Abstract
The largest reported outbreak of type C botulism in fur production animals is described. Epidemiological investigation of 117 out of 157 (response rate, 74.5%) farms revealed that 44,130 animals died or were euthanized, while 8,033 animals with milder symptoms recovered. The overall death rate in all animals at risk was 21.7%. The death rates were significantly higher in blue and shadow foxes (24.2 and 27.8%, respectively) than in silver and blue silver foxes and minks (below 4%). All minks had been immunized against botulinum toxin type C. Deaths were associated with feed manufactured by a local processor, 83 of whose customer farms (70.9%) reported dead or sick animals. Five feedlots out of 19 delivered to the farms on the day preceding the onset of the outbreak (day 2) were associated with a death rate higher than 40%. These feedlots consisted of fresh feed processed on day 2 and feed processed 1 day earlier (day 1). In laboratory analysis, the day 2 feed contained botulinum toxin type C (>600 minimum lethal doses/g), while the day 1 feed did not contain toxin. Toxin was not detected in feed raw-material samples. Clostridium botulinum type C was detected by PCR in some feed components and in feed. However, as the feed temperature was continuously 8°C or below and the pH was continuously 5.6 or below according to the manufacturer, it seems unlikely that spore germination and toxin formation occurred during overnight storage. Hence, the events leading to toxin formation were not determined.
Botulism is a paralytic disease caused by botulinum neurotoxin blocking neurotransmitter release in peripheral nerve endings. Botulinum neurotoxin is produced during exponential growth of the anaerobic bacterium Clostridium botulinum. Based on their serological properties, botulinum neurotoxins are classified into types A to G, of which types C and D, being produced by group III C. botulinum strains, cause disease in animals. Species sensitive to botulinum toxins include birds, horses, cattle, sheep, and fur production animals.
In the fur production industry, botulism outbreaks affecting hundreds or thousands of animals have caused considerable economical losses. Botulism outbreaks in fur-bearing animals are typically feed poisonings due to botulinum toxin formation in improperly chilled slaughter by-products that are used in feed manufacturing. Minks and ferrets are highly susceptible to botulinum toxins, type C toxin in particular, and reports of large outbreaks in these animals are available (3, 5, 7, 10, 19). Unlike minks, foxes are generally considered resistant to botulinum toxins (6, 20), and very few type C botulism outbreaks in foxes have been reported (6, 10, 17, 18). The oral toxicity of type C botulinum toxin to foxes has been reported to vary from 103 to higher than 108 minimum lethal doses (MLD, measured by intraperitoneal injection into mice) per animal (2, 6, 20, 29).
In order to control the microbiological hazards associated with fur animal feed production in Finland, national regulations regarding the safe handling of slaughter by-products have been set (13). The slaughter by-products are to be cut to a maximum final particle size of 50 mm and either acidified with an organic acid (typically formic or acetic acid) to yield a final pH of no higher than 4.0 or heat processed to a minimum internal temperature of 80°C in all parts of the product. In practice, however, all slaughter by-products are acidified rather than heat processed. The pH of the acidified slaughter by-product must be measured upon arrival at the feed processing plant, and it must not exceed 4.3 (13).
In order to inhibit the growth of yeasts and molds, the addition of sodium benzoate to slaughter by-products is also advised (13). Unless used in feed production immediately after arrival at the feed processing plant, the slaughter by-products must be stored at −18°C or below. Fur animal carcasses must be processed at 118°C for 20 min at 2 bars (13). In addition to these control measures, all minks are immunized against botulinum toxin type C at the age of 2 months. Foxes, however, have generally been considered immune to botulinum toxin and thus have not been vaccinated in Finland. Large botulism outbreaks in fur production animals have not been reported in Finland during the past decades.
This paper describes a recent massive outbreak of type C feed poisoning botulism affecting 52,000 farmed foxes in Finland in 2002. To our knowledge, this is the largest botulism outbreak ever reported in fur production animals.
MATERIALS AND METHODS
Background.
The outbreak occurred in October 2002 in an area restricted to 80 by 120 km in the Central Ostrobothnian region in western Finland. On Saturday, 5 October (day 3), and Sunday, 6 October (day 4), regional fur animal veterinarians were notified by farmers about illness and death of farmed foxes and minks (Table 1). By the end of the weekend, it became clear that dozens of farms had thousands of dead or sick animals. All of these farms were customers of the same feed processing plant.
TABLE 1.
Chronological events determining the type C botulism outbreak in October 2002
| Date | Day | Event |
|---|---|---|
| Thursday, 3 October | 1 | Routine feed production; a total of 46,000 kg of leftover feed stored overnight in two trucks and in the loading silo; 3 batches of base mass were prepared (4,300 kg each) |
| Friday, 4 October | 2 | Leftover feed from previous day transported to farms (43,000 kg) or mixed (3,000 kg) in the morning's first feed lot; later tests showed that the morning's first feed lots contained botulinum toxin type C |
| Saturday, 5 October | 3 | First signs of botulism in animals observed |
| Sunday, 6 October | 4 | Sick and dead animals observed at tens of farms |
| Monday, 7 October | 5 | Outbreak mentioned by the media; laboratory and epidemiological investigations of the outbreak begun |
Sixteen processing plants are responsible for fur animal feed production in Finland. The feed is mostly composed of acidified slaughter by-products with an approximate pH of 4.0, heat processed (118°C, 20 min, 2 bars) fur animal carcasses, ensiled fish and fish entrails, barley and soy four, vitamins, and fat. A total of 95% of slaughter by-products are of Finnish origin and thus mainly acidified, while the remaining 5%, imported mainly from Sweden, may be either acidified or frozen without any acid or heat treatment. The temperature of the acidified slaughter by-products is not controlled in the slaughterhouses, and depending on the time of year and thus the ambient temperature, it may vary greatly. However, due to its low pH and the sodium benzoate addition, the final feed is microbiologically very stable at ambient temperature. In practice, feed storage for longer than a few days is uncommon, but in the rare occasions of extended storage, the feed is normally frozen. The feed composition is regulated by the guidelines of the Finnish Fur Breeders' Association, and it may vary slightly with producer, time of year, and availability of food components.
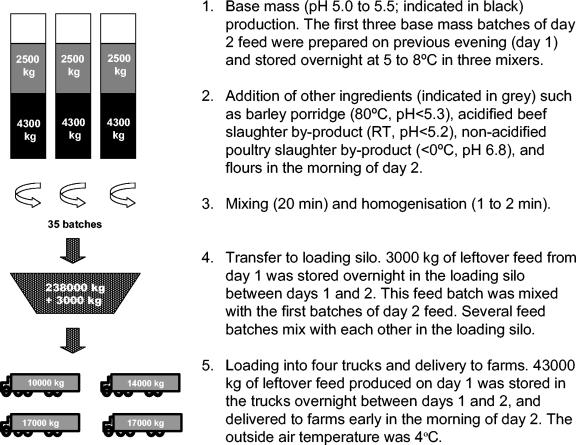
The flow chart of the feed process routinely employed in the feed manufacturing plant associated with the botulism outbreak is presented in Fig. 1. In order to speed up feed production in the early morning of Friday, 4 October (day 2), this was started in the previous afternoon (day 1) at 1600 to 1700 h by the preparation of three batches of feed base mass. These were prepared in three large mixers (each with a capacity of 6,800 kg) by adding frozen acidified beef slaughter by-products, fresh and frozen fish, processed (118°C, 20 min, 2 bar) fur animal carcasses, mink fat, barley and soy flour, vitamins, and meat and bone powder (processed at 133°C, 20 min, 3 bars). The ingredients were roughly mixed but not homogenized at this stage, and the three 4,300-kg batches of such feed base mass were then stored in the mixers overnight. According to the manufacturer, the feed base mass had a typical pH of 5.0 to 5.5 and, due to the large amount of frozen raw material, an internal temperature of 5 to 8°C. No other chilling procedures were employed. The pH and temperature data of the feed base mass, however, were not available upon inspection at the processing plant.
FIG. 1.
Flow chart describing the feed processing practices normally utilized in the feed manufacturing plant associated with the botulism outbreak.
The process continued early in the morning of day 2 with the addition of a total of 2,500 kg of barley porridge (normal pH range, 4.5 to 5.3; internal temperature, 70 to 80°C), crops, acidified beef slaughter by-product, and nonacidified frozen poultry slaughter by-products into each of the three mixers (Table 2). The barley porridge was prepared overnight on-site by heating 6,000 liters of water to an internal temperature of 95°C, after which it was cooled down to 70°C and mixed with barley flour. After boiling for 30 min, this mixture was kept continuously at 70°C overnight and added to the feed mixture the next day. After addition of all ingredients, the complete feed mass was mixed for 20 min, followed by a quick homogenization to a final particle size of 10 mm. A total of 35 similar batches (238,000 kg total) were produced during day 2.
TABLE 2.
Analysis of C. botulinum type and count, presence of botulinum toxin, and pH in the raw materials used in the production of fur animal feed and in completed feeda
| Sample | pH | PCR resultb | Toxin assayc (MLD/g) |
|---|---|---|---|
| Raw materials (country of origin) added to feed base mass produced on day 1 and stored overnight | |||
| Fish entrails (Finland) | 5.3 | 0/20 | — |
| Frozen fish (Finland) | NA | NA | NA |
| Acidified beef slaughter waste material (Finland) | 4.3-5.2 | C, 1/60 (0.02) | — |
| Heat-processed fur animal carcasses (Finland) | ND | 0/90 | — |
| Mink fat (Finland) | ND | 0/20 | — |
| Barley flour (Finland) | NA | NA | NA |
| Soy flour (Germany) | ND | 0/10 | — |
| Feather powder (Germany) | 6.6 | C, 4/20 (0.2) | — |
| Meat and bone powder (Finland) | 6.6 | B, 2/20 (0.1); C, 2/20 (0.1) | — |
| Meat powder (Finland) | ND | 0/10 | — |
| Vitamin preparations (Finland) | ND | C, 1/60 (0.02) | ND |
| Iron solution (Finland) | ND | 0/30 | ND |
| Chalk (Finland) | ND | 0/10 | — |
| Methionine solution (Finland) | ND | 0/20 | ND |
| Raw materials added to base mass on day 2 before mixing | |||
| Barley porridge (Finland) | 4.5-5.3d | NA | NA |
| Acidified beef slaughter waste material (Finland)e | |||
| Nonacidified poultry slaughter waste material (Sweden) | 6.8-6.9 | C, 19/70 (0.3); E, 1/70 (0.01) | — |
| Flour (Finland) | NA | NA | NA |
| Completed fur animal feed | |||
| Feed produced on day 1 | 5.1 | 0/10 | — |
| Feed produced on day 2 | 4.9 | C, 4/10 (0.5) | C (>600) |
| Feed produced on day 3 | 4.6-5.6 | C, 8/270 (0.03); E, 1/270 (0.004) | — |
| Composite sample of feed produced on days 2 and 3 | 4.9 | 0/30 | C |
NA, feed component not available for laboratory investigation; ND, not determined.
C. botulinum type detected, number of positive tubes/number of tubes tested (most-probable-number estimate of cell count [spores/gram of sample material]).
Type of toxin detected and toxin concentration. —, no toxin detected.
Normal pH range as reported by the feed processing plant associated with the outbreak.
Described elsewhere in the table.
After homogenization, the feed was further transferred to a loading silo (capacity, 75,000 kg) so that several different batches from the three mixers were mingled in the large loading silo. From this silo, the feed was loaded into four trucks, indicated as trucks A to D: A, capacity for 10,000 kg of feed; B, 14,000 kg; C, 17,000 kg; and D, 17,000 kg. The trucks transported the feed to the animal farms, and each driving round was up to 120 km long. After each driving round, the trucks returned to the processing plant to refill their tanks and proceed to other farms. The day 2 feed production finished by 1550 h.
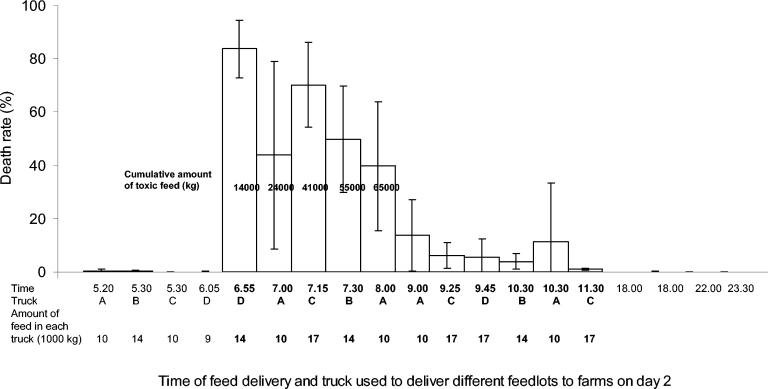
Whereas the loading silo that is located outside of the processing plant building is normally emptied and cleaned at the end of a day, on the night between days 1 and 2 it contained 22,000 kg of leftover feed produced on day 1. In addition, the tanks of trucks A and B were full of this leftover feed on the same night. Before starting feed production early in the morning of day 2, most of the leftover feed from day 1 was delivered to farms by the four trucks, with the exception of a 3,000-kg fraction that remained in the loading silo and was further mixed with the first three feed lots (20,400 kg) produced on day 2. The first trucks (D and A) transporting the day 2 feed loaded their tanks with the mixture of day 1 and day 2 feed at 0655 h. A total of 19 drives were made during day 2, with the last feed lot being transported by 2330 h that night (Fig. 2).
FIG. 2.
Mean animal death rate (error bars indicate standard deviation) observed on farms receiving feed on day 2. The feed lots indicated in normal typeface were produced on day 1, and those indicated in bold were produced on day 2. The truck (A to D) used to deliver each feed lot and the amount of feed in each truck are indicated below the chart. Bold numbers in the columns indicate the cumulative amounts of day 2 feed delivered.
According to the local meteorological observation center, the outside air temperature during the night between day 1 and day 2 was constantly below 4°C.
Epidemiological investigation.
A questionnaire regarding the animal death rates, recovery rates, the time of feeding the suspect feed to the animals, the time of the first observation of clinical symptoms, and the description of the symptoms was sent to all 157 breeders that were customers of the plant that processed the suspect feed. Of these 157 breeders, 117 (74.5%) returned the questionnaire form with complete information. The animals at these farms were considered at risk. The 40 breeders not responding to the questionnaire included farms both with and without dead animals and were not included in the epidemiological investigation. However, an estimate of the number of dead animals on these farms was produced by using the mean farm size (1,736 animals) and the mean death rate of each feed transport lot.
The four truck drivers delivering feed to the farms on day 2 were interviewed, and their exact driving routes and departure times were recollected and analyzed.
Sample analyses.
A total of 59 samples (one to nine samples per sample material) of feed and its raw material components were investigated for the presence of botulinum neurotoxin by the mouse bioassay (15). To determine the toxin type, extracts from 10-g samples were neutralized with type C and D botulinum toxin antisera (Centers for Disease Control and Prevention, Atlanta, Ga.) (15). The presence of C. botulinum types A, B, E, and F in the samples was investigated by botulinum neurotoxin gene-specific multiplex PCR (11), and type C was determined by seminested PCR (28). In addition to the feed samples, gastrointestinal samples from 12 dead foxes were investigated for the presence of C. botulinum and botulinum toxin as described above. Serum samples from five of these animals were also tested for toxin.
In order to detect C. botulinum, 10 1-g aliquots of each sample and 10 ethanol-treated (16) 1-g aliquots of some samples were inoculated into 10-ml tubes containing tryptose-peptone-glucose-yeast extract (TPGY) broth (25), previously boiled for 15 min to remove the oxygen, and cooled to room temperature. The tubes were incubated in an anaerobic cabinet with an internal atmosphere of 85% N2, 10% CO2, and 5% H2 (MK III; Don Whitley Scientific Ltd., Shipley, United Kingdom) at 30 or 37°C for 2 days, followed by subculture into fresh TPGY and overnight incubation under the same conditions. Cells from 1 ml of each overnight culture were washed with Tris-HCl (0.01 M)-EDTA (0.001 M) buffer at 37°C for 1 h and suspended in distilled water. Volumes of 1 μl of each cell suspension were used as PCR templates. The PCR and agarose gel electrophoresis were performed as described earlier (11). The most-probable-number estimates for C. botulinum counts were determined with Thomas' approximation (1).
The pHs of composite feed samples and some feed component samples homogenized with distilled water (1:1 [wt/vol]) were measured (Microprocessor pH 537; Wissenschaflich-Technische Werkstätten, Weilheim, Germany).
Statistical analyses.
The correlation between death rate and the time from feeding animals the suspect feed to the first sign of clinical symptoms (hereafter referred to as the incubation time) was determined with a statistical software package (Statistical Package for Social Sciences, version 11.0.0; SPSS Inc., Chicago, Ill.).
RESULTS
Epidemiological investigation.
A total of 117 out of 157 farms (74.5%) responded to the questionnaire. An average farm included 1,736 animals, ranging from 183 to 6,939 animals. Eighty-three (70.9%) farms reported having dead or sick animals. At the farms responding to the questionnaire, a total of 52,163 animals out of 203,129 were affected, and 44,130 of these died or were euthanized because of clinical symptoms typical of botulism. This yielded an overall death rate for all animals at risk of 21.7% (Table 3). Of all the animals affected, 8,033 (15.4%) recovered. The highest mortality was observed in blue and shadow foxes, while the death rate in silver foxes, blue and silver foxes, and minks remained significantly lower (Table 3). The death rate in adult breeding animals was higher than in the young fur production animals (Table 3). The clinical symptoms observed by farmers are presented in Table 4.
TABLE 3.
Numbers of animals at risk and death rates (DR) at 117 fur farms responding to the epidemiological questionnairea
| Age and sex group | Blue fox | Shadow fox | Silver fox | Blue silver fox | Minks | Total |
|---|---|---|---|---|---|---|
| Young females and males | ||||||
| No. of animals at risk | 140,274 | 8,566 | 1,747 | 4,513 | 13,719 | 168,819 |
| No. of dead animals | 30,951 | 2,364 | 0 | 158 | 87 | 33,560 |
| DR (%) | 22.1 | 27.6 | 0 | 3.5 | 0.6 | 19.9 |
| Adult females | ||||||
| No. of animals at risk | 27,465 | 889 | 557 | 12 | 3,111 | 32,034 |
| No. of dead animals | 9,529 | 240 | 1 | 12 | 142 | 9,924 |
| DR (%) | 34.7 | 27.0 | 0.2 | 100 | 4.6 | 31.0 |
| Adult males | ||||||
| No. of animals at risk | 1,730 | 171 | 222 | 0 | 153 | 2,276 |
| No. of dead animals | 539 | 68 | 5 | 0 | 34 | 646 |
| DR rate (%) | 31.2 | 39.8 | 2.3 | 0 | 22.2 | 28.4 |
| Total | ||||||
| No. of animals at risk | 169,469 | 9,626 | 2,526 | 4,525 | 16,983 | 203,129 |
| No. of dead animals | 41,019 | 2,672 | 6 | 170 | 263 | 44,130 |
| DR (%) | 24.2 | 27.8 | 0.2 | 3.8 | 1.5 | 21.7 |
All minks had been immunized against botulinum toxin type C at the age of 2 months, while foxes were not immunized at all.
TABLE 4.
Clinical symptoms observed in sick animals at 117 farms
| Symptom | Number (%) of farms |
|---|---|
| Paralysis of hind legs | 54 (46) |
| Paraplegia, recumbence | 63 (54) |
| Wobbling | 15 (13) |
| Increased salivation | 12 (10) |
| Complicated breathing | 7 (6) |
| Cramps | 6 (5) |
| Abnormal position | 5 (4) |
| Flaccidness | 5 (4) |
| Decreased appetite | 4 (3) |
| Diarrhea | 1 (1) |
| Ocular discharge | 1 (1) |
| No description | 4 (3) |
Of the farms that did not respond to the questionnaire, 28 received feed from transportation lots known to cause deaths at the farms that responded to the questionnaire, and 12 farms received feed from the feed lots that were not reported to cause deaths. The estimated number of dead animals on the farms not returning the questionnaire was thus 6,546. Taking this estimate into account, the overall death rate on all farms at risk would be 18.6%.
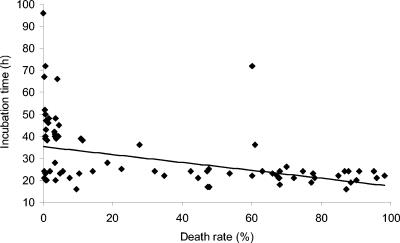
The incubation times at various farms ranged from 8 to 96 h, with an average of 31 h. One-third of the farms reported of an incubation time of 21 to 25 h. On farms with a high death rate, the incubation time tended to be shorter than on the farms with a low death rate (correlation −0.46) (Fig. 3).
FIG. 3.
Correlation between the death rate and incubation times observed at fur animal farms.
Of the 250,000 kg of feed delivered to farms on day 2, five driving lots involving 68,000 kg of feed were associated with a mean death rate of more than 40% in animals. This included 3,000 kg of leftover feed remaining from day 1 production and 65,000 kg of feed freshly produced on day 2 before 0750 h, which was delivered to the farms between 0655 and 0800 h (Fig. 2). The next five feed lots, delivered to the farms between 0900 and 1130 h, were associated with a death rate of 20% or less. Other feedlots, including 19,000 kg of leftover feed produced on day 1 and stored overnight in trucks A and B or in the loading silo, were not associated with an increased death rate.
Sample analyses.
C. botulinum type C organisms and type C botulinum toxin at a level of >600 MLD/g of feed were detected in the feed produced on day 2 (Table 2). Furthermore, a composite sample of feed produced on both days 2 and 3 contained toxin. C. botulinum types B, C, and E were detected in day 3 feed samples and in various feed components at <1 spore/g of feed, but no toxin could be detected in any of these samples. Botulinum toxin type C was also detected in three gastrointestinal samples but not in any of the serum samples. The C. botulinum organism was not detected in any of the clinical samples.
The pH of the toxic feed sample was 4.9, while that in the other feed samples varied from 4.6 to 5.6. The pH of the feed components varied between 4.3 and 6.9 (Table 2).
DISCUSSION
To our knowledge, the type C botulism outbreak described here is the largest botulism outbreak ever reported in fur production animals, affecting more than 52,000 animals in 83 breeding farms. The overall death rate at farms responding to the questionnaire was 21.7%. However, a large number of animals developing typical clinical symptoms were immediately euthanized for the sake of animal protection, and the fact that 8,000 animals with milder clinical symptoms actually recovered indicates that the natural death rate in all animals would have been lower than 21.7%. Also, taking into account the estimated number of dead animals at the farms not responding to the questionnaire, the overall death rate seems slightly lower (18.6%).
The outbreak affected mainly blue foxes and shadow foxes, while the death rate in silver foxes and in the related blue silver foxes as well as in minks was considerably lower. It is possible that the different fox species possess different susceptibilities to botulinum neurotoxins. The blue fox (Alopex lagopus) and its color variant shadow fox are of Arctic origin and consume frozen feedstuffs in wildlife, while the blue silver and related silver fox are related to the wild red fox (Vulpes vulpes), consuming mainly rotted carrion. In evolution, the different feeding habits of the two fox species may thus have resulted in the development of different resistance to botulinum toxin.
The most typical clinical symptoms observed in the sick animals included paralysis of the hind legs, total paralysis, and recumbent position. Foxes with milder symptoms were often seen in a “sitting” position, dragging the rear part of their bodies behind. Such a position is occasionally seen in otherwise healthy foxes and might serve as an indication of a subtle form of type C botulism in these animals. Hind leg paralysis and recumbence have also been observed as the main clinical feature of botulism in cattle (27), while the typical first symptoms of botulism in humans involve the cranial parts of the body. The reason for the different manifestation of botulism in humans and in animals is unknown but might be explained by different distribution of specific receptors for botulinum toxins in humans and animals.
The relatively high death rate in adult foxes compared to young foxes is explained by the lower body weight of the adults. During the off-reproduction period in late summer and autumn, the adult breeding animals are usually fed only once a day with a limited amount of feed, resulting in a body weight of approximately 6 to 8 kg, while the young fur animals fed ad libitum may weigh more than 10 kg. The adults fed a restricted diet also show an increased appetite and thus finish their meal immediately, while the younger fur animals take smaller quantities of feed now and then throughout the day. The low death rate observed in young minks was obviously due to recent immunization against type C botulinum toxin, while the older minks with a higher death rate, having only been immunized in their youth, apparently already had decreased antibody titers in their serum.
The type C toxin level measured in the feed sample investigated was >600 MLD/g of feed. This sample was collected on a farm that had a total death rate of 68% and received feed from truck A at 0700 h. As the death rate for each truck delivery was variable, it is probable that the toxin concentration in different feed lots delivered to the farms varied. Assuming the measured toxin concentration, an animal eating its entire 1-kg feed portion would ingest 6 × 105 MLD of toxin. This is in line with an earlier report on the lethal oral dose of type C toxin in blue foxes (2). However, the earlier findings on the susceptibility of foxes to botulinum toxins are controversial, and silver foxes (20) as well as blue foxes (6) have been reported to survive ingesting 107 MLD of type C toxin. Thus, more information on the pathogenesis and lethality of type C toxin in foxes is required.
The fact that type C botulinum toxin was detected in the feed but not in any of its components leaves us with assumptions on the factors that allowed the outbreak to occur. Theoretically, four different events may explain the outbreak: (i) one or several of the feed raw material components contained preformed toxin; (ii) toxin formation from C. botulinum type C spores present in raw materials occurred in the feed base mass (three times 4,300 kg) during overnight storage; (iii) toxin formation occurred in the leftover feed produced on day 1 and stored overnight in the loading silo; or (iv) toxin was (deliberately) added to the feed during or prior to the day 2 production. These possible events are discussed below.
Botulinum toxin could not be detected in any of the feed raw materials investigated. However, the samples investigated represented only a very small fraction of all the raw materials used, and some of the feed components were not available for laboratory analysis. Therefore, in spite of the negative laboratory analysis results, it is possible that one or several of the feed components contained toxin. One of the most probable components to contain botulinum toxin was the nonacidified poultry slaughter by-product. The pH of this product was nearly neutral and thus theoretically could have allowed botulinal growth and toxin formation before freezing the lot. According to the manufacturer of this feed component, the raw material was collected in a slaughterhouse at daytime and taken elsewhere for freezing at the end of the day. However, documents describing the time elapsed between collecting and freezing the nonacidified poultry lots used in feed processing on day 2 were not available. In the worst case, the component was stored at room temperature for a whole working day.
C. botulinum type C is known to be present in the poultry production environment (4), causing large outbreaks in flocks (21, 24). In the present case, the organism was detected in the nonacidified poultry slaughter by-product at the level of 0.3 spore/g. Type C botulism in birds has been suggested to develop as a consequence of C. botulinum growth and toxin formation in the birds' gastrointestinal tract (22). As the poultry slaughter by-products usually contain the birds' viscera, such as guts, the contamination of poultry slaughter by-products with C. botulinum type C is highly probable. As only one 1,100-kg lot out of 34 similar lots of the suspected poultry by-product was available for laboratory analysis, the possibility that toxin was present in one or several of such batches could not be excluded.
Regarding the other raw material ingredients used in the production of the toxic feed, three components comprising fish entrails, acidified beef slaughter by-products, and barley porridge had a pH range of 4.3 to 5.3. Such a low pH is not likely to support the growth of and toxin formation by C. botulinum. Furthermore, the barley porridge was heated to boiling for 30 min, a heat treatment likely to destroy any possible preformed toxin. The barley and soy flours, feather powder, meat and bone powder, and meat powder would be expected to have such a low water activity that bacterial growth would be inhibited. The frozen fish that theoretically could have contained botulinum toxin were not available for laboratory investigation. However, the most commonly observed C. botulinum type in Finnish fishing waters and fish is nonproteolytic C. botulinum type E (8, 9), while type C strains have not been isolated from these samples. Thus, the source of the high concentration of type C toxin in the feed is likely to be elsewhere.
The growth of and toxin formation by C. botulinum type C present in the feed base mass is possible but improbable. C. botulinum type C cells were detected in the feed raw materials at levels of less than 0.5 spore/g. As a minimum of 500 kg of each type of slaughter by-product was used to prepare the feed, the base mass could theoretically carry 105 spores. Under optimal circumstances, this spore population might lead to toxin formation. However, the growth-inhibitory pH for C. botulinum type C strains has been reported to be 5.1 to 5.6 (23), while the pH measured in most of the base mass components was lower. Also, due to the addition of 1,000 kg of frozen components in each of the 4,300-kg feed base mass lots on the evening of day 1, the feed base mass temperature during the night between days 1 and 2 was constantly kept at 10°C or below according to the feed manufacturer. The growth-limiting temperature for C. botulinum type C is 10 to 15°C (23), thus rendering germination and subsequent toxin formation in the feed unlikely. Whether the feed contained foci with an increased temperature and pH due to possible failure in processing, allowing germination and subsequent toxin formation to occur, remains obscure. Nevertheless, this is improbable, as a large amount of toxin would be required to eliminate such an enormous number of animals.
The possibility of toxin formation in the feed base mass during overnight storage can also be excluded by estimating the amount of toxic feed required to cause such enormous damage. As indicated in Fig. 2, at least five transportation lots containing a total of 65,000 kg of day 2 feed were associated with high death rates. This corresponds to 9 to 10 full mixer lots, and only the base masses of three of these lots were stored overnight. Therefore, in addition to the mixer lots incubated overnight, at least two fresh lots prepared in each of the three mixers in the morning of day 2 still contained a high concentration of toxin. This suggests that toxin was present in one or several raw material components used in the production of the first 10 mixer lots (65,000 kg) between 0630 and 0750 h.
That the leftover feed produced on day 1 contained toxin and contaminated the first 68,000 kg of fresh feed produced on the morning of day 2 is very unlikely. First, the day 1 feed samples investigated were not shown to contain botulinum toxin in the laboratory analysis. Second, the 19,000-kg fraction of the leftover feed lot delivered to the farms early on the morning of day 2 did not cause an increased death rate in animals. Third, to make a 68,000-kg sample contain toxin at a level of 600 MLD/g, the 3,000-kg leftover feed mass mixed with day 2 production should theoretically have contained at least 4 × 107 MLD of toxin. The formation of such a high concentration within one night under the nonoptimal conditions described above is improbable.
The possibility of sabotage, i.e., intentional addition of toxic material to the feed or its components, could not be entirely excluded. However, in order to affect such a large number of animals, an enormous dose of toxin would have to have been added and thoroughly mixed into the feed. As toxin production in group III C. botulinum strains is mediated by a bacteriophage (14), the proper handling and effective preparation of toxic cultures in a laboratory is more challenging than with group I and II strains. Type C botulism has been reported previously in foxes, which might suggest that the outbreak was of natural origin.
The outbreak caused financial losses of 4 million euros to the government, the feed processing plant, and the fur animal farmers. The manufacturing plant was closed on the evening of day 3 for several weeks for thorough cleaning and disinfection. The toxin-containing carcasses and feed were considered high-risk material and subjected to incineration under the control of regional veterinary and environmental health authorities.
In conclusion, the factors leading to the type C botulism outbreak that killed more than 44,000 farmed fur animals remain unclear. However, by careful consideration and exclusion of other possible etiological factors, it may be assumed that one or several raw material components employed in feed production contained preformed toxin. In order to avoid such catastrophes in the future, a careful risk assessment of the factors affecting fur animal feed safety during feed production and storage is warranted.
Acknowledgments
We thank Noora Alanko, Hanna Korpunen, Kirsi Ristkari, and Niina Siiskonen for laboratory assistance.
REFERENCES
- 1.Blodgett, R. January 2001. Most probable number from serial dilutions. In Bacteriological analytical manual online. U.S. Food and Drug Administration, Center for Food and Safety and Applied Nutrition, http://www.cfsan.fda.gov/~ebam/bam-toc.html.
- 2.Borisov, V. N., and Y. I. Vakuev. 1969. Botulism in blue foxes. Krolikovod. Zverovod. 1:34-35. [Google Scholar]
- 3.Dedié, K. 1954. Botulismus beim Nerz. Mn. Vet. Med. 21-22:486-487. [Google Scholar]
- 4.Dohms, J. E. 1987. Laboratory investigation of botulism in poultry, p. 295-314. In M. W. Eklund and V. R. Dowell, Jr. (ed.), Avian botulism: an international perspective. Charles C. Thomas Publisher, Springfield, Ill.
- 5.Gitter, M. 1959. Botulism in mink: an outbreak caused by type C toxin. Vet. Rec. 71:868-871. [Google Scholar]
- 6.Haagsma, J. 1980. Serious outbreak of botulism type C in blue foxes. Proc. 2nd International Congress in Fur Animal Production, Vedbaek, Denmark, 7 p.
- 7.Harrison, S. G., and E. D. Borland. 1973. Deaths in ferrets (Mustela putorius) due to Clostridium botulinum type C. Vet. Rec. 93:576-577. [DOI] [PubMed] [Google Scholar]
- 8.Hielm, S., E. Hyytiä, A.-B. Andersin, and H. Korkeala. 1998. A high prevalence of Clostridium botulinum type E in Finnish freshwater and Baltic Sea sediment samples. J. Appl. Microbiol. 84:133-137. [DOI] [PubMed] [Google Scholar]
- 9.Hyytiä, E., S. Hielm, and H. Korkeala, H. 1998. Prevalence of Clostridium botulinum type E in Finnish fish and fishery products. Epidemiol. Infect. 120:245-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knox, B. 1958. En oversigt over sygdomme hos mink. Medlemsblad for Den danske Dyrlaegeforening 41:1-19. [Google Scholar]
- 11.Lindström, M., R. Keto, A. Markkula, M. Nevas, S. Hielm, and H. Korkeala. 2001. Multiplex PCR assay for detection and identification of Clostridium botulinum types A, B, E, and F in food and fecal material. Appl. Environ. Microbiol. 67:5694-5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loftsgaard, G. 1988. Helsitilstanden i norske pelsdyrfarmer i 1987. Norsk Vet. Tijdskr. 100:34-35. [Google Scholar]
- 13.Ministry of Agriculture and Forestry. 25 May 2001. Turkiseläinten rehun eläintauti-ja hygieniavaatimukset, decree 34/EEO/2001. Ministry of Agriculture and Forestry, Helsinki, Finland, http://www.mmm.fi/el/laki/h/h15.html.
- 14.Nakamura, S., I. Kimura, K. Yamakawa, and S. Nishida. 1983. Taxonomic relationships among Clostridium novyi types A and B, Clostridium haemolyticum and Clostridium botulinum type C. J. Gen. Microbiol. 129:1473-1479. [DOI] [PubMed] [Google Scholar]
- 15.Nordic Committee on Food Analysis. 1991. Botulinum toxin. Detection in foods, blood and other test materials. Method no. 79, 2nd ed. Nordic Committee on Food Analysis, Espoo, Finland.
- 16.Nordic Committee on Food Analysis. 1991. Clostridium botulinum. Detection in foods and other test materials. Method no. 80, 2nd ed. Nordic Committee on Food Analysis, Espoo, Finland.
- 17.Nordstoga, K. 1973. Mistanke om botulisme i en blarevbesetning. Norsk Vet. Tijdskr. 85:654-655. [Google Scholar]
- 18.Pyle, N. J., and R. M. Brown. 1939. Botulism in foxes. J. Am. Vet. Med. Assoc. 94:436-439. [Google Scholar]
- 19.Quortrup, E. R., and A. L. Holt. 1940. A case report on botulism type C in mink. J. Am. Vet. Med. Assoc. 97:167-168. [Google Scholar]
- 20.Quortrup, E. R., and J. R. Gorham. 1949. Susceptibility of furbearing animals to the toxins of Clostridium botulinum types A, B, C, and E. Am. J. Vet. Res. 10:268-271. [Google Scholar]
- 21.Roberts, T. A., A. I. Thomas, and R. J. Gilbert. 1973. A third outbreak of botulism in broiler chickens. Vet. Rec. 92:107-109. [DOI] [PubMed] [Google Scholar]
- 22.Sato, S. 1987. Control of botulism in poultry flocks, p. 349-356. In M. W. Eklund and V. R. Dowell, Jr. (ed.), Avian botulism: an international perspective. Charles C. Thomas Publisher, Springfield, Ill.
- 23.Segner, W. P., C. F. Schmidt, and J. K. Boltz. 1971. Minimal growth temperature, sodium chloride tolerance, pH sensitivity, and toxin production of marine and terrestrial strains of Clostridium botulinum type C. Appl. Microbiol. 22:1025-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smart, J. L., and T. A. Roberts. 1977. An outbreak of type C botulism in broiler chickens. Vet. Rec. 100:378-380. [DOI] [PubMed] [Google Scholar]
- 25.Solomon, H. M., and T. Lilly, Jr. January 2001. Clostridium botulinum. In Bacteriological Analytical Manual Online, U.S. Food and Drug Administration, Center for Food and Safety and Applied Nutrition, http://www.cfsan.fda.gov/~ebam/bam-toc.html.
- 26.Thomas, H. A. 1942. Bacterial densities from fermentation tube tests. J. Am. Waterworks Assoc. 34:572-576. [Google Scholar]
- 27.Whitlock, R. H., and J. M. Williams. 1999. Botulism toxicosis of cattle. Proc. Annu. Conf. Am. Assoc. Bovine Pract. 32:45-53. [Google Scholar]
- 28.Williamson, J. L., T. E. Rocke, and J. M. Aiken. 1999. In situ detection of the Clostridium botulinum type C1 toxin gene in wetland sediments with a nested PCR assay. Appl. Environ. Microbiol. 65:3240-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yndestad, M., A. Helgebostad, and G. Loftsgård. 1977. Susceptibility of foxes to Clostridium botulinum type C and E toxins. Acta Vet. Scand. 18:23-30. [DOI] [PMC free article] [PubMed] [Google Scholar]