Abstract
The impact of circulating plasma cells (CPCs) prior to autologous stem cell transplantation (ASCT) for multiple myeloma has not been defined in the novel agent era. We evaluated the impact of pre-transplant CPCs, detected by six-color flow cytometry in patients undergoing early ASCT on post-transplant response, progression-free survival (PFS) and overall survival (OS). CPCs were detected in 162 out of 840 (19.3%) patients, with the median number of CPCs being 58 per 150 000 events. Ninety-nine percent of patients had received proteasome inhibitor and/or immunomodulator-based induction. The incidence of post-transplant stringent complete response (sCR) in the subgroups with and without CPCs was 15% and 38%, respectively, (P<0.001). The median PFS in the subgroups with and without CPCs was 15.1 (95% confidence interval (CI), 12.5–17.8) and 29.6 months (95% CI, 26.2–32.8), respectively, and the median OS was 41.0 months (95% CI, 32.6–58.2) and not reached (NR) (95% CI, 99.1-NR), respectively, (P<0.001 for both). On multivariate analysis for OS, factors independently predictive of mortality were the presence of CPCs (hazard ratio (HR) 2.5; 95% CI, 1.8–3.6; P<0.001) and sCR post transplant (HR 0.4; 95% CI, 0.2–0.6; P<0.001). Presence of CPCs prior to transplant has a high prognostic impact and should be prospectively validated in clinical trials.
Introduction
The evolving paradigm of circulating plasma cells (CPCs) and its negative prognostic impact in plasma cell disorders has been demonstrated along the entire spectrum of the disease, from monoclonal gammopathy of undetermined significance to relapsed/refractory multiple myeloma (MM).1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 Although the biology of CPCs is not well understood, immunophenotypic and functional characterization has revealed CPCs to be a highly clonogenic side population with functional resemblance to hematopoietic stem cells.1 CPCs have been shown to be quiescent, and hence, can be potentially overlooked by using only M-protein response assessment owing to the absence of monoclonal protein production.1, 12, 13
The therapeutic arena of MM has seen the introduction of new agents in the last decade, including proteasome inhibitors (PIs), immunomodulators (IMiDs), histone deacetylase inhibitors and monoclonal antibodies. However, patients with high-risk disease still experience poor outcomes, and have not been able to completely reap the benefits of novel agents and autologous stem cell transplantation (ASCT).14 The impact of the depth of response prior to transplant on survival in MM has been controversial, with conflicting evidence across various studies.15, 16, 17, 18, 19, 20, 21 In the era predating the widespread use of PIs and IMiDs, the presence of CPCs at transplant by immunohistochemistry or two-color flow cytometry was shown to predict a shorter disease-free survival and overall survival (OS).22, 23 Because of the ease of performing multiparameter flow cytometry (MFC) assays,24, 25 it has become easier to detect clonal CPCs in the peripheral blood with a high sensitivity.
Given the current state of knowledge regarding risk stratification prior to transplant in MM, some questions on CPCs still remain unanswered:1 do CPCs detected prior to transplant have any impact on transplant outcome in the era of novel agent-based induction and post-transplantation therapies?2 Does the identification of CPCs add further prognostic information to the current paradigm of risk stratification, including stage, high-risk cytogenetics and pre-transplant response by the current consensus response criteria?
In this retrospective study, we have investigated the incidence of CPCs prior to transplant using six-color MFC in the era of novel agents, and their impact on best post-transplant response, progression-free survival (PFS) and OS.
Patients and methods
Patients
This study was approved by the Mayo Clinic Institutional Review Board,and was conducted in accordance with federal regulations and the principles of the Declaration of Helsinki. Informed consent was obtained from all patients for review of their medical records. Routine six-color MFC for identification of CPCs was initiated at Mayo Clinic in January 2007 and is performed in all patients as a part of the pre-transplant evaluation. Testing for CPCs was carried out prior to stem cell mobilization. We have included all consecutive patients who underwent ASCT in our institution from January 2007 to May 2015, with an intent for early ASCT (within 12 months of diagnosis).
Mononuclear cells from peripheral blood samples were isolated by Ficoll gradient, and subsequently stained with antibodies to CD19, CD38, CD45, CD138, and cytoplasmic kappa and lambda immunoglobulin light chains.24 The CPCs were detected by the analysis of CD19, CD45, CD38 and CD138, and clonality was assessed by light chain restriction (kappa:lambda expression ratio of 4:1 (kappa restricted) or <1:2 (lambda restricted)). Flow cytometry was performed on BD FACSCanton II instruments (Becton Dickinson, Franklin Lakes, NJ, USA) and data were analyzed using BD FACSDiva software (Becton Dickinson). The target for collection was >150 000 cellular events. The clonal CPCs were reported as the number of clonal events/150 000 total collected events. For samples where <150 000 clonal events were examined, the number of final clonal events was adjusted to 150 000 events.
The primary end point of this study was post-transplant response, PFS and OS. Response was determined according to the current consensus response criteria.26 Best post-transplant response was defined as the best response at any time after ASCT in the first plateau. PFS was defined as time from transplant to disease progression or death due to any cause. OS was defined as time from transplant to death due to any cause. Patients who were alive and free of disease at the last follow-up visit were censored. High-risk cytogenetics by interphase fluorescence in situ hybridization (FISH) on bone marrow plasma cells (BMPCs) was defined by at least one of the following abnormalities: t(4;14), del(17p), t(14;16), t(14;20) or +1q27, 28, 29 detected at any time point from diagnosis to transplant.
Stem cell mobilization, conditioning and transplant management in Mayo Clinic has been previously described.30, 31 All mobilizations were performed with granulocyte colony-stimulating factor subcutaneously (10 mcg/kg per day). Granulocyte colony-stimulating factor was given alone or after cyclophosphamide (1.5 g/m2 daily for 2 consecutive days), or with plerixafor (routinely available since 2009). Full-dose melphalan (200 mg/m2) or reduced-dose melphalan (140 mg/m2) was given at physician discretion depending on general fitness, comorbidities and renal function.
Statistical analysis
Two-sided Fisher's exact tests were used to test for differences between categorical variables. Two-sided Wilcoxon rank sum tests were used to compare continuous variables. Survival analysis was carried out using the method described by Kaplan and Meier.32 Differences in survival between groups were tested for statistical significance using the two-sided log-rank test. Univariate analysis using the Cox proportional hazards model was performed with the following variables: age ⩾65 years, HR cytogenetics, International staging system (ISS) stage 3, CPCs, very good partial response or better at transplant, stringent complete response (sCR) post transplant and melphalan dose. Factors prognostic for PFS and OS with a P-value <0.1 in the univariate analysis were studied in a multivariate analysis. JMP 10.0.0 (SAS Institute Inc., Cary, NC, USA) statistical package was used for all statistical analysis.
Results
The study included a total of 840 patients who met the above-mentioned inclusion criteria. The median age of patients at transplant was 61 years (range, 24–76). Eight hundred and thirty (99%) patients had received novel agent-based induction therapy with PIs and/or IMiDs, including 277 (33%) patients who had received both PI and IMiD.
The median follow-up was 44 months from ASCT (95% confidence interval (CI), 40–47 months). Three hundred and twenty-one (38%) patients had received post-transplant maintenance or consolidation therapy. The median PFS of the entire cohort was 25.3 months (95% CI, 24.0–27.3). The median OS of the entire cohort was 100.0 months (95% CI, 90.7-not reached (NR)) and the 5-year-OS rate was 65% (95% CI, 61–70).
CPCs were detected on flow cytometry in 162 out of 840 (19.3%) patients prior to ASCT. The median number of CPCs among these 162 patients was 58 (range, 1–102 381) per 150 000 events collected. Data on cytogenetics by FISH were available for 742 out of 840 patients. Patients with CPCs had a higher incidence of harboring high-risk cytogenetics by FISH compared with those without CPCs (32.7% vs 20.4%, respectively; P=0.001). Among individual cytogenetic abnormalities, the frequency of t(4;14) was over twofold greater in patients with CPCs compared with those without (16.6% vs 7.3% P<0.001). The frequency of deletion 13q or monosomy 13 was also higher in the subgroup of patients with CPCs (55.6% vs 41.3% P=0.002). There was no significant difference in the frequency of deletion 17p, t(14;16), t(14;20) and +1q. The frequency of individual cytogenetic abnormalities in patients with and without CPCs is depicted in Table 1. On comparing the pre-transplant response, the incidence of very good partial response (VGPR) or better at transplant was lower in patients with CPCs compared with those without (22% vs 47%, respectively; P<0.001). Similarly, only 4.9% of patients with CPCs were in CR prior to transplant compared with 18.9% of patients without CPCs (P<0.001). The incidence of receiving induction therapy with a PI-based regimen was lower in patients with CPCs compared with those without (26% vs 38% P=0.004). There was no difference in the frequency of IMiD-based induction therapy in the two groups. The baseline characteristics of the entire cohort and the two subgroups are shown in Table 2.
Table 1. Frequency of cytogenetic abnormalities by iFISH in different subgroups.
| Cytogenetic abnormalities by iFISH (N=742) | CPCs absent (n=678) | CPCs present (n=162) | P-value |
|---|---|---|---|
| Deletion 17p, n (%) | 65 (11.0) | 24 (15.9) | 0.107 |
| t(4;14), n (%) | 43 (7.3) | 25 (16.6) | <0.001 |
| t(14;16), n (%) | 22 (3.7) | 6 (4.0) | 0.883 |
| t(14; 20), n (%) | 6 (1.0) | 2 (1.3) | 0.747 |
| +1q, n (%) | 29 (4.9) | 8 (5.3) | 0.845 |
| Deletion 13q or monosomy 13 | 244 (41.3) | 84 (55.6) | 0.002 |
Abbreviations: CPC, circulating plasma cell; iFISH, interphase fluorescence in situ hybridization; N, number of patients with available data on iFISH cytogenetics. Bold values indicate statistically significance parameters.
Table 2. Baseline clinical characteristics.
| Baseline characteristics | Overall (n=840) | CPCs absent (n=678) | CPCs present (n=162) | P-value |
|---|---|---|---|---|
| Median age at transplant (range), years | 61.1 (24.4–76.1) | 61.4 (24.4–76.1) | 59.9 (32.2–73.8) | 0.068 |
| Males (%) | 59.6 | 61.2 | 53.1 | 0.060 |
| ISS stage, n (%) | 0.004 | |||
| Stage I | 161 (19.2) | 143 (21.1) | 18 (11.1) | |
| Stage II | 313 (37.3) | 257 (37.9) | 56 (34.6) | |
| Stage III | 204 (24.3) | 156 (23.0) | 48 (29.6) | |
| Missing | 162 (19.3) | 122 (18.0) | 40 (24.7) | |
| Median time from diagnosis to transplant (range), months | 6.1 (1.3–11.9) | 6.1 (1.3–11.9) | 6.3 (2.4–11.9) | 0.215 |
| FISH cytogeneticsa, n (%) | 0.001 | |||
| High risk | 191 (22.7) | 138 (20.4) | 53 (32.7) | |
| Standard risk | 551 (65.6) | 453 (66.8) | 98 (60.5) | |
| Missing | 98 (11.7) | 87 (12.8) | 11 (6.8) | |
| Pre-transplant response, n (%) | <0.001 | |||
| CR | 136 (16.2) | 128 (18.9) | 8 (4.9) | |
| VGPR | 221 (26.3) | 193 (28.5) | 28 (17.3) | |
| PR | 346 (41.2) | 284 (41.9) | 62 (38.3) | |
| MR or SD | 93 (11.1) | 57 (8.4) | 36 (22.2) | |
| PD | 44 (5.2) | 16 (2.4) | 28 (17.3) | |
| Induction regimen | ||||
| PI-based only, n (%) | 297 (35.4) | 255 (37.6) | 42 (25.9) | 0.004 |
| IMiD-based only, n (%) | 256 (30.5) | 206 (30.4) | 50 (30.9) | 0.905 |
| PI- and IMiD-based, n (%) | 277 (33.0) | 211 (31.1) | 66 (40.7) | 0.021 |
| Melphalan dose, n (%) | 0.938 | |||
| Full | 740 (88.1) | 597 (88.1) | 143 (88.3) | |
| Reduced | 100 (11.9) | 81 (11.9) | 19 (11.7) | |
| Post-transplant maintenance or consolidation, n (%) | 321 (38.2) | 262 (38.6) | 59 (36.4) | 0.600 |
Abbreviations: CPC, circulating plasma cell; CR, complete response; FISH, fluorescence in situ hybridization; IMiD, immunomodulator; ISS, International staging system; MR, minimal response; PD, progressive disease; PI, proteasome inhibitor; PR, partial response; SD, stable disease; VGPR, very good partial response.
High-risk FISH cytogenetics was defined by t(4;14), del(17p), t(14;16), t(14;20) and +1q.
A total of 283 (34%) patients had attained sCR after transplant. The incidence of sCR in the subgroups with and without CPCs was 15% and 38%, respectively, (P<0.001). The incidence of post-transplant CR (excluding sCR) in the respective subgroups was 13% and 15%, respectively, (P=0.502), and the incidence of post-transplant VGPR was 32% and 23%, respectively, (P=0.030).
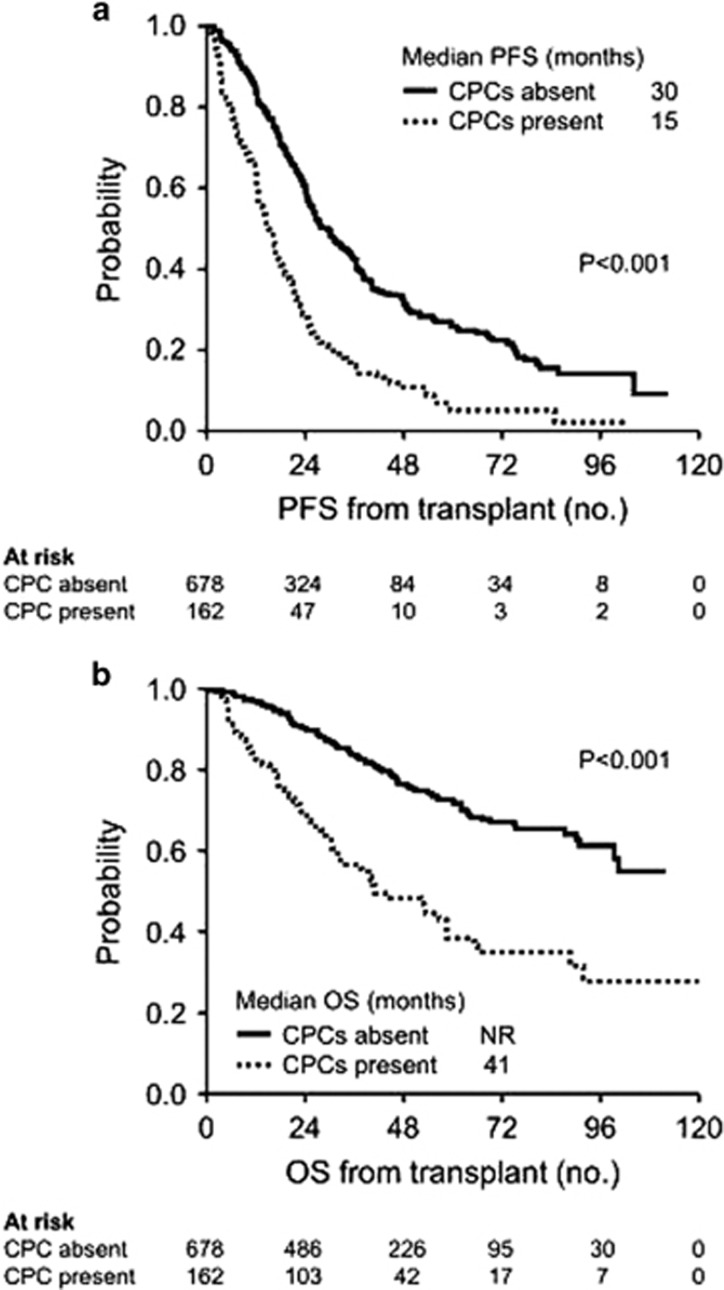
The median PFS in the subgroups with and without CPCs was 15.1 (95% CI, 12.5–17.8) and 29.6 months (95% CI, 26.2–32.8), respectively, and the median OS was 41.0 months (95% CI, 32.6–58.2) and NR (99.1-NR), respectively, (P<0.001 for both comparisons), with the 5-year-OS rates being 39% (95% CI, 30–49%) and 72% (95% CI, 68–77%), respectively. The Kaplan–Meier curves for PFS and OS is depicted in Figures 1a and b.
Figure 1.
(a) Kaplan–Meier curves for PFS from transplant in patients with and without CPCs. (b) Kaplan–Meier curves for OS from transplant in patients with and without CPCs.
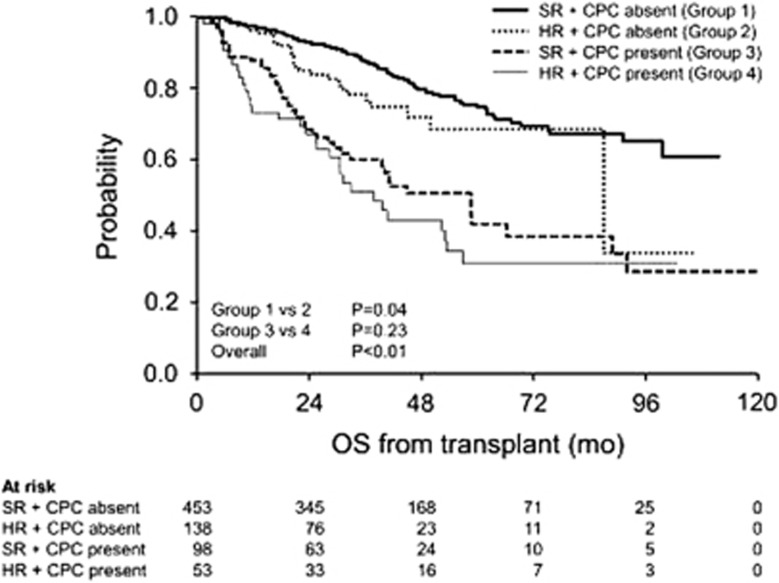
Subsequently, we investigated the median OS of patients stratified by the presence of CPCs and high-risk cytogenetics by FISH (Figure 2). In the subgroup without CPCs, the median OS of patients with high-risk (n=138) and standard-risk (n=453) cytogenetics was 86.8 months (95% CI, 86.8-NR) and NR (99.1-NR), respectively, (P=0.04). In the subgroup of patients with CPCs, the median OS of patients with HR (n=53) and standard risk (n=98) cytogenetics was 37.6 (95% CI, 25.3–53.0) and 58.2 months (32.5–88.3), respectively, (P=0.23).
Figure 2.
Kaplan–Meier curves for OS in patients stratified by the presence of CPCs and HR cytogenetics by FISH.
In the univariate Cox model for PFS (Table 3), the following variables were associated with a significant impact on progression: high-risk FISH cytogenetics (hazard ratio (HR) 1.3; 95% CI, 1.1–1.6; P=0.015), presence of CPCs (HR 2.3; 95% CI, 1.9–2.8; P<0.001), ⩾very good partial response at transplant (HR 0.8; 95% CI, 0.7–0.9; P=0.012) and sCR post transplant (HR 0.5; 95% CI, 0.4–0.6; P<0.001). The factors which retained their significance on multivariate analysis included presence of CPCs (HR 2.0; 95% CI, 1.6–2.5; P<0.001) and sCR post transplant (HR 0.4; 95% CI, 0.3–0.6; P<0.001). In the univariate Cox model for OS (Table 3), the following variables were associated with a significant impact on mortality: high-risk FISH cytogenetics (HR 1.7; 95% CI, 1.2–2.3; P=0.001), presence of CPCs (HR 3.0; 95% CI, 2.2–3.9; P<0.001), sCR post transplant (HR 0.4; 95% CI, 0.3–0.6; P<0.001) and ISS stage 3 at diagnosis (HR 1.5; 95% CI, 1.1–2.0; P=0.015). The factors that retained their significance on multivariate analysis included presence of CPCs (HR 2.5; 95% CI, 1.8–3.6; P<0.001) and sCR post transplant (HR 0.4; 95% CI, 0.2–0.6; P<0.001).
Table 3. Univariate and multivariate analysis for PFS and OS by Cox proportional hazards model.
| Variable |
Progression-free survival (PFS) |
Overall survival (OS) |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | P-value | Multivariate | P-value | Univariate | P-value | Multivariate | P-value | |
| Hazard ratio (95% CI) | Hazard ratio (95% CI) | Hazard ratio (95% CI) | Hazard ratio (95% CI) | |||||
| Age ⩾65 | 0.87 (0.72–1.05) | 0.144 | NA | NA | 1.09 (0.82–1.44) | 0.538 | NA | NA |
| HR FISH cytogenetics | 1.30 (1.05–1.60) | 0.015 | 1.20 (0.97–1.48) | 0.088 | 1.71 (1.24–2.32) | 0.001 | 1.26 (0.86–1.81) | 0.231 |
| CPCs present | 2.28 (1.87–2.76) | <0.0001 | 2.03 (1.64–2.50) | <0.001 | 2.97 (2.25–3.88) | <0.001 | 2.52 (1.78–3.55) | <0.001 |
| ⩾VGPR at transplant | 0.80 (0.67–0.95) | 0.012 | 1.15 (0.92–1.42) | 0.209 | 0.95 (0.72–1.24) | 0.727 | NA | NA |
| sCR post transplant | 0.45 (0.37–0.55) | <0.001 | 0.44 (0.34–0.55) | <0.001 | 0.42 (0.30–0.59) | <0.001 | 0.39 (0.25–0.61) | <0.001 |
| ISS stage 3 | 1.15 (0.94–1.41) | 0.168 | NA | NA | 1.48 (1.08–2.01) | 0.015 | 1.21 (0.86–1.70) | 0.270 |
| Reduced-dose melphalan | 1.03 (0.78–1.32) | 0.829 | NA | NA | 1.40 (0.96–1.99) | 0.076 | 1.27 (0.78–1.98) | 0.322 |
Abbreviations: CI, confidence interval; CPC, circulating plasma cells; FISH, fluorescence in situ hybridization; HR, high risk; ISS, International staging system; NA, not applicable; sCR, stringent complete response; VGPR, very good partial response. Bold values indicate statistically significance parameters.
Discussion
Our study shows the negative prognostic impact of CPCs at transplant on post-transplant depth of response, PFS and OS, irrespective of the presence of high-risk cytogenetic abnormalities, stage at diagnosis and pre-transplant response in patients with newly diagnosed MM undergoing early ASCT. The prognostic impact of CPCs was so strong when present that FISH cytogenetics were no longer predictive of relapse-free or overall survival.
In a study on transplanted myeloma patients at Mayo Clinic who did not receive novel agent-based induction or maintenance, the median PFS and OS of patients with CPCs at transplant was 14 and 33 months, respectively,23 compared with 15 and 41 months, respectively, in our study in the era of PIs and IMiDs. However, their survival is still worse compared with patients with revised-ISS stage III, in whom the median PFS and OS from diagnosis are 29 and 43 months, respectively.27 As revised-ISS staging incorporates ISS stage, HR cytogenetics by FISH and serum lactate dehydrogenase, it is evident that the impact of CPCs is critical and independently defines an ultra-high-risk group, which has a worse survival irrespective of cytogenetic risk status or ISS stage at diagnosis. A phase 2 study of PI-based induction followed by ASCT in patients with primary plasma cell leukemia (defined by the presence of CPCs >2 × 109/l or⩾20% of blood leukocytes) showed a median OS of 36 months,33 indicating poor outcomes compared with patients with standard-risk MM despite novel agent and upfront ASCT. In newly diagnosed multiple myeloma patients, quantification of CPCs at diagnosis has been shown to be a powerful tool to define high-risk disease independent of ISS stage at diagnosis.3
Patients with CPCs had a 50% higher risk of harboring high-risk cytogenetics (performed on BMPCs) compared with those with no CPCs. Upon assessing the frequency of individual cytogenetic abnormalities, the frequency of t(4;14) was over twofold in those with CPCs. Another study on 757 patients with newly diagnosed MM had also shown a significantly higher incidence of t(4;14) in patients with CPCs compared with those without (32% vs 19%, respectively; P=0.038).10 In a study performing FISH on paired samples of BMPCs and extramedullary plasma cells, t(4;14) was more prevalent in the extramedullary compartment, whereas del13q14 and 14q32 was more prevalent in the BM. Of note, the presence of CPCs was predictive of poor OS independent of the presence of t(4;14) in our study, indicating that clonal CPCs might contribute to the unfavorable prognosis in this cytogenetic subgroup. As CPCs have been shown to be unique cytogenetic subclones of BMPCs,1 paired cytogenetic analysis of CPCs and BMPCs should be performed in large cohorts of patients to identify the driver cytogenetic abnormalities, leading to their chemoresistance and replicative immortality.
Immunophenotypic characterization by MFC in CPCs by Paiva et al. has shown downregulation of several surface markers, including integrins, adhesion molecules, CD28, CD38, CD138, CD81 and CD117, among others, as compared with paired BMPCs. Furthermore, CPCs were shown to be mostly quiescent, in the subG0-G1 phase of cell cycle compared with BMPCs, which had also been confirmed in a prior study.12 They had a threefold colony and 16-fold cluster formation potential compared with their counterparts in the marrow. On the basis of these unique characteristics of quiescence and high clonogenic potential, the authors contemplated the possibility of CPCs representing a cancer stem cell-like population in myeloma.1 These findings imply that monoclonal antibodies against surface markers like CD38 might not be effective against CPCs. Myeloma cells with decreased surface expression of CD138 has also been shown to exhibit reduced sensitivity to lenalidomide.34
Myeloma with CPCs has also been proposed as a model for invasion and metastasis owing to intriguing similarities with the epithelial–mesenchymal transition program in solid tumors.2 The BM endosteal niche is quite hypoxic,35 which has been shown to induce quiescence, downregulate surface adhesion molecules and activate transcription factors in myeloma cells.2, 36, 37 BM hypoxia promotes dissemination of myeloma cells in the peripheral blood in a mouse model. It also leads to overexpression of CXCR4 in myeloma cells,36 which is known to have an important role in invasion and homing to new sites in solid tumors, leading to the metastatic cascade. CPCs in humans also overexpress CXCR4 compared with their bone marrow counterparts,1 which is critical in homing to new sites and heralding extramedullary disease.
Differences in DNA methylation patterns leading to epigenetic modulation of gene expression has been shown in both myeloma and plasma cell leukemia.38, 39 Furthermore, significantly increased hypermethylation has been observed in the subset of myeloma cells harboring t(4;14) translocation, which is more common in patients with CPCs compared with those without. The adenomatous polyposis coli gene, which functions as an antagonist of the Wnt signaling pathway, has been shown to be hypermethylated in the t(4;14) subset of myeloma cells.38 As Wnt pathway is known to be activated in both normal and cancer stem cell,40 it would be interesting to explore whether an activated Wnt pathway imparts stemness and self-renewal capacity in CPCs. In the hyperdiploid clones of myeloma cells, DNA methylation arrays have identified two distinct subgroups of patients with different methylation patterns. The subgroup with more heavily methylated genes had a significantly poorer OS compared with the other (45 versus 70 months; P=0.03), despite no difference in the incidence of known FISH-defined high-risk cytogenetic markers in the two groups.38
There are certain limitations of this study. First, this is a retrospective study and the findings need to be prospectively validated in clinical trials. Second, the quantification of CPCs are based on six-color MFC that is routinely performed in our institution. With the advent of more sensitive MFC (⩾eight-color) techniques, the frequency of detection of CPCs will increase and various cutoffs could be explored for further refinement of risk-stratification criteria. Nevertheless, this is the largest study showing the strong prognostic impact of CPCs prior to transplant in predicting survival independent of the currently employed risk-stratification systems in a homogeneously treated cohort of MM patients undergoing early ASCT. The proposed risk stratification with CPCs is easily reproducible and cost-effective. Detailed epigenomic, genomic and transcriptomic analysis of CPCs in large cohorts of patients is urgently needed to uncover the biological basis of their resistance to both high-dose cytotoxic therapy and novel agents, and identify potential avenues of targeted therapy against CPCs. Quantification of CPCs along the entire trajectory of the disease should be actively incorporated in clinical trials to study the clonal evolution and kinetics of CPCs, and its impact on disease outcomes.
Acknowledgments
Author contributions
RC, MAG and EM designed the study, analyzed the data, wrote the first draft and approved the final version of the manuscript. MAG, SKK, DJ, FKB, DD, AD, SRH, WJH, PK, MQL and NL performed patient management, revised the manuscript critically, participated in final data analysis and approved the final version of the manuscript.
The authors declare no conflict of interest.
References
- Paiva B, Paino T, Sayagues JM, Garayoa M, San-Segundo L, Martin M et al. Detailed characterization of multiple myeloma circulating tumor cells shows unique phenotypic, cytogenetic, functional, and circadian distribution profile. Blood 2013; 122: 3591–3598. [DOI] [PubMed] [Google Scholar]
- Ghobrial IM. Myeloma as a model for the process of metastasis: implications for therapy. Blood 2012; 120: 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves WI, Rajkumar SV, Gupta V, Morice WG, Timm MM, Singh PP et al. Quantification of clonal circulating plasma cells in newly diagnosed multiple myeloma: implications for redefining high-risk myeloma. Leukemia 2014; 28: 2060–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peceliunas V, Janiulioniene A, Matuzeviciene R, Zvirblis T, Griskevicius L. Circulating plasma cells predict the outcome of relapsed or refractory multiple myeloma. Leuk Lymphoma 2012; 53: 641–647. [DOI] [PubMed] [Google Scholar]
- Billadeau D, Van Ness B, Kimlinger T, Kyle RA, Therneau TM, Greipp PR et al. Clonal circulating cells are common in plasma cell proliferative disorders: a comparison of monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, and active myeloma. Blood 1996; 88: 289–296. [PubMed] [Google Scholar]
- Kumar S, Rajkumar SV, Kyle RA, Lacy MQ, Dispenzieri A, Fonseca R et al. Prognostic value of circulating plasma cells in monoclonal gammopathy of undetermined significance. J Clin Oncol 2005; 23: 5668–5674. [DOI] [PubMed] [Google Scholar]
- Nowakowski GS, Witzig TE, Dingli D, Tracz MJ, Gertz MA, Lacy MQ et al. Circulating plasma cells detected by flow cytometry as a predictor of survival in 302 patients with newly diagnosed multiple myeloma. Blood 2005; 106: 2276–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez de Larrea C, Kyle RA, Durie BG, Ludwig H, Usmani S, Vesole DH et al. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia 2013; 27: 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves WI, Morice WG, Rajkumar V, Gupta V, Timm MM, Dispenzieri A et al. Quantification of clonal circulating plasma cells in relapsed multiple myeloma. Br J Haematol 2014; 167: 500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An G, Qin X, Acharya C, Xu Y, Deng S, Shi L et al. Multiple myeloma patients with low proportion of circulating plasma cells had similar survival with primary plasma cell leukemia patients. Ann Hematol 2015; 94: 257–264. [DOI] [PubMed] [Google Scholar]
- Bianchi G, Kyle RA, Larson DR, Witzig TE, Kumar S, Dispenzieri A et al. High levels of peripheral blood circulating plasma cells as a specific risk factor for progression of smoldering multiple myeloma. Leukemia 2013; 27: 680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Rajkumar SV, Greipp PR, Witzig TE. Cell proliferation of myeloma plasma cells: comparison of the blood and marrow compartments. Am J Hematol 2004; 77: 7–11. [DOI] [PubMed] [Google Scholar]
- Paiva B, Corchete LA, Vidriales MB, Puig N, Maiso P, Rodriguez I et al. Phenotypic and genomic analysis of multiple myeloma minimal residual disease tumor cells: a new model to understand chemoresistance. Blood 2016; 127: 1896–1906. [DOI] [PubMed] [Google Scholar]
- Chng WJ, Dispenzieri A, Chim CS, Fonseca R, Goldschmidt H, Lentzsch S et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia 2014; 28: 269–277. [DOI] [PubMed] [Google Scholar]
- Lahuerta JJ, Mateos MV, Martinez-Lopez J, Rosinol L, Sureda A, de la Rubia J et al. Influence of pre- and post-transplantation responses on outcome of patients with multiple myeloma: sequential improvement of response and achievement of complete response are associated with longer survival. J Clin Oncol 2008; 26: 5775–5782. [DOI] [PubMed] [Google Scholar]
- Kumar S, Lacy MQ, Dispenzieri A, Rajkumar SV, Fonseca R, Geyer S et al. High-dose therapy and autologous stem cell transplantation for multiple myeloma poorly responsive to initial therapy. Bone Marrow Transplant 2004; 34: 161–167. [DOI] [PubMed] [Google Scholar]
- Singhal S, Powles R, Sirohi B, Treleaven J, Kulkarni S, Mehta J. Response to induction chemotherapy is not essential to obtain survival benefit from high-dose melphalan and autotransplantation in myeloma. Bone Marrow Transplant 2002; 30: 673–679. [DOI] [PubMed] [Google Scholar]
- Gertz MA, Kumar S, Lacy MQ, Dispenzieri A, Dingli D, Hayman SR et al. Stem cell transplantation in multiple myeloma: impact of response failure with thalidomide or lenalidomide induction. Blood 2010; 115: 2348–2353. [DOI] [PubMed] [Google Scholar]
- Moreau P, Attal M, Pegourie B, Planche L, Hulin C, Facon T et al. Achievement of VGPR to induction therapy is an important prognostic factor for longer PFS in the IFM 2005-01 trial. Blood 2011; 117: 3041–3044. [DOI] [PubMed] [Google Scholar]
- Vij R, Kumar S, Zhang MJ, Zhong X, Huang J, Dispenzieri A et al. Impact of pretransplant therapy and depth of disease response before autologous transplantation for multiple myeloma. Biol Blood Marrow Transplant 2015; 21: 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Muchtar E, Kumar SK, Buadi FK, Dingli D, Dispenzieri A et al. Impact of pre-transplant bone marrow plasma cell percentage on post-transplant response and survival in newly diagnosed multiple myeloma. Leuk Lymphoma 2016; 58: 308–315. [DOI] [PubMed] [Google Scholar]
- Gertz MA, Witzig TE, Pineda AA, Greipp PR, Kyle RA, Litzow MR. Monoclonal plasma cells in the blood stem cell harvest from patients with multiple myeloma are associated with shortened relapse-free survival after transplantation. Bone Marrow Transplant 1997; 19: 337–342. [DOI] [PubMed] [Google Scholar]
- Dingli D, Nowakowski GS, Dispenzieri A, Lacy MQ, Hayman SR, Rajkumar SV et al. Flow cytometric detection of circulating myeloma cells before transplantation in patients with multiple myeloma: a simple risk stratification system. Blood 2006; 107: 3384–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Kimlinger T, Morice W. Immunophenotyping in multiple myeloma and related plasma cell disorders. Best Pract Res Clin Haematol 2010; 23: 433–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva B, Almeida J, Perez-Andres M, Mateo G, Lopez A, Rasillo A et al. Utility of flow cytometry immunophenotyping in multiple myeloma and other clonal plasma cell-related disorders. Cytometry B Clin Cytom 2010; 78: 239–252. [DOI] [PubMed] [Google Scholar]
- Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K et al. International uniform response criteria for multiple myeloma. Leukemia 2006; 20: 1467–1473. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L et al. Revised International staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol 2015; 33: 2863–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhael JR, Dingli D, Roy V, Reeder CB, Buadi FK, Hayman SR et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc 2013; 88: 360–376. [DOI] [PubMed] [Google Scholar]
- Rajan AM, Rajkumar SV. Interpretation of cytogenetic results in multiple myeloma for clinical practice. Blood Cancer J 2015; 5: e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz MA, Dingli D. How we manage autologous stem cell transplantation for patients with multiple myeloma. Blood 2014; 124: 882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz MA, Ansell SM, Dingli D, Dispenzieri A, Buadi FK, Elliott MA et al. Autologous stem cell transplant in 716 patients with multiple myeloma: low treatment-related mortality, feasibility of outpatient transplant, and effect of a multidisciplinary quality initiative. Mayo Clin Proc 2008; 83: 1131–1138. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- Royer B, Minvielle S, Diouf M, Roussel M, Karlin L, Hulin C et al. Bortezomib, doxorubicin, cyclophosphamide, dexamethasone induction followed by stem cell transplantation for primary plasma cell leukemia: a prospective phase II study of the Intergroupe Francophone du Myelome. J Clin Oncol 2016; 34: 2125–2132. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Fujiwara S, Wada N, Izaki M, Yuki H, Okuno Y et al. Multiple myeloma cells expressing low levels of CD138 have an immature phenotype and reduced sensitivity to lenalidomide. Int J Oncol 2012; 41: 876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood 1993; 82: 2031–2037. [PubMed] [Google Scholar]
- Azab AK, Hu J, Quang P, Azab F, Pitsillides C, Awwad R et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood 2012; 119: 5782–5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SK, Diamond P, Gronthos S, Peet DJ, Zannettino AC. The emerging role of hypoxia, HIF-1 and HIF-2 in multiple myeloma. Leukemia 2011; 25: 1533–1542. [DOI] [PubMed] [Google Scholar]
- Walker BA, Wardell CP, Chiecchio L, Smith EM, Boyd KD, Neri A et al. Aberrant global methylation patterns affect the molecular pathogenesis and prognosis of multiple myeloma. Blood 2011; 117: 553–562. [DOI] [PubMed] [Google Scholar]
- Dimopoulos K, Gimsing P, Gronbaek K. The role of epigenetics in the biology of multiple myeloma. Blood Cancer J 2014; 4: e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nat Rev Drug Discov 2014; 13: 497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]