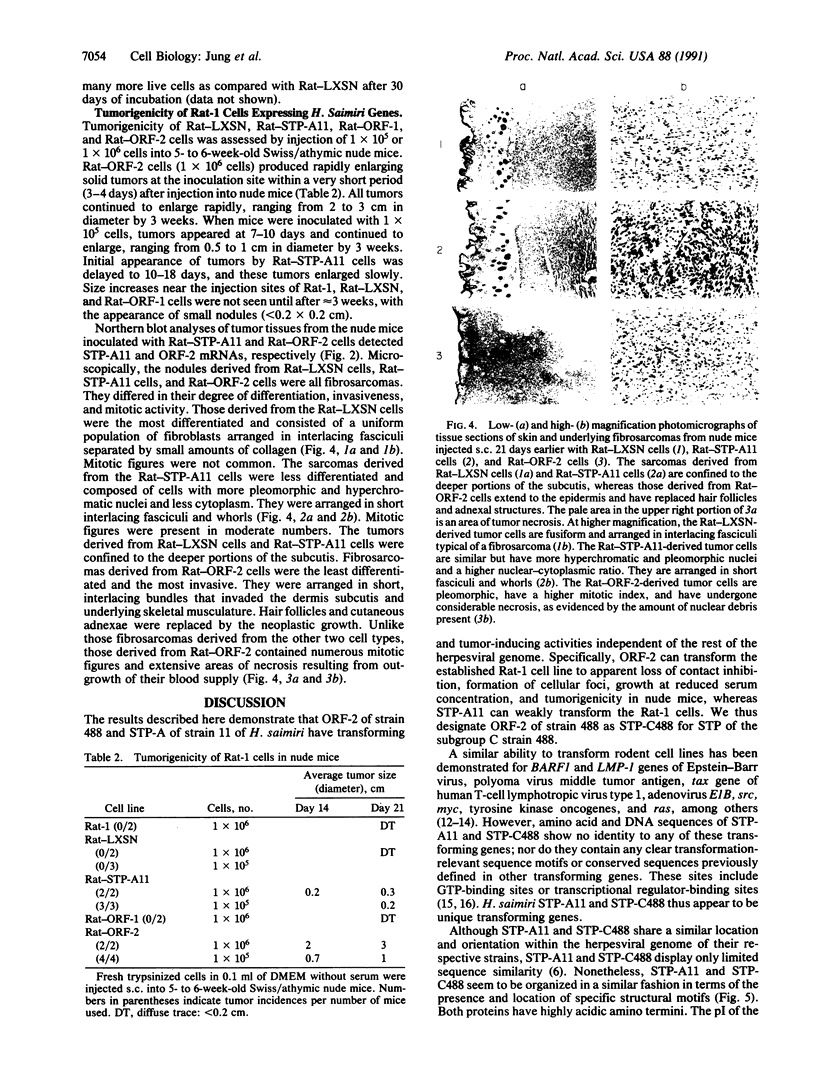

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biesinger B., Trimble J. J., Desrosiers R. C., Fleckenstein B. The divergence between two oncogenic Herpesvirus saimiri strains in a genomic region related to the transforming phenotype. Virology. 1990 Jun;176(2):505–514. doi: 10.1016/0042-6822(90)90020-r. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Compere S. J., Baldacci P. A., Sharpe A. H., Jaenisch R. Retroviral transduction of the human c-Ha-ras-1 oncogene into midgestation mouse embryos promotes rapid epithelial hyperplasia. Mol Cell Biol. 1989 Jan;9(1):6–14. doi: 10.1128/mcb.9.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C., Bakker A., Kamine J., Falk L. A., Hunt R. D., King N. W. A region of the Herpesvirus saimiri genome required for oncogenicity. Science. 1985 Apr 12;228(4696):184–187. doi: 10.1126/science.2983431. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Silva D. P., Waldron L. M., Letvin N. L. Nononcogenic deletion mutants of herpesvirus saimiri are defective for in vitro immortalization. J Virol. 1986 Feb;57(2):701–705. doi: 10.1128/jvi.57.2.701-705.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer K., Dordal M. S., Reynolds L. Mannose-binding proteins isolated from rat liver contain carbohydrate-recognition domains linked to collagenous tails. Complete primary structures and homology with pulmonary surfactant apoprotein. J Biol Chem. 1986 May 25;261(15):6878–6887. [PubMed] [Google Scholar]
- Geck P., Whitaker S. A., Medveczky M. M., Medveczky P. G. Expression of collagenlike sequences by a tumor virus, herpesvirus saimiri. J Virol. 1990 Jul;64(7):3509–3515. doi: 10.1128/jvi.64.7.3509-3515.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock R. A., Miller A. D., Osborne W. R. Expression of human adenosine deaminase from various strong promoters after gene transfer into human hematopoietic cell lines. Blood. 1989 Aug 1;74(2):876–881. [PubMed] [Google Scholar]
- Kodama T., Freeman M., Rohrer L., Zabrecky J., Matsudaira P., Krieger M. Type I macrophage scavenger receptor contains alpha-helical and collagen-like coiled coils. Nature. 1990 Feb 8;343(6258):531–535. doi: 10.1038/343531a0. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Medveczky M. M., Szomolanyi E., Hesselton R., DeGrand D., Geck P., Medveczky P. G. Herpesvirus saimiri strains from three DNA subgroups have different oncogenic potentials in New Zealand white rabbits. J Virol. 1989 Sep;63(9):3601–3611. doi: 10.1128/jvi.63.9.3601-3611.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medveczky P., Szomolanyi E., Desrosiers R. C., Mulder C. Classification of herpesvirus saimiri into three groups based on extreme variation in a DNA region required for oncogenicity. J Virol. 1984 Dec;52(3):938–944. doi: 10.1128/jvi.52.3.938-944.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Murthy S. C., Trimble J. J., Desrosiers R. C. Deletion mutants of herpesvirus saimiri define an open reading frame necessary for transformation. J Virol. 1989 Aug;63(8):3307–3314. doi: 10.1128/jvi.63.8.3307-3314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent M. A., Newman M. J. Inhibition of normal rat kidney cell growth by transforming growth factor-beta is mediated by collagen. J Biol Chem. 1989 Oct 25;264(30):18060–18067. [PubMed] [Google Scholar]
- Reid K. B. Complete amino acid sequences of the three collagen-like regions present in subcomponent C1q of the first component of human complement. Biochem J. 1979 May 1;179(2):367–371. doi: 10.1042/bj1790367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B. Molecular cloning and characterization of the complementary DNA and gene coding for the B-chain of subcomponent C1q of the human complement system. Biochem J. 1985 Nov 1;231(3):729–735. doi: 10.1042/bj2310729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage H. Low molecular weight fibroblast collagen: structure, secretion, and differential expression as a function of fetal and cellular age. Biochemistry. 1985 Dec 3;24(25):7430–7440. doi: 10.1021/bi00346a060. [DOI] [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Waterhouse E. J., Quesenberry P. J., Balian G. Collagen synthesis by murine bone marrow cell culture. J Cell Physiol. 1986 Jun;127(3):397–402. doi: 10.1002/jcp.1041270307. [DOI] [PubMed] [Google Scholar]
- Wei M. X., Ooka T. A transforming function of the BARF1 gene encoded by Epstein-Barr virus. EMBO J. 1989 Oct;8(10):2897–2903. doi: 10.1002/j.1460-2075.1989.tb08438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. T., Damm D., Miller J., Spratt K., Schilling J., Hawgood S., Benson B., Cordell B. Isolation and characterization of the human pulmonary surfactant apoprotein gene. 1985 Sep 26-Oct 2Nature. 317(6035):361–363. doi: 10.1038/317361a0. [DOI] [PubMed] [Google Scholar]







