Abstract
The diagnosis and treatment of multiple myeloma (MM) has changed dramatically in the last decade. The disease definition has been updated to allow highly specific biomarkers in addition to established markers of end-organ damage. The staging system has been revised to combine both measures of tumor burden and disease biology. Advances in therapy have resulted in a significant improvement of overall survival. New drugs introduced in the last few years include carfilzomib, pomalidomide, and panobinostat. In addition, monoclonal antibodies such as elotuzumab and daratumumab have shown promising clinical activity. In this review, we outline the current approach to diagnosis, prognosis, and management of MM.
INTRODUCTION
Multiple myeloma (MM) is a clonal plasma cell malignancy that accounts for approximately 10% of hematologic malignancies.1,2 The annual incidence, age-adjusted to the 2000 United States population, is 4.3 per 100,000, resulting in over 20,000 new patients in the United States each year.3 MM is twice as common in blacks compared to whites, and this racial disparity is related to the higher prevalence of MGUS in blacks.4,5 There is a slight male predominance. The median age of onset is 66 years, and only 2% of patients are less than 40 years of age at diagnosis.6
MM evolves from a pre-malignant condition clinically recognized as monoclonal gammopathy of undetermined significance (MGUS).7 MGUS is present in 3–4% of the general population over the age of 50 years.8,9 Since MGUS is mostly asymptomatic and detected often as an incidental laboratory finding, only 10% of patients with newly diagnosed MM have a history of pre-existing MGUS. However, studies show that MGUS almost always precedes MM, and is associated with a risk of progression to MM of approximately 1% per year.7,10 Smoldering multiple myeloma (SMM) is an intermediate stage between MGUS and MM, and is associated with a higher risk of progression of approximately 10% per year.11
Until 2000, the mainstay of therapy of MM was alkylators and corticosteroids,12 and in selected patients high dose chemotherapy with autologous stem cell transplantation (ASCT).13,14 Subsequently, thalidomide,15 bortezomib,16 and lenalidomide,17 emerged as effective agents and greatly improved clinical outcome.18,19 More recently, carfilzomib, pomalidomide, and panobinostat have been approved in the United States for the treatment of MM, substantially expanding the number of treatment regimens available for patients in all stages of the disease.
DIAGNOSIS
The most common presenting symptoms of MM are fatigue and bone pain.6 Anemia occurs in approximately 75% of patients and contributes to fatigue. Osteolytic skeletal lesions can be detected in approximately 80% of patients. Other common findings at presentation include hypercalcemia (15%), and elevated serum creatinine ≥2 mg/dL (20%).6 Approximately 1 to 2% of patients with MM have extramedullary disease (EMD) at the time of initial diagnosis, while 8% develop EMD later on in the disease course.20
A monoclonal (M) protein in the serum or urine is a cardinal feature of MM, but is seen in only 82% of patients by serum protein electrophoresis.6 The sensitivity increases to 93% when serum immunofixation is added, and to 97% with the addition of either the serum free light chain (FLC) assay or a 24 hour urine studies.21 Thus if MM is suspected, the recommended screening strategy is to order serum protein electrophoresis, serum immunofixation, and either a serum FLC assay or a 24 hour urine protein electrophoresis with immunofixation. The M protein type is IgG in approximately 50%, IgA in 20%, immunoglobulin light chain only in 20%, IgD in 2%, and IgM in 0.5%.6 About 2–3% of MM has no detectable M protein, and is referred to as non-secretory MM.22
The baseline diagnostic work up required for the diagnosis of MM includes complete blood count, serum calcium, serum creatinine, serum and urine protein electrophoresis with immunofixation, serum FLC assay, and bone marrow examination. In addition, low dose whole body computed tomography (CT), or fluoro-deoxyglucose (FDG) positron emission tomography/CT (PET/CT), or at minimum, plain radiographs of the entire skeleton are required to detect osteolytic bone lesions.23 The osteolytic bone lesions in MM exhibit no new bone formation, and nuclear medicine bone scans are therefore not helpful.24 Magnetic resonance imaging (MRI) of the whole body or spine/pelvis is needed in patients with suspected SMM, and whenever the diagnosis of MM is in doubt, to look for focal bone marrow lesions.25 MRI scans are also often needed in patients with osteolytic bone disease involving the spine to rule out cord compression, and to determine need for interventional procedures such as vertebroplasty or kyphoplasty.
DISEASE DEFINITION
In 2014, the International Myeloma Working Group (IMWG) updated the diagnostic criteria for MM and related disorders (Table 1).1 The main revision was to add 3 highly specific biomarkers (clonal bone marrow plasma cells ≥60%, serum FLC ratio ≥100, >1 focal lesion on MRI) to existing markers of end-organ damage (hypercalcemia, renal insufficiency, anemia, or bone lesions) that were used to diagnose the disease. The updated criteria represent a paradigm shift since they allow early diagnosis and initiation of therapy before end-organ damage. As shown on Table 1, the diagnosis of MM requires 10% or more plasma cells on bone marrow examination or a biopsy proven plasmacytoma plus one or more myeloma defining events (MDE). The major differential diagnosis of MM includes MGUS, SMM, immunoglobulin light chain amyloidosis (AL), and solitary plasmacytoma.
Table 1.
International Myeloma Working Group Diagnostic Criteria for Multiple Myeloma and Related Plasma Cell Disorders
| Disorder | Disease Definition |
|---|---|
| Non-IgM monoclonal gammopathy of undetermined significance (MGUS) | All 3 criteria must be met:
|
| Smoldering multiple myeloma | Both criteria must be met:
|
| Multiple Myeloma | Both criteria must be met:
|
| IgM Monoclonal gammopathy of undetermined significance (IgM MGUS) | All 3 criteria must be met:
|
| Light Chain MGUS | All criteria must be met:
|
| Solitary Plasmacytoma | All 4 criteria must be met
|
| Solitary Plasmacytoma with minimal marrow involvement** | All 4 criteria must be met
|
From Lancet Oncol.1
A bone marrow can be deferred in patients with low risk MGUS (IgG type, M protein <15 gm/L, normal free light chain ratio) in whom there are no clinical features concerning for myeloma
Solitary plasmacytoma with 10% or more clonal plasma cells is considered as multiple myeloma
MOLECULAR CLASSIFICATION
Although MM is still considered a single disease, it is in reality a collection of several different cytogenetically distinct plasma cell malignancies (Table 2).26,27 On fluorescent in situ hybridization (FISH) studies of the bone marrow, approximately 40% of MM is characterized by the presence of trisomies in the neoplastic plasma cells (trisomic MM), while most of the rest have a translocation involving the immunoglobulin heavy chain (IgH) locus on chromosome 14q32 (IgH translocated MM).28–31 A small proportion of patients have both trisomies and IgH translocations. Trisomies and IgH translocations are considered primary cytogenetic abnormalities and occur at the time of establishment of MGUS. In addition, other cytogenetic changes termed secondary cytogenetic abnormalities arise along the disease course of MM, including gain(1q), del(1p), del(17p), del(13), RAS mutations, and secondary translocations involving MYC. Both primary and secondary cytogenetic abnormalities can influence disease course, response to therapy, and prognosis (Table 3).27
Table 2.
Primary Molecular Cytogenetic Classification of Multiple Myeloma
| Subtype | Gene(s)/chromosomes affected* | Percentage of myeloma patients |
|---|---|---|
| Trisomic MM | Trisomies of one or more odd-numbered chromosomes | 42 |
| IgH translocated MM | 30 | |
| t(11;14) (q13;q32) | CCND1 (cyclin D1) | 15 |
| t(4;14) (p16;q32) | FGFR-3 and MMSET | 6 |
| t(14;16) (q32;q23) | C-MAF | 4 |
| t(14;20) (q32;q11) | MAFB | <1 |
| Other IgH translocations* | CCND3 (cyclin D3) in t(6;14) MM | 5 |
| Combined IgH translocated/trisomic MM | Trisomies plus any one IgH translocation | 15 |
| Isolated Monosomy 14 | 4.5 | |
| Other cytogenetic abnormalities in absence of IgH translocations or trisomy or monosomy 14 | 5.5 | |
| Normal | 3 |
Adapted from Blood.26
Includes the t(6;14)(p21;q32) translocation, and rarely, other IgH translocations involving uncommon partner chromosomes
Table 3.
Cytogenetic Abnormalities on Clinical Course and Prognosis in Multiple Myeloma
| Cytogenetic Abnormality | Clinical Setting in which Abnormality is Detected | |
|---|---|---|
| Smoldering Multiple Myeloma | Multiple Myeloma | |
| Trisomies | Intermediate-risk of progression, median TTP of 3 years | Good prognosis, standard-risk MM, median OS
7–10 years Most have myeloma bone disease at diagnosis Excellent response to lenalidomide-based therapy |
| t(11;14) (q13;q32) | Standard-risk of progression, median TTP of 5 years | Good prognosis, standard-risk MM, median OS 7–10 years |
| t(6;14) (p21;q32) | Standard-risk of progression, median TTP of 5 years | Good prognosis, standard-risk MM, median OS 7–10 years |
| t(4;14) (p16;q32) | High-risk of progression, median TTP of 2 years | Intermediate-risk MM, median OS 5
years Needs bortezomib-based initial therapy, early ASCT (if eligible), followed by bortezomib-based consolidation/maintenance |
| t(14;16) (q32;q23) | Standard-risk of progression, median TTP of 5 years | High-risk MM, median OS 3
years Associated with high levels of FLC and 25% present with acute renal failure as initial MDE |
| t(14;20) (q32;q11) | Standard-risk of progression, median TTP of 5 years | High-risk MM, median OS 3 years |
| Gain(1q21) | High-risk of progression, median TTP of 2 years | Intermediate-risk MM, median OS 5 years |
| Del(17p) | High-risk of progression, median TTP of 2 years | High-risk MM, median OS 3 years |
| Trisomies plus any one of the IgH translocations | Standard-risk of progression, median TTP of 5 years | May ameliorate adverse prognosis conferred by high risk IgH translocations, and del 17p |
| Isolated Monosomy 13, or Isolated Monosomy 14 | Standard-risk of progression, median TTP of 5 years | Effect on prognosis is not clear |
| Normal | Low-risk of progression, median TTP of 7–10 years | Good prognosis, probably reflecting low tumor burden, median OS >7–10 years |
FISH, fluorescent in situ hybridization; TTP, time to progression; OS, overall survival; SMM, Smoldering multiple myeloma, MM, multiple myeloma; ASCT, autologous stem cell transplantation. From Blood Cancer J.27
PROGNOSIS AND RISK STRATIFICATION
Although median survival is approximately 5–7 years, there is major variation in survival depending on host factors, tumor burden (stage), biology (cytogenetic abnormalities), and response to therapy.32 Tumor burden in MM has traditionally been assessed using the Durie-Salmon Staging (DSS)33 and the International Staging System (ISS).34,35 Disease biology best reflected based on the molecular subtype of MM, and the presence or absence of secondary cytogenetic abnormalities (Table 4).26,36 The Revised International Staging System (RISS) combines elements of tumor burden (ISS) and disease biology (presence of high risk cytogenetic abnormalities or elevated lactate dehydrogenase level) to create a unified prognostic index that and helps in clinical care as well as in comparison of clinical trial data (Table 5).37
Table 4.
Mayo Clinic Risk Stratification for Multiple Myeloma (mSMART)
| Risk Group | Percentage of newly diagnosed patients with the abnormality |
|---|---|
|
| |
| Standard Risk | 75% |
| Trisomies | |
| t(11;14) | |
| t(6;14) | |
| Intermediate Risk | 10% |
| t(4;14) | |
| Gain(1q) | |
| High Risk | 15% |
| t(14:16) | |
| t(14;20) | |
| del(17p) | |
Adapted from Am J Hematol.2
Table 5.
Revised International Staging System for Myeloma
| Stage | Frequency (% of patients) | 5-year survival rate (%) |
|---|---|---|
Stage 1
|
28% | 82 |
Stage II
|
62% | 62 |
Stage III
|
10% | 40 |
From J Clin Oncol.37
It is important to note that to ensure uniform availability only 3 widely available cytogenetic markers are used in the RISS; the Mayo Clinic mSMART risk stratification (www.msmart.org) (Table 4) has additional detail that is valuable in formulating a therapeutic strategy.38 Ideally to complete accurate molecular classification and risk stratification, we recommend FISH probes to detect trisomies, IgH translocations, MYC translocations, and abnormalities of chromosomes 1, 13, and 17.27
TREATMENT
The approach to treatment of newly diagnosed MM is outlined in Figure 1. The most important phases of therapy are initial therapy, stem cell transplantation (if eligible), consolidation/maintenance therapy, and treatment of relapse. Transplant eligible patients typically receive approximately 4 cycles of initial therapy followed by stem cell collection and autologous stem cell transplantation (ASCT). Selected patients with standard risk MM who respond well to induction can opt for delayed ASCT; in this strategy stem cells are collected after 4 cycles of initial therapy and cryopreserved for future use (Figure 1). Transplant ineligible patients are usually treated for 12–18 months. Following initial therapy and/or ASCT, consideration should be given to consolidation/maintenance therapy. The choice of maintenance and duration of therapy is often driven by the presence or absence of high risk cytogenetic features.
Figure 1.
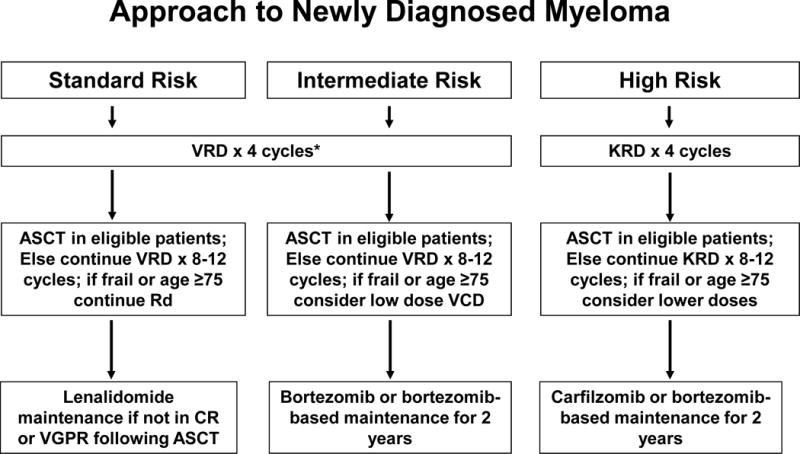
Approach to the treatment of newly diagnosed multiple myeloma
Abbreviations: VRD, bortezomib, lenalidomide, dexamethasone; KRD, carfilzomib, lenalidomide, dexamethasone; Rd, lenalidomide plus dexamethasone; VCD, bortezomib, cyclophosphamide, dexamethasone; ASCT, autologous stem cell transplantation; CR, complete response; VGPR, very good partial response.
Table 6 and 7 list the major drugs used in the treatment of MM. The most common treatment regimens used in MM are listed in Table 8.39–56 Results of recent randomized trials using new active agents for MM are provided in Table 9.57–60
Table 6.
Selected Drugs with significant single-agent activity in multiple myeloma
| Agent | Usual Starting Dose | Postulated Mechanism of Action | Side-effects |
|---|---|---|---|
| Thalidomide | 50–200 mg orally days 1–28 every 4 weeks | Binds to cereblon and activates cereblon E3 ligase activity, resulting in the rapid ubiquitination and degradation of two specific B cell transcription factors, Ikaros family zinc finger proteins Ikaros (IKZF 1) and Aiolos (IKZF3); anti-angiogenesis, immunomodulation, and inhibition of tumor necrosis factor alpha. Direct cytotoxicity by inducing free radical mediated DNA damage. | Sedation, fatigue, skin rash, bradycardia, peripheral neuropathy, and constipation. Deep vein thrombosis is a serious adverse event necessitating routine prophylaxis with aspirin or other anticoagulant in all patients. Teratogen. |
| Bortezomib | 1.3mg/m2 subcutaneously days 1, 8, 15, 22 every 28 days | Inhibits the ubiquitin-proteasome catalytic pathway in cells by binding directly with the 20S proteasome complex. | Gastrointestinal, transient cytopenias, fatigue, and peripheral neuropathy. |
| Lenalidomide | 25 mg orally days 1–21 every 28 days | Cereblon mediated ubiquitination and degradation of Ikaros (IKZF 1) and Aiolos (IKZF3); anti-angiogenesis, immunomodulation, and inhibition of tumor necrosis factor alpha. Direct cytotoxicity by inducing free radical mediated DNA damage. | Fatigue, rash, thrombocytopenia, and neutropenia. Deep vein thrombosis is a serious adverse event necessitating routine prophylaxis with aspirin or other anticoagulant in all patients. Diarrhea and leg cramps with long-term use. Teratogen. |
| Pomalidomide | 4 mg orally days 1–21 every 28 days | Same as thalidomide and lenalidomide | Fatigue, rash, thrombocytopenia, and neutropenia. Deep vein thrombosis is a serious adverse event necessitating routine prophylaxis with aspirin or other anticoagulant in all patients. Teratogen. |
| Carfilzomib | 27 mg/m2 intravenously days 1,2, 8, 9, 15, 16 every 28 days | Proteasome inhibitor | Gastrointestinal, hypokalemia, hypertension, dyspnea. Approximately 5% can get serious cardiac dysfunction |
| Daratumumab | 16 mg/kg intravenously weekly × 8 weeks, every 2 weeks × 16 weeks, then once monthly | Monoclonal antibody targeting CD38 | Infusion related reactions, fatigue, anemia, nausea |
Table 7.
Selected Drugs with activity in combination with other active agents in multiple myeloma
| Agent | Usual Starting Dose | Postulated Mechanism of Action | Side-effects |
|---|---|---|---|
| Elotuzumab | 10 mg/kg intravenously weekly × 8 weeks, and then every 2 weeks | Immunostimulatory monoclonal antibody targeting signaling lymphocytic activation molecule F7 (SLAMF7) | Infusion related reactions, fatigue, infections |
| Panobinostat | 20 mg orally twice weekly 3 weeks on, one week off | Pan-deacetylase inhibitor; blocks aggresome pathway | Diarrhea, thrombocytopenia, fatigue |
Table 8.
Major Treatment Regimens in Multiple Myeloma
| Regimen | Usual Dosing Schedule* |
|---|---|
| Melphalan-Prednisone (MP) (7-day schedule)39 | Melphalan 8–10 mg oral days
1–7 Prednisone 60 mg/day oral days 1–7 Repeated every 6 weeks |
| Thalidomide-Dexamethasone (TD)**40,41 | Thalidomide 200 mg oral days
1–28 Dexamethasone 40 mg oral days 1, 8, 15, 22 Repeated every 4 weeks |
| Lenalidomide-Dexamethasone (Rd)42 | Lenalidomide 25 mg oral days 1–21
every 28 days Dexamethasone 40 mg oral days 1, 8, 15, 22 every 28 days Repeated every 4 weeks |
| Bortezomib-Dex (VD)**43 | Bortezomib 1.3 mg/m2 intravenous
days 1, 8, 15, 22 Dexamethasone 20 mg on day of and day after bortezomib (or 40 mg days 1, 8, 15, 22) Repeated every 4 weeks |
| Melphalan-Prednisone-Thalidomide (MPT)44,45 | Melphalan 0.25 mg/kg oral days 1–4
(use 0.20 mg/kg/day oral days 1–4 in patients over the age of
75) Prednisone 2 mg/kg oral days 1–4 Thalidomide 100–200 mg oral days 1–28 (use 100 mg dose in patients >75) Repeated every 6 weeks |
| Bortezomib-Melphalan-Prednisone (VMP)**46–48 | Bortezomib 1.3 mg/m2 intravenous
days 1, 8, 15, 22 Melphalan 9 mg/m2 oral days 1–4 Prednisone 60 mg/m2 oral days 1 to 4 Repeated every 35 days |
| Bortezomib-Thalidomide-Dexamethasone (VTD)**49 | Bortezomib 1.3 mg/m2 intravenous
days 1, 8, 15, 22 Thalidomide 100–200 mg oral days 1–21 Dexamethasone 20 mg on day of and day after bortezomib (or 40 mg days 1, 8, 15, 22) Repeated every 4 weeks × 4 cycles as pre-transplant induction therapy |
| Bortezomib-Cyclophosphamide-Dexamethasone** (VCD or CyBorD)50,52 | Cyclophosphamide 300 mg/m2 orally
on days 1, 8, 15 and 22 Bortezomib 1.3 mg/m2 intravenously on days 1, 8, 15, 22 Dexamethasone 40 mg orally on days on days 1, 8, 15, 22 Repeated every 4 weeks† |
| Bortezomib-Lenalidomide-Dexamethasone (VRD)**51,52 | Bortezomib 1.3 mg/m2 intravenous
days 1, 8, 15 Lenalidomide 25 mg oral days 1–14 Dexamethasone 20 mg on day of and day after bortezomib (or 40 mg days 1, 8, 15, 22) Repeated every 3 weeks‡ |
| Carfilzomib53 | Carfilzomib 20 mg/m2 (Cycle 1) and
27 mg/m2 (subsequent cycles) intravenously on days 1, 2, 8,
9, 15, 16 Repeated every 4 weeks† |
| Carfilzomib-Cyclophosphamide-Dexamethasone (CCyD) ‡‡54 | Carfilzomib 20 mg/m2 (Cycle 1) and
36 mg/m2 (subsequent cycles) intravenously on days 1, 2, 8,
9, 15, 16 Cyclophosphamide 300 mg/m2 orally on days 1, 8, 15 Dexamethasone 40 mg orally on days on days 1, 8, 15 Repeated every 4 weeks† |
| Carfilzomib-Lenalidomide-Dexamethasone (KRD)55 | Carfilzomib 27 mg/m2 intravenously
on days 1, 2, 8, 9, 15, 16 (Note: Cycle 1, day 1 and 2 carfilzomib dose
is 20 mg/m2) Lenalidomide 25 mg oral days 1–21 Dexamethasone 20 mg on day of and day after bortezomib (or 40 mg days 1, 8, 15, 22) Repeated every 4 weeks |
| Pomalidomide-Dexamethasone (Pom/Dex)56 | Pomalidomide 4 mg days
1–21 Dexamethasone 40 mg orally on days on days 1, 8, 15, 22 Repeated every 4 weeks |
| Carfilzomib-Pomalidomide-Dexamethasone129 | Carfilzomib 27 mg/m2 intravenously
on days 1, 2, 8, 9, 15, 16 (Note: Cycle 1, day 1 and 2 carfilzomib dose
is 20 mg/m2) Pomalidomide 4 mg oral days 1–21 Dexamethasone 40 mg days 1, 8, 15, 22 Repeated every 4 weeks |
All doses need to be adjusted for performance status, renal function, blood counts, and other toxicities
Doses of dexamethasone and/or bortezomib reduced based on subsequent data showing lower toxicity and similar efficacy with reduced doses.
The day 22 dose of all 3 drugs is omitted if counts are low, or after initial response to improve tolerability, or when the regimen is used as maintenance therapy; When used as maintenance therapy for high risk patients, further delays can be instituted between cycles.
Omit day 15 dose if counts are low or when the regimen is used as maintenance therapy; When used as maintenance therapy for high risk patients, lenalidomide dose may be decreased to 10–15 mg per day, and delays can be instituted between cycles as done in total therapy protocols.130,131
Dosing based on trial in newly diagnosed patients; in relapsed patients cycle 2 Carfilzomib dose is 27 mg/m2 consistent with approval summary. Adapted from Am J Hematol.2
Table 9.
Results of Recent Randomized Studies in Newly Diagnosed Myeloma
| Trial | Regimen | No. of patients | Overall response rate (%) | CR plus VGPR (%) | Progression-free survival (Median in months) | P value for progression free survival | Overall survival | P value for overall survival |
|---|---|---|---|---|---|---|---|---|
| Facon57 | MPT | 547 | 62 | 28 | 21.2 | <0.001 | 48 months (median) | 0.016† |
| Rd × 18 months | 541 | 73 | 43 | 20.7 | 53 months (median) | |||
| Rd till progression | 535 | 75 | 44 | 25.5 | 56 months (median) | |||
| Durie et al | Rd | |||||||
| VRd | ||||||||
| Moreau et al | VTD | N/A | N/A | |||||
| VCD | N/A | |||||||
| Attal et al | VRD-ASCT | 88% at 3 years | NS | |||||
| VRD | 88% at 3 years |
Rd until progression versus MPT
Abbreviations: MPT, melphalan plus prednisone plus thalidomide; Rd, lenalidomide plus dexamethasone; VTD, bortezomib, thalidomide, dexamethasone; VCD, bortezomib, cyclophosphamide, dexamethasone; VRD, bortezomib, lenalidomide, dexamethasone; ASCT, autologous stem cell transplantation; N/A, not available; NS, not significant; CR, complete response; VGPR, very good partial response.
Initial Therapy
Initial therapy for MM varies across countries depending on drug availability. The most common regimens used in the treatment of newly diagnosed MM are lenalidomide plus dexamethasone (Rd), bortezomib, lenalidomide, dexamethasone (VRD), bortezomib, thalidomide, dexamethasone (VTD), and bortezomib, cyclophosphamide, dexamethasone (VCD). In a recent randomized trial conducted by the Southwest Oncology Group (SWOG), progression free survival (PFS) and overall survival (OS) were significantly superior with VRD compared with Rd (Table 9).58 Other studies have shown superior response rates and PFS with VTD compared with other doublet regimens.49,61 A recent randomized trial also found that the triplet regimen of VTD which contains a proteasome inhibitor (bortezomib) and an immunomodulatory agent (thalidomide) is superior to VCD (Table 9).59 Based on these data VRD or VTD are the preferred regimens for initial therapy in transplant eligible patients, and in fit transplant ineligible patients (Figure 1).
The low-dose dexamethasone regimen (40 mg once a week) is preferred in all regimens (Rd, VRD, VTD, VCD, etc) to minimize toxicity. In a randomized trial conducted by the Eastern Cooperative Oncology Group (ECOG), the low-dose dexamethasone approach was associated with superior OS and significantly lower toxicity.42 Similarly, the once-weekly subcutaneous schedule of bortezomib is preferred in all regimens. Studies show that the neurotoxicity of bortezomib can be greatly diminished by administering bortezomib once a week instead of twice-weekly,47,48 and by administering the drug subcutaneously instead of the intravenous route.62 The regimens listed on Table 8 reflect these recommendations to lower the dose of dexamethasone and bortezomib from what was used in many of the initial trials. Higher doses of dexamethasone, and twice-weekly bortezomib can be considered if a rapid response is desired as in the case of patients with acute renal failure due to cast nephropathy, extensive extramedullary disease, plasma cell leukemia, or impending cord compression.2
Frail, Elderly Patients
Patients who are ≥75 years of age or are frail, may not tolerate a triplet regimen.63 In these patients, Rd is a reasonable choice for initial therapy, especially for standard risk patients. In a large randomized trial Rd was found to be superior to melphalan, prednisone, thalidomide (MPT).(Table 9)64 The use of melphalan-containing regimens such as MPT and bortezomib, melphalan, prednisone (VMP) has decreased considerably, and they are recommended only if other regimens are not available. If Rd is chosen, data indicate that it needs to be administered until progression.64 This may not be feasible in many countries or in patients with limited insurance or financial means. In these circumstances a limited duration (12–18 months) of a triplet such as VCD can be a reasonable option; in our opinion, VCD is a better tolerated, more predictable alternative to VMP.
High-risk myeloma
The triplet regimen of carfilzomib, lenalidomide, dexamethasone (KRd) has shown high activity in phase II trials, with stringent complete response rates (sCR) and minimal residual disease (MRD) negative rates that appear superior to historical results with VRD.65 However, these are non-randomized comparisons, and there are concerns about cardiac toxicity in a small proportion of patients with carfilzomib. Further, KRD is more cumbersome and expensive compared with VRD. Thus, we recommend the use of KRD at this point only to patients with high risk MM where it may be reasonable to administer a regimen with the highest possible CR rates, and based on data from a relapsed MM trial that suggests a possible advantage of carfilzomib over bortezomib.66
Acute renal failure due to cast nephropathy
The diagnosis of light chain cast nephropathy can be made presumptively if the circulating FLC levels are high in the presence of MM and acute renal failure.1 However, a renal biopsy is required if serum FLC levels are below 500 mg/L. Patients presenting with acute renal failure due to light chain cast nephropathy need urgent treatment to lower circulating FLC levels.67 We recommend a triplet regimen that does not require major dose adjustment such as VCD or VTD.68 The role of plasmapheresis to remove circulating light chains is controversial, and randomized trials indicate a lack of benefit.69 However, the trials so far have had some limitations, and the risk of the intervention is minimal compared with the major impact on prognosis that occurs if renal dysfunction is not reversed.70 Therefore, we recommend plasmapheresis or dialysis using high cut-off filters to rapidly reduce FLCs. Close monitoring of serum FLC levels and creatinine are needed for the first few weeks.
Autologous Stem Cell Transplantation
ASCT improves complete response rates and prolongs median overall survival in MM by approximately 12 months.13,14,71,72 The treatment-related mortality (TRM) rate is 1–2%, and the procedure can be performed entirely as an outpatient in more than 50% of patients.73 Eligibility for ASCT is based on age, performance status, and comorbidities. In the United States the upper age limit is flexible, and patients can be transplanted up to age 75 if they are in good functional status with minimal comorbidities. In contrast, in many other countries, the upper limit for ASCT is 65 years of age. The preferred conditioning regimen is melphalan, 200 mg/m2.74 Studies are ongoing to determine if the conditioning regimen can be improved with the addition of bortezomib or carfilzomib.
Timing of ASCT
Four randomized trials show that survival is similar whether ASCT is done early (immediately following 4 cycles of induction therapy) or delayed (at the time of relapse as salvage therapy).60,75–77 A more recent trial by the Intergroupe Francophone du Myelome (IFM) and the Dana-Farber Cancer Institute (DFCI) compared early versus delayed ASCT in patients treated with VRD followed by lenalidomide maintenance.60 Patients were randomized to receive either VRD (3 cycles) followed by ASCT and then VRD consolidation (2 cycles) versus VRD × 8 cycles with ASCT reserved for relapse. Both arms received lenalidomide maintenance for one year. A significant improvement in PFS was seen as expected with early ASCT, but this has so far not translated into a difference in OS (Table 9). Importantly the trial found that the 3 year OS in both arms was very high which reflects the remarkable improvement that has occurred in MM therapy over the last decade. The trial also demonstrated that patients achieving an MRD negative state had superior OS compared to those who remained MRD positive. Care should be taken in interpretation of these data; they confirm the value of MRD negative state as a prognostic marker, but randomized trials are needed to determine if MRD negativity should be a goal of therapy and if therapy should be altered for patients based on MRD status.
As discussed above there are no data so far that early ASCT prolongs OS compared with delayed ASCT. However, given the inconvenience and the impact on quality of life with prolonged chemotherapy, insurance, and other issues we favor early ASCT if patients do not have a strong preference regarding the timing. We also prefer early ASCT in patients with intermediate and high risk MM based on studies that show that patients with t(4;14) and del(17p) have achieved outcomes closer to standard risk patients in trials that have incorporated early ASCT.78 Delayed ASCT is reasonable in patients with standard risk MM who respond and tolerate initial therapy well and who seek to delay the procedure due to personal preference.
Tandem transplantation
With tandem (double) ASCT, patients receive a second planned ASCT after recovery from the first procedure.79,80 The IFM 94 randomized trial found significantly better event-free and overall survival in recipients of double versus single ASCT.81 A similar benefit was also demonstrated in a randomized trial conducted in Italy.82 These trials were done prior to the arrival of lenalidomide, bortezomib, and other new agents. In both trials, the benefit of a second ASCT was restricted to patients failing to achieve a complete response or very good partial response (>90% reduction in M protein level) with the first transplant. With modern induction regimens and ASCT the vast majority of patients are in VGPR or better status following the first ASCT limiting the role of tandem ASCT. Further, two other randomized trials have not found a significant improvement in OS with tandem ASCT.83,84 The Bone Marrow Transplant Clinical Trials Network (BMT-CTN) 0702 trial will clarify the role of tandem ASCT in patients receiving VRD initial therapy and lenalidomide maintenance. Until these results are available, we typically collect enough stem cells for two transplants in all eligible patients less than 65 years of age. However, rather than performing tandem ASCT, the purpose of collecting additional stem cells is to preserve the possibility of a second ASCT at the time of relapse.
Allogeneic Transplantation
The high TRM and morbidity related to graft versus host disease (GVHD) has made conventional allogeneic transplants unacceptable for most patients with MM. Data from randomized trials regarding the benefit of allogeneic ASCT are conflicting.85,86 Even with a tandem approach of ASCT followed by a HLA identical sibling donor mini-allogeneic transplantation the TRM is high at approximately 10–15%. Given excellent outcomes with current therapy, allogeneic transplantation has a limited role in MM. We recommend it primarily in young patients with high risk MM in first or second relapse who are willing to accept a high TRM and GVHD related morbidity in return for a small chance at long-term OS.
Consolidation/Maintenance Therapy
Numerous trials have been conducted over the years testing maintenance therapy in MM, either following ASCT or following 12–18 months of standard-dose therapy. However, the agents used were either ineffective, toxic, or both, and none of these approaches gained ground in clinical practice. Thalidomide has shown modest PFS and OS benefit as maintenance therapy in two randomized trials, but has drawbacks of significant non-hematologic toxicity.87,88
Post-transplant maintenance
In the post-ASCT setting, maintenance therapy with lenalidomide, and with bortezomib, has shown promise. Two randomized trials have shown better PFS with lenalidomide as post ASCT maintenance therapy, and in one of these trials an OS benefit has also been observed.89,90 The OS benefit is primarily in patients who received lenalidomide as part of initial therapy before ASCT. One concern in the interpretation of these data is that patients in the control arm of these trials lacked uniform access to lenalidomide at relapse, and it is not clear whether the PFS improvement will be neutralized since patients in the control arm can always initiate the same therapy at the time of first relapse.91,92 There is also a clear increased risk of second cancers with lenalidomide maintenance in both trials. The pros and cons of lenalidomide maintenance need to be considered carefully. We recommend lenalidomide maintenance in standard risk patients who did well with lenalidomide-containing initial therapy and fail to achieve a VGPR following ASCT.38
In patients with intermediate and high risk MM we prefer bortezomib-based maintenance. In a randomized trial, patients receiving two years of bortezomib given every other week as post-transplant maintenance had superior outcomes compared with thalidomide maintenance.78 In high risk patients empiric use of a triplet regimen such as VRD as post-transplant therapy may be reasonable.93 Randomized trials with the new proteasome inhibitor ixazomib are ongoing; ixazomib is administered once-weekly orally and is hence ideally suited to the maintenance setting.
Maintenance post-standard dose therapy
The role of maintenance therapy after an initial 12–18 months of treatment for newly diagnosed MM in patients not receiving an ASCT is evolving. There are data that continuous therapy with Rd is superior in terms of PFS to Rd given for 18 months.64 But it is not clear whether this benefit will be seen following 18 months of a triplet such as VRD. In one randomized trial, melphalan, prednisone, lenalidomide (MPR) followed by lenalidomide maintenance had superior PFS compared with MPR alone.94 However in this trial the MPR arm was identical in terms of PFS to melphalan plus prednisone (MP), and no OS differences were seen limiting more definitive conclusions concerning the value of maintenance therapy. If Rd is used as initial therapy, we recommend continuing it until progression. If a triplet regimen is used, we recommend stopping therapy after 12–18 months in patients with standard risk disease, and continuing with bortezomib maintenance in intermediate and high risk disease. Randomized trials with a new oral proteasome inhibitor ixazomib are ongoing in this setting as well.
Treatment of Relapsed Multiple Myeloma
The approach to treatment of relapsed MM is complicated. Numerous effective regimens are available, and the choice of treatment depends on numerous factors such as drug availability, response to prior therapy, aggressiveness of the relapse, eligibility for ASCT, and whether the relapse occurred on or off therapy. In eligible patients, ASCT should be included in the consideration if the patient has never had an ASCT, or if the remission duration with a prior ASCT exceeds 18 months (unmaintained) or 36 months (with maintenance).95 Recent data support the use of a triplet for relapsed MM, but selected patients with indolent relapse can often be treated with a doublet such as Rd, or pomalidomide plus low dose dexamethasone (Pd). MM is characterized by relapses and remissions, with each remission typically lasting less than the previous one.96 In the absence of toxicity, most regimens are continued until progression in the relapsed setting. However, in some regimens such as those employing bortezomib, carfilzomib, or alkylators it may be reasonable to stop therapy with these drugs once a stable plateau has been reached in order to minimize risks of serious toxicity.
New agents approved for the treatment of relapsed MM include carfilzomib, pomalidomide, and panobinostat. The most common regimens and new drugs used in the treatment of relapsed refractory MM are discussed below.
Bortezomib and lenalidomide based regimens
Rd is an effective regimen in relapsed MM, although the dose of dexamethasone must be reduced from the schedules used in the original pivotal trials.97,98 Triplet regimens such as VRD, VCD, and VTD can also be used in the relapsed refractory setting, and are well tolerated when low dose dexamethasone and weekly subcutaneous bortezomib schedules are used.99–101
Carfilzomib and pomalidomide based regimens
Carfilzomib is a keto-epoxide tetrapeptide proteasome inhibitor approved for the treatment of relapsed refractory MM in patients who have been previously treated with lenalidomide and bortezomib.53 In a phase III trial of 792 patients, carfilzomib, lenalidomide, dexamethasone (KRd) was associated with better response rates, PFS, and OS compared with Rd.102 PFS was 26.3 months with KRD versus 17.6 months in the control group; P=0.0001. The 2-year survival rates were 73.3% and 65.0%, respectively, P=0.04. Based on these results, KRd is now an important option for the treatment of relapsed MM. There is debate about whether KRd (or similar carfilzomib based regimen) should be used ahead of bortezomib-based regimens in relapsed MM. Support for carfilzomib as a more potent proteasome inhibitor than bortezomib comes from a randomized trial in which carfilzomib/dexamethasone demonstrated a doubling of PFS compared with bortezomib/dexamethasone in relapsed MM; PFS 18.7 months versus 9.4 months, respectively, P<0.001.66 However, the dose of carfilzomib used in this trial (56mg/m2) was twice the approved dose, and carries a much higher cost compared with bortezomib. Further the dosing of bortezomib used in this trial was suboptimal (twice-weekly schedule) making it difficult to make definitive conclusions. Carfilzomib does have lower risk of neurotoxicity than bortezomib, but a small proportion (5%) of patients may experience serious cardiac side effects.
Pomalidomide is an analog of lenalidomide and thalidomide approved for the treatment of relapsed refractory MM. It has significant activity in relapsed refractory MM, even in patients failing lenalidomide,103,104 or lenalidomide and bortezomib.56,105 In a randomized trial of 302 patients with refractory MM, Pd was found superior to high-dose dexamethasone, median PFS 4.0 months versus 1.9 months, respectively, P<0.0001).106 As with Rd, the doublet regimen of Pd is a reasonable option for patients with indolent relapse. But more often, pomalidomide needs to be administered in combinations such as pomalidomide, cyclophosphamide, prednisone (PCP), pomalidomide, bortezomib, dexamethasone (PVD), or carfilzomib, pomalidomide, dexamethasone (car/pom/dex).
Panobinostat
Panobinostat is a pan-deacetylase inhibitor approved in 2015 for the treatment of relapsed and refractory MM.107 It is the first agent from a new class of drugs with meaningful clinical activity in MM in nearly 15 years. Its putative mechanism of action is to block the aggresome pathway, an alternative route for cells to bypass the lethal effects of proteasome inhibition. By combining bortezomib and panobinostat, there is simultaneous blockade of both proteasome and aggresome pathways.108,109 In a randomized trial of 768 patients, bortezomib/dexamethasone plus panobinostat was associated with superior PFS compared with bortezomib/dexamethasone plus placebo; median PFS 12 months versus 8·1months, respectively, P<0.0001).107 However, panobinostat therapy was associated with grade 3 diarrhea in approximately 25% of patients, and care should be exercised when using this drug. We recommend a lower dose than the approved starting dose. We also recommend that bortezomib be used in the once-weekly subcutaneous schedule rather than the twice weekly regimen used in the pivotal trial.47,48,62
Liposomal Doxorubicin
Anthracyclines have marginal single-agent activity in MM. A phase III randomized trial found that median time to progression (TTP) was superior with bortezomib plus pegylated liposomal doxorubicin compared with bortezomib alone, 9.3 months versus 6.5 months, respectively, P<0.001.110 OS at 15 months was also superior, 76% compared with 65%, respectively, P = 0.03. Despite this study, liposomal doxorubicin is infrequently used in the treatment of relapsed MM given availability of other active agents.
Monoclonal antibodies
Two monoclonal antibodies (daratumumab and SAR650984) targeting CD38 have shown promise in relapsed, refractory MM. In a phase II trial, daratumumab as a single-agent was produced a response rate of approximately 30% in heavily pre-treated patients.111 These are very encouraging results and we are optimistic that daratumumab will be approved for use in relapsed refractory MM based on these data.
Elotuzumab, a monoclonal antibody targeting the signaling lymphocytic activation molecule F7 (SLAMF7), has also shown activity in relapsed MM.112 Unlike anti-CD38 antibodies, elotuzumab does not appear to have any single-agent activity. However, it appears to have synergistic activity when combined with Rd. In a phase III trial of 646 patients, elotuzumab plus Rd was superior to Rd in terms of PFS, median PFS 19.4 months versus 14.9 months, respectively, P<0.001. Elotuzumab is also well tolerated, and is expected to gain approval within the next few months.
Ixazomib
Ixazomib is an oral proteasome inhibitor that is active in both the relapsed refractory setting and in newly diagnosed MM. In a randomized controlled trial in relapsed MM, ixazomib, lenalidomide, dexamethasone (IRd) was found to improve PFS compared with Rd.113 Based on these results it is anticipated that ixazomib will secure regulatory approval soon. It has the advantage of once-weekly oral administration. Compared with bortezomib it has more gastrointestinal adverse events, but lower risk of neurotoxicity.
Other Emerging Options
Other promising agents include marizomib, a new proteasome inhibitor, oprozmib, an oral proteasome inhibitor related to carfilzomib; filanesib, a kinesin spindle protein inhibitor; dinaciclib, a cyclin dependent kinase inhibitor; ABT-199, a selective BCL-2 inhibitor, and LGH-447, pan PIM kinase inhibitor. Each of these has shown single agent activity in relapsed MM.
Supportive Care
Hypercalcemia
The mainstay of therapy is hydration, corticosteroids, and bisphosphonates (pamidronate or zoledronic acid). Pamidronate 60–90 mg intravenously over 2–4 hours, or zoledronic acid (Zometa) 4 mg intravenously over 15 minutes will normalize the calcium levels within 24–72 hours in most patients.114,115 In refractory patients, salmon calcitonin can be used.
Skeletal Lesions
The most important element is the use of bisphosphonates to prevent or reduce the number of skeletal lesions.116–118 Zoledronic acid or pamidronate once-monthly at least for the first 1–2 years is recommended for almost all patients with MM who have evidence of MM bone disease.117,119 Data from a randomized trial that in such patients, there is also a favorable effect on OS.120
In patients with osteolytic bone disease, the use of local radiation should be limited to patients with spinal cord compression from extramedullary tumor extension, and patients with bone pain refractory to analgesics and systemic therapy. Vertebroplasty (injection of methylmethacrylate into a collapsed vertebral body) or kyphoplasty (introduction of an inflatable bone tamp into the vertebral body and after inflation the injection of methylmethacrylate into the cavity) can be used to decrease pain from vertebral fractures.121 Occasional patients with impending fracture may need prophylactic surgical intervention.
Prevention of infections
Patients should receive pneumococcal and influenza vaccinations. Intravenously administered gamma globulin every 3–4 weeks is indicated if patients have recurrent serious infections associated with severe hypogammaglobulinemia. The role of prophylactic antibiotics in patients receiving chemotherapy for MM has not been settled. Randomized trials have not found significant benefit.122 We do recommend acyclovir in all patients receiving bortezomib or carfilzomib to prevent herpes zoster activation. Prophylaxis against pneumocystis jiroveci should be considered in all patients receiving long-term corticosteroids.123 However, there is a risk of serious skin toxicity in patients receiving an immunomodulatory agent (thalidomide, lenalidomide) and trimethoprim/sulfamethoxazole. In such patients, alternative antibiotics (such as levofloxacin) and alternative agents for pneumocystis prophylaxis should be considered.
Hyperviscosity Syndrome
A small proportion of patients with MM, especially of the IgA subtype can develop hyperviscosity syndrome. Plasmapheresis promptly relieves the symptoms and should be done regardless of the viscosity level if the patient has signs or symptoms of hyperviscosity.124
SMOLDERING MULTIPLE MYELOMA
SMM is a stage that is clinically positioned between MGUS and MM.125 It comprises of a heterogenous group of patients, some of whom have MM which has not yet manifested with MDEs, and some who have premalignant MGUS. Patients with SMM have a risk of progression of approximately 10% per year for the first 5 years, 3% per year for the next 5 years, and 1% per year thereafter.11 Patients with the highest risk of progression (ultra-high risk) have now been reclassified as having MM by the new IMWG criteria.1 Within the current definition of SMM (Table 1), there are two groups of patients: high risk (25% per year risk of progression in the first 2 years) and low risk (~ 5% per year risk of progression).125 Criteria for high risk SMM are given on Table 10. Presence of one or more of these factors is associated with a median TTP to MM of approximately 2 years. Early studies in SMM failed to show an advantage to early intervention, but were limited by lack of power, safe and effective drugs, and a risk-adapted strategy.126,127 A recent randomized trial conducted in Spain found that patients with high risk SMM had an OS benefit when treated with Rd compared with observation; 3-year survival rate 94% versus 80%, respectively, P=0.03.128 These are very promising results, and further confirmatory studies are ongoing. Observation is still the standard of care for SMM; however, selected high risk SMM patients with multiple risk factors can be considered for therapy. They are also candidates for clinical trials testing early intervention.
Table 10.
Criteria for High Risk Smoldering Multiple Myeloma*
| Bone marrow clonal plasma cells ≥10% and any one or more of the following: |
| Serum M protein ≥30g/L |
| IgA SMM |
| Immunoparesis with reduction of two uninvolved immunoglobulin isotypes |
| Serum involved/uninvolved free light chain ratio ≥8 (but less than 100) |
| Progressive increase in M protein level (Evolving type of SMM)† |
| Bone marrow clonal plasma cells 50–60% |
| Abnormal plasma cell immunophenotype (≥95% of bone marrow plasma cells are clonal) and reduction of one or more uninvolved immunoglobulin isotypes |
| t (4;14) or del 17p or 1q gain |
| Increased circulating plasma cells |
| MRI with diffuse abnormalities or 1 focal lesion |
| PET-CT with focal lesion with increased uptake without underlying osteolytic bone destruction |
SMM, smoldering multiple myeloma; M, monoclonal; MRI, magnetic resonance imaging; PET-CT, positron emission tomography-computed tomography
Note that the term smoldering multiple myeloma excludes patients without end-organ damage who meet revised definition of multiple myeloma, namely clonal bone marrow plasma cells ≥60% or serum free light chain (FLC) ratio ≥100 (plus measurable involved FLC level ≥100 mg/L), or more than one focal lesion on magnetic resonance imaging. The risk factors listed in this Table are not meant to be indications for therapy; they are variables associated with a high risk of progression of SMM, and identify patients who need close follow up and consideration for clinical trials
Increase in serum monoclonal protein by ≥25% on two successive evaluations within a 6 month period
From Blood.125
Conclusion
Major advances in the diagnosis and treatment of MM have occurred in the last decade. Future trials should address the optimal sequencing of the various treatment regimens available, the incorporation of monoclonal antibodies to existing regimens in a cost-effective and safe manner, the role of MRD as a goal of therapy, optimal treatment of high risk MM and extramedullary disease, and early intervention towards a cure of the disease.
Acknowledgments
SVR and SK conceived of the paper, researched the literature, and wrote the manuscript. All authors reviewed and approved the paper.
Supported in part by grants CA 107476, CA 168762, and CA 186781 from the National Cancer Institute, Rockville, MD, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Contributions and Disclosure of Conflicts of Interest
SVR declares no conflict of interest. SK has obtained research support for clinical trials from Celgene, Millennium, Novartis, Janssen, Sanofi.
References
- 1.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group Updated Criteria for the Diagnosis of Multiple Myeloma. Lancet Oncol. 2014;15:e538–548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar SV. Multiple myeloma: 2014 Update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89:998–1009. doi: 10.1002/ajh.23810. [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Melton LJ., 3rd Incidence of multiple myeloma in Olmsted County, Minnesota: Trend over 6 decades. Cancer. 2004;101:2667–2674. doi: 10.1002/cncr.20652. [DOI] [PubMed] [Google Scholar]
- 4.Landgren O, Gridley G, Turesson I, et al. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood. 2006;107:904–906. doi: 10.1182/blood-2005-08-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landgren O, Graubard BI, Katzmann JA, et al. Racial disparities in the prevalence of monoclonal gammopathies: a population-based study of 12 482 persons from the national health and nutritional examination survey. Leukemia. 2014;28:1537–1542. doi: 10.1038/leu.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1,027 patients with newly diagnosed multiple myeloma. Mayo Clinic Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 7.Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis of monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–569. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 8.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of Monoclonal Gammopathy of Undetermined Significance. N Engl J Med. 2006;354:1362–1369. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 9.Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375:1721–1728. doi: 10.1016/S0140-6736(10)60482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–5417. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyle RA, Remstein ED, Therneau TM, et al. Clinical Course and Prognosis of Smoldering (Asymptomatic) Multiple Myeloma. N Engl J Med. 2007;356:2582–2590. doi: 10.1056/NEJMoa070389. [DOI] [PubMed] [Google Scholar]
- 12.Myeloma Trialists’ Collaborative Group. Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: An overview of 6,633 patients from 27 randomized trials. J Clin Oncol. 1998;16:3832–3842. doi: 10.1200/JCO.1998.16.12.3832. [DOI] [PubMed] [Google Scholar]
- 13.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 14.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 15.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma [see comments] N Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 16.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 17.Richardson PG, Schlossman RL, Weller E, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100:3063–3067. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- 18.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2013 doi: 10.1038/leu.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Short KD, Rajkumar SV, Larson D, et al. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia. 2011;25:906–908. doi: 10.1038/leu.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katzmann JA, Dispenzieri A, Kyle R, et al. Elimination of the Need for Urine Studies in the Screening Algorithm for Monoclonal Gammopathies by Using Serum Immunofixation and Free Light Chain Assays. Mayo Clin Proc. 2006;81:1575–1578. doi: 10.4065/81.12.1575. [DOI] [PubMed] [Google Scholar]
- 22.Chawla SS, Kumar SK, Dispenzieri A, et al. Clinical course and prognosis of non-secretory multiple myeloma. Eur J Haematol. 2014 doi: 10.1111/ejh.12534. [DOI] [PubMed] [Google Scholar]
- 23.Regelink JC, Minnema MC, Terpos E, et al. Comparison of modern and conventional imaging techniques in establishing multiple myeloma-related bone disease: a systematic review. Brit J Haematol. 2013;162:50–61. doi: 10.1111/bjh.12346. [DOI] [PubMed] [Google Scholar]
- 24.Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23:435–441. doi: 10.1038/leu.2008.336. [DOI] [PubMed] [Google Scholar]
- 25.Hillengass J, Fechtner K, Weber MA, et al. Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol. 2010;28:1606–1610. doi: 10.1200/JCO.2009.25.5356. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Fonseca R, Ketterling RP, et al. Trisomies in multiple myeloma: impact on survival in patients with high-risk cytogenetics. Blood. 2012;119:2100–2105. doi: 10.1182/blood-2011-11-390658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajan AM, Rajkumar SV. Interpretation of cytogenetic results in multiple myeloma for clinical practice. Blood Cancer J. 2015;5:e365. doi: 10.1038/bcj.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuehl WM, Bergsagel PL. Multiple myeloma: evolving genetic events and host interactions. Nature Reviews Cancer. 2002;2:175–187. doi: 10.1038/nrc746. [DOI] [PubMed] [Google Scholar]
- 29.Bergsagel PL, Kuehl WM. Chromosome translocations in multiple myeloma. Oncogene. 2001;20:5611–5622. doi: 10.1038/sj.onc.1204641. [DOI] [PubMed] [Google Scholar]
- 30.Fonseca R, Bailey RJ, Ahmann GJ, et al. Genomic abnormalities in monoclonal gammopathy of undetermined significance. Blood. 2002;100:1417–1424. [PubMed] [Google Scholar]
- 31.Seidl S, Kaufmann H, Drach J. New insights into the pathophysiology of multiple myeloma. Lancet Oncology. 2003;4:557–564. doi: 10.1016/s1470-2045(03)01195-1. [DOI] [PubMed] [Google Scholar]
- 32.Russell SJ, Rajkumar SV. Multiple myeloma and the road to personalised medicine. The Lancet Oncology. 2011;12:617–619. doi: 10.1016/S1470-2045(11)70143-7. [DOI] [PubMed] [Google Scholar]
- 33.Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 34.Greipp PR, San Miguel JF, Durie BG, et al. International Staging System for Multiple Myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 35.Hari PN, Zhang MJ, Roy V, et al. Is the international staging system superior to the Durie-Salmon staging system? A comparison in multiple myeloma patients undergoing autologous transplant. Leukemia. 2009;23:1528–1534. doi: 10.1038/leu.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar SK, Mikhael JR, Buadi FK, et al. Management of Newly Diagnosed Symptomatic Multiple Myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) Consensus Guidelines. Mayo Clin Proc. 2009;84:1095–1110. doi: 10.4065/mcp.2009.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol. 2015;33:2863–2869. doi: 10.1200/JCO.2015.61.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mikhael JR, Dingli D, Roy V, et al. Management of Newly Diagnosed Symptomatic Multiple Myeloma: Updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) Consensus Guidelines 2013. Mayo Clin Proc. 2013;88:360–376. doi: 10.1016/j.mayocp.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 39.Kyle RA, Rajkumar SV. Multiple Myeloma. N Engl J Med. 2004;351:1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 40.Rajkumar SV, Blood E, Vesole DH, Fonseca R, Greipp PR. Phase III Clinical Trial of Thalidomide Plus Dexamethasone Compared With Dexamethasone Alone in Newly Diagnosed Multiple Myeloma: A Clinical Trial Coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 41.Rajkumar SV, Rosiñol L, Hussein M, et al. A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study of Thalidomide plus Dexamethasone Versus Dexamethasone as Initial Therapy for Newly Diagnosed Multiple Myeloma. J Clin Oncol. 2008;26:2171–2177. doi: 10.1200/JCO.2007.14.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11:29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harousseau J, Attal M, Leleu X, et al. Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II study. Haematologica. 2006;91:1498–1505. [PubMed] [Google Scholar]
- 44.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 45.Hulin C, Facon T, Rodon P, et al. Efficacy of Melphalan and Prednisone Plus Thalidomide in Patients Older Than 75 Years With Newly Diagnosed Multiple Myeloma: IFM 01/01 Trial. J Clin Oncol. 2009;27:3664–3670. doi: 10.1200/JCO.2008.21.0948. [DOI] [PubMed] [Google Scholar]
- 46.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus Melphalan and Prednisone for Initial Treatment of Multiple Myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 47.Mateos MV, Oriol A, Martinez-Lopez J. Bortezomib/melphalan/prednisone (VMP) versus Bortezomib/Thalidomide/Prednisone (VTP) as induction therapy followed by maintenance treatment with Bortezomib/Thalidomide (VT) versus Bortezomib/Prednisone (VP): A randomised trial in elderly untreated patients with multiple myeloma older than 65 years. The Lancet Oncology. 2010;11:934–941. doi: 10.1016/S1470-2045(10)70187-X. [DOI] [PubMed] [Google Scholar]
- 48.Palumbo A, Bringhen S, Rossi D, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: a randomized controlled trial. J Clin Oncol. 2010;28:5101–5109. doi: 10.1200/JCO.2010.29.8216. [DOI] [PubMed] [Google Scholar]
- 49.Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376:2075–2085. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 50.Reeder CB, Reece DE, Kukreti V, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. 2009;23:1337–1341. doi: 10.1038/leu.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–686. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar S, Flinn I, Richardson PG, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119:4375–4382. doi: 10.1182/blood-2011-11-395749. [DOI] [PubMed] [Google Scholar]
- 53.Siegel DS, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–2825. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bringhen S, Petrucci MT, Larocca A, et al. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: a multicenter, phase 2 study. 2014;124:63–69. doi: 10.1182/blood-2014-03-563759. [DOI] [PubMed] [Google Scholar]
- 55.Jakubowiak AJ. Evolution of carfilzomib dose and schedule in patients with multiple myeloma: A historical overview. Cancer treatment reviews. 2014;40:781–790. doi: 10.1016/j.ctrv.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Richardson PG, Siegel DS, Vij R, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood. 2014;123:1826–1832. doi: 10.1182/blood-2013-11-538835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Facon T, Dimopoulos MA, Dispenzieri A, et al. Initial Phase 3 Results Of The First (Frontline Investigation Of Lenalidomide + Dexamethasone Versus Standard Thalidomide) Trial (MM-020/IFM 07 01) In Newly Diagnosed Multiple Myeloma (NDMM) Patients (Pts) Ineligible For Stem Cell Transplantation (SCT) Blood. 2013;122:2. [Google Scholar]
- 58.Durie Brian GM, Hoering Antje, Rajkumar S Vincent, et al. Bortezomib, Lenalidomide and Dexamethasone vs. Lenalidomide and Dexamethasone in Patients (Pts) with Previously Untreated Multiple Myeloma Without an Intent for Immediate Autologous Stem Cell Transplant (ASCT): Results of the Randomized Phase III Trial SWOG S0777. ASH Annual Meeting Abstracts. 2015 doi: 10.1038/s41408-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Philippe Moreau, Cyrille Hulin, Margaret MACRO, et al. Bortezomib, Thalidomide and Dexamethasone (VTD) Is Superior to Bortezomib, Cyclophosphamide and Dexamethasone (VCD) Prior to Autologous Stem Cell Transplantation for Patients with De Novo Multiple Myeloma. Results of the Prospective IFM 2013-04 Trial. ASH Annual Meeting Abstracts. 2015 [Google Scholar]
- 60.Michel Attal, Valerie Lauwers-Cances, Cyrille Hulin, et al. Autologous Transplantation for Multiple Myeloma in the Era of New Drugs: A Phase III Study of the Intergroupe Francophone Du Myelome (IFM/DFCI 2009 Trial) ASH Annual Meeting Abstracts. 2015 [Google Scholar]
- 61.Moreau P, Facon T, Attal M, et al. Comparison of reduced-dose bortezomib plus thalidomide plus dexamethasone (vTD) to bortezomib plus dexamethasone (VD) as induction treatment prior to ASCT in de novo multiple myeloma (MM): Results of IFM2007-02 study. J Clin Oncol. 2010;28(suppl):15s. doi: 10.1200/JCO.2009.27.9158. (abstr 8014) [DOI] [PubMed] [Google Scholar]
- 62.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–440. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]
- 63.Palumbo A, Bringhen S, Mateos MV, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125:2068–2074. doi: 10.1182/blood-2014-12-615187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. The New England journal of medicine. 2014;371:906–917. doi: 10.1056/NEJMoa1402551. [DOI] [PubMed] [Google Scholar]
- 65.Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120:1801–1809. doi: 10.1182/blood-2012-04-422683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meletios A Dimopoulos, Philippe Moreau, Antonio Palumbo, et al. Carfilzomib and dexamethasone (Kd) vs bortezomib and dexamethasone (Vd) in patients (pts) with relapsed multiple myeloma (RMM): Results from the phase III study ENDEAVOR. J Clin Oncol; 2015 ASCO Annual Meeting; 2015. p. 8509. [Google Scholar]
- 67.Dimopoulos MA, Terpos E, Chanan-Khan A, et al. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:4976–4984. doi: 10.1200/JCO.2010.30.8791. [DOI] [PubMed] [Google Scholar]
- 68.Burnette BL, Leung N, Rajkumar SV. Renal improvement in myeloma with bortezomib plus plasma exchange. N Engl J Med. 2011;364:2365–2366. doi: 10.1056/NEJMc1101834. [DOI] [PubMed] [Google Scholar]
- 69.Clark WF, Stewart AK, Rock GA, et al. Plasma exchange when myeloma presents as acute renal failure: a randomized, controlled trial. Ann Intern Med. 2005;143:777–784. doi: 10.7326/0003-4819-143-11-200512060-00005. [DOI] [PubMed] [Google Scholar]
- 70.Gonsalves WI, Leung N, Rajkumar SV, et al. Improvement in renal function and its impact on survival in patients with newly diagnosed multiple myeloma. Blood cancer journal. 2015;5:e296. doi: 10.1038/bcj.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blade J, Vesole DH, Gertz M. Transplantation for multiple myeloma: who, when, how often? Blood. 2003;102:3469–3477. [Google Scholar]
- 72.Kumar A, Loughran T, Alsina M, Durie BG, Djulbegovic B. Management of multiple myeloma: a systematic review and critical appraisal of published studies. Lancet Oncology. 2003;4:293–304. doi: 10.1016/s1470-2045(03)01077-5. [DOI] [PubMed] [Google Scholar]
- 73.Gertz MA, Ansell SM, Dingli D, et al. Autologous Stem Cell Transplantation in 716 Patients with Multiple Myeloma: Low treatment-related mortality, feasibility of outpatient transplantation, and impact of a multidisciplinary quality initiative. Mayo Clin Proc. 2008;83:1131–1135. doi: 10.4065/83.10.1131. [DOI] [PubMed] [Google Scholar]
- 74.Moreau P, Facon T, Attal M, et al. Comparison of 200 mg/m2 melphalan and 8 Gy total body irradiation plus 140 mg/m2 melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myélome 9502 randomized trial. Blood. 2002;99:731–735. doi: 10.1182/blood.v99.3.731. [DOI] [PubMed] [Google Scholar]
- 75.Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92:3131–3136. [PubMed] [Google Scholar]
- 76.Facon T, Mary JY, Harousseau JL, et al. Front-line or rescue autologous bone marrow transplantation (ABMT) following a first course of high dose melphalan (HDM) in multiple myeloma (MM). Preliminary results of a prospective randomized trial (CIAM) protocol. Blood. 1996;88(Suppl1):685a. [Google Scholar]
- 77.Barlogie B, Kyle R, Anderson K, et al. Comparable Survival in Multiple Myeloma (MM) with High Dose Therapy (HDT) Employing MEL 140 mg/m2 + TBI 12 Gy Autotransplants Versus Standard Dose Therapy with VBMCP and No Benefit from Interferon (IFN) Maintenance: Results of Intergroup Trial S9321. Blood. 2003;102:42a. [Google Scholar]
- 78.Sonneveld P, Schmidt-Wolf IGH, van der Holt B, et al. Bortezomib Induction and Maintenance Treatment in Patients With Newly Diagnosed Multiple Myeloma: Results of the Randomized Phase III HOVON-65/GMMG-HD4 Trial. Journal of Clinical Oncology. 2012;30:2946–2955. doi: 10.1200/JCO.2011.39.6820. [DOI] [PubMed] [Google Scholar]
- 79.Barlogie B, Jagannath S, Vesole DH, et al. Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood. 1997;89:789–793. [PubMed] [Google Scholar]
- 80.Barlogie B, Jagannath S, Desikan KR, et al. Total therapy with tandem transplants for newly diagnosed multiple myeloma. Blood. 1999;93:55–65. [PubMed] [Google Scholar]
- 81.Attal M, Harousseau JL, Facon T, et al. Double Autologous Transplantation Improves Survival of Multiple Myeloma Patients: Final Analysis of a Prospective Randomized Study of the “Intergroupe Francophone du Myelome” (IFM 94) Blood. 2002;100:5a. [Google Scholar]
- 82.Cavo M, Cellini C, Zamagni E, et al. Superiority of Double over Single Autologous Stem Cell Transplantation as First-Line Therapy for Multiple Myeloma. Blood. 2004;104:155a. (A536) [Google Scholar]
- 83.Fermand JP, Alberti C, Marolleau JP. Single versus tandem high dose therapy (HDT) supported with autologous blood stem cell (ABSC) transplantation using unselected or CD34-enriched ABSC: results of a two by two designed randomized trial in 230 young patients with multiple myeloma (MM) Hematol J. 2003;4(Suppl 1):S59. [Google Scholar]
- 84.Goldschmidt H. Single vs. tandem autolgous transplantation in multiple myeloma: the GMMG experience. Hematol J. 2003;4(Suppl 1):S61. [Google Scholar]
- 85.Krishnan A, Pasquini MC, Logan B, et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. The Lancet Oncology. 2011;12:1195–1203. doi: 10.1016/S1470-2045(11)70243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bruno B, Rotta M, Patriarca F, et al. A Comparison of Allografting with Autografting for Newly Diagnosed Myeloma 10.1056/NEJMoa065464. N Engl J Med. 2007;356:1110–1120. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 87.Attal M, Harousseau J-L, Leyvraz S, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma 10.1182/blood-2006-05-022962. Blood. 2006;108:3289–3294. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 88.Spencer A, Prince HM, Roberts AW, et al. Consolidation Therapy With Low-Dose Thalidomide and Prednisolone Prolongs the Survival of Multiple Myeloma Patients Undergoing a Single Autologous Stem-Cell Transplantation Procedure. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.18.8573. JCO.2008.2018.8573. [DOI] [PubMed] [Google Scholar]
- 89.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide Maintenance after Stem-Cell Transplantation for Multiple Myeloma. New England Journal of Medicine. 2012;366:1782–1791. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 90.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after Stem-Cell Transplantation for Multiple Myeloma. N Engl J Med. 2012;366:1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rajkumar SV, Gahrton G, Bergsagel PL. Approach to the treatment of multiple myeloma: a clash of philosophies. Blood. 2011;118:3205–3211. doi: 10.1182/blood-2011-06-297853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rajkumar SV. Haematological cancer: Lenalidomide maintenance[mdash]perils of a premature denouement. Nature reviews. Clinical oncology. 2012;9:372–374. doi: 10.1038/nrclinonc.2012.100. [DOI] [PubMed] [Google Scholar]
- 93.Nooka AK, Kaufman JL, Muppidi S, et al. Consolidation and maintenance therapy with lenalidomide, bortezomib and dexamethasone (RVD) in high-risk myeloma patients. Leukemia. 2014;28:690–693. doi: 10.1038/leu.2013.335. [DOI] [PubMed] [Google Scholar]
- 94.Palumbo A, Hajek R, Delforge M, et al. Continuous Lenalidomide Treatment for Newly Diagnosed Multiple Myeloma. N Engl J Med. 2012;366:1759–1769. doi: 10.1056/NEJMoa1112704. [DOI] [PubMed] [Google Scholar]
- 95.Gertz MA, Lacy MQ, Inwards DJ, et al. Early harvest and late transplantation as an effective therapeutic strategy in multiple myeloma. Bone marrow transplantation. 1999;23:221–226. doi: 10.1038/sj.bmt.1701559. [DOI] [PubMed] [Google Scholar]
- 96.Kumar SK, Therneau TM, Gertz MA, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clinic Proceedings. 2004;79:867–874. doi: 10.4065/79.7.867. [DOI] [PubMed] [Google Scholar]
- 97.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus Dexamethasone for Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 98.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus Dexamethasone for Relapsed Multiple Myeloma in North America. N Engl J Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 99.Pineda-Roman M, Zangari M, van Rhee F, et al. VTD combination therapy with bortezomib-thalidomide-dexamethasone is highly effective in advanced and refractory multiple myeloma. Leukemia. 2008;22:1419–1427. doi: 10.1038/leu.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Richardson PG, Weller E, Jagannath S, et al. Multicenter, Phase I, Dose-Escalation Trial of Lenalidomide Plus Bortezomib for Relapsed and Relapsed/Refractory Multiple Myeloma. Journal of Clinical Oncology. 2009;27:5713–5719. doi: 10.1200/JCO.2009.22.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Richardson PG, Xie W, Jagannath S, et al. A phase 2 trial of lenalidomide, bortezomib, and dexamethasone in patients with relapsed and relapsed/refractory myeloma. Blood. 2014;123:1461–1469. doi: 10.1182/blood-2013-07-517276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–152. doi: 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- 103.Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) Plus Low-Dose Dexamethasone As Therapy for Relapsed Multiple Myeloma. J Clin Oncol. 2009;27:5008–5014. doi: 10.1200/JCO.2009.23.6802. [DOI] [PubMed] [Google Scholar]
- 104.Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) plus low dose dexamethasone (Pom/dex) is active and well tolerated in lenalidomide refractory multiple myeloma (MM) Leukemia. 2010;24:1934–1939. doi: 10.1038/leu.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lacy MQ, Allred JB, Gertz MA, et al. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: comparison of 2 dosing strategies in dual-refractory disease. Blood. 2011;118:2970–2975. doi: 10.1182/blood-2011-04-348896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.San Miguel J, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. The Lancet Oncology. 2013;14:1055–1066. doi: 10.1016/S1470-2045(13)70380-2. [DOI] [PubMed] [Google Scholar]
- 107.San-Miguel MDJF, Hungria VTM, Yoon MDS. Randomized phase 3 trial of the deacetylase inhibitor panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in relapsed or relapsed and refractory multiple myeloma. The Lancet Oncology. 2014;15:1195–1206. doi: 10.1016/S1470-2045(14)70440-1. [DOI] [PubMed] [Google Scholar]
- 108.Hideshima T, Bradner JE, Wong J, et al. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8567–8572. doi: 10.1073/pnas.0503221102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.San-Miguel JF, Richardson PG, Gunther A, et al. Phase Ib study of panobinostat and bortezomib in relapsed or relapsed and refractory multiple myeloma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:3696–3703. doi: 10.1200/JCO.2012.46.7068. [DOI] [PubMed] [Google Scholar]
- 110.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized Phase III Study of Pegylated Liposomal Doxorubicin Plus Bortezomib Compared With Bortezomib Alone in Relapsed or Refractory Multiple Myeloma: Combination Therapy Improves Time to Progression. J Clin Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 111.Sagar Lonial, Brendan M, Weiss Saad, Zafar Usmani, et al. Phase II study of daratumumab (DARA) monotherapy in patients with ≥ 3 lines of prior therapy or double refractory multiple myeloma (MM): MMY2002 (Sirius) J Clin Oncol. 2015;33 (suppl; abstr LBA8512) [Google Scholar]
- 112.Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2015;373:621–631. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 113.Philippe Moreau, Tamás Masszi, Norbert Grzasko, et al. Ixazomib, an Investigational Oral Proteasome Inhibitor (PI), in Combination with Lenalidomide and Dexamethasone (IRd), Significantly Extends Progression-Free Survival (PFS) for Patients (Pts) with Relapsed and/or Refractory Multiple Myeloma (RRMM): The Phase 3 Tourmaline-MM1 Study ( NCT01564537) ASH Annual Meeting Abstracts. 2015 [Google Scholar]
- 114.Gucalp R, Theriault R, Gill I, et al. Treatment of cancer-associated hypercalcemia. Double-blind comparison of rapid and slow intravenous infusion regimens of pamidronate disodium and saline alone. Arch Intern Med. 1994;154:1935–1944. doi: 10.1001/archinte.154.17.1935. [DOI] [PubMed] [Google Scholar]
- 115.Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19:558–567. doi: 10.1200/JCO.2001.19.2.558. [DOI] [PubMed] [Google Scholar]
- 116.Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group [see comments] N Engl J Med. 1996;334:488–493. doi: 10.1056/NEJM199602223340802. [DOI] [PubMed] [Google Scholar]
- 117.Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. [erratum appears in Cancer 2001 May 15;91(10):1956] Cancer. 2001;91:1191–1200. doi: 10.1002/1097-0142(20010401)91:7<1191::aid-cncr1119>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 118.Rosen LS, Gordon D, Kaminski M, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastatses in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase II, double blind, comparative trial. Cancer J. 2001;7:377–387. [PubMed] [Google Scholar]
- 119.Berenson JR, Hillner BE, Kyle RA, et al. American Society of Clinical Oncology Clinical Practice Guidelines: The role of bisphosphonates in multiple myeloma. J Clin Oncol. 2002;20:3719–3736. doi: 10.1200/JCO.2002.06.037. [DOI] [PubMed] [Google Scholar]
- 120.Morgan GJ, Davies FE, Gregory WM, et al. First-line and Ongoing Treatment with Zoledronic Acid Improves Overall Survival in Patients with Multiple Myeloma: Results of the MRC IX Randomised Controlled Trial. Lancet. 2010;376:1989–1999. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. Journal of Neurosurgery. 2003;98:21–30. doi: 10.3171/spi.2003.98.1.0021. [DOI] [PubMed] [Google Scholar]
- 122.Vesole DH, Oken MM, Heckler C, et al. Oral antibiotic prophylaxis of early infection in multiple myeloma: a URCC/ECOG randomized phase III study. Leukemia. 2012;26:2517–2520. doi: 10.1038/leu.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oken MM, Pomeroy C, Weisdorf D, Bennett JM. Prophylactic antibiotics for the prevention of early infection in multiple myeloma. American Journal of Medicine. 1996;100:624–628. doi: 10.1016/s0002-9343(95)00043-7. [DOI] [PubMed] [Google Scholar]
- 124.Gertz MA, Kyle RA. Hyperviscosity syndrome. Journal of Intensive Care Medicine. 1995;10:128–141. doi: 10.1177/088506669501000304. [DOI] [PubMed] [Google Scholar]
- 125.Rajkumar SV, Landgren O, Mateos MV. Smoldering multiple myeloma. Blood. 2015;125:3069–3075. doi: 10.1182/blood-2014-09-568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hjorth M, Hellquist L, Holmberg E, Magnusson B, Rodjer S, Westin J. Initial versus deferred melphalan-prednisone therapy for asymptomatic multiple myeloma stage I–a randomized study. Myeloma Group of Western Sweden. European Journal of Haematology. 1993;50:95–102. doi: 10.1111/j.1600-0609.1993.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 127.Grignani G, Gobbi PG, Formisano R, et al. A prognostic index for multiple myeloma. British journal of cancer. 1996;73:1101–1107. doi: 10.1038/bjc.1996.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mateos M-V, Hernández M-T, Giraldo P, et al. Lenalidomide plus Dexamethasone for High-Risk Smoldering Multiple Myeloma. N Engl J Med. 2013;369:438–447. doi: 10.1056/NEJMoa1300439. [DOI] [PubMed] [Google Scholar]
- 129.Stadtmauer EA, Abonour R, Cohen AD, et al. Phase I/II Dose Expansion Of a Multi-Center Trial Of Carfilzomib and Pomalidomide With Dexamethasone (Car-Pom-d) In Patients With Relapsed/Refractory Multiple Myeloma. Blood. 2013;122:690–690. [Google Scholar]
- 130.Barlogie B, Anaissie E, van Rhee F, et al. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3. British Journal of Haematology. 2007;138:176–185. doi: 10.1111/j.1365-2141.2007.06639.x. [DOI] [PubMed] [Google Scholar]
- 131.van Rhee F, Szymonifka J, Anaissie E, et al. Total therapy 3 for multiple myeloma: prognostic implications of cumulative dosing and premature discontinuation of VTD maintenance components, bortezomib, thalidomide and dexamethasone, relevant to all phases of therapy. Blood. 116:1220–1227. doi: 10.1182/blood-2010-01-264333. [DOI] [PMC free article] [PubMed] [Google Scholar]


