Abstract
Prostate cancer (PCa) is known to develop resistance to chemotherapy. Growth arrest-specific 6 (GAS6), plays a role in tumor progression by regulating growth in many cancers. Here, we explored how GAS6 regulates the cell cycle and apoptosis of PCa cells in response to chemotherapy. We found that GAS6 is sufficient to significantly increase the number and duration of G1 phase in PCa cells. Importantly, GAS6 further increased the number of G1 arrested cells during docetaxel chemotherapy. GAS6 altered the signals of key cell cycle regulators: Cyclin B1 (G2/M phase), CDC25A, Cyclin E1, and CDK2 (S phase entry) were all downregulated, while p27, p21, Cyclin D1, and CDK4 (G0/G1 phase) were upregulated. Importantly, these signaling events were further accentuated during docetaxel treatment in the presence of GAS6. Moreover, the apoptotic response of PCa cells to GAS6 was examined during docetaxel chemotherapy. Docetaxel induced PCa cell apoptosis. However, this apoptotic response was abrogated in PCa cell cultures in the presence of GAS6 or GAS6 secreted from co-cultured osteoblasts. Similarly, the GAS6-expressing bone environment protects PCa cells from apoptosis within primary tumors in vivo studies. In addition, docetaxel induced significant levels of Caspase-3 and PARP cleavages in PCa cells, while GAS6 protected PCa cells from docetaxel-induced apoptotic signaling. Together, these data suggest that GAS6, expressed by osteoblasts in the bone marrow, plays a significant role in the regulation of PCa cell survival during chemotherapy, which may have important implications for targeting metastatic disease.
Keywords: GAS6, PROSTATE CANCER, DOCETAXEL, G1 ARREST/S PHASE DELAY, APOPTOTIC PATHWAY, BONE MARROW
INTRODUCTION
Prostate cancer (PCa) is the second leading cause of cancer deaths in American males [Pienta and Esper, 1993]. Death of most PCa patients is associated with bone metastasis [Koutsilieris, 1993]. PCa cells are known to develop resistance to chemotherapy, particularly within the marrow [Petrioli et al., 2003; Taichman et al., 2007; Sweeney et al., 2015]. Therefore, understanding the mechanisms of tumor cell survival and drug-resistance is a key component in targeting cancer cells more effectively.
The homing and lodging of disseminated tumor cells (DTCs) in the bone marrow and the survival of these cells in this microenvironment are essential steps to establish PCa bone metastases [Jung et al., 2015]. Recent studies suggest that many hematopoietic and mesenchymal cells participate in the cellular and molecular events required for the establishment of metastases and maintenance of tumor progression in marrow [Taichman et al., 2002; Sun et al., 2005; Kucia et al., 2005 ; Shiozawa et al., 2011; Jung et al., 2013]. Once in the marrow, DTC fate including G0-G1 growth arrest or dormancy and reactivation may be governed by the signals from the metastatic microenvironment. As conventional cancer therapeutics largely target proliferating cells, dormant DTCs may be innately protected from chemotherapeutic insults setting the stage for subsequent relapse [Quesnel, 2008; Yumoto et al., 2014]. Previously, we demonstrated that growth arrest-specific 6 (GAS6) secreted by osteoblasts inhibits PCa proliferation [Shiozawa et al., 2010]. These data suggest that once the DTCs enter an environment where GAS6 levels are high, interactions between GAS6 and its receptors may regulate PCa dormancy [Shiozawa et al., 2010]. Consistent with these observations, we reported that GAS6 levels are significantly higher in the femur and tibia vs the humeri of SCID mice corresponding to the prevalence at which metastatic PCa lesions occur following intravenous inoculation [Jung et al., 2012]. We also demonstrated that the binding of PCa cells to osteoblasts in bone marrow induces TANK binding kinase 1 (TBK1) expression, which induces the cell cycle arrest and enhances chemotherapeutic resistance of PCa cells (Kim et al., 2013]. These findings suggest that identifying novel dormancy-associated pathways are crucial to prevent PCa recurrence and provide a more effective therapeutic strategy for PCa.
Chemotherapy using docetaxel is a standard treatment option for patients with metastatic castration-resistant prostate cancer. More recently, docetaxel has also shown an impressive survival benefit when given soon after diagnosis of metastatic hormone-sensitive prostate cancer [Sweeney et al., 2015]. However, all patients eventually develop chemotherapy resistance, which reduces survival in patients with advanced prostate cancer [Hong, 2002; Sweeney et al., 2015]. Docetaxel functions in part by disrupting the microtubule network in cells, which is essential for cell division during mitosis [Yoo et al., 2002; Li et al., 2004]. In addition, docetaxel alters protein targets involved in cell survival, normal physiological functions, and oncogenesis (Li et al., 2004]. Docetaxel also increases cytokine production in PCa cell cultures and circulating cytokines in the castration-resistant PCa patients [Mahon et al., 2015]. CXCL12/CXCR4 signaling is known to prevent docetaxel-induced microtubule stabilization via p21-activated kinase 4 (PAK4)-dependent activation of LIM domain kinase 1 in PCa cells [Bhardwaj et al., 2014]. Further, the inflammatory cytokine CCL2 enhances the development of resistance to docetaxel-induced cytotoxicity in PCa cells [Qian et al., 2010]. Moreover, protein inhibitors of activated signal transducer and activator of transcription (STAT) factors 1 (PIAS1), a crucial survival factor, significantly increased in docetaxel resistant PCa cells and in tissue of patients after docetaxel chemotherapy [Puhr et al., 2014]. Docetaxel also promotes the upregulation of the cell cycle inhibitor (p19) and downregulation of cyclins (cyclin A and cyclin B1) in head and neck cancer cells [Yoo et al., 2002]. Similar results were observed in PCa cells with the upregulation of cyclin-dependent protein kinase (CDK) inhibitors (p21 and p27) and downregulation of cyclins (cyclin A2, cyclin E2, and cyclin F), CDK4, and cell division cycles (CDC2, CDC7, CDC20, and CDC25B) [Li et al., 2004]. Thus, understanding the mechanisms underlying the extrinsic or intrinsic cellular signaling process responsible for docetaxel resistance is urgently needed.
In the present study, we explored that GAS6, expressed by osteoblasts, regulates the cell cycle and apoptosis in PCa cells during chemotherapy in the bone marrow. We demonstrate that GAS6 significantly increases the number of G1 arrested cells by altering signaling networks associated with G1 arrest and S phase delay. Furthermore, we demonstrate that GAS6 contributes to the protection of PCa cells from docetaxel-induced apoptosis in cell culture and similarly the GAS6-expressing bone environment protects PCa cells from apoptosis within primary tumors in vivo studies. In addition, we show that GAS6 can protect PCa cells from apoptotic signaling via Caspase-3 and PARP cleavage. Our results suggest that GAS6 contributes to the regulation of PCa cell survival during chemotherapy in the bone marrow microenvironment.
MATERIALS AND METHODS
CELL CULTURE
Human PCa cell lines (PC3, DU145) were obtained from the American Type Culture Collection (Rockville, MD). GFP expressing PCa cell lines (PC3GFP and DU145GFP) were established by lenti viral transduction. Murine osteoblast cells were established as previously reported [Jung et al., 2011]. All prostate cancer cell lines were routinely grown in RPMI 1640 (Life Technologies, Carlsbad, CA), and murine osteoblast cells were grown in α-MEM (Life Technologies) supplemented with 10% fetal bovine serum (FBS, GEMINI Bio-Products, Sacramento, CA), 1% penicillin-streptomycin (P/S, Life Technologies) and maintained at 37°C, 5% CO2, and 100% humidity.
PROLIFERATION ASSAY
PCa cells (PC3 or DU145) (3 x 103) were seeded onto 96-well culture plates with RPMI 1640, 1% FBS, and 1% P/S and then the cells were treated with human recombinant GAS6 (0–3μg/ml (cat. 885-GSB-050, R&D Systems, Minneapolis, MN) for 3 days. Proliferation was measured by the XTT Assay kit (cat. TOX2, Sigma, St. Louis, MO).
FUCCI-PC3 CELLS
To develop a method to monitor the cell cycle in prostate cancer cells, we transduced a human prostate cancer cell line, PC3 with lentiviruses containing the fluorescent ubiquitination-based cell cycle indicator (Fucci) vectors (Clontech, Mountain View, CA). Cells contain both a chromatin licensing and DNA replication factor 1 (CDT1)-Cherry reporter and a Geminin-Cyan reporter. Early S phase cells are double-positive for the reporters, fluorescing yellow. M phase is colorless due to the destruction of both Geminin-Cyan and CDT1-Cherry [Sakaue-Sawano et al., 2008]. pRetroX-G1-Red vector (cat. 631463, Clontech) and pRetroX-SG2M-Cyan vector (cat. 631462, Clontech) were packaged into lentivirus at the University of Michigan Vector Core Facility. Lentiviral pRetroX-G1-Red vector and lentiviral pRetroX-SG2M-Cyan vector were coinfected into PC3 cells. Infected cells were selected for 7 days in media containing 1μg/ml Puromycin and analyzed by FACS analysis. Cell cycle monitoring was performed in Fucci-PCa cell culture with direct GAS6 (1–2μg/ml) treatment or with the co-culture of osteoblasts (wild-type OB (GAS6+/+ OB) or GAS6 deficient OB (GAS6−/− OB)) following treatment with anticancer drug, docetaxel (Taxotere, 1μg/ml, cat. NDC0409-0201-10, Hospira, Lake Forest, IL). Additionally, Fucci-PC3 cell imaging was captured by video. Fucci-PC3 cells were cultured for 24 hours in RPMI with 10% FBS, 1% P/S and then, treated with Vehicle or GAS6 (2μg/ml) for 24 hours. Five spots of cells in each group were set for tracking. Video images were taken for 24 hours at 15 min intervals using a Deltavision Elite Microscope (GE Healthcare Life Science, Pittsburgh, PA). The duration of G1 phase in single cells was measured (n=20/group).
CELL DEATH ASSAYS
Cell death assays were performed in PCa cell cultures treated with GAS6 or in coculture of PCa cells with osteoblasts. First, PCa cells (1 x 105 cells/well) were seeded onto 12-well culture plates for 24 hours. Cells were cultured for 24–48 hours following GAS6 (2μg/ml) treatment, and then treated with the docetaxcel (1μg/ml) for 24 hours. For the cocultures, GAS6+/+ OB or GAS6−/− OB (1 x 105 cells/well) was seeded onto 12-well culture plates for 24 hours. Thereafter, GFP expressing PCa cells (1 x 105 cells/well) were added to the wells. Cells were cultured together for 48 hours, and then treated with the anticancer drug, docetaxcel (1μg/ml), for 24 hours in the coculture system. Apoptosis was measured by flow cytometry (FACSAria IIu three laser flow-cytometer, Becton Dickinson, Mountainview, CA) using by selecting PCa cells with the GFP reporter and with the PE Annexin V Apoptosis Detection Kit I (cat. 559763, BD Biosciences, San Jose, CA). Assays were performed in triplicate and the results are representative of three independent experiments. Assays were performed in triplicate and the results are representative of three independent experiments. In tumor sections from PCa cells were injected into vertebral bodies (vossicles) from wild-type (GAS6+/+) or GAS6 knockout (GAS6−/−) mice [Jung et al., 2011], apoptosis of PCa cells was measured by Cell Meter TUNEL Apoptosis Assay Kit (cat. 22844, AAT Bioquest, Sunnyvale, CA).
QUANTITATIVE RT-PCR
Total RNA was extracted from cells using the RNeasy mini or micro kit (Qiagen, Valencia, CA) and converted into cDNA using a First-Strand Synthesis Kit (Invitrogen). Quantitative PCR was performed on an ABI 7700 sequence detector (Applied Biosystems) using TaqMan Universal PCR Master Mix Kit (Applied Biosystems) according to the directions of manufacturer. TaqMan MGB probes, PLK1 (Hs00153444_m1) and STK15 (Hs01582072_m1) (Applied Biosystems) were used. β-actin (Hs01060665-g1) was used as an internal control for normalization of target gene expression.
ELISA
An antibody sandwich ELISA was used to evaluate GAS6 expression in bone marrow from GAS6+/+ or GAS6−/− mice as a negative control by following the directions of the manufacturer (cat. DY986; R&D Systems). Bone marrow extracellular fluids were obtained by flushing femora, and tibiae with 500μl of ice-cold PBS, and the supernatant was harvested by centrifugation at 400g for 5 minutes. GAS6 levels were normalized to total protein.
IMMUNOSTAINING
Cells, tumor sections and long bone sections were used for immunostaining. Cells were fixed and permeabilized with Perm/Wash Buffer (cat. 554723, BD Biosciences). Tumor sections were blocked with Image-iT FX signal enhancer for 30 min and incubated for 2 hours at room temperature with primary antibodies combined with reagents of Zenon Alexa Fluor 488 (green) or 555 (red) labeling kit (Invitrogen). GAS6 (cat. AF885, R&D System) antibody was used as primary antibody. After washing with PBS, the slides were mounted with ProLong Gold antifade reagent with DAPI (Invitrogen). Images were taken with Olympus FV-500 confocal microscope.
WESTERN BLOT
PCa cells were cultured in RPMI 1640 with 10% FBS and 1% P/S. Whole cell lysates were prepared from cells, separated on 4–20% Tris-Glycine gels and transferred to PVDF membranes. The membranes were incubated with 5% milk for 1 hour and incubated with primary antibodies overnight at 4°C. Primary antibodies used were as follows: p-CHK2 (1:1,000 dilution, cat. 2197, Cell Signaling), CHK2 (1:1,000 dilution, cat. 2662, Cell Signaling), Cyclin B1 (1:1,000 dilution, cat. 4138, Cell Signaling), Cyclin D1 (1:1,000 dilution, cat. sc-753, Santa Cruz), CDK4 (1:1,000 dilution, cat.12790, Cell Signaling), p27 (1:1,000 dilution, cat. 3686, Cell Signaling), p21 (1:1,000 dilution, cat. 2947, Cell Signalng), p-CDC25A (1:1,000 dilution, cat. sc-101655, Santa Cruz, Santa Cruz, CA), CDC25A (1:1,000 dilution, cat. 3652, Cell Signaling), Cyclin E1 (1:1,000 dilution, cat. sc-481, Santa Cruz), and CDK2 (1:1,000 dilution, 1:1,000 dilution, cat. sc-163, Santa Cruz,), Caspase-3 (1:1,000 dilution, cat. 9662, Cell Signaling), and PARP (1:1,000 dilution, cat. 9542, Cell Signaling). Blots were incubated with peroxidase-coupled anti-rabbit IgG secondary antibody (cat. 7074, 1:2,000 dilution, Cell Signaling) for 1 hour, and protein expression was detected with SuperSignal West Dura Chemiluminescent Substrate (cat. Prod 34075, Thermo Scientific, Rockford, IL). Membranes were reprobed with monoclonal anti-β-actin antibody (1:1,000 dilution; cat. 4970, Cell Signaling) to control for equal loading.
STATISTICAL ANALYSES
Results are presented as mean ± standard deviation (s.d.). Significance of the difference between two measurements was determined by unpaired Student’s t-test, and multiple comparisons were evaluated by the Newman-Keuls multiple comparison test. Values of p < 0.05 were considered significant.
RESULTS
GAS6 EXPRESSED BY OSTEOBLASTS IN BONE MARROW, WHICH INHIBITS PCA CELL PROLIFERATION AND EXPRESSION OF CELL CYCLE MARKERS
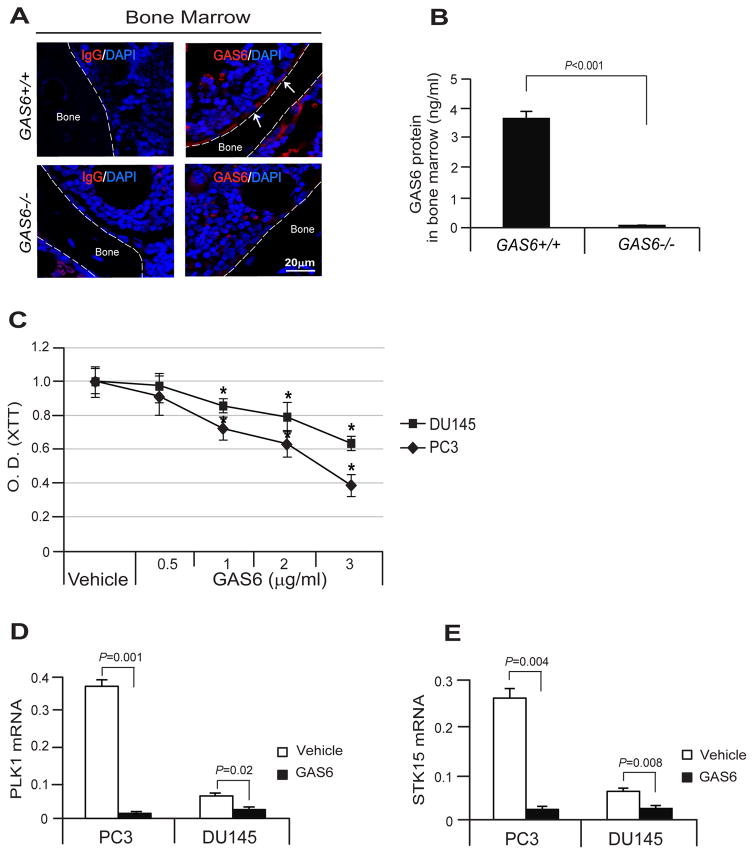
We examined the extent to which GAS6 expressed by osteoblasts in the bone marrow of the long bones of wild-type (GAS6+/+) mice and GAS6 deficient (GAS6−/−) mice. GAS6 was expressed by osteoblasts in the marrow of GAS6+/+ mice, but not in the marrow of GAS6−/− mice (Fig. 1A). GAS6 protein levels in the marrow were also examined. It was determined that the GAS6 protein levels were significantly greater in the marrow of the GAS6+/+ vs GAS6−/− mice (Fig. 1B). Next, to test the effects of Gas6 on PCa cell proliferation, the proliferation assays were performed along with gene expression profiling assays for cell cycle markers. GAS6 inhibited PCa cell proliferation in a dose dependent manner in vitro (Fig. 1C). Levels of mRNA for PLK1 and STK15 indicative of cells in G2/M phase were dramatically decreased in the PCa cells in response to GAS6 (Fig. 1D, E). These findings suggest that GAS6 expressed by osteoblasts in bone marrow inhibits PCa proliferation.
Fig. 1. GAS6 expressed by osteoblasts in bone marrow, which inhibits PCa cell proliferation and the expression of cell cycle markers.
(A) GAS6 expression by osteoblasts (red, white arrows) in the bone marrow of the long bones of GAS6 expressing wild-type (GAS6+/+) mice and GAS6 deficient GAS6 knockout (GAS6−/−) mice as detected by immunofluorescence staining. Blue, DAPI nuclear stain. Bar=20μm. (B) Secretion levels of GAS6 protein in the marrow fluid of the GAS6+/+ and GAS6−/− mice as detected by ELISA. (C) The proliferation assays in PCa cells were performed by XTT. mRNA expression levels of (D) PLK1 and (E) STK15 were measured as qPCR. Data in Fig. 1B–E are representative of mean with s.d. (Student’s t-test).
GAS6 INDUCES G1 CELL CYCLE ARREST IN PCA CELLS IN RESPONSE TO DOCETAXEL CHEMOTHERAPY
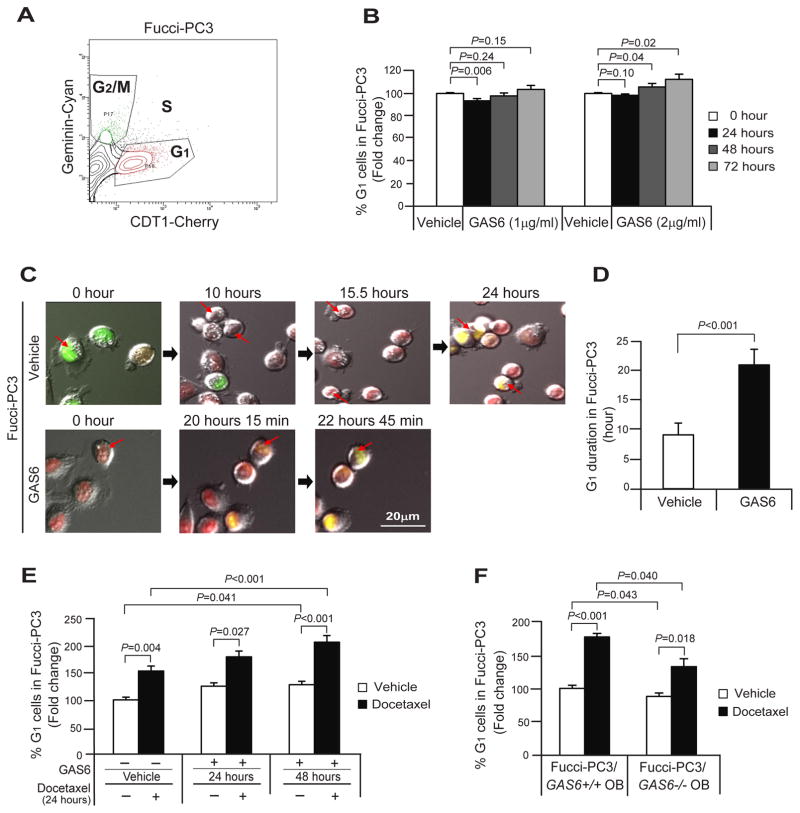
Previously we demonstrated that GAS6 secreted from osteoblasts plays a critical role in establishing PCa cell dormancy [Shiozawa et al., 2010]. Here we further explored how GAS6 regulates the cell cycle in PCa cells. For these investigations, the cell-cycle specific Fucci-vectors were employed in PC3 cells and Fucci expression was used to isolate cells at different stages of the cell cycle [Sakaue-Sawano et al., 2008] by examining FACS profiles of cells in G1, S, and G2/M (Fig. 2A). In standard cultures, we found that 2μg/ml of GAS6 increased the percentage of cells in G1 phase significantly at 48 and 72 hours (Fig. 2B). We also examined that the duration of cell arrested time in G1 phase in live-cell imaging of GAS6 treated Fucci-PC3 cells for 24 hours.
Fig. 2. GAS6 induces G1 cell cycle arrest in PCa cells during docetaxel chemotherapy.
(A) A flow profile of the cell cycle status; G1, S, and G2/M in Fucci-PC3 cells. (B) % G1 cells in Fucci-PC3 cells following treatment of GAS6 (0–2μg/ml) in standard culture conditions for 72 hours. (C) The living cell imaging of Fucci-PC3 (red arrows) following GAS6 (2μg/ml) treatment by video for 24 hours with 15 min interval by Deltavision Elite Microscope. Bar=20μm. (D) Quantification of G1 duration in the single cell from Fig. 2C (n=10/group). (E) % G1 cells in Fucci-PC3 cell culture following the treatment of docetaxel with the presence or absence of GAS6. (F) % G1 cells in cocultured Fucci-PC3 cells with GAS6 expressing wild-type osteoblasts (GAS6+/+ OB) or GAS6 deficient osteoblasts (GAS−/− OB) following the treatment of docetaxel. Data in Fig. 2B, D–F are representative of mean with s.d. (Student’s t-test).
We found that GAS6 treated Fucci-PC3 remained in the G1 phase for 20.8 hours 15 minutes vs 9.1 hours for vehicle treated cells (Fig. 2C, D). The cell cycle monitoring was next performed for docetaxel treated cells with the presence or absence of GAS6. More G1 arrested cells were identified after docetaxel and GAS6 treatments at 24 hours and 48 hours (Fig. 2E). In addition, the cell cycle monitoring was performed in cocultured Fucci-PC3 cells with GAS6 expressing wild-type osteoblasts (GAS6+/+ OB) or GAS6 deficient osteoblasts (GAS−/− OB) following treatment with docetaxel. Importantly, more G1 arrested cells were found in coculture with wild-type osteoblasts (GAS6+/+ OB) compared to GAS6 deficient osteoblasts (GAS−/− OB) in both vehicle and docetaxel treated conditions (Fig. 2F). Collectively, the data suggest GAS6 expressed by osteoblasts may regulate the growth arrest during docetaxel chemotherapy in bone marrow microenvironment.
GAS6 UPREGULATES G1 CELL CYCLE ARREST SIGNALS AND DOWNREGULATES S PHASE ENTRY SIGNALS DURING DOCETAXEL CHEMOTHERAPY IN PCA CELLS
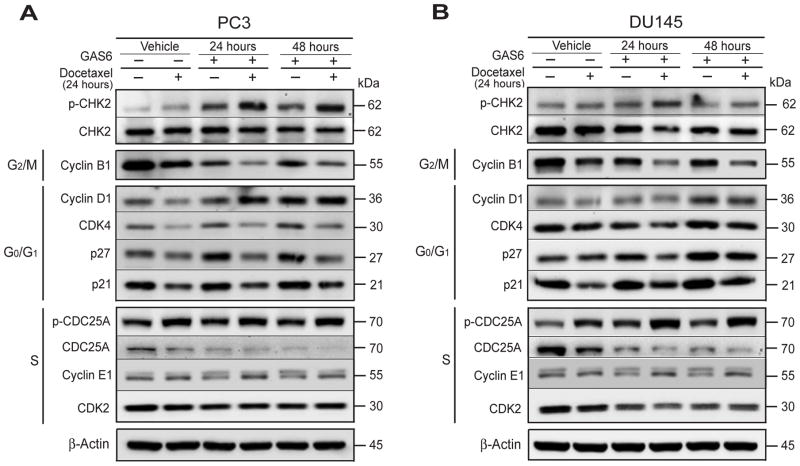
Docetaxel is known to downregulate genes associated with the cell proliferation, while upregulating genes associated with cell cycle arrest and induction of apoptosis [Li et al., 2004]. Therefore we examined the protein expression of several core cell cycle regulators including cyclins, CDKs, CDK inhibitors, and CDCs by Western blot in PCa cell cultures treated with docetaxel and/or GAS6. We found that Cyclin B1 (G2/M marker) and CDC25A, Cyclin E1, CDK2 (S phase entry regulator) were downregulated, while p27, p21, Cyclin D1, and CDK4 (associated with expression at G0/G1) were upregulated in GAS6 treated PCa cells (Fig. 3A, B). These signaling events were further accentuated in PCa cells treated with docetaxel and GAS6. These data suggest that GAS6 promotes G1 cell cycle arrest and a delayed entry into S phase.
Fig. 3. GAS6 regulates signals associated cell cycle during docetaxel chemotherapy in PCa cells.
Examination of expression of the checkpoint and cell cycle related proteins; p-CHK2, CHK2 (checkpoint), Cyclin B1 (G2/M marker), Cyclin D1, CDK4, p27, and p21 (G0/G1 marker), and CDC25A, Cyclin E1, CDK2 (S phase entry marker) in the cultures of (A) PC3 cells and (B) DU145 cells following docetaxel treatment with the presence or absence of GAS6 by Western blots.
GAS6 EXPRESSED BY OSTEOBLASTS CONTRIBUTES TO THE PROTECTION OF PCA CELLS FROM DOCETAXEL-INDUCED APOPTOSIS
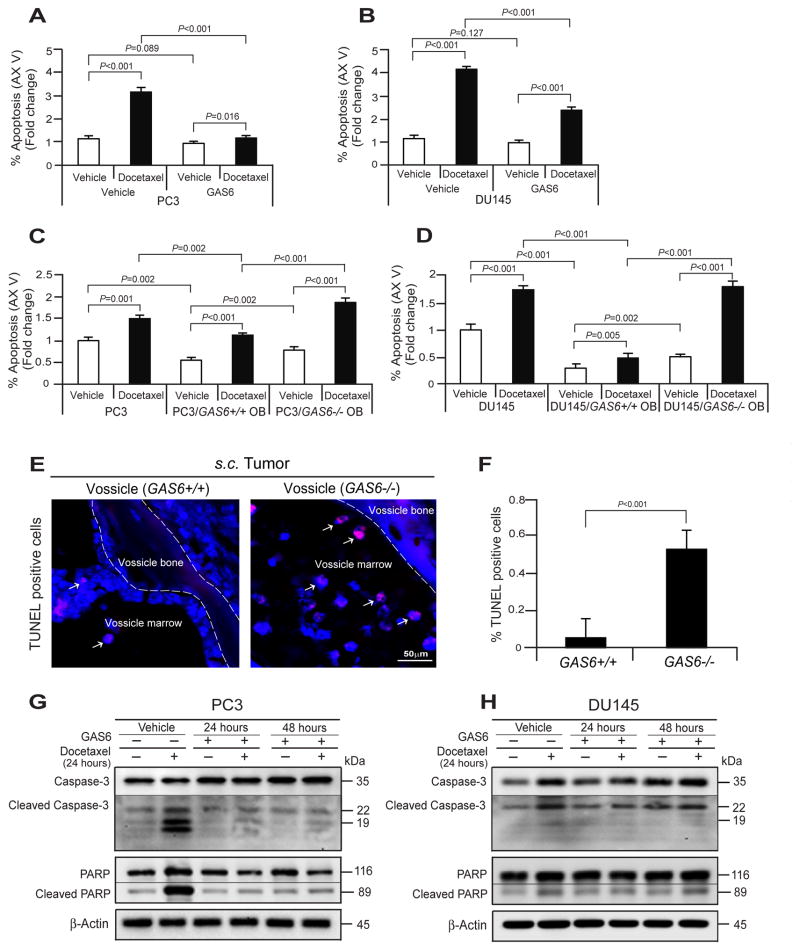
PCa cells are known to develop resistance to chemotherapies, particularly in the marrow. To explore whether GAS6 plays a role in this process, we first examined the percentage of apoptotic cells following treatment with docetaxel and/or GAS6 by Annexin V staining. As expected, more PCa cells underwent apoptosis following docetaxel treatment compared to vehicle treatment (Fig. 4A, B). However, fewer PCa cells underwent apoptosis in the presence of GAS6 for both vehicle and docetaxel treatment (Fig. 4A, B). Additionally, we performed an apoptosis assay on cocultures of PC3 cells with GAS6+/+ OB or GAS−/− OB following docetaxel treatment. Fewer apoptotic cells were identified when PCa cells were cocultured with GAS6+/+ OB vs GAS−/− OBs in both vehicle and docetaxel treatment (Fig. 4C, D). To validate these in vitro studies, next we performed TUNEL staining on tumors grown in a GAS6+/+ or GAS6−/− bone environment (e.g. vossicles) as described in previous studies [Jung et al., 2012]. Fewer apoptotic tumor cells were found in the tumors established by PC3 cells within GAS6+/+ vossicles compared with GAS6−/− vossicles (Fig. 4E, F). Finally we examined the extent to which GAS6 regulates docetaxel-induced apoptosis signaling in PCa cells. For these investigations, Caspase-3 and PARP levels in PCa cells following treatment with docetaxel and/or GAS6 were examined by Western blots. We found that docetaxel induces significant levels of Caspase-3 and PARP cleavages in PCa cells. Importantly, GAS6 protected caspase-3 and PARP from cleavage in the PCa cells (Fig. 4G, H). Collectively, these data suggest that GAS6 expressed by osteoblasts in bone marrow plays a significant role in the regulation of PCa cell survival during docetaxel chemotherapy.
Fig. 4. GAS6 expressed by osteoblasts contributes to the protection of PCa cells from docetaxel-induced apoptosis.
Examination of % apoptosis in (A) PC3 and (B) DU145 cells following the docetaxel treatment with the presence or absence of GAS6 by FACS analysis using Annexin V staining. Examination of % apoptosis in (C) PC3 and (D) DU145 cells in cocultured PC3 cells with GAS6+/+ OB or GAS−/− OB following the treatment of docetaxel. (E) Apoptotic tumor cells (red, white arrows) in the tumor established PC3 cells within GAS6+/+ vossicles or GAS6−/− vossicles as evaluated by TUNEL staining. Blue, DAPI nuclear stain. Bar=50μm. (F) Quantification of % apoptotic cells from Fig. 4E. Docetaxel-induced apoptotic signaling, Caspases-3 and PARP in (G) PC3 cells and (H) DU145 cells following the treatment of docetaxel with the presence or absence of GAS6 were evaluated by Western blots. Data in Fig. 4A–D, F are representative of mean with s.d. (Student’s t-test).
DISCUSSION
Recent studies suggest that signals from the bone marrow microenvironment protect PCa cells from chemotherapy. Here we demonstrate that GAS6 expressed by osteoblasts regulates cell cycle and apoptosis of PCa cells during chemotherapy. We found that GAS6 significantly increased G1 arrested PCa cells by signals associating with G1 cell arrest and S phase delay. Furthermore, we found that GAS6 contributes to the protection of PCa cells from docetaxel-induced apoptosis in vitro cultures and a GAS6-expressing bone environment protects PCa cell apoptosis within the primary tumors in vivo studies. Finally, we found that GAS6 prevents the activation of docetaxel-induced apoptotic signaling, Caspase-3 and PARP. Our results suggest that GAS6 expressed by osteoblasts in the bone marrow plays a significant role in regulation of PCa cell survival during chemotherapy.
PCa frequently takes more than 5 years to progress to lethal metastatic disease or biochemical recurrence after curative surgery or radiation therapy [Amling et al., 2000; Morgan et al., 2009; Roberts and Han, 2009] indicating that DTCs may enter into a state of cellular dormancy for long periods within metastatic sites [Quesnel, 2008; Yumoto et al., 2014]. Yet how DTC cells become dormant, acquire resistance to anticancer drugs, and ultimately cause tumor recurrence/metastatic relapse remain critical questions. Previously, we demonstrated that GAS6 secreted from osteoblasts plays a critical role in establishing PCa cellular dormancy [Shiozawa et al., 2010]. Here, we further demonstrated the mechanism by which GAS6 regulates cell cycle of PCa cells in response to chemotherapy. Indeed, we found that GAS6 increases the number and duration of G1 arrested cells by downregulation of Cyclin B1 (G2/M phase), CDC25A, Cyclin E1, and CDK2 (S phase entry) and upregulation of p27, p21, Cyclin D1, and CDK4 (G0/G1 phase) in PCa cells during docetaxel chemotherapy.
Intensive studies have identified mechanisms of target molecules or signals associated with the anti-proliferative effects and anti-tumorigenic activities for the PCa treatment. Docetaxel-chemotherapy targets proliferating cancer cells, which are associated by core cell cycle regulators including cyclins, CDKs, CDK inhibitors, and CDCs [Kawamata et al., 1995; ELDeiry et al., 1995; Nilsson and Hoffmann, 2000; Poggioli et al., 2001; Erlanson and Landberg, 2001; Yoo et al., 2002; Li et al., 2004; Roy et al., 2008; Chiu et al., 2009]. Double knockdown of the cyclin-dependent kinase inhibitor 1A (p21) and the cyclin-dependent kinase inhibitor 1B (p27) in DU145 cells show higher tumor growth rate than the comparable growth of either p21 or p27 knockdown [Roy et al., 2008]. Another important cell cycle regulator, CDC25A, is highly expressed during S phase entry of the cell cycle in many cancers [Busino et al., 2004; Lavecchia et al., 2009]. CDC25A has oncogenic properties, is highly expressed in human PCa, and its expression level correlates with high Gleason scores and metastatic PCa [Chiu et al., 2009]. The expression of CDC25A in PCa cells suppresses PSA expression and functions as an AR corepressor, suggesting that it may also be a possible anticancer target [Chiu et al., 2009]. Further reports show that docetaxel promotes the upregulation of a CDK inhibitor (p19) and downregulation of the cyclins (cyclin A and cyclin B1) in the head and neck cancer cells [Yoo et al., 2002] and the upregulation of CDK inhibitors (p21 and p27) and downregulation of cyclins (cyclin A2, cyclin E2, and cyclin F), CDK4, and cell division cycles (CDC2, CDC7, CDC20, and CDC25B) in PCa cells [Li et al., 2004]. Despite this evidence, docetaxel continues to be an effective agent of growth inhibition and induction of apoptosis in part by the upregulation of growth arrest signals during the chemotherapy. Yet these actions also lead to the generation of cell cycle arrest signals, which induce cellular dormancy and ultimately increase drug-resistance. Here we found that in docetaxel-chemotherapy treated PCa cells, GAS6 further downregulates active cell cycling phase regulators including Cyclin B1 (G2/M phase), CDC25A, Cyclin E1, and CDK2 (S phase entry). In complement, we saw upregulation of arresting cell cycle phase regulators including p27, p21 Cyclin D1, and CDK4, (G0/G1 phase). Taken together, GAS6 promotes induction of drug-resistant signals in docetaxel-treated PCa cells. Future cancer therapeutic studies will be required to identify selective agents capable of inducing cancer cell apoptosis without inducing dormancy within the context of these complex microenvironmental cues.
The ability of docetaxel to promote cancer cell apoptosis is dependent on its function in cell death signaling [Jin and EL-Deiry, 2005]. During the G2/M phase arrest of cancer cells, docetaxel binds to microtubules with high affinity, activating JNK signaling causing B-cell lymphoma 2 (Bcl-2) phosphorylation, thereby promoting a cascade of events that ultimately leads to apoptotic cell death [Haldar and Basu, 1997]. Increased JNK signaling also increases dephosphorylation of Bad, which associates with Bcl-2, releasing Bcl-2 from Bax. Unbound Bax translocates to the inner mitochondrial membrane forming Bax/Bax pores, allowing release of cytochrome c and activation of caspases leading to apoptosis [Wolter et al., 1997]. Reduction of multidrug resistance-associated protein-1 (MRP-1) by pre-treatment of 1,25-dihydroxy vitamin D3 (1,25-VD) enhanced the docetaxel antitumor activity through Bcl-2 signaling pathway in PC3 cells [Tinga et al., 2007]. Docetaxel is also able to induce apoptosis through different caspase families in PCa cells; Caspase-3 and Caspase-7 in LNCaP and TSU-Pr1 cells vs. Caspase-8 in PC3 cells [Marcelli et al., 1999; Muenchen et al., 2001]. Furthermore, Docetaxel-induced apoptosis has also been associated with the increases of Survivin, GADD45A, Fas/Apo-1, and FOXO3A in PCa cells [Li et al., 2004]. Apoptosis is guided by a complex series of cellular events and initiation of these events is tightly regulated by the balance of cell death and survival signals. The balance of these signaling mediators is not yet fully understood, but is essential in understanding mechanisms that govern cell fate such as apoptosis and cancer cell resistance.
Just as the docetaxel-inducing apoptotic signaling pathways have been elucidated, there is increasing evidence that docetaxel can confer resistance to cancer cells. For example, PIAS1 is a crucial survival factor, which was significantly increased in docetaxel resistant cells and in tissue of patients after docetaxel chemotherapy. Downregulation of PIAS1 results in increased apoptosis, suggesting the importance of PIAS1 in maintaining cell survival [Puhr et al., 2014]. Moreover, GAS6 induces activation of AKT/PKB leading to the phosphorylation of Bad as well as activation of ERK, JNK/SAPK, and p38 MAPK in serum starved NIH3T3 cells [Goruppi et al., 1999]. In addition, GAS6 secreted by osteoblasts rapidly induces the phosphorylation of ERK in PC3 cells in standard culture conditions, which provides evidence that GAS6 can promote PCa cell survival [Shiozawa et al., 2010]. However, the balance between cell survival signals and apoptotic signals in cell fate remains poorly understood. In this investigation, we further demonstrated that docetaxel significantly increases the apoptotic signals, cleaved Caspase-3 and PARP in PCa cells, while GAS6 protected PCa cells from Caspase-3 and PARP cleavages. These data suggest that GAS6 may be a critical molecule in suppressing the activation of key apoptotic signaling mediators, thus promoting cell survival. Therefore, targeting GAS6 or GAS6 signaling during cancer therapy for metastatic diseases may be helpful in elimination of quiescent PCa DTCs, as well.
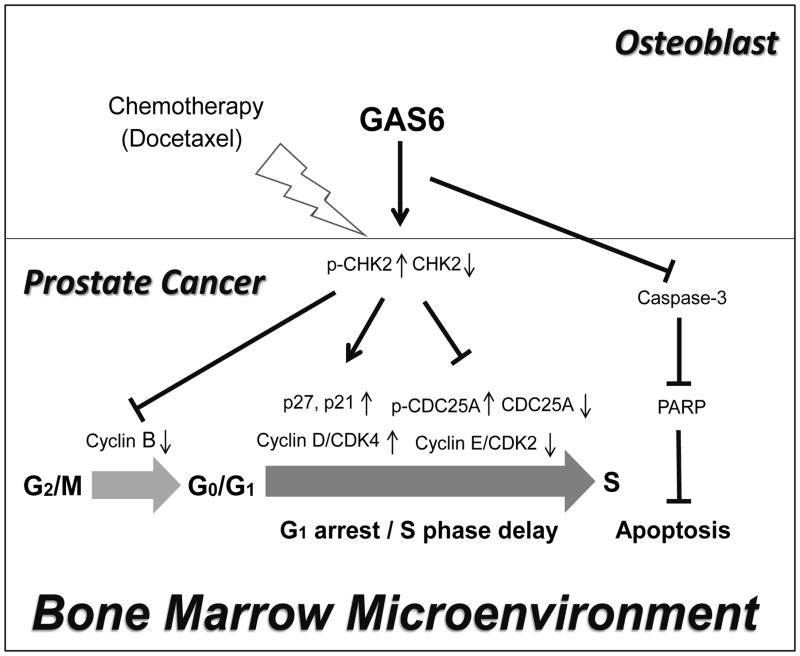
In summary, this work provides evidence that supports a crucial role for GAS6 in PCa cell survival during chemotherapy within the bone marrow microenvironment (Fig. 5). Importantly, this work contributes to the understanding of dormancy signals that facilitate chemotherapeutic resistance in PCa cells, which may have important implications for optimizing therapeutic strategies against metastatic disease and tumor recurrence.
Fig. 5. Model for the role of GAS6 in PCa survival during chemotherapy in the bone marrow.
GAS6 promotes PCa survival by G1 cell cycle arrest/ S phase delay and inhibition of apoptotic signaling pathways during chemotherapy in bone marrow, which may have important implications for targeting metastatic disease.
Acknowledgments
This work is directly supported by the University of Michigan MCubed Project, the National Cancer Institute (CA093900, CA163124), the Department of Defense (W81XWH-11-1-0636 and W81XWH-15-1-0637), and the Prostate Cancer Foundation. R.S. Taichman receives support as the Major McKinley Ash Collegiate Professor.
References
- Amling CL, Blute ML, Bergstralh EJ, Seay TM, Slezak J, Zincke H. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. J Urol. 2000;164(1):101–105. [PubMed] [Google Scholar]
- Bhardwaj A, Srivastava SK, Singh S, Arora S, Tyagi N, Andrews J, McClellan S, Carter JE, Singh AP. CXCL12/CXCR4 signaling counteracts docetaxel-induced microtubule stabilization via p21-activated kinase 4-dependent activation of LIM domain kinase 1. Oncotarget. 2014;5(22):11490–11500. doi: 10.18632/oncotarget.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busino L, Chiesa M, Draetta GF, Donzelli M. CDC25A phosphatase: combinatorial phosphorylation, ubiquitylation and proteolysis. Oncogene. 2004;23(11):2050–1056. doi: 10.1038/sj.onc.1207394. [DOI] [PubMed] [Google Scholar]
- Chiu YT, Han HY, Leung SC, Yuen HF, Chau CW, Guo Z, Qiu Y, Chan KW, Wang X, Wong YC, Ling MT. CDC25A functions as a novel AR corepressor in prostate cancer cells. J Mol Biol. 2009;385(2):446–56. doi: 10.1016/j.jmb.2008.10.070. [DOI] [PubMed] [Google Scholar]
- EL-Deiry WS, Tokino T, Waldman T, Oliner JD, Velculescu VE, Burrell M, Hill DE, Healy E, Rees JL, Hamilton SR. Topological control of p21 WAF1 /CIP1 expression in normal and neoplastic tissues. Cancer Res. 1995;55:2910–2919. [PubMed] [Google Scholar]
- Erlanson M, Landberg G. Prognostic implications of p27 and cyclin E protein contents in malignant lymphomas. Leuk Lymphoma. 2001;40:461–470. doi: 10.3109/10428190109097645. [DOI] [PubMed] [Google Scholar]
- Goruppi S, Ruaro E, Varnum B, Schneider C. Gas6-mediated survival in NIH3T3 cells activates stress signaling cascade and is independent of Ras. Oncogene. 1999;18(29):4224–4236. doi: 10.1038/sj.onc.1202788. [DOI] [PubMed] [Google Scholar]
- Haldar S, Basu A, Croce CM. Bcl2 is the guardian of microtubule integrity. Cancer Res. 1997;57:229–233. [PubMed] [Google Scholar]
- Hong WK. The current status of docetaxel in solid tumors. An M. D. Anderson Cancer Center review. Oncology (Huntington) 2002;16:9–15. [PubMed] [Google Scholar]
- Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biology & Therapy. 2005;4(2):147–171. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, Berry JE, McGee S, Lee E, Sun H, Wang J, Jin T, Zhang H, Dai J, Krebsbach PH, Keller ET, Pienta KJ, Taichman RS. Recruitment of mesenchymal stem cells into prostate tumors promotes metastasis. Nature Communications. 2013;4:e1795. doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Shiozawa Y, Wang J, McGregor N, Dai J, Park SI, Berry JE, Havens AM, Joseph J, Kim JK, Patel L, Carmeliet P, Daignault S, Keller ET, McCauley LK, Pienta KJ, Taichman RS. Prevalence of prostate cancer metastases after intravenous inoculation provides clues into the molecular basis of dormancy in the bone marrow microenvironment. Neoplasia. 2012;14(5):429–439. doi: 10.1596/neo.111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Wang J, Lee E, McGee S, Berry JE, Yumoto K, Dai J, Keller ET, Shiozawa Y, Taichman RS. Annexin 2-CXCL12 Interactions Regulate Metastatic Cell Targeting and Growth in the Bone Marrow. Mol Cancer Res. 2015;13(1):197–207. doi: 10.1158/1541-7786.MCR-14-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Shiozawa Y, Wang J, Patel LR, Havens AM, Song J, Krebsbach PH, Roodman GD, Taichman RS. Annexin-2 is a regulator of stromal cell-derived factor-/CXCL12 function in the hematopoietic stem cell endosteal niche. Exp Hematol. 2011;39(2):151–166. doi: 10.1016/j.exphem.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata N, Morosetti R, Miller CW, Park D, Spirin KS, Nakamaki T, Takeuchi S, Hatta Y, Simpson J, Wilcyznski S. Molecular analysis of the cyclin-dependent kinase inhibitor gene p27/Kip1 in human malignancies. Cancer Res. 1995;55:2266–2269. [PubMed] [Google Scholar]
- Kim JK, Jung Y, Wang J, Joseph J, Mishra A, Hill EE, Krebsbach PH, Pienta KJ, Shiozawa Y, Taichman RS. TBK1 regulates prostate cancer dormancy through mTOR inhibition. Neoplasia. 2013;15(9):1064–74. doi: 10.1593/neo.13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsilieris M. Osteoblastic metastasis in advanced prostate cancer. Anticancer Research. 1993;13(2):443–449. [PubMed] [Google Scholar]
- Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: Pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- Lavecchia A, Di Giovanni C, Novellino E. CDC25A and B dual-specificity phosphatase inhibitors: potential agents for cancer therapy. Curr Med Chem. 2009;16(15):1831–1849. doi: 10.2174/092986709788186084. [DOI] [PubMed] [Google Scholar]
- Li Y, Li X, Hussain M, Sarkar FH. Regulation of microtubule, apoptosis, and cell cycle-related genes by taxotere in prostate cancer cells analyzed by microarray. Neoplasia. 2004;6(2):158–167. doi: 10.1593/neo.03391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon KL, Lin HM, Castillo L, Lee BY, Lee-Ng M, Chatfield MD, Chiam K, Breit SN, Brown DA, Molloy MP, Marx GM, Pavlakis N, Boyer MJ, Stockler MR, Daly RJ, Henshall SM, Horvath LG. Cytokine profiling of docetaxel-resistant castration-resistant prostate cancer. Br J Cancer. 2015;112(8):1340–1348. doi: 10.1038/bjc.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelli M, Cunningham GR, Walkup M, He Z, Sturgis L, Kagan C, Mannucci R, Nicoletti I, Teng B, Denner L. Signaling pathway activated during apoptosis of the prostate cancer cell line LNCaP: overexpression of caspase-7 as a new gene therapy strategy for prostate cancer. Cancer Res. 1999;59(2):382–390. [PubMed] [Google Scholar]
- Morgan TM, Lange PH, Porter MP, Lin DW, Ellis WJ, Gallaher IS, Vessella RL. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res. 2009;15(2):677–683. doi: 10.1158/1078-0432.CCR-08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenchen HJ, Poncza PJ, Pienta KJ. Different docetaxel-induced apoptotic pathways are present in prostate cancer cell lines LNCaP and PC-3. Urology. 2001;57:366–370. doi: 10.1016/s0090-4295(00)00935-3. [DOI] [PubMed] [Google Scholar]
- Nilsson I, Hoffmann I. Cell cycle regulation by the CDC25 phosphatase family. Prog Cell Cycle Res. 2000;4:107–114. doi: 10.1007/978-1-4615-4253-7_10. [DOI] [PubMed] [Google Scholar]
- Petrioli R, Pozzessere D, Messinese S, Sabatino M, Di Palma T, Marsili S, Correale P, Manganelli A, Salvestrini F, Francini G. Weekly low-dose docetaxel in advanced hormone-resistant prostate cancer patients previously exposed to chemotherapy. Oncology. 2003;64:300–305. doi: 10.1159/000070285. [DOI] [PubMed] [Google Scholar]
- Pienta KJ, Esper PS. Risk factors for prostate cancer. Annals of Internal Medicine. 1993;118(10):793–803. doi: 10.7326/0003-4819-118-10-199305150-00007. [DOI] [PubMed] [Google Scholar]
- Poggioli GJ, Dermody TS, Tyler KL. Reovirus-induced sigma1s-dependent G(2)/M phase cell cycle arrest is associated with inhibition of p34(cdc2) J Virol. 2001;75:7429–434. doi: 10.1128/JVI.75.16.7429-7434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhr M, Hoefer J, Neuwirt H, Eder IE, Kern J, Schäfer G, Geley S, Heidegger I, Klocker H, Culig Z. PIAS1 is a crucial factor for prostate cancer cell survival and a valid target in docetaxel resistant cells. Oncotarget. 2014;5(23):12043–12056. doi: 10.18632/oncotarget.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian DZ, Rademacher BL, Pittsenbarger J, Huang CY, Myrthue A, Higano CS, et al. CCL2 is induced by chemotherapy and protects prostate cancer cells from docetaxel-induced cytotoxicity. Prostate. 2010;70:433–442. doi: 10.1002/pros.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesnel B. Dormant tumor cells as a therapeutic target? Cancer Lett. 2008;267:10–17. doi: 10.1016/j.canlet.2008.02.055. [DOI] [PubMed] [Google Scholar]
- Roberts WB, Han M. Clinical significance and treatment of biochemical recurrence after definitive therapy for localized prostate cancer. Surg Oncol. 2009;18(3):268–274. doi: 10.1016/j.suronc.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Singh RP, Agarwal C, Siriwardana S, Sclafani R, Agarwal R. Downregulation of both p21/Cip1 and p27/Kip1 produces a more aggressive prostate cancer phenotype. Cell Cycle. 2008;7(12):1828–1835. doi: 10.4161/cc.7.12.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi H, Imamura T, Ogawa M, Masai H, Miyawaki A. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 2008;132(3):487–498. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
- Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, Pienta MJ, Song J, Wang J, Loberg RD, Krebsbach PH, Pienta KJ, Taichman RS. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121(4):1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa Y, Pedersen EA, Patel LR, Ziegler AM, Havens AM, Jung Y, Wang J, Zalucha S, Loberg RD, Pienta KJ, Taichman RS. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia. 2010;12(2):116–127. doi: 10.1593/neo.91384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YX, Schneider A, Jung Y, Wang J, Dai J, Wang J, Cook K, Osman NI, Koh-Paige AJ, Shim H, Pienta KJ, Keller ET, McCauley LK, Taichman RS. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. 2005;20:318–329. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, Dreicer R, Vogelzang NJ, Picus J, Shevrin D, Hussain M, Garcia JA, DiPaola RS. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. The New England journal of medicine. 2015;373(8):737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62(6):1832–1837. [PubMed] [Google Scholar]
- Taichman RS, Loberg RD, Mehra R, Pienta KJ. The evolving biology and treatment of prostate cancer. J Clin Invest. 2007;117:2351–2361. doi: 10.1172/JCI31791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinga HJ, Hsu J, Bao BY, Lee YF. Docetaxel-induced growth inhibition and apoptosis in androgen independent prostate cancer cells are enhanced by 1a,25-dihydroxy vitamin D3. Cancer Letters. 2007;247:122–129. doi: 10.1016/j.canlet.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo GH, Piechocki MP, Ensley JF, Nguyen T, Oliver J, Meng H, Kewson D, Shibuya TY, Lonardo F, Tainsky MA. Docetaxel induced gene expression patterns in head and neck squamous cell carcinoma using cDNA microarray and PowerBlot. Clin Cancer Res. 2002;8:3910–3921. [PubMed] [Google Scholar]
- Yumoto K, Eber MR, Berry JE, Taichman RS, Shiozawa Y. Molecular pathways: niches in metastatic dormancy. Clin Cancer Res. 2014;20(13):3384–3389. doi: 10.1158/1078-0432.CCR-13-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]