Abstract
Background
Non-starch polysaccharide enzymes (NSPEs) have long been used in monogastric animal feed production to degrade non-starch polysaccharides (NSPs) to oligosaccharides in order to promote growth performance and gastrointestinal (GI) tract health. However, the precise molecular mechanism of NSPEs in the improvement of the mammalian small intestine remains unknown.
Methods
In this study, isobaric tags were applied to investigate alterations of the small intestinal mucosa proteome of growing pigs after 50 days of supplementation with 0.6% NSPEs (mixture of xylanase, β-glucanase and cellulose) in the diet. Bioinformatics analysis including gene ontology annotation was performed to determine the differentially expressed proteins. A protein fold-change of ≥ 1.2 and a P-value of < 0.05 were selected as thresholds.
Results
Dietary supplementation of NSPEs improved the growth performance of growing pigs. Most importantly, a total of 90 proteins were found to be differentially abundant in the small intestinal mucosa between a control group and the NSPE group. Up-regulated proteins were related to nutrient metabolism (energy, lipids, protein and mineral), immunity, redox homeostasis, detoxification and the cell cytoskeleton. Down-regulated proteins were primarily related to transcriptional and translational regulation. Our results indicate that the effect of NSPEs on the increase of nutrient availability in the intestinal lumen facilitates the efficiency of nutrient absorption and utilization, and the supplementation of NSPEs in growing pigs also modulates redox homeostasis and enhances immune response during simulating energy metabolism due to a higher uptake of nutrients in the small intestine.
Conclusions
These findings have important implications for understanding the mechanisms of NSPEs on the small intestine of pigs, which provides new information for the better utilization of this feed additive in the future.
Electronic supplementary material
The online version of this article (doi:10.1186/s12953-016-0109-6) contains supplementary material, which is available to authorized users.
Keywords: Non-starch polysaccharide enzymes, Small intestinal mucosa, Proteomics, Growing pigs
Background
Many cereals such as soybean and wheat contain up to 15% non-starch polysaccharides (NSPs) in their outer or inner cell walls [1]. Monogastric animals lack enzymes to degrade the cell wall and NSP in these feeds. Thus, these anti-nutritive factors may interfere with digestion, nutrient absorption, and intestinal tract health by encapsuling starch and protein, as well as increase the viscosity of the chymus, which may elevate the proliferation of pathological bacteria in the small intestine and reduce the feed conversion ratio of monogastric livestock species [2–4].
The supplementation of exogenous enzymes such as xylanases and β-glucanases in pig diets may facilitate the hydrolysis of the main NSPs and increase the utilization of available raw materials [5, 6]. Adding exogenous enzymes to cereal diets improves both nutrient digestibility and growth performance in pigs [7, 8]. However, the exact molecular mechanisms of NSPEs, particularly in the gastrointestinal (GI) tract, are unknown [9]. There are several indications that exogenous enzymes may function in the GI tract of animals to aid digestion. The supplementation of NSPEs in the diets could increase the activities of certain types of digestive enzymes in vivo including protease, trypsin, and α-amylase [2, 4, 10]. These enzymes reduce the degradation of NSPs within the small intestine, thereby decreasing the viscosity of the digesta, which leads to a reduced bacterial load in the gut, especially potential pathogens [11]. Furthermore, the degradation of NSPs due to the supplementation of NSPEs promotes the higher availability of digestible nutrients such as energy substrates [12]. Additionally, the intestinal morphological structure and some physiological functions in animals benefit from the improvement of the changing intestinal environment due to the supplementation of NSPEs. Some research demonstrated that intestinal morphologies, including the villus height, the ratio of villus height to crypt depth, and the number of crypts and goblet cells, were changed due to the addition of xylanases alone or multiple enzymes [13, 14]. In addition to the effects of NSPEs observed on the GI tract, alterations of blood parameters related to the nutrient metabolism were also noted [15].
Previous studies reported that diet composition affected gene expression in animals [9, 16]. It is assumed that the improvement of the intestinal environment due to the supplementation of NSPEs in the diet may influence the gene expression and subsequent protein expression of epithelial-cell nutrient transporters in the GI tract mucosa, which has not been studied before. However, RNA editing and numerous options for posttranslational modifications should be taken into account [17, 18]. Hence, elucidation protein expression is important [19].
It is impractical to simultaneously measure all protein expression in the GI mucosa by classical method, such as western blotting. More research has yielded high throughput mass spectrometric proteomic technologies that can simultaneously detect hundreds of proteins [20, 21]. A proteomic analysis of the rat small intestinal proteome showed the presence of previously unrecognized proteins involved in various functions including the absorption and transport of nutrients and the maintenance of cell structure, as well as intestinal molecular chaperones [22]. There remains a great need to pursue proteomic technology to elucidate the beneficial effects of NSPEs in the GI tract mucosa. Therefore, we utilized a label-based iTRAQ (isobaric tags for relative and absolute quantitation) method, followed by LC-MS/MS, to quantitate proteins that are differentially induced in the small intestinal mucosa of growing pigs supplemented with NSPEs in the diet.
Methods
Enzyme preparation
The NSP enzyme mixture preparation supplemented in the diet was provided by the State Key Laboratory of Animal Nutrition, Institute of Animal Sciences, Chinese Academy of Agricultural Sciences (Beijing, China); the mixture contained 7 × 105 U/g xylanase activity (EC 3.2.1.8), 1 × 105 U/g β-glucanase activity (EC 3.2.1.6), and 9000 U/g cellulase activity (EC 3.2.1.4). The activities of the enzymes used in the present study was measured according the methods mentioned in previous research [23].
Animals and treatments
Forty-eight crossbred (Duroc × Landrace × Large White) growing pigs had similar initial body weights (39.18 ± 0.98 kg); the pigs were obtained from a commercial farm in Beijing (Shunliang pig farm, Beijing). The pigs were randomly divided into two groups according to their littermates, sex and mean initial body weights with four replicates in each group and six pigs in each replicate (half females and half males). The following two groups were a control group (CTRL, basal diet) and a treatment group (NSPE, basal diet + 0.6% NSP enzymes). The amount of NSPEs supplementation in the present study was based on the previous results from our group [24]. Both diets were formulated to meet NRC (2012) recommendations (Table 1). All pigs were kept in eight adjacent pens covered in a fermentation bed facility. Feed and water were provided ad libitum during the 50 day experimental period. The individual pig weight and feed intake were recorded at the initiation and the termination of the experiment for the measurement of the average daily gain (ADG), average daily feed intake (ADFI) and feed conversion ratio (FCR). All procedures involving animals were evaluated and approved by the Animal Ethics Committee of the Institute of Animal Sciences, Chinese Academy of Agricultural Sciences.
Table 1.
Composition of the basal diet and calculated proximate composition of the diet
| Ingredients | Proportion (%)a |
|---|---|
| Corn | 70.70 |
| Soybean meal | 19.82 |
| Soybean oil | 2.10 |
| Wheat bran | 5.00 |
| Limestone | 0.51 |
| Calcium hydrophosphate | 0.56 |
| L-Lysine | 0.01 |
| Sodium chloride | 0.30 |
| Premixb | 1.00 |
| Total | 100 |
| Nutrient | |
| ME | 13.65 (MJ/kg) |
| Ether extract (EE) | 4.82 |
| Crude protein (CP) | 15.50 |
| Calcium | 0.50 |
| Total phosphorus | 0.45 |
| Available phosphorus | 0.24 |
| Total lysine | 0.75 |
| Total methionine | 0.25 |
aAll data is expressed in g/kg dry weight except for metabolizable energy (ME) in MJ/kg. The amounts of nutrient were estimated based on the NRC 11th ed. swine feedstuff composition table
bProviding the following (g/kg fresh weight), Vitamin A, 8250 IU; Vitamin D3: 825 IU; Vitamin E: 40 IU; Vitamin K3, 4.0 mg; Vitamin B1, 1.0 mg; Vitamin B2, 5.0 mg; Vitamin B6, 2.0 mg; Vitamin B12, 25 μg; choline chloride, 600 mg; nicotinic acid, 35 mg; folic acid, 2.0 mg; biotin, 4.0 mg; Cu, 50.0 mg; Fe, 80.0 mg; Zn, 100.0 mg; Mn, 25.0 mg; Se, 0.15 mg; I, 0.5 mg
Sample collection
At the end of the experiment (Day 50), all pigs were weighted after 12 h of fasting. One pig per replicate, a total of eight pigs (n = 8), were sacrificed by CO2 asphyxiation and then exsanguinated. Blood samples were obtained from the cervical vein by syringe before sacrifice. The whole blood was centrifuged at 2000 g for 30 min at 4 °C, followed by centrifugation at 400 g for 10 min at 4 °C. Then, the resulting supernatant was collected as sera samples, which were stored at −20 °C for further analysis. A 20-cm tissue section was rapidly excised at 50% of the length of the small intestine, rinsed with cold phosphate buffer saline, and blotted dry on paper. Mucosa from this small intestine section was sequentially obtained by careful scraping of the mucosal layer using a glass microscope slide as previously described [25]. Then, the collected mucosal samples were snap-frozen in liquid nitrogen and stored at −80 °C for proteomic analysis.
Serum biochemical analyses
Important serum biochemical parameters, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein (TP), alkaline phosphatase (ALP), glucose (GLU), and creatine kinase (CK), were analyzed using an automatic biochemical analyzer (Hitachi 7020, Tokyo, Japan). Serum levels of total superoxide dismutase (T-SOD) and immunoglobulin G (IgG) were measured using a corresponding kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
Protein extraction and sample preparation
Small intestinal mucosa samples (500 μg) were ground in liquid nitrogen using a Dounce glass grinder. Grinded powder was precipitated with 10% trichloroacetic acid (TCA) (w/v) and 90% ice-cold acetone at −20 °C for 2 h. The precipitate was obtained by centrifugation at 20,000 g for 30 min at 4 °C and subsequently washed with ice-cold acetone. Then, the precipitate was lysed in lysis buffer [8 M urea, 30 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 1 mM phenylmethanesulfonyl fluoride (PMSF), 2 mM ethylene diamine tetraacetic acid (EDTA), and 10 mM dithiothreitol (DTT)]. The crude tissue extracts were centrifuged to remove the remaining debris. The tissue lysates were reduced for 1 h at 56 °C in a water bath using 10 mM DTT and then alkylated with 55 mM iodoacetamide for 1 h in the dark. Afterwards, the lysates were precipitated by adding four volumes of pre-chilled acetone. The pellets were then washed three times with pre-chilled pure acetone and resuspended in the buffer (50% TEAB and 0.1% SDS). The centrifugation was repeated to remove the undissolved pellets. Subsequently, protein quantitation was determined using a Bio-Rad Bradford Protein Assay Kit (Hercules, CA, USA). Each sample was digested with modified sequence grade trypsin (Promega Corporation, Madison, WI) at a 1: 30 ratio (3.3 μg trypsin : 100 μg target) overnight at 37 °C. Each isobaric tag (113, 114, 115, 116, 117, 118, 119, and 121) was solubilized in 70 μL isopropanol and then added to each respective sample (4 samples per group). Incubation continued for 2 h at room temperature.
Strong cation exchange chromatography
The strong cation exchange fractionation was performed according to a previous report [26] with slight modification. Briefly, 800 μg of labeled sample was loaded onto a strong cation exchange column (Phenomenex Luna SCX 100A) installed in an Agilent 1100 (Santa Clara, CA) system and equilibrated with buffer A (25% acetonitrile and 10 mM KH2PO4, pH 3.0). The peptides were separated by a linear gradient of buffer B (25% acetonitrile, 2 M KCl and 10 mM KH2PO4, pH 3.0) according to this procedure (increasing to 5% after 41 min, 50% after 66 min and 100% after 71 min with a flow rate of 1 ml/min). Elution was monitored by setting the absorbance at 214 nm. A total of 10 fractions were obtained, then desalted with a Strata X C18 column (Phenomenex) and dried under a vacuum. The pellets were resuspended by adding 0.1% formic acid before the LC-MS/MS run.
Mass spectrometry
LC-MS/MS was conducted according to a previous report [27], and the detailed process and parameters are shown in Additional file 1.
Data processing and protein quantification
All the detailed parameters are shown in the Supporting Information (Additional file 1). MS/MS data for iTRAQ protein identification and quantitation were analyzed using Proteome Discover 1.3 (Thermo Fisher Scientific, Bremen, Germany) and in-house MASCOT software (Matrix Science, London, UK; Version 2.3.0) against the database Uniprot_pig (Apr. 11th, 2014). Median ratio normalization was performed in intra-sample channels to normalize each channel across all proteins. Protein quantitative ratios for each iTRAQ labeled sample were obtained, using a sample in the control group (sample tagged with 113) as the denominator. Quantitative ratios were then log transformed to base two and presented as the fold change relative to the denominator in the control group for final quantitative testing. Differentially expressed proteins were identified using Student’s t-test corrected for multiple testing using the Benjamini and Hochberg correction [21, 25, 28, 29]. Based on above the relative quantification, statistical analysis, and a number of previous reports regarding to iTRAQ experiments [29–31], we set a 1.2-fold change or greater as the threshold for differentially expressed proteins.
Bioinformatics analysis and validation of protein expression
The databases and software for bioinformatics analysis are shown in Additional file 1. Real-time qPCR was used to verify six small intestinal mucosal proteins of differential abundance at the mRNA level. All detailed procedures are described in the Supporting Information (Additional file 1). The primer sequences used in this study are shown in Additional file 2: Table S1.
Statistical analysis
The data for growth parameters, serum parameters, and gene expression were analyzed by one-way ANOVA using block as a covariate (SAS Version 9.2, SAS institute Inc., Cary, NC) according the previous studies [21, 31], and a t-test was used for independent samples in MS data analysis. A group difference was assumed statistically significant when P < 0.05.
Results
Growth performance of growing pigs
During the entire experimental period (50 days), NSPE pigs had 15.5% greater ADG (P < 0.05) compared with the control group; however, the ADFI between the two groups was not significantly different (P > 0.05). It is notable that pig fed NSPEs had an 8.7% greater FCR compared with the control group (P < 0.05; Table 2).
Table 2.
Effects of NSP enzymes on growth performance of growing pigs
| Groups | |||
|---|---|---|---|
| Control | Treatment | P value | |
| Initial weight (kg) | 38.80 ± 0.99 | 39.55 ± 0.63 | 0.1245 |
| Final weight (kg) | 74.04 ± 1.77b | 78.42 ± 1.06a | 0.0318 |
| ADG (kg/d)c | 0.71 ± 0.05b | 0.82 ± 0.05a | 0.0437 |
| ADFI (kg/d)d | 1.97 ± 0.09 | 2.07 ± 0.06 | 0.0423 |
| FCR (kg feed/kg weight gain)e | 2.77 ± 0.02a | 2.53 ± 0.03b | 0.0352 |
a, b Values within a column having different superscript letters indicate a significant difference at P < 0.05. Numbers are mean ± S.D. (n = 24 for ADG; n = 4 for ADFI and FCR)
c ADG = average daily gain
d ADFI = average daily feed intake
e FCR = feed conversion ratio
Serum parameters of growing pigs
In NSPE pigs, serum concentration of CK was significantly lower (P < 0.05) than the control group (Table 3). Furthermore, the serum concentrations of T-SOD, IgG, and glucose were significantly elevated compared with the control group (P < 0.05) (Table 3). Serum levels of TP, ALT and AST were similar between the two groups (Table 3).
Table 3.
Effect of NSPEs on serum biochemical parameters of growing pigs
| Groups | |||
|---|---|---|---|
| Control | Treatment | P value | |
| ALT (IU/L)c | 49.01 ± 7.96 | 49.00 ± 9.30 | 0.4768 |
| AST (IU/L)d | 79.60 ± 10.70 | 63.80 ± 16.05 | 0.2240 |
| TP (mmol/L)e | 67.31 ± 5.44 | 69.50 ± 2.44 | 0.5331 |
| ALP (U/L)f | 131.83 ± 36.14 | 126.40 ± 22.06 | 0.2565 |
| GLU (mmol/L)g | 6.37 ± 2.24b | 9.73 ± 2.34a | 0.0479 |
| T-SOD (U/mL)h | 61.55 ± 2.67b | 67.44 ± 3.64a | 0.0002 |
| CK (U/L)i | 3117 ± 274a | 2188 ± 218b | 0.0089 |
| IgG (g/L)j | 3.19 ± 0.16b | 3.43 ± 0.20a | 0.0392 |
a, bValues within a column not sharing a common superscript letter indicate significant difference at P < 0.05. Numbers are means ± S.D. (n = 4)
cALT = alanine aminotransferase
dAST = aspartate aminotransferase
eTP = total protein
fALP = alkaline phosphatase
gGLU = glucose
hT-SOD = total superoxide dismutase
iCK = creatine kinase
jIgG = immunoglobulin G
Identification and comparison of proteins of differential abundance
Using iTRAQ analysis, a total of 2634 proteins were identified within the FDR (false discovery rate) of 1% (Additional file 3: Table S2). Following statistical analysis, 104 proteins were found to be differentially expressed in the small intestinal mucosa between CTRL and NSPE pigs, with 43 up-regulated and 61 down-regulated (Additional file 4: Table S3).
A total of 90 proteins of differential abundance were grouped into eight classes based on putative functions: transcriptional and translational regulation (44.4%), miscellaneous (16.7%), redox homeostasis and detoxification (10.0%), immune response and inflammation (8.9%), energy metabolism (7.8%), protein metabolism and modification (5.6%), lipid metabolism (3.3%), and cell cytoskeleton (3.3%) (Fig. 1). Those related to transcriptional and translational regulation, redox homeostasis, and immune response were predominant, accounting for approximately 63% of the differentially expressed proteins. A comparison of proteins of differential abundance with functional groupings between the two groups indicated that a smaller number of protein species were up-regulated in NSPE pigs (36 versus 54) (Table 4).
Fig. 1.
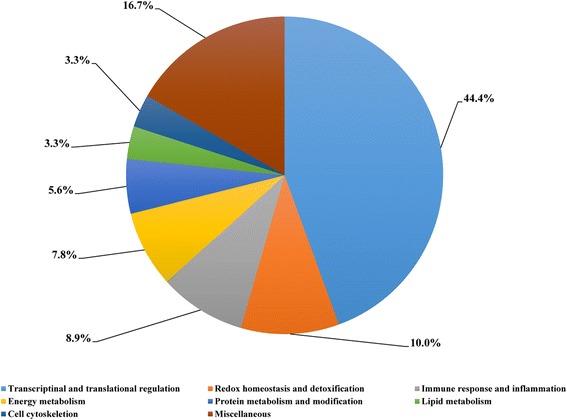
Functional classification of the proteins of differential abundance identified from the small intestinal mucosa of growing pigs supplemented with NSPE
Table 4.
List of differentially expressed proteins in small intestinal mucosal samples from treatment group and control group
| Accessiona | Descriptionb | Gene symbol | Scorec | Pep. Nod | Log2 fold change | P-valuee | Biological process GO term |
|---|---|---|---|---|---|---|---|
| Transcriptional and translational regulation | |||||||
| F1S419 | Uncharacterized protein OS = Sus scrofa GN = SF3B3 PE = 4 SV = 2 - [F1S419_PIG] | None | 85.61 | 3 | −0.37 | 0.0007 | RNA binding |
| K9J4V0 | U5 small nuclear ribonucleoprotein 200 kDa helicase OS = Sus scrofa GN = SNRNP200 PE = 2 SV = 1 - [K9J4V0_PIG] | SNRNP200 | 248.18 | 9 | −0.32 | 0.0012 | Nucleic acid binding |
| F2Z5Q6 | 40S ribosomal protein S6 (Fragment) OS = Sus scrofa GN = RPS6 PE = 3 SV = 2 - [F2Z5Q6_PIG] | RPS6 | 140.42 | 4 | −0.81 | 0.0013 | Structural constituent of ribosome |
| F1SD96 | Uncharacterized protein (Fragment) OS = Sus scrofa GN = RAD23A PE = 4 SV = 1 - [F1SD96_PIG] | RAD23A | 85.25 | 3 | 1.06 | 0.0026 | Nucleotide excision repair |
| F1S8K5 | Uncharacterized protein OS = Sus scrofa GN = SUPT16H PE = 4 SV = 1 - [F1S8K5_PIG] | SUPT16H | 35.43 | 2 | −0.40 | 0.0028 | RNA binding |
| F1RZH4 | Uncharacterized protein OS = Sus scrofa PE = 4 SV = 1 - [F1RZH4_PIG] | ADAM10 | 32.67 | 1 | −0.82 | 0.0048 | Structural constituent of ribosome |
| F1SD98 | Uncharacterized protein OS = Sus scrofa GN = TRMT1 PE = 4 SV = 2 - [F1SD98_PIG] | TRMT1 | 27.72 | 1 | −0.30 | 0.0065 | Poly(A) RNA binding |
| I3LHZ6 | Uncharacterized protein OS = Sus scrofa GN = DHX9 PE = 4 SV = 1 - [I3LHZ6_PIG] | DHX9 | 994.71 | 27 | −0.30 | 0.0075 | ATP-dependent RNA helicase activity |
| F1SDV7 | Uncharacterized protein (Fragment) OS = Sus scrofa GN = TOP1 PE = 4 SV = 1 - [F1SDV7_PIG] | TOP1 | 99.93 | 4 | −0.44 | 0.0075 | DNA binding |
| P62802 | Histone H4 OS = Sus scrofa PE = 1 SV = 2 - [H4_PIG] | None | 358.11 | 7 | −0.67 | 0.0103 | DNA binding |
| F1S1V1 | Uncharacterized protein OS = Sus scrofa GN = SSB PE = 4 SV = 2 - [F1S1V1_PIG] | SSB | 196.5 | 6 | −0.73 | 0.0111 | Nucleotide binding |
| F1RS45 | DNA topoisomerase 2 OS = Sus scrofa PE = 3 SV = 2 - [F1RS45_PIG] | TOP2B | 116.62 | 6 | −0.27 | 0.0117 | DNA binding |
| F1S1X3 | Uncharacterized protein OS = Sus scrofa GN = NARS PE = 3 SV = 2 - [F1S1X3_PIG] | NARS | 262.65 | 7 | −0.30 | 0.0119 | Nucleotide binding |
| F2Z576 | Histone H3 OS = Sus scrofa GN = LOC100525821 PE = 2 SV = 1 - [F2Z576_PIG] | HIST1H3E | 159.44 | 6 | −0.77 | 0.0120 | DNA binding |
| Q29194 | Ribosomal protein S2 (Fragment) OS = Sus scrofa PE = 2 SV = 1 - [Q29194_PIG] | None | 46.59 | 1 | −0.45 | 0.0138 | Structural constituent of ribosome |
| I3LFV4 | Uncharacterized protein OS = Sus scrofa GN = YBX1 PE = 4 SV = 1 - [I3LFV4_PIG] | YBX1 | 157.89 | 4 | 0.41 | 0.0148 | DNA repair |
| I3LIN8 | Histone H2A OS = Sus scrofa GN = H2AFY PE = 3 SV = 1 - [I3LIN8_PIG] | H2AFY | 224.89 | 6 | −0.52 | 0.0149 | Chromatin DNA binding |
| B0FWK5 | Ribosomal protein L5 OS = Sus scrofa GN = RPL5 PE = 2 SV = 1 - [B0FWK5_PIG] | RPL5 | 178.57 | 8 | −0.34 | 0.0165 | Structural constituent of ribosome |
| I3LCI4 | Uncharacterized protein OS = Sus scrofa GN = ZFR PE = 4 SV = 1 - [I3LCI4_PIG] | ZFR | 41.93 | 2 | −0.28 | 0.0167 | Poly(A) RNA binding |
| F1S8A5 | Uncharacterized protein OS = Sus scrofa GN = MRPS26 PE = 4 SV = 1 - [F1S8A5_PIG] | MRPS26 | 38.63 | 1 | −0.36 | 0.0181 | Poly(A) RNA binding |
| A5GFY4 | Negative elongation factor D OS = Sus scrofa GN = NELFCD PE = 3 SV = 1 - [NELFD_PIG] | NELFCD | 43.67 | 1 | −0.32 | 0.0189 | Negative regulation of transcription |
| F1S5A8 | Uncharacterized protein OS = Sus scrofa GN = DHX15 PE = 4 SV = 1 - [F1S5A8_PIG] | DHX15 | 259.43 | 8 | −0.26 | 0.0198 | ATP-dependent RNA helicase activity |
| F1RRG9 | Uncharacterized protein OS = Sus scrofa GN = SMARCA5 PE = 4 SV = 1 - [F1RRG9_PIG] | SMARCA5 | 99.44 | 3 | −0.39 | 0.0201 | DNA binding |
| F1RGP1 | Uncharacterized protein OS = Sus scrofa GN = MYBBP1A PE = 4 SV = 1 - [F1RGP1_PIG] | MYBBP1A | 445.89 | 12 | −0.50 | 0.0208 | Poly(A) RNA binding |
| F2Z5Q8 | Uncharacterized protein OS = Sus scrofa GN = LOC100519675 PE = 4 SV = 1 - [F2Z5Q8_PIG] | RPL35A | 57.33 | 2 | −0.45 | 0.0209 | Structural constituent of ribosome |
| I3L7T6 | Histone H2A OS = Sus scrofa GN = H2AFX PE = 3 SV = 1 - [I3L7T6_PIG] | H2AFX | 357.7 | 7 | −0.56 | 0.0231 | DNA binding |
| F1SMZ9 | Uncharacterized protein (Fragment) OS = Sus scrofa GN = SF3B1 PE = 4 SV = 2 - [F1SMZ9_PIG] | SF3B1 | 267.33 | 9 | −0.26 | 0.0245 | mRNA binding |
| F2Z5K9 | Histone H3 OS = Sus scrofa GN = LOC100622412 PE = 3 SV = 1 - [F2Z5K9_PIG] | LOC100622412 | 178.75 | 6 | −0.76 | 0.0270 | DNA binding |
| P53027 | 60S ribosomal protein L10a (Fragment) OS = Sus scrofa GN = RPL10A PE = 2 SV = 3 - [RL10A_PIG] | RPL10A | 154.25 | 5 | −0.34 | 0.0272 | RNA binding |
| K9IVG8 | DEAD (Asp-Glu-Ala-Asp) box helicase 21 OS = Sus scrofa GN = DDX21 PE = 2 SV = 1 - [K9IVG8_PIG] | DDX21 | 44.62 | 1 | −0.38 | 0.0292 | RNA binding |
| F2Z554 | Uncharacterized protein OS = Sus scrofa GN = RPL30 PE = 3 SV = 1 - [F2Z554_PIG] | RPL30 | 105.87 | 4 | −0.26 | 0.0323 | RNA binding |
| Q29195 | 60S ribosomal protein L10 OS = Sus scrofa GN = RPL10 PE = 2 SV = 3 - [RL10_PIG] | RPL10 | 105.8 | 4 | −0.39 | 0.0350 | Structural constituent of ribosome |
| P67985 | 60S ribosomal protein L22 OS = Sus scrofa GN = RPL22 PE = 2 SV = 2 - [RL22_PIG] | RPL22 | 113.83 | 3 | −0.48 | 0.0355 | Structural constituent of ribosome |
| I7GF95 | Guanine nucleotide binding protein-like 1 OS = Sus scrofa GN = GNL1 PE = 4 SV = 1 - [I7GF95_PIG] | GNL1 | 58.56 | 1 | −0.35 | 0.0371 | Ribosome biogenesis |
| F1S8L9 | Uncharacterized protein OS = Sus scrofa GN = HNRNPU PE = 4 SV = 2 - [F1S8L9_PIG] | HNRNPU | 883.61 | 23 | −0.34 | 0.0377 | Poly(A) RNA binding |
| Q53DY5 | Histone H1.3-like protein OS = Sus scrofa GN = LOC595122 PE = 2 SV = 1 - [Q53DY5_PIG] | HIST1H1D | 251.92 | 7 | 1.29 | 0.0384 | Chromatin DNA binding |
| F1S2G3 | Uncharacterized protein (Fragment) OS = Sus scrofa GN = TBCA PE = 4 SV = 1 - [F1S2G3_PIG] | TBCA | 78.18 | 2 | 0.31 | 0.0389 | Poly(A) RNA binding |
| F2Z5P1 | Histone H2A (Fragment) OS = Sus scrofa GN = H2AFV PE = 3 SV = 1 - [F2Z5P1_PIG] | LOC100512448 | 256.74 | 5 | −0.43 | 0.0427 | DNA binding |
| F2Z553 | Uncharacterized protein OS = Sus scrofa GN = EIF1 PE = 4 SV = 1 - [F2Z553_PIG] | EIF1 | 103.66 | 2 | 0.82 | 0.0437 | Translation initiation factor activity |
| F2Z5L5 | Histone H2A OS = Sus scrofa GN = HIST2H2AC PE = 3 SV = 1 - [F2Z5L5_PIG] | HIST2H2AC | 322.1 | 5 | −0.62 | 0.0448 | DNA binding |
| Redox homeostasis and detoxification | |||||||
| F1SKJ2 | Uncharacterized protein OS = Sus scrofa GN = TXN2 PE = 4 SV = 1 - [F1SKJ2_PIG] | TXN2 | 29.86 | 1 | 0.39 | 0.0043 | Cell redox homeostasis |
| F1SGS9 | Catalase OS = Sus scrofa GN = CAT PE = 3 SV = 1 - [F1SGS9_PIG] | CAT | 923.56 | 23 | 0.58 | 0.0151 | Protect cells from the toxic effects of hydrogen peroxide |
| I3LDJ8 | Uncharacterized protein OS = Sus scrofa PE = 3 SV = 1 - [I3LDJ8_PIG] | None | 303.51 | 10 | 0.77 | 0.0202 | Oxidoreductase activity |
| P12309 | Glutaredoxin-1 OS = Sus scrofa GN = GLRX PE = 1 SV = 2 - [GLRX1_PIG] | GLRX | 277.83 | 6 | 0.64 | 0.0208 | Cell redox homeostasis |
| F1SCF9 | Uncharacterized protein (Fragment) OS = Sus scrofa GN = TECR PE = 4 SV = 2 - [F1SCF9_PIG] | TECR | 38.34 | 1 | −0.37 | 0.0242 | Oxidoreductase activity |
| A5J2A8 | Thioredoxin (Fragment) OS = Sus scrofa GN = TRX PE = 4 SV = 1 - [A5J2A8_PIG] | TRX | 128.36 | 3 | 0.34 | 0.0303 | Cell redox homeostasis |
| F1SMY1 | Uncharacterized protein OS = Sus scrofa GN = TMX3 PE = 4 SV = 2 - [F1SMY1_PIG] | TMX3 | 39.1 | 2 | 0.30 | 0.0345 | Cell redox homeostasis |
| P16549 | Dimethylaniline monooxygenase [N-oxide-forming] 1 OS = Sus scrofa GN = FMO1 PE = 1 SV = 3 - [FMO1_PIG] | FMO1 | 39.55 | 2 | 1.64 | 0.0084 | Oxidative metabolism of a variety of xenobiotics |
| P04178 | Superoxide dismutase [Cu-Zn] OS = Sus scrofa GN = SOD1 PE = 1 SV = 2 - [SODC_PIG] | SOD1 | 459.04 | 9 | 0.35 | 0.0424 | Superoxide dismutase activity |
| Immune response and inflammation | |||||||
| A3FJ41 | MHC class I antigen (Fragment) OS = Sus scrofa GN = SLA-1 PE = 4 SV = 1 - [A3FJ41_PIG] | SLA-1 | 120.03 | 5 | 0.35 | 0.0050 | Immune response |
| F1RGC8 | Uncharacterized protein OS = Sus scrofa GN = NLRP6 PE = 4 SV = 3 - [F1RGC8_PIG] | NLRP6 | 119.58 | 4 | −0.32 | 0.0061 | Activation of NF-κB |
| F1RFM7 | Uncharacterized protein OS = Sus scrofa GN = AIMP2 PE = 4 SV = 1 - [F1RFM7_PIG] | AIMP2 | 232.75 | 6 | −0.29 | 0.0076 | Metabolism of xenobiotics |
| A2SZV5 | Tax1 binding protein 3 (Fragment) OS = Sus scrofa PE = 4 SV = 1 - [A2SZV5_PIG] | None | 55.14 | 1 | 0.29 | 0.0133 | Negative regulation of NF-κB |
| B8XX91 | DNA-dependent activator of IFN-regulatory factor OS = Sus scrofa GN = DAI PE = 2 SV = 1 - [B8XX91_PIG] | DAI | 100.5 | 4 | 0.70 | 0.0137 | Innate immune responses |
| Q8WNQ7 | N-acetylgalactosamine-6-sulfatase OS = Sus scrofa GN = GALNS PE = 2 SV = 1 - [GALNS_PIG] | GALNS | 52.52 | 1 | 0.60 | 0.0311 | Degradation of the glycosaminoglycans keratan sulfate |
| B8XTR8 | Granzyme H OS = Sus scrofa GN = gzmH PE = 2 SV = 1 - [B8XTR8_PIG] | gzmH | 168.84 | 6 | −0.67 | 0.0272 | Serine-type endopeptidase activity |
| A5GFQ5 | Protein canopy homolog 3 OS = Sus scrofa GN = CNPY3 PE = 3 SV = 1 - [CNPY3_PIG] | CNPY3 | 40.13 | 2 | −0.63 | 0.0376 | Receptor binding for proper TLR folding |
| Energy metabolism | |||||||
| Q1ACV5 | Transporter associated with antigen processing 1 OS = Sus scrofa PE = 2 SV = 1 - [Q1ACV5_PIG] | None | 298.67 | 7 | −0.32 | 0.0030 | Triggers ATP hydrolysis |
| F1RIG0 | Uncharacterized protein (Fragment) OS = Sus scrofa PE = 4 SV = 1 - [F1RIG0_PIG] | None | 47.28 | 2 | −0.27 | 0.0169 | ATP binding |
| Q7SIB7 | Phosphoglycerate kinase 1 OS = Sus scrofa GN = PGK1 PE = 1 SV = 3 - [PGK1_PIG] | PGK1 | 850.39 | 23 | 0.30 | 0.0160 | Conversion of 1,3-diphosphoglycerate to 3-phosphoglycerate |
| H9BYW2 | Acyl-coenzyme A oxidase OS = Sus scrofa GN = ACOX1 PE = 2 SV = 1 - [H9BYW2_PIG] | ACOX1 | 370.35 | 10 | 0.91 | 0.0200 | Fatty acid beta-oxidation |
| I3LEN7 | Uncharacterized protein OS = Sus scrofa GN = ALDH1L1 PE = 3 SV = 1 - [I3LEN7_PIG] | ALDH1L1 | 49.04 | 2 | 0.40 | 0.0245 | Formate oxidation |
| F1S0Y8 | Uncharacterized protein OS = Sus scrofa GN = ADH4 PE = 3 SV = 2 - [F1S0Y8_PIG] | ADH4 | 40.7 | 2 | 0.67 | 0.0309 | Oxidation of long-chain aliphatic alcohols |
| A7UIU7 | ATP citrate lyase OS = Sus scrofa GN = ACL PE = 2 SV = 1 - [A7UIU7_PIG] | ACL | 468.98 | 14 | −0.38 | 0.0374 | ATP binding |
| Protein metabolism and modification | |||||||
| F1RIF3 | Uncharacterized protein OS = Sus scrofa GN = FAH PE = 4 SV = 1 - [F1RIF3_PIG] | FAH | 38.37 | 2 | 0.39 | 0.0010 | Catabolism of the amino acid phenylalanine |
| Q9GK25 | Peptidyl-prolyl cis-trans isomerase (Fragment) OS = Sus scrofa PE = 2 SV = 1 - [Q9GK25_PIG] | None | 266.1 | 7 | 1.43 | 0.0025 | Accelerate the folding of proteins |
| I3L739 | Uncharacterized protein OS = Sus scrofa GN = JMJD6 PE = 4 SV = 1 - [I3L739_PIG] | JMJD6 | 39.99 | 1 | −0.29 | 0.0193 | Protein hydroxylases |
| I3LK37 | Uncharacterized protein (Fragment) OS = Sus scrofa PE = 3 SV = 1 - [I3LK37_PIG] | GALNT7 | 33.39 | 2 | −0.30 | 0.0248 | Protein glycosylation |
| F1RNR6 | 4-hydroxyphenylpyruvate dioxygenase OS = Sus scrofa GN = HPD PE = 3 SV = 2 - [F1RNR6_PIG] | HPD | 31 | 1 | 0.35 | 0.0391 | Aromatic amino acid family metabolic process |
| Lipid metabolism | |||||||
| I3LM15 | Uncharacterized protein OS = Sus scrofa GN = AGPS PE = 4 SV = 1 - [I3LM15_PIG] | AGPS | 48.77 | 1 | −0.36 | 0.0019 | Lipid biosynthetic process |
| Q9GJX2 | Diazepam binding inhibitor (Fragment) OS = Sus scrofa GN = DBI PE = 2 SV = 1 - [Q9GJX2_PIG] | DBI | 80.13 | 3 | 0.91 | 0.0057 | Long-chain fatty acyl-CoA binding, triglyceride metabolic process |
| P27917 | Apolipoprotein C-III OS = Sus scrofa GN = APOC3 PE = 1 SV = 2 - [APOC3_PIG] | APOC3 | 226.39 | 7 | 0.78 | 0.0241 | High-density lipoprotein particle receptor binding |
| Cell cytoskeleton | |||||||
| P10668 | Cofilin-1 OS = Sus scrofa GN = CFL1 PE = 1 SV = 3 - [COF1_PIG] | CFL1 | 704.02 | 15 | 0.31 | 0.0059 | Cytoskeleton organization |
| Q5G6W0 | Cofilin-2 (Fragment) OS = Sus scrofa PE = 2 SV = 1 - [Q5G6W0_PIG] | CFL1 | 48.67 | 2 | 0.43 | 0.0073 | Cytoskeleton organization |
| B5APV0 | Actin-related protein 2/3 complex subunit 5 OS = Sus scrofa GN = ARPC5 PE = 2 SV = 1 - [B5APV0_PIG] | ARPC5 | 170.99 | 6 | 0.30 | 0.0167 | Structural constituent of cytoskeleton |
| Miscellaneous | |||||||
| Q9TSA7 | Calmodulin (Fragments) OS = Sus scrofa PE = 4 SV = 1 - [Q9TSA7_PIG] | None | 108.72 | 4 | 1.11 | 0.0008 | Calcium ion binding |
| K7GKQ1 | Uncharacterized protein OS = Sus scrofa GN = RAB9A PE = 3 SV = 1 - [K7GKQ1_PIG] | RAB9A | 26.6 | 1 | −0.40 | 0.0071 | Cytoskeletal signaling |
| F1RKI3 | Uncharacterized protein OS = Sus scrofa GN = HINT1 PE = 4 SV = 1 - [F1RKI3_PIG] | HINT1 | 80.55 | 3 | 0.32 | 0.0073 | Tumor suppressing |
| I3LSY0 | Uncharacterized protein OS = Sus scrofa GN = ACSM4 PE = 4 SV = 1 - [I3LSY0_PIG] | ACSM4 | 21.13 | 1 | 0.86 | 0.0179 | Catalytic activity |
| D0G6R8 | Phosphatidate cytidylyltransferase OS = Sus scrofa GN = CDS2 PE = 2 SV = 1 - [D0G6R8_PIG] | CDS2 | 33.01 | 1 | −0.39 | 0.0192 | Synthesis of phosphatidylglycerol |
| Q95332 | Betaine--homocysteine S-methyltransferase 1 (Fragment) OS = Sus scrofa GN = BHMT PE = 1 SV = 3 - [BHMT1_PIG] | BHMT | 110.41 | 4 | 1.10 | 0.0193 | Regulation of homocysteine metabolism |
| F1RS34 | Uncharacterized protein OS = Sus scrofa GN = GAPVD1 PE = 4 SV = 2 - [F1RS34_PIG] | GAPVD1 | 22.69 | 1 | −0.40 | 0.0207 | Signal transduction |
| F1ST01 | Uncharacterized protein OS = Sus scrofa GN = SELENBP1 PE = 4 SV = 1 - [F1ST01_PIG] | SELENBP1 | 936.42 | 22 | 0.33 | 0.0209 | Selenium binding |
| Q9TV62 | Myosin-4 OS = Sus scrofa GN = MYH4 PE = 2 SV = 1 - [MYH4_PIG] | MYH4 | 192.94 | 7 | −0.83 | 0.0336 | Motor activity |
| F1RN91 | Uncharacterized protein (Fragment) OS = Sus scrofa PE = 4 SV = 2 - [F1RN91_PIG] | MYO18A | 35.04 | 2 | 0.28 | 0.0355 | Cell migration |
| F1RPC8 | Uncharacterized protein OS = Sus scrofa GN = CRYM PE = 4 SV = 2 - [F1RPC8_PIG] | CRYM | 59.33 | 2 | 0.49 | 0.0392 | Thyroid hormone binding |
| F2Z5W6 | Uncharacterized protein OS = Sus scrofa GN = LAMTOR1 PE = 4 SV = 1 - [F2Z5W6_PIG] | LAMTOR1 | 26.54 | 1 | −0.37 | 0.0410 | Guanyl-nucleotide exchange factor activity |
| Q29069 | Myosin light chain OS = Sus scrofa PE = 2 SV = 2 - [Q29069_PIG] | None | 58.61 | 3 | −0.38 | 0.0458 | Calcium ion binding |
| O19175 | Casein kinase I isoform alpha (Fragment) OS = Sus scrofa GN = CSNK1A1 PE = 2 SV = 1 - [KC1A_PIG] | CSNK1A1 | 51.13 | 1 | −0.44 | 0.0473 | Protein kinase activity |
| N0E654 | Casein kinase II b subunit splicing isoform 476 (Fragment) OS = Sus scrofa GN = Csnk2b PE = 2 SV = 1 - [N0E654_PIG] | Csnk2b | 63.97 | 2 | −0.27 | 0.0039 | Cell proliferation and cell differentiation |
aUniprot_ Sus scrofa_9823 database accession number
bThe name of the protein exclusive of the identifier that appears in the database
cThe sum of the scores of the individual peptides
dThe number of distinct peptide sequences in the protein group
eDifferential protein expression in the treatment group was presented as a log2 fold change relative to the control group
GO annotations of proteins of differential abundance
In the cellular component group, the differentially expressed proteins were concentrated in the intracellular part and membrane-bounded organelles (Fig. 2). In the molecular functional group, the differentially expressed proteins that are binding proteins (protein, nucleotide, or nucleic acid binding) and metabolic enzymes (hydrolase, oxidoreductase, or transferase activity) were ranked at the top of the category (Fig. 2). In the biological process category, the proteins that participate in cellular process (organelle organization process), metabolic process (nitrogen compound metabolic and biosynthetic process), and biological regulation (transcriptional and translational regulation, redox homeostasis, and immune response) had the highest ratios among the differentially expressed proteins.
Fig. 2.
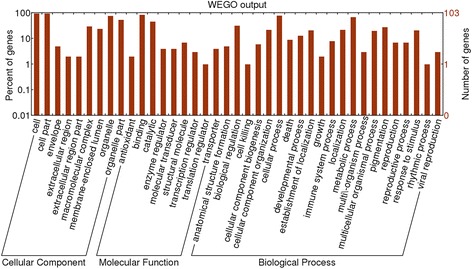
GO distribution analysis of differentially expressed proteins in small intestinal mucosal samples from the NSPE group and control group. The right coordinate axis indicates the number of proteins for each GO annotation, and the left one represents the proportion of proteins for every GO annotation
Validation of proteins of differential abundance
Six differentially expressed proteins superoxide dismutase (SOD1) involved in redox homeostasis; calmodulin (CALM1) involved in calcium ion binding; MHC class I antigen (SLA-1) involved in immune response; acyl-coenzyme A oxidase (ACOX1) involved in energy metabolism; 40S ribosomal protein S6 (RPS6) involved in transcriptional and translational regulation; and apolipoprotein C-III (APOC3) involved in lipid absorption, were selected for the validation of proteomic data at the mRNA level using qPCR (Fig. 3). Most protein levels were consistent with their mRNA expression levels, except for RPS6.
Fig. 3.
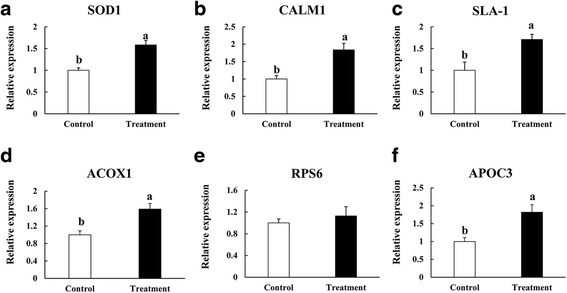
qPCR validation of six proteins of differential abundance from the intestinal mucosa of growing pigs at the mRNA level (a, b, c, d, e and f). Samples were normalized with the reference gene β-actin. Vertical lines represent means ± S.D, and different letters denote significant difference at P < 0.05 (n = 4)
Discussion
The benefit of NSPEs supplementation is well recognized in monogastric animal production; NSPEs supplementation promotes growth performance and GI tract health, including the efficiency of nutrient utilization [2, 3, 8]. A number of studies have proven that the addtion of NSPEs to the diet reduces digesta viscosity by the partial or complete hydrolysis of soluble NSPs, which triggers the changes in microbial composition, especially the reduction of the amount of pathological bacteria within the small intestine [11, 32]. Moreover, the supplementation of NSPEs could increase the nutrient availability in the intestinal lumen (for example, energy substrates and proteins) [12, 33]. All above effects of NSPE supplementation are due to the improvements of the intestinal environment. However, it is still largely unknown how the small intestinal mucosa of the hosts responds to alterations in the luminal environment triggered by the addition of NSPEs. The present study marks the first time that the well-established quantitative iTRAQ label-based technology was applied for the proteomic analysis of the small intestinal mucosa of growing pigs with dietary supplementation of NSPEs. Various functional groupings of differentially expressed mucosal proteins related to nutrient metabolism, transcriptional and translational regulation, immune, and redox homeostasis were identified in response to NSPEs.
In former research, the utilization of β-glucanase and xylanase in the diet demonstrated that enzymes tended to increase the absorptive area and reduce cell proliferation and intraepithelial lymphocytes in the gut of pigs [34]. Both cereal grains and enzymes would affect components of gut health, including intestine morphology, bacteria populations, and microbial metabolites in the gut content [35]. It has been demonstrated that enhanced cell proliferation in the intestinal mucosa is associated with bowel diseases, cellular repair, and apoptosis [36, 37]. As shown in the present study, 89% of proteins related to transcriptional and translational regulation were down-regulated in NSPE pigs. We speculate that supplementation with NSPEs in the diet of growing pigs can reduce the possibility of intestinal infection. This is consistent with the former research result that NSPEs reduce the amount of pathological bacteria within the small intestine by lowering the viscosity of intestinal digesta [11].
The abundance of proteins CFL1 (cofilin-1), CFL2 (cofilin-2) and ARPC5 (actin-related protein 2/3 complex subunit 5), which are classified as cell cytoskeleton proteins relevant to cell structure and mobility, was increased. CFL1 and CFL2 are widely distributed intracellular actin-modulating proteins [38]. These two proteins can cause actin cytoskeleton rearrangement and membrane remodeling to the formation of phagosomes, which are recognized by Fc gamma receptors and beneficial for the host-defense in animals [39]. ARPC5 has a similar function as cofilin in the actin cytoskeleton, which is required for phagocytosis in mammals [40]. The up-regulation of these proteins might reflect the improved integrity of the intestinal mucosa.
As an important immune organ, the small intestine participates in the inflammatory response and the prevention of bacterial infection. SLA-1 (MHC class I antigen), GALNS (N-acetylgalactosamine-6-sulfatase), and DAI (DNA-dependent activator of IFN-regulatory factor) are considered to be involved in the immune response. SLA-1 alerts the immune system to virus-infected cells by presenting peptide fragments derived from intracellular proteins [41]. GALNS is located in lysosomes that digest different types of molecules and engulf viruses or bacteria within cells [42, 43]. DAI selectively enhances the DNA-mediated induction of type I IFN and other genes involved in innate immunity [44, 45]. The abundance of these proteins was up-regulated in NSPE pigs, suggesting that the supplementation of NSPEs may improve potential immunity and reduce the chance of bacterial infection in the small intestine. This is consistent with the elevated serum level of IgG in the NSPE group. However, challenges with exogenous pathogens are still required to verify the effect of NSPEs supplementation on immunity. In contrast, proteins involved in an inflammatory response, including NLRP6 (NLR family, pyrin domain containing 6) and CNPY3 (protein canopy homolog 3), are down-regulated, which indicates that inflammation is attenuated in the small intestinal mucosa due to the supplementation of NSPEs [46]. It has been suggested that one of the performance improvement attributes of NSPEs is due to the reduced local inflammation by controlling pathogens within the small intestine [32].
In addition to affecting the immune response, the up-regulated proteins catalase (CAT), glutaredoxin (GRXS), thioredoxin (TRX), superoxide dismutase (SOD), dimethylaniline monooxygenase [N-oxide-forming] 1 (FMO1) and 4-hydroxyphenylpyruvate dioxygenase (HPPD) are classified as redox homeostasis and detoxification proteins based on their primary functions. The up-regulation of CAT, GRXS, TRX and SOD may suggest that NSPE pigs had more potential to keep redox homeostasis in vivo [47–52]. This is consistent with the increased serum level of T-SOD in the NSPE group of this study. The reason for the up-regulation of these oxidoreductases and immune factors in the present study may be the increased abundance of reactive oxygen species (ROS) and inflammatory factors during stimulating energy metabolism due to a higher uptake of nutrients with NSPEs supplementation. However, further study is required to prove the effect of NSPEs on redox homeostasis. As one of the detoxification enzymes, FMO1 is regulated by xenobiotics, as the enzyme activity markedly increases in response to the invading harmful chemicals [53]. The up-regulation of this protein suggests that the supplementation of NSPEs is helpful to eliminate xenobiotics in the small intestine, which also could be related to the improvement of the intestinal lumen due to NSPEs.
Furthermore, the up-regulated abundance of proteins was observed in the NSPE group, including multiple nutrient metabolism processes such as energy, lipid, amino acid and mineral. These proteins included phosphoglycerate kinase 1 (PGK1), diazepam binding inhibitor (DBI), and acyl-coenzyme A oxidase (ACOX1). PGK1 plays a vital role in glycolysis or gluconeogenesis [54]. The up-regulation of ACOX1 indicates the elevation of glucose synthesis in the small intestine, which is consistent with the increased serum glucose level in the NSPE group. Likewise, higher abundance of DBI and ACOX1 was observed in this study, suggesting the stimulation of lipids β-oxidation for nutrient absorption to meet the energy requirement in the small intestine of NSPE pigs [55, 56]. Apolipoprotein C-III (APOC3) is an important modulator that is secreted from the intestine on the chylomicron upon lipid absorption [57]. The up-regulation of APOC3 implies the enhanced absorption of dietary lipids in the NSPE group.
Two differentially expressed proteins related to the permeability of the tight junction (TJ), including casein kinase II beta subunit splicing isoform 476 (Csnk2b) and myosin-4 (MYH4), were identified in the present study. The tight junctions (TJs) in the small intestine are not only a physical and biological barrier but also a passive diffusion system that depends on the permeability of the TJs [58]. Paracellular transport is one of the passive diffusion systems providing an absorption way for small molecular compounds [59], which are regulated by the permeability of the TJs and are thought to be important for mineral absorption [60]. Additionally, the transepithelial transport of oligosaccharides, but not polysaccharides, also occurs via the paracellular pathway [61]. Previous research has demonstrated that NSPEs are capable of hydrolyzing polysaccharides from the food to oligosaccharides in the gut [62]. Thus, the down-regulation of these two proteins in this study, in addition to former studies, indicates an increased permeability of the TJs in the NSPE group, which is beneficial to small molecular compounds absorption in the small intestine.
Calmodulin regulates cellular calcium concentration as a primary calcium-binding protein [63]. Calcium absorption is reduced if the bioavailability of dietary calcium is lowered by calcium-binding agents like cellulose because nearly all dietary calcium intake occurs in the upper intestine [64]. The up-regulation of this protein observed in this study suggests that calcium absorption in the small intestine is facilitated in the NSPE group by the degradation of calcium-binding agents in the diet, which could be conductive to bone health.
It has been demonstrated that one of the important roles of NSPEs within the small intestine is the elimination of the nutrient-encapsulating effect of cell wall polysaccharides, which increases the availability of starches, amino acids, and minerals. These results are consistent with our results from the present study that the levels of proteins related to nutrient absorption and utilization (energy, lipid, amino acid and mineral) are up-regulated. A fully understanding of the mechanisms of NSPEs supplementation will require the determination of protein modifications and protein regulation such as phosphorylation or glycosylation [65]. However, this part was not involved in the present study due to the technical limitation. Thus, further study is required to prove the effect of NSPEs on regulatory proteins using specific method, for example, the phosphoproteome.
Conclusions
The results of this study provide the first evidence that the small intestinal mucosa proteome is altered in growing pigs supplemented with NSPEs. Growing pigs most likely responded to the increased reactive oxygen species (ROS) and inflammatory factors during stimulating energy metabolism due to NSPEs supplementation by changing the abundance of certain mucosal proteins that modulate redox homeostasis and enhance immune response. Most important of all, the effect of NSPEs on the increase of nutrient availability in the intestinal lumen provided additional benefits to facilitate protein expressions related to the efficiency of nutrient absorption and utilization, such as energy metabolism, amino acid metabolism, mineral metabolism, lipid absorption, and cell structure and mobility. These novel findings show the mechanisms whereby dietary supplementation with NSPEs promotes growth performance and improves the GI health of growing pigs, which also has important implications for the better utilization of this feed additive.
Acknowledgements
This research was supported by the Chinese National Science and Technology Pillar Program (No: 2012BAD39B0), the Special Fund for Innovation Team of the Chinese Academy of Agricultural Sciences (No: ASTTP-IAS07), and the Chinese National Key Basic Research and Development Program (No: 2014CB138804).
Funding
This research was supported by the Chinese National Science and Technology Pillar Program (No: 2012BAD39B0), the Special Fund for Innovation Team of the Chinese Academy of Agricultural Sciences (No: ASTTP-IAS07), and the Chinese National Key Basic Research and Development Program (No: 2014CB138804). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
All relevant data are within the paper and its Additional files.
Authors’ contributions
JZ and HZ designed the study. JZ and YG performed the experiments and analyzed the data. JZ, QL and RS contributed reagents/materials/analysis tools. JZ prepared the manuscript and all of the authors contributed to, read and approved the final manuscript.
Competing interest
The authors declare that there is no competing interest.
Consent for publication
Not applicable.
Ethics approval
This study was conducted in strict accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals of the State Council of the People’s Republic of China. The protocol was approved by the Committee on Experimental Animal Management of the Chinese Academy of Agricultural Sciences.
Abbreviations
- ACOX1
Acyl-coenzyme A oxidase
- ADFI
Average daily feed intake
- ADG
Average daily gain
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- APOC3
Apolipoprotein C-III
- ARPC5
Actin-related protein 2/3 complex subunit 5
- AST
Aspartate aminotransferase
- CALM1
Calmodulin
- CAT
Catalase
- CFL
Cofilin
- CK
Creatine kinase
- CNPY3
Protein canopy homolog 3
- Csnk2b
Casein kinase II beta subunit splicing isoform 476 MYH4: myosin-4
- DAI
DNA-dependent activator of IFN-regulatory factor
- DTT
Dithiothreitol
- FCR
Feed conversion ratio
- FMO1
Dimethylaniline monooxygenase [N-oxide-forming] 1
- GALNS
N-acetylgalactosamine-6-sulfatase
- GI
Gastrointestinal
- GRXS
Glutaredoxin
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HPPD
4-hydroxyphenylpyruvate dioxygenase
- IgG
Immunoglobulin G
- iTRAQ
Isobaric tags for relative and absolute quantitation
- NSPEs
Non-starch polysaccharide enzymes
- PGK1
Phosphoglycerate kinase 1
- PMSF
Phenylmethanesulfonyl fluoride
- RPS6
40S ribosomal protein S6
- SLA-1
MHC class I antigen
- SOD1
Superoxide dismutase
- TP
Total protein
- TRX
Thioredoxin
- T-SOD
Total superoxide dismutase
Additional files
The detailed description of the experiment methods, including mass spectrometric analysis procedures and parameters, bioinformatics analysis softwares, websites and real-time qPCR procedures. (DOCX 15 kb)
qPCR primers used for verification of the differentially expressed genes of the small intestinal mucosa in growing pigs. (DOCX 14 kb)
List of all proteins (n = 2634) identified in the study. (XLSX 199 kb)
List of all differentially expressed proteins (n = 104) identified in the study. (XLSX 31 kb)
Contributor Information
Jize Zhang, Email: jzz2006@126.com.
Yang Gao, Email: hilock305@163.com.
Qingping Lu, Email: luqingping@sina.com.
Renna Sa, Email: sa6289@126.com.
Hongfu Zhang, Email: zhanghf6565@vip.sina.com.
References
- 1.Sterk A, Verdonk JMAJ, Mul AJ, Soenen B, Bezençon ML, Frehner M, et al. Effect of xylanase supplementation to a cereal-based diet on the apparent faecal digestibility in weanling piglets. Livest Sci. 2007;108(1–3):269–71. doi: 10.1016/j.livsci.2007.01.077. [DOI] [Google Scholar]
- 2.Wang MQ, Xu ZR, Sun JY, Kim BG. Effects of enzyme supplementation on growth, intestinal content viscosity, and digestive enzyme activities in growing pigs fed rough rice-based diet. Asian-Aust J Anim Sci. 2008;21(2):270–76. doi: 10.5713/ajas.2008.70289. [DOI] [Google Scholar]
- 3.Lindberg JE. Fiber effects in nutrition and gut health in pigs. J Anim Sci Biotechnol. 2014;5(1):15. doi: 10.1186/2049-1891-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li WF, Feng J, Xu ZR, Yang CM. Effects of non-starch polysaccharides enzymes on pancreatic and small intestinal digestive enzyme activities in piglet fed diets containing high amounts of barley. World J Gastroenterol. 2004;10(6):856–9. doi: 10.3748/wjg.v10.i6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willamil J, Badiola I, Devillard E, Geraert PA, Torrallardona D. Wheat-barley-rye- or corn-fed growing pigs respond differently to dietary supplementation with a carbohydrase complex. J Anim Sci. 2012;90(3):824–32. doi: 10.2527/jas.2010-3766. [DOI] [PubMed] [Google Scholar]
- 6.Susenbeth A, Naatjes M, Blank B, Kühl R, Ader P, Dickhoefer U. Effect of xylanase and glucanase supplementation to a cereal-based, threonine-limited diet on the nitrogen balance of growing pigs. Arch Anim Nutr. 2011;65(2):123–33. doi: 10.1080/1745039X.2010.534896. [DOI] [PubMed] [Google Scholar]
- 7.Prandini A, Sigolo S, Morlacchini M, Giuberti G, Moschini M, Rzepus M, et al. Addition of non-starch polysaccharides degrading enzymes to two hulless barley varieties fed in diets for weaned pigs. J Anim Sci. 2014;92(5):2080–6. doi: 10.2527/jas.2012-6199. [DOI] [PubMed] [Google Scholar]
- 8.Kim JS, Ingale SL, Hosseindoust AR, Lee SH, Lee JH, Chae BJ. Effects of mannan level and β-mannanase supplementation on growth performance, apparent total tract digestibility and blood metabolites of growing pigs. Animal. 2016. doi:10.1017/S1751731116001385. [DOI] [PubMed]
- 9.Guo S, Liu D, Zhao X, Li C, Guo Y. Xylanase supplementation of a wheat-based diet improved nutrient digestion and mRNA expression of intestinal nutrient transporters in broiler chickens infected with Clostridium perfringens. Poult Sci. 2014;93(1):94–103. doi: 10.3382/ps.2013-03188. [DOI] [PubMed] [Google Scholar]
- 10.Lin PH, Shih BL, Hsu JC. Effects of different sources of dietary non-starch polysaccharides on the growth performance, development of digestive tract and activities of pancreatic enzymes in goslings. Br Poult Sci. 2010;51(2):270–7. doi: 10.1080/00071661003779124. [DOI] [PubMed] [Google Scholar]
- 11.Kiarie EG, Slominski BA, Nyachoti CM. Effect of products derived from hydrolysis of wheat and flaxseed non starch polysaccharides by carbohydrase enzymes on net absorption in enterotoxigenic Escherichia coli (K88) challenged piglet jejunal segments. Anim Sci J. 2010;81(1):63–71. doi: 10.1111/j.1740-0929.2009.00716.x. [DOI] [PubMed] [Google Scholar]
- 12.Aulrich K, Flachowsky G. Studies on the mode of action of non-starch-polysaccharides (NSP)-degrading enzymes in vitro. 2. Communication: effects on nutrient release and hydration properties. Arch Tierernahr. 2001;54(1):19–32. doi: 10.1080/17450390109381963. [DOI] [PubMed] [Google Scholar]
- 13.Silva SS, Smithard RR. Effect of enzyme supplementation of a rye-based diet on xylanase activity in the small intestine of broilers, on intestinal crypt cell proliferation and on nutrient digestibility and growth performance of the birds. Br Poult Sci. 2002;43(2):274–82. doi: 10.1080/00071660120121508. [DOI] [PubMed] [Google Scholar]
- 14.Balamurugan R, Chandrasekaran D, Kirubakaran A. Effects of multi-enzyme supplementation on gut morphology and histomorphology in broilers. Indian J Anim Sci. 2011;4(1):15–8. [Google Scholar]
- 15.Ao X, Meng QW, Yan L, Kim YH, Hong SM, Cho JH, et al. Effects of non-starch polysaccharide-degrading enzymes on nutrient digestibility, growth performance and blood profiles of growing pigs fed a diet based on corn and soybean meal. Asian-Aust J Anim Sci. 2010;23(12):1632–8. doi: 10.5713/ajas.2010.10123. [DOI] [Google Scholar]
- 16.Kaput J, Rodriguez RL. Nutritional genomics: the next frontier in the postgenomic era. Physiol Genomics. 2004;16(2):166–77. doi: 10.1152/physiolgenomics.00107.2003. [DOI] [PubMed] [Google Scholar]
- 17.Astle J, Ferguson JT, German JB, Harrigan GG, Kelleher NL, Kodadek T, et al. Characterization of proteomic and metabolomic responses to dietary factors and supplements. J Nutr. 2007;137(12):2787–93. doi: 10.1093/jn/137.12.2787. [DOI] [PubMed] [Google Scholar]
- 18.Zhang JZ, Li DF. Effect of conjugated linoleic acid on inhibition of prolyl hydroxylase 1 in hearts of mice. Lipids Health Dis. 2012;11:22. doi: 10.1186/1476-511X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JJ, Li DF, Dangott LJ, Wu GY. Proteomics and its role in nutrition research. J Nutr. 2006;136(7):1759–62. doi: 10.1093/jn/136.7.1759. [DOI] [PubMed] [Google Scholar]
- 20.Kitteringham NR, Abdullah A, Walsh J, Randle L, Jenkins RE, Sison R, et al. Proteomic analysis of Nrf2 deficient transgenic mice reveals cellular defence and lipid metabolism as primary Nrf2-dependent pathways in the liver. J Proteomics. 2010;73(8):1612–31. doi: 10.1016/j.jprot.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo J, Zheng A, Meng K, Chang W, Bai Y, Li K, et al. Proteome changes in the intestinal mucosa of broiler (Gallus gallus) activated by probiotic Enterococcus faecium. J Proteomics. 2013;91:226–41. doi: 10.1016/j.jprot.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Reichardt F, Habold C, Chaumande B, Ackermann A, Ehret-Sabatier L, Le Maho Y, et al. Interactions between ingested kaolinite and the intestinal mucosa in rat: proteomic and cellular evidences. Fundam Clin Pharmacol. 2009;23(1):69–79. doi: 10.1111/j.1472-8206.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 23.Lowe SE, Theodorou MK, Trinci AP. Cellulase and xylanase of an anaerobic rumen fugus grown on wheat straw, wheat straw holocellulose, cellulose, xylan. Appl Environ Microbiol. 1987;53(6):1216–23. doi: 10.1128/aem.53.6.1216-1223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao Y, Zhou X, Yu JX, Jin YC, Li C, Liu JY, et al. Effects of non-starch polysaccharide enzymes addition on growth performance, carcass traits and meat quality of growing-finishing pigs. Chin J Vet Sci. 2014;34(5):820–4. [Google Scholar]
- 25.Wang X, Yang F, Liu C, Zhou H, Wu G, Qiao S, et al. Dietary supplementation with the probiotic Lactobacillus fermentum I5007 and the antibiotic aureomycin differentially affects the small intestinal proteomes of weanling piglets. J Nutr. 2012;142(1):7–13. doi: 10.3945/jn.111.147074. [DOI] [PubMed] [Google Scholar]
- 26.Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127(3):635–48. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 27.Su L, Cao L, Zhou R, Jiang Z, Xiao K, Kong W, et al. Identification of novel biomarkers for sepsis prognosis via urinary proteomic analysis using iTRAQ labeling and 2D-LC-MS/MS. PLoS One. 2013;8(1):e54237. doi: 10.1371/journal.pone.0054237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hakimov HA, Walters S, Wright TC, Meidinger RG, Verschoor CP, Gadish M, et al. Application of iTRAQ to catalogue the skeletal muscle proteome in pigs and assessment of effects of gender and diet dephytinization. Proteomics. 2009;9(16):4000–16. doi: 10.1002/pmic.200900049. [DOI] [PubMed] [Google Scholar]
- 29.Long B, Yin C, Fan Q, Yan G, Wang Z, Li X, et al. Global liver proteome analysis using iTRAQ reveals AMPK-mTOR-autophagy signaling is altered by intrauterine growth restriction in newborn piglets. J Proteome Res. 2016;15(4):1262–73. doi: 10.1021/acs.jproteome.6b00001. [DOI] [PubMed] [Google Scholar]
- 30.Zhang LZ, Yan WY, Wang ZL, Guo YH, Yi Y, Zhang SW. Differential protein expression analysis following olfactory learning in Apis cerana. J Comp Physiol A. 2015;201(11):1053–61. doi: 10.1007/s00359-015-1042-3. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Li C, Tang X, Lu Q, Sa R, Zhang H. High concentrations of atmospheric ammonia induce alterations in the hepatic proteome of broilers (Gallus gallus): an iTRAQ-based quantitative proteomic analysis. PLoS One. 2015;10(4):e0123596. doi: 10.1371/journal.pone.0123596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiarie E, Romero LF, Nyachoti CM. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr Res Rev. 2013;26(1):71–88. doi: 10.1017/S0954422413000048. [DOI] [PubMed] [Google Scholar]
- 33.Khadem A, Lourenço M, Delezie E, Maertens L, Goderis A, Mombaerts R, et al. Does release of encapsulated nutrients have an important role in the efficacy of xylanase in broilers? Poult Sci. 2016;95(5):1066–76. doi: 10.3382/ps/pew002. [DOI] [PubMed] [Google Scholar]
- 34.Willamil J, Badiola JI, Torrallardona D, Geraert PA, Devillard E. Effect of enzyme supplementation on nutrient digestibility and microbial metabolite concentrations in ileal and caecal digesta of growing pigs. Book of abstracts of 11th International Symposium on Digestive Physiology of Pigs; 2009.
- 35.Zijlstra RT, Owusu-Asiedu A, Simmins PH. Future of NSP-degrading enzymes to improve nutrient utilization of co-products and gut health in pigs. Livest Sci. 2010;134(1–3):255–7. doi: 10.1016/j.livsci.2010.07.017. [DOI] [Google Scholar]
- 36.Bakke-McKellep AM, Penn MH, Salas PM, Refstie S, Sperstad S, Landsverk T, et al. Effects of dietary soyabean meal, inulin and oxytetracycline on intestinal microbiota and epithelial cell stress, apoptosis and proliferation in the teleost Atlantic salmon (Salmo salar L.) Br J Nutr. 2007;97(4):699–713. doi: 10.1017/S0007114507381397. [DOI] [PubMed] [Google Scholar]
- 37.Dehghan-Kooshkghazi M, Mathers JC. Starch digestion, large-bowel fermentation and intestinal mucosal cell proliferation in rats treated with the alpha-glucosidase inhibitor acarbose. Br J Nutr. 2004;91(3):357–65. doi: 10.1079/BJN20031063. [DOI] [PubMed] [Google Scholar]
- 38.Klejnot M, Gabrielsen M, Cameron J, Mleczak A, Talapatra SK, Kozielski F, et al. Analysis of the human cofilin 1 structure reveals conformational changes required for actin binding. Acta Crystallogr D Biol Crystallogr. 2013;69(Pt 9):1780–8. doi: 10.1107/S0907444913014418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakano K, Kanai-Azuma M, Kanai Y, Moriyama K, Yazaki K, Hayashi Y, et al. Cofilin phosphorylation and actin polymerization by NRK/NESK, a member of the germinal center kinase family. Exp Cell Res. 2003;287(2):219–27. doi: 10.1016/S0014-4827(03)00136-8. [DOI] [PubMed] [Google Scholar]
- 40.Insall R, Müller-Taubenberger A, Machesky L, Köhler J, Simmeth E, Atkinson SJ, et al. Dynamics of the Dictyostelium Arp2/3 complex in endocytosis, cytokinesis, and chemotaxis. Cell Motil Cytoskeleton. 2001;50(3):115–28. doi: 10.1002/cm.10005. [DOI] [PubMed] [Google Scholar]
- 41.Hewitt EW. The MHC, class I antigen presentation pathway: strategies for viral immune evasion. Immunology. 2003;110(2):163–9. doi: 10.1046/j.1365-2567.2003.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomatsu S, Orii KO, Vogler C, Nakayama J, Levy B, Grubb JH, et al. Mouse model of N-acetylgalactosamine-6-sulfate sulfatase deficiency (Galns-/-) produced by targeted disruption of the gene defective in Morquio A disease. Hum Mol Genet. 2003;12(24):3349–58. doi: 10.1093/hmg/ddg366. [DOI] [PubMed] [Google Scholar]
- 43.Settembre C, Fraldi A, Medina DL, Ballabio A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. 2013;14(5):283–96. doi: 10.1038/nrm3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448(7152):501–5. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi T, Nishitsuji H, Takamori A, Hasegawa A, Masuda T, Kannagi M. DNA-dependent activator of IFN-regulatory factors enhances the transcription of HIV-1 through NF-κB. Microbes Infect. 2010;12(12–13):937–47. doi: 10.1016/j.micinf.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Levy M, Thaiss CA, Zeevi D, Dohnalová L, Zilberman-Schapira G, Mahdi JA, et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell. 2015;163(6):1428–43. doi: 10.1016/j.cell.2015.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jia M, Jing Y, Ai Q, Jiang R, Wan J, Lin L, et al. Potential role of catalase in mice with lipopolysaccharide/D-galactosamine-induced fulminant liver injury. Hepatol Res. 2014;44(11):1151–8. doi: 10.1111/hepr.12220. [DOI] [PubMed] [Google Scholar]
- 48.Spolarics Z, Wu JX. Role of glutathione and catalase in H2O2 detoxification in LPS-activated hepatic endothelial and Kupffer cells. Am J Physiol. 1997;273(6 Pt 1):G1304–11. doi: 10.1152/ajpgi.1997.273.6.G1304. [DOI] [PubMed] [Google Scholar]
- 49.Lillig CH, Berndt C, Holmgren A. Glutaredoxin systems. Biochim Biophys Acta. 2008;1780(11):1304–17. doi: 10.1016/j.bbagen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Arnér ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267(20):6102–9. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 51.Khadem Ansari MH, Karimipour M, Salami S, Shirpoor A. The effect of ginger (Zingiber officinale) on oxidative stress status in the small intestine of diabetic rats. Int J Endocrinol Metab. 2008;6(3):144–50. [Google Scholar]
- 52.Fattman CL, Schaefer LM, Oury TD. Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med. 2003;35(3):236–56. doi: 10.1016/S0891-5849(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 53.Cashman JR, Zhang J. Human flavin-containing monooxygenases. Annu Rev Pharmacol Toxicol. 2006;46:65–100. doi: 10.1146/annurev.pharmtox.46.120604.141043. [DOI] [PubMed] [Google Scholar]
- 54.Schurig H, Beaucamp N, Ostendorp R, Jaenicke R, Adler E, Knowles JR. Phosphoglycerate kinase and triosephosphate isomerase from the hyperthermophilic bacterium Thermotoga maritima form a covalent bifunctional enzyme complex. EMBO J. 1995;14(3):442–51. doi: 10.1002/j.1460-2075.1995.tb07020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Costa E, Guidotti A. Diazepam binding inhibitor (DBI): a peptide with multiple biological actions. Life Sci. 1991;49(5):325–44. doi: 10.1016/0024-3205(91)90440-M. [DOI] [PubMed] [Google Scholar]
- 56.Mori T, Kondo H, Hase T, Tokimitsu I, Murase T. Dietary fish oil upregulates intestinal lipid metabolism and reduces body weight gain in C57BL/6 J mice. J Nutr. 2007;137(12):2629–34. doi: 10.1093/jn/137.12.2629. [DOI] [PubMed] [Google Scholar]
- 57.Wang F, Kohan AB, Dong HH, Yang Q, Xu M, Huesman S, et al. Overexpression of apolipoprotein C-III decreases secretion of dietary triglyceride into lymph. Physiological Rep. 2014;2(3):e00247. doi: 10.1002/phy2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimizu M. Interaction between food substances and the intestinal epithelium. Biosci Biotechnol Biochem. 2010;74(2):232–41. doi: 10.1271/bbb.90730. [DOI] [PubMed] [Google Scholar]
- 59.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2(4):285–93. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 60.Karbach U. Paracellular calcium transport across the small intestine. J Nutr. 1992;122(Suppl 3):672–7. doi: 10.1093/jn/122.suppl_3.672. [DOI] [PubMed] [Google Scholar]
- 61.Hisada N, Satsu H, Mori A, Totsuka M, Kamei J, Nozawa T, et al. Low-molecular-weight hyaluronan permeates through human intestinal Caco-2 cell monolayers via the paracellular pathway. Biosci Biotechnol Biochem. 2008;72(4):1111–4. doi: 10.1271/bbb.70748. [DOI] [PubMed] [Google Scholar]
- 62.Bao YM, Choct M. Dietary NSP nutrition and intestinal immune system for broiler chickens. World Poultry Sci J. 2010 [Google Scholar]
- 63.Fernández-Fígares I, Wray-Cahen D, Steele NC, Campbell RG, Hall DD, Virtanen E, et al. Effect of dietary betaine on nutrient utilization and partitioning in the young growing feed-restricted pig. J Anim Sci. 2002;80(2):421–8. doi: 10.2527/2002.802421x. [DOI] [PubMed] [Google Scholar]
- 64.Peacock M. Calcium metabolism in health and disease. Clin J Am Soc Nephrol. 2010 doi: 10.2215/CJN.05910809. [DOI] [PubMed] [Google Scholar]
- 65.Potters G. Systems biology of the cell. Nat Educ. 2010;3:33. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Additional files.


