Abstract
Sweet potato peels (SPP) are a major waste generated during root processing and currently have little commercial value. Phenolics with free radical scavenging activity from SPP may represent a possible added-value product for the food industry. The aqueous extraction of phenolics from SPP was studied using a Central Composite Design with solvent to solid ratio (30–60 mL g−1), time (30–90 min) and temperature (25–75 °C) as independent variables. The comparison of response surface methodology (RSM) and artificial neural network (ANN) analysis on extraction modelling and optimising was performed. Temperature and solvent to solid ratio, alone and in interaction, presented a positive effect in TPC, ABTS and DPPH assays. Time was only significant for ABTS assay with a negative influence both as main effect and in interaction with other independent variables. RSM and ANN models predicted the same optimal extraction conditions as 60 mL g−1 for solvent to solid ratio, 30 min for time and 75 °C for temperature. The obtained responses in the optimized conditions were as follow: 11.87 ± 0.69 mg GAE g−1 DM for TPC, 12.91 ± 0.42 mg TE g−1 DM for ABTS assay and 46.35 ± 3.08 mg TE g−1 DM for DPPH assay. SPP presented similar optimum extraction conditions and phenolic content than peels of potato, tea fruit and bambangan. Predictive models and the optimized extraction conditions offers an opportunity for food processors to generate products with high potential health benefits.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-016-2354-1) contains supplementary material, which is available to authorized users.
Keywords: Sweet potato peels, Phenolic content, Radical scavenging activity, Extraction, Response surface methodology, Artificial neural network
Introduction
Sweet potato (Ipomoea batatas L.) is a most used agro product in the world, especially in Asia and Africa where it is used in traditional diets (Bovell-Benjamin 2007). This root has also been profusely included in the composition of innovative applications such as ice-cream (Gurgel et al. 2011) or non-carbonated drink (Wireko-Manu et al. 2010). Chips, flakes, yogurts and juices are same functional products made from sweet potato (Barnes and Sanders 2012). Agro by-products represent an important source of phytochemicals such as phenolic compounds, possessing a wide range of functional activities (Peschel et al. 2006). Peels are accounted as one of the major wastes generated during the processing of sweet potato with currently little commercial value (Maloney et al. 2012) and limited work has been performed for their scale up (Anastácio and Carvalho 2013). Sweet potato peels (SPP) contain a high phenolic content with free radical scavenging activities (Zhu et al. 2010) which were related to every health benefits (Panda et al. 2011). SPP applications in bioethanol (Oyeleke et al. 2012), pharmaceuticals (Manpreet et al. 2005) and biosurfactants (Saharan et al. 2011) have already been revealed. Recycling of agro wastes into high valuable products with potential health benefits could be achieved through extraction. As processing conditions are recognised to influence the removal of phenolics which are located particularly in the peel (Peschel et al. 2006), process modelling and optimization could contribute to the reuse of agro-waste by industry. As many factors such as solvent-to-solid ratio, temperature and time may significantly influence the extraction efficiency, it is necessary to optimise the extraction conditions to obtain the highest phenolics content y and antioxidant activity (Prasad et al. 2011). The optimization of the extraction of bioactive compounds from SPP was previously investigated using Response Surface Methodology (RSM) by Maloney et al. (2012). Artificial neural network (ANN) is also being applied in parallel with RSM method in prediction and modelling of extraction of phenolics with better in data fitting (Cheok et al. 2012). ANN methodology does not require a design to obtain predictive models being considered a better modelling technique for nonlinear data than RSM (Cheok et al. 2012). Phenolics extraction optimization studies were performed on fruit and vegetable peels such as apple, grape (Casazza et al. 2012), jabuticaba (Santos and Meireles 2011), pomegranate (Amyrgialaki et al. 2014; Tabaraki et al. 2012), orange (Dahmoune et al. 2014), tangerine (Londoño-Londoño et al. 2010), bambangan (Prasad et al. 2011), banana, mangosteen (Cheok et al. 2012), rambutan (Prakash Maran et al. 2013), tea fruit (Xu et al. 2012) and potato (Singh et al. 2011; Wijngaard et al. 2012). For the six aforementioned peel materials, the experimental design was based on a Central Composite Design (CCD). The objective of this work was to model the effect of solvent to solid ratio, time and temperature on the extraction of phenolic compounds with radical scavenging activity from SPP through a CCD by RSM and ANN analysis, and define the best conditions to obtain infusions with high phenolic content and biological activity.
Materials and methods
Plant material
Sweet potatoes were purchased at local markets (Faro, Portugal) and transported to the lab at the same day. Roots were washed under tap water and air-dried (18 ± 5 °C) during the night. Peeling was done manually with a cut depth of circa 1.5 mm, and peels were then dried at 60 °C for 48 h. The drying conditions were the same used in a previous study on phenolics extraction key factors screening from sweet potato peels (Anastácio and Carvalho 2013). The dried material was then milled and sieved until all particles were smaller than 600 μm (30 mesh).
Chemicals
2,2-Diphenyl-1-picrylhydrazyl (DPPH), Folin–Ciocalteu reagent, 2-2′-azino-bis (3 ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), gallic acid, sodium persulfate and Trolox were purchased from Sigma-Aldrich Co. Ltd. (Poole, UK). Sodium carbonate was purchased from VWR (West Chester, PA). Absolute ethanol (100 %) was bought from Merck (Nottingham, UK). All reagents were of analytical grade.
Methods
Screening process
The selection of factors and levels to be used in experimental design was based on optimization studies for the extraction of phenolics (measured by the Folin–Ciocalteau assay) and free radical scavenging activity from fruit and vegetable peels (Table 1). Studies on peels valorisation through the extraction of phenolic compounds had been much more focused on fruits than on vegetables. However, only two studies included the measurement of ABTS free radical inhibition and/or DPPH free radical scavenging activities expressed in Trolox equivalent (TE) (Wijngaard et al. 2012). Research on optimization were performed by conventional solid–liquid extraction as well as innovative processes have been investigate such as high pressure carbon dioxide extraction (Santos and Meireles 2011), supercritical fluid extraction (Casazza et al. 2012) and pressurized liquid extraction (Wijngaard et al. 2012). According to Table 1, most of the experimental plans were based on CCD with three independent factors. Solvent to solid ratio was included as an independent variable in four optimization studies and it was considered a fixed factor in the majority of them. On the other hand, time and temperature were considered as independent factors in many investigations done on peels, although not always combined. Regarding the extraction of phenolic compounds from SPP, solvent to solid ratio and depth cut were identified as critical factors in a previous screening study (Anastácio and Carvalho 2013). The most favourable level identified for depth cut, 1.5 mm, was used as a fixed condition in this work. Time and temperature when varied between 60–180 min and 27–40 °C did not influenced the extraction of phenolics from SPP (Anastácio and Carvalho 2013). They were included in this optimization study as independent variables with a wider range of variation. Thus, the design selection for the aqueous extraction process was a three-factor CCD with solvent to solid ratio (X1), time (X2) and temperature (X3) as independent variables and TPC, ABTS and DPPH assays as responses. This design will provide useful information on the body of knowledge regarding extraction of phenolic compounds with antiradical activity from fruit and vegetable peels.
Table 1.
Information on published design of experiments used in extraction optimization studies of phenolics from fruit and vegetables peels
| No. | Peel material | Extraction methoda | Solvent | Design (n/r)b | Solvent to solid ratio (ml/g) | Time (min) | Tempe-rature (°C) | Other variables | TPC | ABTS assay | DPPH assay | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fruits | ||||||||||||
| 1 | Apple | USc | Aqueous ethanol | BBD (3/17) | 57 | 20–40 | 30–50 | Enzyme amount | 7.0 | N | N | |
| 2 | Bambangan | C | Aqueous ethanol | CCD (3/20) | 20–50 | 30 | 30–65 | Ethanol concentration | 14.6 | N | N | Prasad et al. (2011) |
| 3 | Banana | C | Methanol | CCD (3/16) | 6.7 | 21–141 | 25–55 | Number of extractions | 22 | 49 | 31 | |
| 4 | Grape | SF | Carbon dioxide | Fd (3/16) | 30 | 37–46 | Pressure and modifier | 0.44 | N | N | ||
| 5 | Grape | C | Ethanol | F (3/11) | 10–30 | 540-1740 | 25 | – | 3.22 | N | N | Casazza et al. (2012) |
| 6 | Jabuticaba | HP | Carbon dioxide | F (2/10) | 45 | 40–80 | Pressure and solid–liquid/CO2 | 13 | N | N | Santos and Meireles (2011) | |
| 7 | Mangosteen | C | Aqueous methanol | CCD (3/20) | 5.4–200 | 22–382 | 25 | Methanol (%) | 140.6 | N | N | Cheok et al. (2012) |
| 8 | Orange | US | Aqueous acetone | CCD | 250 | 5–15 | 27 | Ultrasound power and acetone concentration | 13.57 | N | N | Dahmoune et al. (2014) |
| 9 | Pomegranate | US | Aqueous ethanol | CCD (3/16) | 50 | 10–30 | 30–60 | Ethanol concentration | 91.98 | N | N | Tabaraki et al. (2012) |
| 10 | Pomegranate | C | Aqueous ethanol | CCD (3/16) | 60 | 60–300 | – | Ethanol concentration and pH | 325 | N | N | Amyrgialaki et al. (2014) |
| 11 | Rambutan | US | Water | CCD (4/30) | 10-20 | 10-30 | 30-50 | Ultrasound power | 5.52 | N | N | Prakash Maran et al. (2013) |
| 12 | Tangerine | US | Water | F (2/4) | 10 | 30–90 | 40 | Peels humidity | 19.6 | N | N | Londoño-Londoño et al. (2010) |
| 13 | Tea fruit | C | Aqueous ethanol | CCD (3/20) | 40 | 10–40 | 30-70 | Ethanol concentration | 47.5 | N | N | Xu et al. (2012) |
| Vegetables | ||||||||||||
| 14 | Potato | MW | Aqueous methanol | F (3/20) | 10 | 5–15 | – | Power and solvent % | 3.94 | N | N | Singh et al. (2011) |
| 15 | Potato | C | Water | BBD (3/17) | 20000 | 40-60 | 40-80 | 3.94 | N | 3.52 | Wijngaard et al. (2012) | |
| 16 | Potato | PL | Carbon dioxide | CCD (2/13) | 65–135 | Ethanol concentration | 3.68 | N | 3.39 | Wijngaard et al. (2012) | ||
| 17 | Potato | C | Aqueous ethanol | CCD (3/20) | 2000 | 5–150 | 25–90 | Ethanol concentration | 10.3 | N | Ye | |
TPC total phenolic content by Folin–Ciocalteau method (mg GAE/g dw), ABTS and DPPH assays (mg TE/g dw), N not included in the study)
a C conventional, HP high pressure carbon dioxide assisted, MW microwave assisted, PL pressurized liquid, SF supercritical fluid, US ultrasound assisted
b BBD Box–Behnken design, CCD Central Composite Design, F factorial design, (n number of factors/r number of runs)
cCellulase enzymolysis extraction
dOrthogonal array design L16:45
eNot expressed in TE units
According to Table 1, the widest range for solvent to solid ratio (X1) was tested for a conventional extraction of phenols from mangosteen peels with a minimum value of 5.4 and maximum value of 200 mL g−1 (Cheok et al. 2012). The time of extraction (X2) was studied at different levels whereas the conventional extraction for grape peels had the highest range, from 540 to 1740 min (Casazza et al. 2012). The lowest level studied was 5 min for phenolics extraction from potato peels (Singh et al. 2011). Regarding temperature (X3) of conventional extractions, the lowest was 25 °C and highest was 90 °C. Thus, minimum and maximum levels established for the independent variables were 30–60 mL g−1 for solvent to solid ratio, 30–90 min for time and 25–75 °C for temperature. These settings were adjusted near to the median of values reported for fruit and vegetable peels studies (Table 1).
Extraction process
Twenty extractions runs were performed according to the conditions presented in Table 2 obtained by a CCD experimental. For each run, 25 mL of distilled water were transferred into a 250 mL screw cap flask with an amount of SPP powder corresponding to the respective solvent to solid ratio. The mixtures were stirred at constant rate of 200 rpm on a hot plate stirrer (HS0707V2, Favorit, Malaysia) during the respective run time and at the respective run temperature measured by digital food thermocouple (Model HI 98501, Hanna Instruments, Bedfordshire, UK). Extracts were filtered through a filter paper with a vacuum pump aspirator (DOA-P604-BN, Cast Manufacturing Inc., USA) and volume filled up to 25 mL. Extracts were hold at −18 °C until analysis.
Table 2.
Actual and coded values of independent variables used for the Central Composite Design and total phenolics content and radical scavenging activity of sweet potato peel extraction trials
| Std Or | Independent variablesa | Results | ||||
|---|---|---|---|---|---|---|
| X1 (mL g−1) | X2 (min) | X3 (°C) | TPC (mg GAE g−1 DM) | ABTS assay (mg TE g−1 DM) | DPPH assay (mg TE g−1 DM) | |
| 1 | 30 (−1) | 30 (−1) | 25 (−1) | 5.77 ± 0.04 | 5.71 ± 0.42 | 20.61 ± 1.28 |
| 2 | 30 (−1) | 30 (−1) | 75 (+1) | 7.89 ± 0.09 | 8.18 ± 0.34 | 19.29 ± 0.43 |
| 3 | 30 (−1) | 90 (+1) | 25 (−1) | 7.01 ± 0.19 | 6.18 ± 0.22 | 21.97 ± 0.75 |
| 4 | 30 (−1) | 90 (+1) | 75 (+1) | 6.09 ± 0.10 | 7.17 ± 0.27 | 23.05 ± 0.35 |
| 5 | 60 (+1) | 30 (−1) | 25 (−1) | 5.72 ± 0.21 | 4.97 ± 0.49 | 31.05 ± 0.59 |
| 6 | 60 (+1) | 30 (−1) | 75 (+1) | 11.35 ± 0.16 | 12.85 ± 0.14 | 45.91 ± 0.52 |
| 7 | 60 (+1) | 90 (+1) | 25 (−1) | 6.51 ± 0.11 | 3.91 ± 0.30 | 37.89 ± 0.70 |
| 8 | 60 (+1) | 90 (+1) | 75 (+1) | 11.11 ± 0.22 | 10.90 ± 0.08 | 45.32 ± 2.13 |
| 9 | 20 (−1.68) | 60 (0) | 50 (0) | 5.64 ± 0.02 | 5.15 ± 0.06 | 13.76 ± 0.33 |
| 10 | 70 (+1.68) | 60 (0) | 50 (0) | 9.36 ± 0.39 | 7.77 ± 0.36 | 49.79 ± 0.69 |
| 11 | 45 (0) | 10 (−1.68) | 50 (0) | 7.08 ± 0.19 | 8.25 ± 0.21 | 30.88 ± 0.98 |
| 12 | 45 (0) | 110 (+1.68) | 50 (0) | 7.24 ± 0.17 | 6.94 ± 0.05 | 31.49 ± 0.17 |
| 13 | 45 (0) | 60 (0) | 8 (−1.68) | 6.47 ± 0.03 | 4.15 ± 0.24 | 31.25 ± 1.57 |
| 14 | 45(0) | 60 (0) | 92 (+1.68) | 11.86 ± 0.29 | 11.94 ± 0.10 | 33.38 ± 2.95 |
| 15 | 45 (0) | 60 (0) | 50 (0) | 8.78 ± 0.25 | 8.06 ± 0.12 | 34.68 ± 0.33 |
| 16 | 45 (0) | 60 (0) | 50 (0) | 8.92 ± 0.75 | 7.87 ± 0.50 | 31.09 ± 0.77 |
| 17 | 45 (0) | 60 (0) | 50 (0) | 8.17 ± 0.17 | 7.97 ± 0.16 | 33.18 ± 0.07 |
| 18 | 45 (0) | 60 (0) | 50 (0) | 8.09 ± 0.20 | 7.66 ± 0.47 | 33.18 ± 1.55 |
| 19 | 45 (0) | 60 (0) | 50 (0) | 7.71 ± 0.24 | 8.00 ± 0.61 | 32.73 ± 1.00 |
| 20 | 45 (0) | 60 (0) | 50 (0) | 8.65 ± 0.11 | 8.39 ± 0.29 | 33.61 ± 1.25 |
Results were expressed as the mean ± SD of three determinations
Std Or standard order, X 1 solvent to solid ratio, X 2 time, X 3 temperature
aCoded values in brackets
Evaluation of extracts
Total phenolic content (TPC), ABTS radical cation scavenging activity (ABTS assay) and DPPH free radical scavenging activity (DPPH assay) were determined regarding the method described previously by Prieto et al. (1999), Re et al. (1999) and Blois (1958), respectively, with the minor modifications indicated in Nunes et al. 2012. In addition, for DPPH assay absolute ethanol (100 %) was used as solvent. TPC was expressed in mg gallic acid equivalent (GAE) per gram of dried material (DM) while the results for ABTS and DPPH assays were reported as mg of Trolox equivalent (TE) g−1 DM. Determinations were carried out in triplicate and data were reported as mean ± SD. All absorbance readings were made by a T70 + UV/Vis Spectrometer (PG Instruments Ltd, United Kingdom).
Statistical analysis
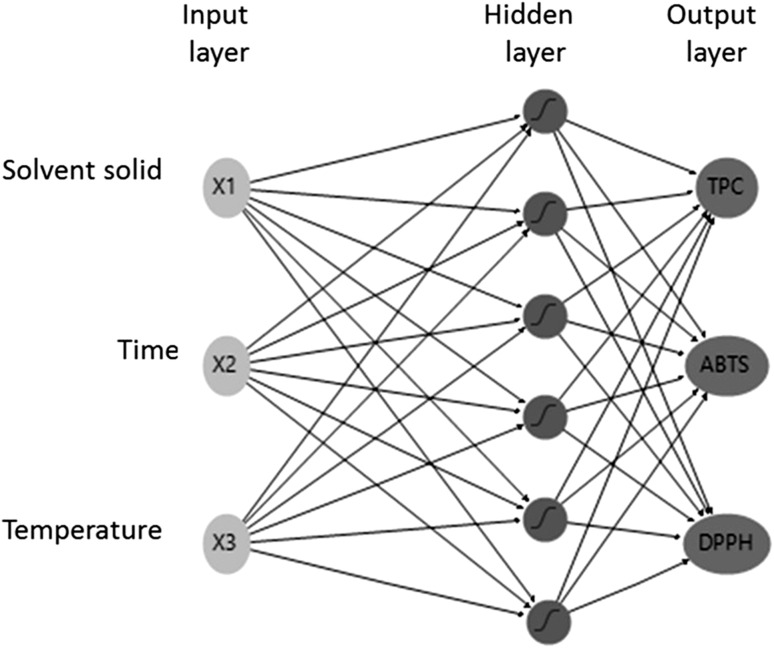
A Central Composite Design (CCD) for the aqueous solid–liquid extraction of SPP was used with three independent variables, solvent to solid ratio (X1), time (X2) and temperature (X3) on three responses, TPC, ABTS and DPPH assays (Table 2). The experimental plan contained 20 runs, with 8 factorial points, 6 axial points and 6 central points. In order to have equal precision of estimation in all directions, design was made rotatable by an axial distance of 1.68, which corresponded to the 4-th root of the number of factorial points. Therefore, variables were coded at five levels (−1.68, −1, 0, 1, 1.68). Pearson correlations were conducted to examine the relationship between the antioxidant assays. A three-dimensional scatter-plot was built to compare the conditions of phenolic compounds extraction among studies performed on fruit and vegetable peels. The software used for the establishment of the experimental design, analysis of the data and creation of the plots was JMP® Pro 10.0.2 (www.jmp.com) provided at no cost by SAS (www.sas.com). Figure 2 were constructed by Microsoft® Excel 2013. For RSM analysis, data were approximated to a second-order polynomial equation by the least-square regression method with the selection of the significant coefficients by the backward method (αout = 0.05) with no rules (model hierarchy was not mandatory). The coefficient of determination (R2), adjusted R2 (R2adj) and root mean square error (RMSE) were used to access the models performance. Fitted models were used to build surface plots and extraction conditions optimization was performed by maximizing the desirability function. For the analysis by ANN, a simple single layer structure with three inputs, X1, X2 and X3, one hidden layer with seven nodes and TPC, ABTS and DPPH assays as outputs was exploited to model the experimental data obtained from CCD design (Fig. 1). The data were divided into two subsets, training and validation and network topology was trained by k-fold cross validation method (k = 6). One hidden layer was considered previously adequate by trial and error for adjusting extraction data (Cheok et al. 2012). The hyperbolic-tangent activation function was used as transfer function in the hidden layer to the output layer. The number of neurons in the hidden layer was adjusted iteratively to maximize performance fitting determined by R2 and RMSE. The rate of change in each predicted output was determined varying a given input while keeping all other input variables constant. Optimization was performed by maximizing the desirability function.
Fig. 2.
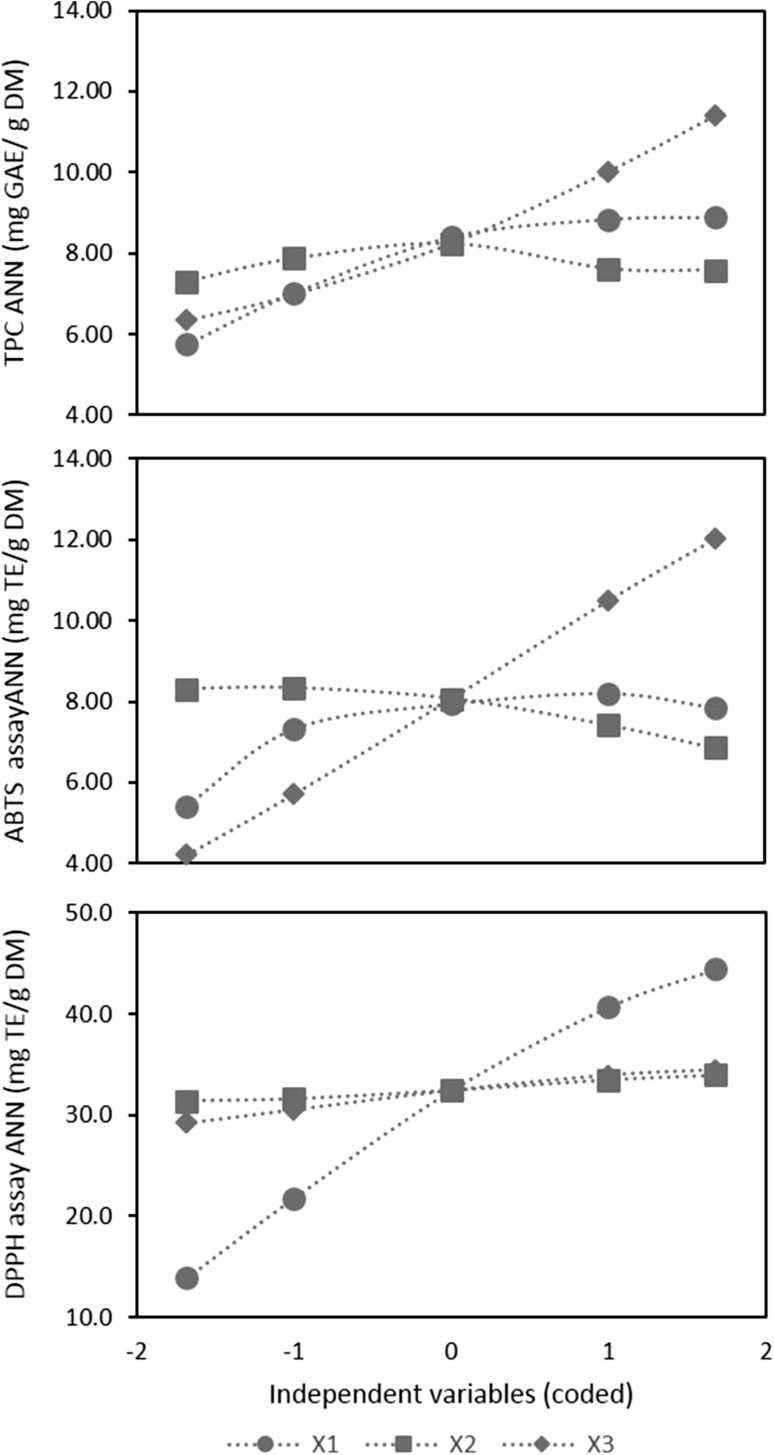
Sensibility analysis of ANN models for TPC, ABTS and DPPH assays. Determinations were performed using coded variables. X 1 solvent to solid ratio, X 2 time, X 3 temperature
Fig. 1.
Diagram of the Artificial Neural Network architecture. X 1 solvent to solid ratio, X 2 time, X 3 temperature
Results and discussion
In this study, total phenolic content and free radical scavenging activities for different extraction conditions were presented, modelled and optimized. A comparison between RSM and ANN methodologies was performed.
Total phenolic content and free radical scavenging activities
TPC results of SPP varied between 5.64 and 11.86 mg GAE g−1 DM (Table 1). This range was reached higher values that those reported in the previous factor screening design, 1.02–6.21 mg GAE g−1 DM (Anastácio and Carvalho 2013). Thus, the conditions tested by the CCD design provided an enhanced extraction of phenolics from SPP in comparison to the conditions tested by the factor screening design. ABTS assay results of SPP presented a similar range of variation to TPC (3.91–12.85 mg TE g−1 DM), so these two variables are strongly associated with a Pearson’s correlation coefficient (R) of 0.957 (p < 0.001). DPPH assay results had values between 13.76 and 49.79 mg TE g−1 DM, and were higher than ABTS. ABTS free radicals could be dissolved in aqueous media whereas DPPH free radicals can only be dissolved in organic media (Wojdylo et al. 2007). The differences observed for the two assays might be justified by a higher antiradical activities of SPP lipophilic compounds in comparison to hydrophilic compounds. Still, DPPH and ABTS assays were moderately correlated (R = 0.521; p < 0.05). A lower correlation of TPC with DPPH assay (R = 0.666; p < 0.01) than with ABTS assay was observed. The higher ability of SPP’s extracts to scavenge DPPH free radicals in comparison to inhibit ABTS free radicals may be related to compounds that are not determined by the Folin–Ciocalteau assay.
Regarding other studies, TPC results were in line with those observed by Zhu et al. (2010) for the SPP methanolic infusions ranged from 8 to 20 mg GAE g−1 DM. However, TPC values was lower than other vegetable wastes such as broccoli (~30 mg GAE g−1 DM) or chicory (~13 mg GAE g−1 DM) (Peschel et al. 2006). Regarding relationships between variables, a moderate correlation coefficient between TPC and ABTS assay was obtained for banana peels (R > 0.55) while DPPH and ABTS assays data were more related (R > 0.70) than what was observed in this work.
Extraction modelling by RSM
Experimental values for TPC, ABTS and DPPH assays were fitted to second order polynomial equations by the RSM analysis. According to ANOVA results for (Table 3), all fitted models were significant at a confidence level of 0.001, and p values of the lack of fit were all higher than 0.05 confirmed suitability of the selected design. Prediction equations were presented in Table 4. The coded regression coefficients sign and magnitude gave a direct measure of the contribution of the various independent variables in each response (see Supplementary Information, Figure S1). For TPC response, temperature of extraction (X3) presented the highest positive effect followed by the interaction term between solvent to solid ratio and temperature (X1X3). In addition, the interaction term time–temperature (X2X3) and quadratic terms for time (X22) and solvent to solid ratio (X21) presented a negative influence in the extraction of phenols. These positive effects were related an enhanced diffusion rate and solubility of antioxidants with temperature (Londoño-Londoño et al. 2010). Solid to solid ratio also presented a positive effect as the higher this ratio more phenolic compounds may permeate into the solvent (Prasad et al. 2011). Extraction Time was not the main effect which may related to the levels tested in the design. Compared to TPC response, ABTS assay fitted model indicated time (X2) as main factor with a negative effect. Main factors and interactions terms with positive effect on ABTS assay were similar to TPC, with a decreasing order of X3 > X1X3 > X1. In general, terms with negative impact presented lower magnitudes than positive terms. A different fitted model was observed for DPPH assay, where all significant terms had a positive effect in the order of X1 > X1X3 > X3. In general, temperature (X3), interaction solvent to solid ratio-temperature (X1X3) and solvent to solid ratio (X1) terms presented a positive influence on TPC, ABTS assay and DPPH assay responses. The significant negative terms observed for TPC and ABTS assay models had much less magnitude than the positive ones. Time (X2) was the least influential variable for SPP extracts and as the main effect it was only significant for ABTS assay. The fitted models explained 96 % of the variability observed for TPC and 99 % for ABTS assay whereas it was slightly lower (94 %) for DPPH assay, as indicated by the coefficients of determination (R2) (see Supplementary Information, Figure S1). Adjusted coefficients of determination (R2Adj) were similar in value with R2 for all three models. Root mean squared error (RMSE) was much higher for DPPH assay than the others responses which demonstrates the existence of a higher variance of the fitted model. To exemplify the combined effects of variables, surface plots of solvent: solid and temperature were constructed for TPC, ABTS and DPPH assays responses (see Supplementary Information, Figure S2). Positive interactions between solvent: solid and temperature could be confirmed. By analysing the slopes for each variable, it is anticipated that the upper level of solvent: solid and temperature will be optimal for the extraction of phenols.
Table 3.
Results of ANOVA for TPC, ABTS and DPPH assays with significant variables obtained by backward stepwise multiple regression method (p < 0.05)
| Source | TPC | ABTS assay | DPPH assay | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DF | MSS | F valuea | DF | MSS | F valuea | DF | MSS | F valuea | |
| Model | 6 | 10.53 | 48.42*** | 7 | 65.60 | 42.80*** | 3 | 487.60 | 96.29*** |
| X1 | 1 | 14.74 | 67.78*** | 1 | 6.95 | 32.48*** | 1 | 1351.25 | 266.84*** |
| X2 | |||||||||
| X3 | 1 | 30.76 | 141.47*** | 1 | 35.19 | 164.46*** | 1 | 48.11 | 9.50** |
| X1X2 | |||||||||
| X1X3 | 1 | 10.19 | 46.88*** | 1 | 12.08 | 59.83*** | 1 | 63.45 | 12.53** |
| X2X3 | 1 | 2.07 | 9.52** | 1 | 1.86 | 8.70* | |||
| X21 | 1 | 1.69 | 7.78* | 1 | 2.21 | 10.34** | |||
| X22 | 1 | 3.71 | 17.10** | 1 | 3.48 | 16.26** | |||
| X23 | 1 | 3.10 | 14.50** | ||||||
| Residual | 13 | 0.22 | 12 | 0.21 | |||||
| Lack of fit | 8 | 0.21 | 0.97ns | 7 | 0.20 | 0.91ns | 5 | 7.39 | 1.84ns |
| Pure error | 5 | 0.22 | 5 | 0.22 | 11 | 4.01 | |||
| Cor total | 19 | 19 | 19 | ||||||
X 1 solvent to solid ratio, X 2 time, X 3 temperature, DF degrees of freedom, MSS mean sum of squares, Cor Total totals corrected from the mean
aSignificance indicated by the p value: ns p > 0.05; * p < 0.05; ** p < 0.01; *** p < 0.001
Table 4.
Predictive expressions for TPC, ABTS and DPPH assays obtained by RSM and ANN analysis
|
RSM methodology
Coded components X 1 = (X1 − 45)/15 X 2 = (X2 − 60)/30 X 3 = (X3 − 50)/50 | |
| TPC = | +8.57911 + 1.03877 X
1 +1.50070 X
3
+1.12875 X 1 X 3 − 0.50875 X 2 X 3 −0.38517 X 21 − 0.50538 X 22 |
| ABTS = | +7.94499 + 0.71732 X
1 − 0.42126 X
2 + 2.30149 X
3
−0.30875 X 1 X 2 + 1.42625 X 1 X 3 − 0.29625 X 2 X 3 −0.50376 X 21 |
| DPPH = | +31.70550 + 9.94702 X
1 +1.87688 X
3
+2.81625 X 1 X 3 |
|
ANN methodology
Input components H 1 = −4.4344 + 0.056085 X1 + 0.022434 X2 + 0.0063245 X3 H 2 = −2.6474 + 0.063560 X1 − 0.019351 X2 + 0.025436 X3 H 3 = −1.7178 + 0.018516 X1 + 0.009443 X2 + 0.006491 X3 H 4 = −0.6297 + 0.056814 X1 + 0.002641 X2 − 0.020855 X3 H 5 = −1.1013 + 0.044306 X1 − 0.026027 X2 − 0.018798 X3 H 6 = +2.5852 − 0.043737 X1 + 0.010170 X2 − 0.019675 X3 | |
| TPC = | 3.0298 − 2.1187 TanH(0.5H 1) − 6.0797 TanH(0.5H 2) − 4.3914 TanH(0.5H 3) + 5.5140 TanH(0.5H 4) − 8.0205 TanH(0.5H 5) − 15.5538 TanH(0.5H 6) |
| ABTS = | 3.5384 − 11.5766 TanH(0.5H 1) − 10.3526 TanH(0.5H 2) + 13.2513 TanH(0.5H 3) + 5.7319 TanH(0.5H 4) − 6.8284 TanH (0.5H 5) − 22.3724 TanH(0.5H 6) |
| DPPH = | 22.5131 − 2.6371 TanH(0.5H 1) − 19.3363 TanH(0.5H 2) + 0.71650 TanH(0.5H 3) + 21.6820 TanH(0.5H 4) − 11.8997 TanH(0.5H 5) − 46.3678 TanH(0.5H 6) |
X1 solvent to solid ratio, X2 time, X3 temperature
Extraction modelling by ANN
The ANN equation models were presented in Table 4. R2 values for ANN models were 0.931 for TPC and 0.936 for both ABTS and DPPH assays. Regarding RMSE, models for TPC, ABTS and DPPH assays presented values of 0.501, 0.458 and 1.77, respectively. The ANN model for DPPH assay presented a higher R2 and lower RMSE than the RSM model. As ANN is not able to provide insights of the models as directly as RSM approach, the rate of change of a response for one independent variable keeping the other fixed at level 0 was computed and represented (Fig. 2). ANN models revealed that temperature (X3) with the highest influence on TPC followed by solvent to solid ratio (X1), both with positive effect. Due to its low slope, time (X2) had minimum impact on TPC response. For ABTS assay, temperature (X3) also presented the higher effect and positive slope. The influence of solvent to solid ratio (X1) was similar to time (X2) but opposite direction, solvent to solid ratio had a positive effect while time presented a negative influence. For DPPH assay, solvent to solid ratio was the main influential factor. Time and temperature presented both a positive slope that was lower than solvent to solid ratio slope. In summary, ANN models revealed that temperature and solvent to solid ratio had a positive effect on responses and the influence of factor time was always smaller than the other two factors. Temperature presented a strong positive effect on both TPC and ABTS assay but less pronounced for DPPH assay.
Extraction optimization
The operational extraction conditions that maximized simultaneously TPC, ABTS and DPPH assays responses by RSM were a solvent to solid ratio of 60 mL g−1, a time of 30 min and a temperature of 75 °C. These optimized settings had a desirability of 0.94. For solvent to solid ratio and temperature, the optimal point was located at the maximum of the variables range. This was consequence of a pronounced effect of these two variables individually and in interaction. An optimized temperature value at the upper limit of the experimental design was also obtained for juticaba (Santos and Meireles 2011), pomegranate (Tabaraki et al. 2012), rambutan (Prakash Maran et al. 2013) and potato peels (Wijngaard et al. 2012). At optimal extraction conditions, the predicted TPC, ABTS and DPPH assays values were 11.87 ± 0.69 mg GAE g−1 DM, 12.91 ± 0.42 mg TE g−1 DM and 46.35 ± 2.71 mg TE g−1 DM, respectively. When each response was maximized individually, the same optimal point was obtained for ABTS and DPPH assays. However, the optimal setting for time changed to 48.1 when TPC response was optimized individually. Predicted responses when maximizing TPC were 11.98 ± 0.61 mg GAE g−1 DM for TPC, 12.29 ± 0.33 mg TE g−1 DM for ABTS assay and 46.35 ± 2.71 mg TE g−1 DM for DPPH assay. These results were not statistically different (p < 0.05) from the optimization for all responses.
The optimal extraction conditions obtained by ANN approach were identical to RSM. ANN predicted responses were 11.44 mg GAE g−1 DM for TPC, 12.84 mg TE g−1 DM for ABTS assay and 47.1 mg TE g−1 DM for DPPH assay. This optimal presented a desirability value of 0.91. When optimized individually, solvent to solid ratio and temperature factors solution did not change from 60 mL g−1 and 75 °C, respectively. However, the optimal extraction time was 42.3, 22.7 and 86.1 min when the desirability function was maximized only for TPC, ABTS and DPHH assays, respectively. Predicted responses were in line with the overall optimum.
Considering that the conventional extraction conditions were different, than those used in in study, SPP water extracts presented a higher optimal TPC value than potato peels but much lower than fruits peels (Table 1). The optimal value of SPP for ABTS assay was higher than the reported for banana peels and optimal DPPH assay was higher than potato and banana peels. When optimal solvent to solid ratio, time and temperature were represented in a scatter 3D plot with TPC as weights for bubble size (see Supplementary Information, Figure S3), SPP was also placed next to potato, forming a group with tea fruit and bambangan peels. Thus, the aqueous extraction of phenols from SPP had a high valorisation potential within agro peels.
Conclusions
Extraction modelling and optimization of phenolic compounds measured by Folin–Ciocalteau assay with ABTS and DPPH antiradical activities from SPP were performed by RSM and ANN based on a CCD experimental plan. Temperature and solvent solid ratio presented a positive impact as both main factor and in interaction for all responses. Time was only significant for ABTS assay and had a negative influence both as main factor and in interaction with other independent variables. RSM and ANN models presented the same optimal extraction conditions by maximization of the desirability function. The optimal settings were a solvent to solid ratio of 60 mL g−1, time of 30 min and temperature of 75 °C with experimental values of 11.87 ± 0.69 mg GAE g−1 DM, 12.91 ± 0.42 mg TE g−1 DM and 46.35 ± 2.71 mg TE g−1 DM for TPC, ABTS and DPPH assays, respectively. SPP optimized conditions for the aqueous extraction of phenolic compounds with antiradical activity may represent an opportunity for food processors to transform this by-product from a liability to an asset. Future research will focus on the development of food applications with potential health benefits based on SPP aqueous extracts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with ethical standard
Conflicts of interest
The authors declare that there is no conflict of interest.
References
- Amyrgialaki E, Makris DP, Mauromoustak A, Kefalas P. Optimisation of the extraction of pomegranate (Punica granatum) husk phenolics using water/ethanol solvent systems and response surface methodology. Ind Crop Prod. 2014;59:216–222. doi: 10.1016/j.indcrop.2014.05.011. [DOI] [Google Scholar]
- Anastácio A, Carvalho IS. Phenolics extraction from sweet potato peels: Key factors screening through a Placket–Burman design. Ind Crop Prod. 2013;43:99–105. doi: 10.1016/j.indcrop.2012.07.011. [DOI] [Google Scholar]
- Barnes SL, Sanders SA. Advances in functional use of sweetpotato, [Ipomoea batatas (L) Lam] Recent Pat Food Nutr Agric. 2012;4:1–7. doi: 10.2174/2212798411204020148. [DOI] [PubMed] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Bovell-Benjamin AC. Sweet potato: a review of its past, present, and future role in human nutrition. Adv Food Nutr Res. 2007;52:1–59. doi: 10.1016/S1043-4526(06)52001-7. [DOI] [PubMed] [Google Scholar]
- Casazza AA, Aliakbarian B, De Faveri D, Fiori L, Perego P. Antioxidants from winemaking wastes: a study on extraction parameters using response surface methodology. J Food Biochem. 2012;36:28–37. doi: 10.1111/j.1745-4514.2010.00511.x. [DOI] [Google Scholar]
- Cheok CY, Chin NL, Yusof YA, Talib RA, Law CL. Optimization of total phenolic content extracted from Garcinia mangostana Linn hull using response surface methodology versus artificial neural network. Ind Crop Prod. 2012;40:247–253. doi: 10.1016/j.indcrop.2012.03.019. [DOI] [Google Scholar]
- Dahmoune F, Moussi K, Remini H, Belbahi A, Aoun O, Spigno G, Madani K. Optimization of ultrasound-assisted extraction of phenolic compounds from Citrus sinensis L peels using response surface methodology. Chem Eng. 2014;37:889–894. [Google Scholar]
- Gurgel CSS, Farias SMdOC, Faria LRG, Moreira RT. Sensory analysis of sweet potato ice cream. Rev Bras Prod Agroindus. 2011;13:21–26. [Google Scholar]
- Londoño-Londoño J, Lima VRd, Lara O, Gil A, Pasa TBC, Arango GJ, Pineda JRR. Clean recovery of antioxidant flavonoids from citrus peel: optimizing an aqueous ultrasound-assisted extraction method. Food Chem. 2010;119:81–87. doi: 10.1016/j.foodchem.2009.05.075. [DOI] [Google Scholar]
- Maloney KP, Truong VD, Allen JC. Chemical optimization of protein extraction from sweet potato (Ipomoea batatas) peel. J Food Sci. 2012;77:E307–E312. doi: 10.1111/j.1750-3841.2012.02921.x. [DOI] [PubMed] [Google Scholar]
- Manpreet S, Sawraj S, Sachin D, Pankaj S, Banerjee UC. Influence of process parameters on the production of metabolites in solid-state fermentation. Malays J Microbiol. 2005;1:1–9. [Google Scholar]
- Nunes R, Anastácio A, Carvalho IS. Antioxidant and free radical scavenging activities of different plant parts from two Erica species. J Food Qual. 2012;35:307–314. doi: 10.1111/j.1745-4557.2012.00459.x. [DOI] [Google Scholar]
- Oyeleke SB, Dauda BEN, Oyewole OA, Okoliegbe IN, Ojebode T. Production of bioethanol from cassava and sweet potato peels. Adv Environ Biol. 2012;6:241–245. [Google Scholar]
- Panda V, Sonkamble M, Patil S. Wound healing activity of Ipomoea batatas tubers (sweet potato) Funct Food Health Dis. 2011;10:403–415. [Google Scholar]
- Peschel W, Sánchez-Rabaneda F, Diekmann W, Plescher A, Gartzía I, Jiménez D, Lamuela-Raventós R, Buxaderas S, Codina C. An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chem. 2006;97:137–150. doi: 10.1016/j.foodchem.2005.03.033. [DOI] [Google Scholar]
- Prakash Maran J, Manikandan S, Vigna Nivetha C, Dinesh R (2013) Ultrasound assisted extraction of bioactive compounds from Nephelium lappaceum L fruit peel using central composite face centred response surface design. Arabian J Chem. doi:10.1016/j.arabjc.2013.02.007
- Prasad KN, Hassan FA, Yang B, Kong KW, Ramanan RN, Azlan A, Ismail A. Response surface optimisation for the extraction of phenolic compounds and antioxidant capacities of underutilised Mangifera pajang Kosterm peels. Food Chem. 2011;128:1121–1127. doi: 10.1016/j.foodchem.2011.03.105. [DOI] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E1. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Saharan B, Sahu R, Sharma D. A review on biosurfactants: fermentation, current developments and perspectives. Gen Eng Biotechnol J. 2011;2011:1–14. [Google Scholar]
- Santos DT, Meireles MAA. Optimization of bioactive compounds extraction from jabuticaba (Myrciaria cauliflora) skins assisted by high pressure CO2. Innov Food Sci Emerg Technol. 2011;12:398–406. doi: 10.1016/j.ifset.2011.02.004. [DOI] [Google Scholar]
- Singh A, Sabally K, Kubow S, Donnelly DJ, Gariepy Y, Orsat V, Raghavan GS. Microwave-assisted extraction of phenolic antioxidants from potato peels. Molecules. 2011;16:2218–2232. doi: 10.3390/molecules16032218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabaraki R, Heidarizadi E, Benvidi A. Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L) peel antioxidants by response surface methodology. Sep Purif Technol. 2012;98:16–23. doi: 10.1016/j.seppur.2012.06.038. [DOI] [Google Scholar]
- Wijngaard HH, Ballay M, Brunton N. The optimisation of extraction of antioxidants from potato peel by pressurised liquids. Food Chem. 2012;133:1123–1130. doi: 10.1016/j.foodchem.2011.01.136. [DOI] [Google Scholar]
- Wireko-Manu FD, Ellis WO, Oduro I. Production of a non-alcoholic beverage from sweet potato (Ipomoea batatas L) Afr J Food Sci. 2010;4:180–183. [Google Scholar]
- Wojdylo A, Oszmianski J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–949. doi: 10.1016/j.foodchem.2007.04.038. [DOI] [Google Scholar]
- Xu P, Bao J, Gao J, Zhou T, Wang Y. Optimization of extraction of phenolic antioxidants from tea (Camellia sinensis L) fruit peel biomass using response surface methodology. BioResources. 2012;7:2431–2443. [Google Scholar]
- Zhu F, Cai YZ, Yang X, Ke J, Corke H. Anthocyanins, hydroxycinnamic acid derivatives, and antioxidant activity in roots of different chinese purple-fleshed sweetpotato genotypes. J Agric Food Chem. 2010;58:7588–7596. doi: 10.1021/jf101867t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.