Abstract
The aim of the present study was to investigate the effect of different ozone doses (2, 5, and 10 mg/L) on shelf life extension of fresh chicken legs, packaged in polyamide/poleyethylene bags and stored at 4 ± 1 °C, for a period of 12 days. Parameters taken into account were: microbiological (Total viable count, Pseudomonas spp., Lactic acid bacteria, Yeasts and molds, and Enterobacteriaceae), physicochemical (pH, colour) and sensory (odor, appearance, texture, and taste) attributes. Results showed that colour parameter values (L*, a*, and b*) were not affected by the gaseous ozone dose, whereas only L* and b* were reduced during storage in all samples. pH was reduced by storage time but was not affected by ozonation dose and packaging. Total viable count and Pseudomonas spp., increased statistically significant with ozonation dose and storage time, but were not affected by packaging. Yeasts, molds, Enterobacteriaceae, and Lactic acid bacteria, were decreased during storage, packaging and ozonation dose. Finally, sensory examination (appearance, texture, odor and taste) showed that samples treated with ozone concentration of 10 mg/L retained the original characteristic features of fresh chicken legs as compared to the control samples. The gaseous ozone treatment of 10 mg/L for 1 h, to chicken legs packaged in plastic containers of polyamide/polyethylene under refrigeration, is appropriate for maintaining freshness and quality of chicken, since their shelf life was extended by 4 days, as compared to the control samples.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-016-2421-7) contains supplementary material, which is available to authorized users.
Keywords: Chicken legs, Shelf-life extension, Ozone treatment, Sensory parameters
Introduction
Poultry covers about 30% of total meat production worldwide, as compared to pork (38%). It is a food commodity being available as fresh or frozen, whole birds or as joints (cuts), bone-in or deboned, seasoned in various ways, raw or ready cooked. Chicken meat is favored by consumers around the world because of its desirable nutritional qualities, such as a low fat content, a relatively high concentration of polyunsaturated fatty acids, its excellent quality protein, and its content in all the essential amino acids needed by humans (Adu-Gyamfi et al. 2012; Zhang et al. 2016). Considering the fact that poultry belongs to perishable foods, the main concern of industries is the shelf-life extension of the poultry products, by ensuring also consumers’ protection from both: spoilage and pathogenic microorganisms (USDA Livestock and Poultry 2014).
Modern trends in packaging technology to achieve this purpose, include minimal processing, the application of hurdle technology concept, the use of natural food preservatives (extracts from herbs and spices in the form of essential oils) in combination with modified atmosphere packaging (MAP), or the combination of MAP with gaseous ozone and gas packaging in fresh meat and poultry (Leistner 1995; Al-Haddad et al. 2005; Chouliara and Kontominas 2006; Karabagias et al. 2011; Zhang et al. 2016).
Ozone (O3) is an allotropic form of oxygen, with very good antimicrobial properties. Ozone is used to increase shelf life of foods and support food safety, as well as in disinfection of drinking water, waste treatment and destruction of pesticide residues (Guzel-Sydin et al. 2004). In fact, despite its antimicrobial activity, it is also used in cold rooms to reduce the ethylene (C2H4) level in air and to extend the storage life of fruits and vegetables (Skog and Chu 2001).
In bacteria, it may act as a protoplasmic oxidant causing progressive oxidation of vital cellular components (Khadre et al. 2001). Ozone is effective against a broad range of Gram-positive and Gram-negative bacteria with Listeria monocytogenes showing high sensitivity to ozone (Khadre et al. 2001; Zeynep 2003; Muthukumar and Muthuchamy 2013).
Ozone (O3) has been approved in the US and classified as a food additive (US FDA 2001). The Food and Drug Administration (FDA) guidance on ingredients and sources of radiation used to reduce microorganisms on carcasses, ground beef, and beef trimmings, ozone is classified as a secondary direct food additive/processing aid allowable for all meat and poultry products (FDA 2001). No specific guidelines are given on levels or dosages of ozone. Ozone can be used in accordance with current industry standards of good manufacturing practice (FSIS Directive 2016).
The level of microbial inactivation by ozone varies according to pH, temperature, relative humidity (RH), additives (surfactants, sugars, etc.) and the amount of organic matter surrounding the cells (Restaino et al. 1995). Given the high reactivity of ozone, it is difficult to predict its reaction in the presence of organic matter. Some other researchers have reported that ozone may oxidize or ionize a substrate or spontaneously decompose to oxygen and free radicals (Manousaridis et al. 2005). The excessive use of ozone is not universally beneficial and in some cases may promote oxidative spoilage in foods, such as meat; discoloration or even development of undesirable odours (Khadre et al. 2001).
Given the extremely limited data available in the literature on the application of ozone to poultry meat products (i.e. fresh chicken legs), the aim of the present study was to investigate the effect of different dose ozone treatments (2, 5, and 10 mg/L) on the shelf life extension of fresh chicken legs, stored at 4 °C, as assessed by microbiological, physicochemical and sensory attributes, in combination with chemometrics.
Materials and methods
Chicken samples and packaging
Fresh chicken legs meat in chunks were provided by a local poultry processing industry (Pindos S.A., Ioannina, Greece) within 1 h after slaughter in insulated polystyrene boxes on ice. Chicken leg samples weighing ca. 300 g were placed in polyamide/polyethylene (PA/PE) barrier pouches 29.5 × 29.5 cm, 90 μm in thickness having an oxygen permeability <15 cm3 m−2 d−1 atm−1, nitrogen permeability <15 cm3 m−2 d−1 atm−1, carbon dioxide permeability <200 cm3 m−2 d−1 atm−1 at 75% relative humidity (RH), 23 °C (Method DIN 53380-2), and a water vapor permeability of <1 g m−2 d−1 at 85% RH, 23 °C (Method DIN 53122-2). Pouches were heat-sealed using a BOSS model N48 vacuum sealer (BOSS, Bad Homburg, Germany) and kept at 4 ± 1 °C. All chicken samples were exposed for 1 h under gaseous ozone treatment [concentrations of 2, 5 and 10 mg/L (ppm)]. System of ozonation was C-Lasky L010 supplied from Air tree company (Taiwan). Sampling was carried out on 0, 2, 4, 6, 8, 10, 12 days of storage.
Microbiological analysis
The following groups of microflora were monitored: Total viable counts (TVC), Pseudomonas spp., Yeasts and molds, Lactic acid bacteria (LAB), as well as Enterobacteriaceae. Ten grams of fresh chicken leg meat were removed aseptically from the package using a spoon, transferred to a stomacher bag (Seward Medical, Worthing, West Sussex, UK), containing 90 mL of sterile buffered peptone water (LAB 204, LAB M), and homogenized using a stomacher (LAB Blender 400, Seward Medical) for 60 s at room temperature. For microbial enumeration, 0.1 mL samples of serial dilutions (1:10, diluent, buffered peptone water) of chicken leg meat homogenates were spread on the surface of the following agar plates prepared. TVC were determined using plate count agar (PCA, LAB 010, LAB M, Lancashire, UK), after incubation for 2 days at 30 °C. Pseudomonads were determined on cetrimide fusidin cephaloridine agar (LAB 108, LAB M, supplemented with X 108, Lancashire, UK) after incubation at 30 °C for 2 days. Yeasts and molds were determined on rose Bengal chloramphenicol agar (LAB 036. LAB M, Lancashire, UK) after incubation at 30 °C for 3–5 days. For members of the Enterobacteriaceae spp., 1.0 mL sample was inoculated into 10 mL of molen (45 °C) violet red bile glucose agar (LAB 088, LAB M, Lancashire, UK) after incubation for 24 h at 37 °C. The large colonies with purple haloes were counted. Lactic acid bacteria were determined on de Man Rogosa Sharpe medium (LAM 093, LAB M, Lancashire, UK) after incubation at 30 °C for 3 days. All plates were examined visually for typical colony types and morphological characteristics associated with each medium.
Physicochemical analysis
pH values of fresh chicken leg meat were measured using a Delta OHM, model HD 3456.2, pH-meter (Padova, Italy) with a precision of ±0.002. Chicken leg samples were thoroughly homogenized with 10 mL of distilled water and the homogenate used for pH determination. Colour determination was carried out on the surface of chicken legs using a Hunter Lab model DP-9000 optical sensor colorimeter (Hunter Associates Laboratory, Reston VA, USA) as described by Karabagias et al. (2011). Each determination was run in triplicate (n = 3).
Sensory evaluation
Chicken leg samples (ca. 100 g) after defrosting, were cooked in a microwave oven set at high power (700 W) for 4 min. A panel of seven judges experienced in chicken evaluation was used for sensory analysis. Panelists were asked to evaluate odor, texture, appearance, and taste intensities of cooked samples. Along with the test samples, the panelists were presented with a freshly thawed chicken leg, stored at −30 °C throughout the experiment, this serving as the reference sample. Acceptability of odor, texture, appearance, and taste was estimated using an acceptability scale ranging from 5 to 0, with 5 corresponding to a most liked sample and 0 corresponding to a least liked sample. A score of 3 was taken as the lower limit of acceptability. Each evaluation was run in triplicate (n = 3).
Statistical analysis
Physicochemical and microbiological data were subjected to analysis of variance (ANOVA) in order to investigate whether ozone treatment and packaging could affect the above collected parameters for 12 days storage period. All statistical treatment was performed using the SPSS v.20.0 statistics software. Experiments were replicated three times on different occasions with different chicken leg samples. Analyses were run in triplicate (three different packaged samples) for each replicate (n = 3×3). Microbiological data were transformed into logarithms of the number of colony forming units (cfu/g).
Results and discussion
Microbiological changes
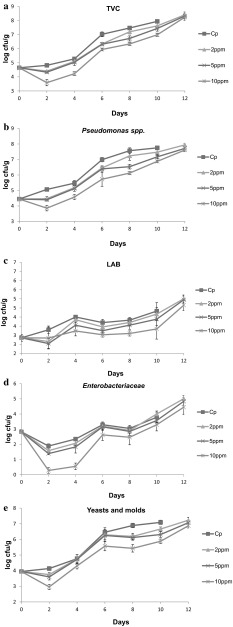
TVC values for chicken leg treated with ozone at different levels under different storage duration are shown in Fig. 1a. TVC values of ca. 4.7 logcfu/g have been reported previously for commercial poultry meat (Chouliara et al. 2007; Ruban and Fairoze 2011; Heetun et al. 2015). TVC reached 7 log cfu/g, which is considered as the microbiological upper limit for good quality poultry meat (ICMSF 1986), on day 5–6 for control (untreated) samples, day 6–8, 8–10 and 12 day, resulted for those samples treated with 2 mg/L, 5 mg/L, and 10 mg/L of ozone. The use of a higher ozone dose (10 mg/L), extended the shelf life of fresh chicken legs by ca. 4–5 days compared to control samples (P < 0.05). A shelf life extension by ca. 2–3 days was reported in aqueous ozone treated (dose of 1 mg/L and exposure time of 60 and 90 min) shucked mussels packaged under vacuum, by Manousaridis et al. (2005).
Fig. 1.
a Combined effect of ozonation and packaging on TVC in chicken leg meat stored at 4 °C as a function of time. b Combined effect of ozonation and packaging on Pseudomonas spp. in chicken leg meat stored at 4 °C as a function of time. c Combined effect of ozonation and packaging on LAB in chicken leg meat stored at 4 °C as a function of time. d Combined effect of ozonation and packaging on Enterobacteriaceae in chicken leg meat stored at 4 °C as a function of time. e Combined effect of ozonation and packaging on Yeasts and Μolds in chicken leg meat stored at 4 °C as a function of time
Pseudomonads (Pseudomonas spp.) are Gram-negative bacteria, being the main spoilage microorganisms in meat (Karabagias et al. 2011). Pseudomonads reached the value of 7 log cfu/g on day 4–6 for control (untreated) samples, day 6–8, 8–10, and 10–12 day for samples treated with 2 mg/L, 5 mg/L and 10 mg/L of ozone respectively. The use of a higher ozone dose (10 mg/L), as in the case of TVC, resulted in keeping Pseudomonads colony forming units below 7 logcfu/g by ca. 4–6 days (P < 0.05) (Fig. 1b). Da Silva et al. (1998) applied ozone treatment and daily ozone exposure (<0.27 mg/L) in fresh scad and reported a decrease (of up to 1 log cfu/cm2) after 10 days of storage in the populations of Pseudomonas putida. Manousaridis et al. (2005) reported that Pseudomonads reached the level of 7 logcfu/g at the 10 day of storage in aqueous ozone treated (1 mg/L) and vacuum packaged shucked mussels compared to that of control samples (day 8). Furthermore, Al-Haddad et al. (2005) applied gaseous ozone of >2000 mg/L for 30 min to control populations of Salmonella infantis and Pseudomonas aeruginosa inoculated on the skin of chicken portions (chilled breasts). Such a high concentration of ozone resulted in reducing the counts of salmonellae by 97% and pseudomonads by 95%. This was not the case for the indigenous coliforms studied.
Lactic acid bacteria (LAB) are facultative anaerobic bacteria (Chouliara et al. 2007). The initial LAB counts (day 0) were ca. 2.9 logcfu/g and never reached 7 logcfu/g (ranging between 4.65 and 5.00) in all treatments, for a 12 days storage period. The lower counts of LAB were monitored on ozonized samples of 10 mg/L followed by 5 mg/L, and 2 mg/L and those of control (Fig. 1c). A similar trend (ranging between 6.5 and 6.6 logcfu/g) was showed in the study of Manousaridis et al. (2005) for a 12 days of storage period, whereas the daily exposure to ozone of fish scad, resulted to a substantially lower logcfu/g value (<3) (Da Silva et al. 1998).
Enterobacteriaceae, are considered a hygiene indicator, the initial counts were ca. 2.8 logcfu/g indicative of good quality chicken meat (Chouliara et al. 2007). Enterobacteriaceae increased (P < 0.05) during storage and air packaging and decreased (P < 0.05) by ozonation dose; while never reached 7 logcfu/g throughout the 12 days of storage in all treatments (Fig. 1d). Da Silva et al. (1998) reported that a higher death rate for certain Gram positive bacteria (Pseudomonas putida, Shewanella putrefaciens, Brochothrix thermosphacta, Enterobacter sp. and Lactobacillus plantarum) on fish scad (Trachurus trachurus) occurred after ozone exposure at 0.25 mg/L for ca.20 min. In the study of Manousaridis et al. (2005), the ozone treatment resulted in controlling Enterobacteriaceae populations below 7 logcfu/g for a 12 days storage period.
Yeasts and molds, showed initial population of ca. 4 logcfu/g, which increased during storage (P < 0.05) in control and ozone treated samples. Ozone treatment at 10 mg/L had a strong effect on Yeasts, since their population never reached 7 logcfu/g throughout the experimental storage (Fig. 1e). The information regarding changes in the population of Yeasts and molds, after application of different ozone dose, was scarce.
Based on the obtained results, ozone treatment had a preservative effect on chicken leg meat, since it kept the populations of spoilage microorganisms at lower levels compared to the control samples (P < 0.05). Present results were similar to those reported in other studies dealing with i.e. pathogenic bacteria: Vaz-Velho et al. (2001), reported that 20 min ozone gas treatment at about 0.1 mg/L was enough to get a 3.5 log reduction of L. monocytogenes; Fisher et al. (2000) suggested that effective inactivation of all L. monocytogenes cells in distilled water, after 5 min exposure to 1 mg/L of ozone. In the purReport (2010) it is remarked that low levels of ozone exposed for 21 days on the table grapes and aluminum surfaces with L. monocytogenes decreased by more than 99.9% (5.02 logs cfu/sample) and 97.5% (approximately 1.5 logs cfu/sample), respectively.
Muthukumar and Muthuchamy (2013) reported that ozone at specific doses of ca. 33 mg/L for 9 min in gaseous phase could be used as an effective method for inactivating 2 × 106 cfu/g of L. monocytogenes in chicken samples before they reach outlets for consumers.
To the best of our knowledge, data on the specific spoilage microorganisms studied in chicken leg meat, after the application of ozone in air packaging are scarce, this constituting the novelty of the present work.
Physicochemical changes
Table 1 shows significant variations (P < 0.05) in pH values according to each treatment and storage period. These variations may be attributed to the production and reduction of lactic acid through the metabolism of LAB (Chouliara et al. 2007). pH varied between 6.85 and 7.16 for the control samples (Cp), 6.85–7.05, 6.85–7.01, and 6.86–7.05 for the 2, 5 and 10 mg/L ozone treated samples, respectively. As it can be observed, the use of ozone at these concentrations did not affect dramatically the pH values of chicken legs stored under refrigeration. This is in agreement with the results of Da Silva et al. (1998) and Manousaridis et al. (2005). Da Silva et al. (1998), reported pH values in the range 6.43–6.58 during storage of fish samples, and using 0.27 mg/L ozone dose. Likewise, Manousaridis et al. (2005) reported pH ranging between 6.3 and 6 for both control and ozonated samples of shucked mussels, at 0.6 and 0.4 mg/L ozone dose throughout a 12 days storage period.
Table 1.
Variations in pH and colour parameters (mean ± SD) according to packaging, ozone treatment and storage time
| Day/parameter | Cp | Cp + 2 mg/L | Cp + 5 mg/L | Cp + 10 mg/L |
|---|---|---|---|---|
| pH | ||||
| 0 | 6.85 ± 0.11 | 6.85 ± 0.11 | 6.85 ± 0.11 | 6.85 ± 0.11 |
| 2 | 7.16 ± 0.03 | 7.03 ± 0.06 | 7.01 ± 0.03 | 6.90 ± 0.04 |
| 4 | 6.86 ± 0.05 | 6.93 ± 0.04 | 7.00 ± 0.02 | 6.78 ± 0.02 |
| 6 | 7.03 ± 0.08 | 6.87 ± 0.07 | 6.94 ± 0.06 | 6.87 ± 0.02 |
| 8 | 6.98 ± 0.07 | 7.05 ± 0.04 | 6.98 ± 0.06 | 6.96 ± 0.06 |
| 10 | 7.09 ± 0.07 | 6.99 ± 0.06 | 6.87 ± 0.05 | 6.75 ± 0.02 |
| 12 | 6.94 ± 0.05 | 7.00 ± 0.06 | 6.96 ± 0.02 | 7.05 ± 0.02 |
| F | 14.654 | 7.377 | 5.838 | 12.599 |
| df | 26 | 26 | 26 | 26 |
| p | <0.001 | <0.001 | <0.001 | <0.001 |
| L* | ||||
| 0 | 60.55 ± 0.33 | 60.55 ± 0.33 | 60.55 ± 0.33 | 60.55 ± 0.33 |
| 2 | 52.44 ± 0.14 | 54.32 ± 0.44 | 51.85 ± 0.06 | 53.61 ± 0.50 |
| 4 | 52.66 ± 0.49 | 59.56 ± 0.37 | 55.85 ± 0.28 | 53.71 ± 0.45 |
| 6 | 54.03 ± 0.22 | 61.68 ± 0.20 | 56.69 ± 0.69 | 53.84 ± 0.27 |
| 8 | 50.03 ± 0.18 | 52.98 ± 0.15 | 55.17 ± 0.12 | 52.91 ± 0.07 |
| 10 | 48.35 ± 0.43 | 50.22 ± 0.63 | 51.59 ± 0.20 | 53.68 ± 0.42 |
| 12 | 51.67 ± 0.70 | 51.60 ± 0.11 | 48.64 ± 0.12 | 52.68 ± 0.42 |
| F | 241.425 | 405.512 | 187.180 | 75.621 |
| df | 26 | 26 | 26 | 26 |
| p | <0.001 | <0.001 | <0.001 | <0.001 |
| a* | ||||
| 0 | 0.91 ± 0. 29 | 0.91 ± 0.29 | 0.91 ± 0.29 | 0.91 ± 0.29 |
| 2 | 2.43 ± 0.13 | 1.37 ± 0.06 | 1.95 ± 0.04 | 0.21 ± 0.48 |
| 4 | 1.95 ± 0.08 | 0.52 ± 0.44 | 0.70 ± 0.25 | 0.46 ± 0.22 |
| 6 | 0.40 ± 0.35 | 0.74 ± 0.31 | 0.51 ± 0.26 | −0.45 ± 0.44 |
| 8 | −0.62 ± 1.26 | 0.69 ± 0.56 | −0.44 ± 0.15 | −0.42 ± 0.18 |
| 10 | −0.38 ± 1.67 | −0.45 ± 1.24 | −0.15 ± 1.17 | −0.11 ± 1.26 |
| 12 | −0.66 ± 0.73 | −1.28 ± 1.50 | −1.17 ± 0.14 | −2.02 ± 0.29 |
| F | 17.464 | 10.518 | 3.137 | 19.958 |
| df | 26 | 26 | 26 | 26 |
| p | <0.001 | <0.001 | 0.021 | <0.001 |
| b* | ||||
| 0 | 9.68 ± 0.39 | 9.68 ± 0.39 | 9.68 ± 0.39 | 9.68 ± 0.39 |
| 2 | 9.91 ± 0.05 | 8.15 ± 0.08 | 8.37 ± 0.06 | 8.73 ± 0.16 |
| 4 | 8.84 ± 0.25 | 9.50 ± 0.17 | 9.00 ± 0.15 | 8.26 ± 0.46 |
| 6 | 8.45 ± 0.41 | 10.05 ± 0.21 | 7.99 ± 0.12 | 9.35 ± 0.31 |
| 8 | 7.81 ± 0.32 | 9.70 ± 0.03 | 8.93 ± 0.17 | 9.78 ± 0.35 |
| 10 | 9.16 ± 0.71 | 9.31 ± 0.19 | 8.65 ± 1.14 | 9.30 ± 0.14 |
| 12 | 9.40 ± 0.25 | 8.71 ± 0.74 | 7.50 ± 0.23 | 8.67 ± 0.48 |
| F | 21.921 | 9.471 | 7.632 | 6.061 |
| df | 26 | 26 | 26 | 26 |
| p | <0.001 | <0.001 | 0.021 | <0.001 |
ANOVA in comparison of means (p < 0.001), mean ± SD: average ± standard deviation values of three replicates (n = 3)
Cp control packaging (air), F variation between sample means/variation within the samples, df degrees of freedom, p probability
Colour values of all chicken leg treatments at selected sampling days are given in Table 1. The L* value which refers to the lightness, decreased up to day 12 of storage, indicative of the fact that the colour of the product became more obscure. This in contrast to the results reported by Pohlman et al. (2002), who reported an increasing in L* values in beef, after using multiple antimicrobial treatments [combined effect of ozonation in a water bath (1%, 7.2 °C) and 0.5% cetylpyridinium chloride or 5% acetic acid, respectively].
Regarding parameter a*, which corresponds to degree of redness when positive and to degree of greenness when negative, as it shown in Table 1 such variations were observed, depending on storage period (P < 0.05) compared to treatments. Variations in colour parameter a* were also reported by Chouliara et al. (2007), in a study involving fresh chicken breast meat stored at 4 °C and treated with MAP and oregano essential oil.
The same pattern holds for parameter b*, the values of which varied among days of storage and treatments. Parameter b* corresponds to yellowness of colour (when positive) and to blueness of colour (when negative). (Commission Internationale de l’ Eclairage, CIE). b* values were not affected (p > 0.05) by the gaseous ozone dose, whereas fluctuations (P < 0.05) depending on storage time were observed in all samples. Such fluctuations are in agreement with the results reported by Chouliara et al. (2007).
Sensory changes
Sensory properties (odor, texture, appearance, and taste) of cooked chicken leg meat are given in Table 2. The lower acceptability score of 3 was reached for odor after 6–8 days for the air packaged samples. The air packaged samples treated with 2 and 5 mg/L of ozone retained a higher score than 3 for 8 days, while those treated with 10 mg/L ozone for 10 days. In the case of texture, for the samples under control packaging 2 mg/L, and 5 mg/L of ozone there was retained an acceptable score of 3 for 6–8 days, while samples treated with 10 mg/L of ozone retained acceptable texture for a period of 8–10 days. Appearance in control packaging and 2 mg/L of ozone retained an acceptable score rate for 6–8 days; samples treated with 5 mg/L of ozone had acceptable appearance for 10 days, while those of 10 mg/L retained acceptability for 10–12 days.
Table 2.
Variations in sensory attributes (mean ± SD) according to packaging, ozone treatment and storage time
| Days/sensory attributes | Cp | Cp + 2 mg/L | Cp + 5 mg/L | Cp + 10 mg/L |
|---|---|---|---|---|
| Odor | ||||
| 0 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 |
| 2 | 4.67 ± 0.15 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 |
| 4 | 4.13 ± 0.12 | 4.17 ± 0.29 | 4.23 ± 0.25 | 4.60 ± 0.17 |
| 6 | 3.27 ± 0.06 | 3.50 ± 0.00 | 3.63 ± 0.32 | 3.57 ± 0.12 |
| 8 | 2.57 ± 0.12 | 3.10 ± 0.17 | 3.13 ± 0.12 | 3.33 ± 0.06 |
| 10 | 2.00 ± 0.00 | 2.27 ± 0.25 | 2.57 ± 0.12 | 3.07 ± 0.12 |
| 12 | 1.30 ± 0.17 | 2.07 ± 0.12 | 2.23 ± 0.06 | 2.47 ± 0.25 |
| F | 799.330 | 338.013 | 260.140 | 460.223 |
| df | 26 | 26 | 26 | 26 |
| p | <0.001 | <0.001 | <0.001 | <0.001 |
| Texture | ||||
| 0 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 |
| 2 | 4.73 ± 0.06 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 |
| 4 | 3.97 ± 0.40 | 4.23 ± 0.25 | 4.30 ± 0.36 | 4.67 ± 0.15 |
| 6 | 3.50 ± 0.00 | 3.67 ± 0.15 | 3.80 ± 0.20 | 3.90 ± 0.17 |
| 8 | 3.00 ± 0.00 | 3.07 ± 0.12 | 3.23 ± 0.21 | 3.40 ± 0.17 |
| 10 | 1.83 ± 0.15 | 2.33 ± 0.29 | 2.63 ± 0.12 | 3.17 ± 0.06 |
| 12 | 1.40 ± 0.17 | 2.00 ± 0.00 | 2.07 ± 0.12 | 2.60 ± 0.17 |
| F | 304.322 | 357.218 | 249.438 | 492.479 |
| df | 26 | 26 | 26 | 26 |
| p | <0.001 | <0.001 | <0.001 | <0.001 |
| Appearance | ||||
| 0 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 |
| 2 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 |
| 4 | 4.30 ± 0.17 | 4.43 ± 0.21 | 4.40 ± 0.35 | 4.87 ± 0.12 |
| 6 | 3.70 ± 0.17 | 3.73 ± 0.25 | 3.77 ± 0.25 | 3.97 ± 0.25 |
| 8 | 3.17 ± 0.06 | 3.20 ± 0.00 | 3.20 ± 0.00 | 3.53 ± 0.15 |
| 10 | 2.67 ± 0.15 | 2.60 ± 0.53 | 3.00 ± 0.00 | 3.20 ± 0.20 |
| 12 | 1.77 ± 0.25 | 2.50 ± 0.00 | 2.70 ± 0.17 | 2.80 ± 0.17 |
| F | 445.500 | 133.874 | 210.223 | 196.709 |
| df | 26 | 26 | 26 | 26 |
| p | <0.001 | <0.001 | <0.001 | <0.001 |
| Taste | ||||
| 0 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 |
| 2 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 |
| 4 | 4.50 ± 0.00 | 4.50 ± 0.00 | 4.60 ± 0.17 | 4.80 ± 0.00 |
| 6 | 3.27 ± 0.06 | 3.67 ± 0.29 | 3.77 ± 0.25 | 4.00 ± 0.00 |
| 8 | ND | 3.00 ± 0.00 | 3.00 ± 0.00 | 3.43 ± 0.12 |
| 10 | ND | ND | 2.67 ± 0.15 | 3.03 ± 0.06 |
| F | 2409 | 139.0 | 160.211 | 783.680 |
| df | 11 | 14 | 17 | 17 |
| p | <0.001 | <0.001 | <0.001 | <0.001 |
ANOVA in comparison of means (p < 0.001), mean ± SD: average ± standard deviation values of three replicates (n = 3)
Cp control packaging (air), ND not determined. F variation between sample means/variation within the samples, df degrees of freedom, p probability
Finally, taste retained an acceptable score rate for control packaging for 6 days, 8 days for chicken leg samples treated with 2 and 5 mg/L of ozone, and 10 days for those treated with a higher dose of 10 mg/L.
Sensory data are in good agreement with microbiological data, based on the TVC populations. For example, odor and taste acceptability scores (6 days) were in excellent agreement with TVC results. Such an observation, is in the same line of reasoning with the aerobic plate count and sensory (odor evaluation) data reported by Pohlman et al. (2002), for ground beef treated with ozone (1%) and 0.5% cetylpyridinium chloride. The combination of ozone and cetylpyridinium chloride resulted to an acceptable score rating of ‘’no off odor’’ for a storage period of 7 days. This was not the case for appearance and texture which had acceptable scores for 8 days. This may be attributed to the fact that it is not only the TVC but specific spoilage microorganisms (SSO) that cause spoilage to the product (Jay 1986; Chouliara et al. 2007). On the other hand, the higher ozone treatment (10 mg/L) contributed positively in sensory properties of chicken legs, since they retained a ‘’fresh score’’ for ca.10 days (P < 0.05).
Conclusion
Based primarily on sensory evaluation, the shelf-life of aerobically packaged fresh chicken leg meat was ca. 6 days. The gaseous ozone treatment of 10 mg/L for 1 h, for chicken legs packaged in plastic containers of PA/PE under refrigeration, was appropriate for maintaining freshness and quality of chicken, since this treatment extended the shelf life for 4 days, as compared to the control samples.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with ethical requirements
Conflicts of interest
All authors declare that they do not have financial, academic, commercial, political or personal interests.
References
- Adu-Gyamfi A, Torgby-Tetteh W, Appiah V. Microbiological quality of chicken sold in Accra and determination of D10-value of E. coli. Food Nutr Sci. 2012;3:693–698. doi: 10.4236/fns.2012.35094. [DOI] [Google Scholar]
- Al-Haddad KSH, Al-Qassemi RAS, Robinson PK. The use of gaseous ozone and gas packaging to control populations of Salmonella infantis and Pseudomonas aeruginosa on the skin of chicken portions. Food Control. 2005;16:405–410. doi: 10.1016/j.foodcont.2004.04.009. [DOI] [Google Scholar]
- Chouliara E, Kontominas MG. Combined effect of thyme essential oil and modified atmosphere packaging to extend shelf life of fresh chicken meat. In: Govil JN, Singh VK, Almad K, Sharma RK, editors. Recent progress in medicinal plants: natural product (15) USA: Studium Press, LCC; 2006. pp. 423–442. [Google Scholar]
- Chouliara E, Karatapanis A, Savvaidis IN, Kontominas MG. Combined effect of oregano essential oil and modified atmosphere packaging on shelf-life extension of fresh chicken breast meat, stored at 4 °C. Food Microbiol. 2007;24(6):607–617. doi: 10.1016/j.fm.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Da Silva MV, Gibbs PA, Kirby RM. Sensorial and microbial effects of gaseous ozone on Fish scad. J Appl Microbiol. 1998;84:802–810. doi: 10.1046/j.1365-2672.1998.00413.x. [DOI] [PubMed] [Google Scholar]
- DIN 53122-2 Water vapor permeability WVTR (electrolysis method), A Mecadi GmbH In der Kolling 966450 Bexbach, Germany
- DIN 53380-2 Gas permeability (plastic films manometric), AMecadi GmbH In der Kolling 966450 Bexbach, Germany
- FDA (2001) Federal Register, Vol. 66 No.123, June 26 (2001) Department of Health and Human Services, Food and Drug Administration, Rules and Regulations, pp 33829–33830
- Fisher CW, Lee D, Dodge B-A, Hamman KM, Robbins JB, Martin SE. Influence of catalase and superoxide dismutase on ozone inactivation of Listeria monocytogenes. Appl Environ Microbiol. 2000;66(4):1405–1409. doi: 10.1128/AEM.66.4.1405-1409.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FSIS Directive (2016) 7120.1, Revision 35, safe and suitable ingredients used in the production of meat, poultry, and egg products, May 24, 2016. United States Department of Agriculture Food Safety and Inspection Service, Washington, DC
- Guzel-Sydin ZB, Greene AK, Seydin AC. Use of ozone in the food industry. LWT-Food Sci Technol. 2004;37(4):453–460. doi: 10.1016/j.lwt.2003.10.014. [DOI] [Google Scholar]
- Heetun I, Goburdhun D, Neetoo H. Comparative microbiological evaluation of raw chicken from markets and chilled outlets of Mauritius. J Worlds Poul Res. 2015;5(1):10–18. [Google Scholar]
- ICMSF . International commission on microbiological specifications for foods. sampling for microbiological analysis: principles and scientific applications. 2. Toronto: University of Toronto Press; 1986. [Google Scholar]
- Jay JM, Loessner MJ, Golden DA. Modern food microbiology. 7. New York: Springer; 2005. [Google Scholar]
- Karabagias I, Badeka A, Kontominas MG. Shelf life extension of lamb meat using thyme or oregano essential oils and modified atmosphere packaging. Meat Sci. 2011;88:109–116. doi: 10.1016/j.meatsci.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Khadre MA, Yousef AE, Kim J. Microbiological aspects of ozone applications in food: A review. J Food Sci. 2001;6:1242–1252. doi: 10.1111/j.1365-2621.2001.tb15196.x. [DOI] [Google Scholar]
- Leistner L. Principles and applications of hurdle technology. In: Gould GW, editor. New methods of food preservation. London: Blackie Academic and Professional; 1995. pp. 1–21. [Google Scholar]
- Manousaridis G, Nerantzaki A, Paleologos EK, Tsiotsias A, Savvaidis IN, Kontominas MG. Effect of ozone on microbial, chemical and sensory attributes of shucked mussels. Food Microbiol. 2005;22:1–9. doi: 10.1016/j.fm.2004.06.003. [DOI] [Google Scholar]
- Muthukumar A, Muthuchamy M. Optimization of ozone in gaseous phase to inactivate Listeria monocytogenes on raw chicken samples. Food Res Int. 2013;54:1128–1130. doi: 10.1016/j.foodres.2012.12.016. [DOI] [Google Scholar]
- Pohlman FW, Stivarius MR, McElyea KS, Johnson ZB, Johnson MG. The effects of ozone, chlorine dioxide, cetylpyridinium chloride and trisodium phosphate as multiple antimicrobial interventions on microbiological, instrumental color, and sensory color and odor characteristics of ground beef. Meat Sci. 2002;61:307–313. doi: 10.1016/S0309-1740(01)00198-X. [DOI] [PubMed] [Google Scholar]
- purReport (2010) Effect of ozone gas on E. coli O157:H7, Salmonella, and L. monocytogenes on fruit and metal surfaces. Purfresh, Inc., Fremont
- Restaino L, Frampton E, Hemphill J, Palnikar P. Efficacy of ozonated water against various food related microorganisms. Appl Environ Microbiol. 1995;61(5):3471–3475. doi: 10.1128/aem.61.9.3471-3475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban SW, Fairoze D. Effect of processing conditions on microbiological quality of marked poultry meats in Bangalore, India. J Anim Vet Adv. 2011;10(2):188–191. doi: 10.3923/javaa.2011.188.191. [DOI] [Google Scholar]
- Skog LG, Chu CL. Effect of ozone on qualities of fruits and vegetables in cold storage. Can J Plant Sci. 2001;81:773–778. doi: 10.4141/P00-110. [DOI] [Google Scholar]
- USDA Livestock & Poultry (2014) World markets & trade. The poultry site. 30 April 2013. Retrieved 24 Feb 2014
- Vaz-Velho M, Duarte G, McLauchlin J, Gibbs P. Characterization of Listeria monocytogenes isolated from production lines of fresh and cold-smoked fish. J Appl Microbiol. 2001;91:556–562. doi: 10.1046/j.1365-2672.2001.01425.x. [DOI] [PubMed] [Google Scholar]
- Zeynep GS. Use of ozone in the food industry. Food Microbiol. 2003;21(4):475–479. [Google Scholar]
- Zhang H, Wu J, Guo X. Effects of antimicrobial and antioxidant activities of spice extracts on raw chicken meat quality. Food Sci Hum Wellness. 2016;5:39–48. doi: 10.1016/j.fshw.2015.11.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.