Abstract
Walnut (Juglans regia L.) contains approximately 20–25 % protein with abundant essential amino acids. The enzymatic hydrolysate of Persian walnut (Chandler) seed proteins was prepared by incubation with three different proteases, including pancreatic chymotrypsin and trypsin, and a microbial enzyme proteinase K. The hydrolysates were found to possess excellent antioxidant capacities. The peptide fractions scavenged the 2, 2′-anizo-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) free radicals and inhibited the activity of reactive oxygen species. Walnut protein hydrolysates were also tested, for the first time, against the viability of human breast (MDA-MB231) and colon (HT-29) cancer cell lines. MTT, [3–(4, 5dimethylthiazolyl)-2,5-diphenyl-tetrazolium bromide], assay was used to assess in vitro cancer cell viability upon treatment with the peptide fractions. The peptide fractions showed cell growth inhibition of 63 ± 1.73 % for breast cancer and 51 ± 1.45 % for colon cancer cells. Thus, a direct correlation between antioxidant and anticancer activities of walnut peptide fractions exists and supports their potential therapeutic benefit.
Keywords: Walnut proteins, Bioactive peptides, Antioxidant, Anticancer, Protease
Introduction
The free radical oxidations in humans, which can be a consequence of aging or environmental factors [1], lead to several diseases such as cancer [2]. Cancer treatments are generally costly and involve drugs with adverse side effects. However, diets rich in antioxidants have been reported to reduce the risk of developing several diseases including many kinds of cancer, and scavenge many free radicals [3]. Several studies have reported that bioactive peptides resulting from various food sources exhibit remarkable properties [4–6]. Bioactive peptides are protein fragments with potential biological activities that have important roles in health promotion [7]. Walnut has received significant attentions for its proteins and protein hydrolysates activities including anti-atherogenic, anti-mutagenic and antioxidant activities [8, 9].
Breast cancer is the most common malignancy in females [10], and colon carcinoma is the second most common cause of death from cancer [11]. These tumors are comprised of phenotypically diverse populations of cells with a wide range of incidence and mortality. MDA-MB231 is an invasive ductal carcinoma and metastatic cell line, and HT-29 is a human colorectal adenocarcinoma cell line with epithelial morphology. The availability of better and more effective therapies for these cancers will have significant clinical implications [11].
The purpose of the present work was to evaluate the potential antioxidant and anticancer activity of the peptides extracted from walnut seeds. We determined the potential protective effects of the walnut proteins and peptide fractions, generated from their enzymatic hydrolysis, against H2O2 induced oxidative damage. In addition, the anticancer activity of the walnut peptides generated from chymotrypsin hydrolysis was evaluated using breast (MDA-MB231) and colon (HT-29) cancer cell lines.
Materials and Methods
Materials
Persian walnut, the Chandler variety, was supplied by Center of Excellence for Walnut Improvement and Technology of Iran at the College of Aburaihan, University of Tehran. Chymotrypsin (EC 3.4.21.1), trypsin (EC 3.4.21.4), proteinase K (EC 3.4.21.64), luminol (5-amino 2,3-dihydro 1,4-phthalazinedione), H2O2 (30 % solution), and gold (III) chloride hydrate (HAuCl4), were purchased from Sigma-Aldrich (Germany).
Extraction of Walnut Proteins
The walnuts were manually cracked, shelled, and their kernels were chopped. The isolation of walnut proteins was carried out using an alkali extraction method [12]. The flour was dispersed in 50 mM Tris-base solution(1:15, w:v) and stirred for 2 h at 4 °C. The mixture was then centrifuged at 13,000 ×g, and the top phase (oil) was discarded. This step was repeated until a transparent solution was obtained. After adjustment of pH to 4, the solution was again centrifuged (4000 ×g for 30 min) to collect the precipitate. The latter was then lyophilized and stored in plastic tubes at −20 °C for further analysis.
Hydrolysis of Walnut Proteins
Enzymatic hydrolysis was performed on fat-free extracts obtained as described above. Proteases used in this study were chymotrypsin, trypsin, and proteinase K, and utilized separately at 37 °C, pH 8. Trypsin and chymotrypsin are digestive enzymes and were selected in order to have the most similarity with physiological conditions. Proteinase K was selected based on our previous work with camel milk casein, where we reported that the peptide fractions resulted from proteinase K digestion had the best antioxidant properties. [13]. Thus, proteinase K was used in the current study to evaluate its effect on walnut proteins.
The walnut protein extracts (50 mg) were dispersed in phosphate buffer (50 mL). The protease was added to the walnut protein mixtures [substrate/enzyme (100/1, w:w)], and it was shaken for certain time intervals. The sampling was done at indicated times, and the fractions were heated at 80 °C for 15 min. The obtained suspensions were centrifuged at 10,000 ×g for 10min, and the supernatants were lyophilized and stored at −20 °C.
In Vitro Digestibility Assessment
For determination of protein hydrolysis, we used the o-Phthaldialdehyde assay. In this assay o-phthaldialdehyde (OPA) and 2-mercaptoethanol react with the amino groups resulting from protein hydrolysis. The adduct has a strong absorbance at 340 nm [14]. A fresh OPA solution was prepared daily using 20 % SDS (w:w), 100 mM sodium tetra hydroborate and 100 µl β-mercaptoethanol. OPA was dissolved in 1 mL of methanol. To assay proteolysis of walnut proteins, an aliquot (10–50 µL) was added directly to 1 mL of OPA reagent in a 1.5 mL quartz cuvette. The solutions were shaken manually and incubated for 2 min at room temperature. Immediately after incubation, the absorbance was recorded using a spectrophotometer at 340 nm (Shimadzu, model UV-3100, Kyoto, Japan).
Determination of Antioxidant Activity
The ABTS+ solution [2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid)] was prepared as described by Re et al. [15]. The solution absorbance was adjusted to 0.70 ± 0.03 at 734 nm by deionized water. The hydrolysate (5 µL) at the concentration of 0.50 mg/mL was mixed with ABTS+ solution (200 µl). The mixture was incubated at 25 °C for 6 min and the absorbance was measured at 734 nm using a plate reader Expert 96. All tests were performed in triplicates. On the basis of the results obtained from these experiments, the hydrolysates for 1 h using chymotrypsin were selected for further studies.
In order to isolate the peptides on the basis of molecular weight, the fractions obtained from 1 h treatment with chymotrypsin, which showed the highest antioxidant activity, were centrifuged at 3000 ×g for 10 min, and the supernatant was passed through the membrane filters of Amicon ultrafiltration system with smaller molecular weight cut-off sizes of 10, 5 and 3 kDa. Permeates from each filtration cycle were collected as <10 kDa, 5–10 kDa, 3–5 kDa and <3 kDa fractions. All the fractions were freeze dried and stored at −20 °C for further analysis.
Determination of ROS Scavenging Activity
Reactive oxygen species (ROS) activity was measured using a chemiluminescence (CL) method. Based on the CL of luminol, increasing the oxidizing agents, as for instance hydrogen peroxide and ROS, causes increased concentration of 3-aminophthlate, which is an excited state molecule. Briefly, in order to perform the experiments, CL signal of luminol was obtained after injecting 5 µL of gold (III) chloride hydrate (HAuCl4) to the sample containing H2O2 using a fluorescence spectrophotometer at 425 nm (Synergy H4 Hybrid Reader; BioTek, USA). Luminol was dissolved in a sodium carbonate buffer (100 mM, pH 11). The stock solution of luminol (10 mM) was stored at 4 °C in the dark [16].
Determination of Anticancer Activity
Determination of anticancer activity was conducted as described by Mosmann [17]. The effect of walnut peptides on HT-29 and MDA-MB231 tumor cells, and normal human umbilical vein endothelial cells (HUVEC) viability was determined using MTT [3-(4, 5-dimethylthiazolyl)-2,5-diphenyltetrazoliumbromide] assay [18]. In this assay, MTT is reduced to blue formazan product that indicates the normal function of mitochondria, and hence reflect cell viability [18]. These tests were performed in triplicates. The rate of growth inhibition was determined using the following formula:
Data Analysis
All data represent the mean value ± S.E. of three independent measurements. Comparison between groups was made using one-way analysis of variance (ANOVA) followed by a specific post-hoc test and t-test. To analyze the differences SPSS software version 22 was used. The statistical significance was achieved when P < 0.05.
Results and Discussion
In Vitro Digestibility Assessment of Walnut Proteins
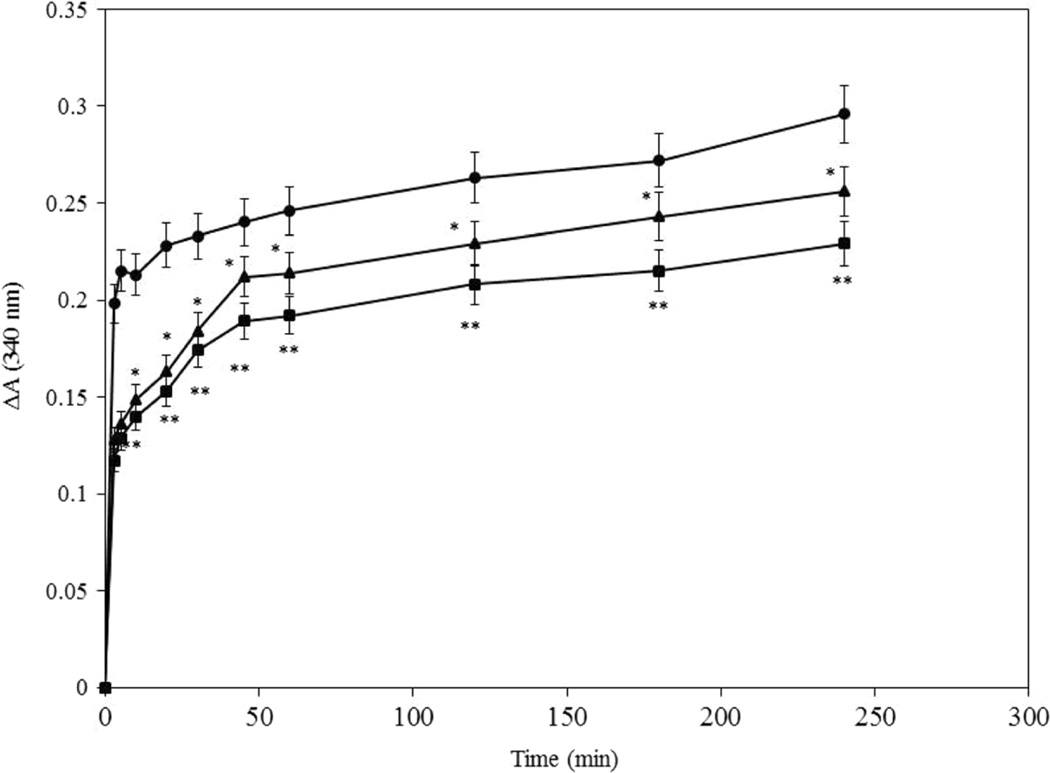
The proteolysis of walnut proteins was characterized using the o-phthaldialdehyde (OPA) test. This method is related to the OPA mechanism of action. During this assay, OPA reacts with the amino terminal of protein and peptide chains. With increased time of hydrolysis, the break in the protein chains as well as the number of free amino termini increases. Thus, this assay can be used as a parameter to determine walnut protein hydrolysis. The extent of proteolysis based on OPA values was determined during the 4 h of proteolysis in the presence of three different proteases (Fig. 1). The analysis of degradation of walnut proteins revealed that the hydrolysis by chymotrypsin was faster than that of trypsin and proteinase K. These results indicated that the maximum level of proteolysis was achieved after approximately 60 min of incubation with these proteases. Thus, the majority of walnut protein degradation occurred during the first hour of hydrolysis.
Fig. 1.
O-phthaldialdehyde spectrophotometric assay to measure proteolysis of walnut proteins by chymotrypsin (●), trypsin (■), and proteinase K (▲) for 4 h. Results are the average of three independent measurements. *P < 0.05 and **P < 0.01 compared with chymotrypsin group (●)
Antioxidant Activity
The antioxidant potential of walnut peptide samples was determined by scavenging activity of ABTS radicals. A higher absorbance represents a higher ABTS scavenging activity. Figure 2 shows the time of hydrolysis, as well as the type of protease used, both of which affected the antioxidant effects. A significantly higher antioxidant activity was observed with chymotrypsin at nearly all time intervals. All samples showed a significant antioxidant activity (p < 0.001) compared to protein sample (time 0). Thus, walnut peptides have substantially higher antioxidant activity than intact proteins. However, since hydrolyzing with chymotrypsin for 1 h showed the highest antioxidant activity among other fractions, chymotrypsin was selected as the protease of choice for further experiments.
Fig. 2.
Measurement of ABTS radical scavenging for 4 h of hydrolysis by chymotrypsin, trypsin and proteinase K. Results are the average of three independent measurements. A significant difference from 0 h treated cells was observed (***P < 0.001)
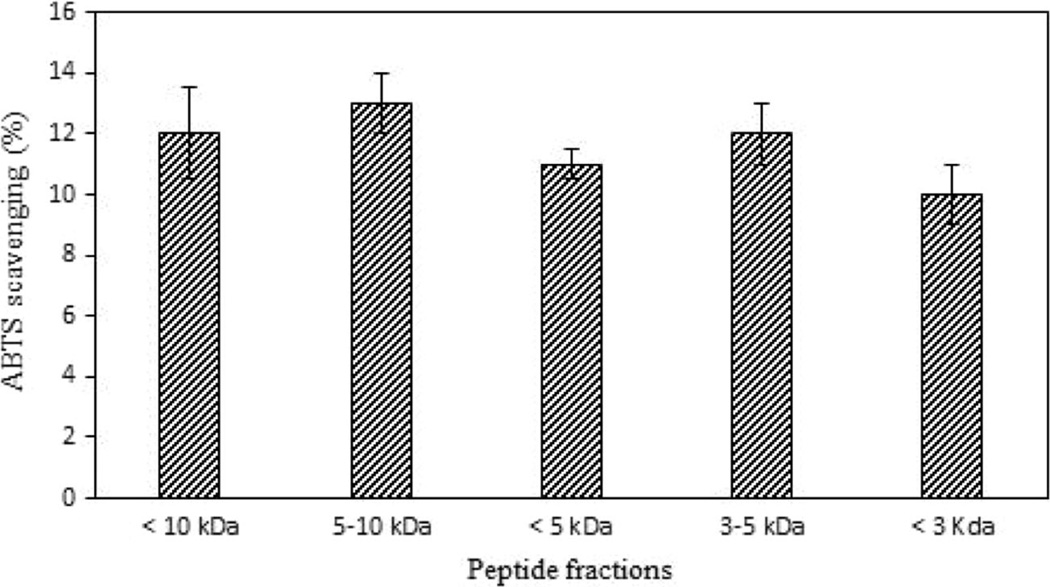
Following partial purification of peptide fractions based on molecular weight by ultrafiltration, we determined the antioxidant activity of <10, 5–10, 3–5 and <3 kDa fractions. These results demonstrated that the antioxidant activity of the peptide fractions was significantly decreased compared with the whole hydrolysate. However, the differences among the fractions were not significant, highlighting the synergistic effect among these peptides (Fig. 3).
Fig. 3.
Measuring ABTS radical scavenging for peptide fractions resulting from ultrafiltration of 1 h chymotrypsin hydrolysate. Results are the average of three independent measurements
Chemiluminescence Spectroscopy
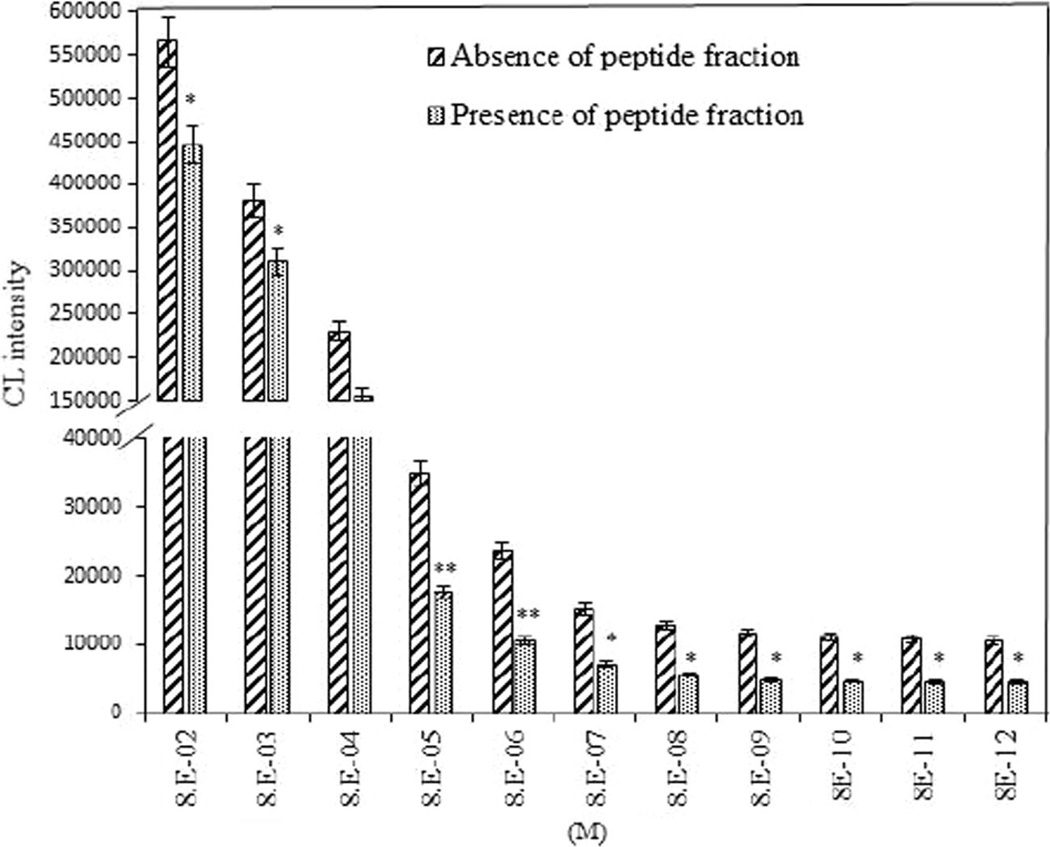
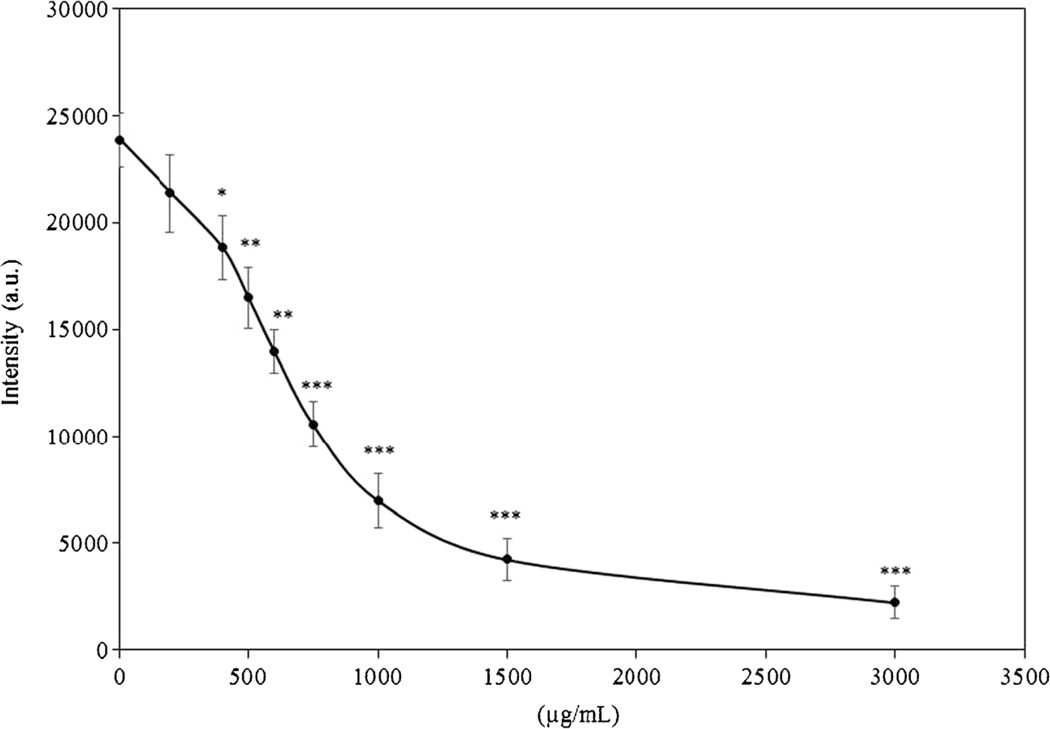
Figure 4 shows CL intensity of luminol in the presence or absence of peptide fractions (5 µL of 750 µg/mL). The different concentration of the H2O2 was tested as blank sample (no peptide fractions). In comparison with blank, CL intensity in the wells containing the peptide fractions significantly decreased, especially at the concentration of 8 µM H2O2. Indeed, this concentration was the lowest concentration with p < 0.01, and thus it was considered for evaluating the effect of peptide concentration on the CL intensity. For optimization of the peptide concentration as a scavenging activity, CL intensity of the different peptide concentrations was measured. Figure 5 shows that when the CL intensity reached near constant level, CL emission was decreased along with increasing concentration of the peptide fraction up to 750 µg/mL. Significant differences were observed in comparison with no added peptides.
Fig. 4.
CL intensity of different hydrogen peroxide concentrations in the absence or presence of peptide fractions. The concentrations of luminol and peptide fraction were 100 µM and 750 µg/mL, respectively. Results represent the average of three independent measurements. A significant difference between two group (absence and presence of peptide) in each concentration of H2O2 was observed (*P < 0.05, and **P < 0.01 and)
Fig. 5.
Optimization of peptide concentration (obtained from chymotrypsin) based on CL intensity of luminol at 8 µM H2O2. The concentrations of luminol and H2O2 were 100 and 8 µM, respectively. Results represent the average of three independent measurements. A significant difference from concentration 0 of peptide was observed (*P < 0.05, **P < 0.01 and ***P < 0.001)
It has been suggested that the ROS scavenging activity is related to hydrophobic amino acids [19]. The presence of hydrophobic amino acids allows direct electron transferring to reactive oxygen species (ROS) [20]. Kumar et al. [21] and Chen et al. [22] found that hydrophobic amino acids play important roles in the antioxidant activity of peptides. Tang et al. [23] also reported that the antioxidant activity of zein peptides was related to the molecular mass and the hydrophobicity of these peptides. Furthermore, there are indications that an increase in hydrophobicity correlates with an increase in antioxidant potential [24]. In the active site of chymotrypsin, there are several reactive groups near the binding sites for the hydrophobic amino acid side chains. These binding sites prefer large hydrophobic residues [25]. Thus, we propose that the chymotrypsin fractions of walnut protein hydrolysates contain a significant amount of hydrophobic amino acids that are responsible for their high antioxidant activity.
Anticancer Activity
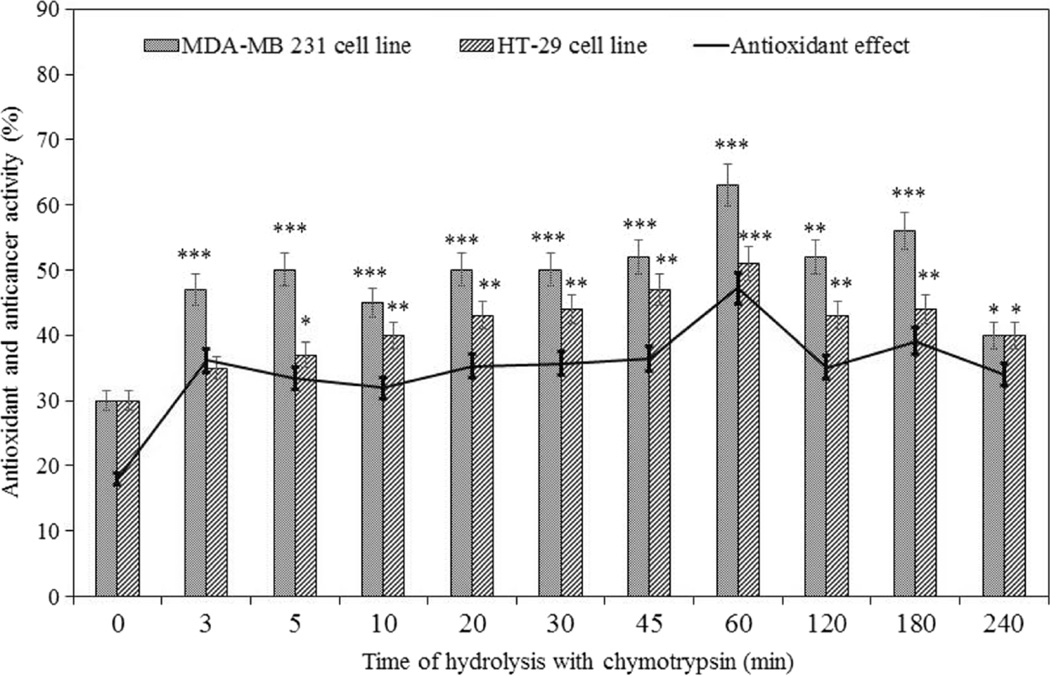
Figure 6 shows that peptide fractions obtained from chymotrypsin hydrolysis exhibit inhibitory activity on survival of the MDA-MB231 and HT-29 cancer cells. The majority of the peptide fractions exhibited strong anticancer activity towards these cells, and among those, the fraction obtained by chymotrypsin hydrolysis in 1 h had the best inhibitory activity. According to antioxidant data, it can be concluded that the antioxidant activity of the peptide fractions and anticancer activity are in line (Fig. 6). The relationship between antioxidant and anticancer activity has been previously reported [26, 27]. In our study the Pearson’s correlation coefficient for antioxidant activity and anticancer activity against MDA-MB231 and HT-29 cells was calculated as 0.93 and 0.85, respectively, demonstrating a high relationship. Since the peptide fractions affected MDA-MB231 more significantly than HT-29 cells, the breast cancer cells were selected as a target for further analysis.
Fig. 6.
MDA-MB231 breast and HT-29 colon cancer cell lines growth inhibition by peptides from walnut chymotrypsin hydrolysate. Control sample is culture medium only. The line chart shows the correlation between inhibition of MDA-NB231 and HT-29 colon cancer cells and antioxidant activity of peptide fractions resulting from chymotrypsin treatment. A significant difference from 0 h treated cells was observed (*P < 0.05, **P < 0.01 and ***P < 0.001)
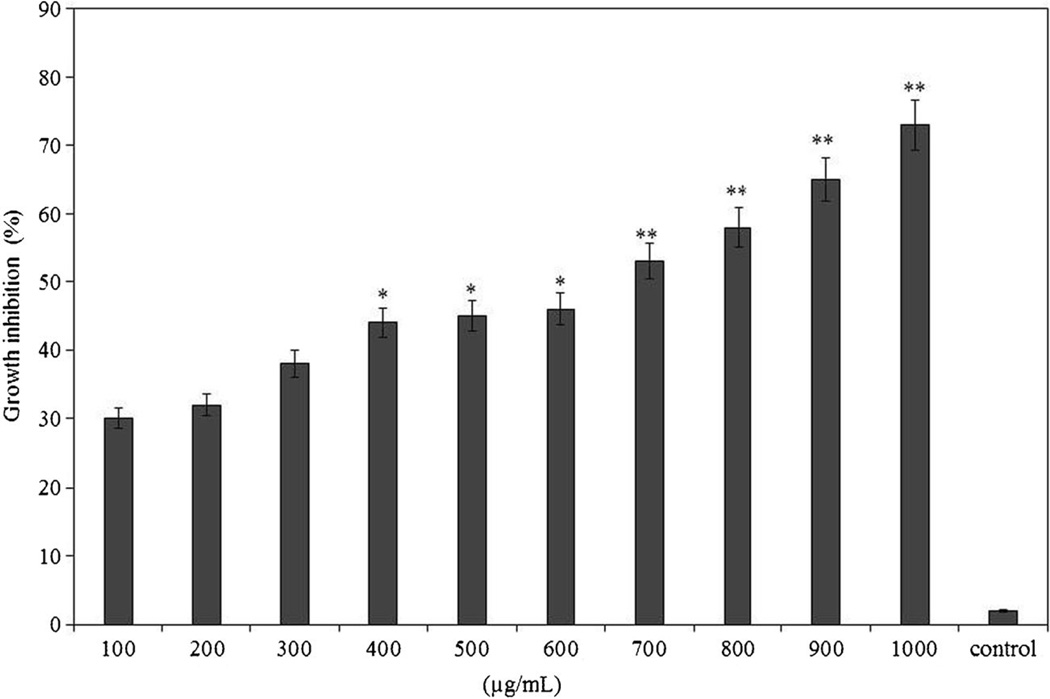
Our results indicated that anticancer activity increased significantly along with increased antioxidant activities. To determine the IC50 of fractions obtained by chymotrypsin hydrolysis in 1 h, a dose dependent test was carried out with MDA-MB231 cells using fractions that exhibited the highest anticancer activity (IC50: 650 µg/mL) (Fig. 7). The normal primary HUVEC was also incubated with the peptide fractions and no toxicity was observed (not shown).
Fig. 7.
Dose response study (100 to 1000 µg/mL) of walnut protein peptide fractions of after 1 h hydrolysis with chymotrypsin on MDA-MB231 breast cancer cells. Control sample is culture medium only. *Significantly different from 0 h treated cells (*P < 0.05, **P < 0.01)
Several efforts have been made during the last decade to advance novel broad-spectrum anticancer peptides into clinical use. The proteins extracted from the walnut and their hydrolysates had cancer cell inhibitory activity (63 ± 1.73 % inhibition using 1 h chymotrypsin hydrolysis). Other fractions may also contribute to inhibition of cancer cell proliferation, but not to the extent observed using fractions obtained from 1 h chymotrypsin hydrolysis. The varying results in inhibition of cancer cell growth may be attributed to the variations of the amino acid sequences of these peptides.
Several studies on the antioxidant and anti-cancer activity of peptide fractions have been previously reported [28, 29]. In some cases, the peptides have been purified and sequenced. According to the majority of these studies the purified peptides are less efficacious than crude fractions [23, 30]. Thus, because of higher efficiency of crude fractions compared with partially purified peptides (Fig. 3), the crude peptides were used in this study.
Conclusion
In the present study, walnut proteins were hydrolyzed by various proteases to extract antioxidant peptides. Among three proteolytic enzymes, chymotrypsin resulted in the production of the peptides with the highest antioxidant and anticancer activity. These crude peptides obtained after 1 h chymotrypsin hydrolysis was used as the optimum natural antioxidant fraction to enhance antioxidant and anticancer properties of functional foods compared with other proteases. Our data in this study showed a significant correlation between antioxidant and anticancer activity of walnut hydrolysate fractions. Ultrafiltration could partially purify the antioxidant peptides. However, the antioxidant activity was significantly decreased by partial size fractionation. Thus, we concluded that the mixtures of peptides are more effective than the purified peptides, and bioactive peptides from walnut proteins can be considered a suitable candidate for a new era of nutraceutical products and cancer therapeutics, with limited side effects, and health-promoting benefits as natural products.
Acknowledgments
The support of University of Tehran, International Scientific Studies & Collaboration (CISSC)-Ministry of Science, Research and Technology in Iran, Center of Excellence in Biothermodynamics (CEBiotherm), Center of Excellence for Walnut Improvement and Technology of Iran, Iran National Science Foundation (INSF) and Iran National Elites Foundation (INEF) and UNESCO Chair on Interdisciplinary Research in Diabetes, Iran Society of Biophysical Chemistry is gratefully acknowledged. The authors also acknowledge the Headquarter of Science and Technology Development in Medicinal Plants of Iran’s Vice-President for Science and Technology Affairs.
Abbreviations
- ABTS
2,2′-anizo-bis-(3-ethylbenzthiazoline-6-sulfonic acid)
- ACE
Angiotensin converting enzyme
- CL
Chemiluminescence
- HUVEC
Human umbilical vein endothelial cells
- MTT
3-(4, 5dimethylthiazolyl)-2,5-diphenyl-tetrazolium bromide
- OPA
o-Phthaldialdehyde
- ROS
Reactive oxygen species
- SDS
Sodium dodecyl sulfate
Footnotes
Compliance with Ethical Standards
Human and Animal Rights This article does not contain any studies with human or animal subjects.
Conflict of Interest The authors declare that there are no conflicts of interest.
References
- 1.Karas M. Anti-free-radical properties of the peptide fractions isolated from string bean by immobilized metal ion affinity chromatography. Protein Pept Lett. 2007;14(5):447–454. doi: 10.2174/092986607780782786. [DOI] [PubMed] [Google Scholar]
- 2.Valko M, Rhodes C, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Borek C. Dietary antioxidants and human cancer. Integre Cancer Ther. 2004;3(4):333–341. doi: 10.1177/1534735404270578. [DOI] [PubMed] [Google Scholar]
- 4.Kitts DD, Weiler K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr Pharm Des. 2003;9(16):1309–1323. doi: 10.2174/1381612033454883. [DOI] [PubMed] [Google Scholar]
- 5.Gu X, Hou Y-K, Li D, Wang J-Z, Wang F-J. Separation, purification, and identification of angiotensin I–converting enzyme inhibitory peptides from walnut (Juglans regia L.) hydrolyzate. Int J Food Prop. 2015;18(2):266–276. [Google Scholar]
- 6.Mojica L, de Mejía EG. Characterization and comparison of protein and peptide profiles and their biological activities of improved common bean cultivars (Phaseolus vulgaris L.) from Mexico and Brazil. Plant Foods Hum Nutr. 2015;70(2):105–112. doi: 10.1007/s11130-015-0477-6. [DOI] [PubMed] [Google Scholar]
- 7.Udenigwe CC, Aluko RE. Food protein-derived bioactive peptides: production, processing, and potential health benefits. J Food Sci. 2012;77(1):R11–R24. doi: 10.1111/j.1750-3841.2011.02455.x. [DOI] [PubMed] [Google Scholar]
- 8.Gu M, Chen H-P, Zhao M-M, Wang X, Yang B, Ren J-Y, Su G-W. Identification of antioxidant peptides released from defatted walnut (Juglans Sigillata Dode) meal proteins with pancreatin. LWT-Food Sci Technol. 2015;60(1):213–220. [Google Scholar]
- 9.Vinson JA, Cai Y. Nuts, especially walnuts, have both anti-oxidant quantity and efficacy and exhibit significant potential health benefits. Food Funct. 2012;3(2):134–140. doi: 10.1039/c2fo10152a. [DOI] [PubMed] [Google Scholar]
- 10.Schreinemachers DM, Everson RB. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiol. 1994;5(2):138–146. doi: 10.1097/00001648-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Jemal A, Siegel R, Ward E, Hao Y, J X, Murray T, Thun MJ. Cancer statistics, 2008. CA. Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 12.Sze-Tao KWC, Sathe SK. Walnuts (Juglans regia L): proximate composition, protein solubility, protein amino acid composition and protein in vitro digestibility. J Sci Food Agric. 2000;80(9):1393–1401. [Google Scholar]
- 13.Rahimi M, Ghaffari SM, Salami M, Mousavy SJ, Niasari-Naslaji A, Jahanbani R, Yousefinejad S, Khalesi M, Moosavi-Movahedi AA. ACE-inhibitory and radical scavenging activities of bioactive peptides obtained from camel milk casein hydrolysis with proteinase K. Dairy Sci Technol. 2016;489(96):1–11. [Google Scholar]
- 14.Church FC, Swaisgood HE, Porter DH, Catignani GL. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J Dairy Sci. 1983;66(6):1219–1227. [Google Scholar]
- 15.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Zhang Z, Yang C. A sensitive chemiluminescence method for the determination of H2O2 in exhaled breath condensate. Anal Sci. 2008;24(2):201–205. doi: 10.2116/analsci.24.201. [DOI] [PubMed] [Google Scholar]
- 17.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 18.Rahman S, Salehin F, Iqbal A. In vitro antioxidant and anti-cancer activity of young Zingiber officinale against human breast carcinoma cell lines. BMC Complement Altern Med. 2011;11(1):76. doi: 10.1186/1472-6882-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Zhou K, Sun S, Canning C. Production and functional characterisation of antioxidative hydrolysates from corn protein via enzymatic hydrolysis and ultrafiltration. Food Chem. 2012;135(3):1192–1197. doi: 10.1016/j.foodchem.2012.05.063. [DOI] [PubMed] [Google Scholar]
- 20.Qian Z-J, Jung W-K, Kim S-K. Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bullfrog skin, Rana catesbeiana Shaw. Bioresour Technol. 2008;99(6):1690–1698. doi: 10.1016/j.biortech.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Kumar NS, Nazeer R, Jaiganesh R. Purification and biochemical characterization of antioxidant peptide from horse mackerel (Magalaspis cordyla) viscera protein. Peptides. 2011;32(7):1496–1501. doi: 10.1016/j.peptides.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 22.Chen C, Chi Y-J, Zhao M-Y, Lv L. Purification and identification of antioxidant peptides from egg white protein hydrolysate. Amino Acids. 2012;43(1):457–466. doi: 10.1007/s00726-011-1102-0. [DOI] [PubMed] [Google Scholar]
- 23.Tang X, He Z, Dai Y, Xiong YL, Xie M, Chen J. Peptide fractionation and free radical scavenging activity of zein hydrolysate. J Agric Food Chem. 2009;58(1):587–593. doi: 10.1021/jf9028656. [DOI] [PubMed] [Google Scholar]
- 24.Amarowicz R, Shahidi F. Antioxidant activity of peptide fractions of capelin protein hydrolysates. Food Chem. 1997;58(4):355–359. [Google Scholar]
- 25.Hedstrom L, Szilagyi L, Rutter WJ. Converting trypsin to chymotrypsin: the role of surface loops. Science. 1992;255(5049):1249–1253. doi: 10.1126/science.1546324. [DOI] [PubMed] [Google Scholar]
- 26.Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74(17):2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15(10):7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mora-Escobedo R, del Carmen R-RM, Ramón-Gallegos E, Reza-Alemán R. Effect of protein hydrolysates from germinated soybean on cancerous cells of the human cervix: an in vitro study. Plant Foods Hum Nutr. 2009;64(4):271–278. doi: 10.1007/s11130-009-0131-2. [DOI] [PubMed] [Google Scholar]
- 29.Xue Z, Gao J, Zhang Z, Yu W, Wang H, Kou X. Antihyperlipidemic and antitumor effects of chickpea albumin hydrolysate. Plant Foods Hum Nutr. 2012;67(4):393–400. doi: 10.1007/s11130-012-0311-3. [DOI] [PubMed] [Google Scholar]
- 30.Das R, Bhattacharjee C. In Vitro Evaluation of Antioxidant Activity and Radical Scavenging Activity of Sesame Bioactive Peptides. Int J Emerging Technol Adv Eng. 2013;3(11):521–527. [Google Scholar]