Abstract
The proximate composition and bioactive components were screened in eight wheat bran cultivars such as UAS (304, 415, 428), DWR (162, 185, 1006) and DDK (1025, 1029). The results showed that carbohydrate content ranged from 62.3 to 73.9%, protein 11–21%, fat 2.4–5.6%, ash content 5–6.5% among the wheat bran genotypes and dietary fiber content was found to be between 21 and 52%. Mineral content vary viz., Fe (0.7–2.45), Mg (4.78–8.36), K (16.47–44.58), Zn (0.78–1.44), Cu (3.35–15.79), Na (1.22–7.14) and Mn (21.77–70.09) μg/gm, highest being in UAS-428 variety, except for Ca content. Linoleic acid was the major fatty acid present to the extent of 47–53%. The antioxidant capacity of wheat bran extract through free radical scavenging showed the IC50 values (mg/mL) of 9.4 for UAS 428 and 10.55 for UAS 415 indicating higher activity. The steryl ferulates, total tocopherols and carotenoids were estimated as fat soluble nutraceuticals. Higher content of steryl ferulate was observed in DWR 185 (477 mg/100 g) followed by DDK 1025 (465 mg/100 g) and the least in UAS 415 (119 mg/100 g) variety. In conclusion genotypic wheat bran is an important source of dietary micronutrients like minerals especially in UAS variety with a potential free radical reducing ability. These varieties have health protective properties and can be incorporated in various food formulations for improved lifestyles.
Keywords: Wheat bran, Steryl ferulate, Tocopherols, Carotenoids, Carbohydrate
Introduction
Wheat (Triticum aestivum) is an ancient staple food crop in the world, and India is the second largest producer of wheat (100 million tons per annum, 2014). Outer part of wheat grain comprises of 15% bran, which is produced as a byproduct of the wheat milling industry and is used as animal fodder. Karnataka is unique in the cultivation of three wheat species, namely, Triticum aestivum (bread wheat), Triticum durum (durum wheat), and Triticum dicoccum (diabetics wheat). Approximate yield of wheat bran (WB) from all varieties ranges from 14 to 19% but, production of WB mainly depends on the choice of milling procedure and physical characteristics of wheat variety (Safdar 2005). Nutritionally, bran fractions are rich in both macro and micronutrients that include carbohydrates, proteins, fats, minerals and other bioactive phytochemicals, in particular antioxidants such as phenolic acids and other fat soluble molecules. Availability of these nutrients in the current food habits is less due to usage of refined wheat flour in most food preparations. Wheat bran has significant health beneficial effects and it is now included in a number of fiber rich food products. The added bran provides nutraceutical effect by providing digestive regularity and ending constipation due to high amounts of fiber.
Reddy et al. (2000) have reported increased colon tumorogenesis after removal of lipids and lipid-soluble components of WB, fortification of de-fatted bran diet with bran oil significantly inhibited tumorogenesis, suggesting that the bioactive compounds present in WB oil possess an inhibitory property against colon carcinogenesis. Some of the fat-soluble bioactives such as tocopherols, sterols, carotenoids, steryl ferulates etc., are known to have health beneficial effects (Agudo et al. 1999). Polyphenols are the most voluminous antioxidants in the human diet, these are naturally occurring compounds having unique structural similarities with carboxylic group as in caffeic acid, gallic acid, p-coumaric acid, vanillic acids, which are well known anti-oxidant compounds which are considered for benefits from their dietary ingestion as well as pharmacological use. Studies have shown that polyphenols from cereal bran serve as antioxidants in food and biological systems (Black 2003). Ingesting products with such added nutraceuticals present in wheat bran are effective tools for overcoming lifestyle disorder in developing countries.
The purpose of this investigation is to screen the bioactive compounds including minerals present in eight wheat bran cultivars of UAS (304, 415, 428), DWR (162, 185, 1006) and DDK (1025, 1029) produced in University of Agricultural Sciences, Dharwad, Karnataka, India and study their chemical composition for their phytochemical rich fat soluble components.
Materials and methods
Wheat bran samples were obtained from University of Agricultural sciences Dharwad, Karnataka. UAS 304, UAS 415, UAS 428, DWR 162, DWR 185, DWR 1006, (T. durum). DDK 1025, DDK 1029 (T. aestivum), CWB-Commercial wheat bran from flour milling and Baking Technology Department, CFTRI.
Standards, α-tocopherols, oryzanol, phenolic acids and 1-1diphenyl-2-picrylhydrazyl (DPPH) were obtained from Sigma-Aldrich Chemical Co., (St. Louis, MO, USA). HPLC grade methanol, acetonitrile, hexane, ethyl acetate and ethanol were purchased from RANKEM Limited, (New Delhi INDIA). All chemicals and reagents were of analytical grade.
Analysis of proximate composition
Moisture (method Ab 2–49), ash (method Bc 5–49), protein (method Ab 4–91), and fat (method Am 2–93) contents in wheat bran of different varieties were determined using standard method (AOCS 1998). Analyses of soluble and insoluble fiber were carried out according to the method AOAC (1992). Mineral content of WB was determined by atomic absorption spectrometry (Shimadzu ASC-6000) according to AOAC (2005) methods. Total carbohydrates was calculated by the difference method [Carbohydrates (%) = 100 − Protein (%) + Fat (%) + Ash (%) + Moisture (%)].
Sample preparations
Analysis of fatty acid composition
Fatty acid composition was determined by the method of Firestone (1998) and methyl esters of the fatty acids were prepared using boron trifluoride/methanol. The samples were analyzed using a gas liquid chromatography GC (model GC-450 Varian Inc., Middelburg, Netherland) equipped with data processor and a flame ionization detector (FID). Conditions: Capillary column, 50 m × 0.25 mm internal diameter from Varian-CP7419, nitrogen flow of 1 mL/min. temperature program: initial column temperature 120 °C/5 min. increased to 260 °C at 5 °C/min and held for 5 min, injection temperature 220 °C, and detector temperature 230 °C. The fatty acids were identified by comparing the retention time of sample with those of reference standard fatty acid methyl esters.
Determination of steryl ferulates, tocopherols and carotenoids
Total oryzanol-like compounds (steryl ferulates) were determined in oil samples of different varieties of wheat bran by HPLC (Gopala Krishna et al. 2001), using Shimadzu LC 10A system coupled with Shimadzu C18 column (150 mm × 4.6 mm, 5 μm, i.d.) and Shimadzu SPD-M10 AVP UV detector. Conditions: Wavelength (λ) 325 nm; mobile phase acetonitrile: methanol: isopropyl alcohol (10:9:1 v/v/v); flow rate 1 mL/min and using γ-oryzanol as standard with LOD 20–100 μg for steryl ferulate quantification.
Analysis of total tocopherol by HPLC
Total tocopherol content was determined and expressed as mg/100 g of oil using HPLC (model LC- 10AVP Shimadzu Corp., Tokyo, Japan) coupled with silica column (250 mm × 4.6 mm × 5 μm 87 i.d.) and fluorescence detector (FLD). The following conditions were used: wavelength Ex 290 nm, Em 330 nm; mobile phase, hexane: isopropanol (99.5:0.5 v/v); flow rate 1 mL/min; and standard tocopherol for identification and quantification.
Carotenoids analysis
β-Carotene and lutein were determined through RP-HPLC analysis by injecting a known amount of oil in acetone using LC-6A, Shimadzu instrument equipped with a Photodiode array detector (PDA) and fitted with C18 column (25 cm × 4.6 mm, 5 μm i.d.), detector set at 450 nm; mobile phase acetonitrile: methanol: dichloromethane (6:2:2 v/v) with 0.1% ammonium acetate; flow rate mL/min, β-carotene with Limit of Detection (LOD) 2–10 μg and lutein with 0.2–0.5 μg were used as reference standards for identification and quantification.
Antioxidant activity as DPPH scavenging capacity
The 1, 1-diphenyl-2- picrylhydrazyl (DPPH) radical scavenging effect of oil was carried out. Wheat bran oil samples were mixed with 900 μm of DPPH solution in toluene. The absorbance of the mixture was measured at 515 nm, after 20 min incubation. Toluene was used as a blank, and antioxidant activity was calculated according to the method of Ramadan et al. (2003).
Determination of individual phenolic acids
Individual phenolic acids in the bran extracts were analyzed by using Simazdu LC,10-A series high performance liquid chromatography equipped with UV as detector and Reversed Phase C18 column (Particle size 5 µm, Pore size 300 Å, 250 × 4.6 mm, Phenomenex). A mobile phase with water: methanol: acetic acid in the ratio 80: 18: 2, and flow rate of 1.0 mL/min was set while detection was carried out at 280 nm. Identification and quantification of phenolic acids in samples were performed by comparing them to the chromatographic retention times and areas of external standards. Phenolic acid standards used for peak identification were GA, PA, CA, VA, p-CA, FA and CA purchased from Sigma Aldrich. Total phenolic acid content was determined by spectrophotometric method using Folin–Ciocalteu reagent using gallic acid as standard and values were expressed in terms of gallic acid equivalents (GAE).
Preparation of alditol acetate and analysis of carbohydrate by Gas Liquid Chromatography
Carbohydrate composition of all the samples (10 mg) was analyzed by hydrolyzing it with 72% sulphuric acid in ice cold temperatures followed by boiling water bath (6–8 h). The hydrolysate was neutralized and deionized using barium carbonate and Amberlite IR 120 resin, respectively. Alditol acetate was prepared with the principle of reduction and acetylation of hydroxyl group of sugars according to Sawardekar et al. (1967). Sodium borohydride (20–30 mg) was added to reduce the hydroxyl group and neutralize excess by acetic acid (2N) followed by co-distillation with methanol till dry. To the dried samples distilled acetic anhydride along with pyridine (0.5 mL) each were added and kept at 100 °C for 2 h in an air tight tube for acetylation reaction. The reagents were removed by co-distilling it with water and toluene and dried. The formed alditol acetates were extracted and filtered using chloroform using glass wool and dried under nitrogen gas. A known (~1.0 mL) volume of chloroform was added and 1μL sample was injected to GLC. Conditions: Capillary column Rtx-2330 (30 m × 0.32 mm i.d × 0.2 µm thickness) fitted to Shimadzu GC (Shimadzu, Kyoto, Japan). Temperature program: 175 °C (2 min), 8 °C/min↑, 240 °C(1 min), 8 °C/min↑, 265 °C (12 min). Injector and Detector temperature; 275 °C, Nitrogen flow; 1 mL/min. The column was calibrated and the response factors calculated by injecting a sugar standard mixture containing Rha, Ara, xyl, Man, Gal, Glu and Inositol (internal standards) (mixture contained 10 mg of each standard). Sugars present in the samples were identified by comparing their relative retention times with standards and were expressed in terms of relative percent.
Statistical analysis
All measurements were carried out in triplicates. The results are expressed as the mean ± standard deviation (SD) and the data analyzed using analysis of variance (ANOVA) and Duncan’s multiple range tests (DMRT), using SPSS Version15 software. Statistical significance was accepted at a level of P < 0.05.
Result and discussion
Proximate compositions
The analysis of different wheat bran varieties for composition of vital nutrient includes macro and micro-nutrients along with other fat soluble bioactives. The moisture content of the samples varied from 4.47 to 7.29%. The ash content ranged from 4.97 to 6.50% among the different varieties. The protein content of all other varieties ranged between 11.52 and 19.74%, the highest being in UAS 304 followed by DDK 1029 and in other varieties ranged between 4.97 and 6.50%. The fat content of wheat bran ranged between 2.7 and 5.8%, while the carbohydrate content was between 62.03 and 73.95% (Table 1).
Table 1.
Proximate analysis of bran from genotypic wheat varieties (%)
| Wheat genotypes | Moisture | Ash | Protein | Fat | Carbohydrate | IDF | SDF | TDF |
|---|---|---|---|---|---|---|---|---|
| CWB | 5.02 ± 0.28b | 6.00 ± 0.08b | 16.79 ± 0.82d | 2.70 ± 0.16a | 69.49 ± 1.24d | 42.1 ± 1.98ef | 4.01 ± 0.86a | 46.01 ± 2.28e |
| UAS-304 | 5.75 ± 0.35bc | 5.85 ± 0.05b | 21.21 ± 0.97h | 3.70 ± 0.13bc | 63.49 ± 2.23ab | 42.0 ± 2.03ef | 4.0 ± 0.71a | 46.0 ± 2.33e |
| UAS-415 | 6.28 ± 0.36c | 5.44 ± 0.03ab | 14.14 ± 0.92b | 3.07 ± 0.14b | 71.11 ± 2.52d | 35.0 ± 1.62c | 4.97 ± 0.55a | 39.95 ± 1.87c |
| UAS-428 | 4.47 ± 0.12a | 6.50 ± 0.04c | 18.56 ± 0.85f | 5.58 ± 0.19c | 64.89 ± 3.26b | 44.5 ± 1.81f | 7.92 ± 0.64c | 52.47 ± 1.44f |
| DWR-162 | 7.09 ± 0.34d | 5.00 ± 0.04a | 11.55 ± 0.79a | 2.41 ± 0.11a | 73.95 ± 1.36e | 38.6 ± 3.96d | 3.96 ± 0.57a | 42.57 ± 1.59d |
| DWR-185 | 4.71 ± 0.12a | 6.37 ± 0.06bc | 15.22 ± 0.86c | 5.68 ± 0.23e | 68.02 ± 3.01cd | 46.5 ± 1.80g | 4.95 ± 0.84b | 51.48 ± 2.14f |
| DWR-1006 | 7.29 ± 0.53d | 4.97 ± 0.06a | 16.74 ± 0.73d | 3.76 ± 0.21bc | 67.24 ± 2.15c | 40.5 ± 1.42de | 4.97 ± 0.92b | 45.54 ± 1.74e |
| DDK-1025 | 6.00 ± 0.21c | 5.88 ± 0.07b | 17.27 ± 0.95de | 4.05 ± 0.24d | 66.80 ± 1.98c | 17.8 ± 1.95a | 3.96 ± 0.66a | 21.74 ± 2.11a |
| DDK-1029 | 5.89 ± 0.25bc | 6.46 ± 0.05c | 19.74 ± 0.64g | 5.88 ± 0.18e | 62.03 ± 2.32a | 22.0 ± 2.01b | 4.0 ± 2.13a | 26.0 ± 2.13b |
CWB commercial wheat bran, IDF insoluble dietary fiber, SDF soluble dietary fiber, TDF total dietary fiber mean values bearing different superscripts in columns are significantly different (p ≤ 0.05)
Dietary fibre is often classified as soluble dietary fiber (SDF) and insoluble dietary fiber (IDF). Because, the term solubility refers simply to fibers that are dispersible in water, the soluble–insoluble ratio is important for both dietary and functional properties derived from DF (Gorinstein et al. 2001). The varieties DWR 185 and UAS 428 showed higher IDF values of 46.53 and 44.55%, respectively followed by UAS 304 and CWB both of which have 42%. The least value of 17.82% was observed in the DDK 1025 variety (Table 1).
Soluble dietary fiber SDF and TDF was highest in the UAS 428 variety and others showed values ranging between 3.96 and 4.97%. It was observed that among the various varieties, UAS 428 scored the highest for all types of (IDF, SDF and TDF) fibers. This increase might be due to a higher proportion of pericarp and aleuronic layer in durum wheat. Eposito et al. (2005) have reported that the soluble fiber content of durum wheat ranged between 0.9 and 4.1%, while IDF was 21–64% (Table 1). These values are similar to those observed in the varietal differences studied by Nandini and Salimath (2010), who reported that the dietary fiber contents varied between cereals and that wheat bran had the highest among the wheat, sorghum and bajra studied.
Mineral composition of wheat bran genotypes
All the bran components were highly influenced by the locations and genotypes, similarly, results from our study showed concentration of eight elements in different wheat cultivars (Table 2). Calcium content in the wheat traits studied varied from 0.65 to 2.34 μg/g, highest being in UAS 415 and UAS 304, both of these varieties were genetically similar from the durum family. The magnesium content ranged from 4.7 to 8.3 μg/g of which highest amount being in UAS 304, lowest amount in CWB. Butt et al. (2004) studied the effect of incorporating bran to flour in order to fortify mineral content, for instance iron content was observed in wheat bran i.e. 64.6 mg/Kg whereas that in fortified brown flour ranged from 16.8 to 29.2 mg/Kg. Chapattis prepared by brown flour with 10% followed by 15% bran were of the best quality and quite comparable with chapattis prepared with whole wheat flour. Thus it was concluded that wheat bran is a good source of minerals and could be used for supplementing foods.
Table 2.
Mineral composition (µg/g) of genotypic wheat bran
| Wheat genotypes | Fe | Mg | Ca | K | Zn | Cu | Na |
|---|---|---|---|---|---|---|---|
| CWB | 0.70 ± 0.02a | 4.78 ± 0.04a | 0.97 ± 0.04b | 16.47 ± 1.02a | 1.01 ± 0.04b | 3.35 ± 0.06a | 5.52 ± 0.02e |
| UAS-304 | 2.45 ± 0.06e | 8.36 ± 0.01d | 2.34 ± 0.03d | 41.57 ± 1.64d | 1.36 ± 0.03cd | 15.79 ± 0.06e | 4.49 ± 0.02d |
| UAS-415 | 0.80 ± 0.02a | 6.19 ± 0.03c | 2.07 ± 0.02d | 21.60 ± 1.20b | 0.78 ± 0.03a | 05.66 ± 0.04a | 1.22 ± 0.03a |
| UAS-428 | 2.09 ± 0.03de | 5.25 ± 0.02ab | 0.87 ± 0.04b | 28.56 ± 1.56c | 1.29 ± 0.05c | 11.38 ± 0.08d | 3.53 ± 0.03bc |
| DWR-162 | 1.32 ± 0.02c | 6.40 ± 0.02c | 1.68 ± 0.02c | 28.25 ± 1.36c | 1.28 ± 002c | 6.78 ± 0.02b | 4.68 ± 0.03de |
| DWR-185 | 1.78 ± 0.03d | 6.93 ± 0.04c | 1.21 ± 004c | 44.58 ± 1.95d | 1.44 ± 0.04d | 14.66 ± 0.04e | 3.96 ± 0.03c |
| DWR-1006 | 1.39 ± 0.06c | 6.38 ± 0.02c | 1.78 ± 0.03cd | 28.47 ± 1.21c | 1.32 ± 0.04c | 10.29 ± 0.08d | 4.50 ± 0.02d |
| DDK-1025 | 1.31 ± 0.04bc | 6.14 ± 0.03c | 0.65 ± 0.02a | 28.20 ± 1.01c | 1.06 ± 0.02b | 8.43 ± 0.02c | 2.92 ± 0.02b |
| DDK-1029 | 1.10 ± 0.04b | 5.49 ± 0.03ab | 1.12 ± 0.06c | 21.55 ± 1.32b | 1.20 ± 0.04c | 6.85 ± 0.03b | 7.14 ± 0.04f |
Mean values bearing different superscripts in a columns are significantly different (p ≤ 0.05) (n = 3)
Among all cultivars, UAS 304 and DWR 185 were predominantly rich in macro and micro nutrients. Similar results were observed by Rokhade and Rao (2005), who studied the effect of location on the composition of wheat and acceptability of traditional product.
Fatty acid composition of different varieties of wheat bran
The fatty acid compositions of the lipids extracted from different varieties of wheat bran are given in Table 3. The saturated fatty acids ranged from 17.9 to 36.7% while the monounsaturated fatty acids ranged between 13.4 and 28.1%. Wheat bran rich in PUFA, essential fatty acids were 56% in DWR 1006 variety, followed by CWB at 55%, which are comparable with earlier reports of Chang et al. (2010). Presence of high amount of linoleic acid (18:2) as observed in WB oil is a good source of ω-6 fatty acids along with the small amount of ω-3 fatty acids. ω-6 and ω-3 fatty acids are required for the normal growth, health and development of body (Cunnane and Anderson 1997). Suresh Kumar et al. (2014) studied the nutraceuticals composition of both wheat bran oil yield 3.35% and germ oil yield 7.35%, containing polyunsaturated fatty acids of 64 and 61.2% respectively. Also it has been reported that the fat soluble nutraceuticals retained after treatment with different temperature in the commercial wheat bran (Suresh Kumar and Gopala Krishna. 2015).
Table 3.
Fatty acid profile of genotypic wheat bran oil cultivars (%)
| Wheat genotypes | C14:0 | C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 |
|---|---|---|---|---|---|---|---|
| CWB | ND | 17.44 ± 1.6e | 2.25 ± 0.2a | 25.15 ± 1.2c | 51.88 ± 1.3cd | 3.24 ± 0.02c | ND |
| UAS-304 | 0.87 ± 0.4b | 27.16 ± 0.6f | 7.06 ± 0.3e | 20.38 ± 0.3a | 40.11 ± 1.2a | 2.76 ± 0.03b | 1.65 ± 0.2c |
| UAS-415 | 6.47 ± 0.2f | 16.74 ± 0.8cd | 2.83 ± 0.4c | 23.23 ± 0.2b | 47.69 ± 1.6b | 3.01 ± 0.02c | ND |
| UAS-428 | 1.05 ± 0.3b | 15.44 ± 0.8b | 2.65 ± 0.2bc | 25.65 ± 0.1c | 52.20 ± 2.8d | 1.78 ± 0.02a | 1.18 ± 0.2b |
| DWR-162 | 1.60 ± 0.2c | 17.04 ± 0.4de | 3.75 ± 0.4d | 23.86 ± 0.1b | 47.03 ± 3.2b | 3.15 ± 0.02c | 3.69 ± 0.8d |
| DWR-185 | 2.23 ± 0.4d | 14.25 ± 0.4a | 2.40 ± 0.2a | 28.14 ± 0.2d | 49.29 ± 2.6c | 2.29 ± 0.03b | 0.74 ± 0.04a |
| DWR-1006 | 0.57 ± 0.02a | 17.54 ± 0.2e | 2.43 ± 0.1a | 21.85 ± 0.2a | 53.78 ± 1.5d | 3.10 ± 0.04c | 0.70 ± 0.06a |
| DDK-1025 | 3.07 ± 0.2e | 16.07 ± 0.3c | 2.76 ± 0.1c | 25.70 ± 0.5c | 48.01 ± 2.4bc | 2.53 ± 0.04b | 1.83 ± 0.4c |
| DDK-1029 | 0.92 ± 0.3b | 14.85 ± 0.8ab | 2.49 ± 0.2ab | 27.63 ± 0.5d | 49.94 ± 1.4c | 2.88 ± 0.02bc | 1.25 ± 0.1b |
C14:0, Myristic acid.C16:0, Palmitic acid.C18:0, Stearic acid.C18:1, Oleic acid. C18:2, Linoleic acid. C18:2, Linolenic acid C20:0, Arachidonic acid. ND not detected, Mean values bearing different superscripts in a columns are significantly different (p ≤ 0.05) (n = 3)
Steryl ferulate content of wheat bran varieties
Steryl ferulates are the esters of sterol and ferulic acid, their content in wheat bran varieties chosen for the study are given in Table 4 and Fig. 1a–c. In general among the varieties it was observed that the content of campestanyl ferulate and β-sitosteryl ferulate dominant steryl ferulate, followed by sitostanyl ferulate and campesteryl ferulate. Higher content was observed in DWR 185 (477 mg/100 g) followed by DDK 1025 (465 mg/100 g) and least in UAS 415 (119 mg/100 g) variety. This study is in accordance with the results of Nystrom et al. (2007) wherein two Finnish wheat varieties were studied. In another study, rye bran and wheat bran steryl ferulates were compared and yields obtained were different (Nyström et al. 2007). Suresh Kumar and Gopala Krishna (2015) and Suresh Kumar et al. (2014) have also reported the changes in wheat bran oil steryl ferulates before and after heating at different temperatures. Steryl ferulates has significant effects in reducing serum cholesterol levels, inhibiting tumorigenesis, anti-diabetic properties etc. (Wilson et al. 2007).
Table 4.
Bioactive compounds in (mg/100 g) genotypic wheat bran varieties
| Wheat genotypes | Campesteryl ferulate | Campestanyl ferulate and β-sitosteryl ferulate | Sitostanyl ferulate | T. Tocopherol | Lutein | β-Carotene | Total carotenoids | IC 50 value |
|---|---|---|---|---|---|---|---|---|
| CWB | 65.2 ± 3.2f | 307.3 ± 12.2 g | 157.3 ± 6.2f | 230 ± 18.2d | 3.55 ± 0.20f | ND | 3.55 ± 0.2e | 14.6 ± 2.0b |
| UAS-304 | 05.1 ± 1.6a | 273.7 ± 10.2f | 177.1 ± 5.1g | 202 ± 20.4c | 0.18 ± 0.01b | ND | 0.20 ± 0.0b | 10.5 ± 1.9a |
| UAS-415 | 15.4 ± 1.4b | 119.7 ± 12.2c | 047.8 ± 6.4b | 183 ± 10.3b | 0.45 ± 0.10d | 2.7 ± 0.4b | 3.20 ± 0.5e | 18.0 ± 3.2e |
| UAS-428 | 25.7 ± 2.1d | 157.8 ± 2.4e | 063.0 ± 4.2c | 270 ± 20.6e | 0.10 ± 0.00ab | 2.0 ± 0.1a | 2.10 ± 0.1cd | 09.4 ± 1.6a |
| DWR-162 | 20.9 ± 1.8c | 137.5 ± 10.4d | 086.4 ± 4.3d | 245 ± 11.9d | 4.07 ± 0.20g | ND | 4.00 ± 0.2e | 26.6 ± 2.9g |
| DWR-185 | 56.7 ± 3.4e | 477.1 ± 10.6h | 315.1 ± 8.6h | 249 ± 22.3d | 0.27 ± 0.00c | 1.8 ± 0.2a | 2.10 ± 0.2c | 15.6 ± 1.8b |
| DWR-1006 | 06.8 ± 0.3a | 175.8 ± 8.4e | 131.1 ± 4.0e | 113 ± 11.8a | 0.70 ± 0.20e | 4.0 ± 0.3c | 4.70 ± 0.5f | 25.5 ± 3.2f |
| DDK-1025 | 24.0 ± 1.4cd | 465.2 ± 16.4h | 146.6 ± 4.0e | 206 ± 21.2c | 0.35 ± 0.00d | 2.4 ± 0.3b | 2.70 ± 0.3de | 18.0 ± 2.4e |
| DDK-1029 | 15.2 ± 1.6b | 170.4 ± 11.8e | 069.8 ± 4.6c | 256 ± 16.9de | 0.07 ± 0.00a | ND | 0.07 ± 0.0a | 16.1 ± 1.6d |
Mean values bearing different superscripts in a columns are significantly different (p ≤ 0.05) (n = 3)
ND not detect
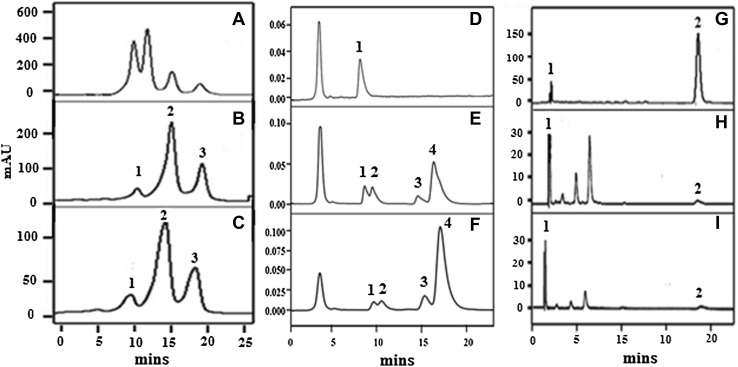
Fig. 1.
HPLC Chromatogram of various bioactives. Oryzanol like compounds (a Std. oryzanol, b UAS 428, c UAS 304), total tocols (d Std. α tocopherol e DDK 1025, f DWR 1006) and carotenoids (g Std. Mix (lutein, β carotenoid), h UAS 428, I; DWR 185) present in wheat bran oil: Wheat bran oil was extracted from the different varieties of wheat bran using soxhlet method and subjected to HPLC using different solvent system, columns and detector as provided in the materials and method. Quantifications and identifications were done by respective standards
Total tocopherol and carotenoid contents
Tocopherols having vitamin E activity ranged between 113 and 270 mg/100 g among different varieties, highest amount being in UAS-428 (270 mg/100 g) and the least in DWR-1006 (113 mg/100 g) (Table 4; Fig. 1d–f).The total carotenoid content varied between (mg/100 g) 0.07 (UAS-304) to 4.7 (DWR-1006), with an average of 2.5 mg/100 g among different varieties (Table 4; Fig. 1g–i). Lutein an antioxidant carotenoid and a pigmented nutrient that is responsible for the yellow colors of fruits and vegetables, was found to be 1.02%. Suresh Kumar et al. (2014) have also reported that total tocols content ranged from 63 to 210 mg/100 g in wheat bran oil.
Antioxidant activity of wheat bran
Antioxidant activity in wheat bran was carried out using free radical DPPH method and expressed as IC50 values. UAS 428 and 304 varieties showed the least value IC50 values of 9.4 and 10.5 indicating higher antioxidant activities compared to the other varieties (Table 4). Kim et al. (2006) have reported antioxidant activities of wheat bran extract, phenolic acids were being the major components present in the wheat bran. The main portion of the total ferulic acid was from alkaline hydrolysis. Baublis et al. (2000) studied the potential of wheat- based breakfast cereals and bran as a source of dietary antioxidants. They concluded that phenolic acids from breakfast cereals possess strong antioxidant activity in in vitro, at concentrations that would be obtained from a normal serving of whole wheat cereal. In addition, acidic conditions and enzymatic hydrolysis increased the solubility and activity of wheat phenolics suggesting that the digestive process could be important in altering the antioxidant potential of wheat-based foods.
Determinations of individual phenolic acids by HPLC
Results of polyphenols analyzed in methanol extracts are shown in Table 5; Fig. 2a–c. Significant differences were observed among samples for different phenolic acids; however, phenolic acid concentrations varied according to wheat genotype (Gelinas and McKinnon 2006). Regarding the phenolic acid profile, GA, CA, VA and CiA were observed in different wheat bran varieties. VA, CA and GA were present in all the genotypes. While, CiA was not observed in DWR varieties but in UAS genotype 304 and 428 a higher content was observed. In methanolic extract of genotype 415 CiA was not found. Hatcher and Kruger (1997) have also reported similar results in canadian wheat flours. The major constituents found in their studies were sinapic, ferulic, and vanillic acids, with minor amounts of coumaric, caffeic, and syringic acids. Verma et al. (2009) showed the release of vanillic, caffeic and other phenolic acid after processing wheat bran with acid and alkali treatments. Finally, total phenolic acid content observed to be more in DDK (~74 mg/100 g) when compared to UAS (40–65 mg/100 g) and DWR (50–60 mg/100 g) varieties.
Table 5.
Carbohydrate compositions and individual/total phenolic acid content of different wheat bran varieties
| Wheat genotypes | Carbohydrate composition (relative %) | Phenolic acid composition and content (mg/100 g) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ara | Xyl | Man | Glu | GA | CA | VA | CiN | Total phenolic acid cont. as GAE | |
| CWB | 21.3 ± 1.2e | 34.12 ± 3.2d | ND | 44.54 ± 3.8g | 4.40 ± 1.0e | ND | 13.30 ± 3.2b | 1.03 ± 0.6b | 64.20 ± 8.2c |
| UAS-304 | 13.10 ± 0.8a | 49.70 ± 1.2d | 3.68 ± 0.2c | 32.52 ± 1.2a | 1.79 ± 0.2b | 1.49 ± 0.8b | 14.84 ± 1.2cd | 2.95 ± 0.2c | 43.07 ± 6.4a |
| UAS-415 | 18.45 ± 1.2d | 53.15 ± 4.2e | 1.89 ± 0.2b | 26.49 ± 2.4a | 3.77 ± 1.2d | 4.24 ± 1.2c | 15.42 ± 4.2d | ND | 68.34 ± 7.5 |
| UAS-428 | 21.20 ± 1.8c | 25.89 ± 1.4a | 4.46 ± 0.2cd | 47.54 ± 3.2c | 1.39 ± 0.1a | 0.49 ± 0.8a | 21.30 ± 1.4g | 2.00 ± 0.2c | 46.67 ± 4.3a |
| DWR-162 | 12.32 ± 0.6b | 42.56 ± 1.4d | 4.00 ± 0.2d | 41.12 ± 1.4d | 2.95 ± 1.6c | 0.11 ± 0.6a | 06.50 ± 1.4b | ND | 50.05 ± 5.6a |
| DWR-185 | 20.94 ± 1.2d | 28.13 ± 1.2c | ND | 50.75 ± 3.4fg | 4.99 ± 1.2f | 1.24 ± 1.2b | 19.59 ± 1.2f | ND | 52.69 ± 6.2b |
| DWR-1006 | 09.20 ± 0.6a | 52.40 ± 3.6d | ND | 38.40 ± 2.3b | 0.95 ± 0.8a | 0.25 ± 0.6a | 17.52 ± 3.6e | ND | 62.66 ± 4.8b |
| DDK-1025 | 18.18 ± 1.4c | 24.36 ± 1.4b | 0.66 ± 0.1a | 49.55 ± 2.1e | 3.40 ± 0.2d | 1.09 ± 1.4b | 14.60 ± 1.4cd | 0.40 ± 0.1a | 74.61 ± 8.3c |
| DDK-1029 | 14.87 ± 1.4b | 23.50 ± 1.4a | 0.88 ± 0.1a | 59.49 ± 3.6f | 4.56 ± 0.8e | 1.19 ± 1.4b | 10.19 ± 1.4a | 0.75 ± 0.1e | 72.89 ± 6.6c |
Mean values bearing different superscripts in a columns are significantly different (p ≤ 0.05) (n = 3)
Ara arabinose, Xyl xylose, Man mannose, Glu glucose, GA gallic acid, CA caffeic acid, VA vanillic acid, CiN cinnamic acid, ND not detected, GAE gallic acid equivalent
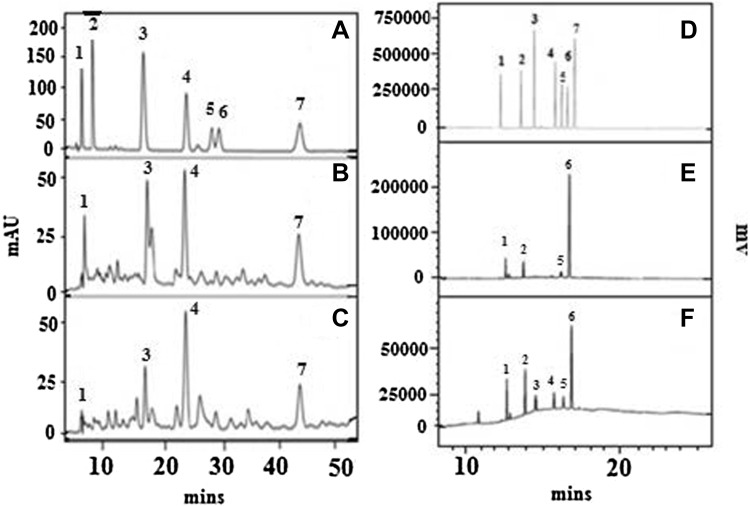
Fig. 2.
HPLC and GLC Chromatograms of phenolic acid and carbohydrate composition of selected wheat bran varieties. Conditions used for phenolic acid: λ; 280 nm, mobile phase; water: methanol: acetic acid (80:18:2), isocratic, flow rate; 1 mL/min. Standards of various phenolic acids; GA, PC, CA, VA, pCA, FA and CiA were used for the identification of phenolic acids (a Std Mix, b UAS304, c DWR162). Carbohydrate composition of samples (10 mg) hydrolyzed with 72% H2SO4, neutralized and deionized with barium carbonate and Amberlite IR 120 resin, respectively. Sodium borohydride (20–30 mg), dry and distilled acetic anhydride and pyridine (0.5 mL each) were used for reduction and acetylation reactions for sugars followed by alditol acetate extracted using chloroform and dried under nitrogen gas. Before injection, the sample was diluted with known volume of chloroform and injected (1 μL). Conditions: Capillary column Rtx-2330 (30 m × 0.32 mm i.d × 0.2 µm thickness). Temperature program: 175 °C (2 min), 8 °C/min↑, 240 °C(1 min), 8 °C/min↑, 265 °C (12 min). Injector temp 275 °C, Detector Temp 275 °C, Nitrogen flow mL/min, Standard alditol acetate containing (Peaks 1. Rha, 2. Ara, 3. Xyl, 4. Man, 5. Gal and 6. Glu.). (d Std Mix, e DDK1029, f UAS428)
Carbohydrate composition of different wheat bran varieties
The carbohydrate composition of wheat bran is presented in Table 5, major sugars in both durum and aestivum varieties were arabinose, glucose, xylose and to a lesser extent of galactose and mannose (Table 5; Fig. 2d–f). However, sugar composition varied between different traits. The high concentration of arabinose, xylose and glucose could be attributed to the presence of carbohydrate polymers like arabinoxylans in brans of many cereals and pulses. Revanappa et al. (2010) have reported sugar composition of native and purified fractions of DWR-162 and MACS wheat bran varieties, arabinose and xylans were higher and glucose was the major sugar, The present results are similar to the work carried out by Barrett and Gibson (2012), who have estimated the sugars in wheat bran. In another study Nandini and Salimath (2010) reported carbohydrate composition of commercial wheat bran, whose major sugars were arabinose, xylose and glucose and the results are in agreement with present work.
Conclusion
Bran from eight wheat varieties were screened for their bioactive compounds, dietary fiber (DF) was found to be between 21 and 52% among the varieties chosen for the study. Minerals (Fe, Mg, K, Zn, Cu, Na and Mn) content was highest in UAS 428 varieties, while Ca content was comparatively lower. The total sterol ferulates, tocopherols and carotenoids were found in higher amounts in UAS-428 and least in DWR 1006. The antioxidant capacity of extracts of wheat bran from UAS-428 and 304 had the highest activity. The results of the study showed that genotypic wheat bran may be a valuable source of nutraceuticals with health protective/promoting potential and suitable for preparation of nutra-enriched food products. The leading food manufacturers have already started fortifying food products with bran with over whelming health-promoting potentials. Thus the wheat bran promises to be a potential ingredient in functional foods.
Acknowledgements
The authors are thankful to the Director, CSIR-CFTRI, Mysore, for providing the necessary infrastructure. We are grateful for financial support under the fellowship scheme of University Grant Commission, New Delhi, INDIA. The authors also express their thanks to Dr. S. Yella Reddy for his suggestions and timely help with the manuscript and Mr. Lokesh, Dept. PCT, CSIR-CFTRI, Mysore, for his help in the extraction of oil.
Abbreviations
- GA
Gallic acid
- PC
Protocatechuic acid
- CA
Caffeic acid
- VA
Vanillic acid
- pCA
p-Coumaric acid
- FA
Ferulic acid
- CiA
Cinnamic acid
- Rha/Fuc
Rhamnose/fucose
- Ara
Arabinose
- Xyl
Xylose
- Man
Mannose
- Gal
Galactose
- Glu
Glucose
- TDF
Total dietary fiber
- SDF
Soluble dietary fiber
- IDF
Insoluble dietary fiber
Compliance with ethical standards
Conflict of interest
All the authors declare that there is no conflict of interest in publishing this manuscript.
References
- Agudo A, Amiano P, Barcos A, Barricarte A, Beguiristain JM, Chirlaque MD, Dorronsoro M, Gonzalez CA, Lasheras C, Martinez C, Navarro C, Pera G, Quiros JR, Rodriguez M, Tormo MJ. Dietary intake of vegetables and fruits among adults in five regions of Spain. EPIC Group of Spain. European Prospective Investigation into Cancer and Nutrition. Eur J Clin Nutr. 1999;53:74–80. doi: 10.1038/sj.ejcn.1600694. [DOI] [PubMed] [Google Scholar]
- AOAC (1992) Method 991.43. Total, soluble and insoluble dietary fiber in foods. Enzymatic-gravimetric method, MES-TRIS buffer. Official Method of Analysis of the Association of official Analytical chemists, 15th ed., 3rd suppl. Association: Arlington, VA
- AOAC . Official methods of analysis of AOAC International. 18. USA: AOAC Int Maryland; 2005. [Google Scholar]
- AOCS (1998) Official methods and recommended practices of the American Oil Chemists’ Society. American Oil Chemists’ Society, Champaign
- Barrett JS, Gibson PR. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols and non-allergic food intolerance: FODMAPs or food chemicals? Ther Adv Gastroenterol. 2012;4:261–268. doi: 10.1177/1756283X11436241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baublis AJ, Lu C, Clydesdale FM, Decker EA. Potential of wheat-based breakfast cereals as a source of dietary antioxidants. J Am Coll Nutr. 2000;19:308S–311S. doi: 10.1080/07315724.2000.10718965. [DOI] [PubMed] [Google Scholar]
- Black R. Micronutrient deficiency: an underlying cause of morbidity. Bull World Health Organ. 2003;81(2):79. [PMC free article] [PubMed] [Google Scholar]
- Butt MS, Ihsanullah QM, Anjum FM, Abdul A, Randhawa AM. Development of minerals enriched brown flour by utilizing wheat milling by- products. Int J Food Saf. 2004;3:15–20. [Google Scholar]
- Chang JM, Cheng CM, Hung LM, Chung YS, Wu RY. Potential use of plectranthus amboinicus in the treatment of rheumatoid arthritis. Evid Based Complement Altern Med. 2010;7:115–120. doi: 10.1093/ecam/nem168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnane SC, Anderson MJ. The majority of dietary linoleate in growing rats is beta-oxidized or stored in visceral fat. J Nutr. 1997;127:146–152. doi: 10.1093/jn/127.1.146. [DOI] [PubMed] [Google Scholar]
- Eposito F, Arlottib G, Bonifatib AM, Napolitanoa A, Vitalec D, Foglianoa V. Antioxidant activity and dietary fibre in durum wheat bran by-products. Food Res Int. 2005;38:1167–1173. doi: 10.1016/j.foodres.2005.05.002. [DOI] [Google Scholar]
- Firestone D (1998) Official methods and recommended practices of the American oil chemists Society (AOCS) method no. Ce 1–62, 5th edn, Champaign, 3:38
- Gelinas P, McKinnon CM. Effect of wheat variety, farming site, and bread-baking on total phenolics. Int J Food Sci Technol. 2006;41:329–332. doi: 10.1111/j.1365-2621.2005.01057.x. [DOI] [Google Scholar]
- Gopala Krishna AG, Khatoon S, Shiela PM, Sarmandal CV, Indira TN, Mishra A. Effect of refining of crude rice bran oil on the retention of oryzanol in the refined oil. J Am Oil Chem Soc. 2001;78:127–131. doi: 10.1007/s11746-001-0232-0. [DOI] [Google Scholar]
- Gorinstein S, Zachwieja Z, Folta M, Barton H, Piotrowicz J, Zember M. Comparative content of dietary fibre, total phenolics, and minerals in persimmons and apples. J Agric Food Chem. 2001;49:952–957. doi: 10.1021/jf000947k. [DOI] [PubMed] [Google Scholar]
- Hatcher DW, Kruger JE. Simple phenolic acids in flours prepared from Canadian wheat: relationship to ash content, color, and polyphenol oxidase activity. Cereal Chem. 1997;74(3):337–343. doi: 10.1094/CCHEM.1997.74.3.337. [DOI] [Google Scholar]
- Kim KH, Tsao R, Yang R, Cui SW. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006;95:466–473. doi: 10.1016/j.foodchem.2005.01.032. [DOI] [Google Scholar]
- Nandini CD, Salimath PV. Carbohydrate composition of wheat bran, sorghum and bajra with good chapati/roti (Indian flat bread) making quality. Food Chem. 2010;73:197–203. doi: 10.1016/S0308-8146(00)00278-8. [DOI] [Google Scholar]
- Nystrom L, Paasonen A, Lampi A, Piironen V. Total plant sterols, steryl ferulates andsteryl glycosides in milling fractions of wheat and rye. J Cereal Sci. 2007;45:106–115. doi: 10.1016/j.jcs.2006.08.003. [DOI] [Google Scholar]
- Nyström L, Lampi AM, Rita H, Aura AM, Oksman-Caldentey KM, Piironen V. Effects of processing on availability of total plant sterols, steryl ferulates and steryl glycosides from wheat and rye bran. J Agric Food Chem. 2007;55:9059–9065. doi: 10.1021/jf071579o. [DOI] [PubMed] [Google Scholar]
- Ramadan MF, Kroh LW, Moersel JT. Radical scavenging activity of black cumin (Nigella sativa L.), coriander (Coriandrumsativum L.) and Niger (Guizotiaabyssinica Cass) crude seed oils and oil fractions. J Agric Food Chem. 2003;51:6961–6969. doi: 10.1021/jf0346713. [DOI] [PubMed] [Google Scholar]
- Reddy BS, Hirose Y, Cohen LA, Simi B, Cooma I, Rao CV. Preventive potential of wheat bran fractions against experimental colon carcinogenesis: implications for human colon cancer prevention. Cancer Res. 2000;17:4792–4797. [PubMed] [Google Scholar]
- Revanappa SB, Nandini CD, Salimath PV. Structural characterisation of pentosans from hemicellulose B of wheat varieties with varying chapati-making quality. Food Chem. 2010;119:27–33. doi: 10.1016/j.foodchem.2009.04.064. [DOI] [Google Scholar]
- Rokhade CJ, Rao M. Effect of location on the composition of wheat and acceptability of traditional products. Karnataka J Agric Sci. 2005;18:1044–1047. [Google Scholar]
- Safdar MN. Composition of wheat grain, wheat milling news, physiochemical quality assessment of wheat grown in different regions of Punjab. Pak J Agric Res. 2005;22:1–2. [Google Scholar]
- Sawardekar JS, Slonekar LS, Jeanes A. Quantitative determination of monosaccharides as their alditol acetates by gas liquid chromatography. Anal Chem. 1967;59:430–435. [Google Scholar]
- Suresh Kumar G, Gopala Krishna AG. Studies on the nutraceuticals composition of wheat derived oils wheat bran oil and wheat germ oil. J Food Sci Technol. 2015;52:1145–1151. doi: 10.1007/s13197-013-1119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh Kumar G, Swathi R, Gopala Krishna AG. Fat-soluble nutraceuticals and their composition in heat-processed wheat germ and wheat bran. Int J Food Sci Nutr. 2014;65(3):327–334. doi: 10.3109/09637486.2013.866640. [DOI] [PubMed] [Google Scholar]
- Verma B, Hucl P, Chibbar RN. Phenolic acid composition and antioxidant capacity of acid and alkali hydrolysed wheat bran fractions. Food Chem. 2009;116:947–954. doi: 10.1016/j.foodchem.2009.03.060. [DOI] [Google Scholar]
- Wilson TA, Nicolosi RJ, Woolfrey B, Kritchevsky D. Rice bran oil and oryzanol reduce plasma lipid and lipoprotein cholesterol concentrations and aortic cholesterol ester accumulation to a greater extent than ferulic acid in hypocholesterolemic hamsters. J Nutr Biochem. 2007;18:105–112. doi: 10.1016/j.jnutbio.2006.03.006. [DOI] [PubMed] [Google Scholar]