Abstract
Proper surveillance of virus activity and a timely response to viral outbreaks depend upon the rapid diagnosis of viral infections. The immunoglobulin M (IgM) antibody-capture enzyme-linked immunosorbent assay (MAC-ELISA) is a fast, sensitive test routinely used for the diagnosis of the medically important West Nile and St. Louis encephalitis flaviviruses. However, the suckling mouse brain-derived (SMB) antigen used in this assay is tedious to prepare and has a risk of exposing personnel to live virus and hazardous chemicals. We report the development of a St. Louis encephalitis virus (SLEV) noninfectious recombinant antigen that is a safe and easily produced alternative antigen for use in diagnostic assays. The expression plasmid pCB8SJ2, containing the premembrane and envelope structural protein-encoding regions of SLEV, was constructed to express secreted extracellular virus-like particles (VLPs) from CHO cells. Blind-coded human serum panels were assembled from patients having recent SLEV, West Nile virus (WNV), Powassan virus, or La Crosse encephalitis virus infections to assess the sensitivity and specificity of assays with SLEV VLP or SMB antigen. MAC-ELISAs with either antigen had comparable sensitivity for the detection of IgM antibodies against SLEV. Importantly, when these two antigens were tested against a human serum panel from patients having recent WNV or Powassan virus infections, the SLEV VLPs were less likely than SMB antigen to detect flavivirus cross-reactive IgM antibodies. An optimized IgG antibody capture ELISA (GAC-ELISA) with both WNV and SLEV VLPs was developed to circumvent the frequently observed higher background in the antigen-capture IgG-ELISA (ACG-ELISA). For the detection of IgG antibodies against WNV, the GAC-ELISA resulted in a statistically significant higher performance accuracy (P = 0.003) than the ACG-ELISA when the WNV VLP antigen was used in both assays. However, no statistical difference was observed in the assay performance of the GAC-ELISA with SLEV VLP or the ACG-ELISA with SLEV SMB antigen.
St. Louis encephalitis virus (SLEV), a member of the genus Flavivirus in the family Flaviviridae, was the leading cause of epidemic flaviviral encephalitis in the United States prior to the introduction of West Nile virus (WNV) in late 1999. This mosquito-borne arbovirus, transmitted primarily by Culex species, is distributed throughout the lower 48 states, with periodic epidemics occurring mostly in the Midwest and Southeast. Between 1964 and 2002, over 4,500 human cases of SLEV infection were reported, with a national average of 118 reported cases per year (G. L. Campbell, Centers for Disease Control and Prevention, personal communication). The last major outbreak, from 1974 to 1977, involved 35 states and over 2,500 reported cases (16, 17). More recent outbreaks occurred in Florida in 1990 (226 reported human cases) (2, 9), Louisiana in 1998 and 2001 (18 and 71 cases, respectively) (3, 4), and Texas in 2002 (19 cases; G. L. Campbell, Centers for Disease Control and Prevention, personal communication). Most SLEV infections are not clinically apparent or only present as a nonspecific flu-like illness; therefore, the vast majority remain undiagnosed. Infection may progress to severe disease, such as meningoencephalitis with a case fatality rate of 5 to 20% (24). The elderly are especially at risk for progression to severe disease and death.
Intensive surveillance of flaviviral outbreaks provides valuable information applicable to the control and prevention of virus activity. A standardized IgM antibody-capture enzyme-linked immunosorbent assay (MAC-ELISA) protocol was previously developed for the rapid screening of human serum samples for various arboviral infections (15). This assay serves as a valuable tool for the presumptive diagnosis of acute flaviviral infections and facilitates the processing of numerous serum samples. However, the use of arbovirus antigens prepared from virus-infected suckling mouse brains in this diagnostic assay may expose the personnel to infectious agents and hazardous chemicals during antigen production. In addition, the procedure for this antigen preparation is time consuming, costly and tedious. During natural flavivirus infection, empty virus-like particles consisting of viral premembrane, membrane (M), and envelope (E) structural proteins are produced in addition to infectious virus (21). These noninfectious virus-like particles (VLPs) do not contain nucleocapsid but are structurally and physiochemically similar to infectious virion particles. We have developed an expression system to produce secreted extracellular VLPs for several flaviviruses in mammalian cell culture. African green monkey kidney (COS-1) cells transformed with expression plasmids containing the premembrane and E structural proteins of either Japanese encephalitis virus (JEV) or WNV secreted VLPs into tissue culture fluid at high antigen capture (AC) enzyme-linked immunosorbent assay (ELISA) titers (6, 8, 12). Replacement of the C terminus of dengue virus type 2 E protein (amino acids E-397 to E-495) with the JEV homologous region was necessary, however, for high-level secretion of dengue virus type 2 VLPs (7). This region is believed to contain a putative membrane retention signal located between amino acids E-397 and E-436 that prevents efficient budding of VLPs from the cytoplasmic membrane.
Noninfectious recombinant antigen prepared by polyethylene glycol precipitation of VLPs secreted into tissue culture fluid by a stably transformed COS-1 cell line was shown to be a safe and sensitive alternative to virus-infected suckling mouse brain-derived (SMB) antigens for diagnosis of JEV and WNV infections (8, 12). The WNV VLP antigen was shown to have sensitivity comparable to that of WNV SMB antigen for detecting IgM and IgG antibodies against WNV (8). In 2001, public health service laboratories in the United States replaced the traditional WNV SMB antigen with WNV VLPs for use in diagnostic assays (8, 19).
Prior to the introduction of WNV in 1999, SLEV was the only mosquito-borne flavivirus resulting in significant human morbidity and mortality in the United States Both WNV and SLEV are members of the JEV serocomplex. The recent expansion of WNV across the United States and the subsequent cocirculation with SLEV in some regions has complicated serodiagnosis due to the presence of flavivirus group-reactive antibodies elicited by either viral infection. However, antibodies produced during SLEV infection were generally found to be more cross-reactive than those produced during WNV infection (14). Simultaneous testing of these human serum samples for WNV and SLEV antibodies, thus, is necessary in order to identify the virus responsible for the most recent infection.
We present here a procedure for the production and purification of SLEV VLPs in tissue culture. A SLEV premembrane and E protein expression plasmid, pCB8SJ2, was constructed for transient expression of viral structural proteins in Chinese hamster ovary (CHO) cells, and the secreted VLPs were partially purified and concentrated by ultracentrifugation. Four human serum panels were assembled to compare the sensitivity and specificity of SLEV VLPs and SMB antigen in detecting IgM and IgG antibody. These serum panels consisted of sera from patients infected with two members of the JEV serocomplex, SLEV and WNV, as well as two North American arboviruses, Powassan virus (a member of the tick-borne flaviviruses), and La Crosse virus, a member of the family Bunyaviridae). Additionally, an optimized IgG antibody capture (GAC)-ELISA for both SLEV and WNV was developed in order to circumvent frequently observed higher background absorbance against normal CHO cell antigen in the antigen capture IgG ELISA (ACG-ELISA).
MATERIALS AND METHODS
Cell culture and virus strain.
CHO-K1 cells (CCL-61; American Type Culture Collection, Mannassas, Va.) were grown in Dulbecco's modified Eagle's medium/F12 (Gibco Laboratories, Grand Island, N.Y.). Human embryonic kidney (293) cells (ATCC CRL-1573), African green monkey kidney (COS-1) cells (ATCC CRL-1650), and C6/36 mosquito cells were grown in Dulbecco's modified Eagle's medium (Gibco Laboratories). All media were supplemented with 10% heat-inactivated fetal bovine serum (HyClone Laboratories, Inc., Logan, Utah), 1 mM sodium pyruvate, 0.1 mM minimal essential medium nonessential amino acids, penicillin (100 U/ml), and 100 μg of streptomycin per ml, and all cells were grown at 37°C with 5% CO2. SLEV (strain MSI-7) propagated in C6/36 cell culture was used for RNA extraction, reverse transcription-PCR (RT-PCR), cDNA cloning, and protein analysis. For use in the Western blot, virus was purified by precipitation with 7% polyethylene glycol 8000 (Fisher Scientific, Fair Lawn, N.J.), followed by ultracentrifugation on 30% glycerol-45% potassium tartrate gradients (18).
Construction of expression plasmids.
The pCDSLE4MU4 plasmid was constructed by inserting the SLEV premembrane and E genes into pCDNA-3 (Invitrogen Corp., Carlsbad, Calif.) as previously described for other flaviviruses (5, 6, 8, 12). Briefly, the QIAamp Viral RNA kit (Qiagen Inc., Valencia, Calif.) was used according to the manufacturer's recommended procedures to extract genomic RNA from 140 μl of C6/36 cell culture supernatant containing SLEV, MSI-7 strain. Extracted RNA was used as the template for production of cDNA containing the SLEV premembrane and E protein-encoding sequences with the Qiagen OneStep RT-PCR kit (Qiagen Inc.).
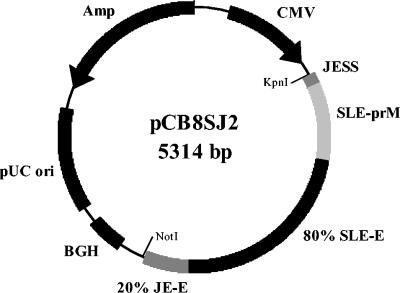
Primer sequences containing the appropriate restriction enzyme sites for cloning (Table 1) were designed based on the published sequence (23). The cDNA was digested with AfeI and NotI and then ligated into AfeI- and NotI-digested pCDNA-3. This SLEV premembrane-E plasmid and the pCDJE2-7 (6) plasmid were used as the PCR templates to amplify SLEV and JEV cDNA sequences, respectively, with the appropriate primer sets listed in Table 1. The PCR products for the SLEV premembrane and 80% E and 20% JEV E protein-encoding sequences were digested with BsmBI and ligated. This ligation product was then PCR amplified with primers T7 and cSP6. This amplicon was digested with KpnI and NotI and then inserted into the KpnI and NotI sites of the pCBamp plasmid (6) to create pCB8SJ2 (Fig. 1).
TABLE 1.
Oligonucleotides used for cloning of 100% and 80% SLEV premembrane-E protein-encoding cDNA into expression plasmids
| Construct and primers | Oligonucleotide sequencea |
|---|---|
| pCDSLE4MU4 | |
| SLE-AfeI-463 | 5′ AAAAGAAAAAGCGCTTTGCAGTTATCAACCTATCAGGGGAA |
| cSLE-NotI-2447 | 5′ AAAAGAAAAAGCGGCCGCTCAGGCTTGCACGCTGGTTGCCA |
| pCB8SJ2 | |
| T7 | 5′ CTTATCGAAATTAATACGACTCACTATAGG |
| cSLE-BsmBI-2150 | 5′ AATGCTCGTCTCGCTTCCCTCTTTGTGCCAGTGGTAGTTA |
| JE-BsmBI-2175 | 5′ GGAATTCGTCTCGGAAGCACGCTGGGCAAGG |
| cSP6 | 5′ GCGAGCTCTAGCATTTAGGTGACACTATAG |
Restriction endonuclease sites are indicated by bold, italicized, and underlined font.
FIG. 1.
pCB8SJ2 plasmid map for expressing SLEV structural proteins. This plasmid contains the human cytomegalovirus (CMV) early gene promoter, Japanese encephalitis virus (JEV) signal sequence (JESS), SLEV premembrane (prM) and envelope (E, N-terminal 80%) gene regions, and JEV E (C-terminal 20%), and bovine growth hormone poly(A) signal (BGH) for expression of premembrane and recombinant E proteins in tissue culture cells.
Automated DNA sequencing was performed on a CEQ 8000 genetic analysis system (Beckman Coulter, Fullerton, Calif.) according to the manufacturer's recommended procedures. The recombinant plasmids that had the correct sequence were identified.
Electroporation of tissue culture cells with plasmid DNA.
For transformation, tissue culture cells were grown to 90 to 100% confluence in 150-cm2 culture flasks, trypsinized, and resuspended in ice-cold phosphate-buffered saline (PBS) to a final density of 1.5 × 107 cells/ml. For each reaction, 0.5 ml of this cell suspension was electroporated with 30 μg of plasmid DNA in a 0.4-cm electrode gap cuvette with a Bio-Rad Gene Pulser II (Bio-Rad Laboratories, Hercules, Calif.) set at 250 V and 975 μF. Two separate electroporation reactions were combined and seeded onto a single 150-cm2 culture flask containing 40 ml of growth medium. Tissue culture medium was harvested 48 h following electroporation, clarified by centrifugation at 10,000 rpm for 30 min at 4°C in a Sorval F-16/250 rotor, and stored at 4°C for further analysis. The AC-ELISA was performed with flavivirus E-specific, group-reactive monoclonal antibodies 4G2 (11) and 6B6C-1 conjugated to horseradish peroxidase (20) (Jackson Immunological Laboratories, Inc., West Grove, Pa.) to capture and detect secreted VLPs, respectively, as previously described (6, 12).
Characterization of SLEV extracellular VLPs.
The SLEV VLPs were concentrated and partially purified from clarified tissue culture medium by ultracentrifugation at 19,000 rpm for 8 to 16 h in a Beckman Coulter type 19 rotor at 4°C. The pellet was resuspended in TN buffer (50 mM Tris, 100 mM NaCl, pH 7.5) to 1/50th the original volume, aliquoted into 1-ml samples, and stored at −70°C. For use in assays, aliquots were thawed once and stored at 4°C for up to 1 week, then discarded. For comparison of noninfectious recombinant antigen purification and concentration protocols, SLEV VLPs were also precipitated from clarified tissue culture medium with 10% polyethylene glycol 8000 for 4 to 12 h at 4°C (12). The precipitant was collected by centrifugation at 10,000 rpm for 30 min at 4°C in a Sorval F-16/250 rotor, and the pellet was resuspended as above. SLEV VLPs and gradient-purified virions were analyzed under nondenaturing conditions on a NuPAGE 4 to 12% Bis-Tris gradient gel in an XCell SureLock Mini-Cell electrophoresis apparatus (Invitrogen Corp.), followed by electroblotting onto nitrocellulose membranes with an XCell II Blot module (Invitrogen Corp.). SLEV proteins were detected by Western blot with mouse hyperimmune ascitic fluid (HIAF) against SLEV (MSI-7) and flavivirus E-protein cross-reactive monoclonal antibody 4G2. Total protein was detected with the Owl Pro-Blue kit (Owl Separation Systems, Portsmouth, N.H.) as per the manufacturer's recommended protocol.
Human serum.
Serum specimens were obtained from the Diagnostic and Reference Laboratory, Arbovirus Diseases Branch, Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention. Panels were assembled by selecting serum specimens collected from 1999 to 2003 having neutralizing antibody titers to WNV (n = 18), SLEV(n = 12), Powassan virus (n = 5), or La Crosse virus (n = 5), as determined by the gold standard 90% plaque-reduction neutralization test. The flavivirus responsible for the most recent infection was defined as that virus having a neutralizing antibody titer at least fourfold greater than any other virus tested. Positive-to-negative absorbance ratios of these specimens for MAC- or ACG-ELISA with SLEV SMB, WNV SMB, or WNV VLPs were determined by Arbovirus Diseases Branch's diagnostic laboratory and are listed in Table 2.
TABLE 2.
ELISA detection of IgM and IgG antibodies in human serum samples with various diagnostic antigens
| Panel | Specimen
|
PRNT90a
|
Positive/negative ratiob
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgM
|
IgG
|
||||||||||||
| No. | Day post onset | SLEV | WNV | SLEV
|
WNV
|
SLEV
|
WNV
|
||||||
| VLP | SMBc | VLP | SMBc | VLP | SMBd | VLP | SMBd | VLPd | |||||
| SLEV | 1 | 8 | 1,280 | 160 | 8.27 | 2.64 | 10.80 | 3.08 | 9.82 | 4.69 | 23.10 | 7.89 | |
| 2 | 0 | 320 | 10 | 7.33 | 9.03 | 2.41 | 2.23 | 1.83 | 2.58 | 1.17 | 2.42 | ||
| 3 | 0 | 1,280 | 20 | 8.42 | 8.28 | 4.29 | 4.61 | 1.68 | 3.23 | 1.49 | 4.07 | ||
| 4 | 5 | 2,560 | 40 | 10.70 | 7.82 | 3.50 | 2.46 | 2.67 | 3.45 | 1.66 | 2.70 | ||
| 5 | 0 | 160 | 10 | 9.96 | 8.97 | 2.92 | 2.25 | 2.59 | 2.74 | 1.75 | 3.28 | ||
| 6 | 6 | 10,240 | 80 | 10.60 | 9.11 | 2.89 | 2.67 | 5.30 | 2.49 | 3.37 | 3.61 | ||
| 7 | 18 | 160 | 40 | 6.40 | 10.50 | 6.68 | 8.60 | 3.93 | 19.80 | 5.20 | 14.20 | ||
| 8 | 4 | 640 | 20 | 12.40 | 9.82 | 14.90 | 7.54 | 2.29 | 4.56 | 2.14 | 6.72 | ||
| 9 | 0 | 80 | <10 | 8.11 | 8.47 | 2.79 | 2.31 | 1.45 | 3.53 | 1.13 | 2.16 | ||
| 10 | 0 | 1,280 | 20 | 9.81 | 8.68 | 11.10 | 7.00 | 5.06 | 5.35 | 2.67 | 3.63 | ||
| 11 | 0 | 320 | 20 | 8.56 | 9.08 | 8.27 | 5.32 | 2.04 | 5.85 | 1.90 | 5.61 | ||
| 12 | 21 | 1,280 | 10 | 11.80 | 8.71 | 43.70 | 8.71 | 2.08 | 3.94 | 2.96 | 5.13 | ||
| No. positive | 12 | 11 | 8 | 7 | 4 | 9 | 3 | 9 | |||||
| WNV | 1 | 10 | 80 | 320 | 2.73 | 2.00 | 8.38 | 13.60 | 6.80 | 21.00 | 3.90 | ||
| 2 | 0 | 40 | 160 | 3.37 | 2.80 | 7.88 | 9.65 | 5.70 | 17.50 | 2.30 | |||
| 3 | 12 | 40 | 160 | 1.70 | 2.00 | 18.66 | 11.20 | 4.70 | 19.90 | 2.80 | |||
| 4 | 6 | 1,280 | 1.28 | 2.10 | 9.27 | 1.66 | 2.40 | 7.16 | 2.70 | ||||
| 5 | 0 | 40 | 1,280 | 2.62 | 3.40 | 34.70 | 1.84 | 2.90 | 14.30 | 3.00 | |||
| 6 | 10 | 320 | 1.29 | 2.50 | 8.61 | 5.77 | 3.60 | 13.60 | 3.60 | ||||
| 7 | 10 | 640 | 1.98 | 2.70 | 18.60 | 4.09 | 4.70 | 9.60 | 3.00 | ||||
| 8 | 14 | 40 | 640 | 2.02 | 2.70 | 19.50 | 1.67 | 3.10 | 5.32 | 3.70 | |||
| 9 | 24 | 320 | 1,280 | 4.36 | 9.80 | 33.00 | 13.00 | 5.00 | 23.60 | 5.70 | |||
| 10 | 9 | 40 | 5,120 | 3.45 | 4.30 | 33.80 | 2.66 | 3.00 | 11.60 | 4.80 | |||
| 11 | 3 | 80 | 1,280 | 3.34 | 3.60 | 23.90 | 2.95 | 7.44 | 18.20 | 7.76 | |||
| 12 | 10 | 1,280 | 2.66 | 4.20 | 33.40 | 2.36 | 7.05 | 14.90 | 5.27 | ||||
| 13 | 0 | 40 | 2,560 | 2.12 | 2.70 | 26.30 | 2.64 | 2.40 | 18.40 | 4.80 | |||
| 14 | 4 | 20 | 640 | 2.16 | 4.10 | 37.10 | 2.20 | 2.60 | 12.50 | 4.44 | |||
| 15 | 9 | 80 | 320 | 3.69 | 3.40 | 8.13 | 11.30 | 4.10 | 21.30 | 2.60 | |||
| 16 | 16 | 40 | 1,280 | 2.14 | 5.00 | 28.80 | 3.01 | 3.00 | 15.30 | 5.10 | |||
| 17 | 160 | 2,560 | 1.48 | 2.30 | 5.76 | 12.80 | 8.40 | 20.30 | 6.20 | ||||
| 18 | 4 | 20 | 640 | 1.49 | 2.70 | 16.50 | 1.71 | 5.57 | 6.66 | 5.87 | |||
| No. positive | 5 | 8 | 18 | 9 | 14 | 18 | 14 | ||||||
| Powassan virus | 1 | 19 | 20 | 1.81 | 2.00 | 1.25 | 0.92 | 1.11 | 2.70 | 1.32 | 1.70 | ||
| 2 | 15 | <10 | <10 | 2.41 | 5.00 | 1.83 | 3.10 | 1.37 | 4.30 | 1.62 | 3.00 | ||
| 3 | 1.12 | 0.60 | 1.05 | 0.51 | 0.90 | * | 0.84 | 1.50 | |||||
| 4 | 1.62 | 3.10 | 1.90 | 2.90 | 1.09 | 2.80 | 1.05 | 1.90 | |||||
| 5 | 5 | 1.23 | 1.10 | 1.13 | 0.77 | 1.90 | * | 2.17 | * | ||||
| No. positive | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 1 | |||||
| La Crossre Virus | 1 | 30 | 1.03 | 0.88 | 0.97 | 0.47 | 1.14 | 0.89 | 1.35 | 0.90 | |||
| 2 | 34 | 1.06 | 0.86 | 1.02 | 0.94 | 1.06 | 1.50 | 1.06 | 1.40 | ||||
| 3 | 24 | 1.12 | 1.00 | 2.29 | 9.60 | 0.97 | 1.20 | 0.96 | 1.10 | ||||
| 4 | 62 | 0.99 | 0.88 | 1.05 | 0.52 | 0.89 | 0.82 | 0.92 | 0.90 | ||||
| 5 | 10 | 1.08 | 1.10 | 1.03 | 1.30 | 1.15 | 0.92 | 1.05 | 1.00 | ||||
| No positive | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |||||
PRNT90, plaque reduction neutralization test; titers represent 90% plaque reduction endpoints reported by the reference laboratory.
Values represent ratios calculated by the method indicated in Materials and Methods. Unless specified, the IgM or IgG capture ELISA protocol was used. Positive ratios of ≥3 are shown in bold.
Ratios reported by the reference laboratory.
Ratios reported by the reference laboratory. The antigen capture IgG-ELISA (ACG-ELISA) protocol was used. *, specimens failed the test validation due to high background absorbance against the normal cell antigen.
ELISA protocols.
SLEV VLPs and normal CHO cell culture antigen were prepared as described above. Lyophilized preparations of WNV VLP antigen and normal COS-1 cell culture antigen, prepared as previously described (8), were resuspended in 0.5 ml of distilled water. Antigens were independently titrated against a positive control serum sample with a twofold dilution series and standardized by selecting a dilution that yielded an absorbance of 0.8 to 1.2 at 450 nm (A450).
For detecting the presence of virus-specific IgM in serum panels with the VLPs, the MAC-ELISA was performed as described previously (15) with some modifications. Briefly, the inner 60 wells of Immulon II HB flat-bottomed 96-well plates (Dynatech Industries, Inc., Chantilly, Va.) were coated overnight at 4°C in a humidified chamber with 75 μl of goat anti-human IgM (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) diluted 1:2,000 in coating buffer (0.015 M sodium carbonate, 0.035 M sodium bicarbonate, pH 9.6). Wells were blocked with 300 μl of StartingBlock (PBS) blocking buffer (Pierce, Rockford, Ill.) according to the manufacturer's recommended procedure. Dried plates were stored with desiccant at 4°C until use. In our hands, the use of plates that had been coated with capture antibody, blocked, and then dried as per the manufacturer's recommended procedure had similar activity in detecting antiviral IgM and IgG antibodies after storage at 4°C for up to 2 weeks.
Patient sera and positive and negative antibody controls were diluted appropriately in wash buffer (PBS with 0.5% Tween 20), added to wells (50 μl/well), and incubated at 37°C for 1 h in a humidified chamber. Test and negative human control sera were diluted 1:400 as recommended (13). Positive control sera for SLEV and WNV were diluted 1:3,000 and 1:200, respectively. Positive and negative control antigens were tested with each patient serum sample in triplicate by diluting appropriately in wash buffer and adding 50 μl to appropriate wells for incubation at 4°C overnight in a humidified chamber. Horseradish peroxidase-conjugated 6B6C-1 was diluted 1:6,000 in PBS containing 0.5% Tween 20 and 5% nonfat dry milk, and 50 μl was added to each well and incubated at 37°C for 1 h in a humidified chamber. Bound conjugate was detected by adding 75 μl of 3,3′5,5′-tetramethylbenzidine (TMB; Neogen Corp., Lexington, Ky.) substrate and incubating at room temperature for 10 min. The substrate reaction was stopped with 50 μl of 1 N H2SO4 and reactions were measured at an A450 with an EL 312e Bio-Kinetics microplate reader (Bio-Tek Instruments, Inc., Winooski, Vt.).
For detection of virus-specific IgG in serum samples with VLPs, the GAC-ELISA was performed essentially as described above for the modified MAC-ELISA with the following exception: plates were initially coated with goat anti-human IgG (Kirkegaard & Perry Laboratories), diluted 1:2,000, to capture IgG antibodies from patient serum.
Test validation and calculation of positive/negative absorbance ratio values.
Test validation and positive-to-negative ratio values were determined according to the procedure of Martin et al. (14). Briefly, an internal positive and negative serum control were included in each 96-well plate for test validation. For a testing plate to be considered valid, the average A450 for the positive serum control reacted with positive viral antigen must be at least two times greater than the average A450 for the same positive serum control reacted with the negative tissue culture fluid antigen. Each patient serum sample was validated in the same manner. This verifies that significant A450 values against viral antigen are not due to nonspecific binding of serum antibodies to tissue culture fluid components.
Positive values for each specimen were determined as the average A450 for the patient serum sample reacted with positive viral antigen. Negative values were determined for individual 96-well plates as the average A450 for the normal human serum control reacted with the positive viral antigen. A specimen was classified as a validated-positive sample if it had a positive/negative ratio of ≥3.0.
Statistical analysis.
The receiver operating characteristic (ROC) curve, a plot of sensitivity versus specificity, was applied to determine the discriminatory accuracy of tests in question with MedCalc statistical package (Berkley, Calif.) (22). The accuracy is measured by the area under the ROC curve (AUC). A parametric method with a maximum likelihood estimator was used to calculate the AUC and 95% confidence interval (26). The AUC measures the discriminatory power, that is, the ability of a test to correctly classify those with or without disease. An AUC approaching 1 indicates that the test is highly sensitive as well as highly specific; an AUC approaching 0.5 indicates that the test is neither sensitive nor specific. We used the following criteria to define the disease state and the assigned numerical value was applied to transform the positive/negative ratio value for ROC analysis: 0 = positive/negative absorbance ratio of <2 (definitive negative), 1 = positive/negative ratio of ≥2 and <3 (presumptive positive), 2 = positive/negative ratio of ≥3 and <4 (positive), and 3 = positive/negative ratio of ≥4 (definitive positive). The comparative ROC was used to calculate the significance level and determine an assay's performance.
Calculation of positive and negative prediction value.
A positive/negative ratio of <3 or ≥3 for a given specimen was classified as assay negative or positive, respectively. A 2 by 2 table was prepared which categorized four quadrants as true positive, true negative, false negative, and false positive. The positive prediction value is the percentage of true positives/(true positives + false positives), and the negative prediction value is the percentage of true negatives/(false negatives + true negatives).
RESULTS
Transient expression, concentration, and characterization of SLEV VLPs.
Tissue culture cells transiently transformed by electroporation with plasmid DNA, pCDSLE4MU4, encoding the full-length SLEV premembrane and E proteins expressed these proteins intracellularly, as determined by the indirect immunofluorescence antibody assay (data not shown), but did not secrete VLPs into the tissue culture fluid efficiently. The AC-ELISA titers for SLEV antigen in tissue culture fluid were less than 1:4 at 48 h after plasmid transformation for multiple attempts (data not shown). However, transient expression with pCB8SJ2 (Fig. 1), containing a replacement of the C-terminal 20% of the SLEV E protein with that of JEV, resulted in the dramatically enhanced secretion of SLEV VLPs. Three types of tissue culture cells were compared for optimal secretion when transformed with pCB8SJ2. CHO cells were found to be the optimal cell line for this purpose. VLP secretion measured by the AC-ELISA was higher in CHO cells (1:32) than COS-1 cells (1:4). Plasmid-transformed CHO and 293 cells secreted VLPs to similar titers; however, CHO cells attached to tissue culture flasks more tightly than 293 cells, a characteristic that is preferable for process and production purposes. All further production of SLEV VLPs was therefore conducted with CHO cells.
A comparison of two noninfectious recombinant antigen purification protocols was made to determine if a simpler ultracentrifugation procedure could be used to prepare VLP antigen. Equal volumes of transiently transformed CHO cell tissue culture fluid were prepared by either precipitation with 10% polyethylene glycol or ultracentrifugation. Both concentration methods yielded similar titers of VLPs. However, the SLEV antigens concentrated by ultracentrifugation were cleaner than by polyethylene glycol precipitation. In a total protein staining gel, a continuous smear of protein was observed for samples prepared by polyethylene glycol precipitation, making the identification of discrete individual bands difficult, whereas discrete protein bands could be easily discerned from samples prepared by ultracentrifugation (data not shown). Therefore, the ultracentrifugation was used to prepare the SLEV VLP antigen.
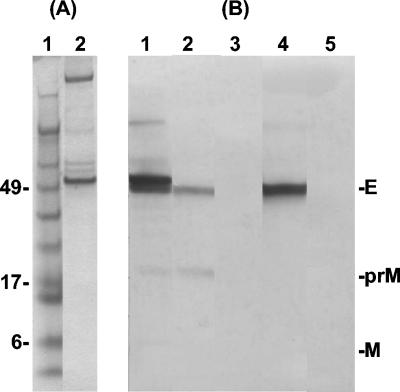
The purity and protein identity of the resuspended preparation were analyzed by a total protein stain (Fig. 2A) and a Western blot with SLEV HIAF and the flavivirus group-reactive, E-specific monoclonal antibody 4G2. SLEV HIAF detected protein bands corresponding to the size for SLEV premembrane and E proteins (≈20 and 55 kDa, respectively) for both purified SLEV (MSI-7) and SLEV VLPs (Fig. 2B, lanes 1 and 2, respectively). Monoclonal antibody 4G2 detected the E protein as an appropriately sized band in the SLEV VLP sample (Fig. 2B, lane 4). Nonspecific binding was not detected with either antibody preparation when reacted with CHO negative antigen prepared identically to VLPs (Fig. 2B, lanes 3 and 5).
FIG. 2.
Detection of SLEV structural proteins in virions and extracellular virus-like particles (VLPs). (A) Total protein stain of SLEV VLPs partially purified and concentrated by ultracentrifugation (lane 2). Lane 1 contains SeeBlue Plus2 prestained protein standards with molecular masses indicated to the left (in kilodaltons). (B) Reactivity of purified SLE virion protein (lane 1), pelleted SLEV VLPs (lanes 2 and 4), and pelleted normal CHO tissue culture fluid (lanes 3 and 5) with mouse hyperimmune ascitic fluid against SLEV strain MSI-7 (lanes 1-3) or flavivirus group-reactive E-protein specific monoclonal antibody 4G2 (lane 4 and 5). Bands corresponding to envelope (E), premembrane (prM), and cleaved membrane (M) proteins are indicated to the right.
Comparison of ELISA formats for detection of IgG in WNV diagnosis.
Several attempts to utilize ACG-ELISA with SLEV and WNV VLPs in this study resulted in a high background absorbance against normal CHO cell antigens (13). A panel of 18 serum samples (Table 2), collected between 2002 and 2003 from WNV-infected patients, was used to develop the GAC-ELISA for detection of WNV IgG antibody (Table 2). For this panel, 14 of 18 samples were previously determined to be positive (positive/negative ratio values of ≥3.0; range, 3.0 to 7.76; average, 4.80) by the ACG-ELISA. Testing this panel by GAC-ELISA resulted in 18 positive samples (positive/negative ratio values of ≥3.0; range, 5.32 to 23.6; average, 15.1).
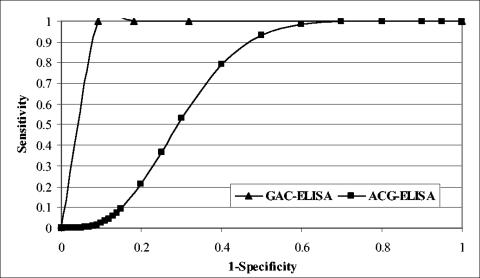
The ROC analysis was applied to compare the performance accuracy of two protocols. The WNV panel was designated the disease panel and the remaining three serum panels, SLEV, Powassan virus, and La Crosse virus, were combined as the nondisease panel (Table 3). The AUCs of 0.95 and 0.70 for GAC- and ACG-ELISA, respectively, indicated that the GAC-ELISA protocol had higher sensitivity and specificity than the ACG-ELISA (Table 3 and Fig. 3). The test performance between the two protocols was statistically significant at the level of 0.003. The GAC-ELISA had a higher positive prediction value than the ACG-ELISA (86 versus 64%, respectively) and, additionally, the GAC-ELISA had a negative prediction value of 100%, compared to 78% with ACG-ELISA.
TABLE 3.
ROC curve determination of sensitivity and specificity of WNV IgG detection by antigen-capture IgG or IgG antibody- capture ELISA
| ELISA | AUC (95% CI) [P] | Predictive value (%)
|
|
|---|---|---|---|
| Positive | Negative | ||
| ACG | 0.70 (0.53-0.84) [0.003] | 64 | 78 |
| GAC | 0.95 (0.83-0.99) | 86 | 100 |
FIG. 3.
Comparative ROC curves corresponding to the GAC- and ACG-ELISA results with WNV VLPs as the antigen, as shown in Table 3.
Detection of SLE viral antibody by ELISA.
A panel of 12 SLEV-infected serum samples, confirmed by the plaque reduction neutralization test, was used in this study (Table 2). The IgM antibody was tested by the MAC-ELISA with SMB antigen or VLPs. Use of SMB antigens in the MAC-ELISA detected 11 of 12 positive samples (positive/negative ratio values of ≥3.0; range, 7.82 to 10.5; average, 8.95). Replacing SMB antigens with SLEV VLPs in the MAC-ELISA detected 12 of 12 positive samples (positive/negative ratio values of ≥3.0; range, 6.40 to 12.4; average, 9.36). ROC analysis with various combined panels as the nondisease panel indicated that there was no statistically significant difference in the performance of MAC-ELISA with either VLPs or SMB antigen, with the single exception of SLEV against the combined WNV and Powassan virus panel (Table 4, P = 0.05). Compared to SMB antigen, this analysis also indicated that SLEV VLP had a higher positive predictive value and negative predictive value (Table 4).
TABLE 4.
IgM antibody detection by the IgM antibody-capture ELISA with either SLEV VLP or suckling mouse brain-derived antigens
| Seraa | Antigen | AUC (95% CI) [P] | Predictive value (%)
|
||
|---|---|---|---|---|---|
| Disease panel | Nondisease panel | Positive | Negative | ||
| SLEV | WNV + POWV + LACV | SMB | 0.86 (0.71-0.95) [0.08] | 52 | 95 |
| VLP | 0.98 (0.88-0.99) | 71 | 100 | ||
| SLEV | WNV + POWV | SMB | 0.82 (0.66-0.93) [0.05] | 52 | 93 |
| VLP | 0.98 (0.86-0.99) | 71 | 100 | ||
| SLEV | POWV + LACV | SMB | 0.93 (0.74-0.99) [0.23] | 85 | 89 |
| VLP | 1.00 (0.84-1.00) | 100 | 100 | ||
| SLEV | WNV | SMB | 0.81 (0.63-0.93) [0.06] | 58 | 91 |
| VLP | 0.97 (0.84-0.99) | 71 | 100 | ||
| SLEV | POWV | SMB | 0.87 (0.62-0.98) [0.14] | 85 | 75 |
| VLP | 1.00 (0.80-1.00) | 100 | 100 | ||
| SLEV + WNV | POWV + LACV | SMB | 0.87 (0.72-0.95) [0.81] | 90 | 42 |
| VLP | 0.88 (0.74-0.96) | 100 | 43 | ||
| SLEV + WNV + POWV | LACV | SMB | 0.97 (0.86-1.00) [0.09] | 100 | 26 |
| VLP | 0.86 (0.71-0.95) | 100 | 22 | ||
POWV, Powassan virus; LACV, LaCrosse virus.
When the same panel was tested for the presence of IgG antibody by ACG-ELISA, use of SLEV SMB antigen detected nine positive specimens (75%, Table 2). However, the higher absorbance against normal CHO cell antigens prohibited the use of VLPS with the ACG-ELISA protocol. Thus, the GAC-ELISA protocol developed and verified with the WNV panel was applied to test IgG antibodies in the SLEV panel. This protocol greatly reduced the background absorbance against normal cell antigen but also reduced the number of IgG-positive specimens to 33% (4 out of 12; Table 2). However, ROC analysis with various combined panels as the nondisease panel indicated that there was no statistically significant differences between the performance of GAC- and ACG-ELISA (Table 5).
TABLE 5.
Comparison of IgG antibody detection with either SLEV VLP or suckling mouse brain-derived antigens
| Seraa | ELISA | Antigen | AUC (95% CI) [P] | Predictive value (%)
|
||
|---|---|---|---|---|---|---|
| Disease panel | Nondisease panel | Positive | Negative | |||
| SLEV | WNV + POWV + LACV | ACG | SMB | 0.59 (0.43-0.75) [0.89] | 38 | 81 |
| GAC | VLP | 0.58 (0.41-0.73) | 31 | 70 | ||
| SLEV | WNV + POWV | ACG | SMB | 0.51 (0.33-0.68) [0.92] | 38 | 73 |
| GAC | VLP | 0.52 (0.34-0.69) | 31 | 64 | ||
| SLEV | POWV + LACV | ACG | SMB | 0.87 (0.66-0.97) [0.97] | 90 | 75 |
| GAC | VLP | 0.88 (0.67-0.97) | 100 | 56 | ||
| SLEV | WNV | ACG | SMB | 0.56 (0.37-0.74) [0.84] | 39 | 57 |
| GAC | VLP | 0.58 (0.39-0.76) | 31 | 53 | ||
| SLEV | POWV | ACG | SMB | 0.74 (0.48-0.92) [0.34] | 90 | 57 |
| GAC | VLP | 0.88 (0.63-0.98) | 100 | 38 | ||
| SLEV | LACV | ACG | SMB | 1.00 (0.80-1.00) [0.15] | 100 | 63 |
| GAC | VLP | 0.88 (0.63-0.98) | 100 | 38 | ||
| SLEV + WNV + POWV | LACV | ACG | SMB | 1.00 (0.91-1.00) [0.03] | 100 | 31 |
| GAC | VLP | 0.83 (0.68-0.93) | 100 | 18 | ||
| SLEV + WNV | LACV | ACG | SMB | 1.00 (0.90-1.00) [0.07] | 100 | 42 |
| GAC | VLP | 0.88 (0.73-0.97) | 100 | 22 | ||
| SLEV + WNV | POWV | ACG | SMB | 0.76 (0.58-0.89) [0.24] | 96 | 36 |
| GAC | VLP | 0.88 (0.73-0.97) | 100 | 23 | ||
See Table 4, footnote a.
Detection of cross-reactive antibodies.
As shown in Table 2, use of SLEV SMB antigen in either MAC- or ACG-ELISA for WNV-infected patients detected cross-reactive IgM and IgG antibodies in 8 (44%) and 14 (78%), respectively, of the 18 WNV-positive serum samples. However, the SLEV VLPs detected cross-reactive antibody in only 5 (28%) and 9 (50%) of the same 18 samples by MAC- and GAC-ELISA, respectively. Cross-reactive IgM antibodies in 8 of 12 (67%) SLEV-infected serum samples were detected by WNV VLPs, compared to 7 of 12 (58%) detected by WNV SMB antigen. The WNV VLPs detected cross-reactive IgG antibody in 3 of 12 (25%) SLEV-infected samples by GAC-ELISA and WNV SMB antigen detected 9 of 12 (75%) of these samples by ACG-ELISA.
SLEV and WNV VLPs were used to test against WNV, SLEV, and Powassan virus panels to determine the extent of IgM and IgG cross-reactivity against flaviviruses commonly associated with human disease in North America in MAC- and GAC-ELISAs, respectively. The comparative ROC analysis (Table 6) indicated that SLEV VLPs performed significantly better than WNV VLPs in MAC-ELISA (AUCs of 0.97 and 0.79, respectively), and WNV VLPs performed significantly better than SLEV VLPs in GAC-ELISA (AUCs of 0.94 and 0.58, respectively) in detecting flavivirus cross-reactive antibodies.
TABLE 6.
Flavivirus cross-reactive IgM and IgG antibody detection by IgM and IgG antibody-capture ELISAs with SLEV and WNV VLP antigens
| ELISA | Seraa
|
Antigen | AUC (95% interval) | Predictive value (%)
|
||
|---|---|---|---|---|---|---|
| Panel 1 | Panel 2 | Positive | Negative | |||
| MAC | SLEV | WNV + POWV | SLEV | 0.97 (0.84-0.99) | 70.6 | 100 |
| WNV | SLEV + POWV | WNV | 0.79 (0.62-0.91) | 69.2 | 100 | |
| GAC | SLEV | WNV + POWV | SLEV | 0.58 (0.39-0.76) | 30.7 | 63.6 |
| WNV | SLEV + POWV | WNV | 0.94 (0.81-0.99) | 85.7 | 100 | |
See Table 4, footnote a.
DISCUSSION
An expression plasmid was constructed for the extracellular secretion of SLEV VLPs by CHO cells. As reported previously in our dengue virus type 2 study (7), use of the native viral full-length premembrane and E protein-encoding sequence resulted in accumulation of these proteins intracellularly, but VLPs were not efficiently secreted. Replacement of the C-terminal 20% of the SLEV E protein (amino acids E-405 to E-501) with the corresponding JEV E region increased secretion of VLPs by at least eightfold. This result substantiates the previous observation and suggests the presence of a putative membrane retention signal in the C-terminal region of the E protein. This region consists of two α-helical segments (H1 and H2) and two transmembrane α-helices (T1 and T2) involved in the proper E-E and premembrane-E hetero- or homodimer interactions necessary for secretion. The finding that the dengue virus type 2 90%-JEV 10% E protein construct did not secrete VLPs implicates H1 and/or H2 in forming the proper interactions required for efficient VLP formation and budding (7). Identification of the specific amino acid(s) involved in these interactions is currently being investigated for SLEV and all four dengue virus serotypes.
Three tissue culture cell lines were compared for their ability to secrete SLEV VLPs when transiently transformed with pCB8SJ2 plasmid DNA. COS-1 cells, previously shown to efficiently secrete JEV, WNV, and dengue virus type 2 VLPs (7, 8, 12), did not secrete SLEV VLPs efficiently in this study. Both CHO and 293 cells secreted SLEV VLPs at equally high titers, but 293 cells were found to be impractical for production and preparation due to their weak adherence to tissue culture flasks. CHO cells had additional advantages over COS-1 cells for their use in production of flaviviral VLPs, including their shorter generation time and higher cell density per 150-cm2 culture flask. These factors decrease both the time and amount of medium and materials required to produce the large quantities of antigen necessary for supporting nationwide surveillance.
Secretion of VLPs into tissue culture fluid and ultracentrifugation as the only purification and concentration procedure additionally reduced the time and effort required for noninfectious recombinant antigen production compared to previously described protocols with polyethylene glycol precipitation. This simple pelleting procedure provided a product of higher purity which could be used as a diagnostic antigen as well as a protein preparation readily examined by immunochemical techniques without the prerequisite ethanol extraction necessary for removal of residual polyethylene glycol (12). The presence of polyethylene glycol in a sample results in aberrant protein band migration in gel electrophoresis. Additionally, polyethylene glycol precipitates much of the serum protein present in tissue culture fluid and results in a continuous smear in a total protein stain, making identification of discrete bands difficult.
Electron microscopy, density gradient analysis, and hemagglutination assays indicated that JEV VLPs secreted from a stably transformed COS-1 cell line mimicked the virion structure of JEV (12). Epitope mapping of the E proteins of JEV and dengue virus type 2 VLPs with panels of E-specific monoclonal antibodies, including the conformation-dependent antibodies 4G2 and 6B6C-1, suggested that the E protein present in these particles was properly folded and similar to the E protein of virion particles (6, 7). In the current study, Western blot examination of pelleted tissue culture fluid preparations from pCB8SJ2-transformed CHO cells with SLEV HIAF revealed protein bands corresponding in size to native virus premembrane and E proteins, and the E-specific monoclonal antibody 4G2 detected a single E protein band. These results supported the expectation that SLEV premembrane and E proteins were expressed and secreted by CHO cells and that these proteins presumably self-assembled into particle form.
Several flaviviruses, including JEV, SLEV, and dengue virus type 2, contain N-linked glycosylation sites in the E proteins. SLEV MSI-7 has two potential glycosylation sites in the E protein, and the degree of glycosylation in the proteins may affect their migration in acrylamide gels. The Western blot analysis of SLEV MSI-7 virions with HIAF detected a doublet (Fig. 2B, lane 1) at ≈50 kDa. These bands were previously investigated and found to correspond to slow-migrating glycosylated and fast-migrating nonglycosylated forms of the E protein, designated E and E0, respectively (25). Heterogeneous glycosylation of SLE virions has been demonstrated, and glycosylation does not appear to be essential for epitope conformation and recognition by monoclonal antibodies (25). Comparison of SLEV VLPs with purified virus in a Western blot with either HIAF or monoclonal antibody 4G2 (Fig. 2) revealed that the fast-migrating, potentially nonglycosylated E0 form is apparently the dominant form present in SLEV VLPs. This presumptive nonglycosylated Eo was readily detected by SLEV HIAF, monoclonal antibody 4G2, or virus-specific IgM and IgG antibodies present in human serum.
The IgG-ELISA developed previously by Johnson et al. (13) has been employed successfully to detect antiflaviviral IgG antibody in patient specimens (8, 13). In our experiments, however, use of this antigen capture format with both WNV and SLEV VLPs resulted in a high background absorbance against normal CHO cell antigens, precluding the screening of serum panels at the recommended serum dilution of 1:400. Additionally, as noted in Table 2, ACG-ELISA results were not interpretable for two Powassan virus-infected serum samples tested on SMB antigens. Johnson et al. noted that this background absorbance of antibody-positive serum reacted on normal mouse brain antigen became a significant problem when specimens were tested at a dilution of less than 1:400.
To circumvent this problem, we employed the GAC-ELISA, a protocol similar in format to MAC-ELISA (15). This IgG antibody capture ELISA format, described by Erdman et al. (10), uses plate-bound goat anti-human IgG to capture serum IgG antibody to reduce nonspecific background absorbance. One potential disadvantage of the GAC-ELISA is the capture of IgG antibodies of unknown specificity as well as virus-specific antibodies. High concentrations of IgG antibodies of unknown specificity may compete with and inhibit the capture of the relatively small amounts of virus-specific IgG antibodies, thereby reducing the sensitivity of the assay. Although there was no statistically significant difference between the performance of either test, concerns about the lower number of positivity detected by the GAC-ELISA with SLEV VLPs compared to the ACG-ELISA with SLEV SMB antigen (Tables 2 and 5) were raised. However, the GAC-ELISA had higher sensitivity and specificity than the ACG-ELISA when WNV VLPs were used to detect antiviral IgG antibody in WNV-infected patient serum samples (Tables 2 and 3).
These apparently contradictory results might indicate that the detection of a greater number of IgG-positive serum samples in the ACG-ELISA contributed to the presence of cross-reactive nonstructural proteins in the SMB preparation. Testing of the Powassan virus and La Crosse virus serum panels with the SLEV and WNV VLPs in both MAC- and GAC-ELISAs resulted in specificities of 100%, whereas the specificities of the corresponding ELISA format with the SMB antigens with these panels were lower. Both IgM and IgG serum antibodies contribute to the neutralization of virus in the plaque reduction neutralization test; therefore, for samples which are found to have a recent viral infection by plaque reduction neutralization test but which do not have detectable titers of specific IgG antibody by ELISA, neutralization must be due to IgM antibodies. For the two SLEV-infected samples (2 and 5 in Table 2) that tested negative for SLEV-specific IgG antibody by either GAC-ELISA or ACG-ELISA, SLEV-specific IgM antibody was detected with either the SLEV SMB or VLP antigen.
MAC-ELISAs with either VLP or SMB antigen as the serodiagnostic antigen had comparable sensitivity and specificity (Table 4) for the detection of serum IgM antibody produced in response to SLEV infection. The positive predictive value as well as the negative predictive value for a given test are important determinants when the assay is implemented as the tool for disease surveillance. The positive and negative predictive values determine the true positive and negative disease states, respectively, for a given specimen under the specified assay condition. In general, VLPs had a higher positive predictive value and negative predictive value than SMB antigens, and therefore selecting VLPs as the antigen of choice should enhance the performance of disease surveillance to determine the disease prevalence.
The ease of production, safety, adequate sensitivity for detection of true positives, and low cross-reactivity with anti-WNV IgM antibody make the use of SLEV VLPs in MAC-ELISA screening of patient serum samples preferable to that of antigens derived from suckling mouse brain preparations. The use of a stably transformed cell clone to constitutively secrete VLPs would also allow standardization and large-scale production of this flaviviral noninfectious recombinant antigen. A cell line stably transformed with the SLEV premembrane-E (80% SLEV-20% JE) protein-coding region is currently being developed in our laboratory.
Acknowledgments
We thank Brent Davis and Roselyn Hochbein for excellent technical assistance. We are also grateful to Ann R. Hunt for providing insightful comments on the manuscript and Day-Yu Chao for providing statistical analysis.
REFERENCES
- 1.Aizawa, C., S. Hasegawa, C. Chih-Yuan, and I. Yoshioka. 1980. Large-scale purification of Japanese encephalitis virus from infected mouse brain for preparation of vaccine. Appl. Environ. Microbiol. 39:54-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1990. Update: St. Louis encephalitis—Florida and Texas, 1990. Morb. Mortal. Wkly. Rep. 39:756-759. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1999. Summary of notifiable diseases, United States, 1998. Morb. Mortal. Wkly. Rep. 47:ii-92. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2003. Summary of notifiable diseases, United States, 2001. Morb. Mortal. Wkly. Rep. 50:2-14. [PubMed] [Google Scholar]
- 5.Chang, G. J., B. S. Davis, A. R. Hunt, D. A. Holmes, and G. Kuno. 2001. Flavivirus DNA vaccines: current status and potential. Ann. N. Y. Acad. Sci. 951:272-228. [PubMed] [Google Scholar]
- 6.Chang, G. J., A. R. Hunt, and B. Davis. 2000. A single intramuscular injection of recombinant plasmid DNA induces protective immunity and prevents Japanese encephalitis in mice. J. Virol. 74:4244-4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, G. J., A. R. Hunt, D. A. Holmes, T. Springfield, T. S. Chiueh, J. T. Roehrig, and D. J. Gubler. 2003. Enhancing biosynthesis and secretion of premembrane and envelope proteins by the chimeric plasmid of dengue virus type 2 and Japanese encephalitis virus. Virology 306:170-180. [DOI] [PubMed] [Google Scholar]
- 8.Davis, B. S., G. J. Chang, B. Cropp, J. T. Roehrig, D. A. Martin, C. J. Mitchell, R. Bowen, and M. L. Bunning. 2001. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J. Virol. 75:4040-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day, J. F. 2001. Predicting St. Louis encephalitis virus epidemics: lessons from recent, and not so recent, outbreaks. Annu. Rev. Entomol. 46:111-138. [DOI] [PubMed] [Google Scholar]
- 10.Erdman, D. D., and L. J. Anderson. 1990. Monoclonal antibody-based capture enzyme immunoassays for specific serum immunoglobulin G (IgG), IgA, and IgM antibodies to respiratory syncytial virus. J. Clin. Microbiol. 28:2744-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henchal, E. A., M. K. Gentry, J. M. McCown, and W. E. Brandt. 1982. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am. J. Trop. Med. Hyg. 31:830-836. [DOI] [PubMed] [Google Scholar]
- 12.Hunt, A. R., C. B. Cropp, and G. J. Chang. 2001. A recombinant particulate antigen of Japanese encephalitis virus produced in stably transformed cells is an effective noninfectious antigen and subunit immunogen. J. Virol. Methods 97:133-149. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, A. J., D. A. Martin, N. Karabatsos, and J. T. Roehrig. 2000. Detection of anti-arboviral immunoglobulin G by using a monoclonal antibody-based capture enzyme-linked immunosorbent assay. J. Clin. Microbiol. 38:1827-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin, D. A., B. J. Biggerstaff, B. Allen, A. J. Johnson, R. S. Lanciotti, and J. T. Roehrig. 2002. Use of immunoglobulin m cross-reactions in differential diagnosis of human flaviviral encephalitis infections in the United States. Clin. Diagn. Lab. Immunol. 9:544-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin, D. A., D. A. Muth, T. Brown, A. J. Johnson, N. Karabatsos, and J. T. Roehrig. 2000. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J. Clin. Microbiol. 38:1823-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monath, T. P. 1980. Epidemiology, p. 239-312. In T. P. Monath (ed.), St. Louis encephalitis. American Public Health Association, Washington, D.C.
- 17.Monath, T. P., and T. F. Tsai. 1987. St. Louis encephalitis: lessons from the last decade. Am. J. Trop. Med. Hyg. 37:40S-59S. [DOI] [PubMed] [Google Scholar]
- 18.Obijeski, J. F., D. H. Bishop, E. L. Palmer, and F. A. Murphy. 1976. Segmented genome and nucleocapsid of La Crosse virus. J. Virol. 20:664-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prince, H. E., and W. R. Hogrefe. 2003. Detection of West Nile virus (WNV)-specific immunoglobulin M in a reference laboratory setting during the 2002 WNV season in the United States. Clin. Diagn. Lab. Immunol. 10:764-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roehrig, J. T., J. H. Mathews, and D. W. Trent. 1983. Identification of epitopes on the E glycoprotein of Saint Louis encephalitis virus using monoclonal antibodies. Virology 128:118-126. [DOI] [PubMed] [Google Scholar]
- 21.Russell, P. K., W. E. Brandt, and J. M. Dalrymple. 1980. Chemical and antigenic structure of flaviviruses, p. 503-529. In R. W. Schlesinger (ed.), The Togaviruses. Academic Press, New York, N.Y.
- 22.Shapiro, D. E. 1999. The interpretation of diagnostic tests. Stat. Methods Med. Res. 8:113-134. [DOI] [PubMed] [Google Scholar]
- 23.Trent, D. W., R. M. Kinney, B. J. Johnson, A. V. Vorndam, J. A. Grant, V. Deubel, C. Rice, and C. Hahn. 1987. Partial nucleotide sequence of St. Louis encephalitis virus RNA: structural proteins, NS1, ns2a, and ns2b. Virology 156:293-304. [DOI] [PubMed] [Google Scholar]
- 24.Tsai, T. F., and C. J. Mitchell. 1989. St. Louis encephalitis, p. 431-458. In T. P. Monath (ed.), The arboviruses: epidemiology and ecology. CRC Press, Boca Raton, Fla.
- 25.Vorndam, V., J. H. Mathews, A. d. Barrett, J. T. Roehrig, and D. W. Trent. 1993. Molecular and biological characterization of a non-glycosylated isolate of St Louis encephalitis virus. J. Gen. Virol. 74:2653-2660. [DOI] [PubMed] [Google Scholar]
- 26.Zweig, M. H., and G. Campbell. 1993. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin. Chem. 39:561-577. [PubMed] [Google Scholar]