Abstract
In this study native starches as ingredients (corn, rice, wheat, tapioca and potato) were characterized for microstructure, physicochemical, functional and thermal properties, in vitro digestibility and glycemic index. There was a significant variation in the granule shape and size distribution of the starches. Particle size monomodal (corn, tapioca, potato) and bimodal (rice, wheat) distribution was observed amongst the starches. The potato starch showed the biggest size granules while the rice showed the smallest. The examined properties and nutritional characteristics of starches were significantly different. Thermal properties were studied using Differential Scanning Calorimeter (DSC). DSC results showed that the transition temperatures (58.8–78.7 °C) and enthalpies of gelatinization (2.3–8.2 J/g) of the starches appeared to be greatly influenced by microstructure and chemical composition (e.g. resistant starch). Nutritional properties such as slowly digestible starch and expected glycemic index values followed the order: rice > wheat > tapioca > corn > potato. In particular, the highest resistant starch was recorded for potato starch.
Keywords: Starch, Food structure, Functional properties, Physicochemical properties, Resistant starches, Expected glycemic index
Introduction
Commercial native starches are mainly obtained from cereals, such as corn, wheat and rice in most developed countries, and from tubers or roots, such as potato and tapioca (or cassava) in countries as India, Brazil, Thailand, Indonesia, Philippines and China (Moorthy 2002). In particular corn is the main raw material for starch production, accounting for about 80 % of the world market. Native starch and its derivatives are widely used in food production and other branches of industry. The widespread application of starches as a group is due to their availability, cost, specific physico- chemical properties (Leszczyński 2004) and, recently, to their nutritional characteristics as slow digestibility (Rake et al. 2002). For nutritional purposes, starch has been in fact classified according to their rate of digestion into rapidly digestible starch (RDS), slowly digestible starch (SDS), and resistant starch (RS) (Englyst et al. 1992). Nowadays much attention is being given to SDS of starches for the development of foods low in rapidly digestible starch and hence of low glycemic index (GI). SDS is in fact considered to be beneficial for the slow and prolonged release of glucose in metabolic disorders such as diabetes or glycogen storage diseases (GSDs, in particular type I) (Bjorck and Asp 1994; Zhang and Hamaker 2009), and satiety (Lehmann and Robin 2007). The rate and extent to which starch is digested and absorbed (in vitro and in vivo hydrolysis) vary considerably depending on the structure, the conditions and the degree of food processing (i.e. cooking or baking starch under hydrating conditions), the physico-chemical characteristics of the starch (Englyst et al. 1999; Kaur et al. 2010). Progress towards correlating structural and physical properties of the starch granule to its digestibility, is almost evident (Tester et al. 2006; Dona et al. 2010). Furthermore, there is still a deficiency of information on in vitro starch digestibility including nutritionally important starch fractions (rapidly digestible, slowly digestible, and resistant starches and GI) and properties of different botanical sources. Thus, the aim of this study was to investigate the microstructure, the some physical and functional properties of commercial starches obtained from cereals (corn, rice and wheat) and from roots and tubers (tapioca and potato), with the intent to provide the baseline information for their potential use as uncooked ingredients for starchy tailored foods. Moreover, the in vitro digestibility and expected GI of selected starches were evaluated.
Materials and methods
Materials
Commercial starches from corn (Giglio 2TBS, Roquette Italia S.p.A., Italy), rice (Le Farine Magiche Lo Conte, IPAFOOD s.r.l., Italy), wheat (Frumina Paneangeli, Cameo S.p.A., Italy), tapioca (Novation® 3300, Ingredion™, United Kingdom) and potato (Roquette Italia S.p.A., Italy) were provided by the CAISIAL—Centre for Food Innovation and Development in the Food Industry, University of Naples Federico II, Italy.
The main nutritional characteristics (%w/w) indicated by the producers can be reassumed as follows: corn starch—proteins 0.3, fiber 0, fat <0.1, minerals <0.1; wheat starch—proteins 0.35, fiber 0, fat 0.1, minerals <0.1; rice starch—proteins 1.0, fiber 0, fat <0.1, minerals 0.6; tapioca starch: proteins <0.5, fiber <1.0, fat <0.15, minerals <0.15; potato starch—proteins 0, fiber 0, fat <0.1, minerals 0.19.
Scanning Electron Microscopy analysis
Starches were dried at the critical point and coated with gold particles in an automated critical point drier (model SCD 050, Leica Vienna). Microstructure of starches was examined by means of Scanning Electron Microscopy (SEM) (LEO EVO 40, Zeiss, Germany) with a 20 kV acceleration voltage and a magnification of 4000×.
Particle size analysis
Particle size distributions of the starches were measured by light scattering (Mastersizer 3000, Malvern Instruments, UK) in deionized water. The measurement range of the equipment was 0.01–3500 µm. The Fraunhofer diffraction model, assuming a standardized spherical shape, was used to analyse all samples. The results obtained were diameters of equivalent spheres expressed in volume. Each average value represents the mean of 3–7 independent measurements.
Physicochemical properties
The moisture content of each sample was determined by the AACC method (number 44-15.02, 1999). Three samples, weighing approximately 3 g, were dried for 24 h at 105 °C. Samples were removed from the oven and immediately placed in a desiccator prior to weighing after cooling and within 30 min. The dried samples weight was subtracted to the respective initial weight. The results were calculated as percentage of water per sample weight (%).
The pH of starches was measured by using a digital pHmeter (MP220, Mettler, Toledo) according to the AACC method (number 02-52.01, 1999).
Functional properties
The solubility of starches was determined at 20 and 70 °C by the method of Mishra and Rai (2006).
Water holding capacity (WHC) was determined at 20 °C by the method of Xu et al. (2013). WHC was calculated as:
Gelatinization thermal properties
The gelatinization properties of the starches were assessed by means of a Differential Scanning Calorimeter (DSC) (Q200, TA Instruments, Milan, Italy). Samples of approximately 6 mg were hydrated in the Tzero aluminium hermetic pans at about 70 % moisture. The pan was closed with a lid and weighed. All samples were heated from 30 to 100 °C at 10 °C min−1 using an empty pan as the reference. The gelatinisation properties, as enthalpy of gelatinization (ΔH), onset (To) and peak (Tp) temperatures, were measured. In addition, Peak Height Index (PHI) was calculated as described by Krueger et al. (1987):
| 1 |
Average values of four measurements were calculated for each sample.
In vitro starch digestibility and expected glycemic index
Measurement of resistant starch (RS) and non- resistant starch (solubilised, Non- RS) were determined using an enzymatic assay kit (Resistant Starch Assay Kit, Megazyme International Ireland) by AACC method (number 32-40.01, 2009). The total starch (TS) was expressed as the sum of RS and Non- RS. All these results were expressed as percentage weight/weight on dry basis.
In vitro kinetic of starch digestion was determined using AACC (2000) method 32-40.01 with minor modifications. Each starch sample (25 ± 0.1 mg) was incubated with pancreatic α-amylase (0.1 mg) and amyloglucosidase (3U) in 1 mL of 100 mM sodium maleate buffer (pH 6.0) in a shaking water bath (200 strokes/min, horizontal agitation) at 37 °C for 30, 60, 90, 120, 150 and 180 min, so six incubation times in duplicate were employed for each starch. The enzymatic mixture added to each tube was set to 34 ± 3 °C. After incubation at each time, 1 mL of ethanol (99 % v/v) was added to inactivate the enzymes, prior to centrifugation at 3500 rpm for 10 min. The supernatant was carefully decanted and the pellet was re-suspended with 2 mL of aqueous ethanol (50 % v/v) and centrifuged again at 3500 rpm for 10 min. This ethanol washing was repeated one more time. The three supernatant solutions were combined together and the volume was adjusted to 25 mL with 100 mM sodium acetate buffer (pH 4.5). Aliquots (0.1 mL) of each solutions (in duplicate) were incubated with 10 μL of dilute amyloglucosidase solution (300 U/mL) in 100 mM sodium maleate buffer (pH 6.0) to hydrolyse the digested starch into glucose after 20 min at 50 °C. The glucose content of the supernatant was measured using a glucose oxidase/peroxidase (GODOP) kit. The rate of starch digestion was expressed as the percentage of TS hydrolysed at each time (30, 60, 90, 120, 150 and 180 min).
Rapidly digestible starch (RDS) and slowly digestible starch (SDS) were measured after incubation for 30 min and a further 120 min, respectively.
The digestion kinetics were described by means of a non- linear model following the equation found by Goñi et al. (1997):
| 2 |
where C was the hydrolysis degree at each time, C∞ was the maximum hydrolysis extent and k was the kinetic constant. The hydrolysis index (HI) was calculated as the relation (as percentage) between the area under the hydrolysis curve (AUC, 0–180 min) of each sample and the AUC of white bread as reference food. Previous research has shown HI to be a good predictor of glycemic response (Goni et al. 1997). Lastly, expected glycemic index (eGI) was calculated using the equation proposed by Goñi et al. (1997):
| 3 |
Statistical analysis
All experimental results are reported as means and standard deviation of at least three independent experiments. One way ANOVA with Duncan’s multiple comparison test at the 95 % confidence level (P ≤ 0.05) were performed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) on all experimental data.
Results and discussion
Microstructural and particle size analyses
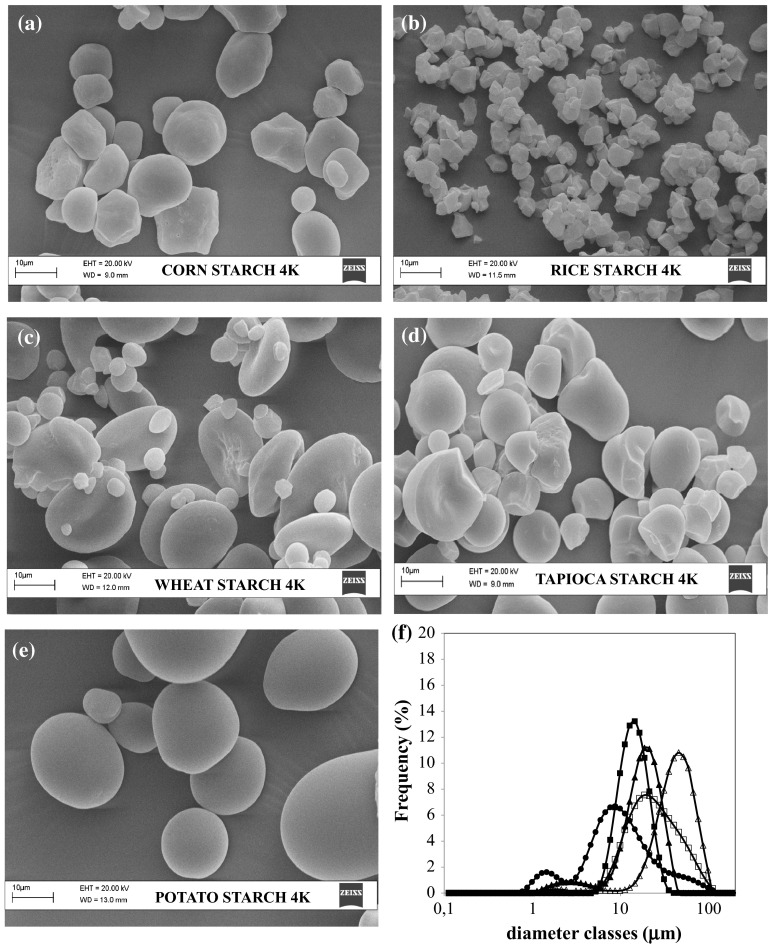
In order to study differences among starches at microstructural level, the characterisation of the microstructure by means of SEM and of light scattering was performed. The SEM images and particle size distribution of corn, rice, wheat, tapioca and potato starches are shown in Fig. 1.
Fig. 1.
SEM images of starch granules at magnification ×4000 of: a corn, b rice, c wheat, d tapioca, e potato and f Particle size distribution of starches: (filled square) corn, (filled circle) rice, (filled triangle) wheat, (open square) tapioca and (open triangle) potato
The shape and size of starch granules differed amongst the different starches. Microstructural results of corn starch (Fig. 1a) showed polyhedral, round granules with small (~5 μm) and large (~18 μm) granules. Rice starch (Fig. 1b) presented characteristic clusters of very small granules, with an angular and polygonal shape and well- defined edges; the size varied in a limited range (~3 to 8 μm). Wheat starch (Fig. 1c) contained small (<10 μm, B- type) as well as large (>10 μm, A- type) granules of round, spherical or polygonal shapes. Tapioca starch (Fig. 1d) seemed similar in size to corn starch, as also observed by Mishra and Rai (2006), with small (~7 μm) and large (~21 μm) elements; the shape was spherical, with some roundish or truncated granules.
Potato starch (Fig. 1e) showed large oval with flattened and ellipsoid granules, in the range between 15 and 50 μm. The surface analysis revealed a slight roughness with the presence of holes and cracks for corn starch; sufficiently smooth surface with the presence of some fractures for rice starch; considerable roughness with the presence of holes and protuberances for wheat starch; little wrinkled granules and often characterized by the presence of holes and small grooves for tapioca starch; a very smooth surface for potato starch.
The size distributions of starch granules (Fig. 1f) were monomodal for corn, tapioca and potato starches and bimodal for rice and wheat. In particular, the granule size distribution of corn, tapioca and potato starches displayed only one peak around 15, 20 and 50 µm, respectively. Wheat starch instead exhibited a bimodal distribution of small (1–2.8 µm) and large (19 µm) granules, likely due to small B granules and large A granules, respectively, while the bimodal distribution of rice samples showed a first population around 1.5 µm diameter and a second population around 10 µm size, due to the small granules clustered together to minimize the surface area. Among all the starches, the potato starch showed the biggest size granules while the rice showed the smallest. These observations agreed with Lindeboom et al. (2004) results of the distribution of starch granule size in different biological sources. The differences in granular size and proportion of starch granules were related to the botanical source from which the starch was isolated and even it could be associated with differences in genotypes as reported by Shevkani et al. (2011) that studied the granule size distribution of starches from Indian wheat lines.
Chemical composition and properties
The resistant (RS) and non- resistant starch (non-RS) compositions of starches were analysed and compared with corn (Table 1). ANOVA indicates that significant differences (P < 0.05) existed for different fractions of starches, RS (w/w d. b.) and non-RS (w/w d. b.) for each of the samples tested. As shown in Table 1, potato starch contained the highest RS content (69.6 %) followed by tapioca (1.8 %), corn (1.4 %), wheat (1.1 %) and rice (0.3 %). The differences of structural characteristics of different starches may account for the differences of RS. RS is considered a dietary fibre because of its indigestibility by body enzymes. Raw potato starch is an enzyme resistant starch which is in fact associated with the large granule size (Fig. 1e), B-type cristallinity, as well as different molecular weight and weight distribution (Liu et al. 2006), as compared to cereals and other starches. The low RS content of cereal samples was in line with the expected results from their polymorph starch type: cereal starches are A- type starches, always having a low RS fraction of total starch, while most of the tuber and root starches exhibit the B- type diffractometric pattern; tapioca can show A, C or a mixed pattern (Moorthy 2004; Themeier et al. 2005). Therefore, RS results appeared in agreement with dimensional characteristics of samples (Fig. 1a–e). The granule size distribution and shape are considered important for the functional and nutritional properties of the starches (Tester et al. 2006). When developing slowly digestible or resistant starches for human nutrition, the morphology and the particle size of the starch granules, as the relationship between surface area and starch volume, has to be evaluated. Although the method could be not suitable for samples with less than 2 %w/w RS, these values agreed with the reported results by McCleary et al. (2002), McCleary and Monaghan (2002) for corn and potato starches, by Themeier et al. (2005) for corn and wheat samples and by Liu et al. (2006) for potato one.
Table 1.
Nutritional fractions and properties of starches, expressed as mean ± SD
| Starch source | RS | Non RS | pH | Moisture content | Solubility (%) | WHC | |
|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | 20 °C | 70 °C | (%) | ||
| Corn | 1.44b ± 0.03 | 91.11b ± 1.2 | 6.43b ± 0.1 | 10.09b ± 0.8 | 0.50a ± 0.2 | 3.81a ± 0.2 | 69.24a ± 1.2 |
| Rice | 0.31a ± 0.05 | 92.05c ± 0.7 | 6.83d ± 0.0 | 10.03b ± 0.2 | 0.75a ± 0.1 | 3.53a ± 0.4 | 99.99d ± 1.3 |
| Wheat | 1.07a ± 0.07 | 90.07b ± 1.2 | 6.62c ± 0.3 | 12.68c ± 0.9 | 1.28b ± 0.4 | 3.27a ± 0.1 | 82.75b ± 1.5 |
| Tapioca | 1.77b ± 0.05 | 91.61b ± 1.1 | 4.86a ± 0.1 | 9.29a ± 0.8 | 2.43c ± 0.2 | 16.46c ± 0.1 | 91.42c ± 1.2 |
| Potato | 69.59c ± 1.77 | 22.21a ± 1.3 | 7.26e ± 0.5 | 18.06d ± 1.4 | 1.18b ± 0.1 | 11.21b ± 0.9 | 83.60b ± 1.6 |
a–eMeans within the same column with different letters are significantly different (P < 0.05; Duncan test)
The levels of non-RS varied in a limited range for cereal starches and tapioca starch (90.1–92.1 %) whilst the Non-RS in potato starch was only 22.2 %. These results showed similar behaviour to that reported for RS results, as expected.
The pH and moisture content are shown in Table 1. The pH analysis of starches showed significant differences (P < 0.05) among samples, discriminating into five statistical groups (P < 0.05). pH is an important property in starch industrial applications, being used generally to indicate the acidic or alkaline properties of liquid media (Ashogbon and Akintayo 2012). The pH of the potato starch (7.26) was observed to be significantly the highest (P < 0.05), this shows that it has low acid content, while tapioca starch (4.86) was the lowest (P < 0.05). Significant differences (P < 0.05) were detected for moisture content of starches. The moisture content ranged between 9.3 % (tapioca starch) to 18.1 % (potato starch). Rice starch showed slightly lower moisture content (10.0 %) than corn starch (10.1 %). The trend of moisture content results was similar with pH results. Overall, the average pH and moisture content results of starches can be considered consistent with previous literature (Muazu et al. 2011; Ashogbon and Akintayo 2012).
The solubility at 20 °C (cold) and at 70 °C (hot) and water holding capacity (WHC) are shown in Table 1. The solubility of starches showed significant differences (P ≤ 0.05) among samples. It was observed that all starches exhibited very low solubility at 20 °C and formed only a temporary suspension when stirred in water: this phenomenon can depend on the starch source (presence of hydroxyl groups in the starch molecules able to form hydrogen bonds with the aqueous medium) and the granule structure (semi-crystalline at low temperatures). Tapioca starch showed the highest cold solubility value compared to the other starches (P < 0.05), in dependence of botanical properties of the starch molecules such as amylose/amilopectin ratio, chain length and molecular weight distribution, degree/length of branching and conformation (Moorthy 2004). The solubility results for all starches showed that their solubility increases with increase in temperature. The order of hot solubility was tapioca > potato > corn, rice, wheat (P < 0.05). The results derived from the solubility at 70 °C confirmed the trend of the cold solubility values, obviously with higher values. The phenomenon is due to the fact that the crystalline structure is disrupted when starch molecules are heated in excess water, and water molecules become linked by hydrogen bonding to the exposed hydroxyl groups of amylose and amylopectin (Singh et al. 2003). Cereal starches (corn, rice and wheat) did not differ significantly each other (P < 0.05) and exhibited a lower solubility than the tuber and root starches (potato and tapioca) at 70 °C, which differed significantly each other (11.2 and 16.5 %, respectively). This behaviour could be due firstly to the fact that cereal starches have a more compact structure and different crystallinity than tuber starches; secondarily the temperature used (70 °C) was higher than the gelatinization temperature of tubers but lower than the required for cereal starches(Mishra and Rai 2006). The differences in the physical state of the amylose component in native wheat and potato starches observed in the past by Kulp and Lorenz (1981) could justify the significant difference (P < 0.05) among the values of their hot solubilities: in wheat starch amylose is present in a helical form whilst it is in an amorphous form in potato granule. Singh et al. (2003) also reported that the higher value of potato starch than corn, rice and wheat starch could be probably due to a higher content of phosphate groups on amylopectin, responsible of a repulsion effect and of a consequent hydration increase by weakening the extent of bonding within the crystalline domain.
In order to use uncooked starches as ingredient for novel food, tapioca starch improved techno- functional properties in terms of cold solubility; therefore it could be used for shaken liquid or semi- liquid products.
Results of WHC ranged from 69.2 % for corn starch to 100 % for rice starch and discriminated starches into four statistical groups (P < 0.05). WHC is a room temperature measurement and does not indicate the behaviour of the starches when heated, but is an important parameter for food processing applications, such as texture of semi- liquid products, custards and doughs, because this is supposed to imbibe water thereby attaining body thickening and viscosity. The differences (P < 0.05) in WHC (Table 1) of the starches among the different species, similarly among the different cultivars, could be attributed to the starch structure (differences in amylose/amylopectin content and size) and extent of interactions between starch chains and water. It can be deduced from WHC results that rice starch has more proportion of amorphous regions within its small granules than corn starch. Results appeared in agreement with those reported in literature (Mishra and Rai 2006; Olayemi et al. 2008; Xu et al. 2013).
Gelatinization thermal properties
Starch gelatinization is the collapse (disruption) of molecular orders within the starch granule manifested in irreversible changes in properties such as granular swelling, native crystalline melting, loss of birefringence, and starch solubilization (Atwell et al. 1988). It is an important factor contributing to starch functionality and is widely exploited in the food industry (Ratnayake and Jackson 2007). The thermal properties of starches were studied by means of DSC, and related DSC characteristics (∆H—transition enthalpy, To—transition onset temperature, Tp—transition peak temperature and PHI—Peak height index) are summarized in Table 2.
Table 2.
Gelatinization thermal parameters of the analyzed starches, expressed as mean ± SD
| Starch source | To (°C) | Tp (°C) | ∆H (J/g) | PHI |
|---|---|---|---|---|
| Corn | 67.15d ± 0.9 | 73.26d ± 0.8 | 3.75d ± 0.2 | 0.64c ± 0.2 |
| Rice | 60.28b ± 0.9 | 67.85b ± 0.6 | 2.29a ± 0.1 | 0.17a ± 0.0 |
| Wheat | 58.77a ± 0.7 | 65.41a ± 0.4 | 2.64b ± 0.1 | 0.41a,b ± 0.1 |
| Tapioca | 65.14c ± 0.1 | 72.14c,d ± 0.7 | 3.11c ± 0.0 | 0.45b,c ± 0.0 |
| Potato | 69.18e ± 0.6 | 78.71e ± 0.4 | 8.23e ± 0.4 | 0.86d ± 0.0 |
To, onset temperature; Tp, peak temperature; ΔH, transition enthalpy; PHI, peak height index
a–eMeans within the same column with different letters are significantly different (P < 0.05; Duncan test)
A single endothermic transition, corresponded mainly to the gelatinization transition of the starch, was observed in the DSC profiles of all tested starches (data not shown) as the high level of moisture (70 %) at which samples were scanned. The transition temperatures (To and Tp) varied significantly (P < 0.05) among the examined starches. These fall within the range of gelatinization temperatures commonly observed for starches: To from 58.8 to 69.2 °C and Tp from 65.4 to 78.7 °C (Table 2). In particular, wheat samples exhibited the lowest To (58.8 °C) and Tp (65.4 °C) values as compared to other starches. Differences in the transition temperature range were also highlighted by the values of the PHI, which indicate the shape and degree of symmetry of endotherms. A low PHI indicates a broad transition range and suggests structural heterogeneity in starch granules. The PHI values of potato starch were the highest (0.9), followed by corn and tapioca, wheat and finally by rice, indicative of a narrow transition range and of a resistance to gelatinization process. According to Kruerger et al. (1987), gelatinization of starch is a cooperative process, such that structural relations between amorphous and crystalline regions within the starch granules are responsible for the sharpness of thermal transition and the temperature at which it occurs. Moreover, the gelatinization temperature provides an indication of the minimum temperature required to cook a given sample and it has implications on the stability of other components in a formula and also indicates energy cost. Thus, potato starch, with the major To, Tp and PHI values (P < 0.05), will require the most energy for it to be cooked than other starches. Tapioca and corn starches showed a similar thermal behavior about Tp and PHI parameters, while the transition temperatures of rice and wheat starches were lower as compared to others, and the endothermic peak of rice was the broadest (PHI = 0.2).
The ∆H values obtained in this study (Table 2) ranged between 2.3 to 8.2 J/g and discriminated significantly starches into five statistical groups (P < 0.05). The order of enthalpy was potato > corn > tapioca > wheat > rice (P < 0.05). The ∆H primarily reflects the loss of molecular (double-helical) order within the starch granules and it refers to the energy required to disrupt the native structure of the starch granules (Cooke and Gidley 1992). In fact starches with higher ΔH show higher crystallinity and vice versa (Singh et al. 2010). Furthermore the highest ∆H of potato starch suggests that the starch granules that melt during gelatinization are resistant and strongly associated within its native structure, in agreement with granule size results of potato (Fig. 1) and RS values (Table 1). Likewise the low value of transition temperatures and the lowest ∆H of rice starch may be attributed to the reduced thermal granule stability of this cereal characterized by small size granules (Fig. 1f) and the lowest RS content (Table 1). Tapioca showed a middle thermal behavior that may be due to some degree of starch modification, which occurred during processing and storage. ∆H values obtained for tapioca starch were in fact less than values reported for tapioca by Sun and Yoo (2015) (15.4 J/g) and less than corn starch results (3.8 J/g). From these observations, it seems that the higher RS content of starch, the higher the ∆H of the sample.
In vitro starch digestibility and expected glycemic index
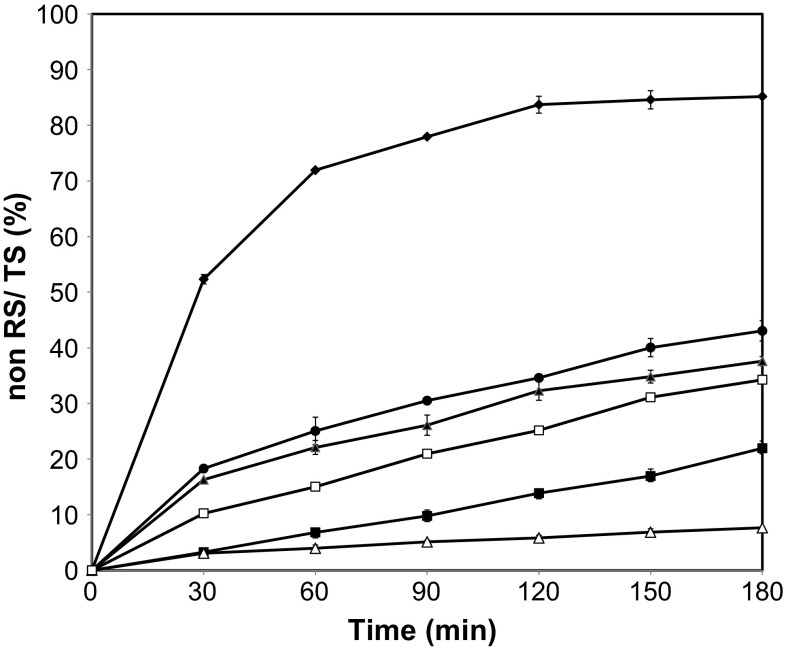
The effects of botanical origin on in vitro starch digestion were investigated by measuring the released glucose content during starch digestion. Figure 2 shows the hydrolysis curves of starches that were compared with those of the reference food (white bread).The hydrolysis kinetics of starches showed similar hydrolysis trend, where the percentage of digested starch increased gradually. As expected, all of the starches selected in this study were found to depress starch hydrolysis by digestive enzymes, compared with the control bread. The rate and extent of starch digestion were the highest in the rice, followed by wheat, tapioca, corn and potato (Fig. 2).
Fig. 2.
Total starch hydrolysis rate of (filled diamond) white bread and of starches: (filled square) corn, (filled circle) rice, (filled triangle) wheat, (open square) tapioca, (open triangle) potato
Starch and starchy food can be classified according to their digestibility. Rapidly digestible starch (RDS) and slowly digestible starch (SDS) of different starches are shown in Table 3. RDS is rapidly and completely digested in the small intestine and is associated with more rapid elevation of postprandial plasma glucose whereas SDS is more slowly digested in the small intestine and is generally the most desirable form of dietary starch (Jenkins et al. 1981). The RDS and SDS fractions of starches differed significantly (P < 0.05) with botanical source. The RDS content was in the range of 2.8–16.9 %, while the SDS contents ranged from 5.4 ± 0.4 % of potato starch to 12.8 ± 0.6 % of rice one. Rice starch showed significantly higher RDS, SDS (Table 3) in comparison to other starches. Rice had the highest digestibility because of its small granules, as described in microstructural and particle results (Fig. 1b, f). In fact the digestibility properties are directly related to the particle size, the smaller the particle the higher the digestibility. Since enzyme hydrolysis took place first on the surface of starch granules, the particle size of the starch and the surface area to starch ratio play in fact an important role for the digestibility: a smaller granule size as rice (Fig. 1b, f) shows higher enzymatic susceptibility, while a large, compact and smooth surface as for example potato granules (Fig. 1e, f) can explain significantly the lowest results of nutritional fractions: 2.8 % (RDS) and 5.4 % (SDS) and the resistance of potato granules against enzymatic digestion. The SDS present in raw starch agree with the ∆H results (Table 2) too. Samples with the lowest SDS contents, as potato and corn, had in fact higher enthalpy values than other starches. The relationship between thermal properties and digestibility of starches seems concrete for all the selected starches in this study.
Table 3.
Effect of botanical origin on starch nutritional fraction (RDS, rapidly digestible starch, and SDS, slowly digestible starch) and expected glycemic index (eGI) of the analyzed starches
| Starch source | RDS (%) | SDS (%) | eGI |
|---|---|---|---|
| Corn | 2.95a ± 0.9 | 12.84b ± 0.8 | 47.9b ± 0.1 |
| Rice | 16.90d ± 0.9 | 31.95e ± 0.6 | 61.3e ± 0.2 |
| Wheat | 14.85c ± 0.7 | 29.41d ± 0.4 | 58.8d ± 0.1 |
| Tapioca | 9.55b ± 0.1 | 23.49c ± 0.7 | 55.2c ± 0.0 |
| Potato | 2.83a ± 0.6 | 5.35a ± 0.4 | 43.5a ± 0.1 |
Each value is expressed as mean ± SD
a–eMeans within the same column with different letters are significantly different (P < 0.05; Duncan test)
The expected glycemic index (eGI) for different starches are shown in Table 3. eGI results differed significantly varying between 61.3 of rice to 43.5 % of potato (Table 3). The order of eGI starch was rice > wheat > tapioca > corn > potato. The eGI was affected by the amount of RS (Table 1) and RDS present. In particular, the RS content had an inverse relationship with eGI, while RDS had positive and significant influence on eGI. Higher percentages of RDS in starch generally relate to a higher degree of starch digestion and consequently with a higher degree of eGI (Englyst et al. 1999; Rosin et al. 2002). eGI is related to nutritional quality of food and a product with a low GI is preferable not only in individuals with diabetes, but also in healthy individuals (Björck and Asp 1994). Considering the in vitro digestibility results of tapioca, it might be a potential alternative to corn starch in the formulation of products for diabetics and weight management and could lead to the formulation of novel foods characterized by the slow release of glucose, that is to say low glycemic index and prevention of fasting hypoglycemia. Moreover, the bland taste of the tapioca could represent an advantage over the cereal starches as uncooked ingredient because of the absence of the specific cereal flavour due to the lipids present in these starches (Moorthy 2004).
Conclusion
Significant differences in microstructure, properties, in vitro digestibility and expected glycemic index were observed among starches of different botanical sources (cereal, tubers and roots). Structural characterization of starches by means of SEM and particle size analyses showed characteristic differences concerning shape and size of granules attributed to the botanical origin, which determines morphology and crystalline organization. The examined (physicochemical, functional, thermal and nutritional) properties of starches were significantly different and correlated to the internal structure of starches. In particular, gelatinization thermal properties might be an important parameter for the indication of starch digestibility in processed foods. The in vitro digestibility and expected glycemic index of potato, corn, tapioca were significantly lower than wheat and rice. Among the studied starches, tapioca was well comparable to corn starch in physicochemical and thermal properties and in nutritional potentials (resistant starch, slowly digestible starch and expected glycemic index), therefore it could serve as an alternative to uncooked corn starch.
The information gathered through this investigation may help the food industry to choose suitable starches for the development of novel foods or native starch blends with altered digestibility (slow) and tailored functional properties.
Acknowledgments
This work was supported by a Campania Region fund (Grant Number/CUP: B25B09000080007) within the program “POR CAMPANIA FSE 2007/2013”—Project CARINA (Safety sustainability and competitiveness of the agro-food production in Campania). A Mackie was supported through BBSRC research Grant BB/J004545/1.
References
- AACC International Approved Methods of Analysis (1999) Method 44-15.02. Moisture—Air-Oven Methods. Method 02-52.01. Hydrogen- Ion Activity (pH)—Electrometric Method. St. Paul, MN, USA
- AACC International Approved Methods of Analysis (2009) Method 32-40.01. St. Paul, MN, USA
- Ashogbon AO, Akintayo ET. Morphological, functional and pasting properties of starches separated from rice cultivars grown in Nigeria. Int Food Res J. 2012;19(2):665–671. [Google Scholar]
- Atwell WA, Hood LF, Lineback DR, Varriano-Marston E, Zobel HF. The terminology and methodology associated with basic starch phenomena. Cereal Foods World. 1988;33:306–311. [Google Scholar]
- Bjorck I, Asp NG. Controlling the nutritional properties of starch in foods—a challenge to the food-industry. Trends Food Sci Technol. 1994;5:213–218. doi: 10.1016/0924-2244(94)90251-8. [DOI] [Google Scholar]
- Cooke D, Gidley MJ. Loss of crystalline and molecular order during starch gelatinization: origin of the enthalpic transition. Carbohydr Res. 1992;227:103–112. doi: 10.1016/0008-6215(92)85063-6. [DOI] [Google Scholar]
- Dona AC, Pages G, Gilbert RG, Kuchel PW. Digestion of starch: In vivo and in vitro kinetic models used to characterise oligosaccharide or glucose release. Carbohydr Polym. 2010;80:599–617. doi: 10.1016/j.carbpol.2010.01.002. [DOI] [Google Scholar]
- Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr. 1992;46:S33–S50. [PubMed] [Google Scholar]
- Englyst KN, Englyst HN, Hudson GJ, Cole TJ, Cummings JH. Rapidly available glucose in foods: an in vitro measurement that reflects the glycemic response. Am J Clin Nutr. 1999;69(3):448–454. doi: 10.1093/ajcn/69.3.448. [DOI] [PubMed] [Google Scholar]
- Goni I, Garcia-Alonso A, Saura-Calixto F. A starch hydrolysis procedure to estimate glycemic index. Nutr Res. 1997;17:427–437. doi: 10.1016/S0271-5317(97)00010-9. [DOI] [Google Scholar]
- Jenkins DJA, Wolever TMS, Taylor RH, Barker HM, Fielden H, Baldwin JM, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- Kaur M, Sandhu KS, Lim ST. Microstructure, physicochemical properties and in vitro digestibility of starches from different Indian lentil (Lens culinaris) cultivars. Carbohydr Polym. 2010;79(2):349–355. doi: 10.1016/j.carbpol.2009.08.017. [DOI] [Google Scholar]
- Krueger BR, Knutson CA, Inglett GE, Walker CE. A differential scanning calorimetry study on the effect of annealing on gelatinization behavior of corn starch. J Food Sci. 1987;52:715–718. doi: 10.1111/j.1365-2621.1987.tb06709.x. [DOI] [Google Scholar]
- Kulp K, Lorenz K. Heat-moisture treatment of starches. I. Physicochemical properties. Cereal Chem. 1981;58(1):46–48. [Google Scholar]
- Lehmann U, Robin F. Slowly digestible starch – its structure and health implications: a review. Trends Food Sci Technol. 2007;18(7):346–355. doi: 10.1016/j.tifs.2007.02.009. [DOI] [Google Scholar]
- Leszczyński W. Resistant starch–classification, structure, production. Pol J Food Nutr Sci. 2004;13(54):37–50. [Google Scholar]
- Lindeboom N, Chang PR, Tyler RT. Analytical, biochemical and physicochemical aspects of starch granule size, with emphasis on small granule starches: a review. Starch/Stärke. 2004;56:89–99. doi: 10.1002/star.200300218. [DOI] [Google Scholar]
- Liu Q, Donner E, Yin Y, Huang RL, Fan MZ. The physicochemical properties and in vitro digestibility of selected cereals, tubers and legumes grown in China. Food Chem. 2006;99(3):470–477. doi: 10.1016/j.foodchem.2005.08.008. [DOI] [Google Scholar]
- McCleary BV, Monaghan DA. Measurement of resistant starch. J AOAC Int. 2002;85(3):665–675. [PubMed] [Google Scholar]
- McCleary BV, McNally M, Rossiter P. Measurement of resistant starch by enzymatic digestion in starch and selected plant materials: collaborative study. J AOAC Int. 2002;85(5):1103–1111. [PubMed] [Google Scholar]
- Mishra S, Rai T. Morphology and functional properties of corn, potato and tapioca starches. Food Hydrocoll. 2006;20(5):557–566. doi: 10.1016/j.foodhyd.2005.01.001. [DOI] [Google Scholar]
- Moorthy SN. Physicochemical and functional properties of tropical tuber starches: a review. Starch/Stärke. 2002;54(12):559–592. doi: 10.1002/1521-379X(200212)54:12<559::AID-STAR2222559>3.0.CO;2-F. [DOI] [Google Scholar]
- Moorthy W. Starch in food: structure, functions and applications. Boca Raton: Eliasson; 2004. [Google Scholar]
- Muazu J, Musa H, Isah AB, Bhatia PG, Tom GM. Extraction and characterization of Kaffir potato starch: a potential source of pharmaceutical raw material. J Nat Prod Plant Resour. 2011;1(2):41–49. [Google Scholar]
- Olayemi OJ, Oyi AR, Allagh TS. Comparative evaluation of maize, rice and wheat starch powders as pharmaceutical excipients. Niger J Pharm Sci. 2008;7(1):131–138. [Google Scholar]
- Rake JP, Visser G, Labrune P, Leonard JV, Ullrich K, Smit GPA. Guidelines for management of glycogen storage disease type I-European Study on Glycogen Storage Disease Type I (ESGSD I) Eur J Pediatr. 2002;161(1):112–119. doi: 10.1007/BF02680007. [DOI] [PubMed] [Google Scholar]
- Ratnayake WS, Jackson DS. A new insight into the gelatinization process of native starches. Carbohydr Polym. 2007;67:511–529. doi: 10.1016/j.carbpol.2006.06.025. [DOI] [Google Scholar]
- Rosin PM, Lajolo FM, Menezes EW. Measurement and characterization of dietary starches. J Food Compos Anal. 2002;15:367–377. doi: 10.1006/jfca.2002.1084. [DOI] [Google Scholar]
- Shevkani K, Singh N, Singh S, Ahlawat AK, Singh AM. Relationship between physicochemical and rheological properties of starches from Indian wheat lines. Int J Food Sci Technol. 2011;46:2584–2590. doi: 10.1111/j.1365-2621.2011.02787.x. [DOI] [Google Scholar]
- Singh N, Singh J, Kaur L, Singh Sodhi N, Singh Gill B. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003;81(2):219–231. doi: 10.1016/S0308-8146(02)00416-8. [DOI] [Google Scholar]
- Singh S, Singh N, Isono N, Noda T. Relationship of granule size distribution and amylopectin structure with pasting, thermal, and retrogradation properties in wheat starch. J Agric Food Chem. 2010;58:1180–1188. doi: 10.1021/jf902753f. [DOI] [PubMed] [Google Scholar]
- Sun D, Yoo B. Effect of tapioca starch addition on rheological, thermal, and gelling properties of rice starch. LWT-Food Sci Technol. 2015;64:205–211. doi: 10.1016/j.lwt.2015.05.062. [DOI] [Google Scholar]
- Tester RF, Qi X, Karkalas J. Hydrolysis of native starches with amylases. Anim Feed Sci Technol. 2006;130:39–54. doi: 10.1016/j.anifeedsci.2006.01.016. [DOI] [Google Scholar]
- Themeier H, Hollmann J, Neese U, Lindhauer MG. Structural and morphological factors influencing the quantification of resistant starch II in starches of different botanical origin. Carbohydr Polym. 2005;61(1):72–79. doi: 10.1016/j.carbpol.2005.02.017. [DOI] [Google Scholar]
- Xu Y, Grizzard C, Sismour EN, Bhardwaj HL, Li Z. Resistant starch content, molecular structure and physicochemical properties of starches in Virginia-grown corn, potato and mung bean. J Cereals Oilseeds. 2013;4(1):10–18. doi: 10.5897/JCO2012.0097. [DOI] [Google Scholar]
- Zhang GY, Hamaker BR. Slowly digestible starch: concept, mechanism, and proposed extended glycemic index. Crit Rev Food Sci Nutr. 2009;49:852–867. doi: 10.1080/10408390903372466. [DOI] [PubMed] [Google Scholar]