Abstract
Oat emulsion gels and oil-free oat gels were formulated with varying proportions of oat bran/olive oil (from 12/40 to 28/0) without or with alginate or gelatin used as animal fat replacers and/or to provide β-glucan and MUFA for meat products. Composition, technological properties (thermal stability, colour, texture, etc.) and the effects of chilled and frozen storage of samples were evaluated. Depending on the proportion, samples developed for use as animal fat replacers in meat products may endow these with properties qualifying them for nutrition and health claims. No samples showed any noticeable syneresis and all showed good thermal stability. Increasing of oat bran/olive oil increased a* and reduced b* values, while differences in L* depended on the gelling agent. Penetration force (PF) and gel strength increased when the oat bran/oil ratio increased, with the highest values in the samples containing alginate or gelatin. Thermal losses and PF generally increased during chilled and frozen storage, and no significant differences were observed in colour or pH over storage.
Keywords: Oat emulsion gel, Oil-free oat gel, Alginate, Gelatin, Olive oil
Introduction
Reformulation is one of the most important approaches to improving the fat content of meat products and developing healthy and functional foods. Different vegetable and marine oils, or combinations of these, have been used to partially replace animal fat for a better (healthy) fatty acid profile and lower fat content in meat products. However, these liquid lipid materials have different physicochemical characteristics from habitually used meat fats, which may have a negative effect on the desired quality attributes in the reformulated product (Jimenez-Colmenero 2007). Novel proposals for liquid-phase oil stabilization and structuring have recently been reported based on the modification or structuring of oils to create a plastic fat which retains solid-like properties while possessing a healthier fatty acid profile (Jimenez-Colmenero et al. 2015). In this context, it should be highlight the possibilities offered by the formation of structured emulsions such as hydrogelled emulsions (emulsion gels). Such a solid-like emulsion gel may be generated from a stable liquid-like emulsion by gelling (by thermal, enzymatic or chemical means) the continuous phase and/or aggregating the emulsion droplets (Jimenez-Colmenero et al. 2015).
Oat bran is a potentially useful ingredient in reformulation to produce healthy and functional meat products, since it contains healthy bioactive compounds and possesses useful technological properties. Oat bran contains high-quality proteins, vitamins and unsaturated fatty acids; however, the chief nutritional attribute of oat bran is that it provides soluble dietary fibre, primarily β-glucan (Arendt and Zannini 2013) which is associated with several health benefits (Wolever et al. 2010). A health claim has been associated with this component: oat beta-glucan can actively lower/reduce blood LDL and total cholesterol (EFSA 2010).
The proteins and β-glucans (cell-wall polysaccharides) present in oats have useful emulsifying and gelling properties depending on the conditions. In this regard, chemically modified oat protein (mainly globulins) has been described as an emulsifier (Laine et al. 2011). Additionally, oat protein has been used as a gelling agent, being its gel properties conditioned by various different treatments such as pH, thermal processing, enzymatic hydrolysis or addition of microbial transglutaminase, inulin, etc. (Siu et al. 2002). Then again, β-glucans extracted from barley and oats had good emulsion capacity, offering useful applications such as thickening and stabilizing of emulsions (Brummer et al. 2014; Burkus and Temelli 2000; Kontogiorgos et al. 2004; Lazaridou and Biliaderis 2007; Santipanichwong and Suphantharika 2009). Cereal β-glucan hydrogels with diverse molecular characteristics and properties have been produced under various isothermal conditions (5–45 °C), molecular sizes and polymer concentrations (Lazaridou and Biliaderis 2007). In light of all these statements, the use of oat bran would seem a promising option for the development of structured emulsions (particularly emulsions gels) and gels with solid-like properties. An interesting alternative could be to use oat bran with a cold gelling agent to reinforce their solid-like characteristic.
Of vegetable oils used as animal fat replacers, olive is the one that has received most attention because of its high biological value, including high MUFA content and antioxidants (Jimenez-Colmenero 2007). The combination of olive oil and oat bran could confer attractive additional nutritional and healthy properties. Structured emulsions and gels containing oat bran and olive oil could be excellent carriers for delivery of healthy bioactive compounds in consumer diets, such as β-glucans, dietary fibre, unsaturated fatty acids (mainly MUFAs from olive oil), antioxidants, etc. In this regard, previous studies have shown the potential of emulsion gels containing a variety of bioactive compounds formulated with olive oil, chia (source of α-linolenic fatty acids, dietary fibre, minerals, etc.) and cold gelling agents with suitable technological and nutritional properties, for use as healthier fat ingredients (Pintado et al. 2015).
Strategies for structuring healthy oils based on the use of oat bran do then seem a reasonable approach, but it has not yet been explored. The objective of this work was therefore to assess the possibility of developing oat bran structured emulsions (hydrogelled emulsions) and gels to obtain a new solid-like lipid material for use as an animal fat replacer and provide certain bioactive compounds in healthier-lipid reformulation of meat products. To that end, various proportions of oat bran/olive oil and alginate or gelatin were assayed to produce stabilized and structured (oil-in-water, O/W) emulsions in hydrogels (emulsion gels) and oil-free gels with appropriate nutritional (mainly as sources of β-glucans and MUFAs) and technological properties. The technological properties of the new lipid material were estimated in different formulation conditions. Additionally, the effects of chilling and frozen storage were evaluated in the structured emulsions and gels since these can influence their technological characteristics, hence affecting the quality of meat products containing them, which are typically stored chilled or frozen.
Materials and methods
Design and preparation of samples
Various proportions of oat bran, olive oil and gelling agents (alginate or gelatin) were assayed to develop a lipid material with a solid-like structure. Oat bran used containing 20% protein, 20% carbohydrates, 44% total dietary fibre (of which 22% β-glucan soluble fibre) and 5.1% fat according to supplier. Oat bran and olive oil concentrations employed, as well as the presence of alginate or gelatin in the new lipid material in the form of structured O/W oat emulsions in hydrogel-emulsion gels, were prepared based on both technological considerations (formation of the different solid-like structures) and compositional aspects (high concentration of bioactive compounds). This last aspect include assessing the possibility of using nutritional and health claims when these lipid materials can be included in foods such as meat products (Parliament 2006, 2012). Indeed, the main purpose of this work was to modulate the solid-like properties of structured emulsions and gels so as to mimic animal fat as nearly as possible, since they are designed for use as fat replacers, ideally without detrimental changes in quality properties of the final product in which they are included. Additionally, oil-free gels were prepared as olive oil-free samples. Alginate and gelatin concentrations (Table 1) were based on previous studies (Pintado et al. 2015).
Table 1.
Formulation (%) of different oat emulsion gels and oil-free gels
| Samplesa | Oat bran | Olive oil | Water | Alginate | Gelatin |
|---|---|---|---|---|---|
| OEW 12/40 | 12 | 40 | 48 | 0 | 0 |
| OEA 12/40 | 12 | 40 | 46 | 2 | 0 |
| OEG 12/40 | 12 | 40 | 46 | 0 | 2 |
| OEW 14/35 | 14 | 35 | 51 | 0 | 0 |
| OEA 14/35 | 14 | 35 | 49 | 2 | 0 |
| OEG 14/35 | 14 | 35 | 49 | 0 | 2 |
| OEW 16/30 | 16 | 30 | 54 | 0 | 0 |
| OEA 16/30 | 16 | 30 | 52 | 2 | 0 |
| OEG 16/30 | 16 | 30 | 52 | 0 | 2 |
| OEW 18/25 | 18 | 25 | 57 | 0 | 0 |
| OEA 18/25 | 18 | 25 | 55 | 2 | 0 |
| OEG 18/25 | 18 | 25 | 55 | 0 | 2 |
| OEW 20/20 | 20 | 20 | 60 | 0 | 0 |
| OEA 20/20 | 20 | 20 | 58 | 2 | 0 |
| OEG 20/20 | 20 | 20 | 58 | 0 | 2 |
| OEW 22/15 | 22 | 15 | 63 | 0 | 0 |
| OEA 22/15 | 22 | 15 | 61 | 2 | 0 |
| OEG 22/15 | 22 | 15 | 61 | 0 | 2 |
| OEW 24/10 | 24 | 10 | 66 | 0 | 0 |
| OEA 24/10 | 24 | 10 | 64 | 2 | 0 |
| OEG 24/10 | 24 | 10 | 64 | 0 | 2 |
| OEW 26/5 | 26 | 5 | 69 | 0 | 0 |
| OEA 26/5 | 26 | 5 | 67 | 2 | 0 |
| OEG 26/5 | 26 | 5 | 67 | 0 | 2 |
| OGW 28/0 | 28 | 0 | 72 | 0 | 0 |
| OGA 28/0 | 28 | 0 | 70 | 2 | 0 |
| OGG 28/0 | 28 | 0 | 70 | 0 | 2 |
aSample denominations for oat emulsion gels (OE): without added gelling agent (OEW), containing alginate (OEA) and gelatin (OEG). Sample denominations for oil-free oat gels (OG): without added gelling agent (OGW), containing alginate (OGA) and gelatin (OGG)
Three types of olive oil-in-water structured (hydrogelled) emulsions (emulsion gels) were formulated: an oat emulsion gel without added gelling agent (OEW) and two types of oat emulsion gels with added gelling agent, one with alginate (OEA) and the other with gelatin (OEG). From each of these types of emulsion gel, various formulations were made up containing different oat bran (12–28% w/w) and olive oil (5–40% w/w) concentrations (Table 1). Additionally, three types of oil-free oat gels were prepared (Table 1), one without a gelling agent (OGW) and two with alginate (OGA) or gelatin (OGG).
Samples of 700 g were prepared (in duplicate) for each formulation by mixing the ingredients in a homogenizer (Thermomix TM 31, Vorwerk España M.S.L., S.C, Madrid, Spain). To prepare OEW, firstly the oat bran powder (OatWell® 22%, Zeus Química, S.A. Barcelona, Spain) was mixed with water in different proportions as indicated in Table 1, for 30 s at high speed (approx. 5600 rpm). The final mixture was mixed at approx. 5600 rpm with gradual addition of the corresponding olive oil (Carbonell Virgen Extra, SOS Cuétara SA, Madrid, Spain) (Table 1). In OEA or OEG the gelling agent, alginate and gelatin (Tradissimo, TRADES S.A., Barcelona Spain), was added and the sample mixed for 15 s (at approx. 5600 rpm) before the olive oil was added. The alginate gelling agent was prepared beforehand by mixing sodium alginate (0.73%), CaSO4 (0.73%) and tetra-sodium pyrophosphate anhydrous (0.54%) (the last two previously dissolved in water). Oil-free oat gels were prepared by mixing oat bran and water (OGW), or oat bran, water and the gelling agent; alginate (OGA) or gelatin (OGG) (Table 1). Finally, each sample was placed in a metal container (designed for cooked ham) (IMPOTUSA, SA, Navarra, Spain) under pressure to compact it and prevent air bubbles, and stored in a chilled room at 2 °C for 24 h. Similar amounts of samples (about 700 g) were added to each metal container. The same maximum pressure mark level which permits this amount of sample was chosen in this container for all samples to obtain similar pressure (aprox. maximum mark three for this amount of sample).
The weight of sample to metal container and these containers have characteristics marks identified that permit chose the same pressure level for all samples.
The different formulations (Table 1) were divided into pieces of approximately 150–190 g and packed (after weighing) in plastic bags (Cryobac ® BB3050) in aerobic conditions. For each formulation part of the samples was stored in a chilled room at 2 °C (±1) for 15 days and another part were frozen in an air blast freezer (Frigoscandia, Aga, Freezer Division, Helsingborg, Sweden), until the thermal centre reached −20 °C (within 1 h 30 min), and the resulting frozen samples were stored at −20 ± 2 °C for 15 days. Analyses were carried out at 0, 6 and 15 days for chilled storage and 1 and 15 days to evaluate the effect of frozen storage (including freezing, frozen storage and thawing). The frozen samples were thawed for analysis (20 h at 4 °C). The entire experimental procedure was performed in duplicate.
Technological properties
Syneresis
Syneresis, evaluated as content of exudate, including fat and water losses, was determined in samples by weight difference (%) between initial, chilled, freezing/frozen storage.
Thermal stability
Thermal stability, in terms of water and fat binding properties, of the samples was determined (in triplicate) by measuring water and fat loss during heating. Whole portions (25–30 g, accurately weighed) of appropriate dimensions were placed in cylindrical plastic tubes (FalconTM 50 ml, diam. 30 mm × 115 mm), which were hermetically sealed and heated in a water bath for 30 min at 70 °C. They were then opened and left to stand upside down (for 50 min) to release the separated fat and water on to a plate. Matrix stability, as total fluid release (TFR), was expressed as % of initial sample weight. Water release (WR) was determined as % weight loss after heating the total released fluid (fat and water) for 16 h on a stove at 100 °C. Fat release (FR) was calculated as the difference between total loss (measured as % of initial sample weight) and water loss (Jimenez-Colmenero et al. 1995).
pH
Sample pH was measured in quadruplicate using a 827 Metrohm pH-meter (MetrohmAG, Switzerland) at room temperature on homogenates of samples in distilled water in a ratio of 1:10 w/v.
Colour measurement
Surface colour determinations were evaluated (Konica Minolta CM-3500d colorimeter. Konica Minolta, Madrid, Spain) with the samples placed in glass Petri dishes (9 mm diameter). Colour, CIE-LAB tristimulus values, lightness, L*; redness, a* and yellowness, b* of the samples were evaluated. Nine determinations were carried out for each sample.
Textural properties
Textural properties were determined (six measurements) by penetration test performed in a TA-XT plus Texture Analyzer (Texture Technologies Corp., Scarsdale, NY). Penetration test was performed with a load cell of 5 kg and a 4 mm diameter cylindrical stainless steel plunger at a velocity of 0.8 mm/s and force exerted at 10 mm. The textural parameters evaluated were: (a) penetration force (PF, N), which is the maximum force in the curve, and (b) gel strength (GS, mJ) defined as the area enclosed by the force–deformation curve at the point of gel rupture. When no point of rupture was found, PF and GS were not calculated.
Statistical analysis
Analyses of variance (ANOVA) were performed to evaluate the statistical significance (p < 0.05) of the effect of sample formulation and storage time using IBM SPSS Statistics 22 (SPSS Inc., Chicago, Ill., USA). Least squares differences were used for comparison of mean values among formulations and Tukey’s HSD test to identify significant differences (p < 0.05) between formulations and storage time. Pearson product moment correlation (R) was performed using Statgraphics Plus version 5.0 to determine the relationships between oat bran, olive oil, water and gelling agents added (alginate or gelatin) and technological properties data.
Results and discussion
Considerations regarding sample composition
In order to understand differences in sample properties, as well as sample behaviour during processing, some considerations need to be set out regarding their composition (estimated from Table 1). Thus, protein content of the samples ranged between 2.4 and 5.6%, derived from oat bran, although the samples containing 2% gelatin (84% protein content) additionally contained 1.68% protein. Oat bran proteins consist mainly of globulins, although they also contain smaller proportions of albumins, glutelins and prolamines (Klose and Arendt 2012). Their low prolamine content (4–15%) makes them suitable for inclusion in gluten-free diets (Storsrud et al. 2003). Compared to other cereal proteins, oat protein also provides a better amino acid balance as it contains higher amounts of limiting amino acids lysine and threonine, which are essential for humans and other monogastric animals (Klose and Arendt 2012).
Oat bran contain the bulk of the dietary fibre (Malkki and Virtanen 2001). In our experimental conditions, the total dietary fibre (TDF) content in samples varied from 5.3 to 12.3% depending on oat bran content. Dietary fibre has been recognized as an important part of the complete diet and a fibre intake of 28–36 g/day is recommended for adults (Mehta et al. 2015). β-glucan content in samples ranged from 2.6 to 6.2% depending on the amount of oats added. β-glucans are linear homopolysaccharides of d-glucopyranosyl residues linked via β-(1 → 3) and β-(1 → 4) linkages, mainly forming (1 → 3)-linked cellotriosyl and (1 → 3)-linked cellotetraosyl units (Wood et al. 1994). β-glucans extracted from cereals such as barley or oats play a significant role in reduce cardiovascular diseases or the symptoms of diabetes (Tapola et al. 2005; Wolever et al. 2010).
Emulsion gel fat content ranged between 40.2% (OEW, OEA and OEG 12/40) and 6.2% (OEW, OEA and OEG 26/5); for all oil-free gels (OGW, OGA and OGG 28/0) fat content was 1.4%. The fat source in emulsion gels (OEW, OEA and OEG) was mainly olive oil, while in oat gels (OGW, OGA and OGG) it was only oat bran. It is important to note that olive oil containing 14.87% SFA, 75.32% MUFA and 8.97% PUFA) (Delgado-Pando et al. 2010) and oat bran 5.1% fat (0.9% SFA; 2.1% MUFA and 1.9% PUFA). Thus, maximum values of MUFAs, PUFAs and SFA were 30.1, 3.8 and 0.1% for emulsion gels OEW, OEA and OEG 12/40, while minimum values were registered in OEW, OEA and OEG 26/5 with 4.3% MUFAs, 0.9% PUFAs and 1.0% SFA. In oil-free gels (OGW, OGA and OGG 28/0) the fatty acid profiles were 0.2%; 0.6 and 0.5% for SFA, MUFA and PUFA contents respectively.
Oat emulsion gels and oil-free gels showed energy values between 61.6 and 393.12 kcal/100 g. These energy values were based on 9 kcal/g for fat; 4 kcal/g for protein and carbohydrates and 2 kcal/g for dietary fibre (Moreiras et al. 2013). If we compare, for example, the calorie content of these oat emulsion gels and oil-free gels (as healthier fat ingredients) with the pork backfat normally used in making meat products, whose energy value is about 673 kcal/100 g (Moreiras et al. 2013), the calorie count could be reduced by over 30%.
Additionally, in view of the health benefits of olive oil and oat bran, oat emulsion gels and oil-free oat gels would seem promising as healthier ingredients in food products. With the appropriate composition, their use as animal fat replacers in meat products could, depending on the amount used, warrant some nutrition and health claims under the EU Regulations 432/2012 and 1924/2006 (Parliament 2006, 2012). Possible nutritional claims could include labelling as sources of fibre and unsaturated fat (Parliament 2006). As for potential health claims, for instance given the amount of β-glucan that can be delivered in the meat product, it could be labelled helps to maintain normal blood cholesterol levels (Parliament 2012).
Technological properties
On-line preparation and use of these emulsion gels and oil-free gels is not always possible, and often prepared beforehand and used as ingredients in food product reformulation. In this regard, chilled and frozen storage are both habitually used, among other things to organize production tasks. However, the kinds of chilling and freezing/thawing processes typically used in the production of foods such as meat products can influence their characteristics, affecting the quality properties of reformulated products containing them. A clear understanding of the behaviour of oat emulsion gels and oil-free oat gels under different processing and storage conditions is important to guarantee the quality of the product containing them upon completion of production and throughout the commercialization process.
Syneresis and thermal stability
No noticeable release of exudates (syneresis) was observed in any of the samples immediately after preparation. Oat emulsion gels and oil-free gels were generally thermally stable since there was no appreciable phase separation or disruption of the gel’s physical structure after heating. All samples showed good water and fat binding ability after heating. None of the samples containing gelatin showed any noticeable TFR at day 0 (Table 2). However, emulsion gels OEW and OEA with oat/oil proportions from 12/40 to 16/30 registered TFR at day 0, with the highest (p < 0.05) values recorded for OEA 12/40 and OEW 12/40 (Table 2). These TFR proportions were due mainly to fat loss (around 94–99.8%); this behaviour seems consistent with the fact that there was no noticeable TFR in samples with lower proportions of oil and oil-free gels containing mainly water and oats (Table 1). The better thermal stability observed for oat emulsion gels and oil-free gels may be attributed to both the presence of oat bran and where applicable also to the concentration of gelling agent, mainly gelatin. Both the proteins and the β-glucans present in oat bran have been found to possess water and fat binding capacity as well as emulsion stabilizing capabilities (Burkus and Temelli 2000; Klose and Arendt 2012; Kontogiorgos et al. 2004; Lazaridou and Biliaderis 2007; Santipanichwong and Suphantharika 2009; Siu et al. 2002). It has been reported that in whey protein concentrate emulsions with added barley β-glucan the phase separation substantially decreased due to a decrease in droplet size (Burkus and Temelli 2000). Also, the inclusion of β-glucan in egg-yolk model emulsions improved their stability extending time to manifestation of creaming by network formation in the continuous phase (Kontogiorgos et al. 2004). Also, Laine et al. (2011) showed that when oat bran content in an emulsion increases, the creaming rate decreases, because the movement of oil droplets is restricted due to the large increase in viscosity of the water phase. Then again, the thermal stability of oat emulsion gels and oil-free gels was influenced by the presence of alginate and gelatin since both possess useful low-temperature gel-forming properties whereby a structural network is formed containing a large water fraction (Roopa and Bhattacharya 2010; Sala et al. 2009). Some authors have evaluated gelled emulsions stabilized by gelatin, alginate and a mixture of both according to their phase separation stability, reporting that none of the emulsions formed a bottom aqueous phase, thus showing that they were stable (Sato et al. 2014). Additionally, optimal binding (water and fat) properties have been reported in oil-in-water emulsion gels stabilized with chia (Salvia hispanica L.) and alginate or gelatin (Pintado et al. 2015).
Table 2.
Total fluid release (%) of oat emulsion gels and oil-free gels during chilled and frozen storage
| Samplesa | Day 0 | Chilled storage | Frozen storage | ||
|---|---|---|---|---|---|
| Day 6 | Day 15 | Day 1 | Day 15 | ||
| OEW12/40 | 5.38 ± 0.07bAX | 9.84 ± 0.29dB | 6.43 ± 0.91dB | 9.02 ± 0.55d Y | 5.82 ± 0.44cdX |
| OEA 12/40 | 7.13 ± 0.76bAX | 6.35 ± 0.51cA | 8.88 ± 0.24eB | 9.66 ± 0.73deY | 10.35 ± 0.26eY |
| OEG 12/40 | ND | ND | ND | ND | 3.08 ± 0.90b |
| OEW 14/35 | 3.33 ± 0.44aAX | 8.51 ± 0.68dB | 6.51 ± 0.98dB | 8.67 ± 1.35cdY | 6.93 ± 0.50dY |
| OEA 14/35 | 2.73 ± 0.16aAX | 5.53 ± 1.21cB | 5.09 ± 0.34bcdB | 11.16 ± 0.64eZ | 9.17 ± 0.65eY |
| OEG 14/35 | ND | ND | ND | ND | 3.03 ± 0.45b |
| OEW 16/30 | 2.28 ± 0.37aAX | 6.26 ± 0.54cB | 4.75 ± 0.45bcB | 4.79 ± 0.57bY | 5.56 ± 0.29cY |
| OEA 16/30 | 2.04 ± 0.60aAX | 2.89 ± 0.32bAB | 4.00 ± 0.56bB | 8.60 ± 0.45cdY | 7.02 ± 0.16dY |
| OEG 16/30 | ND | ND | ND | ND | 1.73 ± 0.09a |
| OEW 18/25 | ND | 1.59 ± 0.21bA | 1.34 ± 0.16aA | 4.54 ± 0.32bX | 3.50 ± 0.27bX |
| OEA 18/25 | ND | 0.30 ± 0.18aA | 1.69 ± 0.01aB | 6.47 ± 0.73bcY | 5.06 ± 0.16cX |
| OEG 18/25 | ND | ND | ND | ND | ND |
| OEW 20/20 | ND | ND | ND | 1.43 ± 0.23a | ND |
| OEA 20/20 | ND | ND | ND | 1.55 ± 0.24aY | 0.91 ± 0.10aX |
| OEG 20/20 | ND | ND | ND | ND | ND |
| OEW 22/15 | ND | ND | ND | ND | ND |
| OEA 22/15 | ND | ND | ND | ND | ND |
| OEG 22/15 | ND | ND | ND | ND | ND |
| OEW 24/10 | ND | ND | ND | ND | ND |
| OEA 24/10 | ND | ND | ND | ND | ND |
| OEG 24/10 | ND | ND | ND | ND | ND |
| OEW 26/5 | ND | ND | ND | ND | ND |
| OEA 26/5 | ND | ND | ND | ND | ND |
| OEG 26/5 | ND | ND | ND | ND | ND |
| OGW 28/0 | ND | ND | ND | ND | ND |
| OGA 28/0 | ND | ND | ND | ND | ND |
| OGG 28/0 | ND | ND | ND | ND | ND |
Mean ± standard deviation. Different lowercase letters in superscript (a, b, c) in the same column indicate significant (p < 0.05) differences. Different capital letters in subscript for chilled storage (A, B) and frozen storage (X, Y, Z) indicate significant (p < 0.05) differences for each type of oat emulsion gel or oil-free gel with the same oat/olive oil proportion
ND non detectable total fluid release
aFor sample description see Table 1
During chilled storage syneresis values in oat emulsion gels and oil-free oat gels were generally <5%, except for sample OEW 12/40, where it was 10%. Samples with thermal loss at day 0 (OEW and OEA from 12/40 to 16/30) showed an increase in TFR during chilled storage (Table 2). Additionally, TFR values in OEW 18/25 and OEA 18/25, which were not noticeable at day 0, increased significantly at day 6 of storage and then decreased at day 15, although values never exceeded a maximum of 1.6% (Table 2). During chilled storage, it was still observed that TFR was fat release mainly with values between 76 and 100%. None of the samples containing gelatin registered any noticeable TFR during chilled storage. According with these results, Pintado et al. (2015) reported high thermal stability of oil-in-water chia emulsion gels formulated with alginate and gelatin during chilled storage.
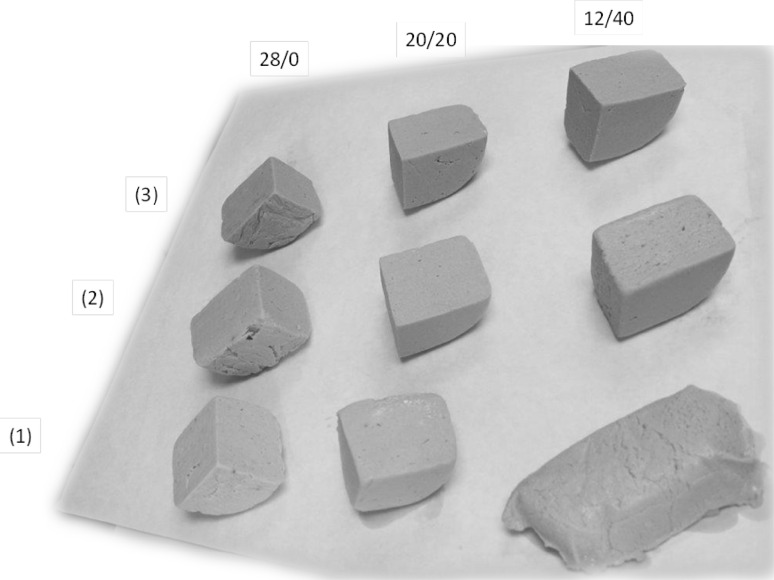
Figure 1 shows the typical appearance of the frozen/thawed oat emulsion gels and oil-free gels reported here. In samples OEW and OEA from 12/40 to 16/30, which registered some initial thermal losses (TFR), TFR increased significantly during frozen storage, with the greatest increase at day 1 in sample OEW 12/40 and OEA 14/35 (Table 2). Also, TFR increased significantly in samples OEW and OEA 18/25 and 20/20 over frozen storage, although no TFR registered at day 0. TFR values were less than 6.5% for an oat/oil proportion of 18/25 and <1.6% for 20/20 (Table 2). In general, samples containing gelatin registered no noticeable TFR during frozen storage except at day 15 in samples with an oat/oil proportion of 12/40–16/30, although values were <3%. About 95% of TFR was fat, since water losses were less than 0.5% (data not shown) in all samples, with no significant variation (p > 0.05) over storage.
Fig. 1.
Oat emulsion gels and oil-free gels freezing/thawed at day 15: 1 without gelling agent added, 2 with alginate and 3 with gelatin. Oat bran/olive oil proportions: 28/0, 20/20 and 12/40
pH
pH values ranged between 6.48 and 7.21 and were influenced (p < 0.05) by the oat/oil proportion and the presence of gelling agent (Table 3). It was found that when the oat concentration increased (and the oil concentration decreased), there was a significant reduction in pH except in the samples containing gelatin, where the effect was the opposite (Table 3). If we compare oat emulsion gels and oil-free gels formulated with the same oat/oil proportion, it was found that the samples made with gelling agents registered lower (p < 0.05) pH values, especially (p < 0.05) the samples with gelatin. Some authors have observed that dairy products fortified with β-glucan concentrate exhibit significantly lower pH (Volikakis et al. 2004) which would explain why pH decreased when the oat concentration was increased in samples containing only oat bran (OEW) and the ones containing oat bran and alginate (OEA) (Table 3). Then again, previous studies have indicated that chia emulsion gels formulated with gelatin exhibited lower pH values than ones formulated with alginate (Pintado et al. 2015). These findings are consistent with the lower pH values found in oat emulsion gels and oil-free gels made with gelatin. For purposes of using these samples as fat replacers in foods such as meat products, it is important to bear in mind that all pH values (Table 3) are within the ranges reported in pork backfat (Jimenez-Colmenero et al. 2012), which implies that any of these samples show limitations for use as fat replacers in the reformulation of meat products.
Table 3.
pH values and colour parameters (L* lightness. a* redness and b* yellowness) of oat emulsion gels and oil-free gels
| Samplesa | pH | Colour parameters | ||
|---|---|---|---|---|
| L* | a* | b* | ||
| OEW 12/40 | 7.20 ± 0.00kl | 62.66 ± 1.34a | 0.83 ± 0.07bc | 19.93 ± 0.57m |
| OEA 12/40 | 7.16 ± 0.00k | 65.68 ± 0.90bcde | 0.88 ± 0.08bc | 19.57 ± 0.22m |
| OEG 12/40 | 6.48 ± 0.04a | 76.76 ± 1.01m | 0.37 ± 0.09a | 17.85 ± 0.19ghij |
| OEW 14/35 | 7.17 ± 0.02kl | 62.95 ± 1.17a | 0.86 ± 0.07bc | 19.44 ± 0.79m |
| OEA 14/35 | 7.05 ± 0.01ij | 67.46 ± 1.18fgh | 1.22 ± 0.25ef | 18.70 ± 0.29l |
| OEG 14/35 | 6.59 ± 0.01b | 76.58 ± 0.49m | 0.52 ± 0.07a | 17.50 ± 0.31efgh |
| OEW 16/30 | 7.18 ± 0.01kl | 63.18 ± 0.56a | 0.95 ± 0.08bcd | 18.48 ± 0.38jkl |
| OEA 16/30 | 6.96 ± 0.01g | 67.11 ± 2.16def | 1.19 ± 0.30def | 18.68 ± 0.31 l |
| OEG 16/30 | 6.63 ± 0.02b | 73.97 ± 0.84 l | 0.78 ± 0.08b | 17.35 ± 0.38defg |
| OEW 18/25 | 7.19 ± 0.01kl | 64.09 ± 0.46ab | 1.07 ± 0.08cde | 17.61 ± 0.40fgh |
| OEA 18/25 | 7.01 ± 0.01h | 70.91 ± 0.51k | 1.87 ± 0.09i | 18.55 ± 0.33kl |
| OEG 18/25 | 6.71 ± 0.11c | 70.10 ± 1.42jk | 1.41 ± 0.10fg | 18.04 ± 0.29hijk |
| OEW 20/20 | 7.21 ± 0.01l | 65.30 ± 1.01bcd | 1.31 ± 0.22f | 17.67 ± 0.23fghi |
| OEA 20/20 | 7.03 ± 0.04hi | 68.23 ± 1.56fghi | 1.60 ± 0.23gh | 18.29 ± 0.30ijkl |
| OEG 20/20 | 6.76 ± 0.01d | 68.19 ± 1.13fghi | 1.74 ± 0.11hi | 17.91 ± 0.50ghij |
| OEW 22/15 | 7.08 ± 0.01j | 66.97 ± 0.52cdef | 1.24 ± 0.09ef | 16.59 ± 0.27abc |
| OEA 22/15 | 6.94 ± 0.01g | 69.69 ± 0.77ijk | 1.77 ± 0.11hi | 17.48 ± 0.26efgh |
| OEG 22/15 | 6.79 ± 0.01de | 68.38 ± 0.60fghij | 1.73 ± 0.04hi | 17.21 ± 0.35cdef |
| OEW 24/10 | 7.06 ± 0.01ij | 67.42 ± 0.94efg | 1.23 ± 0.12ef | 16.23 ± 0.22ab |
| OEA 24/10 | 6.95 ± 0.00g | 69.44 ± 1.20hijk | 1.94 ± 0.13ij | 17.55 ± 0.14fgh |
| OEG 24/10 | 6.80 ± 0.01de | 68.09 ± 0.57fghi | 1.94 ± 0.08ijk | 17.12 ± 0.28cdef |
| OEW 26/5 | 7.07 ± 0.01j | 67.49 ± 0.85efg | 1.86 ± 0.12i | 16.58 ± 0.29abc |
| OEA 26/5 | 6.94 ± 0.01g | 68.99 ± 0.81ghij | 1.97 ± 0.08ijk | 17.51 ± 0.12efgh |
| OEG 26/5 | 6.85 ± 0.01f | 66.92 ± 0.83cdef | 1.88 ± 0.12i | 16.13 ± 0.25ab |
| OGW 28/0 | 7.08 ± 0.01i | 66.30 ± 0.63cdef | 2.16 ± 0.10jkl | 16.76 ± 0.18bcd |
| OGA 28/0 | 6.94 ± 0.01g | 67.73 ± 0.32fgh | 2.36 ± 0.24 l | 16.90 ± 0.46cde |
| OGG 28/0 | 6.83 ± 0.02ef | 65.20 ± 1.33bc | 2.19 ± 0.10kl | 16.03 ± 0.27a |
Mean ± standard deviation. Different lowercase letters in superscript for each parameter indicate significant (p < 0.05) differences
aFor sample description see Table 1
There were no significant changes in pH values (ranging between 6.48 and 7.21 as noted above) during chilled and freezing/frozen storage, and therefore the data are not shown.
Colour measurement
Colour is essential in the development of fat analogues since it is one of the main factors determining the consumer’s choice of foods, including meat products. Colour parameters, lightness (L*), redness (a*) and yellowness (b*), depend significantly on the oat/oil proportion and the presence of a gelling agent, with interaction (p < 0.05) between these factors (Table 3). There was an increase (p < 0.05) in lightness when the proportion of oat bran increased (and that of olive oil decreased) in samples without an added gelling agent and with alginate (Table 3). However, the samples containing gelatin registered lower (p < 0.05) lightness when oat bran content increased. A comparison of emulsion gels with similar oat/oil proportions showed that only from 12/40 to 22/15 did the presence of alginate or gelatin produce an increase in lightness (Table 3). Increasing oat bran and reducing olive oil produced an increase in a* and a decrease in b* in all samples (Table 3). A significant positive (r = 0.855, p < 0.0000) and negative (r = −0.735, p < 0.0000) correlation was observed between addition of oat bran and a* and b* respectively; these correlations were the reverse in the case of olive oil, while non-significant correlations were found between a* and b* and the presence of a gelling agent. Increasing redness with the addition of oat bran may be related to a higher concentration of β-glucans, which are reported to confer a darker colour and greater redness in certain bakery end-products (Lazaridou et al. 2003). The decrease in b* could be attributed both to the yellowish-green hue of olive oil (Pintado et al. 2015) and to the addition of barley β-glucans, which have been found to result in products with lower brightness and yellowness (Lazaridou and Biliaderis 2007). Compared to animal fat, the oat emulsion gels registered similar values of L* (Jimenez-Colmenero et al. 2012), but less redness and more yellowness, probably due to the olive oil and oat bran. However, oil-free oat gels registered similar redness to pork back fat.
There were no significant variations in the colour parameters (L*, a* and b*) of oat emulsion gels and oil-free gels during chilled and freezing/frozen storage, and therefore the data included in Table 3. Pintado et al. (2015) also observed that lightness in chia emulsion gels formulated with alginate or gelatin did not vary during storage, but at the end of storage there was a significant increase in a* in oat emulsion gel containing these gelling agents.
Textural properties
Tables 4 and 5 show PF and GS of oat bran emulsion gels (OEW, OEA and OEG) and oil-free oat gels (OGW, OGA and OGG). Only emulsions OEW with the lowest oat/oil ratio OEW 12/40 and OEW 14/35 behaved like a viscous material, with no breaking point. All the other samples had a typical gel structure with a clear breaking point. PF were affected (p < 0.05) by oat/oil proportions and gelling agent, with interaction (p < 0.05) between these factors (Table 4). PF and GS increased in all samples when the proportion of oat bran increased and that of oil decreased. In all gelling systems (OEW, OEA or OEG) (Tables 4, 5) both PF and GS were also significantly dependent on the presence of alginate or gelatin; in the case of samples containing the same proportion of oat/oil, PF and GS were greater (p < 0.05) in the samples containing alginate or gelatin (Tables 4, 5). Oat bran emulsion gels between 12/40 and 18/25 with gelatin (OEG) registered higher (p < 0.05) PF than those containing alginate (OEA), while from 22/15 to 26/5 PF was the highest (p < 0.05) in the samples containing alginate (Tables 4, 5). The pattern was similar for GS except in 18/25 samples. Oil-free oat gels registered the highest (p < 0.05) PF and GS values, particularly the ones containing alginate (OGA 28/0) (Tables 4, 5). Textural properties of these oat emulsion gels and oil-free gels are consistent (in the same range) with other animal fat replacers (emulsions, emulsion gels, oil bulking agent, etc.) (Jimenez-Colmenero et al. 2012; Pintado et al. 2015) used in the reformulation of meat products with optimal texture behaviour (Jiménez-Colmenero et al. 2010; Pintado et al. 2016).
Table 4.
Penetration force of oat emulsion gels and oil-free gels during chilled and freezing/frozen storage
| Samplesa | Day 0 | Chilled storage | Frozen storage | ||
|---|---|---|---|---|---|
| Day 6 | Day 15 | Day 1 | Day 15 | ||
| OEW 12/40 | − | – | – | – | – |
| OEA 12/40 | 0.24 ± 0.01bcAX | 0.36 ± 0.01bC | 0.31 ± 0.04abB | 0.33 ± 0.03aY | 0.23 ± 0.01aX |
| OEG 12/40 | 0.67 ± 0.04ghAX | 0.92 ± 0.03fgB | 1.04 ± 0.02hiC | 0.73 ± 0.05bcX | 0.70 ± 0.06cX |
| OEW 14/35 | – | – | – | – | – |
| OEA 14/35 | 0.30 ± 0.03cAX | 0.59 ± 0.03dB | 0.57 ± 0.03cdB | 0.44 ± 0.03aZ | 0.38 ± 0.02bY |
| OEG 14/35 | 0.64 ± 0.01efAX | 1.07 ± 0.03hiB | 1.25 ± 0.08jkC | 0.90 ± 0.08dZ | 0.77 ± 0.03cdY |
| OEW 16/30 | 0.13 ± 0.00aAX | 0.25 ± 0.01aB | 0.21 ± 0.01aB | 0.41 ± 0.02aZ | 0.38 ± 0.02bY |
| OEA 16/30 | 0.48 ± 0.03dAX | 0.75 ± 0.03eB | 0.77 ± 0.05efB | 0.61 ± 0.04bY | 0.53 ± 0.03bX |
| OEG 16/30 | 0.76 ± 0.02fgAX | 1.09 ± 0.03hiC | 0.98 ± 0.08ghB | 0.89 ± 0.06cdY | 1.01 ± 0.05efgZ |
| OEW 18/25 | 0.20 ± 0.01abAX | 0.34 ± 0.01abB | 0.31 ± 0.02abB | 0.61 ± 0.03bZ | 0.53 ± 0.04bY |
| OEA 18/25 | 0.64 ± 0.06efAX | 0.99 ± 0.02ghB | 1.00 ± 0.08ghB | 0.83 ± 0.04cdY | 0.98 ± 0.06efZ |
| OEG 18/25 | 0.75 ± 0.11ghAX | 1.04 ± 0.04hB | 1.16 ± 0.05ijC | 0.75 ± 0.05bcdX | 0.78 ± 0.05cdX |
| OEW 20/20 | 0.27 ± 0.01bcAX | 0.49 ± 0.02cB | 0.43 ± 0.05bcB | 0.79 ± 0.10cdY | 0.74 ± 0.04cdY |
| OEA 20/20 | 0.78 ± 0.05hAX | 1.33 ± 0.06jB | 1.31 ± 0.02kB | 1.27 ± 0.05fZ | 1.16 ± 0.05gY |
| OEG 20/20 | 0.76 ± 0.02ghAX | 1.06 ± 0.03hB | 1.25 ± 0.04jkC | 0.87 ± 0.03cdY | 0.88 ± 0.03deY |
| OEW 22/15 | 0.42 ± 0.01dAX | 0.64 ± 0.01dB | 0.66 ± 0.02deB | 1.07 ± 0.03eZ | 0.99 ± 0.05efY |
| OEA 22/15 | 1.23 ± 0.04kAX | 1.81 ± 0.03mB | 1.79 ± 0.06mB | 1.47 ± 0.04ghY | 1.51 ± 0.09iY |
| OEG 22/15 | 0.92 ± 0.03iAX | 1.31 ± 0.05jB | 1.46 ± 0.04C l | 1.18 ± 0.16efY | 1.12 ± 0.08fgXY |
| OEW 24/10 | 0.58 ± 0.00eAX | 0.86 ± 0.03jB | 0.89 ± 0.03fgB | 1.46 ± 0.08ghZ | 1.31 ± 0.03hY |
| OEA 24/10 | 1.78 ± 0.02mnAX | 1.97 ± 0.10nB | 2.48 ± 0.16C | 1.57 ± 0.03hX | 1.77 ± 0.04jX |
| OEG 24/10 | 1.10 ± 0.01jAX | 1.65 ± 0.04lB | 1.77 ± 0.03mB | 1.31 ± 0.07fgY | 1.40 ± 0.06hiY |
| OEW 26/5 | 0.69 ± 0.04fghAX | 1.17 ± 0.04iB | 1.17 ± 0.02ijkB | 2.09 ± 0.15jY | 1.99 ± 0.07kY |
| OEA 26/5 | 1.86 ± 0.03nAX | 2.72 ± 0.08pC | 2.50 ± 0.10oB | 2.62 ± 0.04kY | 2.84 ± 0.05nZ |
| OEG 26/5 | 1.41 ± 0.01lAX | 1.86 ± 0.03mB | 2.18 ± 0.03nC | 1.74 ± 0.04iY | 1.86 ± 0.06jkZ |
| OGW 28/0 | 1.01 ± 0.04jAX | 1.52 ± 0.03kB | 1.45 ± 0.06lB | 2.60 ± 0.05kY | 2.66 ± 0.07mY |
| OGA 28/0 | 2.25 ± 0.05oAX | 2.64 ± 0.09pB | 3.03 ± 0.06pC | 3.35 ± 0.10lY | 3.80 ± 0.11oZ |
| OGG 28/0 | 1.72 ± 0.05mAX | 2.35 ± 0.03oB | 2.40 ± 0.12oB | 2.25 ± 0.02jY | 2.42 ± 0.17lY |
Mean ± standard deviation. Different lowercase letters in superscript (from a to p) in the same column indicate significant (p < 0.05) differences. Different capital letters in subscript for chilled storage (A, B, C) and frozen storage (X, Y, Z) indicate significant (p < 0.05) differences for each type of oat emulsion gel or oil-free gel with the same oat/olive oil proportion
aFor sample description see Table 1. Puncture force not shown for samples OEW 12/40 and OEW 14/35
Table 5.
Gel strength (mJ) of oat emulsion gels and oil-free gels during chilled and freezing/frozen storage
| Samplesa | Day 0 | Chilled storage | Frozen storage | ||
|---|---|---|---|---|---|
| Day 6 | Day 15 | Day 1 | Day 15 | ||
| OEW 12/40 | – | – | – | – | – |
| OEA 12/40 | 0.45 ± 0.03aAX | 0.81 ± 0.01aB | 0.71 ± 0.12aB | 0.94 ± 0.30aY | 0.56 ± 0.07aX |
| OEG 12/40 | 1.79 ± 0.20deAY | 2.06 ± 0.20cdefB | 2.33 ± 0.24cdeC | 1.27 ± 0.12aX | 1.24 ± 0.15abcX |
| OEW 14/35 | – | – | – | – | – |
| OEA 14/35 | 0.65 ± 0.16abAX | 1.41 ± 0.12abcB | 1.40 ± 0.29abcB | 1.01 ± 0.10aY | 0.83 ± 0.05abXY |
| OEG 14/35 | 1.99 ± 0.26defgAX | 2.28 ± 0.04defB | 2.77 ± 0.36defgB | 1.60 ± 0.19abX | 1.64 ± 0.13cdeX |
| OEW 16/30 | 0.28 ± 0.04aAX | 0.68 ± 0.14aB | 0.49 ± 0.04aAB | 1.14 ± 0.17aY | 1.49 ± 0.15bcY |
| OEA 16/30 | 1.28 ± 0.19bcdAX | 1.91 ± 0.16bcdeB | 1.90 ± 0.6bcdB | 1.44 ± 0.21abX | 1.33 ± 0.13bcX |
| OEG 16/30 | 2.05 ± 0.06efgAY | 2.07 ± 0.12cdefA | 2.09 ± 0.23cdA | 1.40 ± 0.10abX | 1.87 ± 0.12cdefXY |
| OEW 18/25 | 0.42 ± 0.03aAX | 0.84 ± 0.10aA | 0.59 ± 0.04aA | 1.87 ± 0.07abcY | 1.75 ± 0.05cdeY |
| OEA 18/25 | 1.50 ± 0.12cdeAX | 2.54 ± 0.26efB | 2.58 ± 0.56defB | 2.07 ± 0.10abcY | 2.21 ± 0.11defgY |
| OEG 18/25 | 1.63 ± 0.25cdeAX | 2.17 ± 0.36cdefA | 3.23 ± 0.78efgB | 1.37 ± 0.09aX | 1.59 ± 0.13cdX |
| OEW 20/20 | 0.59 ± 0.03abAX | 1.17 ± 0.04abB | 0.86 ± 0.19abB | 2.93 ± 0.44cdZ | 2.33 ± 0.16efgY |
| OEA 20/20 | 1.75 ± 0.16deAX | 3.42 ± 0.45ghB | 3.50 ± 0.37fgB | 3.36 ± 0.19deZ | 2.89 ± 0.21ghY |
| OEG 20/20 | 1.61 ± 0.23cdeAX | 1.72 ± 0.22bcdA | 2.53 ± 0.29defB | 1.93 ± 0.18abcY | 1.73 ± 0.12cdeX |
| OEW 22/15 | 0.96 ± 0.06abcAX | 1.85 ± 0.24bcdeB | 1.98 ± 0.28cdB | 3.69 ± 0.17deY | 3.37 ± 0.17ghY |
| OEA 22/15 | 3.38 ± 0.35hiAX | 5.26 ± 0.40ijB | 5.05 ± 0.34jB | 4.22 ± 0.25efY | 3.50 ± 0.43hiX |
| OEG 22/15 | 1.94 ± 0.28defAX | 2.72 ± 0.36fgB | 3.34 ± 0.65efgB | 2.59 ± 0.23bcdY | 2.54 ± 0.33fgY |
| OEW 24/10 | 1.57 ± 0.11cdeAX | 2.64 ± 0.10efgB | 2.68 ± 0.18defgB | 5.08 ± 0.51fgZ | 4.05 ± 0.10iY |
| OEA 24/10 | 4.87 ± 0.53jAY | 5.67 ± 0.36jB | 7.11 ± 0.52kC | 5.75 ± 0.29gZ | 3.88 ± 0.05iX |
| OEG 24/10 | 1.99 ± 0.15efgAX | 3.70 ± 0.36hB | 3.55 ± 0.14fghB | 2.93 ± 0.40cdY | 2.51 ± 0.23fgY |
| OEW 26/5 | 1.43 ± 0.09cdeAX | 3.56 ± 0.13hB | 3.65 ± 0.23ghiB | 7.85 ± 1.02hY | 6.83 ± 0.22kY |
| OEA 26/5 | 5.43 ± 0.78jAX | 8.45 ± 0.84kC | 7.19 ± 1.08kB | 8.70 ± 0.72hZ | 6.90 ± 0.44kY |
| OEG 26/5 | 2.51 ± 0.37fgAX | 3.57 ± 0.34hB | 4.68 ± 0.35ijC | 4.32 ± 0.80efY | 5.19 ± 0.32jZ |
| OGW 28/0 | 2.69 ± 0.53ghAX | 4.68 ± 0.37iB | 4.61 ± 0.32hijB | 12.11 ± 0.95iZ | 9.11 ± 0.28lY |
| OGA 28/0 | 6.72 ± 0.36kAX | 10.67 ± 0.79lC | 9.08 ± 0.47lB | 11.83 ± 1.36iY | 10.79 ± 0.35mY |
| OGG 28/0 | 3.93 ± 0.41iAX | 5.16 ± 0.18ijB | 5.40 ± 0.80jB | 6.23 ± 0.32gY | 6.85 ± 0.97kY |
Mean ± standard deviation. Different lowercase letters in superscript (from a to m) in the same column indicate significant (p < 0.05) differences. Different capital letters in subscript for chilled storage (A, B, C) and frozen storage (X, Y, Z) indicate significant (p < 0.05) differences for each type of oat emulsion gel or oil-free gel with the same oat/olive oil proportion
aFor sample description see Table 1. Gel textural behavior not shown for samples OEW 12/40 and OEW 14/35
These results indicate that oat bran, olive oil, and alginate or gelatin in the case of emulsion gels or oat bran and these gelling agents in the case of oil-free gels, influence sample textural properties. For instance, there was a significant positive correlation between PF (r = 0.712, p < 0.0000) and GS (r = 0.631, p < 0.0000) and the proportion of added oat bran. These correlations were negative for olive oil content in oat emulsion gels [PF (r = −0.650, p < 0.0000); GS (r = −0.535, p < 0.0000)]. A positive correlation was also found between gelling agent and PF (r = 0.641, p < 0.0001) and GS (r = 0.600, p < 0.0007). And again, there was a positive correlation between PF (r = 0.641, p < 0.0000) and GS (r = 0.570, p < 0.0000) and added water, since these factors affect technological properties of the sample components, mainly oats (proteins and β-glucan), such as solubility, emulsifying ability and gel-forming ability (Lazaridou and Biliaderis 2007).
The increase in sample firmness with increased oat bran content (Tables 4, 5) could be due to the potential of oat β-glucan and oat globulin protein as stabilizers in emulsion-type food products, and to their good gelling properties. In that respect some authors have shown that barley β-glucan contribute to the formation of gelled network (Burkus and Temelli 2000). Lazaridou and Biliaderis (2007) reported an increase in strength and a decrease in brittleness of oat gel with increasing concentration and molecular weight of polymer β-glucan. Other authors have reported an increase in the gelation rate with cereal β-glucan concentration but as a function of gel cure temperature, peaking at 31 °C (Vaikousi et al. 2004). It has been reported that addition of β-glucan affects textural behaviour, for instance in low-fat cake where the addition of cereal β-glucan concentrates increased hardness (Kalinga and Mishra 2009), or in low-fat white-brined cheese where the texture of the product was stronger (Volikakis et al. 2004).
Differences in oat protein/fat ratios affect the characteristics of the matrix formed in each sample. In this regard, a decrease in fat content and an increase in oat will raise the “effective” concentration of the protein that acts to form the gel/emulsion matrix. The increase in protein concentration in the continuous phase of the emulsion generally gave rise to an increase in the number of locations on the polypeptide chains that are capable of interacting to form a gel, a phenomenon associated with products having harder structures (Tables 4, 5) and with higher binding properties (Table 2). A similar explanation has been proposed for the effect produced by differing fat content on meat product characteristics (Jimenez-Colmenero et al. 1995). The increase in firmness observed with an increasing oat/oil ratio and with the presence of alginate or gelatin may also be related to emulsion droplet size. In this connection, some authors working with emulsion gels have posited that increased firmness and gel strength indicates the presence of small emulsion droplets which can reinforce the network structure by acting as anchor points, consequently increasing gel strength (Dickinson 2012).
Also relevant is the role of alginate or gelatin, since samples containing these gelling agents exhibited the greatest firmness (Table 4). Alginate is of particular interest due to its ability to form gels consisting of polymeric molecules cross-linked to form a three-dimensional macromolecular network containing a large water fraction in the structure and displaying mechanical rigidity (Roopa and Bhattacharya 2010). Additionally, gelatin also possesses gel-forming capacity through which to create a structural network with relatively higher gel strength (Sala et al. 2009).
During chilled storage PF and GS increased (p < 0.05) in all samples except OEG 16/30 and OEW 18/25. This increase in PF and GS during storage was generally more pronounced in samples containing alginate or gelatin (Tables 4, 5); in the samples containing gelatin the effect on PF was particularly marked. It has been reported that β-glucan addition considerably improved the creaming stability of emulsions during storage, possibly due to an increase in viscosity of the continuous phase and/or formation of a three-dimensional droplet network (Santipanichwong and Suphantharika 2009). This could account for the increase in PF and GS with storage time in the oat emulsion gels. Increased PF and GS have also been reported during chilled storage in emulsion gels formulated with chia and different cold gel agents (transglutaminase, alginate and gelatin) (Pintado et al. 2015). Some authors have reported increased hardness of cakes containing β-glucan concentrates over time under refrigeration conditions (4 °C) (Kalinga and Mishra 2009).
PF generally increased significantly during freezing/frozen storage (Table 4). Similarly, GS tended generally to increase during freezing/frozen storage, but in some cases the changes were not significant (Table 5). Increased hardness in frozen storage (−20 °C) has been reported in foods such as cakes containing β-glucan concentrates and it was indicative of staling (Kalinga and Mishra 2009).
Conclusion
This research pointed to the possibilities of oat emulsion gels and oil-free oat gels for use as solid-like healthier ingredients which could replace fat and at the same time supply bioactive compounds, mainly β-glucan and MUFA, and specific technological properties which depend on the oat/olive oil proportions used.
The oat emulsion gels and oil-free oat gels have a considerable potential for use as ingredients in the development of nutritious foods, for example meat products, when used as animal fat replacers, depending on the amount used products containing them may qualify for nutrition and health claims under the EU Regulations.
The oat emulsion gels and oil-free oat gels offer a range of possible advantages, such as imparting appropriate texture colour and thermal stability to product. These technological properties could be modulated by the oat/oil proportions used in oat emulsion gels and oil-free oat gels. On a general note it is also important to highlight that during chilled and frozen storage while there were slight changes in some technological properties like stronger texture or lower thermal stability (in samples OEW and OEA from 12/40 to 16/30) other properties such as pH or colour were unchanged. This makes them particularly suitable for addition to a food matrix in the development of novel functional foods such as healthier meat products which are habitually stored in these conditions.
Acknowledgements
The authors wish to thank MINECO, CAM and CSIC for financial support of this investigation, Projects AGL2014-53207-C2-1-R, S2013/AGR-2913 (MEDGAN), 2014470E073 and 201470E056.
References
- Arendt EK, Zannini E. Oats. In: Zannini E, Arendt EK, editors. Cereal grains for the food and beverages industries. Cambridge: Woodhead; 2013. pp. 238e–243e. [Google Scholar]
- Brummer Y, Defelice C, Wu Y, Kwong M, Wood PJ, Tosh SM. Textural and rheological properties of oat beta-glucan gels with varying molecular weight composition. J Agric Food Chem. 2014;62:3160–3167. doi: 10.1021/jf405131d. [DOI] [PubMed] [Google Scholar]
- Burkus Z, Temelli F. Stabilization of emulsions and foams using barley beta-glucan. Food Res Int. 2000;33:27–33. doi: 10.1016/S0963-9969(00)00020-X. [DOI] [Google Scholar]
- Delgado-Pando G, Cofrades S, Ruiz-Capillas C, Solas MT, Jimenez-Colmenero F. Healthier lipid combination oil-in-water emulsions prepared with various protein systems: an approach for development of functional meat products. Eur J Lipid Sci Technol. 2010;112:791–801. doi: 10.1002/ejlt.200900234. [DOI] [Google Scholar]
- Dickinson E. Emulsion gels: the structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 2012;28:224–241. doi: 10.1016/j.foodhyd.2011.12.017. [DOI] [Google Scholar]
- EFSA Scientific opinion on the substantiation of a health claim related to oat beta-glucan and lowering blood cholesterol and reduced risk of (coronary) heart disease pursuant to article 14 of regulation (EC) no 1924/2006. EFSA J. 2010;8:1885. doi: 10.2903/j.efsa.2010.1885. [DOI] [Google Scholar]
- Jimenez-Colmenero F. Healthier lipid formulation approaches in meat-based functional foods. Technological options for replacement of meat fats by non-meat fats. Trends Food Sci Technol. 2007;18:567–578. doi: 10.1016/j.tifs.2007.05.006. [DOI] [Google Scholar]
- Jimenez-Colmenero F, Carballo J, Solas MT. The effect of use of freeze-thawed pork on the properties of Bologna sausages with two fat levels. Int J Food Sci Technol. 1995;30:335–345. doi: 10.1111/j.1365-2621.1995.tb01382.x. [DOI] [Google Scholar]
- Jimenez-Colmenero F, Cofrades S, Herrero AM, Fernandez-Martín F, Rodriguez-Salas L, Ruiz-Capillas C. Konjac gel fat analogue for use in meat products: comparison with pork fats. Food Hydrocoll. 2012;26:63–72. doi: 10.1016/j.foodhyd.2011.04.007. [DOI] [Google Scholar]
- Jiménez-Colmenero F, Herrero A, Pintado T, Solas MT, Ruiz-Capillas C. Influence of emulsified olive oil stabilizing system used for pork backfat replacement in frankfurters. Food Res Int. 2010;43:2068–2076. doi: 10.1016/j.foodres.2010.06.010. [DOI] [Google Scholar]
- Jimenez-Colmenero F, Salcedo-Sandoval L, Bou R, Cofrades S, Herrero AM, Ruiz-Capillas C. Novel applications of oil-structuring methods as a strategy to improve the fat content of meat products. Trends Food Sci Technol. 2015;44:177–188. doi: 10.1016/j.tifs.2015.04.011. [DOI] [Google Scholar]
- Kalinga D, Mishra VK. Rheological and physical properties of low fat cakes products by addition of cereal beta-glucan concentrates. J Food Process Preserv. 2009;33:384–400. doi: 10.1111/j.1745-4549.2008.00260.x. [DOI] [Google Scholar]
- Klose C, Arendt EK. Proteins in oats; their synthesis and changes during germination: a review. Crit Rev Food Sci Nutr. 2012;52:629–639. doi: 10.1080/10408398.2010.504902. [DOI] [PubMed] [Google Scholar]
- Kontogiorgos V, Biliaderis CG, Kiosseoglou V, Doxastakis G. Stability and rheology of egg-yolk-stabilized concentrated emulsions containing cereal beta-glucans of varying molecular size. Food Hydrocoll. 2004;18:987–998. doi: 10.1016/j.foodhyd.2004.04.003. [DOI] [Google Scholar]
- Laine P, Toppinen E, Kivela R, Taavitsainen VM, Knuutila O, Sontag-Strohm T, Jouppila K, Loponen J. Emulsion preparation with modified oat bran: optimization of the emulsification process for microencapsulation purposes. J Food Eng. 2011;104:538–547. doi: 10.1016/j.jfoodeng.2011.01.014. [DOI] [Google Scholar]
- Lazaridou A, Biliaderis CG. Molecular aspects of cereal beta-glucan functionality: physical properties, technological applications and physiological effects. J Cereal Sci. 2007;46:101–118. doi: 10.1016/j.jcs.2007.05.003. [DOI] [Google Scholar]
- Lazaridou A, Biliaderis CG, Izydorczyk MS. Molecular size effects on rheological properties of oat beta-glucans in solution and gels. Food Hydrocoll. 2003;17:693–712. doi: 10.1016/S0268-005X(03)00036-5. [DOI] [Google Scholar]
- Malkki Y, Virtanen E. Gastrointestinal effects of oat bran and oat gum—a review. Lebensm Wiss Technol. 2001;34:337–347. doi: 10.1006/fstl.2001.0795. [DOI] [Google Scholar]
- Mehta N, Ahlawat SS, Sharma DP, Dabur RS. Novel trends in development of dietary fiber rich meat products—a critical review. J Food Sci Technol. 2015;52:633–647. doi: 10.1007/s13197-013-1010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreiras O, Carbajal A, Cabrera L, Cuadrado C. Tablas de composición de alimentos. Guía de prácticas. Madrid: Pirámide; 2013. [Google Scholar]
- Parliament E. Regulation (EC) of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Eur Comm. 2006;1924/2006:3–18. [Google Scholar]
- Parliament E. Regulation (EU) of the European Parliament and of the Council of 16 May 2012 establishing a list of permitted health claims made on foods other than those referring to the reduction of disease risk and to children’s development and health. Eur Comm. 2012;432/2012:1–40. [Google Scholar]
- Pintado T, Ruiz-Capillas C, Jimenez-Colmenero F, Carmona P, Herrero AM. Oil-in-water emulsion gels stabilized with chia (Salvia hispanica L.) and cold gelling agents: technological and infrared spectroscopic characterization. Food Chem. 2015;185:470–478. doi: 10.1016/j.foodchem.2015.04.024. [DOI] [PubMed] [Google Scholar]
- Pintado T, Herrero AM, Jiménez-Colmenero F, Ruiz-Capillas C. Strategies for incorporation of chia (Salvia hispanica L.) in frankfurters as a health-promoting ingredient. Meat Sci. 2016;114:75–84. doi: 10.1016/j.meatsci.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Roopa BS, Bhattacharya S. Texturized alginate gels: screening experiments to identify the important variables on gel formation and their properties. LWT Food Sci Technol. 2010;43:1403–1408. doi: 10.1016/j.lwt.2010.04.008. [DOI] [Google Scholar]
- Sala G, van Vliet T, Stuart MAC, van Aken GA, van de Velde F. Deformation and fracture of emulsion-filled gels: effect of oil content and deformation speed. Food Hydrocoll. 2009;23:1381–1393. doi: 10.1016/j.foodhyd.2008.11.016. [DOI] [Google Scholar]
- Santipanichwong R, Suphantharika M. Influence of different beta-glucans on the physical and rheological properties of egg yolk stabilized oil-in-water emulsions. Food Hydrocoll. 2009;23:1279–1287. doi: 10.1016/j.foodhyd.2008.10.006. [DOI] [Google Scholar]
- Sato ACK, Moraes KEFP, Cunha RL. Development of gelled emulsions with improved oxidative and pH stability. Food Hydrocoll. 2014;34:184–192. doi: 10.1016/j.foodhyd.2012.10.016. [DOI] [Google Scholar]
- Siu NC, Ma CY, Mock WY, Mine Y. Functional properties of oat globulin modified by a calcium-independent microbial transglutaminase. J Agric Food Chem. 2002;50:2666–2672. doi: 10.1021/jf011163p. [DOI] [PubMed] [Google Scholar]
- Storsrud S, Hulthen LR, Lenner RA. Beneficial effects of oats in the gluten-free diet of adults with special reference to nutrient status, symptoms and subjective experiences. Br J Nutr. 2003;90:101–107. doi: 10.1079/BJN2003872. [DOI] [PubMed] [Google Scholar]
- Tapola N, Karvonen H, Niskanen L, Mikola M, Sarkkinen E. Glycemic responses of oat bran products in type 2 diabetic patients. Nutr Metab Cardiovasc. 2005;15:255–261. doi: 10.1016/j.numecd.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Vaikousi H, Biliaderis CG, Izydorczyk MS. Solution flow behavior and gelling properties of water-soluble barley (1 → 3, 1 → 4)-beta-glucans varying in molecular size. J Cereal Sci. 2004;39:119–137. doi: 10.1016/j.jcs.2003.09.001. [DOI] [Google Scholar]
- Volikakis P, Biliaderis CG, Vamvakas C, Zerfiridis GK. Effects of a commercial oat-beta-glucan concentrate on the chemical, physico-chemical and sensory attributes of a low-fat white-brined cheese product. Food Res Int. 2004;37:83–94. doi: 10.1016/j.foodres.2003.07.007. [DOI] [Google Scholar]
- Wolever TMS, Tosh SM, Gibbs AL, Brand-Miller J, Duncan AM, Hart V, Lamarche B, Thomson BA, Duss R, Wood PJ. Physicochemical properties of oat beta-glucan influence its ability to reduce serum LDL cholesterol in humans: a randomized clinical trial. Am J Clin Nutr. 2010;92:723–732. doi: 10.3945/ajcn.2010.29174. [DOI] [PubMed] [Google Scholar]
- Wood PJ, Weisz J, Blackwell BA. Structural studies of (1–3), (1–4)-beta-d-glucans by c(13)-nuclear magnetic-resonance spectroscopy and by rapid analysis of cellulose-like regions using high-performance anion-exchange chromatography of oligosaccharides released by lichenase. Cereal Chem. 1994;71:301–307. [Google Scholar]