Abstract
Recent case reports describe patients receiving piperacillin-tazobactam who were found to have circulating galactomannan detected by the double sandwich enzyme-linked immunosorbent assay (ELISA) system, leading to the false presumption of invasive aspergillosis. Since this property of piperacillin-tazobactam and galactomannan ELISA is not well understood, we investigated the in vitro, in vivo, and clinical properties of this interaction. Among the 12 reconstituted antibiotics representing four classes of antibacterial compounds that are commonly used in immunocompromised patients, piperacillin-tazobactam expressed a distinctively high level of galactomannan antigen in vitro (P = 0.001). After intravenous infusion of piperacillin-tazobactam into rabbits, the serum galactomannan index (GMI) in vivo changed significantly (P = 0.0007) from a preinfusion mean baseline value of 0.27 to a mean GMI of 0.83 by 30 min to slowly decline to a mean GMI of 0.44 24 h later. Repeated administration of piperacillin-tazobactam over 7 days resulted in accumulation of circulating galactomannan to a mean peak GMI of 1.31 and a nadir of 0.53. Further studies revealed that the antigen reached a steady state by the third day of administration of piperacillin-tazobactam. Twenty-six hospitalized patients with no evidence of invasive aspergillosis who were receiving antibiotics and ten healthy blood bank donors were studied for expression of circulating galactomannan. Patients (n = 13) receiving piperacillin-tazobactam had significantly greater mean serum GMI values (0.74 ± 0.14) compared to patients (n = 13) receiving other antibiotics (0.14 ± 0.08) and compared to healthy blood bank donors (0.14 ± 0.06) (P < 0.001). Five (38.5%) of thirteen patients receiving piperacillin-tazobactam had serum GMI values > 0.5 compared to none of thirteen subjects receiving other antibiotics (P = 0.039) and to none of ten healthy blood bank donors (P = 0.046). These data demonstrate that among antibiotics that are commonly used in immunocompromised patients, only piperacillin-tazobactam contains significant amounts of galactomannan antigen in vitro, that in animals receiving piperacillin-tazobactam circulating galactomannan antigen accumulates in vivo to significantly increased and sustained levels, and that some but not all patients receiving this antibiotic will demonstrate circulating galactomannan above the threshold considered positive for invasive aspergillosis by the recently licensed double sandwich ELISA.
The recent development and introduction of the double sandwich enzyme-linked immunosorbent assay (ELISA) for the detection of galactomannan antigenemia is an important advance in the nonculture diagnosis of invasive aspergillosis (3, 5-8, 14, 15). Depending on the patient population, several studies have demonstrated a sensitivity ranging from 50 to 95% and a specificity ranging from 87 to 99% for the diagnosis of invasive aspergillosis. However, recent clinical reports describing results of circulating galactomannan antigenemia in patients receiving piperacillin-tazobactam prompted our investigation of this observation (11, 13, 16). Since properties of this piperacillin-tazobactam-associated galactomannan antigenemia are not well understood, we investigated the in vitro, in vivo, and clinical properties of this interaction. We initially studied the cross-reactivity of piperacillin-tazobactam and other antibiotics that are likely to be used in seriously ill medical, surgical, and pharmacologically immunocompromised patients. We further evaluated the kinetics of expression of this antigen in an animal model receiving piperacillin-tazobactam intravenously. We then further prospectively evaluated hospitalized patients who had no risk for or evidence of invasive aspergillosis but who were receiving different types of antibiotics in order to further evaluate the expression of galactomannan antigenemia in a general medical and surgical patient population receiving piperacillin-tazobactam.
MATERIALS AND METHODS
Serum galactomannan assay.
Serum galactomannan concentrations were determined by the Platelia Aspergillus enzyme immunoassay one-stage immunoenzymatic sandwich microplate assay (Platelia Aspergillus ELISA; Bio-Rad, Marnes-la-Coquette, France). The assay uses the rat monoclonal antibody (MAb) EB-A2, which is directed against Aspergillus galactomannan (12). The MAb is used to sensitize the wells of the microplate and to bind the antigen. Peroxidase-linked rat MAb is used as the detector antibody.
A 300-μl sample of rabbit or human serum was mixed with 100 μl of 4% EDTA treatment solution and boiled for 3 min in order to dissociate immune complexes and to precipitate serum proteins. After centrifugation at 7,000 × g for 10 min, 50 μl of the supernatant was added to 50 μl of a reaction mixture containing peroxidase-conjugated anti-galactomannan MAb EB-A2. The 100-μl mixture was added to the wells of a microtitration plate coated with the same MAb EB-A2, followed by incubation for 90 min at 37°C. An MAb-galactomannan-MAb/peroxidase complex was formed in the presence of Aspergillus antigen. The microtiter plates were washed to remove any unbound material, and 200 μl of substrate solution, which reacted with the complexes bound to the well to form a blue reaction, was added to each well. Plates were incubated for another 30 min in darkness at room temperature. The enzymatic reaction was stopped by the addition of 100 μl of stopping solution (1.5 N sulfuric acid). The optical absorbance of specimens and controls was determined by using a Titertek Multiscan microplate spectrophotometer (MMC/340; Titertek, Huntsville, Ala.) equipped with 450- and 620-nm filters. Optical density data from the enzyme immunoassay were expressed as a galactomannan index (GMI). The GMI for each test sample is equal to the optical density (OD) of the clinical sample divided by the OD of a threshold serum provided in the test kit.
In vitro studies of antibiotics.
The level of galactomannan antigen was quantified for different antibiotics representative of different classes of antibiotics (beta-lactams, fluoroquinolones, aminoglycosides, and glycopeptides) used in hospitalized patients. Galactomannan antigen was studied in the following antibiotics reconstituted in the vial according to the manufacturer's instructions: beta-lactams (penicillin G and ampicillin [125 mg/ml], ampicillin-sulbactam [125 mg/ml], piperacillin-tazobactam [125 mg/ml], nafcillin [167 mg/ml], oxacillin [167 mg/ml], ceftazidime [200 mg/ml], fluoroquinolones (ciprofloxacin [10 mg/ml], and levofloxacin [25 mg/ml]), aminoglycoside (gentamicin [40 mg/ml]), and glycopeptide (vancomycin [50 mg/ml]). The high concentrations of antimicrobial agents were used as a sensitive screen for detection of galactomannan. An aliquot of 300 μl was processed by the same procedure as that applied to serum. Samples were run in triplicate, and the entire experiment was repeated.
In vivo studies. (i) Animals.
Healthy female New Zealand White rabbits (Hazleton, Research Products, Inc., Denver, Pa.) weighing 2.8 to 3.3 kg were used in an experiment (n = 6). Rabbits were selected in these experiments because of the similarity between their patterns of expression of circulating galactomannan in experimental pulmonary aspergillosis and those found in humans with invasive aspergillosis (4, 8, 10, 18). As in noninfected humans, rabbits have a normal mean GMI level well below the threshold of a positive value of 0.5. Rabbits infected with Aspergillus spp. demonstrate a predictable rise in circulating galactomannan in association with progressive infection. Circulating galactomannan levels in rabbits also decline in response to antifungal therapy in parallel with a reduction in residual fungal burden and an improvement in survival.
All rabbits were housed and monitored under conditions for humane care and use in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and according to National Institutes of Health guidelines for animal care and in fulfillment of the guidelines of the National Research Council (9). Vascular access was established in each rabbit by the surgical placement of a silastic tunneled central venous catheter (17). The silastic catheter permitted nontraumatic venous access for intravenous (i.v.) administration of piperacillin-tazobactam and for repeated blood sampling for the study of serum galactomannan antigen levels.
(ii) Treatment with piperacillin-tazobactam.
Rabbits received i.v. piperacillin-tazobactam prepared according to the manufacturer's instructions at 150 mg/kg given twice daily i.v. via a 10-min steady infusion for each of 7 days. All i.v. infusions of piperacillin-tazobactam were well tolerated.
(iii) Serum sampling for galactomannan levels.
In order to examine the kinetics of circulating galactomannan antigen associated with piperacillin-tazobactam, serial blood samples from rabbits receiving the antibiotic were collected on day 1 and on day 7 at baseline (preinfusion); at the end of infusion (Cmax) (0.17 h); and at 0.5, 1, 2, 4, and 8 h postinfusion. Peak and trough samples also were collected on days 2 through 6.
Clinical studies.
Serum samples were measured in 26 hospitalized patients receiving antibiotics at the Washington Hospital Center in Washington, D.C. None of these patients had a history of invasive aspergillosis or had evidence for invasive aspergillosis at the time of serum collection. Serum samples were drawn from patients who were receiving piperacillin-tazobactam (n = 13) or control antibiotics (n = 13), consisting of imipenem, ampicillin, nafcillin, ciprofloxacin, or ceftriaxone. Patient age, gender, and underlying disease were similar between both treatment and control groups. As additional internal controls, sera from 10 healthy blood bank donors were also analyzed for circulating galactomannan antigen.
Statistical analysis.
GMI values are expressed as means ± the standard errors of the mean (SEM). Differences between the means of galactomannan levels from antibiotics studied in vitro were evaluated by one-way analysis of variance (ANOVA). Serial galactomannan levels measured in rabbit sera were analyzed by using one-way ANOVA or Student t test, as appropriate. Differences in proportions of patients with or without elevated galactomannan levels were analyzed by Fisher exact test. Differences in mean GMI values among patients receiving antibiotics were analyzed by Kruskal-Wallis test (nonparametric ANOVA). A two-tailed P value of <0.05 was considered to be statistically significant.
RESULTS
In vitro studies.
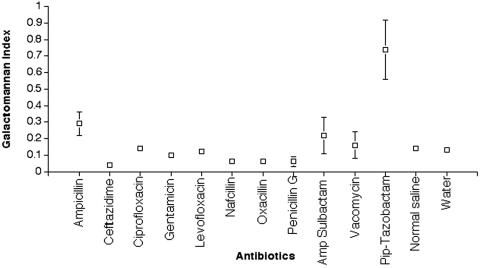
The comparative galactomannan levels in the wells containing each of the reconstituted antibiotics are presented in Fig. 1. Among the 12 reconstituted antibiotics representing four classes of antibacterial compounds commonly used in immunocompromised patients, piperacillin-tazobactam expressed a distinctively high level of galactomannan antigen in vitro (P = 0.001). The levels for the other antibiotics for the detection of galactomannan ranged from 0.04 (for ceftazidime) to 0.29 (for ampicillin).
FIG. 1.
In vitro comparative galactomannan levels in the wells of reconstituted antibiotics that are frequently used in hospitalized patients. Piperacillin-tazobactam expressed a significantly elevated level of galactomannan antigen compared to other antibiotics (P = 0.001). Values are presented as means ± the SEM.
In vivo studies.
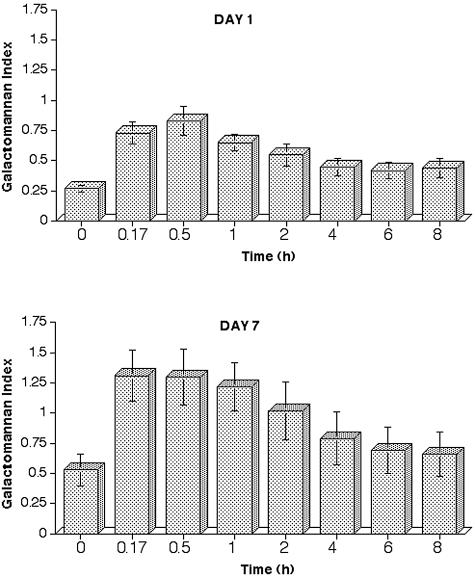
The in vivo expression of galactomannan antigen after infusion of piperacillin-tazobactam are presented in Fig. 2, which depicts the kinetics on day 1 (upper panel) and on day 7 (lower panel) after 7 days of piperacillin-tazobactam therapy. After i.v. infusion of piperacillin-tazobactam on day 1, the serum GMI underwent significant changes over time (P = 0.0007 [one-way ANOVA]). The serum GMI value increased from a preinfusion mean baseline value of 0.27 to more than double the original value, and by 30 min it attained a mean of 0.83. The galactomannan antigen persisted in serum after infusion for the entire dosing interval, and the GMI declined from a peak of 0.83 to a trough of 0.44.
FIG. 2.
Expression of galactomannan antigen after a 10-min steady infusion of piperacillin-tazobactam in normal rabbits on day 1 (upper panel; P = 0.0007 [ANOVA]) and on day 7 (lower panel; P = 0.045 [ANOVA]). Values are presented as means ± the SEM.
Repeated administration of piperacillin-tazobactam over 7 days resulted in an accumulation of circulating GM to a mean peak GMI of 1.31 and a mean nadir of 0.53. The mean preinfusion GMI value on day 7 of 0.53 had virtually doubled in comparison to that of day 1 (0.27). All other values have also had increased in comparison to those of day 1, again indicating an accumulation of galactomannan antigen. The GMI value now exceeded on day 7 a value of 1 for more than an hour, and the value exceeded 0.5 throughout the dosing interval (P = 0.045).
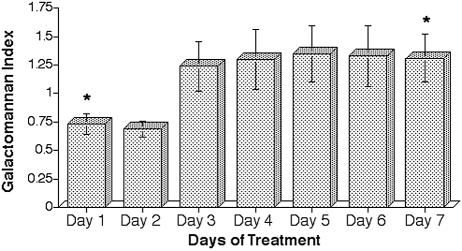
Figure 3 reveals that there was an accumulation of galactomannan antigen after infusion of piperacillin-tazobactam that appeared to reach a steady state after day 3. The peak GMI after administration of piperacillin-tazobactam was significantly higher on day 7 than on day 1 (P = 0.035).
FIG. 3.
Expression of serial peak GMI values each day over 7 days of treatment with piperacillin-tazobactam in normal rabbits (✽, P = 0.035). Values are presented as means ± the SEM.
Clinical studies.
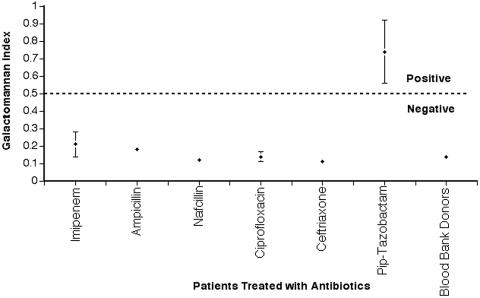
The mean GMI for each patient group receiving a different antibiotic is presented in Fig. 4. The cohort of patients receiving piperacillin-tazobactam had significantly greater mean serum GMI values (0.74 ± 0.14) compared to patients receiving other antibiotics (0.14 ± 0.08) and healthy blood bank donors (0.14 ± 0.06) (P < 0.001). There was no difference between the mean serum GMI values of patients receiving other antibiotics and those of healthy blood bank donors (P > 0.05). Five of thirteen patients receiving piperacillin-tazobactam had serum GMI values of >0.5 compared to none of thirteen receiving other antibiotics (P = 0.039) and to none of ten healthy blood bank donors (P = 0.046) (Table 1). Of the 26 patients receiving different antibiotics, only those receiving piperacillin-tazobactam demonstrated a significantly increased mean GMI compared to other values and to those of healthy blood bank donors.
FIG. 4.
Mean GMI expression in patients receiving different antibiotics. The last column demonstrates expression of galactomannan in healthy blood bank donors. The cohort of patients receiving piperacillin-tazobactam had mean serum GMI values significantly greater than those of patients receiving other antibiotics and those of blood bank donors (P < 0.001).
TABLE 1.
Expression of circulating galactomannan antigen in patients receiving piperacillin-tazobactam and in control populationsa
| Study cohort | n | Mean GMI ± SEM | 95% confidence interval (range) | No. of cases (%) with a GMI of >0.5 |
|---|---|---|---|---|
| Patients receiving piperacillin-tazobactam | 13 | 0.74 ± 0.14A | 0.35-1.13 | 5 (38.4)D |
| Patients receiving other antibioticsb | 13 | 0.14 ± 0.06B | 0.10-0.18 | 0 (0)E |
| Blood bank donors | 10 | 0.14 ± 0.08C | 0.10-0.18 | 0 (0)F |
Superscripts: A, P < 0.001 (Kruskal-Wallis test for comparison of A and B values and of A and C values); E, P = 0.039 (Fisher exact test for comparison of D and E values); F, P = 0.046 (Fisher exact test for comparison of D and F values).
That is, patients receiving imipenem, ampicillin, nafcillin, ciprofloxacin, or ceftriaxone.
DISCUSSION
We found that among antibiotics representing four classes of antibacterial compounds that are commonly used in immunocompromised patients, piperacillin-tazobactam expressed a distinctively high level of galactomannan antigen in vitro. When piperacillin-tazobactam was administered to rabbits, the mean serum GMI rose and fell significantly on day 1. After repeated administrations of piperacillin-tazobactam over 7 days circulating galactomannan further accumulated, as measured by both peak and nadir GMI levels, reaching a steady state by the third day of administration of piperacillin-tazobactam. Among hospitalized patients with no evidence of invasive aspergillosis who were receiving antibiotics and healthy blood bank donors, patients receiving piperacillin-tazobactam had significantly greater mean serum GMI values compared to those receiving other antibiotics and compared to healthy blood bank donors. Five (38.5%) of the thirteen patients receiving piperacillin-tazobactam had sufficiently elevated serum GMI values to exceed the threshold of >0.5 for a presumptive diagnosis of invasive aspergillosis. Recent case reports describe patients receiving piperacillin-tazobactam who were found to have circulating galactomannan detected by the double sandwich ELISA system, leading to the false presumption of invasive aspergillosis.
The present study demonstrates that piperacillin-tazobactam contains galactomannan antigen in significantly higher levels than other antibiotics that are widely used in hospitalized patients. Although galactomannan antigen is expressed by Penicillium spp. and some Penicillium spp. may also yield penicillin, we have no evidence of similar levels of galactomannan antigen in penicillin G or semisynthetic penicillins (ampicillin, ampicillin-sulbactam, nafcillin, and oxacillin). Nor was there any evidence of excess galactomannan antigen in the semisynthetic cephalosporin ceftazidime. Since galactomannan antigen may be introduced in the manufacturing process, we also examined other synthetic and semisynthetic classes of antibiotics represented by ciprofloxacin, vancomycin, and gentamicin, where again the presence of galactomannan antigen was comparatively minimal. These findings are compatible with an earlier study of Ansorg et al., who reported in 1997 a series of compounds that may contain galactomannan antigen and yield a positive reaction for the galactomannan immunodiagnostic assays (2). At that time, piperacillin-tazobactam was noted to be positive for galactomannan antigen. However, no further studies were conducted to explore the significance of this observation.
This cross-reactivity has not surfaced as an issue for clinical microbiology laboratories until the recent report from Western Europe (13). Two more recent reports from Western Europe underscore the increasing recognition of this observation. Little is known, however, about the expression and kinetics of galactomannan antigen associated with administration of piperacillin-tazobactam.
In order to better understand the expression and kinetics of circulating galactomannan antigen associated with the administration of piperacillin-tazobactam, we investigated serial galactomannan antigen levels in serum when was the drug was administered i.v. to rabbits. Previous studies of experimental invasive pulmonary aspergillosis with rabbit models have demonstrated patterns of circulating galactomannan that are similar to those of humans for both detection and correlation with therapeutic response (1, 4, 8, 10, 16).
The galactomannan antigen reactivity observed in vitro with piperacillin-tazobactam correlated with the in vivo expression of galactomannan antigenemia after the infusion of piperacillin-tazobactam.
These studies found that circulating galactomannan antigen persists well beyond the known levels of piperacillin-tazobactam. The plasma half-life of piperacillin-tazobactam is <1 h, indicating that virtually all of the drug will be eliminated from the circulation within <5 h. By comparison, the galactomannan antigen accumulates over time, and the level rises and falls as a function of time after infusion. A steady state between piperacillin-tazobactam-associated galactomannan accumulation and galactomannan clearance appears to be attained by day 3 of infusion. These in vivo studies indicate that a clinically significant level of galactomannan antigen (GMI > 0.5) depends upon when the specimen was drawn in relation to the infusion of piperacillin-tazobactam, as well as the length of time over which the patient had been receiving piperacillin-tazobactam.
Consistent with the in vitro observations, of the patients who received different antibiotics, only patients receiving piperacillin-tazobactam had a significantly elevated level of galactomannan. Consistent with the in vivo observations, this level of circulating galactomannan in some patients significantly exceeded the threshold of detection for positive galactomannan antigen considered to indicate invasive aspergillosis. To our knowledge, the present study is the first to demonstrate an in vitro, in vivo, and clinical correlation of cross reactivity between galactomannan antigen and piperacillin-tazobactam.
For clinical microbiology laboratories performing the galactomannan double sandwich ELISA, an awareness of antibiotics that the patient may be receiving is important in interpretation of the results of a positive result. For patients receiving piperacillin-tazobactam who have a GMI of >0.5, further confirmation of the presence of invasive aspergillosis through direct examination and/or culture of clinical specimens of bronchoalveolar lavage, fine needle aspiration, or biopsy may be necessary. The in vivo results of our studies suggest that drawing a serum sample prior to the next dose of piperacillin-tazobactam may minimize but not eliminate the presence of piperacillin-tazobactam-associated circulating galactomannan. Our clinical findings reveal that while patients receiving piperacillin-tazobactam tend to have higher levels of circulating galactomannan, only a minority of patients will demonstrate a GMI of >0.5.
Thus, among antibiotics that are commonly used in immunocompromised patients, we found that only piperacillin-tazobactam contained significant amounts of galactomannan antigen in vitro, that circulating galactomannan antigen accumulates in animals receiving piperacillin-tazobactam in vivo to significantly increased and sustained levels, and that some but not all patients receiving this antibiotic will demonstrate circulating galactomannan above the threshold considered positive for invasive aspergillosis by the recently licensed double sandwich ELISA. An increased understanding of piperacillin-tazobactam-associated galactomannan antigenemia will enhance the clinical microbiology laboratory's capacity to interpret the results of this assay.
REFERENCES
- 1.Adam, O., A. Auperin, F. Wilquin, J. H. Bourhis, B. Gachot, and E. Chachaty. 2004. Treatment with piperacillin-tazobactam and false-positive Aspergillus galactomannan antigen test results for patients with hematological malignancies. Clin. Infect. Dis. 38:917-920. [DOI] [PubMed] [Google Scholar]
- 2.Ansorg, R., R. van den Boom, and P. M. Rath. 1997. Detection of Aspergillus galactomannan antigen in foods and antibiotics. Mycoses 40:353-357. [DOI] [PubMed] [Google Scholar]
- 3.Bretagne, S., A. Marmorat-Khuong, M. Kuentz, J. P. Latge, E. Bart-Delabesse, and C. Cordonnier. 1997. Serum Aspergillus galactomannan antigen testing by sandwich ELISA: practical use in neutropenic patients. J. Infect. 35:7-15. [DOI] [PubMed] [Google Scholar]
- 4.Francis, P., J. W. Lee, A. Hoffman, J. Peter, A. Francesconi, J. Bacher, J. Shelhamer, P. A. Pizzo, and T. J. Walsh. 1994. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar d-mannitol and serum galactomannan as markers of infection. J. Infect. Dis. 169:356-368. [DOI] [PubMed] [Google Scholar]
- 5.Herbrecht, R., V. Letscher-Bru, C. Oprea, B. Lioure, J. Waller, F. Campos, O. Villard, K. L. Liu, S. Natarajan-Ame, P. Lutz, P. Dufour, J. P. Bergerat, and E. Candolfi. 2002. Aspergillus galactomannan detection in the diagnosis of invasive aspergillosis in cancer patients. J. Clin. Oncol. 20:1898-1906. [DOI] [PubMed] [Google Scholar]
- 6.Kwak, E. J., S. Husain, A. Obman, L. Meinke, J. Stout, S. Kusne, M. M. Wagener, and N. Singh. 2004. Efficacy of galactomannan antigen in the Platelia Aspergillus enzyme immunoassay for diagnosis of invasive aspergillosis in liver transplant recipients. J. Clin. Microbiol. 42:435-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maertens, J., J. Verhaegen, H. Demuynck, P. Brock, G. Verhoef, P. Vandenberghe, J. Van Eldere, L. Verbist, and M. Boogaerts. 1999. Autopsy-controlled prospective evaluation of serial screening for circulating galactomannan by a sandwich enzyme-linked immunosorbent assay for hematological patients at risk for invasive aspergillosis. J. Clin. Microbiol. 37:3223-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marr, K. A., S. A. Balajee, L. McLaughlin, M. Tabouret, C. Bentsen, T. Sein, and T. J. Walsh. 2004. Detection of galactomannan antigenemia by enzyme immunoassay for the diagnosis of invasive aspergillosis: variables that affect performance. J. Infect. Dis. 190:641-649. [DOI] [PubMed]
- 9.National Research Council Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 10.Petraitiene, R., V. Petraitis, A. H. Groll, T. Sein, S. Piscitelli, M. Candelario, A. Field-Ridley, N. Avila, J. Bacher, and T. J. Walsh. 2001. Antifungal activity and pharmacokinetics of posaconazole (SCH 56592) in treatment and prevention of experimental invasive pulmonary aspergillosis: correlation with galactomannan antigenemia. Antimicrob. Agents Chemother. 45:857-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinel, C., H. Fricker-Hidalgo, B. Lebeau, F. Garban, R. Hamidfar, P. Ambroise-Thomas, and R. Grillot. 2003. Detection of circulating Aspergillus fumigatus galactomannan: value and limits of the Platelia test for diagnosing invasive aspergillosis. J. Clin. Microbiol. 41:2184-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stynen, D., J. Sarfati, A. Goris, M. C. Prevost, M. Lesourd, H. Kamphuis, V. Darras, and J. P. Latge. 1992. Rat monoclonal antibodies against Aspergillus galactomannan. Infect. Immun. 60:2237-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sulahian, A., S. Touratier, and P. Ribaud. 2003. False positive test for Aspergillus antigenemia related to concomitant administration of piperacillin and tazobactam. N. Engl. J. Med. 349:2366-2367. [DOI] [PubMed] [Google Scholar]
- 14.Verweij, P. E., E. C. Dompeling, J. P. Donnelly, A. V. Schattenberg, and J. F. Meis. 1997. Serial monitoring of Aspergillus antigen in the early diagnosis of invasive aspergillosis: preliminary investigations with two examples. Infection 25:86-89. [DOI] [PubMed] [Google Scholar]
- 15.Verweij, P. E., Z. Erjavec, W. Sluiters, W. Goessens, M. Rozenberg-Arska, Y. J. Debets-Ossenkopp, H. F. Guiot, J. F. Meis, et al. 1998. Detection of antigen in sera of patients with invasive aspergillosis: intra- and interlaboratory reproducibility. J. Clin. Microbiol. 36:1612-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viscoli, C., M. Machetti, P. Cappellano, B. Bucci, P. Bruzzi, M. T. Van Lint, and A. Bacigalupo. 2004. False-positive galactomannan Platelia Aspergillus test results for patients receiving piperacillin-tazobactam. Clin. Infect. Dis. 38:913-916. [DOI] [PubMed] [Google Scholar]
- 17.Walsh, T. J., J. Bacher, and P. A. Pizzo. 1988. Chronic silastic central venous catheterization for induction, maintenance and support of persistent granulocytopenia in rabbits. Lab. Anim. Sci. 38:467-471. [PubMed] [Google Scholar]
- 18.Walsh, T. J., V. Petraitis, R. Petraitiene, A. Field-Ridley, D. Sutton, M. Ghannoum, T. Sein, R. Schaufele, J. Peter, J. Bacher, H. Casler, D. Armstrong, A. Espinel-Ingroff, M. G. Rinaldi, and C. A. Lyman. 2003. Experimental pulmonary aspergillosis due to Aspergillus terreus: pathogenesis and treatment of an emerging fungal pathogen resistant to amphotericin B. J. Infect. Dis. 188:305-319. [DOI] [PubMed] [Google Scholar]