Abstract
The processed drinks produced from grass of three varieties of rice (Jasmine, Sukhothai 1 and Sukhothai 2) and one each from wheat and barley grasses were analyzed for chlorophyll and total phenolic contents, and antioxidant activities [ABTS radical cation decolorisation assay and ferric reducing/antioxidative power (FRAP) assay]. The consumer acceptance of all the drinks was also evaluated. One serving size (200 mL) of these contained 82–958 μg of chlorophyll, 5.60–26.14 mg Gallic acid equivalent for total phenolic content and their antioxidant activities, ABTS and FRAP values were 17.88–35.18 mg Vitamin C equivalent and 5.66–23.70 mg FeSO4 equivalent, respectively. The overall consumer acceptability of drinks was significantly correlated to their aroma intensity and consumer preference on aroma and flavor. Jasmine and Sukhothai 1 rice grass drinks were most preferred, however, one subgroup preferred grass drink from Sukhothai 1 rice, while the second subgroup preferred the drinks from Sukhothai 2 rice and Jasmine rice. Wheat and barley grass drinks were not preferred by both subgroups.
Keywords: Grass drinks, Chlorophyll, Total phenolic content, Antioxidant activities, Internal preference map, Cluster analysis
Introduction
Functional beverages are the fastest growing segment in the functional food category (Gruenwald 2009). In Thailand, functional drink market grew from Bt1.8 billion in 2009 to Bt6.6 billion in 2014 (Ketnil 2014). The consumer interest in natural functional drinks, with anti-aging, energy supplying, relaxing, or beauty enhancing effects is increasing. To avoid intake of chemical substances, natural substances from plant, which are preferred than animal sources, have been increasingly used as functional botanical ingredients in beverages (Gruenwald 2009).
The cereal grasses (young leaves of grain-bearing plants), including wheat, barley, alfalfa, rye, oat, and kamut, are interesting ingredients for functional drinks. They contain substantial concentrations of phytochemicals and vitamins (Gruenwald 2009). Especially wheatgrass juice, obtained from young wheat plant was first used for promoting human health by Ann Wigmore, founder of the Hippocrates Health Institute in Boston (Wigmore 1985). It is commonly known as the “green blood” due to its high chlorophyll content (Padalia et al. 2010). Chlorophyll bears structural similarity to hemoglobin and has been found to regenerate or act as a substitution for hemoglobin in hemoglobin deficiency conditions. This might be the reason behind the utility of wheatgrass in clinical conditions like thalassemia and hemolytic anemia (Marwaha et al. 2004; Padalia et al. 2010). In addition, chlorophyll together with the vital enzymes like superoxide dismutase, the plant hormone abscisic acid or dormin, and its alkalinity play their roles in the anticancer function (Livingston 1976; Pokhrel 1999; Mates et al. 2000; Jed et al. 2005; Padalia et al. 2010).
Antioxidants in wheatgrass juice such as (pro) vitamins C, E, β-carotene and zinc are responsible for anti-allergic and anti-asthmatic treatment, while bioflavonoids account for many clinical utilities such as management of inflammatory bowel disease and as a general detoxifier. Wheatgrass juice is safe and the incidence of side effects is very low. It may cause nausea and headache if excessive quantities are consumed. Throat swelling may occur in hyper-sensitive individuals (Padalia et al. 2010). In case of barley grass, there is a report on the contents of vitamin C, total polyphenols, ferulic acid, monosaccharides and amino acids indicating that it is a valuable plant material (Paulíčková et al. 2007). For other species of cereal grasses, more research is needed to uncover additional unknown benefits (Gruenwald 2009).
Rice is an edible cereal grain eaten by more than half of the world’s population (Zronik 2006). Thailand is one of the world’s biggest rice producers, with paddy output of 19.4 million metric tons in marketing year 2014/2015, while wheat production is insignificant in Thailand due to unfavourable climate conditions, lack of seed development, and unattractive returns (USDA Foreign Agricultural Service 2015). With increasing of functional drink demand in Thailand (Ketnil 2014) and emerging of green botanicals in beverages including cereal grasses (Gruenwald 2009), processed drinks from grasses of rice, wheat and barley have been developed.
This study was aimed to evaluate some chemical properties related to health benefits, including chlorophyll content, total phenolic content and antioxidant activities (improved ABTS radical cation decolorisation assay and ferric reducing/antioxidative power (FRAP) assay), of processed grass drinks produced from three varieties of rice (Jasmine, Sukhothai 1 and Sukhothai 2), wheat and barley. Consumer acceptance of products was studied and the preference direction of this consumer group was identified by internal preference mapping and cluster analysis.
Materials and methods
Chemicals
Acetone, Folin–Ciocalteau phenol reagent, sodium bicarbonate, gallic acid, 2,2’-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), potassium persulphate, vitamin C, 2,4,6-tripyridyl-s-triazine (TPTZ), ferric chloride, glacial acetic acid, hydrochloric acid, sodium acetate, and ferrous sulphate were obtained from Sigma-Aldrich and all of them were of analytical grade.
Grass production
Seeds were washed and soaked in water for 24 h. Floating seeds were discharged. Soaked seeds were then covered by wet cheesecloth for 12–48 h to let them sprout. Sprouts were placed on the wet soil and were covered by wet cheesecloth for 3 days. After that, they were watered twice a day. Grasses of wheat and barley were harvested after 7 days, while those of rice were harvested after 20 days. Harvesting was done by cutting it about ½ inch from the soil.
Samples
Five processed drinks produced from young grasses of three rice varieties (Jasmine, Sukhothai 1 and Sukhothai 2), wheat, and barley were produced. In brief, the grasses with optimum ages were collected, extracted, and filled in 200 mL clear glass bottles before processing under the same sterilizing condition. The samples were kept at room temperature for a month before all chemical analysis and consumer acceptance test.
Chemical analysis
Chlorophyll content
Chlorophyll contents in all sample drinks were determined with some modifications of Arnon (1949). One mL of sample and 4 mL of acetone (80% acetone) were well-mixed prior to absorbance (A) measurements at 663 and 645 nm. Total chlorophyll content was reported as μg chlorophyll per 200 mL of product (one serving size) and was calculated by this equation:
Total phenolic content
The Folin–Ciocalteau micro method of Pinsirodom and Changnoi (2002) was used with some modifications. In brief, 500 µL of sample, 9.5 mL of distilled water and 0.5 mL of Folin–Ciocalteau reagent were thoroughly mixed in a test tube and incubated at room temperature for 5 min. Two mL of 10% (w/v) sodium bicarbonate was added and incubated for 10 min before reading an absorbance at 730 nm. Gallic acid solution (0–150 µg) was used to generate a standard line. Results were reported as mg Gallic equivalent per 200 mL of product (one serving size).
Improved ABTS radical cation decolorization assay
The method based on the ability of antioxidant molecules to quench the long-lived ABTS radical cation (ABTS·+) of Re et al. (1999) was modified. The ABTS·+ was produced by reacting 7 mM ABTS stock solution with 2.45 mM potassium persulphate (final concentration) and allowing the mixture to stand in the dark at room temperature for 12 h before use. The ABTS·+ solution was diluted with deionized water and 95% ethanol (1:1) to an absorbance of 0.70 (±0.02) at 734 nm. Twenty μL of sample was mixed with 3 mL of diluted ABTS·+ solution. The decrease of absorbance was measured at 1 min after mixing. Vitamin C (0–15 µg) was used as a standard, and results were reported as mg Vitamin C equivalent per 200 mL of product (one serving size).
Ferric reducing antioxidant power (FRAP) assay
The FRAP, a method for measuring total reducing power of electron donating substances, was assessed according to Benzie and Strain (1999) with some modifications. Briefly, 3 mL of working FRAP reagent (300 mM acetate buffer:20 mM FeCl3:10 mM TPTZ = 10:1:1, daily prepared) was mixed with 40 μL of sample and incubated at 37 °C for 30 min before measuring the absorbance at 593 nm. FeSO4 (0–15 µg) was used as a standard and results were reported as mg FeSO4 equivalent per 200 mL of product (one serving size).
Sensory evaluation
One hundred and sixty staffs of Maejo University, Chiang Mai, Thailand were used as a consumer sample group (118 women and 42 men at the age of 23–60). They were instructed to evaluate the aroma intensity of products and express their preference on aroma, color, flavor and overall liking of products by using 10 cm line scale (Fig. 1; Lawless and Heymann 1998).
Fig. 1.
A 10 cm line scale used for sensory evaluation
Statistical analysis
Results of chemical analysis (3 replications) were analyzed by completely randomized design, while sensory results were analyzed by randomized complete block design. Mean comparisons were done by Tukey’s HSD (honestly significant difference) test. Bivariate correlation was applied for the correlation test. Internal preference mapping and cluster analysis were used for identifying the preference direction of consumers from overall liking data. All statistical analysis was done by R version 3.2.1 (R Core Team 2015).
Results and discussion
Chemical analysis
Chlorophyll content, total phenolic content and antioxidant activities of five processed grass drinks are shown in Table 1. Their chlorophyll contents were significantly different (p < 0.05). Barley grass drink contained more chlorophyll content than grass drinks of Jasmine rice, Sukhothai 2 rice, wheat, and Sukhothai 1 rice, respectively. These chlorophyll contents, 82–958 μg/200 mL (0.06 °Brix) or 0.07–0.8% of solids, were less when compared to those of wheatgrass juice, 70% of solids, which has been used for hemoglobin deficiency treatment (Marwaha et al. 2004; Padalia et al. 2010).
Table 1.
Chlorophyll content, total phenolic content (TPC) and antioxidant activities (ABTS and FRAP) of 5 processed grass drinks (Mean ± SD)
| Grass drinks | Chlorophyll (μg/200 mL) | TPC (mg Gallic acid equivalent/200 mL) | Antioxidant activities | |
|---|---|---|---|---|
| ABTS (mg Vitamin C equivalent/200 mL) | FRAP (mg FeSO4 equivalent/200 mL) | |||
| Rice | ||||
| Jasmine | 286b ± 8 | 10.50d ± 1.06 | 35.18a ± 0.74 | 22.62a ± 0.62 |
| Sukhothai 1 | 82e ± 2 | 18.92c ± 0.38 | 25.44b ± 0.64 | 16.00b ± 0.40 |
| Sukhothai 2 | 128c ± 8 | 23.04b ± 0.46 | 34.87a ± 0.80 | 23.70a ± 0.50 |
| Wheat | 90d ± 2 | 5.60e ± 0.44 | 22.15c ± 0.54 | 10.26c ± 0.16 |
| Barley | 958a ± 4 | 26.14a ± 0.52 | 17.88d ± 0.62 | 5.66d ± 0.22 |
a,b,c,d Means with different letters in the same column are significantly different (p < 0.05)
Total phenolic contents were also significantly different (p < 0.05). Barley grass drink contained more phenolic compounds than grass drinks of three rice varieties (Sukhothai 2, Sukhothai 1 and Jasmine) and wheat, respectively. Previous studies showed that total phenolic contents of culinary plants were significantly correlated (p < 0.05) to their antioxidant activities (Wangcharoen and Morasuk 2007a, b, c), however such correlations were not observed in these five (p > 0.05). Grass drinks of Jasmine and Sukhothai 2 rice had significantly higher antioxidant activities, ABTS and FRAP values, (p < 0.05) than those of Sukhothai 1 rice, wheat, and barley, respectively. This difference between total phenolic contents and antioxidant activities may be attributed to difference in antioxidant activity in each grass type. For example, apigenin, quercitin and luteolin were reported in wheat grass (Padalia et al. 2010), while ferulic acid was reported in barley grass (Paulíčková et al. 2007), and gallic acid, catechin, rutin, isoquercetin, epicatechin, and epigallocatechin-3-gallate were reported in Sukhothai 1 rice (Phimphilai et al. 2013). The antioxidant potential of phenolic compounds depends on the number and position of hydroxyl groups attached to ring structures of their molecules (Pratt 1992; Rice-Evans et al. 1996).
Antioxidant activities of processed grass drinks, especially of ABTS values was 17.88–35.18 mg Vitamin C equivalent/200 mL (one serving size), it could be considered that these five processed grass drinks had antioxidant potential because one serving size of them had antioxidant activities at least equal to 17.88 mg of Vitamin C or 29.8% of Thai recommended daily intake at 60 mg of Vitamin C (Notification of the Ministry of Publish Health 1998). However, such claim are not permitted.
Sensory evaluation
Consumer acceptability results are shown in Table 2. Bivariate analysis showed that overall liking of products was significantly correlated to aroma intensity, preference on aroma and flavor of products (r = 0.320, 0.543 and 0.735, respectively, p < 0.05). Aroma seemed to be highly influenced to overall liking because any sugar or additives was not used in the drinks. The grass drinks of Jasmine and Sukothai 1 rice were most accepted products (p < 0.05), followed by those of Sukhothai 2 rice and wheat, and barley, depending on their aroma intensity.
Table 2.
Sensory evaluation of 5 processed grass drinks (Mean ± SD) by 160 consumers
| Grass drinks | Aroma intensity | Acceptance (preference) test | |||
|---|---|---|---|---|---|
| Aroma | Color | Flavor | Overall liking | ||
| Rice | |||||
| Jasmine | 5.63a ± 2.28 | 5.79a ± 2.18 | 5.61a ± 2.31 | 5.51a ± 2.30 | 5.90a ± 2.08 |
| Sukhothai 1 | 5.53a ± 2.36 | 5.86a ± 2.26 | 5.61a ± 2.36 | 5.63a ± 2.35 | 5.87a ± 2.31 |
| Sukhothai 2 | 4.58b ± 2.20 | 5.04b ± 2.38 | 5.89a ± 2.22 | 5.18ab ± 2.51 | 5.25b ± 2.34 |
| Wheat | 4.81b ± 2.48 | 5.18b ± 2.32 | 5.07b ± 2.13 | 4.83b ± 2.40 | 4.79b ± 2.29 |
| Barley | 3.89c ± 2.56 | 3.08c ± 2.24 | 3.08c ± 2.24 | 2.84c ± 2.15 | 3.00c ± 2.20 |
0 = Min, 10 = Max
a,b,c,d Means with different letters in the same column are significantly different (p < 0.05)
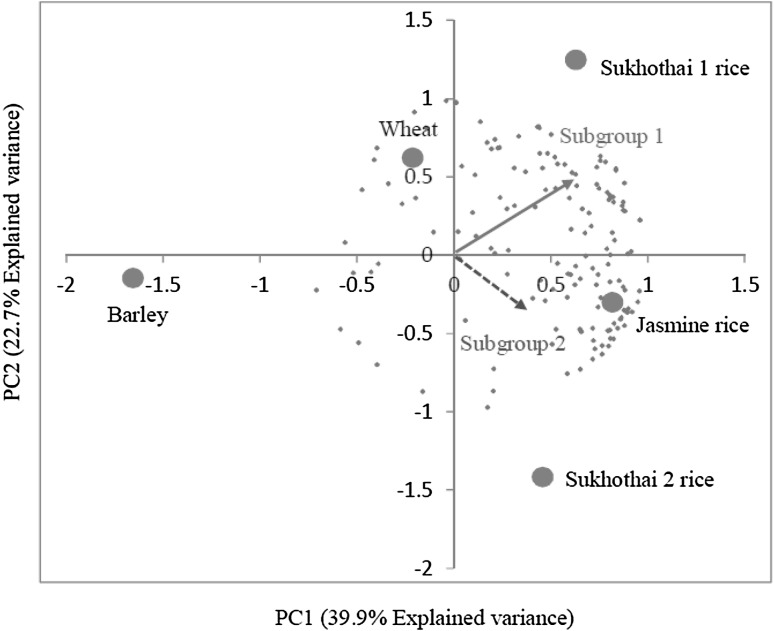
Internal preference mapping is the construction of a multidimensional product map based on acceptance data. The major source of variation within the preference data was extracted by principal component analysis to create preference dimensions (Greenhoff and MacFie 1994). For overall liking data of five processed grass drinks, 4 principal components (PCs) or preference dimensions were created but the first 2 PCs explained 62.6% variance and were used to create an internal preference map as shown in Fig. 2. Product positions (·) expressed the difference of product acceptance and consumer positions (+) expressed their preference direction.
Fig. 2.
An internal preference map of 5 processed grass drinks
Cluster analysis is a technique used to identify groups of individuals or objects that are similar to each other but different from individuals in other groups (Norušis 2011). In this study, it could separate all consumer positions on the internal preference map into 3 subgroups, but the member of the third subgroup was too small for further investigation because the minimum number of assessors for acceptance test was recommended to 64 (Wangcharoen et al. 2005) and 70 (Lyon et al. 1992). The first 2 subgroups with 77 and 60 consumers close to the recommended minimum values were examined and two preference directions were found.
The first subgroup (n1 = 77) preferred grass drink of Sukhothai 1 rice than of Jasmine rice, wheat, Sukhothai 2 rice, and barley, while the second subgroup (n2 = 60) preferred grass drinks of Sukhothai 2 and jasmine rice than those of Sukhothai 1 rice, wheat, and barley, as shown in Table 3. The difference of two preference directions showed that there was evidently difference in aroma and flavor between grass drinks of Sukhothai 1 and Sukhothai 2 rice which affected consumer acceptance of both the drinks. Jasmine rice grass drink was accepted by all consumers, while wheat grass drink was neither liked or disliked but barley grass drink was disliked (Tables 2, 3). These results explained the similarity of aroma and flavor in jasmine rice and the non similar with wheat, and barley.
Table 3.
Overall liking of 5 processed grass drinks (Mean ± SD) by 2 subgroups of consumers
| Subgroup 1 (n1 = 77) | Subgroup 2 (n2 = 60) | |
|---|---|---|
| Rice | ||
| Jasmine | 5.68b ± 2.03 | 6.58a ± 1.76 |
| Sukhothai 1 | 7.14a ± 1.66 | 4.56b ± 2.03 |
| Sukhothai 2 | 4.07c ± 1.92 | 7.04a ± 1.70 |
| Wheat | 5.35b ± 2.40 | 4.09b ± 1.93 |
| Barley | 2.72d ± 1.79 | 2.08c ± 1.43 |
0 = Min, 10 = Max
a,b,c,d Means with different letters in the same column are significantly different (p < 0.05)
Conclusion
This work showed some chemical properties related to health benefits of processed grass drinks. Their chlorophyll contents might be very low when compared to wheatgrass juice but they could be considered as functional drinks with antioxidant potential because of their total phenolic content and other natural antioxidants. Consumer acceptance depended on aroma intensity and preference for aroma and flavor of products. The preference mapping and cluster analysis, the difference in consumer acceptance of grass drinks from Sukhothai 1 and Sukhothai 2 rice was recorded.
Acknowledgements
Authors would like to thank The National Research Council of Thailand for the financial support, and Natural Rice Co., Ltd. for product supply. We also thank Ms. Jantima Thongboon, Ms. Suphannipa Kantima, Ms. Supika Kaewmuangma and Ms. Saranya Suwanpium for very useful helps on collecting data.
References
- Arnon DI. Copper enzymes in isolated chloroplasts; polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. Ferric reducing/antioxidative power assay: direct measure of total antioxidant activity of biological fluids and modified version of simultaneous measurement of antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. doi: 10.1016/S0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- Greenhoff K, MacFie HJH. Preference mapping in practice. In: MacFie HJH, Thomson DMH, editors. Measurement of food preferences. Glasgow: Chapman & Hall; 1994. pp. 137–166. [Google Scholar]
- Gruenwald J. Novel botanical ingredients for beverages. Clin Dermatol. 2009;27:210–216. doi: 10.1016/j.clindermatol.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Jed WF, Katherine KS, Dinkova-Kostova AT, Egner PA, Kensler TW, Talalay P. Chlorophyll, chlorophyllin and related tetrapyrroles are significant inducers of mammlian phase 2 cytoprotective genes. Carcinogenesis. 2005;26(7):1247–1255. doi: 10.1093/carcin/bgi068. [DOI] [PubMed] [Google Scholar]
- Ketnil N (2014) Thailand food market report. National Food Institute. http://fic.nfi.or.th/broadcast/May-2014-OIE.pdf. Accessed 20 Oct 2015
- Lawless HT, Heymann H. Sensory evaluation of food: principles and practices. New York: Chapman & Hall; 1998. [Google Scholar]
- Livingston WCV (1976) Abscisic acid tablets and process. US Patent 3958025
- Lyon DH, Francombe MA, Hasdell TA, Lawson K. Guidelines for sensory analysis in food product development and quality control. London: Chapman & Hall; 1992. [Google Scholar]
- Marwaha RK, Bansal D, Kaur S, Trehan A. Wheat grass juice reduces transfusion requirement in patients with thalassemia major: a pilot study. Indian Pediatr. 2004;41:716–720. [PubMed] [Google Scholar]
- Mates MJ, Jimenez S, Fransisca M. Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell Biol. 2000;32(2):157–170. doi: 10.1016/S1357-2725(99)00088-6. [DOI] [PubMed] [Google Scholar]
- Norušis MJ (2011) Chapter 17 Cluster analysis. IBM SPSS Statistics Guides Web. http://www.norusis.com/pdf/SPC_v19.pdf. Accessed Dec 10 2015
- Notification of the Ministry of Publish Health (No. 182) B.E.2541 (1998) RE: nutrition labeling. Thailand
- Padalia S, Drabu S, Raheja I, Gupta A, Dhamija M. Multitude potential of wheatgrass juice (green blood): an overview. Chron Young Sci. 2010;1(2):23–28. [Google Scholar]
- Paulíčková I, Ehrenbergerová J, Fiedlerová V, Gabrovská D, Havlová P, Holasová M, Kopáček J, Ouhrabková J, Pinkrová J, Rysová J, Vaculová K, Winterová R. Evaluation of barley grass as a potential source of some nutritional substances. Czech J Food Sci. 2007;25:65–72. [Google Scholar]
- Phimphilai S, Topoonyanont N, Srichairatanakool S, Wangcharoen W, Teerawutgulrag A, Poonnoy P, Charoenrath S. Research and development of rice grass products for Thalassemia patients, Final report. Bangkok: National Research Council of Thailand; 2013. [Google Scholar]
- Pinsirodom P, Changnoi W. Comparison of total polyphenol content and antioxidant potential of extracts obtained from seeds of different citrus cultivated in Thailand. Food. 2002;34(4):300–307. [Google Scholar]
- Pokhrel N. Use of natural foods in preventing bone marrow depression in cancer patients who are under chemotherapy. Research report. Kathmandu: Institute of Medicine, Tribhuvan University; 1999. [Google Scholar]
- Pratt DE. Natural antioxidants from plant materials. In: Huang MT, Ho CT, Lee CY, editors. Phenolic compounds in food and their effects on health II: antioxidants & cancer prevention. Washington: American Chemical Society; 1992. pp. 54–71. [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice- Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20(7):933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org. Accessed 12 Aug 2015
- USDA Foreign Agricultural Service (2015) Thailand gain and feed annual 2015. http://gain.fas.usda.gov/Recent%20GAIN%20Publications/Grain%20and%20Feed%20Annual_Bangkok_Thailand_3-19-2015.pdf. Accessed 20 Oct 2015
- Wangcharoen W, Morasuk W. Antioxidant capacity and phenolic content of some Thai culinary plants. Maejo Inter J Sci Technol. 2007;1(2):100–106. [Google Scholar]
- Wangcharoen W, Morasuk W. Antioxidant capacity and phenolic content of holy basil. Songklanakarin J Sci Technol. 2007;29(5):1407–1415. [Google Scholar]
- Wangcharoen W, Morasuk W. Antioxidant capacity and phenolic content of chilies. Kasetsart J (Natural Sci) 2007;41(3):561–569. [Google Scholar]
- Wangcharoen W, Ngarmsak T, Wilkinson BH. Snack product consumer surveys: large versus small samples. Food Qual Pref. 2005;16:511–516. doi: 10.1016/j.foodqual.2004.10.005. [DOI] [Google Scholar]
- Wigmore A. The wheatgrass book. New Jersey: Avery Publishing; 1985. [Google Scholar]
- Zronik J. The biography of rice. Ontario: Crabtree Publishing; 2006. [Google Scholar]