Abstract
In eukaryotic cells, the organization of genomic DNA into chromatin regulates many biological processes, from the control of gene expression to the regulation of chromosome segregation. The proper maintenance of this structure upon cell division is therefore of prime importance during development for the maintenance of cell identity and genome stability. The chromatin assembly factor 1 (CAF-1) is involved in the assembly of H3-H4 histone dimers on newly synthesized DNA and in the maintenance of a higher order structure, the heterochromatin, through an interaction of its large subunit with the heterochromatin protein HP1a. We identify here a conserved domain in the large subunit of the CAF-1 complex required for its interaction with HP1a in the Drosophila fruit fly. Functional analysis reveals that this domain is dispensable for viability but participates in two processes involving heterochromatin: position-effect variegation and long range chromosomal interactions during meiotic prophase. Importantly, the identification in the large subunit of CAF-1 of a domain required for its interaction with HP1 allows the separation of its functions in heterochromatin-related processes from its function in the assembly of H3-H4 dimers onto newly synthesized DNA.
Keywords: heterochromatin, variegation, HP1, CAF-1, Drosophila
IN eukaryotic cells, the chromatin is partitioned into two cytologically and functionally distinct structures: heterochromatin and euchromatin. Heterochromatin was initially defined as the part of the genome that remains condensed during the whole cell cycle and stains intensively with DNA dyes. Heterochromatin is generally gene poor; rich in repeated sequences and transposable elements (Hoskins et al. 2007). Initially considered to correspond to “junk DNA,” heterochromatin contains essential protein-coding genes whose expression depends on the neighboring heterochromatin structure (Schulze et al. 2005). It encodes essential chromosomal structures such as centromeres (Sun et al. 1997) or telomeres (Mason et al. 2008) and is required for essential chromosomal functions such as homolog pairing during meiosis (Dernburg et al. 1996; Karpen et al. 1996) . While essential for the biology of the genome, many of these structures are not directly encoded in the sequence of these regions and epigenetic mechanisms are likely required for their maintenance through generations.
The chromatin assembly factor-1 (CAF-1) is a heterotrimeric complex first isolated as a histone chaperone able to deposit H3-H4 dimers onto newly synthesized DNA during replication or repair (Smith and Stillman 1989; Gaillard et al. 1996). Its large subunit interacts directly with PCNA (Shibahara and Stillman 1999; Moggs et al. 2000) and the CAF-1 complex is found in vivo at the replication foci (Krude 1995; Taddei et al. 1999). The large subunit of CAF-1 has also been associated to the maintenance of heterochromatin: it was shown to be essential for the stable inheritance of gene silencing in subtelomeric regions in Saccharomyces cerevisiae (Monson et al. 1997); its absence in fission yeast led to defective maintenance of silencing at both the centromeres and mating type loci, accompanied with a decrease of HP1 ortholog Swi6p binding in these regions (Dohke et al. 2008); and in mice, it is required for the duplication and maintenance of pericentric heterochromatin (Quivy et al. 2004, 2008). This function of mouse P150 is independent of the known function of CAF-1 in histone deposition and has been linked to its ability to interact with HP1 proteins (Quivy et al. 2008). The large subunit of CAF-1 is therefore an important and conserved factor required for maintenance of multiple levels of chromatin organization. It however remains to be determined whether the two apparently separate biochemical activities of CAF-1 ensure common or independent functions during development.
In Drosophila, the large subunit of CAF-1, P180 (Tyler et al. 2001; Song et al. 2007; Klapholz et al. 2009), is essential for larval development (Song et al. 2007; Klapholz et al. 2009) and is required for the following: (i) proliferation of mitotic and endocycling cells (Tyler et al. 2001; Song et al. 2007; Klapholz et al. 2009), (ii) assembly of nucleosomes on newly synthesized DNA (Klapholz et al. 2009; Tyler et al. 2001), and (iii) replication of euchromatic regions in larval endocycling cells (Klapholz et al. 2009). These properties together with genetic interactions between mutant alleles of Caf1-180 (hereafter referred to as p180), which encodes P180, and asf1 (Klapholz et al. 2009), encoding the histone chaperone ASF1, suggest that the function of P180 essential for viability in Drosophila is related to CAF-1-dependent histone deposition. Whether P180 is also required for proper maintenance of heterochromatic regions was initially less clear: although two mutant alleles of p180 were shown to act as dominant suppressors of position-effect variegation (PEV) (Song et al. 2007), quantitative analysis of the efficiency of the replication of heterochromatic regions did not show, in contrast to euchromatic regions, major defects upon the loss of P180 activity in larval endocycling cells (Klapholz et al. 2009). More recently, an RNA interference approach showed that P180 regulates, in a dose-dependent manner, the structure of pericentric heterochromatin by affecting H3K9Me and H4K20Me levels together with the recruitment of HP1a on polytene chromosomes; thereby conclusively showing the conservation of this function in flies (Huang et al. 2010). Moreover, as the artificial targeting of HP1a to chromosomes induces the accumulation of P180 at these ectopic positions (Huang et al. 2010), it was proposed that the role of CAF-1 in heterochromatin maintenance in flies was also likely mediated by an interaction between P180 and HP1a.
In this study, we identify a conserved domain in the Drosophila CAF-1 large subunit required for its in vitro interaction with HP1a. We show that this domain is not essential for viability but is required for proper heterochromatin maintenance in germ cells and participates in two processes that require proper heterochromatin structure in flies: PEV and persistence of pairing between heterochromatic chromosomal regions in developing oocytes. Our results therefore demonstrate that the interaction between the large subunit of CAF-1 and HP1a is not essential for Drosophila larval development and viability, but participates in the maintenance of heterochromatin organization.
Materials and Methods
Fly stocks
Fly stocks include the p1803 [caf1-180(3)] (Klapholz et al. 2009), Su(var)2055 [Su(var)205(5)] (Eissenberg et al. 1992), and Mei-W681 [Mei-W68(1)] (McKim et al. 1998) alleles; the Dp(1;Y)B[S]-marked Y chromosome; the P{mw,HSBGHb3Bcd3-lacZ} insertion on the X chromosome (Simpson-Brose et al. 1994); the FM7i, P{ActGFP}JMR3, TM3,Sb, TM3,Sb Ser and SM6b balancer chromosomes; the T(2;3)SbV (T(2;3)Sb[V]) translocation; and the C(2)EN and C(4)RM, ci[1], ey[R] compound chromosomes. Ubiquitous expression was obtained using the Gal4/UAS system (Brand and Perrimon 1993) with P{Act5C-Gal4}25FO1 insertion as a Gal4 driver. Upstream activation sequence (UAS) transgenes include UAS-p180 (Klapholz et al. 2009) and UAS-p180ΔHIM, which were obtained by germline transformation of the pUASp vector (Rorth 1998) containing the p180 or p180ΔHIM complementary DNA (cDNA) (see the Vectors and cloning section).
Genetics
PEV was assessed by analyzing the progeny of females that were or were not carrying mutant alleles of p180 and Su(var)205 crossed with SbV/TM3,Sb,Ser (Figure 4C) or SbV/TM3,Ser (Supplemental Material, Figure S3) males. The 12 macrochaetae in notum and scutellum of SbV offspring were scored to quantify the Sb phenotype.
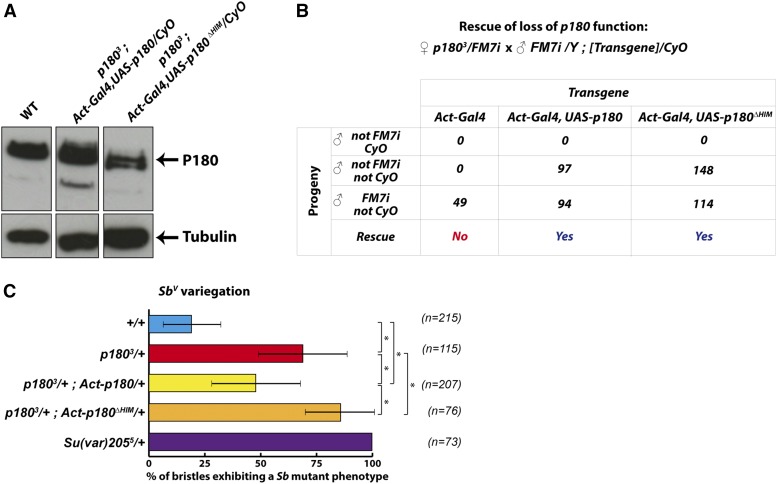
Figure 4.
The HIM domain is not essential for viability but contributes to p180-induced PEV. (A) Expression of P180 in larvae of the indicated genotype assessed by western blotting on larval extracts using an anti-tubulin antibody and an α-P180 antibody raised against a peptide covering residues 198–421 of P180. WT, wild type. (B) Rescue efficiency of the various transgenes was quantified using a test cross between p1803/FM7i females and CyO males carrying the indicated combination of transgenes. Rescue was evaluated in the progeny of the test cross by the presence of rescued males (non-FM7, non-CyO) only expressing the transgenic P180. (C) Rescue of SbV variegation induced by p1803 heterozygosity by transgenic expression of P180. Flies heterozygous for the SbV chromosomal aberration and expressing either the wild-type or the HIM-deleted P180 were scored for the number of macrochaetae showing a Sb phenotype in p1803/+ background. n specifies the number of flies analyzed for each genotype and * indicates a significant difference (P ≤ 0.0001) using Mann–Whitney–Wilcoxon test.
Meiotic segregation defects of X chromosomes were monitored using dominant markers located on the paternal X (mw from P{mw,HSBGHb3Bcd3-LacZ}) and Y (Bar from Dp(1;Y)B[S]) chromosomes as described in McKim et al. (2009). Briefly, females that were or were not carrying mutant alleles of p180 were also heterozygous for the y marker to allow the discrimination of meiosis I from meiosis II segregation defects. When y+,p180+or−/FM7,y,B1 females were crossed to y,{mw}/BSY males, regular progeny include females with normal or mildly Bar (B1 heterozygotes) eyes and males with severe Bar (BS) eyes. X chromosome segregation defects during female meiosis generate oocytes with two X chromosomes or none: the corresponding exceptional progeny are females with severe Bar eyes (XXY, BS female) or males with normal or mildly Bar eyes (XO male). Segregation defects during meiosis I lead to nonyellow-bodied XXY females that are heterozygous for the y marker, whereas segregation defects in meiosis II lead to an equal number of yellow-bodied and nonyellow-bodied XXY females. Because of increased meiotic segregation defects in XXY females when compared to wild type (Xiang and Hawley 2006), each female parent and each exceptional progeny was tested by PCR to detect the presence of the Y chromosome Pp1-Y2 gene (primers Pp1-Y21, GCGAATTATTGACACTCGCCG; and Pp1-Y22, ATCGTTGTCGCTCCATCCCAT) and of the paternal X-linked transgenic sequences (primers HSBG407, CAGCGCTGACTTTGAGTGGAA; and LacZ-seq, AGACCAATGCCTCCCAGACC). The frequency of segregation defects was normalized to account for unviable XXX and OY genotypes, and calculated as 2×(exceptional progeny)/[2× (exceptional progeny) + (regular progeny)].
Statistical tests
The low level of exceptional progeny observed did not allow us to use the classical χ2 test. Rates of segregation defects were therefore compared using Fisher’s exact test which is more suitable to low effectives. For all tests, a two-tailed P-value of lower than 5% was considered statistically significant. A recent study provided tools to compare rates of segregation defects of the X chromosome using a multinomial Poisson hierarchy model (Zeng et al. 2010). Treatment of our data with the Fisher’s exact test or the Poisson hierarchy model reached similar conclusions.
Sequence alignment and conservation
Sequences were aligned using the MUSCLE algorithm (Edgar 2004a,b). Conservation at any given position was computed as the average of a numerical index reflecting the conservation across the selected species of physico-chemical properties (Livingstone and Barton 1993) on 11 residues centered on the position: for example the value at position 15 reflects the conservation of residues 10–21. When <11 values were available for a given position, conservation was computed on the available values within the +5/−5 distance.
Vectors and cloning
A cDNA library of Drosophila embryos (Clontech) was used to generate the three Drosophila HP1a GST constructs using three pairs of primers: ssHP1-a (TTAAGAATTCATGGGCAAGAAAATCGACAACCC) and ssHP1-b (TTAAGAATTCGCTCGCCTCGTACTGCTGG) to amplify the sequence encoding the first 72 residues of HP1a; ssHP1-a and ssHP1-c (TTAAGAATTCGCCGCGATCGAATCCGGTAGATCC) to amplify the sequence encoding the first 146 residues of HP1a; ssHP1-a and ssHP1-d (TTAAGAATTCATCTTCAATATCAGAGTACCAGG) to amplify the full-length HP1a coding sequence. These PCR products were then cloned into the EcoRI restriction site of the pGEX-4T-1 plasmid (GE Healthcare). The wild-type and mutant P180-His proteins were produced in bacteria using the pET30A+ plasmid (Novagen), in which the corresponding cDNA was cloned in frame with the sequence encoding the histidine tag. The various cDNAs encoding the truncated P180 proteins used to identify the HP1-interacting motif (HIM) were obtained using standard cloning methods as follows: for p180ΔNt, ligation of LG1 (TATGCACGCTGGCGTAGTTG) and LG2 (GATCCAACTACGCCAGCGTGCA) oligonucleotides in the p180 cDNA digested with NdeI and BamHI; for p180ΔNtΔ(883–1101), ligation of PSKP1 (GCAGGAGTTCGCTGATGCG) and PSKP2 (GTACCGCATCAGCGAACTCCTGCTGCA) oligonucleotides in the p180ΔNt cDNA digested with PstI and KpnI; for p180ΔNtΔ(1110–1183), ligation of KPNO1 (GTACCAGCCGCCTCGCCCGC) and KPNO2 (GTACCGCATCAGCGAACTCCTGCTGCA) oligonucleotides in the p180ΔNt cDNA digested with NotI and KpnI; for p180ΔNtΔ(883–959), self-ligation of the p180ΔNt cDNA digested with PstI; for p180ΔNtΔ(883–986), ligation in the p180ΔNt cDNA digested with PstI and KpnI of the product obtained by PCR amplification of the p180 cDNA with the PSKP DELTA2959 (CCGCTGCAGGAGTACCTGAAAACCCAAGCGA) and p180-3′KpnI (GCGGCTGGTACCGCATCCGCTG) primers; for p180ΔNtΔ(883–1010), ligation in the p180ΔNt cDNA digested with PstI and KpnI of the product obtained by PCR amplification of the p180 cDNA with the PSKP DELTA3030 (CCGCTGCAGAAATTCGACGAGCTTGCCAG) and p180-3′KpnI (GCGGCTGGTACCGCATCCGCTG) primers; for p180ΔNtΔ(883−1040), ligation in the p180ΔNt cDNA digested with PstI and KpnI of the product obtained by PCR amplification of the p180 cDNA with the PSKP DELTA3130 (CCGCTGCAGAAACCGAAGAAGCGCCTCTG) and p180-3′KpnI (GCGGCTGGTACCGCATCCGCTG) primers; for p180ΔNtΔHIM, ligation in the PstI digested p180ΔNtΔ(883–986) cDNA of the fragment obtained by PstI digestion of the p180 cDNA; for p180Δ(959–986), ligation in the p180 cDNA digested with PstI and KpnI of the product obtained by PCR amplification of the p180 cDNA with the PSKP DELTA2959 (CCGCTGCAGGAGTACCTGAAAACCCAAGCGA) and p180-3′KpnI (GCGGCTGGTACCGCATCCGCTG) primers; and for p180 ΔHIM, ligation of the fragment obtained by PstI digestion of the p180 cDNA in the p180Δ(959–986) cDNA digested with PstI. The P180-His was produced in bacteria using the pET30A+ plasmid (Novagen), in which the p180 cDNA was cloned in frame with the sequence encoding the histidine tag.
Co-immunoprecipitation on Schneider S2 cells
Schneider S2 cells were incubated for 15 min on ice in a lysis buffer containing 10 mM HEPES, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF, 5 µg/ml leupeptin, 2 µg/ml pepstatin, and 15 µg/ml aprotinin. NP-40 was added to a final concentration of 0.5% and the cells were strongly agitated. Nuclei were centrifuged for 5 min at 100 × g. The nuclear pellet was resuspended in nuclear extraction buffer (NEB) 0.42 (20 mM HEPES, 25% glycerol, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.5 mM PMSF, 5 µg/ml leupeptin, 2 µg/ml pepstatin, and 15 µg/ml aprotinin) with 0.42 M NaCl and incubated for 20 min at 4° on a wheel before a centrifugation of 30 min at 20,000 × g. Immunoprecipitation (IP) was performed on the supernatant with a 1:100 dilution of a polyclonal rabbit anti-P180, kindly provided by Tyler et al. (2001), and Sepharose-Protein A beads (Upstate) in NEB 0.2 (same as NEB 0.42 but containing 0.2 M NaCl). The detection of co-immunoprecipitated proteins was performed by western blots using a 1:8000 dilution of the same anti-P180, a 1:1000 dilution of polyclonal rabbit anti-P105 kindly provided by Tyler et al. (2001), or a 1:25,000 dilution of a mouse monoclonal anti-HP1a antibody (#C1A9, Developmental Studies Hybridoma Bank). Secondary antibodies were either a 1:50,000 dilution of an HRP-conjugated donkey anti-rabbit (#711-036-152, Jackson ImmunoResearch Laboratories) or a 1:10,000 dilution of a goat anti-mouse (#115-036-062, Jackson ImmunoResearch Laboratories).
Recombinant proteins and GST pull downs
GST-fusion and His-tagged proteins were produced in Escherichia coli BL21-DE3 upon induction with 0.4 mM isopropyl 1-thio-β-D-galactopyranoside. Pellets were suspended in buffer Y (1× PBS, 1 mM EDTA, 1% Triton X-100, 1 mM PMSF, 5 µg/ml leupeptin, 2 µg/ml pepstatin, and 10 µg/ml aprotinin) and sonicated. Lysates were centrifuged at 20,000 × g for 30 min to remove aggregates. GST fusions were purified using Glutathione Sepharose 4B beads (GE Healthcare). After washes in buffer Y, bound proteins were suspended in buffer (PBS/EDTA, 0.5 M, pH 8.0). The relative concentration of proteins was estimated by Coomassie staining of SDS-PAGE for GST fusion and by western blotting for His-tagged proteins. For GST pull-down assays, beads of Glutathione Sepharose bound to GST fusions were suspended in immuno-precipitation (IP) buffer (50 mM Tris-HCl, pH 7.0, 0.25 mM EDTA, 0.03% NP-40, 5 µg/ml leupeptine, 2 µg/ml pepstatine, and 10 µg/ml aprotinine) and incubated with lysates containing the His-tagged proteins at 4° for 3 hr under gentle agitation. The Sepharose beads were collected by centrifugation at 800 × g and washed four times with IPTAG buffer containing 150 mM NaCl. Pulled-down proteins were detected by western blotting with a 1:500 dilution of a His-probe mouse monoclonal antibody (H-3, #sc-8036, Santa Cruz Biotechnology), a 1:10,000 dilution of a secondary goat peroxidase-conjugated anti-mouse antibody (#115-036-062, Jackson ImmunoResearch), and SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific).
Detection of P180 in larval extracts
Larval protein extracts were prepared as described in Klapholz et al. (2009). P180 proteins were detected using an antibody raised in rabbit against a peptide corresponding to the residues 198–421 fused to GST. As this peptide is located in the amino-terminal region of the protein, the deletion of the HIM is unlikely to affect recognition of this epitope in denaturing conditions.
Immunodetection on salivary gland polytene chromosomes
Staining was performed as described in Paro (2000). P180 was detected using a FLAG-tag fused to the P180 protein in the UAS-p180 transgene using either a monoclonal mouse (M2, Sigma Aldrich, Figure 2B) or a polyclonal rabbit antibody (F7425, Sigma-Aldrich, Figure 2, A and C). Using the M2 antibody, a nonspecific staining was recurrently observed at the chromocenter, even in flies not expressing the P180-Flag protein. The other antibodies used were: mouse monoclonal PC10 (Dako) specific for PCNA, rabbit polyclonal R20/12 specific for acetylated H4K12 (Turner et al. 1992), and mouse monoclonal C1A9 (Developmental Studies Hybridoma Bank) detecting HP1a.
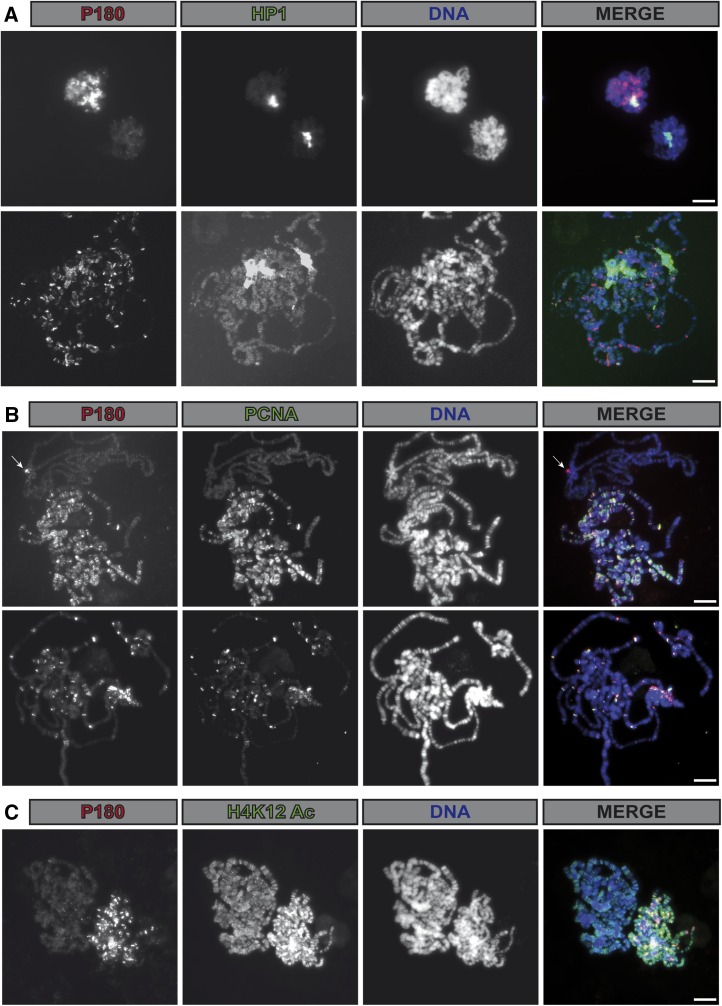
Figure 2.
Binding of P180 on polytene chromosomes occurs in a replication-dependent manner and only poorly correlates with HP1a localization. (A–C) Immunofluorescent staining allows detection of (A–C) P180-FLAG fusion protein, (A) HP1a, (B) PCNA, or (C) H4 acetylated on lysine 12 on salivary gland polytene chromosome in p1803/p1803; Act-Gal4,UAS-p180-FLAG/CyO. Under the staining conditions used for (B), a nonspecific staining (arrow) was recurrently observed at the chromocenter, even in flies not expressing the P180-Flag protein (see Materials and Methods for details). The two genomes shown in each panel exemplify the diversity of staining observed and likely represent different replication timings. Scale bar on all panels represents 10 μm.
Immunodetection on ovaries
Ovaries of 4- to 5-day-old females were dissected in PBS and fixed in 4% paraformaldehyde for 15 min. Samples were then permeabilized in PBS with 0.2% Triton X-100 (PBT) for at least 30 min, incubated at 4° overnight with primary antibodies diluted in PBT (rabbit anti-P180, 1:1000, described in the section Detection of P180 in larval extracts; and mouse anti-HP1 C1A9, Developmental Studies Hybridoma Bank, 1:100), washed three times for 15 min in PBT, incubated for 2 hr with secondary antibodies diluted in PBT (Alexa-568 conjugated anti-rabbit and Alexa-488 conjugated anti-mouse, Life Technologies, 1:1000), and washed three times for 15 min in PBT. For DNA staining, samples were subsequently stained with DAPI (0.8 µg/ml in PBT) for 10 min. Samples were mounted in Vectashield (Vector Laboratories, Burlingame, CA).
Imaging of germaria and egg chambers was performed with a Carl Zeiss (Thornwood, NY) LSM780 confocal microscope using identical acquisition settings for all analyzed genotypes. Image projections were performed with six to eight images containing the upper half of the germarium or five to six images containing the oocytes for the egg chambers. Images were separated by 0.8–1 µm along the z-axis.
Quantification of HP1a signal in oocytes
The total HP1a signal in oocytes was evaluated using Fiji, by (i) identifying the stacks containing the oocyte nucleus based on the DAPI staining, (ii) performing a sum-intensity projection of the typically five to six stacks containing the oocyte’s nucleus, and (iii) summing the value of every pixel contained within a region of interest designed to encompass the oocyte nucleus.
FISH staining and imaging
The 359-bp repeat probe was obtained by PCR amplification of genomic DNA using primers (359 bp-1, CGGTCATCAAATAATCATTTATTTTGC; and 359 bp-2, CGAAATTTGGAAAAACAGACTCTGC) designed from the published sequence (Hsieh and Brutlag 1979). Probes were digested with AluI and then tailed with digoxigenin using the DIG-oligonucleotide tailing kit (Roche). FISH was performed as described (Dernburg et al. 1996). After hybridization, probes were detected using a sheep anti-digoxigenin primary antibody (1:1500, Roche) and the Alexa Fluor 568 donkey anti-sheep (1:1000, Molecular Probes, Eugene, OR), DNA was stained with DAPI, and the samples were mounted in Vectashield. Image acquisitions and processing were performed using a Delta Vision microscope (GE Healthcare Life Sciences) with 40× and 100× objectives and the SoftWorx software. The settings for acquisition and deconvolution were constant for each staining. Each quantification was performed on at least two independent experiments. Image projections were performed with 4–10 images separated by 0.2 µm along the z-axis.
Data availability
Strains are available upon request. Table S1 contains accession numbers for P180 orthologs used for conservation analysis.
Results
The Drosophila large subunit of CAF-1 and HP1a interact directly
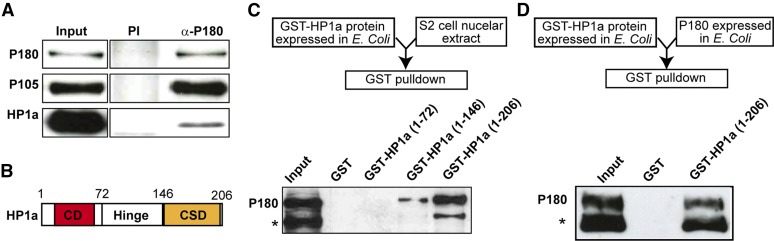
P180 and HP1a have been shown to genetically interact in Drosophila and to co-immunoprecipitate in embryonic extracts (Huang et al. 2010). Given the potential importance of this interaction in the maintenance of heterochromatic structure, we aimed to characterize its molecular details. We first recapitulated this interaction by performing an IP of P180 from a nuclear extract of Drosophila Schneider S2 cells. P180 coprecipitated with P105, the medium subunit of CAF-1, and, in agreement with a study by Jiao and colleagues (Huang et al. 2010), with HP1a (Figure 1A). Additional GST pull-down assays on S2 nuclear extracts with truncated forms of HP1a (Figure 1B) fused at their N-terminal end to the GST indicated that (a) P180 does not interact with the chromodomain of HP1a, (b) the hinge of HP1a is able to interact weakly with P180, and (c) the full interaction between P180 and HP1a requires both the hinge and the chromo shadow domain of HP1a (Figure 1C). Finally, GST pull-down assays using recombinant P180 and GST-HP1a proteins showed that HP1a was able to pull-down P180 on its own (Figure 1D) and that the interaction between the two proteins was therefore direct.
Figure 1.
P180 and HP1a interact directly. (A) IP of P180 in nuclear extracts from cultured Schneider S2 cells. The large (P180) and medium (P105) subunits of CAF-1, as well as the Drosophila HP1a protein, were detected by western blot on nuclear extracts (Input), after IP with a preimmune serum (PI) or with an anti-P180 antibody (α-P180). (B) Representation of the HP1a protein, carrying a chromodomain (CD, red) in the N-terminal and a chromo shadow domain (CSD, orange) in the C-terminal. The positions of the first and last HP1a amino acids contained in the GST-fusion proteins used hereafter are indicated. (C) GST pull-down assay in nuclear extracts from cultured Schneider S2 cells, using different forms of HP1a fused to GST in N-terminal. Bottom panel: western blot anti-P180 on the nuclear extract (Input) and after the pull-down assay using the different fusion proteins. (D) GST pull-down assay using recombinant proteins expressed in E. coli. The presence of P180 in the bound fraction was analyzed by western blotting against a poly-histidine motif fused to its C-terminal. * designates a putative degradation product of the P180 protein.
Colocalization between P180 and HP1a is only observed on replicating polytene chromosomes
In the experiment described Figure 1A, we noticed that only a minor fraction of HP1a was coprecipitated with P180. This could be due to the fact that HP1 proteins interact with other partners that could potentially compete with P180 for binding to HP1 in nuclear extracts (Kellum 2003). In addition, as CAF-1 is mostly required during S phase of the cell cycle, this interaction might also be restricted during this phase of the cell cycle in vivo. We tested this hypothesis by analyzing the localization of both P180 and HP1a on salivary gland polytene chromosomes. As described previously (James and Elgin 1986; James et al. 1989), HP1a was detected at the chromocenter, a DAPI-dense region containing the pericentric and centric regions (Figure 2A). Codetection of P180 using a FLAG-tagged transgene indicated that localization of P180 only poorly correlated with the binding of HP1a but was detected on polytene chromosomes also stained by PCNA (Figure 2B) or showing high levels of newly incorporated histones (H4K12Ac, Figure 2C); two markers of chromatin undergoing replication. Accordingly, polytene chromosomes not undergoing replication and therefore not stained by PCNA, or with lower signals of H4K12Ac, were poorly bound by P180. This suggests that binding of P180 at the HP1a bound loci might occur in a replication-dependent manner and raises the possibility that the interaction between these two factors might be cell-cycle regulated.
A 27-residue domain is required for the direct interaction between recombinant P180 and HP1a proteins
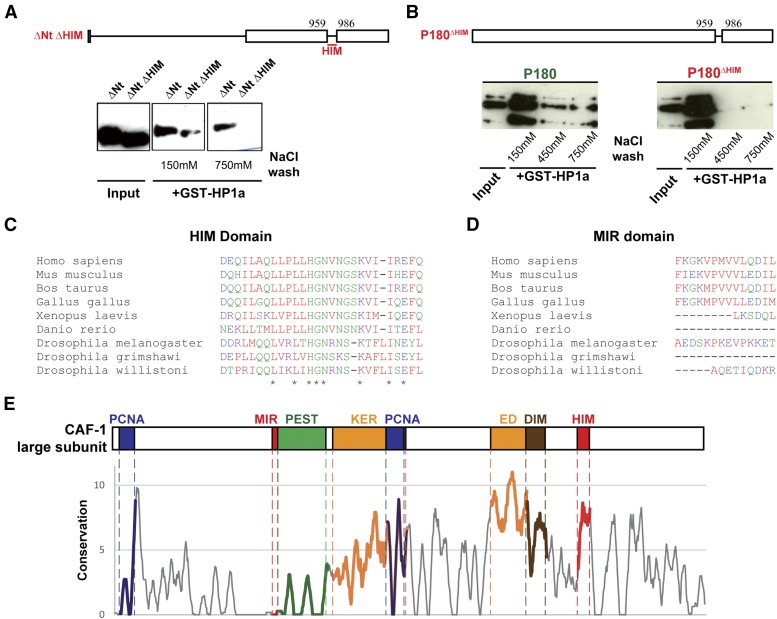
We next searched for a peptide motif in the P180 protein sequence which could mediate its interaction with the chromo shadow domain of HP1a. Surprisingly, P180 lacks the canonical consensus PXVXL motif found in many HP1-interacting proteins, including vertebrate CAF-1 large subunits (Murzina et al. 1999; Smothers and Henikoff 2000). P180 also does not contain the HP1-interacting peptide motif of the heterochromatin protein HP2 (Stephens et al. 2005), indicating that the direct interaction between P180 and HP1a in Drosophila was mediated through a domain not previously described. As we were able to detect a direct interaction between recombinant P180 and HP1a, we performed additional GST pull-down experiments on truncated versions of P180 expressed in bacteria and identified, in the C-terminal, a 27-amino acid motif likely involved in this interaction (Figure S1 and Figure 3A). Further experiments using a full-length P180 protein lacking only the identified 27 residues confirmed that this domain, the HIM domain, was required for P180 to interact with HP1a in vitro under stringent conditions (450 mM NaCl) (Figure 3, A and B). Of note, these stringent conditions are commonly used to detect specific interactions with HP1 proteins (Maison et al. 2002). Furthermore, many cells and tissues have been reported to have a high nuclear sodium and potassium concentrations, up to 250 mM NaCl and 280 mM KCl in frog oocyte nuclei (Naora et al. 1962; Hooper and Dick 1976; Moore and Morrill 1976), and such stringent salt conditions can therefore be achieved in the nucleus. Altogether, these observations indicate that the robust interaction we observed in vitro between the CAF-1 Drosophila large subunit and HP1a involves both the hinge and chromo shadow domain of HP1a and requires the HIM domain, a short domain of 27 residues localized in the C-terminal part of the CAF-1 large subunit.
Figure 3.
A conserved 27-residue domain is required for CAF-1 large subunit interaction with HP1a. (A) The same GST pull-down strategy described in Figure 1D was used to identify the residues critical for P180 interaction with HP1a. Truncation of the HIM, comprising residues 959–986, abolished the interaction between the C-terminal part of P180 and GST-HP1a after washing at high salt concentration. (B) The specificity of the interaction between GST-HP1a and the full length P180 with or without the HIM was then challenged by increasing the concentration of NaCl (150, 450, and 750 mM) in the washes. The presence of wild-type and mutant P180 proteins containing a poly-histidine tag at their C-terminal was determined in the bound fraction by western blotting using an anti-polyhistidine antibody. (C and D) Alignment of the (C) HIM and (D) MIR domains in three mammalian, three vertebrate nonmammalian, and three Drosophila species. Alignment was initially calculated on the whole orthologous protein sequences using the MUSCLE algorithm (Edgar 2004a,b). Coordinates were determined based on reported coordinates in Mus musculus for the MIR domain (Murzina et al. 1999) and D. melanogaster for the HIM (this study). (E) Conservation was computed across the selected species mentioned in (C and D) by averaging a numerical index reflecting the conservation of physico-chemical properties (Livingstone and Barton 1993) on an 11-residue-wide sliding window centered on the plot x coordinate. Coordinates of the domains identified in CAF-1 large subunit are based on the human sequence for the PEST, KER, ED (Kaufman et al. 1995), and dimerization domains (Quivy et al. 2001); on the mouse sequence for the MIR domain (Murzina et al. 1999) and PCNA interacting motifs (Rolef Ben-Shahar et al. 2009); and on D. melanogaster sequence for the HIM (this study).
The HIM is conserved in vertebrates and insects
We next assessed the HIM conservation by aligning available sequences of CAF-1 large subunits in various species (Figure S2 and Table S1). We noticed that the HIM was well conserved in insects and vertebrates (Figure 3C). The MIR domain, which contains the PXVXL motif and was identified as the domain mediating the interaction between the murine large subunit of CAF-1 and HP1β protein (Murzina et al. 1999), is conserved in mammals and the chicken (Takami et al. 2007), but is lacking in Xenopus or zebrafish (Figure 3D). In Drosophila species, the MIR domain is either completely lacking (Drosophila grimshawi) or highly divergent (D. willistoni and D. melanogaster). Interestingly, when conservation was assessed along the whole length of the protein using a method computing the conservation of physico-chemical properties in the alignment (Livingstone and Barton 1993), the HIM domain clearly corresponded to a local maximum of conservation with a score comparable to other domains previously identified as important for the function of the large subunit of CAF-1 in replication-coupled nucleosome assembly, such as the acidic KER and ED domains, which are thought to interact with the histones (Kaufman et al. 1995); the dimerization domain (Quivy et al. 2001); or the PCNA interacting motifs (Shibahara and Stillman 1999; Moggs et al. 2000) (Figure 3E). In contrast, in this same analysis, the MIR domain was not highlighted, confirming its poor conservation.
The HIM domain of P180 is not essential for viability
We then intended to test the functional significance of the HIM in vivo and constructed, using the Gal4/UAS system (Brand and Perrimon 1993), a strain expressing a mutant version of P180, P180ΔHIM, deleted for the 27 residues identified previously (Figure 4A). Interestingly, ubiquitous expression of P180ΔHIM using an Act5C-Gal4 driver rescued the viability of p1803 mutant males at least as efficiently as the wild-type P180 (Figure 4B). We had previously reported that maternally provided P180 is undetectable by the end of embryonic development (Klapholz et al. 2009) and therefore the transgene is the sole source of P180 in the rescued p1803 hemizygous males at least from larval development onwards. Importantly, the rescued individuals showed no obvious developmental defects and in particular none that could resemble the defects previously described upon downregulation of any of the CAF-1 subunits (Huang et al. 2010; Yu et al. 2013). These observations indicate that the HIM domain of P180 is largely dispensable for Drosophila larval development and supports a model in which the ability to interact with HP1a is not an essential feature of P180, at least after embryogenesis. The P180ΔHIM construct can therefore be used to directly test in vivo the role of the interaction between the large subunit of CAF-1 and HP1a during these stages, without affecting CAF-1 essential functions.
The HIM domain of P180 is required for P180-induced modification of PEV
In a previous study, mutant alleles of p180 were shown to act as dominant suppressors of PEV, thereby highlighting the role of P180 in the maintenance of heterochromatin (Huang et al. 2010). We therefore wondered whether transgenic expression of P180 or its mutant version, P180ΔHIM, was able to counteract this effect. As a reporter of PEV, we used the T(2;3)SbV translocation (hereafter referred to as SbV) which juxtaposes the dominant Sb1 mutation and a portion of the centric heterochromatin of the second chromosome. This induces mosaic flies with short (stubble) or long (normal) bristles which can be rigorously quantified. Changing the genetic context from wild type to p1803/+ in SbV flies induced a significant increase in the frequency of short bristles (Figure 4C, compare the blue and the red histograms), indicating that this allele also behaves as a dominant suppressor of PEV. Interestingly, this suppression of PEV was rescued, albeit partially, by Gal4-induced expression of wild-type P180 (Figure 4C, compare red and yellow histograms), while Gal4-induced expression of the P180ΔHIM mutant did not rescue and indeed further suppressed the variegation in a p1803/+ background (compare the red and orange histograms). In contrast, expression of either the wild-type or the HIM-depleted versions of P180 in flies with two wild-type copies of p180 induced only a mild suppression of SbV variegation of similar magnitude (Figure S3). Altogether, these observations support a role for the HIM domain of P180 in p1803-induced suppression of variegation.
The HIM domain of P180 contributes to heterochromatin-mediated pairing in oocytes
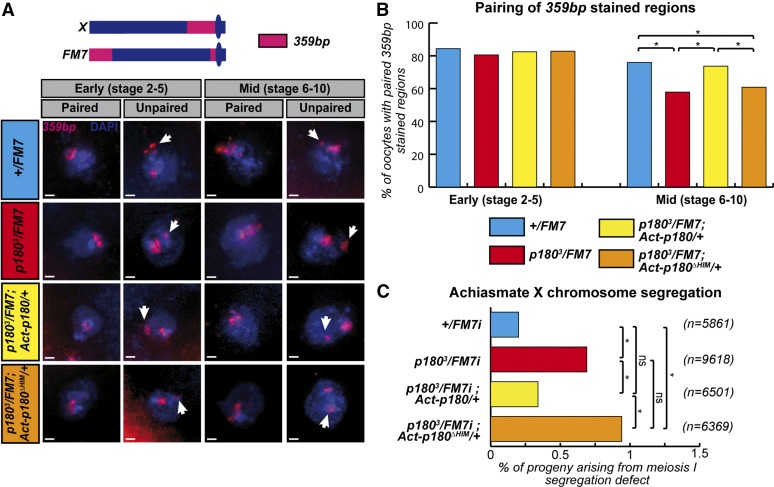
In Drosophila oocytes, homologous chromosomes remain associated along their pericentromeric regions throughout meiotic prophase and, when homologs have failed to form crossovers during meiotic recombination, this persisting association promotes their correct segregation according to the homologous achiasmate segregation system (Dernburg et al. 1996; Karpen et al. 1996). This pairing process relies on the integrity of pericentric regions that are heterochromatic and, accordingly, mutations in components of heterochromatin have altered levels of achiasmate segregation (Verni et al. 2000; Peng and Karpen 2009; Subramanian and Bickel 2009). More specifically, a recent study was able to demonstrate that partial depletion of HP1a, or its paralog Rhino in germ cells, induced defects in maintenance of pericentric associations of a pair of achiasmate X chromosomes, which resulted in elevated segregation defects (Giauque and Bickel 2016). We therefore aimed to test the functional impact of the loss of the HIM domain of P180 in this process and analyzed the pairing status of heterochromatic repeats of an achiasmate pair composed of a wild-type X and a FM7 balancer chromosome in females expressing various doses of wild-type or HIM-deleted versions of P180.
We first compared pairing efficiencies between wild-type and p1803 heterozygous females. FISH using a probe specific for the pericentric heterochromatin sequences of the wild-type X chromosome (359-bp repeats) indicated that oocytes produced by females carrying one wild-type and one p1803 allele were significantly impaired in maintaining the pairing of X chromosome-heterochromatic 359-bp sequences during oogenesis when compared to their wild-type counterparts (Figure 5, A and B, compare red and blue histograms in midstage egg chambers). In the FM7 chromosome, the heterochromatic 359-bp repeats are split in a large subtelomeric region and a smaller pericentromeric region (Figure 5A). The defects in pairing could thus involve only one of those sequences or both. By assessing the size of the unpaired sequence, which allows distinguishing defects in pairing involving either the subtelomeric (large FISH signal) or the pericentric sequences (small FISH signal), we observed that the defects in pairing observed upon reduction in the dose of P180 affect both sequences in proportions correlated to their respective sizes (see Figure S4 for quantification).
Figure 5.
The HIM domain participates in heterochromatin-mediated pairing in germ cells. (A) FISH staining on oocytes allows the detection of X pericentric regions (359 bp) and total DNA (DAPI) in females of the indicated genotype. Based on previously described detection of synaptonemal complex components (Resnick et al. 2009), two different regions of the ovariole were analyzed: the early region from vitelline stage 2–5 in which the synaptonemal complex, maintaining aligned homologs, is fully assembled; and the middle region containing egg chambers from stage 6–10, in which the synaptonemal complex is undergoing disassembly and therefore likely requires additional mechanisms to maintain pericentric interactions. Scale bar represents 1 μm. (B) Pairing of 359-bp regions of homologous X chromosomes was quantified in females of the indicated genotype. Numbers of oocytes scored for stages 2–5 (early): +/FM7i, 64; p1803/FM7i, 68; p1803/FM7;Act-p180/+, 80; and p1803/FM7i;Actp180ΔHIM/+, 126. Number of oocytes scored for stages 6–10 (late): +/FM7i, 75; p1803/FM7i, 63; p1803/FM7;Act-p180/+, 57; and p1803/FM7i;Actp180ΔHIM/+, 99. (C) Rate of exceptional oocytes arising from X chromosome-segregation defects during meiosis I, measured in females of the indicated genotype. In this experiment, due to heterozygosity of the females for the FM7i balancer chromosome, X chromosomes fail to recombine and therefore segregate according to the homologous achiasmate system. Differences are indicated to be statistically significant (*) or not significant (ns) using Fisher’s exact test (P ≤ 0.05).
As some of these pairing events have been proposed to contribute to the correct segregation of homologous achiasmate chromosomes, we analyzed this process in p1803 heterozygous females. In agreement with their pairing defects, these females produced oocytes with a modest but significant increase in the segregation defects of this pair of nonrecombining chromosomes (Figure 5C, compare red and blue histograms). These segregation defects are specific to nonrecombining chromosomes: similar defects were observed with achiasmate chromosomes II and IV but were not observed with chiasmate chromosomes II (Figure S5).
Importantly, ubiquitous expression of wild-type P180 but not P180ΔHIM, using the Gal4/UAS system, was able to rescue both the pairing maintenance defects and segregation defects of oocytes produced by p1803 heterozygous females (Figure 5, A–C, compare yellow and orange histograms). This argues that the HIM domain, which is central to the direct interaction between HP1a and the large subunit of CAF-1, is also important for the maintenance of pericentric pairing during meiotic prophase; a process relying on proper HP1a loading and function (Giauque and Bickel 2016).
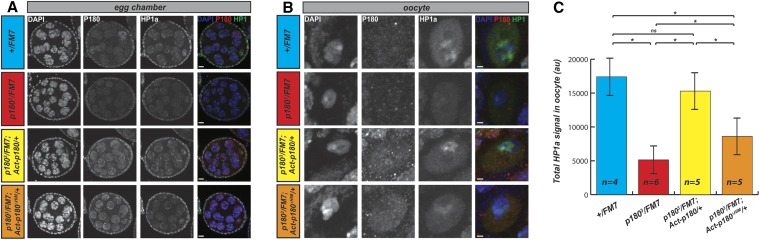
The HIM domain of P180 contributes to the loading of HP1 at the oocyte chromocenter
One possible way P180 could contribute to heterochromatin maintenance would be by regulating the loading of HP1a to chromosomes. As we identified the HIM as an important domain for the interaction between P180 and HP1a, we tested this possibility by analyzing the in vivo distribution of P180 and HP1a in egg chambers isolated from females expressing a wild-type dose of p180. We observed a robust P180 signal in nurse cell nuclei (Figure 6A, second column, blue genotype) but not in oocytes (Figure 6B, second column, blue genotype). As nurse cells are actively replicating and oocytes are postreplicative and undergoing the meiotic prophase program, this observation is consistent with staining on salivary gland polytene chromosomes indicating that P180 localizes to the chromatin during S phase. In contrast, P180 expression was detected at similar levels in oocytes from females expressing the wild-type or the HIM-depleted transgene (Figure 6B, second column, yellow and orange genotypes); indicating that at least some degree of P180 accumulation during S phase is due to transcriptional regulation, which is not recapitulated by the ubiquitous Act5C-Gal4 driver. As the samples were prepared, stained, and imaged under identical conditions, we could also compare the overall levels of HP1a accumulated in the oocyte nucleus in the different genotypes. We could robustly observe that levels of HP1a were reduced in oocytes of p1803 heterozygous females when compared to females with two wild-type alleles of p180 (Figure 6, B and C, third column, compare blue and red genotypes). This decrease was rescued by transgenic expression of the wild-type P180 (Figure 6, B and C, third column, yellow genotype) whereas expression of the HIM-depleted P180 protein resulted in only partial rescue (Figure 6, B and C, third column, orange genotype). Similar trends were observed on the relative level of HP1a in nurse cells, depending on the genotype (Figure S6). Thus the HIM-depleted P180 appears defective in loading HP1 in the DAPI-dense regions of the oocyte nucleus. Altogether, these experiments indicate that P180 is required for the loading or maintenance of HP1a in germ cells and that the HIM domain contributes to this effect.
Figure 6.
Altered loading of HP1a in germ-cells of females expressing either low dose of P180 or P180ΔHIM. (A and B) Immunodetection of P180, HP1a, and DNA (DAPI) on (A) egg chambers and with higher magnification on the (B) oocytes obtained from females of the indicated genotype. Images are maximum intensity projections of all the stacks containing the oocyte. Scale bar represents 5 μm (A) and 1 μm (B). (C) Quantification of total HP1a signal in n oocytes nuclei of the indicated genotype. Settings for acquisition and processing were similar between all analyzed genotypes and fluorescence is therefore quantified using a scale of arbitrary unit (au) that is identical across all genotypes. Differences are indicated to be statistically significant (*) using Fisher’s exact test (P < 0.05) and Holm P-value correction for multiple comparisons. ns, nonsignificant.
Discussion
The CAF-1 complex was originally isolated and characterized as a histone chaperone that assembles histone H3-H4 dimers onto newly synthesized DNA (Smith and Stillman 1989). Further studies showed that the interaction between the large subunit of CAF-1 and the HP1 proteins was required for the proper duplication and maintenance of heterochromatin regions in mitotically dividing cells (Dohke et al. 2008; Quivy et al. 2008). These two functions of CAF-1's large subunit, which involve different levels of chromatin organization, are also conserved in Drosophila. First, the large subunit of CAF-1 is involved in H3-H4 dimer deposition during DNA replication (Tyler et al. 2001): this function was shown to be essential for larval development and viability (Song et al. 2007; Klapholz et al. 2009). Second, CAF-1's large subunit interacts with HP1a (Huang et al. 2010). We have shown that this interaction is direct and have identified the HIM domain in CAF-1's large subunit required for this interaction. Interestingly, flies expressing only a mutant form of CAF-1's large subunit deleted for the HIM are viable. This indicates that the ability of CAF-1's large subunit to interact with HP1a is not strictly required for viability in flies and that additional mechanisms, such as the one involving the interaction between HP1a and the H3.3 chaperone XNP (Bassett et al. 2008), might contribute to proper heterochromatin formation or maintenance and ensure successful development. As this function is essential in mammalian cells (Houlard et al. 2006; Quivy et al. 2008), D. melanogaster represents a unique model to understand its role during development.
Interestingly, although we could obtain individuals, males and females, expressing only the HIM-deleted forms of CAF-1 large subunit, we realized that the HIM deletion resulted in female sterility (Marie Clémot, unpublished data). This could indicate either that the HIM domain is strictly required for some aspect of fly reproduction, either via its role in heterochromatin maintenance or via some other functions of CAF-1's large subunit, or that the HIM is required during embryogenesis for the initial formation of heterochromatin structure, a possibility we could not directly test because of the important maternal contribution and the inability of p1803 germline clones to develop into mature oocytes (Klapholz et al. 2009).
Our work also provides new insights into the mechanisms of heterochromatin maintenance: previous studies in S. pombe and mammalian cells had led to propose a model in which CAF-1 is recruited to the replication forks through its interaction with PCNA and transfers HP1 to the replicated chromatin at these sites to locally maintain the chromatin structure (Quivy et al. 2004; Dohke et al. 2008; Quivy et al. 2008). Consistently, we only detected colocalization of CAF-1's large subunit with HP1a on salivary gland polytene chromosomes upon replication of the HP1a-enriched regions. However, although it is now clearly established that CAF-1 participates in the maintenance of heterochromatin in eukaryotes, very little is known on the contribution of the different domains to this function. In mammals, the MIR domain, which contains a PXVXL motif, is essential for heterochromatin maintenance (Quivy et al. 2004; Dohke et al. 2008; Quivy et al. 2008). The MIR domain is not conserved in the yeasts S. cerevisiae and S. pombe, although a role in heterochromatin maintenance has been reported for CAF-1's large subunits (Monson et al. 1997; Dohke et al. 2008). Similarly, in chicken cultured cells, the MIR domain does not seem to be involved in heterochromatin maintenance since a point mutant in the PXVXL motif could support normal cell proliferation with no detectable defects in heterochromatin structure (Takami et al. 2007). In this study, we coupled biochemistry, genetics, and cytology to identify the first domain of Drosophila CAF-1's large subunit involved in heterochromatin maintenance. Although our data suggest a similar role for the MIR and the HIM domains in transferring the HP1 protein upon replication of heterochromatin regions, further studies are required to elucidate the precise molecular mechanisms by which the HIM domain contributes to the maintenance of heterochromatin and to determine whether it is functionally conserved in other species.
Acknowledgments
We thank Geneviève Almouzni, Valérie Borde, Jean-Pierre Quivy, Edith Heard, Angela Taddei, Aude Porcher, and all members of the Unité Mixte de Recherche 3664 for discussions. We are grateful to the Bloomington Stock Center, Developmental Studies Hybridoma Bank, Stéphane Ronsseray, and Jessica Tyler for providing reagents. We also gratefully acknowledge Fabiana Heredia for the polytene chromosome staining and Patricia Le Baccon and the Plateforme d'Imagerie Cellulaire et Tissulaire - Infrastructures en Biologie Santé et Agronomie PICT-IBiSA of the Institut Curie. This work was supported by funds from Association pour la Recherche Contre le Cancer (ARC) (3735); Electricité de France (EDF,32030) Ligue Nationale Contre le Cancer (LNCC, 24059); the “Investissements d'Avenir” launched by the French Government and implemented by ANR with the references ANR-11-LABX-0044 Development, Epigenesis, Epigenetics and life-time Potential Laboratoire d’Excellence and Paris Sciences et Lettres ANR-10-IDEX-0001-02. B.R. was supported by Fondation pour la Recherche Médicale (FRM); B.K. was supported by ARC and FRM.
Footnotes
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.190785/-/DC1.
Communicating editor: J. Sekelsky
Literature Cited
- Bassett A. R., Cooper S. E., Ragab A., Travers A. A., 2008. The chromatin remodelling factor dATRX is involved in heterochromatin formation. PLoS One 3: e2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Dernburg A. F., Sedat J. W., Hawley R. S., 1996. Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell 86: 135–146. [DOI] [PubMed] [Google Scholar]
- Dohke K., Miyazaki S., Tanaka K., Urano T., Grewal S. I., et al. , 2008. Fission yeast chromatin assembly factor 1 assists in the replication-coupled maintenance of heterochromatin. Genes Cells 13: 1027–1043. [DOI] [PubMed] [Google Scholar]
- Edgar R. C., 2004a MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C., 2004b MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg J. C., Morris G. D., Reuter G., Hartnett T., 1992. The heterochromatin-associated protein HP-1 is an essential protein in Drosophila with dosage-dependent effects on position-effect variegation. Genetics 131: 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard P. H., Martini E. M., Kaufman P. D., Stillman B., Moustacchi E., et al. , 1996. Chromatin assembly coupled to DNA repair: a new role for chromatin assembly factor I. Cell 86: 887–896. [DOI] [PubMed] [Google Scholar]
- Giauque C. C., Bickel S. E., 2016. Heterochromatin-associated proteins HP1a and Piwi collaborate to maintain the association of achiasmate homologs in Drosophila oocytes. Genetics 203: 173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper G., Dick D. A., 1976. Nonuniform distribution of sodium in the rat hepatocyte. J. Gen. Physiol. 67: 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins R. A., Carlson J. W., Kennedy C., Acevedo D., Evans-Holm M., et al. , 2007. Sequence finishing and mapping of Drosophila melanogaster heterochromatin. Science 316: 1625–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlard M., Berlivet S., Probst A. V., Quivy J. P., Hery P., et al. , 2006. CAF-1 is essential for heterochromatin organization in pluripotent embryonic cells. PLoS Genet. 2: e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T., Brutlag D., 1979. Sequence and sequence variation within the 1.688 g/cm3 satellite DNA of Drosophila melanogaster. J. Mol. Biol. 135: 465–481. [DOI] [PubMed] [Google Scholar]
- Huang H., Yu Z., Zhang S., Liang X., Chen J., et al. , 2010. Drosophila CAF-1 regulates HP1-mediated epigenetic silencing and pericentric heterochromatin stability. J Cell Sci. 123: 2853–2861. [DOI] [PubMed] [Google Scholar]
- James T. C., Elgin S. C., 1986. Identification of a nonhistone chromosomal protein associated with heterochromatin in Drosophila melanogaster and its gene. Mol. Cell. Biol. 6: 3862–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James T. C., Eissenberg J. C., Craig C., Dietrich V., Hobson A., et al. , 1989. Distribution patterns of HP1, a heterochromatin-associated nonhistone chromosomal protein of Drosophila. Eur. J. Cell Biol. 50: 170–180. [PubMed] [Google Scholar]
- Karpen G. H., Le M. H., Le H., 1996. Centric heterochromatin and the efficiency of achiasmate disjunction in Drosophila female meiosis. Science 273: 118–122. [DOI] [PubMed] [Google Scholar]
- Kaufman P. D., Kobayashi R., Kessler N., Stillman B., 1995. The P150 and P60 subunits of chromatin assembly factor I: a molecular link between newly synthesized histones and DNA-replication. Cell 81: 1105–1114. [DOI] [PubMed] [Google Scholar]
- Kellum R., 2003. HP1 complexes and heterochromatin assembly. Curr. Top. Microbiol. Immunol. 274: 53–77. [DOI] [PubMed] [Google Scholar]
- Klapholz B., Dietrich B. H., Schaffner C., Heredia F., Quivy J. P., et al. , 2009. CAF-1 is required for efficient replication of euchromatic DNA in Drosophila larval endocycling cells. Chromosoma 118: 235–248. [DOI] [PubMed] [Google Scholar]
- Krude T., 1995. Chromatin assembly factor 1 (CAF-1) colocalizes with replication foci in HeLa cell nuclei. Exp. Cell Res. 220: 304–311. [DOI] [PubMed] [Google Scholar]
- Livingstone C. D., Barton G. J., 1993. Protein sequence alignments: a strategy for the hierarchical analysis of residue conservation. Comput. Appl. Biosci. 9: 745–756. [DOI] [PubMed] [Google Scholar]
- Maison C., Bailly D., Peters A. H., Quivy J. P., Roche D., et al. , 2002. Higher-order structure in pericentric heterochromatin involves a distinct pattern of histone modification and an RNA component. Nat. Genet. 30: 329–334. [DOI] [PubMed] [Google Scholar]
- Mason J. M., Frydrychova R. C., Biessmann H., 2008. Drosophila telomeres: an exception providing new insights. Bioessays 30: 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim K. S., Green-Marroquin B. L., Sekelsky J. J., Chin G., Steinberg C., et al. , 1998. Meiotic synapsis in the absence of recombination. Science 279: 876–878. [DOI] [PubMed] [Google Scholar]
- McKim K. S., Joyce E. F., Jang J. K., 2009. Cytological analysis of meiosis in fixed Drosophila ovaries. Methods Mol. Biol. 558: 197–216. [DOI] [PubMed] [Google Scholar]
- Moggs J. G., Grandi P., Quivy J. P., Jonsson Z. O., Hubscher U., et al. , 2000. A CAF-1-PCNA-mediated chromatin assembly pathway triggered by sensing DNA damage. Mol. Cell. Biol. 20: 1206–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson E. K., de Bruin D., Zakian V. A., 1997. The yeast Cac1 protein is required for the stable inheritance of transcriptionally repressed chromatin at telomeres. Proc. Natl. Acad. Sci. USA 94: 13081–13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. D., Morrill G. A., 1976. A possible mechanism for concentrating sodium and potassium in the cell nucleus. Biophys. J. 16: 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murzina N., Verreault A., Laue E., Stillman B., 1999. Heterochromatin dynamics in mouse cells: interaction between chromatin assembly factor 1 and HP1 proteins. Mol. Cell 4: 529–540. [DOI] [PubMed] [Google Scholar]
- Naora H., Naora H., Izawa M., Allfrey V. G., Mirsky A. E., 1962. Some observations on differences in composition between the nucleus and cytoplasm of the frog oocyte. Proc. Natl. Acad. Sci. USA 48: 853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paro R., 2000. Mapping protein distributions on polytene chromosomes by immunostaining, pp. 131–139 in Drosophila Protocols, edited by Sullivan W., Ashburner M., Hawley R. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Peng J. C., Karpen G. H., 2009. Heterochromatic genome stability requires regulators of histone H3 K9 methylation. PLoS Genet. 5: e1000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quivy J. P., Grandi P., Almouzni G., 2001. Dimerization of the largest subunit of chromatin assembly factor 1: importance in vitro and during Xenopus early development. EMBO J. 20: 2015–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quivy J. P., Roche D., Kirschner D., Tagami H., Nakatani Y., et al. , 2004. A CAF-1 dependent pool of HP1 during heterochromatin duplication. EMBO J. 23: 3516–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quivy J. P., Gerard A., Cook A. J., Roche D., Almouzni G., 2008. The HP1-p150/CAF-1 interaction is required for pericentric heterochromatin replication and S-phase progression in mouse cells. Nat. Struct. Mol. Biol. 15: 972–979. [DOI] [PubMed] [Google Scholar]
- Resnick T. D., Dej K. J., Xiang Y., Hawley R. S., Ahn C., et al. , 2009. Mutations in the chromosomal passenger complex and the condensin complex differentially affect synaptonemal complex disassembly and metaphase I configuration in Drosophila female meiosis. Genetics 181: 875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolef Ben-Shahar T., Castillo A. G., Osborne M. J., Borden K. L., Kornblatt J., et al. , 2009. Two fundamentally distinct PCNA interaction peptides contribute to chromatin assembly factor 1 function. Mol. Cell. Biol. 29: 6353–6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorth P., 1998. Gal4 in the Drosophila female germline. Mech. Dev. 78: 113–118. [DOI] [PubMed] [Google Scholar]
- Schulze S. R., Sinclair D. A., Fitzpatrick K. A., Honda B. M., 2005. A genetic and molecular characterization of two proximal heterochromatic genes on chromosome 3 of Drosophila melanogaster. Genetics 169: 2165–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara K., Stillman B., 1999. Replication-dependent marking of DNA by PCNA facilitates CAF-1-coupled inheritance of chromatin. Cell 96: 575–585. [DOI] [PubMed] [Google Scholar]
- Simpson-Brose M., Treisman J., Desplan C., 1994. Synergy between the hunchback and bicoid morphogens is required for anterior patterning in Drosophila. Cell 78: 855–865. [DOI] [PubMed] [Google Scholar]
- Smith S., Stillman B., 1989. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell 58: 15–25. [DOI] [PubMed] [Google Scholar]
- Smothers J. F., Henikoff S., 2000. The HP1 chromo shadow domain binds a consensus peptide pentamer. Curr. Biol. 10: 27–30. [DOI] [PubMed] [Google Scholar]
- Song Y., He F., Xie G., Guo X., Xu Y., et al. , 2007. CAF-1 is essential for Drosophila development and involved in the maintenance of epigenetic memory. Dev. Biol. 311: 213–222. [DOI] [PubMed] [Google Scholar]
- Stephens G. E., Slawson E. E., Craig C. A., Elgin S. C., 2005. Interaction of heterochromatin protein 2 with HP1 defines a novel HP1-binding domain. Biochemistry 44: 13394–13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian V. V., Bickel S. E., 2009. Heterochromatin-mediated association of achiasmate homologs declines with age when cohesion is compromised. Genetics 181: 1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Wahlstrom J., Karpen G., 1997. Molecular structure of a functional Drosophila centromere. Cell 91: 1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei A., Roche D., Sibarita J. B., Turner B. M., Almouzni G., 1999. Duplication and maintenance of heterochromatin domains. J. Cell Biol. 147: 1153–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami Y., Ono T., Fukagawa T., Shibahara K., Nakayama T., 2007. Essential role of chromatin assembly factor-1-mediated rapid nucleosome assembly for DNA replication and cell division in vertebrate cells. Mol. Biol. Cell 18: 129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B. M., Birley A. J., Lavender J., 1992. Histone H4 isoforms acetylated at specific lysine residues define individual chromosomes and chromatin domains in Drosophila polytene nuclei. Cell 69: 375–384. [DOI] [PubMed] [Google Scholar]
- Tyler J. K., Collins K. A., Prasad-Sinha J., Amiott E., Bulger M., et al. , 2001. Interaction between the Drosophila CAF-1 and ASF1 chromatin assembly factors. Mol. Cell. Biol. 21: 6574–6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verni F., Gandhi R., Goldberg M. L., Gatti M., 2000. Genetic and molecular analysis of wings apart-like (wapl), a gene controlling heterochromatin organization in Drosophila melanogaster. Genetics 154: 1693–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y., Hawley R. S., 2006. The mechanism of secondary nondisjunction in Drosophila melanogaster females. Genetics 174: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Wu H., Chen H., Wang R., Liang X., et al. , 2013. CAF-1 promotes Notch signaling through epigenetic control of target gene expression during Drosophila development. Development 140: 3635–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Li H., Schweppe N. M., Hawley R. S., Gilliland W. D., 2010. Statistical analysis of nondisjunction assays in Drosophila. Genetics 186: 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains are available upon request. Table S1 contains accession numbers for P180 orthologs used for conservation analysis.