Abstract
We analyzed the population structure of the anthropophilic dermatophyte species Trichophyton violaceum, which mainly causes tinea capitis, and T. rubrum, the most frequently isolated agent of dermatophytosis worldwide. A microsatellite marker (T1) was developed by using the enrichment technique for microsatellites. The T1 marker containing a (GT)8-10 repeat was proven to specifically amplify both species, underlining their close kinship. Four polymorphic alleles were detected within a set of about 130 strains by using polyacrylamide gel electrophoresis with this marker. An association with geographic origin of the isolates was apparent. Given the close relatedness of both species, these data suggest an African origin of the entire T. rubrum complex, followed by the emergence of a new genotype (B) in Asia with subsequent spread of this genotype over Europe and the United States.
Despite large phenetic differences, Trichophyton rubrum and T. violaceum are closely related dermatophytes, composing a single, robust clade in ribosomal DNA (rDNA) internal transcribed spacer (ITS) phylogeny (5). T. rubrum is remarkable because its molecular variability is minimal. Some polymorphisms among clinical isolates have been detected in the copy number of a repetitive element (TRS) in the nontranscribed spacer (NTS) of the ribosomal operon (7), but no clear correlation with either clinical picture or geographic origin was apparent. Detection of additional markers therefore remains necessary.
Trichophyton rubrum mostly causes mild tinea pedis and onychomycosis. Its transmission from human to human has particularly been promoted by the general use of closed footwear in urban populations. Gräser et al. (3), by using PCR fingerprinting, amplified fragment length polymorphism, and anonymous DNA markers, proved that T. rubrum evolved only very recently, possibly from a single clone. A detectable degree of polymorphism, combined with the rapid transmission from human to human, would enable the localization of the origin of this species before it emerged on a worldwide scale. In older literature (14) the species has been hypothesized to originate from the Far East and subsequently to have been spread by soldiers during the First World War.
In contrast, the T. violaceum complex seems to have gone through speciation processes in Africa and the Middle East. The species comprises some variants or sister species which all mainly cause inflammatory tinea capitis or tinea corporis but differ slightly in cultural characteristics and production of extracellular metabolites: T. gourvilii, T. soudanense, and T. yaoundei. These taxa were reduced to synonymy of T. violaceum on the basis of ITS sequence data (5), but this unification conceals possible evolutionary diversification. Microsatellite markers developed for the model species T. rubrum are likely to produce polymorphisms in the entire T. rubrum clade that includes T. violaceum. This provides significant possibilities for diagnostic and epidemiological typing studies.
The aim of the present study was therefore twofold: (i) to develop polymorphic microsatellite markers to explore the population structure and epidemiology of T. rubrum and (ii) to test these markers for their potential of identification of species and variants of the T. rubrum-T. violaceum clade, eventually directly from clinical samples. Microsatellites were chosen because they are known to evolve at a high mutation rate (10−2 to 10−5). Single-locus markers are superior to multicopy markers, and thus problems due to the variability in single copies can be avoided.
MATERIALS AND METHODS
Fungal strains.
In all, 130 clinical and reference strains from several geographic regions and causing different clinical pictures were analyzed (Table 1). For DNA extraction, strains were cultured on Sabouraud glucose agar (Difco Laboratories, Detroit, Mich.). Morphological identification was done at the host laboratories.
TABLE 1.
Strains examined in this studya
| Trichophyton sp. | Reference no. or status | Strain-dependent deviation | Clinical picture | Geography | T1 pattern |
|---|---|---|---|---|---|
| T. violaceum | CBS 319.31 | Folliculitis | Unknown | C | |
| T. violaceum | CBS 374.92 | Skin | Unknown | C | |
| T. violaceum | Tv2 | Unknown | Africa | C | |
| T. violaceum | CBS 499.48 | Skin | Unknown | C | |
| T. violaceum (yaoundei) | CBS 730.88 | Skin | Unknown | D | |
| T. violaceum (yaoundei) | CBS 305.60 | Tinea capitis (child) | Cameroon | D | |
| T. rubrum (gourvilii) | CBS 360.62 | Onychomycosis | Togo | A | |
| T. rubrum (soudanense) | CBS 452.61 | Reflexive hyphae | Skin | Zaire | A |
| T. rubrum (soudanense) | Tsd1 | Reflexive hyphae | Tinea corporis | Africa | A |
| T. rubrum (soudanense) | Tsd2 | Reflexive hyphae | Tinea corporis | Africa | A |
| T. rubrum (soudanense) | 1135/99 | Reflexive hyphae | Tinea capitis/tinea corporis | West Africa | A |
| T. rubrum (soudanense) | 110043/2002 | Reflexive hyphae | Tinea corporis | Senegal | A |
| T. rubrum | CBS 286.30 | Unknown | Africa | A | |
| T. rubrum | CBS 592.68 | Skin | Span. Guinea | A | |
| T. rubrum | CBS 517.63 | Tinea capitis (child) | Congo | A | |
| T. rubrum | CBS 422.67 | Tinea capitis | Zaire | A | |
| T. rubrum (raubitschekii) | 2911 | Abundant MC; urease+ | Tinea corporis | Ghana | A |
| T. rubrum | CBS 376.49 | Tinea cruris | Congo | A | |
| T. rubrum | CBS 303.38 | Tinea capitis (child) | D'Adrar Sahara, Mauretania | A | |
| T. rubrum (raubitschekii) | 576 | Abundant MC; urease+ | Tinea corporis | Cameroon | A |
| T. rubrum | R17 | Tinea pedis | Angola | A | |
| T. rubrum (megninii) | CBS 735.88 | l-Histidine requirement | Skin of chin | Unknown | A |
| T. rubrum | CBS 191.69 | Skin | Unknown | A | |
| T. rubrum (raubitschekii) | IFM 45885 | Abundant MC; urease+ | Tinea corporis | Kagoshima, Japan | A |
| T. rubrum | ATU TR16 | Unknown | Japan | A | |
| T. rubrum | J11 | Tinea corporis | Hyougo, Japan | A | |
| T. rubrum | J17 | Unknown | Nagasaki, Japan | A | |
| T. rubrum | J23 | Unknown | Gifu, Japan | A | |
| T. rubrum | J25 | Otitis externa | Kanagawa, Japan | A | |
| T. rubrum | J27 | Tinea pedis | Ishikawa, Japan | A | |
| T. rubrum | J29 | Tinea capitis | Ishikawa, Japan | A | |
| T. rubrum | J31 | Tinea pedis | Ishikawa, Japan | A | |
| T. rubrum | J35 | Tinea corporis | Ishikawa, Japan | A | |
| T. rubrum | J37 | Tinea corporis | Ishikawa, Japan | A | |
| T. rubrum | J42 | Tinea pedis | Ishikawa, Japan | A | |
| T. rubrum (raubitschekii) | IFM 46113 | Abundant MC; urease+ | Unknown | Kagoshima, Japan | A |
| T. rubrum (raubitschekii) | CBS 100084 | Abundant MC; urease+ | Skin | Toronto, Canada | A |
| T. rubrum (kanei) | CBS 289.86 | Microconidia absent | Buttock | Canada (Asian) | A |
| T. rubrum (raubitschekii) | CBS 287.86 | Abundant MC; urease+ | Skin | Toronto, Canada | A |
| T. rubrum | CBS 304.60 | Skin | Unknown | A | |
| T. rubrum (megninii) | CBS 734.88 | l-Histidine requirement | Skin of chin | Unknown | A |
| T. rubrum | CBS 189.69 | Onychomycosis | Unknown | B | |
| T. rubrum | CBS 392.58 | Tinea pedis | Unknown | B | |
| T. rubrum (fischeri) | CBS 100081 | No red reverse on CEA | Contaminant | Unknown | B |
| T. rubrum (fischeri) | CBS 288.86 | No red reverse on CEA | Contaminant | Toronto, Canada | B |
| T. rubrum (raubitschekii) | CBS 202.88 | Abundant MC; urease+ | Tinea pedis | Canada (Caucasian) | B |
| T. rubrum, 21 strains | R18, U1-4, U6-21 | Tinea corporis, tinea pedis, onycho- mycosis | United States | B | |
| T. rubrum, 48 strains | 14, R1-16, R19-49 | Tinea corporis, tinea pedis, Tinea manuum, Tinea capitis, onycho- mycosis | Berlin, Germany | B | |
| T. rubrum (raubitschekii) | IFM 45887 | Abundant MC; urease+ | Unknown | Kagoshima, Japan | B |
| T. rubrum | J15 | Tinea pedis | Tokyo, Japan | B | |
| T. rubrum | J16 | Unknown | Nagasaki, Japan | B | |
| T. rubrum | J24 | Tinea corporis | Shizuoka, Japan | B | |
| T. rubrum | J22 | Unknown | Gifu, Japan | B | |
| T. rubrum | J28 | Tinea pedis | Ishikawa, Japan | B | |
| T. rubrum | J30 | Tinea pedis | Ishikawa, Japan | B | |
| T. rubrum | J32 | Tinea pedis | Ishikawa, Japan | B | |
| T. rubrum | J33 | Tinea pedis | Ishikawa, Japan | B | |
| T. rubrum | J34 | Tinea pedis | Ishikawa, Japan | B | |
| T. rubrum | J36 | Tinea pedis | Ishikawa, Japan | B | |
| T. rubrum | J26 | Tinea pedis | Ishikawa, Japan | B | |
| T. rubrum | J38 | Tinea pedis | Ishikawa, Japan | B | |
| T. rubrum | J39 | Unknown | Ishikawa, Japan | B | |
| T. rubrum | J40 | Tinea pedis | Ishikawa, Japan | B | |
| T. rubrum | J41 | Tinea pedis | Ishikawa, Japan | B |
Species identification is based on rDNA ITS profiles according to the method of Graser et al. (5). Strains that deviate consistently from species descriptions given by de Hoog et al. (2) are listed. Abbreviations: MC, macroconidia; CEA, Casamino Acids erythritol albumin agar; ATU, Faculty of Agriculture, University of Tokyo, Tokyo, Japan; CBS, Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; IFM, Culture Collection of Research Center for Pathogenic Fungi and Microbial Toxicoses, Chiba, Japan.
Isolation of microsatellites (enrichment method).
Repetitive sequences were captured with biotinylated (CA)15 probes and immobilized on avidin-coated beads. The captured DNA was subjected to washing steps and then eluted, amplified, and cloned to produce a library enriched for the target sequence. These procedures were modified as described by Kandpal et al. (8). Briefly, genomic DNA was isolated from a clinical isolate of T. rubrum (R4) by using a standard CTAB (cetyltrimethylammonium bromide) method (4). About 10 μg of the DNA was digested with Sau3A and size-fractionated by using Microspin columns (Amersham Biosciences, Piscataway, N.J.). Linkers (Sau-A, 5′-GCGGTACCCGGGAAGCTTGG; Sau-B, 5′-GATCCCAAGCTTCCCGGGTACCGC) were ligated to both ends of the fragments by using T4 DNA ligase (NEB, Beverly, Mass.). After purification via columns, prehybridization PCR was performed with the Sau-A linker only (annealing temperature of 56°C, 35 cycles). For enrichment, the PCR product was denatured and hybridized to the biotinylated (CA) probe in a solution of 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate. The mixture was denatured at 95°C and cooled slowly (15 to 20 min) to room temperature. The probe was then captured with avidin beads (VECTREX Avidin D; Vector Laboratories, Burlingame, Calif.) in TBT buffer (100 mM Tris [pH 7.5], 0.1% Tween 20) at 50°C for 30 min. Several washing steps followed (three times with TBT plus 150 mM NaCl and three times with 0.2× SSC-0.1% SDS). Afterward, the DNA was denatured from the beads in 10 mM Tris (pH 8)-0.1 mM EDTA at 95°C for 5 min and again PCR amplified with Sau-A. The resulting PCR product was cloned and transformed by using the TOPO TA cloning kit (Invitrogen, Carlsbad, Calif.). White selection colonies were picked, and the corresponding insert was directly amplified with M13 primers. Inserts 300 to 600 bp in size were chosen for sequencing. Then specific primers were designed to amplify PCR fragments in the range of 100 to 300 bp containing more than eight GT repeats. Amplification of each primer pair was tested on a panel of strains representing the species of the T. rubrum complex, including the genomic DNA of the isolate the library was generated from.
PCR amplification with the developed primers.
Standard PCR conditions were as follows: reactions were performed in 35-μl volumes containing 10 mM Tris-HCl, 50 mM KCl, 3 mM MgCl2, 21 pmol of each primer (T1.forward [5′-GTAAGGATGGCTAGTTAGGGGG] and T1.reverse [5′-TGGTCTGGCCTTGACTGACC]), 50 μM concentrations of each deoxynucleotide triphosphate, 1.75 U of Taq polymerase, and 30 to 50 ng of template DNA. Samples were amplified through 30 cycles as follows: initial denaturation for 10 min at 95°C, followed by denaturation for 30 s at 95°C, annealing for 30 s at 60°C, and extension for 45 s at 72°C. This was followed by a final extension step of 3 min at 72°C.
The sensitivity of the primer pair T1 was determined with serial dilutions (35 ng to 35 fg) of purified DNA (T. rubrum) at 3 days, successively. The limit of detection was determined as the minimum concentration where all three replicates still amplified.
Screening for length polymorphisms.
We loaded 10 to 15 μl of PCR products (depended on the intensity of the product) onto 9% acrylamide gels (Rotiphorese Gel 29:1, 40%; Carl Roth, Karlsruhe, Germany) to run the microsatellites for 7 h at 45 W or overnight at 22 W for 17 h (constant power). Gels were silver stained and dried for documentation. Representatives of distinctive acrylamide patterns were sequenced to locate and characterize the polymorphisms detected.
RESULTS
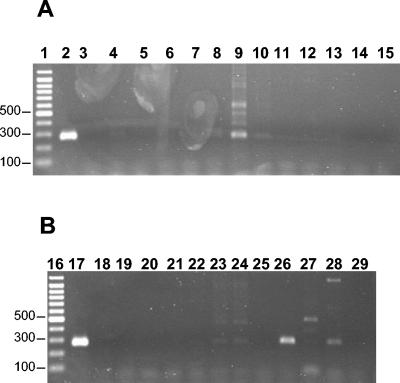
For 11 of 24 clones, primer pairs were designed. Only four of these (36%) successfully amplified the genomic DNA of the panel of representative strains selected, including the genomic DNA of the library. The specificity of the primer pair T1 was evaluated in PCR with 130 clinical and reference strains of the T. rubrum complex (Table 1), as well as with 22 isolates of phylogenetically related species or causative agents of similar clinical pictures (Fig. 1). The genomic DNA of dermatophyte species outside the T. rubrum complex or other fungi did not produce specific PCR products but rarely produced a nonspecific banding pattern or faint bands of different sizes (Fig. 1). The detection limit of the specific primer pair was 35 pg (data not shown).
FIG. 1.
Detection of specific PCR products by using the microsatellite primer pair T1. Lanes 1 and 16, molecular weight markers in base pairs; lanes 2 and 17, Trichophyton rubrum CBS 392.58 (genotype B); lanes 3 and 18, negative control; lanes 4 to 8, clinical isolates of T. tonsurans; lanes 9 to 12 and 14, T. interdigitale CBS 558.66, CBS 165.66, CBS 501.46, CBS 435.73, and CBS 100378; lane 13, T. verrucosum CBS 562.50; lanes 15 to 19, T. schoenleinii VKPGF 231/16 and VKPGF 232/181; lane 20, T. mentagrophytes CBS 388.58; lanes 21 and 22, T. erinacei CBS 677.86 and CBS 511.73; lane 23, T. radicosum CBS 511.73; lane 24, T. immergens CBS 338.37; lane 25, T. abyssinicum CBS 126.34; lane 26, T. violaceum CBS 319.31 (C type); lane 27, Microsporum canis CBS 495.86; lane 28, M. vanbreuseghemii CBS 243.66; lane 29, Scopulariopsis brevicaulis B99079.
One of the four primer pairs generated PCR products (280 bp) that yielded four polymorphic patterns among the isolates studied (Table 1). Types A to D (Table 1) were due to different numbers of GT repeats (8-10), and to three substitutions and one indel of four nucleotides (GGCC) within the flanking region of the repeat (accession no. AJ745081-84).
The polymorphisms in the flanking regions discriminated T. violaceum and T. yaoundei from the remaining species, including T. soudanense, T. gourvilii, and the T. rubrum complex. With the help of the numbers of GT repeats T. violaceum was discriminated from T. yaoundei (8 versus 10 repeats, types C and D, Table 1). Among the strains of the T. rubrum complex, two polymorphisms were found (8 versus 9 GT repeats, types A and B, Table 1). T. rubrum type A comprised a few strains with reflexive branching hyphae. Colonies that either remained red on all media or changed their color during subculturing were culturally and morphologically identified as T. soudanense and T. gourvilii, respectively. Two recent segregants of T. rubrum had genotype A: T. kanei lacking microconidia and T. raubitschekii described for strains with abundant macroconidia in addition to microconidia, while both segregants show a positive urease test. Type B included T. fischeri, described by Kane et al. (9) as a nonpathogenic fungus that in contrast to T. rubrum lacks the red colony reverse on CEA medium.
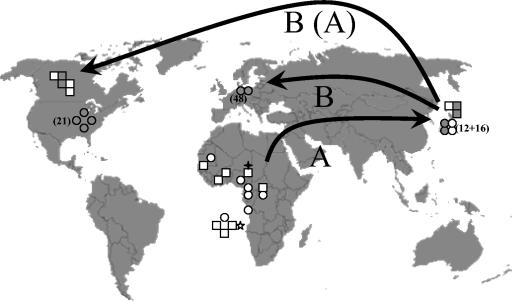
About 50% (15) of the strains of T. rubrum genotype A were collected on the African continent (Fig. 2 and Table 1). Of four isolates with genotype A, the geographic origin is unknown. However, it is very likely that at least CBS 191.69 originated from Africa, since it was identified as T. fluviomuniense of which the type strain came from the same area. Twelve isolates were collected in Japan. The 90 T. rubrum strains displaying the B type were derived from Germany (48 strains), the United States (21 strains), Japan (16 strains), Canada (2 strains), and the origin of 3 strains was unknown, including the neotype strain of T. rubrum (CBS 392.58).
FIG. 2.
Distribution of strains analyzed (genotypes A to D) with known geographic origin. Arrows represent the proposed migration of genotypes A and B. Strains with the unspecified origin (Africa or West Africa) were placed adjacent to the continent. Symbols: squares, strains with morphological and/or physiological features that differ from T. rubrum (T. fischeri, T. soudanense, T. gourvilii, T. raubitschekii, and T. kanei); circles, isolates morphologically described as T. rubrum; open, genotype A; solid, genotype B; star, genotype C; +, genotype D.
DISCUSSION
The low success rate of PCR primers derived from the library suggested that the majority of the clones were chimeric and not representative of contiguous stretches of genomic DNA. To reduce the number of recombinant clones, it may be necessary to run fewer cycles of PCR amplifications before and after the enrichment procedure (1).
The marker developed in our study amplified species of the T. rubrum-T. violaceum clade only (Fig. 1) and proved to be polymorphic within the clade. T. violaceum s. str. and T. yaoundei each showed characteristic profiles, whereas no differences have been found with taxonomic markers such as rDNA ITS (5). The striking phenetic deviations from T. violaceum despite uniformity at the molecular level indicate that speciation processes in dermatophytes are of recent date. Microsatellite markers are commonly species specific (15) but have never been used for the discrimination of species; therefore, we refrained from the restoration of T. yaoundei as a formal taxon. It should be noted that the name was invalidly introduced by Cochet and Doby-Dubois in 1957.
The T1 microsatellite developed in our study is the second DNA marker displaying polymorphisms among strains that are otherwise genetically homogenous. Using the TRS marker, a repeat region within the NTS region of the rDNA, several authors were also able to detect variability among isolates of this species (7, 6, 15). However, no clear association was found with the clinical picture or the geographic origin. The only conclusion their data allowed was that the predominant NTS type 1 found in the United Kingdom and most other countries was almost absent among the Japanese isolates (only 1 of 11 isolates). Indeed, the T1 marker also did not show an association with the clinical picture, but our data suggest that geographic isolation is the driving force for subpopulations.
Despite the multicopy gene structure of the NTS region, it was suggested by Yazdanparast et al. (16) that multiple strains can be involved in an infection of a single patient. With the T1 marker, no indication (detection of more than one allele per isolate by superimposition of patterns in the polyacrylamide gel electrophoresis gel) for the involvement of more than one strain was found. Therefore, polymorphic single-locus markers are better suited for population genetic and epidemiological studies, as well as for monitoring relapse and reinfection. To clarify the population structure of T. rubrum in detail, the analysis of a larger number of (microsatellite) markers is needed.
Although the T1 marker was polymorphic within local populations of T. rubrum, e.g., among strains from a single city in Japan, none of the polymorphisms (A or B) showed a clear correlation to any of the taxonomic entities maintained in the older literature. These results confirm the synonymization of these species—i.e., T. fischeri, T. kanei, T. megninii, and T. raubitschekii—on the basis of rDNA ITS data (5). In disagreement with ITS data, the microsatellite marker grouped the isolates of T. soudanense and T. gourvilii with T. rubrum and not with T. violaceum. This grouping was also detected with other DNA markers, e.g., ATP9/CYTII, which is located within the mitochondrial DNA (13), and TR1 developed by using arbitrarily primed PCR (11). The discrepancy with ITS data may be due to a repeated “TA” motif at the end of ITS2 that might not been properly edited in the two strains used.
T. rubrum, as now recircumscribed on the basis of microsatellite data, occurs in Canada and Japan with polymorphic populations (A/B). Polymorphism is noted on a very small geographic scale, since several cities in Japan, as well as Toronto in Canada, harbor both genotypes. In Japan genotypes A and B are nearly equally frequent (45% versus 55%). Rippon (14) suggested that T. rubrum has evolved from a chronic case of tinea corporis in the late 19th century in an area of endemicity in Southeast Asia. If that hypothesis is correct, we have to assume that the A/B polymorphism in Toronto is due to repeated import of strains, most probably from Asia (10). T. rubrum populations in the United States, Germany, and Southern Europe (i.e., Bulgaria [unpublished data]) are nearly monomorphic (Fig. 2), with only genotype B being represented in our large collections of strains. In contrast, the African population is monomorphic in genotype A. However, phenetic variation is well established on the latter continent. Several strains tend to lose sporulation and become waxy, with an abundant production of colored metabolites. Such cultures are phenotypically similar to T. gourvilii and T. soudanense. If we take into account that T. violaceum and T. yaoundei also have a degenerate, nonsporulating morphology, it may be that these phenotypic transitions in the fungus have taken place in tropical Africa, since they are not observed on other continents. Local transmission may take place in resident populations by contaminated skin flakes, either directly from human to human or via environmental propagules. The emerged diversity underlines the probability of an ancient African history of the T. rubrum complex. The widespread occurrence of genotype A in (rural) Africa, with phenetic variants already known since the early 20th century, suggests that the African origin of the species must antedate the supposed pandemic that started in Asia. This seems in conflict with the low degree of molecular variation in Africa, i.e., with only genotype A being present. If we consider T. rubrum to form a single species complex with T. violaceum (C) and T. yaoundei (D), the highest degree of molecular variation in T1 is indeed found in Africa. The Asian genotype A then probably originated from Africa through an early bottleneck.
Uniformity in T. rubrum populations (A in Africa and B in the United States and Europe) would suggest either a recent emergence of genotypes favored by natural selection of, e.g., virulent strains, or of recent bottlenecks in population size provided that our nonrandom sampling is representative for each of the continents. In clonal (nonrecombining) organisms we would expect to have similar genetic population signatures for both situations. In the case of a recently emerged pathogen, however, we would expect no or low geographical structuring combined with an absence of host specificity of the genotypes (12), since the species is supposed to have a low degree of adaptation to any new host. T. rubrum is known to have a longstanding relationship with the human host and is only rarely encountered in animals. The United States is known to have only a recent history of import, and this circumstance is therefore not indicative for evolutionary processes in the dermatophytes. A certain degree of geographical clustering (Africa versus Europe and North America) is evident from our data. This suggests a bottleneck of small population size, with a subsequent, relatively recent emergence of genotype B in Asia. The prevalence of genotype B in Europe and North America is of very recent date, resulting from epidemic emergence. If this genotype is not markedly different in virulence, other, newly provided favorable conditions enhancing rapid transmission, such as the increasing use of closed footware (especially boots), concomitant with large-scale movements of people under poor hygienic conditions, may have allowed its rapid spread. As suggested in earlier literature (14), the First World War may have played a major role in this process.
Acknowledgments
We thank H.-J. Tietz, Department of Dermatology, Charité Hospital (Germany); M. Kawasaki, Department of Dermatology, Kanazawa Medical University (Japan); Y. Kitamura and H. Ishizaki, National Kanazawa Hospital, Ishikawa (Japan); and V. Chaturvedi, New York State Department of Health (United States), for providing and identification of the clinical strains,
We thank Kristin Ebert for excellent technical assistance.
REFERENCES
- 1.Carleton, K. L., J. T. Streelman, B. Y. Lee, N. Garnhart, M. Kidd, and T. D. Kocher. 2002. Rapid isolation of CA microsatellites from the tilapia genome. Anim. Genet. 33:140-144. [DOI] [PubMed] [Google Scholar]
- 2.de Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 3.Gräser, Y., J. Kühnisch, and W. Presber. 1999. Molecular markers reveal exclusively clonal reproduction in Trichophyton rubrum. J. Clin. Microbiol. 37:3713-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gräser, Y., M. El Fari, R. Vilgalys, A. F. Kuijpers, G.S. de Hoog, W. Presber, and H. J. Tietz. 1999. Phylogeny and taxonomy of the family Arthrodermataceae (dermatophytes) using sequence analysis of the ribosomal ITS region. Med. Mycol. 37:105-114. [PubMed] [Google Scholar]
- 5.Gräser, Y., A. F. Kuijpers, W. Presber, and G. S. de Hoog. 2000. Molecular taxonomy of the Trichophyton rubrum complex. J. Clin. Microbiol. 38:3329-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta, A. K., Y. Kohli, and R. C. Summerbell. 2001. Variation in restriction fragment length polymorphisms among serial isolates from patients with Trichophyton rubrum infection. J. Clin. Microbiol. 39:3260-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson, C. J., R. C. Barton, S. L. Kelly, and E. G. Evans. 2000. Strain identification of Trichophyton rubrum by specific amplification of subrepeat elements in the ribosomal DNA nontranscribed spacer. J. Clin. Microbiol. 38:4527-4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kandpal, R. P., G. Kandpal, and S. M. Weissman. 1994. Construction of libraries enriched for sequence repeats and jumping clones, and hybridization selection for region-specific markers. Proc. Natl. Acad. Sci. USA 91:88-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kane, J. 1977. Trichophyton fischeri sp. nov.: a saprophyte resembling Trichophyton rubrum. Sabouraudia 15:231-241. [PubMed] [Google Scholar]
- 10.Kane, J., S. Krajden, R. C. Summerbell, and R. G. Sibbald. 1990. Infections caused by Trichophyton raubitschekii: clinical and epidemiological features. Mycoses 33:499-506. [DOI] [PubMed] [Google Scholar]
- 11.Liu, D., L. Pearce, G. Lilley, S. Coloe, R. Baird, and J. Pedersen. 2002. PCR identification of dermatophyte fungi Trichophyton rubrum, T. soudanense, and T. gourvilii. J. Med. Microbiol. 51:117-122. [DOI] [PubMed] [Google Scholar]
- 12.Morehouse, E. A., T. Y. James, A. R. Ganley, R. Vilgalys, L. Berger, P. J. Murphy, and J. E. Longcore. 2003. Multilocus sequence typing suggests the chytrid pathogen of amphibians is a recently emerged clone. Mol. Ecol. 12:395-403. [DOI] [PubMed] [Google Scholar]
- 13.Nenoff, P., Y. Gräser, H. Neubauer, and G. R. Filz. 2003. Trichophyton soudanense: aus Afrika “importierter” Erreger bei Tinea capitis et corporis. Mikrobiologe 13:1-3. [Google Scholar]
- 14.Rippon J.W. 1988. Medical mycology: the pathogenic fungi and pathogenic Actinomycetes, p. 178. W. B. Saunders Co., Philadelphia, Pa.
- 15.Wickstead, B., K. Ersfeld, and K. Gull. 2003. Repetitive elements in genomes of parasitic protozoa. Microbiol. Mol. Biol. Rev. 67:360-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yazdanparast, A., C. J. Jackson, R. C. Barton, and E. G. Evans. 2003. Molecular strain typing of Trichophyton rubrum indicates multiple strain involvement in onychomycosis. Br. J. Dermatol. 148:51-54. [DOI] [PubMed] [Google Scholar]