Abstract
Shiga toxin-producing Escherichia coli belonging to serotype O26:H11 was isolated from a 2-month-old guanaco with severe watery diarrhea. E. coli colonies carried the stx1 and eae genes, showed localized adherence to HEp-2 cells, and produced enterohemolysin. A serological response to lipopolysaccharide O26 was observed at the onset of diarrhea.
Shiga toxin (Stx)-producing Escherichia coli (STEC) is recognized as a cause of diarrhea, hemorrhagic colitis, and hemolytic uremic syndrome in human beings (17). Besides producing Stx, some STEC strains express intimin, a protein responsible for the strong attachment of bacteria to intestinal epithelial cells, causing a cytopathic attaching and effacing (A/E) lesion. Intimin is encoded by the eae gene, located in a chromosomal pathogenicity island referred to as the locus of enterocyte effacement (16). Localized adherence to epithelial cells, an adhesive property of enteropathogenic E. coli (EPEC) (8) and some STEC strains (6, 30), is closely related to the ability to produce intimin, though the mere presence of the eae gene does not confer the adherence phenotype upon any E. coli strain (16). A subset of STEC isolates with virulence-associated traits, such as a defined serotype, production of enterohemolysin (Ehly), and the presence of the eae gene, are recognized as enterohemorrhagic E. coli (EHEC) strains.
Ruminants, particularly cattle and sheep, are reservoirs of STEC. Although healthy animals normally carry and shed a variety of serotypes (3, 4, 5), natural outbreaks and experimental infections have demonstrated that STEC strains that induce the A/E lesion can also cause diarrhea and dysentery in young calves (13, 19, 23, 24, 29, 30).
Serotypes of frequently implicated STEC strains were O5:H−, O26:H11, O103:H2, O111:H−, and O145:H−. These and other virulence-associated traits, such as production of Ehly and localized and diffuse adherence patterns to epithelial cells, are shared with human STEC strains. The purpose of this study was to report the isolation and characterization of an STEC strain from a diarrheic guanaco (Lama guanicoe) cria and the concomitant increase of lipopolysaccharide (LPS) antibody levels.
A group of 18 1-month-old guanaco crias belonging to a breeding farm located in the Rio Negro Province (Patagonia region), Argentina, were transported to the Instituto Nacional de Tecnología Agropecuaria Castelar Experimental Station, Buenos Aires Province, Argentina, according to proper management practices for this species. The animals were dewormed at entry and placed in a corral with access to shelter. They were fed with bovine pasteurized milk three times a day and received potable water ad libitum. One month later, two crias were housed in an isolation facility for physiological studies. Eight days after confinement, one of the crias developed a severe case of green, watery diarrhea. The animal was orally rehydrated with an electrolyte solution. The diarrhea was autolimited 3 days after onset without antimicrobial therapy.
Fecal specimens of the confined crias were collected daily and screened for the presence of rotavirus (RV) antigen by enzyme-linked immunosorbent assay (ELISA) as described previously (7). In the case of the diarrheic animal, feces collected 1 day before diarrhea onset, during the peak of severe diarrhea, and 4 days after the onset of diarrhea were screened for E. coli, Yersinia enterocolitica, Salmonella enterica, and Clostridium perfringens. Isolations of E. coli and Y. enterocolitica were performed with MacConkey agar, whereas Salmonella organisms were isolated with salmonella-shigella and Wilson-Blair agars after an enrichment step in tetrathionate broth. C. perfringens was investigated by isolating it under an anaerobic atmosphere in Brewer and egg yolk agars. Standard biochemical tests were used for confirmation of suspected colonies (10). The stx1, stx2, and eae sequences were sought by PCR according to the protocol of Blanco et al. (3). Bacterial strains harboring stx sequences were tested on Vero cells to determine Stx production, as described previously (18). The HEp-2 cell adhesion assay and detection of the Ehly phenotype were previously described elsewhere (19). O and H antigens were examined according to standard procedures (20). Antisera for O26, O111, O157, H7, and H11 antigens were provided by the ANLIS Institute of Microbiology “Dr. C. G. Malbrán,” Buenos Aires, Argentina.
Serum samples from all animals were collected upon arrival to the station and monthly up to 3 months postarrival. ELISA techniques were adapted for RV antibody detection in serum by previously described assays (21). Each sample was assayed in serial fourfold dilutions. Anti-LPSO26 titers in serum were determined by a dot blot ELISA as described previously (9). We used purified LPS obtained from the isolated STEC O26 strain by the method of Westphal and Jann (28). Ten micrograms of LPS and serial twofold serum dilutions ranging from 1:15 to 1:480 were applied per well. Peroxidase-labeled goat anti-llama immunoglobulin G (IgG) (H+L; Bethyl Laboratories, Inc.) was used as conjugate for both assays.
All animals were seropositive for RV at arrival, and two crias were shedding the virus asymptomatically, indicating its circulation in the herd. However, RV was not detected in the feces of the diarrheic animal, which also showed the lowest anti-RV titer of the group of camelids.
Among all pathogens investigated, the only one detected in the diarrheic animal was E. coli. Four out of six selected colonies were positive for the stx1 and eae genes (Fig. 1), produced Stx according to the Vero cell culture assay, and showed the Ehly-positive phenotype. In agreement with the detection of the eae gene, the Stx-producing E. coli strains showed localized adherence (Fig. 2) in the HEp-2 cell adhesion assay. The isolates belonged to serotype O26:H11. Fecal samples collected 4 days after the onset of diarrhea were negative for STEC O26:H11, and three of six colonies corresponded to STEC O26:H11 on the sample collected 1 day before the animal became ill. Only the sample collected at the peak of diarrhea showed a watery aspect. An eightfold increase in the level (15 to 120 [the titer was expressed as the inverse of the maximal dilution with a positive signal]) of LPSO26 antibodies was detected in the serum sample collected during the acute period, and the level remained constant in the samples taken 1 and 2 months later. Further searching for LPSO26 antibodies in sera collected upon arrival at the Experimental Station from eight selected healthy animals showed titers equal to or higher than 80, which continued unaltered on the subsequent serum samples.
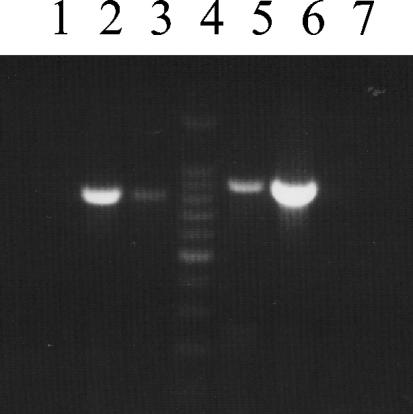
FIG. 1.
PCR amplification products corresponding to the eae gene from E. coli strains O26:H11 (lane 3), EDL933 (eae positive control) (31) (lane 2), and DH5α (eae negative control) (lane 1) and to the stx1 gene from E. coli O26:H11 (lane 5), C600(933J) (stx1 positive control) (31) (lane 6), and DH5α (stx1 negative control) (lane 7). Sizes of eae and stx1 amplicons are 815 and 894 bp, respectively. Lane 4 contains 100-bp molecular mass marker.
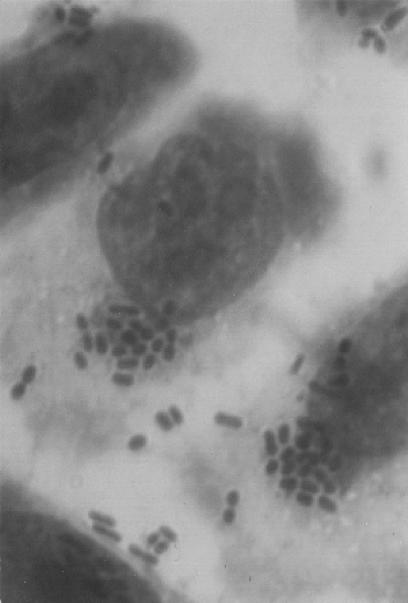
FIG. 2.
Microphotography showing the localized adherence of E. coli O26:H11 to HEp-2 cells (magnification, ×1,250).
A limited number of reports concerning infectious diseases of South American camelids (SAC) has been published in recent years (1, 11, 14, 21, 22, 26, 27). Enteric pathogens, such as C. perfringens, Salmonella sp., RV, coronavirus, and certain parasites, such as Cryptosporidium sp., Giardia duodenalis, and coccidia, have been reported to be involved in outbreaks or sporadic cases of diarrhea in these animals (1, 21, 22). In particular, there is one report of a diarrheic outbreak where RV associated with E. coli or Salmonella sp. was detected in the feces of newborn guanacos from the Argentinean Patagonia region (21).
In this study, an STEC strain was isolated from the feces of a 2-month-old SAC with diarrhea. Remarkably, we found STEC colonies in samples collected during the diarrheic episode and 1 day before the onset of diarrhea but not later. This isolate presented the typical characteristics of O26:H11 EHEC strains and seemed to lead to a seroconversion in the diseased guanaco. Surprisingly, a high titer of LPSO26 antibodies was observed in some of the young guanacos when they arrived at the research station. While these antibodies could be of colostral origin, an active immune response to previous infection in 1-month-old animals could not be discounted. It is well known that colostrum-deprived neonate animals, such as calves and pigs, are able to develop an active immune response against a natural or experimental infection early in life (2, 15). As the goat anti-llama IgG (H+L) used here was reported by the manufacturer to react also with light chains common to other llama immunoglobulins (IgM and IgA), the antibodies detected during the acute phase could be a primary IgM response or a secondary IgG response. However, because no reagent to detect camelid IgM and IgA antibodies was available, the isotype-related response to E. coli LPS could not be discriminated in the present study.
The source of contamination with STEC O26:H11 of the young camelid remains unclear. Previous contamination of the environment with bovine fecal bacteria could have served as a source of infection for the diarrheic animal. It is documented that some serotypes of STEC, such as O157:H7, O26:H11, and O111:H−, can survive in bovine feces for a long time, especially at 15°C (12). However, we cannot discount the existence of a resident population of serologically diverse STEC strains in guanacos, including serotypes such as O26:H11, which could be responsible for the LPSO26 antibodies detected in the animals when they arrived at the Experimental Station. Similarly, high levels of specific LPSO157 antibodies have been observed with human E. coli O157 disease, and a serological test was proposed to provide evidence of infection (25).
Taking into account that we were not able to demonstrate the presence of any other enteric pathogen in the stools of the diarrheic guanaco, our data suggest that STEC O26:H11 was responsible for the symptoms observed in the affected animal.
Although isolation of STEC strains from several animal species has been reported, most of these strains were not identified as typical EHEC strains. According to the virulence markers investigated, the present isolate was indistinguishable from EHEC associated with severe human and bovine disease. To our knowledge, this is the first documented isolation of E. coli O26:H11 with genetic determinants corresponding to Stx-producing strains with the ability to form A/E lesions in a SAC.
Since cattle and sheep, the principal reservoirs of STEC, are pregastric fermentors, there is a high risk that SAC, their close relatives, will become an important source of harmful human STEC, as well as EHEC, in the future. The housing of guanacos in the same environment as cattle will probably facilitate the transmission of pathogens from domestic species to SACs. Implementation of management practices will change living conditions for camelids. Indeed, factors such as diet, direct contact with soil and water contaminated with bovine feces, housing, and other activities or situations causing stress could give rise to carriage of STEC by these animals.
Acknowledgments
We thank L. Beutin and I. Scaletsky for providing the reference strains, O. Zabal for generously supplying cell cultures, M. L. Martìnez and L. González for their technical assistance, and members of the microscopy department of A. Bolondi for their kind assistance in microscopy analysis.
REFERENCES
- 1.Adams, R., and F. B. Garry. 1992. Gram-negative bacterial infection in neonatal New World camelids: six cases (1985-1991). J. Am. Vet. Med. Assoc. 201:1419-1424. [PubMed] [Google Scholar]
- 2.Barrington, G. M., and S. M. Parish. 2001. Bovine neonatal immunology. Vet. Clin. North Am. Food Anim. Pract. 17:463-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanco, M., J. E. Blanco, J. Blanco, A. Mora, C. Prado, M. P. Alonso, M. Mouriño, C. Madrid, C. Balsalobre, and A. Juárez. 1997. Distribution and characterization of faecal verotoxin-producing Escherichia coli (VTEC) isolated from healthy cattle. Vet. Microbiol. 54:309-319. [DOI] [PubMed] [Google Scholar]
- 4.Cerqueira, A. M. F., B. E. C. Guth, R. M. Joaquim, and J. R. C. Andrade. 1999. High occurrence of Shiga toxin-producing Escherichia coli (STEC) in healthy cattle in Rio de Janeiro State, Brazil. Vet. Microbiol. 70:111-121. [DOI] [PubMed] [Google Scholar]
- 5.Cobbold, R., and P. Desmarchelier. 2000. A longitudinal study of Shiga-toxigenic Escherichia coli (STEC) prevalence in three Australian dairy herds. Vet. Microbiol. 71:125-137. [DOI] [PubMed] [Google Scholar]
- 6.Cookson, A. L., and M. J. Woodward. 2003. The role of intimin in the adherence of enterohaemorrhagic Escherichia coli (EHEC) O157: H7 to HEp-2 tissue culture cells and to bovine gut explant tissues. Int. J. Med. Microbiol. 292:547-553. [DOI] [PubMed] [Google Scholar]
- 7.Cornaglia, E. M., M. Barrandeguy, N. Fijtman, and A. Schudel. 1989. Enzyme link immunosorbent assay, immunofluorescent test and electrophoretic analysis of rotaviral RNA in the diagnosis and characterization of the bovine rotavirus. Rev. Latinoam. Microbiol. 31:59-62. [Google Scholar]
- 8.Cravioto, A., R. J. Gross, S. M. Scotland, and B. Rowe. 1979. An adhesive factor found in strains of Escherichia coli belonging to the traditional infantile enteropathogenic serotypes. Curr. Microbiol. 3:95-99. [Google Scholar]
- 9.Dean-Nystrom, E. A., L. J. Gansheroff, M. Mills, H. W. Moon, and A. D. O'Brien. 2002. Vaccination of pregnant dams with intiminO157 protects suckling piglets from Escherichia coli O157:H7 infection. Infect. Immun. 70:2414-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwing, W. H. 1986. The genus Escherichia, p. 93-134. In W. H. Ewing (ed.), Identification of Enterobacteriaceae, 4th ed. Elsevier Science Publishing Co., Inc., New York, N.Y.
- 11.Frank, N., L. L. Couetil, and K. A. Clarke. 1998. Listeria monocytogenes and Escherichia coli septicemia in a 7-day-old llama. Can. Vet. J. 39:100-102. [PMC free article] [PubMed] [Google Scholar]
- 12.Fukushima, H., K. Hoshina, and M. Gomyoda. 1999. Long-term survival of Shiga toxin-producing Escherichia coli O26, O111, and O157 in bovine feces. Appl. Environ. Microbiol. 65:5177-5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall, G. A., D. J. Reinolds, N. Chanter, J. H. Morgan, K. R. Parsons, T. G. Debeney, A. P. Bland, and J. C. Bridger. 1985. Dysentery caused by Escherichia coli (S102-9) in calves: natural and experimental disease. Vet. Pathol. 22:156-163. [DOI] [PubMed] [Google Scholar]
- 14.Hewson, J., and C. K. Cebra. 2001. Peritonitis in a llama caused by Streptococcus equi subsp. zooepidemicus. Can. Vet. J. 42:465-467. [PMC free article] [PubMed] [Google Scholar]
- 15.Hodgins, D. C., S. Y. Kang, L. deArriba, V. Parreño, L. A. Ward, L. Yuan, T. To, and L. J. Saif. 1999. Effects of maternal antibodies on protection and development of antibody responses to human rotavirus in gnotobiotic pigs. J. Virol. 73:186-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 87:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karmali, M. A. 1989. Infection by verocytotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 2:15-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konowalchuk, J., J. I. Speirs, and S. Stavric. 1977. Vero response to a cytotoxin of Escherichia coli. Infect. Immun. 18:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mercado, E. C., A. Gioffré, S. M. Rodríguez, A. Cataldi, K. Irino, A. M. Elizondo, A. L. Cipolla, M. I. Romano, R. Malena, and M. A. Méndez. 2004. Non-O157 Shiga toxin-producing Escherichia coli isolated from diarrhoeic calves in Argentina. J. Vet. Med. B 51:82-88. [DOI] [PubMed] [Google Scholar]
- 20.Ørskov, I., and F. Ørskov. 1984. Serotyping of Escherichia coli. Methods Microbiol. 14:43-112. [Google Scholar]
- 21.Parreño, V., V. Constantini, S. Cheetham, J. Blanco Viera, L. Saif, F. Fernández, L. Leoni, and A. Schudel. 2001. First isolation of rotavirus associated with neonatal diarrhoea in guanacos (Lama guanicoe) in the Argentinean Patagonia region. J. Vet. Med. B 48:713-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rulofson, F. C., E. R. Atwill, and C. A. Holmberg. 2001. Fecal shedding of Giardia duodenalis, Cryptosporidium parvum, Salmonella organisms, and Escherichia coli O157: H7 from llamas in California. Am. J. Vet. Res. 62:637-642. [DOI] [PubMed] [Google Scholar]
- 23.Sandhu, K. S., and C. L. Gyles. 2002. Pathogenic Shiga toxin-producing Escherichia coli in the intestine of calves. Can. J. Vet. Res. 66:65-72. [PMC free article] [PubMed] [Google Scholar]
- 24.Schoonderwoerd, M., R. C. Clarke, A. A. van Dreumel, and S. A. Rawluk. 1988. Colitis in calves: natural and experimental infection with a verotoxin-producing strain of Escherichia coli O111: NM. Can. J. Vet. Res. 52:484-487. [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas, A., T. Cheasty, J. A. Frost, H. Chart, H. R. Smith, and B. Rowe. 1996. Vero cytotoxin-producing Escherichia coli, particularly serogroup O157, associated with human infections in England and Wales: 1992-4. Epidemiol. Infect. 117:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsur, I., A. Harmelin, I. Dvir, and J. Yanai. 1996. Meningoencephalitis and brain abscessation due to Escherichia coli in a 2 week old alpaca cria. Aust. Vet. J. 74:437-438. [DOI] [PubMed] [Google Scholar]
- 27.Uzal, F. A., R. A. Assis, and E. C. Reissig. 2000. Malignant oedema in a guanaco (Lama guanicoe). Vet. Rec. 147:336. [DOI] [PubMed] [Google Scholar]
- 28.Westphal, O., and K. Jann. 1965. Bacterial lipopolysacharides, p. 83-91. In R. L. Whistler (ed.), Methods in carbohydrate chemistry. Academic Press, New York, N.Y.
- 29.Wieler, L. H., R. Bauerfeind, and G. Baljer. 1992. Characterization of Shiga-like toxin producing Escherichia coli (SLTEC) isolated from calves with and without diarrhoea. Zentbl. Bakteriol. 276:243-253. [DOI] [PubMed] [Google Scholar]
- 30.Wieler, L. H., E. Vieler, C. Erpenstein, T. Schlapp, H. Steinrück, R. Bauerfeind, A. Byomi, and G. Baijer. 1996. Shiga toxin-producing Escherichia coli strains from bovines: association of adhesion with carriage of eae and other genes. J. Clin. Microbiol. 34:2980-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu, J., and J. B. Kaper. 1992. Cloning and characterization of the eae gene of enterohemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 6:411-417. [DOI] [PubMed] [Google Scholar]