Abstract
We report a case of eumycetoma due to Cladophialophora bantiana in a 3-year-old male Siberian Husky living in France. The dog presented a tumefaction on the thorax and deformity of the second and third subjacent ribs, which were surgically removed. Macroscopic black granules were visible on the ribs, and direct microscopic examination revealed their fungal origin. Cultures yielded pure colonies of C. bantiana. The identification of the causative agent was confirmed after amplification and sequence analysis of fungal internal transcribed spacers 1 and 2 and 5.8S ribosomal DNA regions. Surgery and antifungal treatment with oral itraconazole associated with flucytosine allowed apparent cure after a 10-month follow-up. Envenomation with pine processionary caterpillars (Thaumetopoea pityocampa) and subsequently intensive corticotherapy were considered as possible predisposing factors. This is, to the best of our knowledge, the first case in which C. bantiana is identified as the causative agent of eumycetoma.
CASE REPORT
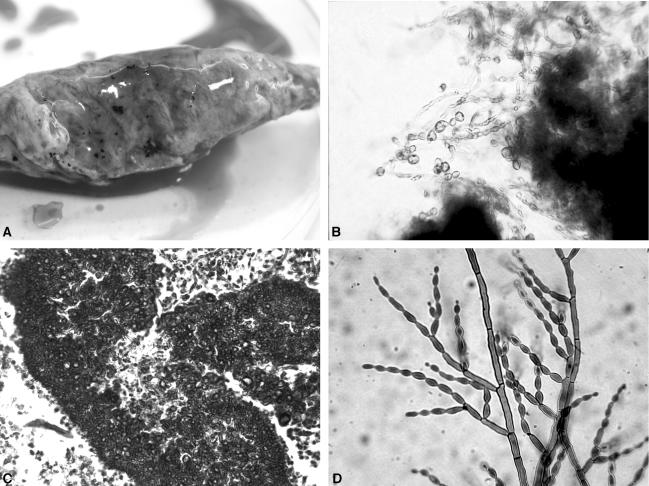
A 3-year-old male Siberian Husky, born in and still living in the South of France (Montpellier), was first examined in December 2002. At physical examination, the dog, which had suffered from lameness at the right foreleg for 5 weeks, presented a fistulated tumefaction of the subcutis on the right side of the thorax. The animal was in otherwise good health. After surgical excision of the cutaneous lesion, the dog was treated with antibiotics (clindamycin) for 3 weeks but mycetoma was not identified at that time, despite the presence of black granules inside the lesion. In May 2003, the tumefaction recurred and deformity of a rib below the tumefaction was detected. As radiographic examination identified lytic lesions, the third right rib was surgically removed in June 2003. Mycological cultures were not performed at that time, but the histological examination identified a eumycetoma. The animal received oral ketoconazole (10 mg/kg of body weight/day) for 6 weeks, but the tumefaction and subsequent lameness recurred in November 2003. Radiographic and tomodensitometric examinations confirmed that the second right rib was affected. The rib, surgically removed in February 2004, contained and was covered by numerous black grains, which were hard in consistency and irregular in size and shape (Fig. 1, top left). Direct microscopic examination of the grains in Amann lactophenol revealed their fungal origin with a dense rim of brown septate hyphae with vesicles, toruloid filaments, and swollen granular thick-walled cells (5 to 11 μm in diameter), sometimes germinating (Fig. 1, top right). Histological examination after hematoxylin and eosin stain confirmed the presence of many fungal black grains (300 to 500 μm in diameter) containing no cement, and the evolution of a chronic osteomyelitis with inflammatory tissue composed of many macrophages and altered polymorphonuclear neutrophils (Fig. 1, bottom left). Grains were washed in sterile water and then seeded directly onto Sabouraud dextrose agar supplemented with chloramphenicol (0.5 g/liter) and incubated at 37°C. A pure filamentous fungus was isolated from all the grains after 5 days of incubation. The colonies were moderately expanding with a velvety aspect and an olivaceous-green color. Microscopic examination revealed dark septate hyphae with sparsely branched conidiophores producing long chains of pale olivaceous lemon-shaped conidia (Fig. 1, bottom right). No chlamydospores were observed, and growth was obtained at 40 and 42°C. According to these macroscopic and microscopic characteristics, the isolate was identified as Cladophialophora bantiana (Sacc.) de Hoog et al. To confirm the specific identification, the isolate was subjected to internal transcribed spacer (ITS) sequencing using primers ITS1 and ITS4 (18). Sequencing reaction was performed in a 10-μl volume containing 50 ng of fungal DNA, 4 pmol of primers, and 4 μl of BigDye mix (Applied Biosystems). The DNA products were analyzed on an ABI Prism genetic analyzer (Applied Biosystems). The sequence was directly compared to other fungal sequences with BLAST. The P-1 and P-2 sequences showed 100% similarity with those of C. bantiana reference strain CBS 173.52 (GenBank accession no. AB091211) isolated from human brain abscess. MICs were determined by a broth microdilution method derived from the National Committee for Clinical Laboratory Standards standards for itraconazole (0.25 μg/ml), voriconazole (0.125 μg/ml), flucytosine (<0.125 μg/ml), caspofungin (2 μg/ml), and terbinafine (0.06 μg/ml). The isolate was considered susceptible to all antifungal drugs tested, except caspofungin. Of note were the low MICs of flucytosine already reported by de Hoog et al. (6).
FIG. 1.
(Top left) One of the two ribs surgically removed. Note the deformity of the bone and the presence of numerous black grains. (Top right) Direct examination of one grain in Amann lactophenol. Brown septate hyphae with vesicles, toruloid filaments, and swollen granular thick-walled and budding cells are visible. (Bottom left) Hematoxylin and eosin stain showing a fungal grain in histological section of the bone. Fungal hyphae growing toward the periphery of the black grain and vesicles are clearly seen. (Bottom right) Microscopic aspect of C. bantiana. Lateral and terminal conidiophores of various sizes. Unicellular long chains of smooth, lemon-shaped conidia are produced.
The dog received oral itraconazole (5 mg/kg/day) for 6 weeks, followed by oral flucytosine (100 mg/kg/day) and itraconazole (5 mg/kg/day) for 4 weeks, after which the medical treatment was stopped. No side effect was reported by the owners. At the last follow-up in March 2004, no evidence of recurrence was observed in the dog.
A great variety of fungal species are able to form granules in vivo but only a few species are regularly reported as causative agents of mycetomas in humans (9). The main fungal agents responsible for black grain mycetomas are Madurella mycetomatis, Magnaporthe grisea, Leptosphaeria senegalensis, and Exophiala jeanselmei (9). To our knowledge, C. bantiana has never been described as an agent of mycetoma in humans or in animals. However, clinical presentations of C. bantiana-related infections may greatly differ according to the host and the route of infection. In immunocompetent humans, C. bantiana is considered as a neurotropic species, which causes cerebral phaeohyphomycosis. The distribution is worldwide, and the fungus is probably introduced by inhalation (13). More rarely, subcutaneous infections are also reported in humans (10). In dogs, C. bantiana-related meningo-encephalitis has rarely been described (3, 14, 15, 16), and in most cases, it was identified on postmortem examination. Clinical presentations look more diverse in domestic cats, as C. bantiana (formerly reported as Cladosporium bantianum and sometimes Cladosporium trichoides) has been isolated from cerebral lesions (4, 12, 17), ulcerated nodules (1), and a systemic infection without cerebral involvement (8). In all of these animal cases, the portal of infection could not be identified with certainty. Interestingly, most of the cases in dogs and cats were reported in males. The same predominance is observed in humans with C. bantiana-related infections (13).
The present isolate of C. bantiana had typical colonial and microscopic aspects, and the molecular tools we used confirmed the specific identification. The isolate was deposited in the Collection of the National Reference Center for Mycoses and Antifungals at the Pasteur Institute of Paris (CNRMA 2004/222).
Eumycetomas principally occur in tropical and subtropical regions, and only a very few cases have been observed in dogs. Two canine cases of eumycetoma were caused by Scedosporium apiospermum (2, 11), one was caused by Madurella mycetomatis (5), and another was caused by Curvularia lunata (7). The organisms have been traumatically implanted into the deep dermis or subcutaneous tissue. In the present case, the infection formed an abscess that first discharged onto the skin surface through a fistula. The abscess did not heal after surgical excision. Recurrence 5 months later may be explained because of an inappropriate or incomplete treatment or because of a reinfection. Chronic evolution of the disease, swelling of soft tissues which became fistulated, and complication of osteomyelitis in subjacent bones observed in that dog are typical clinical signs of eumycetoma. Surprisingly, the owners of the dog did not recall any trauma, puncture, or wound at the site of the infection. Nevertheless, the dog developed an acute dermatitis a few weeks before the onset of lameness due to envenomation after contact with pine processionary caterpillars (Thaumetopoea pityocampa), which are very abundant near Montpellier. A subsequent treatment with high dosages of corticosteroids (methyl-prednisolone) was necessary. The possible traumatic inoculation of the fungus through pine caterpillars and the administration of corticosteroids may be considered as predisposing factors.
Treatment of eumycetomas is sometimes difficult, and a few antifungals are efficient. Amphotericin B, ketoconazole, and itraconazole have been used in human cases with success, but the response rate looks variable (9). In one case of canine eumycetoma caused by Curvularia lunata (7), itraconazole was administrated for 5 consecutive months. However, recurrence of the lesions was observed after interruption of the treatment. Surgery is also a method used to stop the evolution of a fungal mycetoma, but it must be sufficiently radical to prevent the relapses. In the present case, the surgical resection of the two affected ribs and combined therapy with itraconazole and flucytosine gave satisfactory results.
REFERENCES
- 1.Abramo, F., F. Bastelli, S. Nardoni, and F. Mancianti. 2002. Feline cutaneous phaeohyphomycosis due to Cladophialophora bantiana. J. Feline Med. Surg. 4:157-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison, N., R. K. McDonald, S. R. Guist, and J. Bentinck-Smith. 1989. Eumycotic mycetoma caused by Pseudallescheria boydii in a dog. J. Am. Vet. Med. Assoc. 194:797-799. [PubMed] [Google Scholar]
- 3.Anor, S., B. K. Sturges, L. Lafranco, S. S. Jang, R. J. Higgins, P. D. Koblik, and R. A. LeCouteur. 2001. Systemic phaeohyphomycosis (Cladophialophora bantiana) in a dog, clinical diagnosis with stereotactic computed tomographic-guided brain biopsy. J. Vet. Intern. Med. 15:257-261. [DOI] [PubMed] [Google Scholar]
- 4.Bouljihad, M., C. J. Lindeman, and D. W. Hayden. 2002. Pyogranulomatous meningoencephalitis associated with dematiaceous fungal (Cladophialophora bantiana) infection in a domestic cat. J. Vet. Diagn. Investig. 14:70-72. [DOI] [PubMed] [Google Scholar]
- 5.Brodey, R. S., H. F. Schryver, M. J. Deubler, W. Kaplan, and L. Ajello. 1967. Mycetoma in a dog. J. Am. Vet. Med. Assoc. 151:442-451. [PubMed] [Google Scholar]
- 6.de Hoog, G. S., J. Guarro, J. Gene, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 7.Elad, D., U. Orgad, B. Yakobson, S. Perl, P. Golomb, R. Trainin, I. Tsur, S. Shenkler, and A. Bor. 1991. Eumycetoma caused by Curvularia lunata in a dog. Mycopathologia 116:113-118. [DOI] [PubMed] [Google Scholar]
- 8.Eliès, L., V. Balandraud, L. Boulouha, F. Crespeau, and J. Guillot. 2003. Fatal systemic phaeohyphomycosis in a cat due to Cladophialophora bantiana. J. Vet. Med. Assoc. 50:50-53. [DOI] [PubMed] [Google Scholar]
- 9.Hay, R. J. 2000. Agents of eumycotic mycetomas, p. 487-496. In L. Ajello and R. J. Hay (ed.), Topley and Wilson's microbiology and microbial infections, vol. 4. Mycology. Arnold, London, United Kingdom.
- 10.Jacyk, W. K., J. H. Bruyn, N. du Holm, H. Gryffenberg, and V. O. Karusseit. 1997. Cutaneous infection due to Cladophialophora bantiana in a patient receiving immunosuppressive therapy. Br. J. Dermatol. 136:428-430. [PubMed] [Google Scholar]
- 11.Jang, S. S., and J. A. Popp. 1970. Eumycotic mycetoma in a dog caused by Allescheria boydii. J. Am. Vet. Med. Assoc. 157:1071-1076. [PubMed] [Google Scholar]
- 12.Jang, S. S., E. L. Biberstein, M. G. Rinaldi, A. M. Henness, G. A. Boorman, and R. F. Taylor. 1977. Feline brain abscesses due to Cladosporium trichoides. Sabouraudia 15:115-123. [PubMed] [Google Scholar]
- 13.Kwon-Chung, K. J., and J. E. Bennett. 1992. Phaeohyphomycosis, p. 620-677. In C. Cann (ed.), Medical mycology. Lea & Febiger, Malvern, Pa.
- 14.Leisewitz, A. L., C. Rademeyer, and J. Picard. 2002. The use of liposomal amphotericin B in the management of Xylohypha bantiana mycosis in a dog, J. S. Afr. Vet. Assoc. 73:79-82. [DOI] [PubMed] [Google Scholar]
- 15.Lobetti, R. G. 1996. Leukogram and serum globulin values in two dogs with systemic Xylohypha bantiana infection. J. S. Afr. Vet. Assoc. 67:91-92. [PubMed] [Google Scholar]
- 16.Schroeder, H., J. E. Jardine, and V. Davis. 1994. Systemic phaeohyphomycosis caused by Xylohypha bantiana in a dog. J. S. Afr. Vet. Assoc. 65:175-178. [PubMed] [Google Scholar]
- 17.Shinwari, M. W., A. D. Thomas, and J. S. Orr. 1985. Feline cerebral phaeohyphomycosis associated with Cladosporium bantianum. Aust. Vet. J. 62:383-384. [DOI] [PubMed] [Google Scholar]
- 18.White, T. J., T. Burns, S. Lee, and J. Taylor. 1996. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to method and applications. Academic Press, San Diego, Calif.