Abstract
The outcome of adult patients with Philadelphia chromosome-negative acute lymphoblastic leukemia (Ph− ALL) relapsing after pediatric-inspired front-line therapy is ill known. Here 229 relapsing Ph− ALL younger adults (18–63 years) treated within the Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL)-2003/-2005 trials were considered. Salvage regimens consisted of potentially curative therapies in 194 cases, low-intensity therapies in 21, allogeneic stem cell transplant (allo-SCT) in 6 and best supportive care in 8. Overall, 77 patients received allo-SCT after relapse. The median follow-up was 3.1 years. A second complete remission (CR2) was achieved in 121 patients (53%). In multivariate analysis, only younger age <45 years (P=0.008) and CR1 duration ⩾18 months (P=0.009) predicted CR2. Overall survival (OS) at 2 and 5 years was 19.3% (14–24%) and 13.3% (8–18%), respectively. In CR2 patients, disease-free survival (DFS) at 2 and 5 years was 29.0% (21–38%) and 25% (17–33%). In multivariate analysis, CR1 duration ⩾18 months and allo-SCT after relapse were associated with longer DFS (P<0.009 and P=0.004, respectively) and longer OS (P=0.004 and P<0.0001, respectively). In conclusion, although younger adults relapsing after pediatric-inspired ALL therapies retain a poor outcome, some of them may be cured if CR1 duration ⩾18 months and if allo-SCT can be performed in CR2. New therapies are definitely needed for these patients.
Introduction
The prognosis of adult patients with relapsed Philadelphia-negative (Ph−) acute lymphoblastic leukemia (ALL) is dismal.1, 2, 3, 4, 5 A frequent option in such circumstances is to obtain a new complete remission (CR) as a bridge to perform allogeneic stem cell transplantation (allo-SCT), which is the best prospect for cure.5 This strategy mainly applies to younger and fit patients who can receive aggressive salvage regimens. Second CR (CR2) rates in such patients have been shown to range between 30 and 45% and median survival between 6 and 9 months only.1, 2, 3, 4, 5, 6, 7 These data are mostly issued from studies incorporating patients treated before the era of pediatric-inspired strategies. The latter have yielded significant advances in this group of patients as demonstrated by the results of the two Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL)-2003 and -2005 trials.8, 9, 10, 11, 12, 13, 14 The GRAALL-2005 trial only differed from the 2003 trial by the addition of a randomized evaluation of hyperfractionated cyclophosphamide during induction and late intensification and by the randomization of rituximab addition during all phases of therapy in CD20+ B-cell precursor (BCP) ALL patients.14 High-risk patients8, 12 were candidate for allo-SCT in CR1.
The outcome of patients who relapsed after specific pediatric-like protocols in first-line therapy has to be further analyzed. The hypothesis was that as pediatric-based regimens have significantly improved survival, this might lead to a selection of patients with a more refractory disease/subclone. Here we retrospectively describe such results from a cohort of patients with relapsed Ph− ALL initially treated in the GRAALL-2003 and -2005 trials.
Patients and methods
Patients
Between 2003 and 2011, 955 younger adults with de novo Ph− ALL aged between 15 and 59 years from 70 French, Belgian and Swiss centers were treated within the multicenter prospective French GRAALL-2003 (N=225, period: 2003–2005) and GRAALL-2005 (N=730, period: 2006–2011) trials (clinicaltrials.gov, nos. NCT00222027 and NCT00327678, respectively). Of these 955 patients, 860 (548 BCP-ALL and 312 T-cell ALL (T-ALL)) reached CR1 and 311 further received allo-SCT in first CR (204 BCP-ALL and 107 T-ALL).13 Overall, 264 patients (30%) relapsed, including 58 after allo-SCT. For the purpose of this study, postrelapse information was collected retrospectively. First relapse and its issue was part of the information to be collected in the e-case report forms of the trials. Based on an extraction of these events, additional necessary information was retrieved from the patients' files.
Genetic/molecular status at diagnosis
Patients were assigned to risk groups according to definitions applied to previous GRAALL trials.8, 12 Some relapsed patients were informative for their genetic/molecular status at diagnosis according to data published by the GRAALL11, 12 MLL (KMT2A) gene rearrangements and IKZF1 gene deletion, for BCP-ALL, and NOTCH1/FBXW7/RAS/PTEN gene mutations/deletions, for T-ALL, were thus considered as potential prognostic factors. There were no analyses of minimal residual disease levels in CR2 patients.
Statistical analyses
The primary objective of the study was to assess the outcome in younger Ph− ALL patients relapsing after having being treated in first-line therapy as part of the GRAALL-2003/-2005 trials. This was evaluated by assessing the CR2 rate as well as overall (OS) and disease-free survivals (DFS). Secondary objectives were to determine prognostic factors for CR2, DFS and OS.
CR2 was defined by a neutrophil count >1.0 × 109/, a platelet count >100 × 109/l and bone marrow blasts <5% while all extramedullary disease had resolved. Relapse after CR2 was defined as the reappearance of leukemic cells in the bone marrow or extramedullary. Patient characteristics and CR rates were compared using Fisher's exact test. Comparisons of medians were performed using the Mann–Whitney U-test.
OS was defined as the time from the day of first relapse to death or last follow-up. DFS was defined from the date of CR2 to that of second relapse or death or last follow-up. Survival outcomes were not censored at allo-SCT. OS and DFS were estimated using the Kaplan–Meier method and then compared using the log-rank test.15
Characteristics considered for univariate analysis were age (⩽45 years), ALL lineage (B vs T), ALL risk classification (high vs standard),8, 12 CR1 duration (⩽18 months), prior allo-SCT, relapse type (central nervous system vs others), relapse treatment type (intensive vs non-intensive, allo-SCT after relapse or not) and response to salvage regimen (CR2 or not). To evaluate the prognostic value of CR2 achievement, a landmark period of 82 days (75th percentile of the achievement of CR2) was used. Some lineage-specific cytogenetic or molecular features present at diagnosis were also considered, including t(4;11)(q21;q23)/MLL-AF4(KMT2A-AFF1), low hypodiploidy/near triploidy and IKZF1 gene deletion for BCP-ALL and complex karyotype (⩾5 anomalies) or NOTCH1/FBXW7/RAS/PTEN mutational status for T-ALL.
Postrelapse allo-SCT was analyzed as a time-dependent event using Mantel–Byar estimations.16
Factors associated with a P-value <0.10 on univariate analysis were included in the final logistic regression hazard model for multivariate analysis. Multivariable regressions were performed with the Cox model.17 Hazard ratios (HRs) are given with 95% confidence interval (CI).
STATA/SE 10.1 software (STATA, College Station, TX, USA) was used. All tests were two-sided, with a type I error at 5%.
Results
Patients
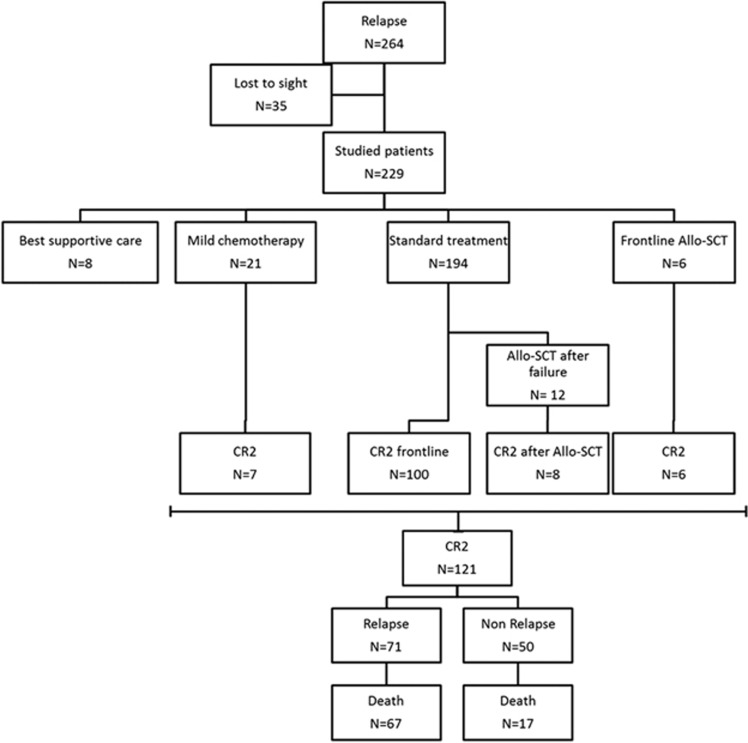
Data were available for 229 of the 264 patients who relapsed after prior CR1 achievement. In this cohort, the median age at relapse was 35.7 years. Allo-SCT preceded relapse for 54 patients (24%) and the median duration of CR1 was 10 months (range, 0.5–74). One hundred and fifty-one patients (66%) had BCP-ALL and 165 patients (72%) carried high-risk characteristics8, 12 at diagnosis. The main site of relapse was bone marrow alone (n=181, 79%). Characteristics of the patients are given in Table 1. Details of the patients' evolution are given in the flow chart (Figure 1).
Table 1. Patients' characteristics.
| Patients | N=229 |
|---|---|
| Median follow-up (range), years | 3.1 (2.7–4.15) |
| First-line trial, GRAALL-2003/-2005 | 65/164 |
| Gender, male/female | 148/81 |
| Median age (range), years | 35.6 (17.2–63.1) |
| <45 years/>45 years | 166/63 |
| Lineage, BCP-ALL/T-ALL | 151/78 |
| High-risk cytogenetics at diagnosis | |
| t(4;11)(q21;q23)/MLL-AF4(KMT2A-AFF1) | 21 |
| t(1;19)(q23;p13)/E2A-PBX1 (TCF3-PBX1) | 6 |
| Complex | 18 |
| Low hypodiploidy/near triploidy | 10 |
| Molecular status at diagnosis | |
| IKZF1 gene deletion (BCP-ALL), yes/no | 34/87 |
| No NOTCH1/FBXW7 mutation and/or N/K-RAS mutation and/or PTEN gene alteration (T-ALL), yes/no | 42/56 |
| Risk stratification at diagnosis, standard/high/unclassifieda | 45/65/19 |
| Response to treatment in first-line therapy | |
| Corticoresistance,b yes/no/not determined | 62/165/2 |
| Chemoresistance,c yes/no/not determined | 108/113/8 |
| Salvage regimen needed to obtain CR1, yes/no | 10/219 |
| Allo-SCT in CR1 | 54 |
| Duration of CR1, median, months (range) | 10 (0.5–74) |
| <18 months/>18 months | 179/50 |
| Site of relapse | |
| Bone marrow | 181 |
| Central nervous system | 19 |
| Both | 20 |
| Other | 9 |
| Unknown | 2 |
Abbreviations: ALL, acute lymphoblastic leukemia; allo-SCT, allogeneic stem cell transplantation; BCP, B-cell precursor; CR1, first complete remission; GRAALL, Group for Research on Adult Acute Lymphoblastic Leukemia; T-ALL, T-cell acute lymphoblastic leukemia.
High-risk factors were a WBC count of ⩾30 × 109/l for B-lineage ALL, clinical and/or morphological CNS involvement, t(4;11) and/or MLL-AF4 fusion transcript, t(1;19) and/or E2A-PBX1 fusion transcript, low hypodiploidy and/or near triploidy.8
Poor peripheral blood blast clearance after the corticosteroid prephase.
Poor bone marrow blast clearance after one additional week of induction chemotherapy.
Figure 1.
Flow chart.
Salvage regimens
All salvage regimens were permitted after relapse and details are given in Table 2. Most patients (n=194, 85%) were retreated intensively with a variety of reported regimens,18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 while 21 patients received less-intensive chemotherapy (mainly vincristine and corticosteroids), 6 upfront allo-SCT and 8 only best supportive care. The main novelties compared with previously reported salvage regimens were the use of second-generation purine analogs, such as clofarabine for BCP-ALL18 (34 patients) or nelarabine for T-ALL19 (12 patients), and a regimen based on L-asparaginase encapsulated in erythrocytes21 (12 patients). Only a few patients received immunotherapy with rituximab40 because of CD20 expression or gemtuzumab ozogamicin because of CD33 expression.41
Table 2. Salvage regimens.
| Total patients | N=229 |
|---|---|
| Best supportive care | N=8 |
| Upfront allo-SCT | N=6 |
| Less-intensive chemotherapy | N=21 |
| Vincristine/corticosteroids | 8 |
| Vincristine/low-dose methotrexate | 4 |
| Vincristine/6-mercaptopurine | 3 |
| 6-Mercaptopurine+low-dose methotrexate | 3 |
| Intrathecal therapy (liposomal Ara-C)18 | 3 |
| Intensive chemotherapy | N=194 |
| Newer regimens | N=60 |
| Clofarabine based19 | 34 |
| Vandevol | 27 |
| Endevol | 2 |
| Other combinations | 5 |
| Nelarabine based20 | 12 |
| L-asparaginase encapsulated in erythrocyte based21 | 12 |
| Rapamycin based22 | 2 |
| Older regimens | N=134 |
| Pediatric like | |
| Vanda23 | 19 |
| Cooprall24 | 6 |
| Fralle-9325 | 1 |
| Hyper-CVAD26 | 27 |
| Anthracycline based | |
| Idarubicine–Ara-C27 | 27 |
| Flag-Idarubicine28 | 2 |
| Idarubicine–etoposide29 | 1 |
| VAD30 | 5 |
| Idarubicine/vincristine/L-asparaginase31 | 2 |
| Daunorubicine/Ara-C+Cyclophosphamide32 | 4 |
| Mitoxantrone/Ara-C (HAM)33 | 10 |
| Mitoxantrone/Ara-C/etoposide/corticosteroid (PAME)34 | 1 |
| Amsacrine/Ara-C35 | 1 |
| Methotrexate based | |
| High-dose methotrexate+Ara-C36 | 6 |
| Copadem37 | 2 |
| GRAALL-2005 re-induction12 | 5 |
| LALA-94 re-induction38 | 1 |
| Capizzi re-induction39 | 4 |
| Miscellaneous (etoposide/DEX, Ara-C/DEX, Ara-C/GO41) | 4 (2,1,1) |
| Unknown | 5 |
Abbreviations: allo-SCT, allogeneic stem cell transplantation; DEX, dexamethasone; GO, gemtuzumab ozogamicin; GRAALL, Group for Research on Adult Acute Lymphoblastic Leukemia.
Only the 215 patients who received an intensive (frontline allo-SCT excluded) or a less-intensive salvage regimen were considered for the analysis of prognostic factors of CR2. Only the 108 patients having obtained CR2 after intensive salvage regimen were considered for DFS analyses, and only the 221 who had received a treatment at relapse were considered for OS analyses.
Factors predicting CR2, relapse and death after CR2
CR2 was achieved in 121 cases (53%), including 108 out of the 194 (56%) after intensive salvage regimen and 7 out of the 21 (33%) after a less-intensive salvage regimen. In addition, all of the six patients who received allo-SCT upfront as salvage regimen achieved CR2. Eight of the 108 patients in the intensive group achieved CR2 only after receiving allo-SCT as second-line salvage therapy. Risk stratification at diagnosis was not predictive of CR2 achievement (standard risk, 53% high risk, 50% unclassified, 58%). There was no statistically significant difference in the CR rates (51% for BCP-ALL vs 56% for T-ALL). Of the 14 T-ALL patients who received Nelarabine, 7 reached CR2. Of the 37 patients with central nervous system involvement at relapse, 26 reached CR2, 16 relapsed and 30 died. Among the 54 patients who had received AlloSCT before relapsing, 50% reached CR2.
In the univariate analysis, a younger age (<45 years; HR=0.48 (95% CI, 0.27–0.87); P=0.015) and a longer CR1 duration (>18 months; HR=0.45 (95% CI, 0.23–0.86); P=0.017) were associated with CR2 achievement. In BCP-ALL, the presence of t(4;11) (n=21) was also associated with failure to reach CR2 with only 20% of these patients reaching CR2.
In multivariate analysis, younger age (<45 years old; HR=0.44 (95% CI, 0.24–0.80); P=0.008) and longer CR1 duration (>18 months; HR=0.40 (95% CI, 0.20–0.80); P=0.009) were independently associated with CR2 achievement.
Among the 121 patients who reached CR2, 71 (59%) relapsed and 67 (94%) died after relapse. Of the 50 patients who did not relapse after CR2 (n=50), only 17 (34%) died. Of these 17 patients, 7 died of infectious complications, 6 of allo-SCT-related mortality, 2 of multivisceral organ failure and 2 of secondary hematological malignancy (1 AML, 1 NHL). Overall, 84 patients died (69%) after achieving CR2.
Allo-SCT after first relapse
A total of 77 patients received allo-SCT after relapse: 6 (8%) upfront, 59 (77%) after reaching CR2 after intensive (n=55) or less-intensive (n=4) chemotherapy, and 12 (15%) after failure of the intensive salvage regimen. Among the 18 patients who received allo-SCT while with active disease, 14 (78%) achieved CR2 (6/6 upfront, and 8/12 refractory). The median time between relapse and allo-SCT was 111 days (range, 5–311). Seventeen patients received a reduced intensity conditioning (RIC) and 60 a myeloablative conditioning. For 26 patients, the donor was a matched relative, for 2 a haploidentical relative and for 40 a matched 10/10 or mismatched 9/10 unrelated donor. Nine patients received a cord-blood allo-SCT. Of the 77 patients who received allo-SCT, 13 received a RIC regimen (median age 42 years old, range 19–63) and 64 a myeloablative regimen (median age 28 years, range 18–60, P=0.003). Of the 13 RIC patients, 8 died, including 4 after second relapse, and another one relapsed but was still alive at last news. By comparison, of the 64 patients with myeloablative conditioning, 27 relapsed and 38 died. These differences were not statistically significant.
DFS and factors predicting DFS
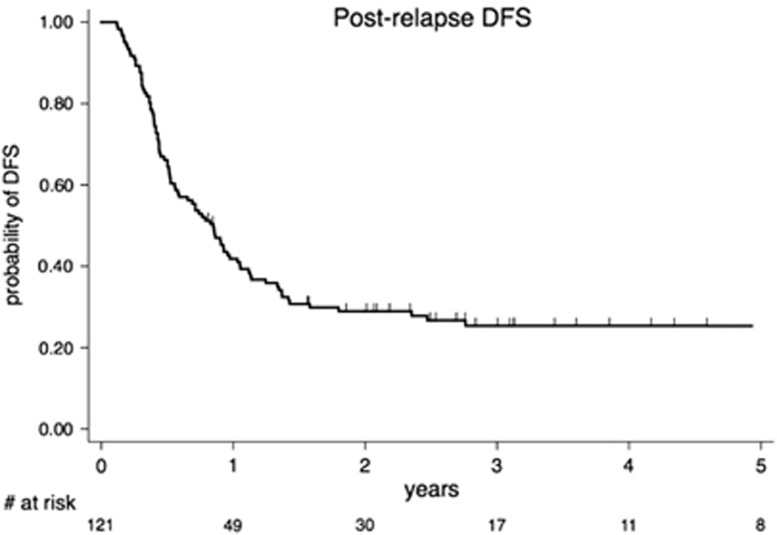
With a median DFS of 10.2 months (95% CI, 6.7–12.4), overall 1-, 2- and 5-year DFS for the 121 CR2 patients were 42% (95% CI, 0.32–0.50), 29% (95% CI, 0.21–0.37) and 25% (95% CI, 0.17–0.33), respectively (Figure 2).
Figure 2.
DFS for patients achieving second complete remission (CR2; n=121).
In the univariate analysis, a significantly longer DFS was observed for patients with a CR1 duration ⩾18 months (HR=0.36 (95% CI, 0.21–0.62); P<0.0001) and for those who received allo-SCT after reaching CR2 (HR=0.45 (95% CI, 0.27–0.73); P=0.001). Multivariate analysis confirmed the independent favorable impact on DFS of longer CR1 duration (HR=0.41 (95% CI, 0.21–0.80); P<0.009) and of receiving allo-SCT after CR2 (HR=0.40 (95% CI, 0.22–0.75); P=0.004).
OS and factors predicting OS
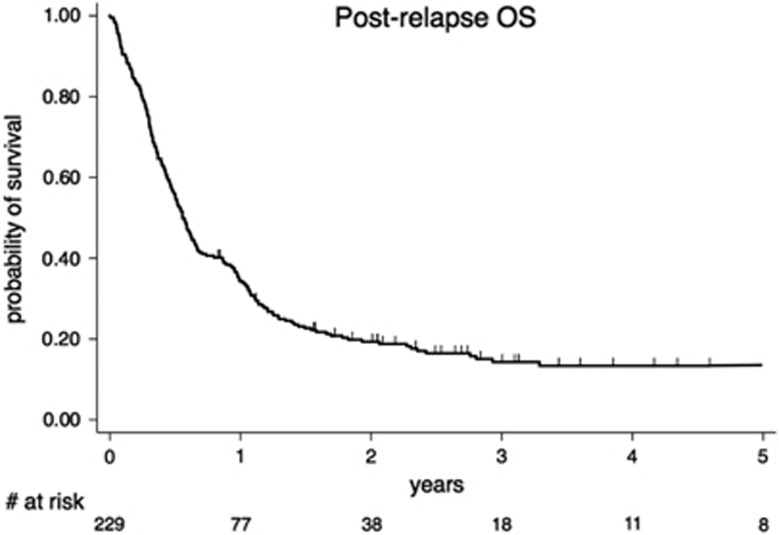
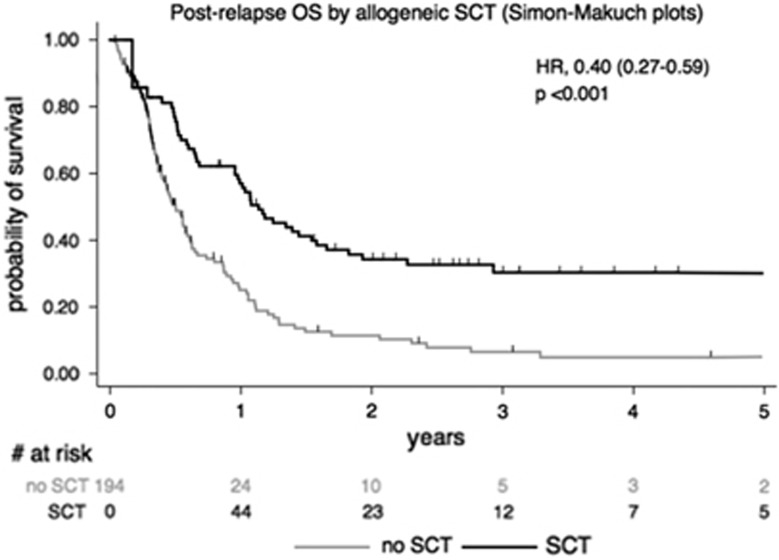
The median follow-up for alive patients was 3.1 years. For the entire cohort, the median OS was 6.8 months (95% CI, 5.8–7.9) and 1-, 2- and 5-year OS were 34.4% (95% CI, 0.28–0.40), 19.3% (95% CI, 0.14–0.24) and 13.3% (95% CI, 0.08–0.18), respectively (Figure 3).
Figure 3.
OS, (N=229).
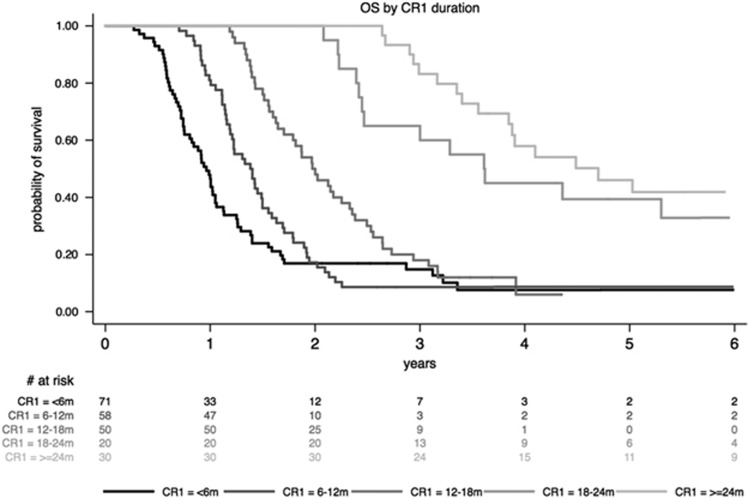
In the univariate analysis, factors associated with better OS were: younger age (⩽45 years; HR=0.68 (95% CI, 0.50–0.93); P=0.01), CR1 duration >18 months (HR=0.42 (95% CI, 0.29–0.63); P<0.0001; Figure 4), achievement of CR2 (HR=0.19 (95% CI, 0.16–0.27); P<0.001), and allo-SCT after first relapse (HR=0.40 (95% CI, 0.27–0.59); P<0.001; Figure 5). A worse OS was observed in BCP-ALL with t(4;11) (HR=2.13 (95% CI, 1.30–3.47); P=0.002) or low hypodiploidy/near triploidy (HR=2.96 (95% CI: 1.52–5.72); P=0.001) and in T-ALL with complex karyotype (HR=5.37 (95% CI, 2.18–13.23); P<0.001). Neither IKZF1 gene deletion for BCP-ALL nor NOTCH1/FBXW7/RAS/PTEN mutational status for T-ALL did influence OS.
Figure 4.
OS according to CR1 duration (m: months).
Figure 5.
OS according to allo-SCT performed or not at relapse.
In the multivariate analysis, longer CR1 and allo-SCT after first relapse were the two factors that remained associated with prolonged OS (HR=0.53 (95% CI, 0.34–0.82); P=0.004; and HR=0.43 (95% CI, 0.27–0.66); P<0.0001, respectively).
Discussion
The objective of this study was to assess the main outcomes (CR2 achievement, DFS, OS) of younger adults with Ph− ALL having relapsed after the pediatric-inspired GRAALL protocol. The main issue was to know whether any progress has been achieved in terms of survival regarding this relapsed population in recent years. Indeed, applying more intensive regimen could select, for relapse, patients with a more severe initial disease. This is partly supported by the lower rate of relapse observed in the GRAALL trials8, 12 compared with previous studies.1, 2, 3, 4, 5
Although the rate of CR2 (53%) was slightly higher, both the median DFS (10.2 months) and OS (6.8 months) remain similar to what has been previously reported.1, 2, 3, 4, 5 As in the past, a longer CR1 duration (>18 months) and allo-SCT after relapse (upfront or not) remain the two factors predicting a better outcome.1, 2, 3, 4, 5, 6, 7 These results suggest that no major breakthrough has been made in the recent years in the management of younger adults with Ph− ALL after relapse although increased survival has been obtained using pediatric-inspired therapy in first line.8, 9, 10, 11, 12, 13, 14 This also confirms that initial therapy does not really influence the outcome after relapse.2, 3
One striking point in the present study, confirming also previous reports,1, 2, 3, 4, 5, 6, 7 was the variety of salvage regimens used to treat patients at relapse. This emphasizes that still there is no standard-of-care treatment in this setting. The availability of new drugs such as clofarabine,18 nelarabine19 or L-asparaginase encapsulated in erythrocytes21 could have participated in the slightly higher CR2 rate (53%) observed in our series compared with previous reports.1, 2, 3, 4, 5 Yet, the longer CR1 duration possibly explained by the higher intensity of first-line treatment must also be taken into account. Emerging therapies, such as blinatumomab,42 inotuzumab ozogamicin,43 chimeric antigen receptor T cells44 or radio-labeled monoclonal antibodies,45 may soon increase the number of patients who will achieve CR2, in a non-intensive manner, which is of high interest in patients too frail to receive a standard salvage regimen.
The question remains of how to improve the results of these patients. Considering the factors predicting better OS, if we cannot influence such a parameter as CR1 duration, it remains that everything has probably still to be made to consider allo-SCT in all relapsing patients. The lower toxicity of RIC and the availability of alternative donors (cord blood or haplo-identical) may allow to proceed in time with allo-SCT for all these patients, even in case of comorbidities. However, it has to be recalled that patients who received allo-SCT after relapse in this series were in majority those who achieved CR2 (~80%) and that post-allo-SCT survival of patients with active disease at transplant (even if they finally achieved CR2) remains poor,46, 47 suggesting that upfront allo-SCT may not be an option in these circumstances. As a consequence, it appears crucial to obtain CR2, whatever the salvage regimen used, intensive or not. Interestingly, some prognostic genetic features observed at diagnosis, such as IKZF1 gene deletion or NOTCH1/FBXW7/RAS/PTEN mutation status, were taken into account for their impact of postrelapse outcome. This has only been investigated in some pediatric publications.48, 49, 50 None of these molecular features had any impact in terms of postrelapse outcome in our study, perhaps because of the small number of informative cases. It may also be, although this was not investigated, that different clones were involved at relapse which did not express mutations or deletions found at diagnosis.50
Finally, the impact of minimal residual disease levels in adults reaching CR2 remains to be determined.The MRD level may help to discriminate patients who may benefit from additional therapy/maintenance, such as blinatumomab,51 before transplant.
In conclusion, younger adult patients relapsing after current ALL therapies still display a poor outcome. Yet, a minority of them may be cured, especially if CR1 duration has exceeded 18 months and if they are able to receive allo-SCT in CR2. New therapies have to be evaluated prospectively in these patients.
Acknowledgments
We thank all data managers for their help in collecting and updating data.
Author contributions
AD, PC and HD designed, performed and coordinated the research; collected, analyzed and interpreted the data and wrote the manuscript. HD performed statistical analyses, produced the figures and edited the manuscript. MCB provided research support, collected, analyzed and interpreted the data and helped writing the manuscript. XT, FH, TL, MB, JOB, ET, AC, FI, MH, PT, MR, JNB, CH, SL, BL, VL, VA, KB, MLP and NI included patients, contributed data and commented on the manuscript.
The authors declare no conflict of interest.
References
- Thomas DA, Kantarjian H, Smith TL, Koller C, Cortes J, O'Brien S et al. Primary refractory and relapsed adult acute lymphoblastic leukemia: characteristics, treatment results, and prognosis with salvage therapy. Cancer 1999; 86: 1216–1230. [DOI] [PubMed] [Google Scholar]
- Tavernier E, Boiron JM, Huguet F, Bradstock K, Vey N, Kovacsovics T et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia 2007; 21: 1907–1914. [DOI] [PubMed] [Google Scholar]
- Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 2007; 109: 944–950. [DOI] [PubMed] [Google Scholar]
- Oriol A, Vives S, Hernández-Rivas JM, Tormo M, Heras I, Rivas C et al. Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA Study Group. Haematologica 2010; 95: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gökbuget N, Stanze D, Beck J, Diedrich H, Horst HA, Hüttmann A et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood 2012; 120: 2032–2041. [DOI] [PubMed] [Google Scholar]
- Spyridonidis A, Labopin M, Schmid C, Volin L, Yakoub-Agha I, Stadler M et al. Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia 2012; 26: 1211–1217. [DOI] [PubMed] [Google Scholar]
- Poon LM, Hamdi A, Saliba R, Rondon G, Ledesma C, Kendrick M et al. Outcomes of adults with acute lymphoblastic leukemia relapsing after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2013; 19: 1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguet F, Leguay T, Raffoux E, Thomas X, Beldjord K, Delabesse E et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol 2009; 27: 911–918. [DOI] [PubMed] [Google Scholar]
- Asnafi V, Buzyn A, Le Noir S, Baleydier F, Simon A, Beldjord K et al. NOTCH1/FBXW7 mutation identifies a large subgroup with favorable outcome in adult T-cell acute lymphoblastic leukemia (T-ALL): a Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) study. Blood 2009; 113: 3918–3924. [DOI] [PubMed] [Google Scholar]
- Ben Abdelali R, Asnafi V, Leguay T, Boissel N, Buzyn A, Chevallier P et al. Pediatric-inspired intensified therapy of adult T-ALL reveals the favorable outcome of NOTCH1/FBXW7 mutations, but not of low ERG/BAALC expression: a GRAALL study. Blood 2011; 118: 5099–5107. [DOI] [PubMed] [Google Scholar]
- Trinquand A, Tanguy-Schmidt A, Ben Abdelali R, Lambert J, Beldjord K, Lengliné E et al. Toward a NOTCH1/FBXW7/RAS/PTEN-based oncogenetic risk classification of adult T-cell acute lymphoblastic leukemia: a Group for Research in Adult Acute Lymphoblastic Leukemia study. J Clin Oncol 2013; 31: 4333–4342. [DOI] [PubMed] [Google Scholar]
- Beldjord K, Chevret S, Asnafi V, Huguet F, Boulland ML, Leguay T et al. Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood 2014; 123: 3739–3749. [DOI] [PubMed] [Google Scholar]
- Dhédin N, Huynh A, Maury S, Tabrizi R, Beldjord K, Asnafi V et al. Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood 2015; 125: 2486–2496. [DOI] [PubMed] [Google Scholar]
- Maury S, Chevret S, Thomas X, Heim D, Leguay T, Huguet F et al. Rituximab in B-Lineage adult acute lymphoblastic leukemia. N Engl J Med 2016; 375: 1044–1053. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- Delgado J, Pereira A, Villamor N, López-Guillermo A, Rozman C. Survival analysis in hematologic malignancies: recommendations for clinicians. Haematologica 2014; 99: 1410–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D. Regression models and life tables. J R Stat Soc B 1972; 34: 187–220. [Google Scholar]
- Parasole R, Menna G, Marra N, Petruzziello F, Locatelli F, Mangione A et al. Efficacy and safety of intrathecal liposomal cytarabine for the treatment of meningeal relapse in acute lymphoblastic leukemia: experience of two pediatric institutions. Leuk Lymphoma 2008; 49: 1553–1559. [DOI] [PubMed] [Google Scholar]
- Huguet F, Leguay T, Raffoux E, Rousselot P, Vey N, Pigneux A et al. Clofarabine for the treatment of adult acute lymphoid leukemia: the Group for Research on Adult Acute Lymphoblastic Leukemia intergroup. Leuk Lymphoma 2015; 56: 847–857. [DOI] [PubMed] [Google Scholar]
- Forcade E, Leguay T, Vey N, Baruchel A, Delaunay J, Robin M et al. Nelarabine for T cell acute lymphoblastic leukemia relapsing after allogeneic hematopoietic stem cell transplantation: an opportunity to improve survival. Biol Blood Marrow Transplant 2013; 19: 1124–1126. [DOI] [PubMed] [Google Scholar]
- Domenech C, Thomas X, Chabaud S, Baruchel A, Gueyffier F, Mazingue F et al. l-asparaginase loaded red blood cells in refractory or relapsing acute lymphoblastic leukaemia in children and adults: results of the GRASPALL 2005-01 randomized trial. Br J Haematol 2011; 153: 58–65. [DOI] [PubMed] [Google Scholar]
- Annesley CE, Brown P. Novel agents for the treatment of childhood acute leukemia. Ther Adv Hematol 2015; 6: 61–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelken B, Cave H, Leverger G, Galambrun C, Plat G, Schmitt C et al. A phase I study of clofarabine with multiagent chemotherapy in childhood high risk relapse of acute lymphoblastic leukemia (VANDEVOL Study of the French SFCE Acute Leukemia Committee). Pediatr Blood Cancer 2016; 63: 270–275. [DOI] [PubMed] [Google Scholar]
- Domenech C, Mercier M, Plouvier E, Puraveau M, Bordigoni P, Michel G et al. First isolated extramedullary relapse in children with B-cell precursor acute lymphoblastic leukaemia: results of the Cooprall-97 study. Eur J Cancer 2008; 44: 2461–2469. [DOI] [PubMed] [Google Scholar]
- Schaison G, Auclerc MF, Baruchel A, Leblanc T, Leverger G. [Prognosis of acute lymphoblastic leukemia in children. Results of the French protocol FRALLE 93]. Bull Acad Natl Med 2001; 185: 149–160. [PubMed] [Google Scholar]
- Kantarjian HM, O'Brien S, Smith TL, Cortes J, Giles FJ, Beran M et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol 2000; 18: 547–561. [DOI] [PubMed] [Google Scholar]
- Arcese W, Meloni G, Giona F, Vegna ML, Testi AM, Annino L et al. Idarubicin plus ARA-C followed by allogeneic or autologous bone marrow transplantation in advanced acute lymphoblastic leukemia. Bone Marrow Transplant 1991; 7((Suppl 2)): 38. [PubMed] [Google Scholar]
- Specchia G, Pastore D, Carluccio P, Liso A, Mestice A, Rizzi R et al. FLAG-IDA in the treatment of refractory/relapsed adult acute lymphoblastic leukemia. Ann Hematol 2005; 84: 792–795. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee KH, Kim S, Seol M, Kim SH, Kim WK et al. Combination chemotherapy of intermediate-dose cytarabine, idarubicin, plus etoposide and subsequent mobilized donor leukocyte infusion for relapsed acute leukemia after allogeneic bone marrow transplantation. Leuk Res 2001; 25: 305–312. [DOI] [PubMed] [Google Scholar]
- Kantarjian HM, Walters RS, Keating MJ, Barlogie B, McCredie KB, Freireich EJ. Experience with vincristine, doxorubicin, and dexamethasone (VAD) chemotherapy in adults with refractory acute lymphocytic leukemia. Cancer 1989; 64: 16–22. [DOI] [PubMed] [Google Scholar]
- Bassan R, Battista R, Viero P, Pogliani E, Rossi G, Lambertenghi-Deliliers G, Rambaldi A et al. Intensive therapy for adult acute lymphoblastic leukemia: preliminary results of the idarubicin/vincristine/L-asparaginase/prednisolone regimen. Semin Oncol 1993; 20: 39–46. [PubMed] [Google Scholar]
- Willemze R, Peters WG, van Hennik MB, Fibbe WE, Kootte AM, van Berkel M et al. Intermediate and high-dose ARA-C and m-AMSA (or daunorubicin) as remission and consolidation treatment for patients with relapsed acute leukaemia and lymphoblastic non-Hodgkin lymphoma. Scand J Haematol 1985; 34: 83–87. [DOI] [PubMed] [Google Scholar]
- Hiddemann W, Kreutzmann H, Donhuijsen-Ant R, Planker M, Wendt FC, Büchner T. High-dose cytosine arabinoside and mitoxantrone (HAM) for the treatment of refractory acute lymphoblastic leukemia. Onkologie 1987; 20: 11–12. [DOI] [PubMed] [Google Scholar]
- Milpied N, Gisselbrecht C, Harousseau JL, Sebban C, Witz F, Troussard X et al. Successful treatment of adult acute lymphoblastic leukemia after relapse with prednisone, intermediate-dose cytarabine, mitoxantrone, and etoposide (PAME) chemotherapy. Cancer 1990; 66: 627–631. [DOI] [PubMed] [Google Scholar]
- Arlin ZA, Feldman E, Kempin S, Ahmed T, Mittelman A, Savona S et al. Amsacrine with high-dose cytarabine is highly effective therapy for refractory and relapsed acute lymphoblastic leukemia in adults. Blood 1988; 72: 433–435. [PubMed] [Google Scholar]
- Moe PJ, Wesenberg F, Kolmannskog S. Methotrexate infusions in poor prognosis acute lymphoblastic leukemia: II. High-dose methotrexate (HDM) in acute lymphoblastic leukemia in childhood: a pilot study from April 1981. Med Pediatr Oncol 1986; 14: 189–190. [DOI] [PubMed] [Google Scholar]
- Blay JY, Bouhour D, Carrie C, Bouffet E, Brunat-Mentigny M, Philip T et al. The C5R protocol: a regimen of high-dose chemotherapy and radiotherapy in primary cerebral non-Hodgkin's lymphoma of patients with no known cause of immunosuppression. Blood 1995; 86: 2922–2929. [PubMed] [Google Scholar]
- Thomas X, Boiron JM, Huguet F, Dombret H, Bradstock K, Vey N et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol 2004; 22: 4075–4086. [DOI] [PubMed] [Google Scholar]
- Capizzi RL, Keiser LW, Sartorelli AC. Combination chemotherapy--theory and practice. Semin Oncol 1977; 4: 227–253. [PubMed] [Google Scholar]
- Chevallier P, Pigneux A, Robillard N, Ayari S, Guillaume T, Delaunay J et al. Rituximab for the treatment of adult relapsed/refractory CD20 positive B-ALL patients: a pilot series. Leuk Res 2012; 36: 311–315. [DOI] [PubMed] [Google Scholar]
- Zwaan CM, Reinhardt D, Jürgens H, Huismans DR, Hählen K, Smith OP et al. Gemtuzumab ozogamicin in pediatric CD33-positive acute lymphoblastic leukemia: first clinical experiences and relation with cellular sensitivity to single agent calicheamicin. Leukemia 2003; 17: 468–470. [DOI] [PubMed] [Google Scholar]
- Topp MS, Gökbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 2015; 16: 57–66. [DOI] [PubMed] [Google Scholar]
- Yilmaz M, Richard S, Jabbour E. <the clinicl potential ofintouzumab ozogamycin in relapsed and refractory acute lymphocytic leukemia. Ther Adv Hematol 2015; 6: 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015; 385: 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier P, Eugene T, Robillard N, Isnard F, Nicolini F, Escoffre-Barbe M et al. (90)Y-labelled anti-CD22 epratuzumab tetraxetan in adults with refractory or relapsed CD22-positive B-cell acute lymphoblastic leukaemia: a phase 1 dose-escalation study. Lancet Haematol 2015; 2: 108–117. [DOI] [PubMed] [Google Scholar]
- Mohty M, Labopin M, Tabrizzi R, Theorin N, Fauser AA, Rambaldi A et al. Reduced intensity conditioning allogeneic stem cell transplantation for adult patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Haematologica 2008; 93: 303–306. [DOI] [PubMed] [Google Scholar]
- Chevallier P, Labopin M, Milpied N, Bilger K, Socié G, Yakoub-Agha I et al. Outcomes of adults with active or progressive hematological malignancies at the time of allo-SCT: a survey from the Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC). Bone Marrow Transplant 2014; 49: 361–365. [DOI] [PubMed] [Google Scholar]
- Kuiper RP, Waanders E, van der Velden VH, van Reijmersdal SV, Venkatachalam R, Scheijen B et al. IKZF1 deletions predict relapse in uniformly treated pediatric precursor B-ALL. Leukemia 2010; 24: 1258–1264. [DOI] [PubMed] [Google Scholar]
- Krentz S, Hof J, Mendioroz A, Vaggopoulou R, Dörge P, Lottaz C et al. Prognostic value of genetic alterations in children with first bone marrow relapse of childhood B-cell precursor acute lymphoblastic leukemia. Leukemia 2013; 27: 295–304. [DOI] [PubMed] [Google Scholar]
- Yang JJ, Bhojwani D, Yang W, Cai X, Stocco G, Crews K et al. Genome-wide copy number profiling reveals molecular evolution from diagnosis to relapse in childhood acute lymphoblastic leukemia. Blood 2008; 112: 4178–4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topp MS, Gökbuget N, Zugmaier G, Degenhard E, Goebeler ME, Klinger M et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood 2012; 120: 5185–5187. [DOI] [PubMed] [Google Scholar]