Abstract
Repetitive mild traumatic brain injury (rmTBI) is an important medical concern for adolescent athletes that can lead to long-term disabilities. Multiple mild injuries may exacerbate tissue damage resulting in cumulative brain injury and poor functional recovery. In the present study, we investigated the increased brain vulnerability to rmTBI and the effect of hyperbaric oxygen treatment using a juvenile rat model of rmTBI. Two episodes of mild cortical controlled impact (3 days apart) were induced in juvenile rats. Hyperbaric oxygen (HBO) was applied 1 hour/day × 3 days at 2 atmosphere absolute consecutively, starting at 1 day after initial mild traumatic brain injury (mTBI). Neuropathology was assessed by multi-modal magnetic resonance imaging (MRI) and tissue immunohistochemistry. After repetitive mTBI, there were increases in T2-weighted imaging-defined cortical lesions and susceptibility weighted imaging-defined cortical microhemorrhages, correlated with brain tissue gliosis at the site of impact. HBO treatment significantly decreased the MRI-identified abnormalities and tissue histopathology. Our findings suggest that HBO treatment improves the cumulative tissue damage in juvenile brain following rmTBI. Such therapy regimens could be considered in adolescent athletes at the risk of repeated concussions exposures.
Keywords: magnetic resonance imaging, susceptibility weighted imaging, T2-weighted imaging, diffusion weighted imaging, concussion, gliosis, adolescent, rat
Introduction
Mild traumatic brain injury (mTBI, concussion) is an important global public health problem in the pediatric population and accounts for 80–90% of all treated traumatic brain injury (TBI) cases (Cassidy et al., 2004). Large numbers of children and adolescents participate in a variety of sports (1.6 to 3.6 million/year) with a significant risk for these young athletes to be exposed to repeated episodes of mTBI (Leibson et al., 2011; Mutch et al., 2016). After an initial mTBI event, a complex cascade of metabolic disturbances may occur at the cellular level in the absence of overt clinical symptoms (Giza and Hovda, 2001). It is during this initial post-injury period, when cellular metabolism is already stretched to its limits, that the cell is most vulnerable to further insults. Clinical studies and data from experimental models have suggested that repeated mild head injuries exacerbate outcomes (Longhi et al., 2001; Vagnozzi et al., 2005; Kim et al., 2015; Yang et al., 2015; Bajwa et al., 2016). Compared to the adult brain, the immature brain is unique in its response and vulnerability to TBI, due in part, to the developmental and structural differences of the brain's response to injury (Giza and Hovda, 2001). A previous preliminary report showed that the repetitive mTBI (rmTBI) in the infant rat pup brain may accelerate the development of diffuse axonal injury (DAI) accompanied by the cortical and white matter atrophy (Huh et al., 2007).
Hyperbaric oxygen (HBO) has been explored as a therapeutic treatment approach for management of adult TBI (Huang and Obenaus, 2011). HBO has also been shown to decrease oxidative stress and inflammation in children with autism and cerebral palsy with improved outcomes (Rossignol et al., 2007; Mukherjee et al., 2014). Thus, HBO therapy may provide a potential treatment strategy that is clinically available and safe to treat children exposed to rmTBI. Magnetic resonance imaging (MRI) is widely used clinically to evaluate children and adults with neurological diseases and is predictive of long-term neurological outcomes (Ashwal et al., 2006; Galloway et al., 2008; Niogi et al., 2008; Yang et al., 2015). T2-weighted imaging (T2WI) is used to evaluate brain edema and hemorrhage (Tate et al., 2012). Susceptibility weighted imaging (SWI) is sensitive for detection of micro-hemorrhagic lesions associated with diffuse axonal injury (Ashwal et al., 2006). Diffusion weighted imaging (DTI) is known to detect subtle microstructural white matter lesions that correlate with persistent cognitive deficits in adult mTBI (Niogi et al., 2008; Yang et al., 2015). Our early study showed that repeated mild impacts significantly increased the MRI-identifiable tissue vulnerability in a adult rat model of rmTBI (Huang et al., 2013).
In the current study, a multi-modal MRI was used to characterize the cumulative brain injury in a juvenile rat model of rmTBI (rmjTBI). We assessed whether the HBO treatment delivered after the initial concussion could reduce the brain tissue vulnerability to the following repetitive mild insult.
Material and Methods
All protocols were approved by the Animal Health and Safety Committees of Loma Linda University (LLU) and were in compliance with Federal regulations.
A total of 24 male juvenile Sprague-Dawley rats (Harlan, Indianapolis, IN, USA), 30 days old (equivalent to human age of 12 years), were randomized into 4 experimental groups with 6 rats in each group: 1) sham, 2) HBO + sham, 3) rmjTBI 3 days apart, 4) rmjTBI 3 days apart + HBO. TBI was induced as previously described (Huang et al., 2013). Briefly, a craniotomy was performed in isoflurane anesthetized rats, followed by a mild controlled cortical impact (CCI) delivered to the right cortical surface using an electromagnetic driven piston with 3 mm diameter tip at a depth of 0.8 mm, speed of 5.0 m/s, and 200 ms contact duration (dwell) (Leica Biosystems Inc., Richmond, IL, USA). For animals in the rmjTBI groups, a second identical impact was delivered 3 days after the first CCI event. HBO was applied 1 hour/day × 3 days at 2 atmosphere absolute (ATA; 1 ATA = 50.66 kPa) consecutively using HBO chamber for small animal research (1300B, Sechrist USA, Anaheim, CA, USA), starting at 1 day after the initial CCI and ending right before the 2nd CCI. In vivo multi-modal MRIs including T2WI and SWI were acquired 24 hours after each surgery and at final 14 days. Ex vivo DTI was acquired prior to histological examination. Imaging parameters were identical to those previously published (Huang et al., 2013; Donovan et al., 2014). Regions of interest on cortical signal abnormalities (hyper- and hypo-intensities) were drawn manually on T2WI's. An operator-guided thresholding method was used to segment hemorrhages on SWI's. For DTI analysis, the right (ipsilateral) corpus callosum was outlined on the MRI slice at the level of the maximal lesion (M). There were no significant differences between shams and HBO treated shams in any of the MRI derived parameters, thus we combined the two groups into a single sham group for quantitative data statistics.
Immunohistochemistry was performed on free floating sections at the level of the maximal cortical lesion. The sections were incubated in the primary antibodies of 1) mouse glial fibrillary acidic protein (GFAP, Millipore, Temecula, CA, USA; 1:400); 2) rabbit ionized calcium binding adaptor molecule 1 (IBA1, Dako, Carpinteria, CA, USA; 1:400), followed by the secondary antibody conjugated to goat anti-mouse Alexafluor AF488 (Invitrogen, Carlsbad, CA, USA; 1:400) and goat anti-rabbit rhodamine (Millipore, Temecula, CA, USA; 1:200). Stain intensity were calculated using Imaging J software (National Institute of Health, Bethesda, MD, USA).
Quantitative data are presented as the mean ± SEM. SigmaPlot 11.0 (Systat software Inc, San Jose, CA, USA) was used for statistical analysis. One-way analysis of variance (ANOVA) for multiple comparisons and Student-Newman-Keuls post hoc test were used. A P value less than 0.05 was considered statistically significant.
Results
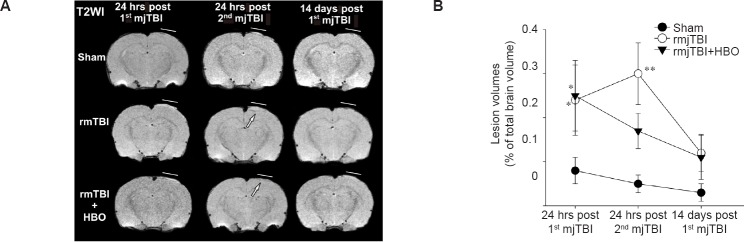
All animals survived in the surgical procedure and HBO treatment. The overall cortical lesion volume, defined as abnormal T2WI signal intensities (hyperintensity = edema, hypointensity = blood) was 0.25 ± 0.08% of the total brain volume at 24 hours following the initial impact, confirming the mild nature of our TBI model. rmjTBI delivered 3 days apart exacerbated cortical injury; however, hyperbaric oxygen therapy (HBO) right after the initial mjTBI decreased the lesion volumes compared to non-treated rmjTBI (Figure 1).
Figure 1.
T2-weighted imaging (T2WI) identified lesion at 24 hours (hrs) post repetitive mild juvenile traumatic brain injury (rmjTBI).
Note: (A) Representative T2WI images revealed hyperbaric oxygen (HBO) protection against the cumulative brain tissue damages including edema (hyperintensity) and hemorrhage (hypointensity) 24 hrs after repetitive injury (arrows). Lines indicate the locations of craniotomy. (B) Quantifying T2WI-derived lesion showed the significant increases in cortical lesion volumes at 24 hrs after the rmjTBI, which was reduced by HBO treatment. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, vs. sham group (one-way analysis of variance followed by Student-Newman-Keuls post hoc test). rmTBI: Repetitive mild traumatic brain injury.
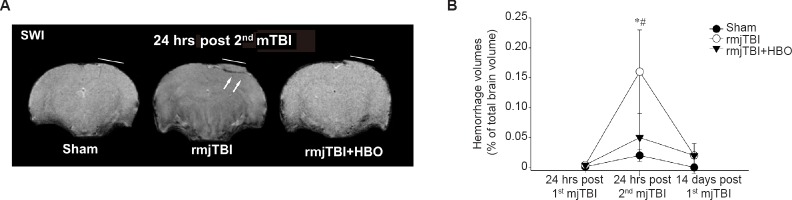
The evaluation of blood within tissues can be readily visualized with SWI, which is uniquely sensitive to the iron content of extravascular blood and can be visualized as dark regions on MRI. The initial impact did not result in significant hemorrhage compared to shams. However, repetitive mTBI at 3 days apart significantly enhanced the tissue bleeding at 24 hours post 2nd mTBI compared to that of first mjTBI (P = 0.034). Consistent with the findings from T2WI, HBO protected rat juvenile pups from the rmjTBI-induced hemorrhage transformation. There was less SWI derived-hemorrhagic lesion volume in HBO treated rmjTBI animals (Figure 2).
Figure 2.
Susceptibility weighted imaging (SWI) identified hemorrhage lesion at 24 hours (hrs) post repetitive mild juvenile traumatic brain injury (rmjTBI).
Note: (A) Representative SWI images revealed that hyperbaric oxygen (HBO) treatment resulted in less extravascular bleeding (hypointensity) within the traumatized brain tissue 24 hours after repetitive injury (arrows). Lines indicate the locations of craniotomy; (B) Quantifying SWI-derived hemorrhages volumes revealed that HBO decreased hemorrhagic susceptibility of mild injured brains to repetitive mild controlled cortical impact. Data are presented as the mean ± SEM. *P < 0.05, vs. sham group, #P < 0.05, vs. rmjTBI + HBO group (one-way analysis of variance followed by Student-Newman-Keuls post hoc test). mTBI: Mild traumatic brain injury.
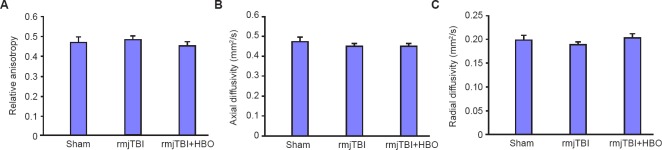
White matter integrity within corpus callosum can be non-invasively assessed using DTI, in which water moves preferentially along intact fiber bundles. At 14 days post-injury, our final time point, however, there were no significant difference of ex vivo DTI measures of relative anisotropy, axial or radial diffusivity among sham, rmjTBI and HBO treated rmjTBI (Figure 3).
Figure 3.
Diffusion weighted imaging (DTI) measures within ipsilateral corpus callosum at 14 days post repetitive mild juvenile traumatic brain injury (rmjTBI).
Note: There were no significant DTI identified alterations in relative anisotropy, axial or radial diffusivity. HBO: Hyperbaric oxygen.
At 14 days after the initial mTBI (i.e., 11 days after rmTBI), the rmjTBI animals exhibited increased GFAP and IBA1 immunostaining intensity at the impact site within the ipsilateral cortex relative to sham animals, which was reduced by HBO treatment (Figure 4).
Figure 4.
Tissue histopathology at 14 days post repetitive mild juvenile traumatic brain injury (rmjTBI).
Note: There was increased glial fibrillary acidic protein (GFAP) staining intensity (A) and ionized calcium binding adaptor molecule 1 (IBA1) staining intensity (B), indicating gliosis of astrocyte and microglia following rmjTBI. Hyperbaric oxygen (HBO) treatment alleviated the astrocyte hypertrophy and microglial activation. Scale bars: 10 μm. Data are presented as the mean ± SEM. *P < 0.05, vs. sham group, #P < 0.05, vs. rmjTBI + HBO group (one-way analysis of variance followed by Student-Newman-Keuls post hoc test).
Results
Using a rmjTBI, we found that: 1) Two episodes of mjTBI with 3 days apart significantly exacerbated T2WI- and SWI-identified brain injury, consistent with increased brain edema and bleeding; 2) Daily HBO treatment during the 3 days interval of two rmjTBI significantly improved the neuroimaging outcomes, which was efficacious in reducing edema and extravascular blood following the second mTBI; 3) DTI-defined corpus callosum injury was not observed in this rmjTBI model.
In the absence of evident structural damage (Gordon et al., 1998), human mTBI is typically associated with post-traumatic edema formation (Tokutomi et al., 1997), altered cerebral blood flow (Golding et al., 1999; Mutch et al., 2016), as well as alterations in cellular metabolism (Masdeu et al., 1994; Vagnozzi et al., 2010). After concussion, a complicated cascade of cellular metabolic disturbances may exist in the absence of overt clinical symptoms (Giza and Hovda, 2001), which can enhance brain vulnerability to a secondary insult (Jenkins et al., 1989; Hovda et al., 1991; Gennarelli et al., 1993). Second-impact syndrome has been associated with athletes who suffer repeated concussions when playing contact sports. Before symptoms from an earlier concussive event have subsided, a second concussion can result in massive brain swelling, subdural hematoma, increased intracranial pressure, and occasionally death (Kelly et al., 1991; Cantu, 1998; Bailes and Cantu, 2001). A higher prevalence of pathological lesions was reported in athletes with a history of multiple concussions (McKee et al., 2009).
We found a single episode of mTBI resulted in the negligible T2WI-identifiable edema or SWI-derived micro-hemorrhages at the site of impact 24 hours post injury, validating the mild nature of our model. However, there was an increased vulnerability to a second mild impact following an initial mTBI in juvenile rats, where there were significantly worse MRI-defined pathologies. These findings are consistent to the previous study of ours and the others in adult rats (Huang et al., 2013; Yang et al., 2015).
Among a collection of therapeutic strategies against neurological diseases (Dock et al., 2015; Li et al., 2015; Lioutas et al., 2015; Merali et al., 2015; Ploughman et al., 2015; Qi et al., 2015; Reuter et al., 2015; Schlunk et al., 2015; Soliman et al., 2015; Zhu et al., 2015), medical gas regimens have been extensively explored with the advantage of administration feasibility (Harch, 2015; Herrera et al., 2015; Hu et al., 2015; Ichihara et al., 2015; Langston and Toombs, 2015; Miller et al., 2015; Parra et al., 2015; Stoller, 2015; Weaver and Liu, 2015; Yan et al., 2015). Emerging evidence has shown HBO induces neuroprotection against a variety of neurological disorders including severe TBI (Huang and Obenaus, 2011). In the present study, multiple HBO treatments starting at 24 hours after the first mTBI event significantly improved repetitive impact-induced MRI-defined brain injuries including brain edema and microhemorrhages. During HBO therapy, the increased partial pressure of oxygen within the blood and subsequent improved mitochondrial metabolism/tissue oxygenation constitutes the net effect of HBO (Huang and Obenaus, 2011). Metabolic disturbances have been shown to occur after concussions which render the brain vulnerable to subsequent impact (Vagnozzi et al., 2005, 2010). Thus, HBO could induce brain tolerance against subsequent injury acutely due to its antioxidant properties and maintenance of mitochondrial redox status (Li et al., 2008).
Preclinical and clinical studies have demonstrated that DTI was able to identify acute integrity changes of the white matter tracts following single mTBI or rmTBI (Babcock et al., 2015; Herrera et al., 2016). However, the significant changes at ipsilateral corpus callosum were not observed at 14 days after injury in our present study. The possible reasons could be very mild nature of our model or younger age at injury leading to no apparent white matter impairments at the end time point we selected in this study.
Pathological TBI insults to the brain trigger astrocytic and microglial reactions (Bye et al., 2011). In an adult rat model of rmTBI, cortical astrocyte hypertrophy and microglial activation were observed around the site of impact, which correlated with the MRI-identified brain tissue abnormalities (Huang et al., 2013). The astroliosis progression was also found in a mouse model of repetitive concussive head injury, correlated with MRI pathological characterization (Yang et al., 2015). Activated glial cells release proinflammatory mediators and chemokines to attract inflammatory cells to the site of injury, resulting in further tissue damage (Csuka et al., 2000; Semple et al., 2010). We observed the similar correlation in terms of ex vivo brain tissue histopathology and in vivo MRI changes. HBO treatment improved the gliosis at site of cortical impact following rmTBI, consistent to the neuroprotection identified by acute MRI.
In conclusion, the neuroimaging and immunohistochemistry profiles of a rmjTBI rat model suggested that HBO treatment was neuroprotective to juvenile brains subjected to two episodes of mild impact. Such therapy regimens could be considered in adolescent athletes at the risk of repeated concussion exposures.
Acknowledgments
The authors wish to acknowledge the assistance of Kamalakar Ambadipudi and Sonny Kim (both from the Department of Basic Sciences, Loma Linda University, CA, USA) for MRI, and Katherine Tsai (summer student from University of California, Riverside) for imaging data analysis.
Footnotes
Funding: This study was supported by funding from Loma Linda University GRASP award (LH and AO, 699314-4526).
Conflicts of interest
None.
Plagiarism check
This paper was screened twice using CrossCheck to verify originality before publication.
Peer review
This paper was double-blinded and stringently reviewed by international expert reviewers.
References
- Ashwal S, Holshouser BA, Tong KA. Use of advanced neuroimaging techniques in the evaluation of pediatric traumatic brain injury. Dev Neurosci. 2006;28:309–326. doi: 10.1159/000094157. [DOI] [PubMed] [Google Scholar]
- Babcock L, Yuan W, Leach J, Nash T, Wade S. White matter alterations in youth with acute mild traumatic brain injury. J Pediatr Rehabil Med. 2015;8:285–296. doi: 10.3233/PRM-150347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailes JE, Cantu RC. Head injury in athletes. Neurosurgery. 2001;48:26–45. doi: 10.1097/00006123-200101000-00005. discussion 45-26. [DOI] [PubMed] [Google Scholar]
- Bajwa NM, Halavi S, Hamer M, Semple BD, Noble-Haeusslein LJ, Baghchechi M, Hiroto A, Hartman RE, Obenaus A. Mild concussion, but not moderate traumatic brain injury, is associated with long-term depression-like phenotype in mice. PloS One. 2016;11:e0146886. doi: 10.1371/journal.pone.0146886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bye N, Carron S, Han X, Agyapomaa D, Ng SY, Yan E, Rosenfeld JV, Morganti-Kossmann MC. Neurogenesis and glial proliferation are stimulated following diffuse traumatic brain injury in adult rats. J Neurosci Res. 2011;89:986–1000. doi: 10.1002/jnr.22635. [DOI] [PubMed] [Google Scholar]
- Cantu RC. Second-impact syndrome. Clin Sports Med. 1998;17:37–44. doi: 10.1016/s0278-5919(05)70059-4. [DOI] [PubMed] [Google Scholar]
- Cassidy JD, Carroll LJ, Peloso PM, Borg J, von Holst H, Holm L, Kraus J, Coronado VG. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO collaborating centre task force on mild traumatic brain injury. J Rehabil Med. 2004:28–60. doi: 10.1080/16501960410023732. [DOI] [PubMed] [Google Scholar]
- Csuka E, Hans VH, Ammann E, Trentz O, Kossmann T, Morganti-Kossmann MC. Cell activation and inflammatory response following traumatic axonal injury in the rat. Neuroreport. 2000;11:2587–2590. doi: 10.1097/00001756-200008030-00047. [DOI] [PubMed] [Google Scholar]
- Dock H, Theodorsson A, Theodorsson E. DNA Methylation inhibitor zebularine confers stroke protection in ischemic rats. Transl Stroke Res. 2015;6:296–300. doi: 10.1007/s12975-015-0397-7. [DOI] [PubMed] [Google Scholar]
- Donovan V, Kim C, Anugerah AK, Coats JS, Oyoyo U, Pardo AC, Obenaus A. Repeated mild traumatic brain injury results in long-term white-matter disruption. J Cerebr Blood F Met. 2014;34:715–723. doi: 10.1038/jcbfm.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway NR, Tong KA, Ashwal S, Oyoyo U, Obenaus A. Diffusion-weighted imaging improves outcome prediction in pediatric traumatic brain injury. J Neurotrauma. 2008;25:1153–1162. doi: 10.1089/neu.2007.0494. [DOI] [PubMed] [Google Scholar]
- Gennarelli TA, Tipperman R, Maxwell WL, Graham DI, Adams JH, Irvine A. Traumatic damage to the nodal axolemma: an early, secondary injury. Acta Neurochir Suppl (Wien) 1993;57:49–52. doi: 10.1007/978-3-7091-9266-5_7. [DOI] [PubMed] [Google Scholar]
- Giza CC, Hovda DA. The Neurometabolic cascade of concussion. J Athl Train. 2001;36:228–235. [PMC free article] [PubMed] [Google Scholar]
- Golding EM, Steenberg ML, Contant CF, Jr, Krishnappa I, Robertson CS, Bryan RM., Jr Cerebrovascular reactivity to CO(2) and hypotension after mild cortical impact injury. Am J Physiol. 1999;277:H1457–1466. doi: 10.1152/ajpheart.1999.277.4.H1457. [DOI] [PubMed] [Google Scholar]
- Gordon WA, Brown M, Sliwinski M, Hibbard MR, Patti N, Weiss MJ, Kalinsky R, Sheerer M. The enigma of “hidden” traumatic brain injury. J Head Trauma Rehabil. 1998;13:39–56. doi: 10.1097/00001199-199812000-00006. [DOI] [PubMed] [Google Scholar]
- Harch PG. Hyperbaric oxygen in chronic traumatic brain injury: oxygen pressure and gene therapy. Med Gas Res. 2015;5:9. doi: 10.1186/s13618-015-0030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera BS, Coimbra LS, da Silva AR, Teixeira SA, Costa SK, Wallace JL, Spolidorio LC, Muscara MN. The H2S-releasing naproxen derivative ATB-346 inhibits alveolar bone loss and inflammation in rats with ligature-induced periodontitis. Med Gas Res. 2015;5:4. doi: 10.1186/s13618-015-0025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera JJ, Bockhorst K, Kondraganti S, Stertz L, Quevedo J, Narayana PA. Acute white matter tract damage after frontal mild traumatic brain injury. J Neurotrauma. 2016 doi: 10.1089/neu.2016.4407. doi:10.1089/neu.2016.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovda DA, Yoshino A, Kawamata T, Katayama Y, Becker DP. Diffuse prolonged depression of cerebral oxidative metabolism following concussive brain injury in the rat: a cytochrome oxidase histochemistry study. Brain Res. 1991;567:1–10. doi: 10.1016/0006-8993(91)91429-5. [DOI] [PubMed] [Google Scholar]
- Hu Q, Manaenko A, Guo Z, Huang L, Tang J, Zhang JH. Hyperbaric oxygen therapy for post concussion symptoms: issues may affect the results. Med Gas Res. 2015;5:10. doi: 10.1186/s13618-015-0033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Obenaus A. Hyperbaric oxygen therapy for traumatic brain injury. Med Gas Res. 2011;1:21. doi: 10.1186/2045-9912-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Coats JS, Mohd-Yusof A, Yin Y, Assaad S, Muellner MJ, Kamper JE, Hartman RE, Dulcich M, Donovan VM, Oyoyo U, Obenaus A. Tissue vulnerability is increased following repetitive mild traumatic brain injury in the rat. Brain Res. 2013;1499:109–120. doi: 10.1016/j.brainres.2012.12.038. [DOI] [PubMed] [Google Scholar]
- Huh JW, Widing AG, Raghupathi R. Basic science; repetitive mild non-contusive brain trauma in immature rats exacerbates traumatic axonal injury and axonal calpain activation: a preliminary report. J Neurotrauma. 2007;24:15–27. doi: 10.1089/neu.2006.0072. [DOI] [PubMed] [Google Scholar]
- Ichihara M, Sobue S, Ito M, Ito M, Hirayama M, Ohno K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen - comprehensive review of 321 original articles. Med Gas Res. 2015;5:12. doi: 10.1186/s13618-015-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins LW, Moszynski K, Lyeth BG, Lewelt W, DeWitt DS, Allen A, Dixon CE, Povlishock JT, Majewski TJ, Clifton GL, et al. Increased vulnerability of the mildly traumatized rat brain to cerebral ischemia: the use of controlled secondary ischemia as a research tool to identify common or different mechanisms contributing to mechanical and ischemic brain injury. Brain Res. 1989;4 doi: 10.1016/0006-8993(89)91409-1. [DOI] [PubMed] [Google Scholar]
- Kelly JP, Nichols JS, Filley CM, Lillehei KO, Rubinstein D, Kleinschmidt-DeMasters BK. Concussion in sports. Guidelines for the prevention of catastrophic outcome. Jama. 1991;266:2867–2869. doi: 10.1001/jama.266.20.2867. [DOI] [PubMed] [Google Scholar]
- Kim JW, Lee HD, Jang SH. Severe bilateral anterior cingulum injury in patients with mild traumatic brain injury. Neural Regen Res. 2015;10:1876–1878. doi: 10.4103/1673-5374.170321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston JW, Toombs CF. Defining the minimally effective dose and schedule for parenteral hydrogen sulfide: long-term benefits in a rat model of hindlimb ischemia. Med Gas Res. 2015;5:5. doi: 10.1186/s13618-015-0027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibson CL, Brown AW, Ransom JE, Diehl NN, Perkins PK, Mandrekar J, Malec JF. Incidence of traumatic brain injury across the full disease spectrum: a population-based medical record review study. Epidemiology. 2011;22:836–844. doi: 10.1097/EDE.0b013e318231d535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liu W, Ding S, Xu W, Guan Y, Zhang JH, Sun X. Hyperbaric oxygen preconditioning induces tolerance against brain ischemia-reperfusion injury by upregulation of antioxidant enzymes in rats. Brain Res. 2008;1210:223–229. doi: 10.1016/j.brainres.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Li L, Tao YH, Tang J, Chen QW, Yang Y, Feng Z, Chen YJ, Yang LM, Yang YF, Zhu G, Feng H, Chen Z. A cannabinoid receptor 2 agonist prevents thrombin-induced blood-brain barrier damage via the inhibition of microglial activation and matrix metalloproteinase expression in rats. Transl Stroke Res. 2015;6:467–477. doi: 10.1007/s12975-015-0425-7. [DOI] [PubMed] [Google Scholar]
- Lioutas VA, Alfaro-Martinez F, Bedoya F, Chung CC, Pimentel DA, Novak V. Intranasal insulin and insulin-like growth factor 1 as neuroprotectants in acute ischemic stroke. Transl Stroke Res. 2015;6:264–275. doi: 10.1007/s12975-015-0409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhi L, Saatman KE, Raghupathi R, Laurer HL, Lenzlinger PM, Riess P, Neugebauer E, Trojanowski JQ, Lee VM, Grady MS, Graham DI, McIntosh TK. A review and rationale for the use of genetically engineered animals in the study of traumatic brain injury. J Cereb Blood Flow Metab. 2001;21:1241–1258. doi: 10.1097/00004647-200111000-00001. [DOI] [PubMed] [Google Scholar]
- Masdeu JC, Van Heertum RL, Kleiman A, Anselmi G, Kissane K, Horng J, Yudd A, Luck D, Grundman M. Early singlephoton emission computed tomography in mild head trauma. A controlled study. J Neuroimaging. 1994;4:177–181. doi: 10.1111/jon199444177. [DOI] [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Leung J, Mikulis D, Silver F, Kassner A. Longitudinal assessment of imatinib's effect on the blood-brain barrier after ischemia/reperfusion injury with permeability MRI. Transl Stroke Res. 2015;6:39–49. doi: 10.1007/s12975-014-0358-6. [DOI] [PubMed] [Google Scholar]
- Miller RS, Weaver LK, Bahraini N, Churchill S, Price RC, Skiba V, Caviness J, Mooney S, Hetzell B, Liu J, Deru K, Ricciardi R, Fracisco S, Close NC, Surrett GW, Bartos C, Ryan M, Brenner LA, Team HT. Effects of hyperbaric oxygen on symptoms and quality of life among service members with persistent postconcussion symptoms: a randomized clinical trial. JAMA Intern Med. 2015;175:43–52. doi: 10.1001/jamainternmed.2014.5479. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Raison M, Sahni T, Arya A, Lambert J, Marois P, James PB, Parent A, Ballaz L. Intensive rehabilitation combined with HBO 2 therapy in children with cerebral palsy: a controlled longitudinal study. Undersea Hyperb Med. 2014;41:77–85. [PubMed] [Google Scholar]
- Mutch WA, Ellis MJ, Ryner LN, Ruth Graham M, Dufault B, Gregson B, Hall T, Bunge M, Essig M, Fisher JA, Duffin J, Mikulis DJ for The Canada North Concussion Network, and; for The University Health Network Cerebrovascular Reactivity Research Group. Brain magnetic resonance imaging CO2 stress testing in adolescent postconcussion syndrome. J Neurosurg. 125:648–660. doi: 10.3171/2015.6.JNS15972. [DOI] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P, Ghajar J, Johnson C, Kolster RA, Sarkar R, Lee H, Meeker M, Zimmerman RD, Manley GT, McCandliss BD. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol. 2008;29:967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra RS, Lopes AH, Carreira EU, Feitosa MR, Cunha FQ, Garcia SB, Cunha TM, da Rocha JJ, Feres O. Hyperbaric oxygen therapy ameliorates TNBS-induced acute distal colitis in rats. Med Gas Res. 2015;5:6. doi: 10.1186/s13618-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploughman M, Austin MW, Glynn L, Corbett D. The effects of poststroke aerobic exercise on neuroplasticity: a systematic review of animal and clinical studies. Transl Stroke Res. 2015;6:13–28. doi: 10.1007/s12975-014-0357-7. [DOI] [PubMed] [Google Scholar]
- Qi ZF, Dong W, Shi WJ, Wang RL, Zhang CC, Zhao YM, Ji XM, Liu KJ, Luo YM. Bcl-2 phosphorylation triggers autophagy switch and reduces mitochondrial damage in limb remote ischemic conditioned rats after ischemic stroke. Transl Stroke Res. 2015;6:198–206. doi: 10.1007/s12975-015-0393-y. [DOI] [PubMed] [Google Scholar]
- Reuter B, Rodemer C, Grudzenski S, Meairs S, Bugert P, Hennerici MG, Fatar M. Effect of simvastatin on MMPs and TIMPs in human brain endothelial cells and experimental stroke. Transl Stroke Res. 2015;6:156–159. doi: 10.1007/s12975-014-0381-7. [DOI] [PubMed] [Google Scholar]
- Rossignol DA, Rossignol LW, James SJ, Melnyk S, Mumper E. The effects of hyperbaric oxygen therapy on oxidative stress, inflammation, and symptoms in children with autism: an open-label pilot study. BMC Pediatr. 2007;7:36. doi: 10.1186/1471-2431-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlunk F, Schulz E, Lauer A, Yigitkanli K, Pfeilschifter W, Steinmetz H, Lo EH, Foerch C. Warfarin pretreatment reduces cell death and MMP-9 activity in experimental intracerebral hemorrhage. Transl Stroke Res. 2015;6:133–139. doi: 10.1007/s12975-014-0377-3. [DOI] [PubMed] [Google Scholar]
- Semple BD, Bye N, Rancan M, Ziebell JM, Morganti-Kossmann MC. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2-/- mice. J Cereb Blood Flow Metab. 2010;30:769–782. doi: 10.1038/jcbfm.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman S, Ishrat T, Fouda AY, Patel A, Pillai B, Fagan SC. Sequential therapy with minocycline and candesartan improves long-term recovery after experimental stroke. Transl Stroke Res. 2015;6:309–322. doi: 10.1007/s12975-015-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller KP. All the right moves: the need for the timely use of hyperbaric oxygen therapy for treating TBI/CTE/PTSD. Med Gas Res. 2015;5:7. doi: 10.1186/s13618-015-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate DF, Shenton ME, Bigler ED. Introduction to the brain imaging and behavior special issue on neuroimaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012;6:103–107. doi: 10.1007/s11682-012-9185-0. [DOI] [PubMed] [Google Scholar]
- Tokutomi T, Hirohata M, Miyagi T, Abe T, Shigemori M. Posttraumatic edema in the corpus callosum shown by MRI. Acta Neurochir Suppl. 1997;70:80–83. doi: 10.1007/978-3-7091-6837-0_25. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R, Signoretti S, Tavazzi B, Cimatti M, Amorini AM, Donzelli S, Delfini R, Lazzarino G. Hypothesis of the postconcussive vulnerable brain: experimental evidence of its metabolic occurrence. Neurosurgery. 2005;57:164–171. doi: 10.1227/01.neu.0000163413.90259.85. discussion 164-171. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R, Signoretti S, Cristofori L, Alessandrini F, Floris R, Isgro E, Ria A, Marziali S, Zoccatelli G, Tavazzi B, Del Bolgia F, Sorge R, Broglio SP, McIntosh TK, Lazzarino G. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133:3232–3242. doi: 10.1093/brain/awq200. [DOI] [PubMed] [Google Scholar]
- Weaver J, Liu KJ. Does normobaric hyperoxia increase oxidative stress in acute ischemic stroke. A critical review of the literature? Med Gas Res. 2015;5:11. doi: 10.1186/s13618-015-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Liang T, Cheng O. Hyperbaric oxygen therapy in China. Medical gas research. 2015;5:3. doi: 10.1186/s13618-015-0024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Wang P, Morgan D, Lin D, Pan J, Lin F, Strang KH, Selig TM, Perez PD, Febo M, Chang B, Rubenstein R, Wang KK. Temporal MRI characterization, neurobiochemical and neurobehavioral changes in a mouse repetitive concussive head injury model. Sci Rep. 2015;5:11178. doi: 10.1038/srep11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WB, Casper A, Libal NL, Murphy SJ, Bodhankar S, Offner H, Alkayed NJ. Preclinical evaluation of recombinant t cell receptor ligand rtl1000 as a therapeutic agent in ischemic stroke. Transl Stroke Res. 2015;6:60–68. doi: 10.1007/s12975-014-0373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]